Abstract
Background
Middle meningeal artery (MMA) embolization is an apparently efficacious minimally invasive treatment for nonacute subdural hematomas (NASHs), but how different embolisates affect outcomes remains unclear. Our objective was to compare radiographic and clinical outcomes after particle or liquid MMA embolization.
Methods
Patients who had MMA embolization for NASH were retrospectively identified from a multi-institution database. The primary radiographic and clinical outcomes—50% NASH thickness reduction and need for surgical retreatment within 90 days, respectively—were compared for liquid and particle embolizations in patients treated 1) without surgical intervention (upfront), 2) after recurrence, or 3) with concomitant surgery (prophylactic).
Results
The upfront, recurrent, and prophylactic subgroups included 133, 59, and 16 patients, respectively. The primary radiographic outcome was observed in 61.8%, 61%, and 72.7% of particle-embolized patients and 61.3%, 55.6%, and 20% of liquid-embolized patients, respectively (p = 0.457, 0.819, 0.755). Hazard ratios comparing time to reach radiographic outcome in the particle and liquid groups or upfront, recurrent, andprophylactic timing were 1.31 (95% CI 0.78–2.18; p = 0.310), 1.09 (95% CI 0.52–2.27; p = 0.822), and 1.5 (95% CI 0.14–16.54; p = 0.74), respectively. The primary clinical outcome occurred in 8.0%, 2.4%, and 0% of patients who underwent particle embolization in the upfront, recurrent, and prophylactic groups, respectively, compared with 0%, 5.6%, and 0% who underwent liquid embolization (p = 0.197, 0.521, 1.00).
Conclusions
MMA embolization with particle and liquid embolisates appears to be equally effective in treatment of NASHs as determined by the percentage who reach, and the time to reach, 50% NASH thickness reduction and the incidence of surgical reintervention within 90 days.
Keywords: Chronic subdural hematoma, liquid embolization, middle meningeal artery embolization, nonacute subdural hematoma, particle embolization
Introduction
The incidence of nonacute subdural hematomas (NASHs) is rising as the population ages and the use of antiplatelet and anticoagulant treatment grows concomitantly.1,2 Middle meningeal artery (MMA) embolization has gained traction as an off-label minimally invasive treatment alternative with reportedly similar efficacy to conventional surgical management in retrospective studies.3–7
Many questions remain regarding technical aspects of MMA embolization for NASH treatment, including whether a particular embolisate is most effective. Most studies of MMA embolization to date have shown success using polyvinyl alcohol (PVA) microparticles3,4 (or other particle-based embolisates), whereas fewer small series have reported efficacious treatment with commercially available liquid embolisates.8–10 The choice between particle or liquid embolisates is highly subjective because they have not yet been compared directly in a clinical study. In this exploratory study, our objective was to characterize radiographic and clinical outcomes in patients treated with either particle or liquid embolisates for MMA embolization of NASHs to determine whether either led to better patient outcomes. We hypothesized that there would be no difference in radiographic or clinical outcomes as a result of the type of embolisate used.
Methods
We retrospectively reviewed a prospectively maintained multi-institutional database (15 US academic centers) of patients who had MMA embolization of the frontal and/or parietal branches to treat NASH in November 2017-August 2021. Each center submits all patients who undergo MMA embolization to the database, which thus is continually accruing patients. As such, some patients may have been included in more than one retrospective study from this database. The criteria for database inclusion are presence of NASH and treatment with MMA embolization. No patients that underwent MMA embolization from these centers were excluded. Each center had Institutional Review Board approval to collect and review data with waivers of patient consent.
The type of embolisate used was at the discretion of the treating provider. Particle agents included PVA Foam Particles (Cook Medical, Bloomington, IN), Contour PVA Particles (Boston Scientific, Marlborough, MA), and Embosphere Microspheres (Merit Medical, South Jordan, UT), whereas liquid agents included Trufill N-butyl cyanoacrylate glue (NBCA, Cerenovus, Raynham, MA) and the Onyx Liquid Embolic System (Medtronic Neurovascular, Irvine, CA). Detailed descriptions of MMA embolization have been reported elsewhere.3,7 The radiographic and clinical follow-up varied somewhat, but follow-up at 1 day, 2 weeks, 6 weeks, and 90 days was used as the standard.
The entire patient cohort was divided into three subgroups, depending on treatment stage, to eliminate the effect of treatment timing on outcome. Subgroups based on treatment stage were defined as: 1) MMA embolization as the first NASH treatment without concomitant surgical intervention (upfront), 2) MMA embolization for recurrence after prior surgical evacuation of the same NASH (>7 days; recurrent), and 3) MMA embolization concomitantly with surgical evacuation (<7 days; prophylactic). Baseline patient demographics, hematoma radiographic variables, preoperative use of anticoagulation and antiplatelet agents, and procedural factors were recorded for each subgroup. The three subgroups were then assessed to determine the effect of embolisate used on radiographic and clinical outcomes at follow-up.
Outcomes
The primary radiographic outcome measure was 50% radiographic reduction in maximal NASH thickness on computed tomography (CT) imaging at 90 days. This has previously been reported as a metric of treatment success.4,6,11–14 NASH size was measured on each CT (preoperative and at the follow-up intervals) retrospectively in an unblinded fashion by a single independent evaluator at each center. A survival analysis was performed to identify whether the time from treatment to reaching 50% radiographic reduction occurred more quickly with either type of embolisate. The primary clinical outcome was need for subsequent surgical intervention for NASH retreatment because of clinical deterioration, defined as any change in neurologic or functional status that led to retreatment of the NASH, or NASH radiographic worsening, defined as any increase in NASH size that led to retreatment, within 90 days. Clinical and radiographic deterioration were based solely on assessment by the treating physician. Complications within 90 days were assessed as a secondary outcome.
Statistical analysis
The primary outcomes were treated as dichotomous variables. Comparisons between the treatment types within each of the three treatment-stage subgroups were done using the Chi-square test or Fisher's exact test, with a priori α = 0.05 used to determine significance. The survival analysis was performed using a Cox proportional hazards model in which time to primary radiographic outcome was compared between the treatments within each of the three treatment-stage subgroups. All statistical analyses were performed with Stata IC v 15.1 (StataCorp; College Station, TX). The study followed STROBE guidelines.
Results
Data from 208 patients, including 133, 59, and 16 patients treated with MMA embolization upfront, for recurrence, or prophylactically, respectively, were analyzed (Table 1).
Table 1.
Characteristics of patients who underwent MMA embolization with particle or liquid embolic agents.
| Variable | Upfront MMA embolization | Prior surgical evacuation | Concurrent surgery | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Particles (n = 102) | Liquid (n = 31) | P value | Particles (n = 41) | Liquid (n = 18) | P value | Particles (n = 11) | Liquid (n = 5) | P value | |
| Sex (male) | 65.7%, n = 67/102 | 77.4%, n = 24/31 | 0.218 | 80.5%, n = 33/41 | 88.9%, n = 16/18 | 0.428 | 100%, n = 11/11 | 80%, n = 4/5 | 0.126 |
| Age in years | 70.0 ± 13.3 (33–94) | 70.8 ± 15.3 (38–96) | 0.726 | 73.5 ± 11.7 (37–98) | 61.8 ± 17.7 (1.5–82) | 0.004 | 72.1 ± 11.8 (47–87) | 68.6 ± 4.4 (62–74) | 0.528 |
| White race | 69.2%, n = 65/94 | 79.3%, n = 23/29 | 0.289 | 79.4%, n = 27/34 | 100%, n = 18/18 | 0.039 | 72.7%, n = 8/11 | 80%, n = 4/5 | 0.755 |
| Radial access | 10.8%, n = 11/102 | 22.6%, n = 7/31 | 0.093 | 19.5%, n = 8/41 | 38.9%, n = 7/18 | 0.116 | 9.09%, n = 1/11 | 40%, n = 2/5 | 0.142 |
| General anesthesia | 47.1%, n = 48/102 | 35.5%, n = 11/31 | 0.256 | 46.3%, n = 19/41 | 27.8%, n = 5/18 | 0.181 | 72.7%, n = 8/11 | 20.0%, n = 1/5 | 0.049 |
| Duration (min) | 65.7 ± 26.0 (19–140) | 75 ± 35.6 (30–140) | 0.116 | 53.0 ± 18.0 (19–90) | 74.7 ± 26.1 (34–128) | <0.001 | 65.3 ± 29.9 (33–140) | 70.0 ± 46.4 (23–140) | 0.808 |
| Use of coils additionally | 65.7%, n = 67/102 | 3.23%, n = 1/31 | <0.001 | 53.7%, n = 22/41 | 11.1%, n = 2/18 | 0.003 | 45.5%, n = 5/11 | 0%, n = 0/5 | 0.119 |
| Patient on anticoagulation therapy | 22.6%, n = 23/102 | 22.6%, n = 7/31 | 0.997 | 22.0%, n = 9/41 | 11.1%, n = 2/18 | 0.325 | 0%, n = 0/11 | 40%, n = 2/5 | 0.025 |
| Patient on antiplatelet therapy | 35.6%, n = 36/101 | 25.8%, n = 8/31 | 0.309 | 25%, n = 10/40 | 23.5%, n = 4/17 | 0.805 | 45.5%, n = 5/11 | 40%, n = 2/5 | 0.838 |
| Embolization of both MMA branches | 76.2%, n = 77/101 | 82.8%, n = 24/29 | 0.457 | 75%, n = 30/40 | 77.8%, n = 14/18 | 0.819 | 72.7%, n = 8/11 | 80%, n = 4/5 | 0.755 |
| Technical success | 97.1%, n = 99/102 | 100%, n = 31/31 | 1.000 | 100%, n = 41/41 | 94.4%, n = 17/18 | 0.305 | 100%, n = 11/11 | 100%, n = 5/5 | 1.000 |
| Complications | 11.6%, n = 11/102 | 3.2%, n = 1/31 | 0.465 | 7.3%, n = 3/41 | 0%, n = 0/18 | 0.549 | 0%, n = 0/11 | 0%, n = 0/5 | 1.000 |
| Retreatments | 8.0%, n = 8/100 | 0%, n = 0/31 | 0.197 | 2.4%, n = 1/41 | 5.6%, n = 1/18 | 0.521 | 0%, n = 0/11 | 0%, n = 0/5 | 1.00 |
| 50% improvement at 90 days | 61.8%, n = 63/102 | 61.3%, n = 19/31 | 0.962 | 61.0%, n = 25/41 | 55.6%, n = 10/18 | 0.696 | 72.7%, n = 8/11 | 20%, n = 1/5 | 0.106 |
Data reported as % with n, or mean ± SD (range). Boldface type indicates statistical significance at p < 0.05.
MMA, middle meningeal artery.
Upfront MMA embolization
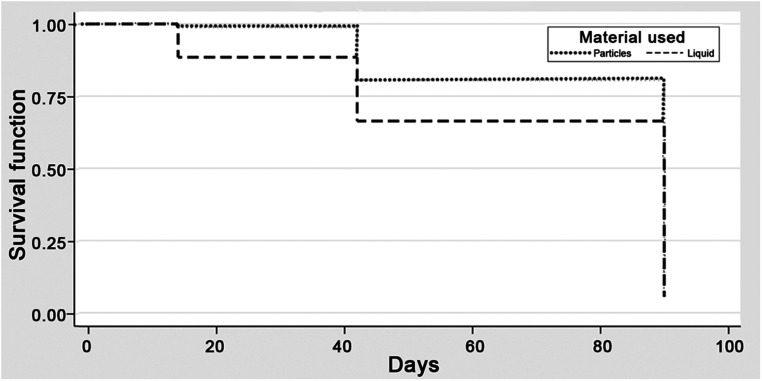
In the upfront subgroup, the radiographic primary outcome was reached in 63/102 (62%) and 19/31 (61%) patients in particle and liquid groups, respectively (p = 0.962, power 0.05) (Table 1). The hazard ratio (1.31, 95% CI 0.78–2.18, p = 0.310) demonstrated no difference in time to 50% reduction in NASH size between groups (Figure 1). Among patients who had clinical follow-up, surgical intervention for NASH retreatment occurred in 8/100 (8%) and 0/31 (0%) patients in the particle and liquid groups, respectively (p = 0.197, power 0.27).
Figure 1.
The Kaplan-Meier survival curve for the radiographic outcome—50% reduction in NASH size at 90 days—in the upfront subgroup comparing the particle and liquid embolic groups; there was no significant difference in the curves.
There were no differences in the age of the patients (p = 0.726), type of access used (radial vs. transfemoral) (p = 0.093), use of general anesthetic (p = 0.261), or procedure duration (p = 0.116). Both branches of the MMA were embolized in 77/101 particle-embolized patients (76.2%) and 24/29 liquid-embolized patients (82.8%) (p = 0.457) with data available. The liquid group was significantly less likely (1/31, 3%) to have coils used than the particle group (67/102, 65.7%) (p < 0.001).
Recurrent MMA embolization
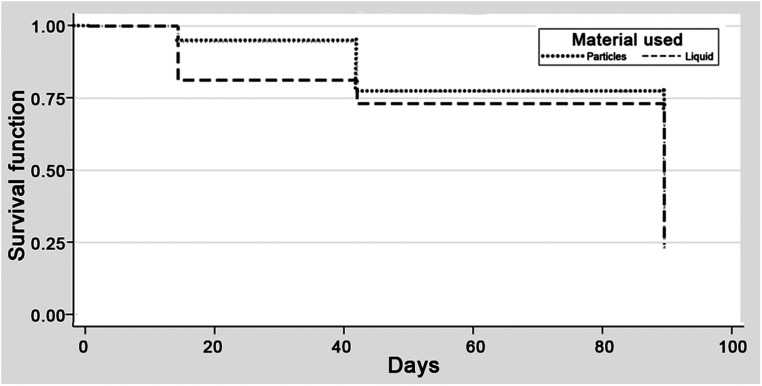
In the recurrent subgroup, the radiographic outcome was reached in 25/41 (61%) and 10/18 (56%) patients in particle and liquid groups, respectively (p = 0.696, power 0.07) (Table 1). The hazard ratio (1.09, 95% CI 0.52–2.27, p = 0.82) indicated no difference in time to primary radiographic outcome (Figure 2). Subsequent surgical intervention occurred in 1/41 (2.4%) and 1/18 (5.6%) patients, respectively (p = 0.521, power 0.14).
Figure 2.
The Kaplan-Meier survival curve for the radiographic outcome—50% reduction in NASH size at 90 days—in the recurrent subgroup demonstrating no significant difference between the particle and liquid embolic groups.
We found no differences in type of access (p = 0.116) or use of general anesthetic (p = 0.181). The procedure was significantly longer among patients treated with liquid embolisates (74.7 vs. 53.0 min, p < 0.001), and patients treated with particles were older (73.5 vs. 61.8 years, p = 0.004). Both MMA branches were embolized in 30/40 particle-embolized patients (75.0%) and 14/18 liquid-embolized patients (77.8%) with data available (p = 0.819). Coils were used significantly more often in the particle group (53.7%) than in the liquid group (11.1%) (p = 0.003).
Prophylactic MMA embolization
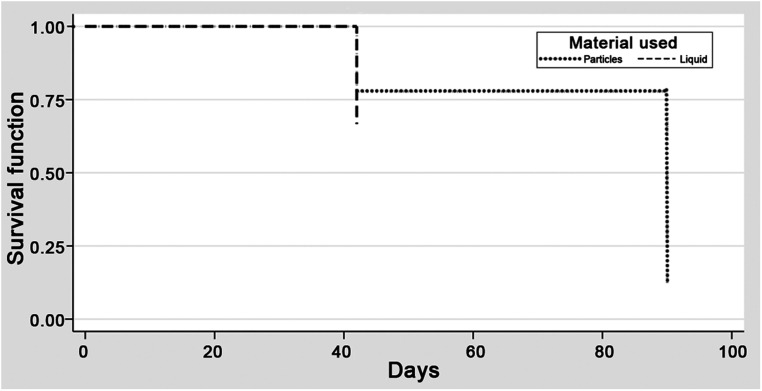
In the prophylactic subgroup, the radiographic primary outcome was reached in 8 (73%) and 1 (20%) patients in particle and liquid groups, respectively (p = 0.106, power 0.51) (Table 1). The hazard ratio (1.5, 95% CI 0.14–16.54, p = 0.74) demonstrated no difference in the time to primary radiographic outcome (Figure 3). No patient in either group required subsequent surgical intervention (p = 1.0, power 0.0). Survival analysis was limited to 40 days, which was the maximum follow-up in the liquid group.
Figure 3.
The Kaplan-Meier survival curve for the radiographic outcome—50% reduction in NASH size at 90 days—in the prophylactic subgroup demonstrating similar survival curves for the particle and liquid embolic groups.
There were no differences in the age of patients (p = 0.126), type of access (p = 0.142), or procedure duration (p = 0.596). General anesthesia was used more frequently in particle-embolized patients (72.7% vs. 20.0%. p = 0.049). Both MMA branches were embolized in 8/11 (72.7%) particle-embolized patients and 4/5 (80%) liquid-embolized patients with data available (p = 0.755).
Complications
Overall, there was no difference in the proportion of complications in each treatment group when compared at the subgroup level (Table 1). There were 9 neurologic complications: 1 patient each with stroke due to subdural compression, increased lethargy, increased balance difficulties, intermittent aphasia, and numbness; 2 patients with worsening headaches; and 2 patients with new-onset seizures. There were 4 technical complications: 1 patient each with MMA rupture, external carotid artery spasm, postprocedural facial droop from Onyx infiltrating the meningeal branches of the skull base and resulting in facial nerve damage; and 1 uncategorized technical complication. Interestingly, the patient that had MMA rupture was treated with particles and achieved the primary radiographic outcome by 42 days. Further outcome was unavailable because the patient died within the 90-day follow-up period from unrelated causes. There were 2 postsurgical infections that necessitated surgical treatment.
Discussion
The current study represents a large multicenter retrospective study of patients undergoing MMA embolization. Our analysis was divided into cohorts for analysis to isolate any bias that surgical evacuations could have introduced. The statistical analysis demonstrates that there was no significant difference in the radiographic outcomes of patients treated with particle or liquid embolisates within any of the cohorts studied. There was also no difference in the time to primary radiographic outcome in any of the cohorts as demonstrated in the Cox proportional hazard models. Interestingly, within the larger cohorts of upfront embolization and recurrent embolization, regardless of embolisate used, 56–63% of patients reached a 50% reduction in subdural hematoma size at 90 days; furthermore, only 0–8% of patients required retreatment, which compares favorably to published recurrence rates of chronic subdural hematomas (5–33%). 15 The prophylactic MMA embolization with concomitant surgery cohort, which contained only 16 patients, was most likely too small to identify a significant result. Our data demonstrate that MMA embolization is efficacious in the management of NASH as a primary treatment modality and as a rescue treatment.
In this study, the choice to use liquid or particle embolisates when performing MMA embolization was at the discretion of the treating physician. Results of particle-based treatments, especially PVA, have been reported more frequently in the literature to date,3,4,10,16,17 but comparable outcomes have been reported when liquid embolisates have been used.7,9,18 The advantages and disadvantages of particle versus liquid embolisates have been described previously. Particle agents are cheaper, easy-to-use, and more comfortable for patients, and they can be used under local anesthesia with conscious sedation. 7 However, the mechanism of action of particle embolization, in which blood flow directs the particles to their target location, may require a more proximal location of the microcatheter for delivery than for liquid embolisates, which has been shown to increase the potential for inadvertent off-target embolization and complications. 3 Liquid embolisates are permanent, can be better visualized during the procedure, and potentially allow for better embolization of distal neovasculature of the NASH,3,7,9,18 consistent with the histologic results observed with Onyx-18 for preoperative tumor embolization. 19 The ideal microcatheter location for delivery of liquid embolics is a distal, “wedged” location in which there is no antegrade blood flow, minimizing concern for reflux around the catheter tip. In addition, liquid embolisates putatively have a twofold mechanism of action, in which the proximal vessel leading to the NASH is blocked along with the neovascular channels within the NASH. This concept likely explains the significantly higher concomitant use of coils among particle-embolized patients in our series (45.5–65.7%), inasmuch as operators likely believed that coils would allow for proximal blockage of perfusion. Greater use of coils among the particle cohort undoubtedly narrows the cost difference between the two strategies; however, this was not examined here. One major disadvantage of liquid embolics is their possible penetration into meningeal arteries in the skull base, which can lead to permanent cranial nerve damage resulting in facial droop and blindness. To avoid such complications, the anatomical anastomoses between the extracranial and intracranial arteries should be avoided. 11
Embolization with liquid embolics is often done under general anesthesia, especially when using Onyx LES, or lidocaine must be injected for patient comfort under conscious sedation. 9 We observed a nonsignificant trend toward longer procedures when liquid embolisates were used (70–75 min in the liquid group vs. 53–65.7 min in the particle group) and also noted that liquid embolic embolization was more common among younger patients (61.7–70.8 years in the liquid group vs. 70.0–73.51 years in the particle group). We find these observations noteworthy, because concerns for longer procedural time and intubation, with consequent potential for intra- and postprocedural cardiopulmonary complications, are particularly germane to the elderly population. 6
Data relating to the differences in outcomes associated with the use of particle versus liquid embolisates for MMA embolization remain limited. Catapano et al. 18 recently published a retrospective single-center series detailing the treatment of NASH with 41 MMA embolizations in 35 patients (34 treated with liquid embolisates and 7 with particle embolisates). Their results showed no significant statistical difference in mean preoperative and postoperative size of the subdural hematoma with various liquid and particle embolisates. They reported a 76% resolution rate when both branches of the MMA were successfully embolized. There was also no difference in the distal penetration of the agents or the technical success of the embolization. Earlier, Waqas et al. 10 found no difference between liquid and particle embolisates when comparing efficacy and complications in a smaller cohort of patients in a systematic review. Our findings corroborate the findings of these reports, demonstrating no difference in efficacy, technical success, and complications between liquid and particle-based embolisates.
Furthermore, it has been demonstrated that NASHs have a higher chance of resolution when both the anterior and posterior branches of the MMA are targeted and successfully embolized. 18 We observed no differences in the rate of embolization of both branches between the particle- and liquid-based subgroups within each cohort, thus minimizing the influence of this key confounding factor. Most patients had embolization of both MMA branches, but 17.2–28.3% had a selected branch embolized, primarily because of technical feasibility or provider preference. It has also been suggested that more distal embolization of MMA branches increases the chance of successful NASH resolution.7,9,18
Numerous prospective studies currently underway have been designed to determine outcomes after MMA embolization for treatment of NASHs. The ELIMINATE study requires the use of particle-based embolisates, with the primary outcome measure being reoperation. EMBOLISE follows 90-day recurrence progression requiring reintervention after Onyx embolization. The STEM trial is designed to identify treatment failure after embolization with a nonadherent ethylene vinyl alcohol copolymer dissolved in dimethyl sulfoxide and suspended in tantalum powder. This embolisate is opaque like Onyx but potentially decreases pain associated with its use. These technical questions have spurred the debate about the appropriate embolisate. It will be interesting to see the results of these studies; however, none of the trials directly compares outcomes based on the type of embolisate used.
Limitations
A major limitation of this study is inherent in its design as a retrospective database analysis. Selection bias is most likely present within each cohort because the treatment arms are not balanced in all demographics, likely because of the small number of patients. This was particularly a concern in the liquid embolic groups and is likely due to physician preference for a certain treatment modality. The presence of missing data, including patients erroneously omitted from the database, is also a real limitation of a retrospectively collected database. The small sample sizes overall in conjunction with the small effect size between the treatment arms resulted in lower power, but because this study is exploratory in nature, these results will help generate hypotheses and could aid in the creation of a well-powered prospective trial studying the radiographic and clinical outcomes of NASH treated with particles or liquid. As such, all conclusions should be interpreted with caution. Furthermore, the retrospective nature of the study means that there was variation in the choices of particle and liquid embolisates, but this represents a real-world experience at multiple institutions. As with all new technologies and procedures, a learning curve could influence the outcomes of the study if patients treated earlier were more likely to have complications or worse outcomes; this study did not specifically control for that. However, the multi-institutional nature of the study attempts to overcome these biases, which increases the generalizability and external validity of our results.
Conclusions
In this retrospective review of 208 patients treated with MMA embolization for a NASH, the choice of embolisate—particle or liquid—did not affect the proportion of patients reaching 50% hematoma thickness reduction at 90 days in patients treated upfront, for recurrence, or prophylactically. Similarly, NASH retreatment within 90 days did not differ among subgroups when comparing embolisates. In conclusion, MMA embolization with particle and liquid embolisates appear to be equally effective in treatment of NASHs.
Acknowledgements
We would like to acknowledge Kristin Kraus, MS, for her help in editing this paper.
Footnotes
Authorship confirmation statement: All of the authors have contributed significantly to the preparation of this article.
Howard Riina is a consultant for Medtronic. Elad Levy has shares/ownership interest in NeXtGen Biologics, RAPID Medical, Claret Medical, Cognition Medical, Imperative Care, Rebound Therapeutics, StimMed, and Three Rivers Medical; has a patent for Bone Scalpel; receives honoraria for training and lectures from Medtronic, Penumbra, MicroVention, and Integra; is a consultant for Clarion, IRRAS AB, GLG Consulting, Guidepoint Global, Imperative Care, Medtronic, StimMed, Misionix, and Mosiac; is the Chief Medical Officer of Haniva Technology; is a national principal investigator for Medtronic; is on the steering committees of SWIFT Prime and SWIFT Direct trials; is the site PI for the CONFIDENCE study (MicroVention) and sub-PI for the STRATIS study (Medtronic); serves on the advisory board for Stryker (AIS Clinical Advisory Board), NeXtGen Biologics, MEDX, Cognition Medical; Endostream Medical, and IRRAS AB; and provides medical/legal opinions for medical legal review; and has leadership or fiduciary roles in CNS, ABNS, and UBNS. Alejandro Spiotta has research support from Penumbra, Stryker, Medtronic, and RapidAI and is a consultant for Penumbra, Stryker, Terumo, and RapidAI. Bradley Gross is a consultant for Medtronic and Medvision. Adita Pandey has stock in NextGen Biologics and FlexDex Surgical. Ricardo Hanel is a consultant for Medtronic, Stryker, Cerenovous, Microvention, Balt, Phenox, Rapid Medical, and Q'Apel; he is on advisory board for MiVI, eLum, Three Rivers, Shape Medical and Corindus; he has received unrestricted research grants from NIH, Interline Endowment, Microvention, Stryker, CNX; he is an investor/stockholder for InNeuroCo, Cerebrotech, eLum, Endostream, Three Rivers Medical Inc, Scientia, RisT, BlinkTBI, and Corindus. David Langer is a consultant of ORBEYE. Michael Levitt has grants from the National Institutes of Health, The Aneurysm and AVM Foundation, Congress of Neurological Surgeons Society of NeuroInterventional Surgery, is a consultant for Medtronic, has investigator-initiated unrestricted educational grants from Medtronic and Styker, is a shareholder of Proprio, Cerebrotech, Synchron, Hyperion Surgical, has equity interest in Fluid Biomed and Stereotaxis, is an advisor for Aeaean Advisers, and is on the Journal of NeuroInterventional Surgery editorial board. Philipp Taussky is a consultant for Avail Medsystems, Johnson & Johnson, Medtronic, and Stryker Neurovascular. Peter Kan is a consultant for Stryker Neurovascular, MicroVention, and Imperative Care. Ramesh Grandhi is a consultant for Balt Neurovascular, Cerenovus, Integra, and Medtronic Neurovascular.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Elad I. Levy https://orcid.org/0000-0002-6208-3724
Aditya S. Pandey https://orcid.org/0000-0003-0789-4273
Ricardo Hanel https://orcid.org/0000-0001-7195-5806
Ramesh Grandhi https://orcid.org/0000-0001-9000-6083
References
- 1.Yang W, Huang J. Chronic subdural hematoma: epidemiology and natural history. Neurosurg Clin N Am 2017; 28: 205–210. [DOI] [PubMed] [Google Scholar]
- 2.Balser D, Farooq S, Mehmood T, et al. Actual and projected incidence rates for chronic subdural hematomas in United States veterans administration and civilian populations. J Neurosurg 2015; 123: 1209–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ban SP, Hwang G, Byoun HS, et al. Middle meningeal artery embolization for chronic subdural hematoma. Radiology 2018; 286: 992–999. [DOI] [PubMed] [Google Scholar]
- 4.Link TW, Boddu S, Paine SM, et al.: Middle meningeal artery embolization for chronic subdural hematoma: a series of 60 cases. Neurosurgery 2019;85:801–807. [DOI] [PubMed] [Google Scholar]
- 5.Kan P, Maragkos GA, Srivatsan A, et al. Middle meningeal artery embolization for chronic subdural hematoma: a multi-center experience of 154 consecutive embolizations. Neurosurgery 2021; 88: 268–277. [DOI] [PubMed] [Google Scholar]
- 6.Joyce E, Bounajem MT, Scoville J, et al. Middle meningeal artery embolization treatment of nonacute subdural hematomas in the elderly: a multiinstitutional experience of 151 cases. Neurosurg Focus 2020; 49(4): E5. [DOI] [PubMed] [Google Scholar]
- 7.Fiorella D. Arthur AS: middle meningeal artery embolization for the management of chronic subdural hematoma. J Neurointerv Surg 2019; 11: 912–915. [DOI] [PubMed] [Google Scholar]
- 8.Saito H, Tanaka M, Hadeishi H. Angiogenesis in the septum and inner membrane of refractory chronic subdural hematomas: consideration of findings after middle meningeal artery embolization with low-concentration n-butyl-2-cyanoacrylate. NMC Case Rep J 2019; 6: 105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rajah GB, Waqas M, Dossani RH, et al. Transradial middle meningeal artery embolization for chronic subdural hematoma using Onyx: case series. J Neurointerv Surg 2020; 12: 1214–1218. [DOI] [PubMed] [Google Scholar]
- 10.Waqas M, Vakhari K, Weimer PV, et al. Safety and effectiveness of embolization for chronic subdural hematoma: systematic review and case series. World Neurosurg 2019; 126: 228–236. [DOI] [PubMed] [Google Scholar]
- 11.Motiei-Langroudi R, Stippler M, Shi S, et al. Factors predicting reoperation of chronic subdural hematoma following primary surgical evacuation. J Neurosurg 2018; 129: 1143–1150. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman H, Ziechmann R, Beutler T, et al. First-line management of chronic subdural hematoma with the subdural evacuating port system: institutional experience and predictors of outcomes. J Clin Neurosci 2018; 50: 221–225. [DOI] [PubMed] [Google Scholar]
- 13.Singla A, Jacobsen WP, Yusupov IR, et al. Subdural evacuating port system (SEPS)--minimally invasive approach to the management of chronic/subacute subdural hematomas. Clin Neurol Neurosurg 2013; 115: 425–431. [DOI] [PubMed] [Google Scholar]
- 14.Rughani AI, Lin C, Dumont TM, et al. A case-comparison study of the subdural evacuating port system in treating chronic subdural hematomas. J Neurosurg 2010; 113: 609–614. [DOI] [PubMed] [Google Scholar]
- 15.Yadav YR, Parihar V, Namdev H, et al. Chronic subdural hematoma. Asian J Neurosurg 2016; 11: 330–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Link TW, Rapoport BI, Paine SM, et al. Middle meningeal artery embolization for chronic subdural hematoma: endovascular technique and radiographic findings. Interv Neuroradiol 2018; 24: 455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Link TW, Schwarz JT, Paine SM, et al. Middle meningeal artery embolization for recurrent chronic subdural hematoma: a case series. World Neurosurg 2018; 118: e570–e574. [DOI] [PubMed] [Google Scholar]
- 18.Catapano JS, Ducruet AF, Nguyen CL, et al. Middle meningeal artery embolization for chronic subdural hematoma: an institutional technical analysis. J Neurointerv Surg 2021; 13: 657–660. [DOI] [PubMed] [Google Scholar]
- 19.Grandhi R, Hunnicutt CT, Harrison G, et al. Comparing angiographic devascularization with histologic penetration after preoperative tumor embolization with onyx: what indicates an effective procedure? J Neurol Surg A Cent Eur Neurosurg 2015; 76: 309–317. [DOI] [PubMed] [Google Scholar]