Abstract
Objective
To describe in detail the clinical profile of Charcot-Marie-Tooth disease subtype 3 (CMTX3) to aid appropriate genetic testing and rehabilitative therapy.
Methods
We reviewed the clinical and neurophysiologic profile and CMT Pediatric Scale (CMTPedS) assessments of 11 children with CMTX3.
Results
Compared with the more common forms of CMT, CMT1A and CMTX, CMTX3 was characterized by early onset with early and progressive hand weakness. Most affected children were symptomatic within the first 2 years of life. The most common presentation was foot deformity in the first year of life. CMTPedS analysis in these children revealed that CMTX3 progressed more rapidly (4.3 ± 4.1 points over 2 years, n = 7) than CMT1A and CMTX1. Grip strength in affected boys was 2 SDs below age- and sex-matched normative reference values (z score −2.05 ± 1.32) in the second decade of life. The most severely affected individual was wheelchair bound at 14 years of age, and 2 individuals had no movement in the small muscles of the hand in the second decade of life. Nerve conduction studies showed a demyelinating sensorimotor neuropathy with motor conduction velocity ≤23 m/s.
Conclusions
CMTX3 had an earlier onset, severe hand weakness, and more rapidly progressive disability compared to the more common forms of CMT. Understanding the unique phenotype of CMTX3 is essential for directing genetic testing because the CMTX3 insertion will not be seen on a routine microarray or neuromuscular gene panel. Early diagnosis will enable rehabilitation to be started early in this rapidly progressive neuropathy.
Charcot-Marie-Tooth disease (CMT) is the most common inherited neuromuscular disorder, with a prevalence of 1 in 1,214 individuals.1 X-linked CMT accounts for 10% to 15% of all CMT. CMTX1 is the most common form of X-linked CMT and is caused by mutations in GJB1. The genetic abnormality responsible for CMTX3, a rarer form of X-linked CMT, has recently been identified.2 With whole-genome sequencing, a 78-kb interchromosomal insertion from chromosome 8q24.3 into the CMTX3 locus was identified in 2 closely related families with CMTX3. This was the first report of an interchromosomal insertion causing CMT. CMTX3 is reported to have a late childhood onset with hand weakness.3,4 However, the literature on the clinical and neurophysiologic characteristics of CMTX3, especially in childhood, is limited. A detailed understanding of the clinical and neurophysiologic profile will enable early diagnosis and appropriate rehabilitation.
Methods
The study was a retrospective review in a tertiary pediatric peripheral neuropathy clinic. The diagnosis of CMTX3 was confirmed by the multiplex PCR CMTX3 breakpoint assay.2 Affected children were evaluated on the CMTPedS, a linearly weighted and responsive clinical outcome measure to assess disease severity, evaluating fine and gross motor function, strength, sensation, and balance. It provides an age- and sex-adjusted disability score (0 = unaffected, 44 = severely affected) based on normative reference values, allowing comparison within and between participants with different CMT types.5 Paired-sample t tests were used to evaluate differences between baseline and 2 years. Student t tests were used to evaluate differences between CMTX3, CMT1A, and CMTX1. A level of α = 0.05 was used for statistical significance.
Standard protocol approvals, registrations, and patient consents
The study was approved by the hospital ethics committee (CCR2016/19), and written informed consent was obtained from patients or guardians.
Data availability
Anonymized data will be shared on request by any qualified investigator.
Results
Clinical profile
We assessed 11 children with CMTX3, all from the original Australian family (CMT193-ext), including 10 boys and 1 symptomatic girl (table 1), over an 11-year period. Individuals 3, 4, 5, 6, and 10 have previously been reported as part of a genetically unclassified non-CMTX1 X-linked CMT cohort.4 Age at onset ranged from birth to 5 years in boys and 12 years for the only female. Six boys had foot deformity at presentation, while 4 boys presented with a gait abnormality. Equinovarus foot deformity was evident in 5 children before 1 year of age, while the other child presented with mild hind foot valgus deformity at 2 years of age.
Table 1.
Clinical profile of children with CMTX3 (n = 11, 10 boys)
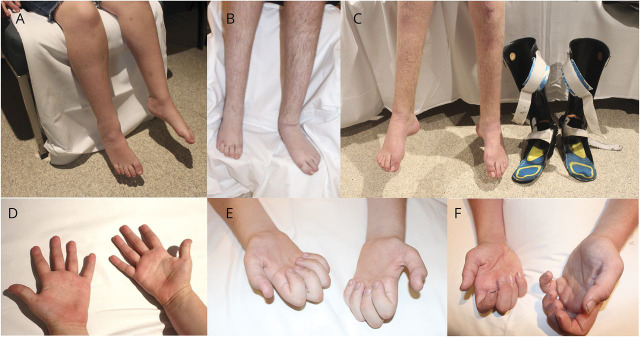
Hand function was affected in 7 children and was identified between 4 months and 12 years of age. Five children had wasting of the hand intrinsics, and 2 children (individuals 1 and 7) had severe hand weakness, with no movement in the small muscles of the hand and severe forearm wasting noted at 12 and 16 years of age (figure 1). The most severely affected child (individual 1) also had proximal lower limb weakness when assessed at 11 years of age, had mild restrictive lung disease identified at 12 years of age, and lost independent ambulation at 14 years of age. None of the other children in this cohort had proximal upper or lower limb weakness. None had cranial nerve involvement (including optic atrophy, facial or bulbar weakness, deafness), upper motor neuron signs, or cognitive involvement. One individual, initially diagnosed with cerebral palsy, had an MRI of the brain in the first year of life, which was normal.
Figure 1. Lower limb and hand weakness in affected boys with CMTX3.
(A and D) Individual 2 at 8 years of age, (B and E) individual 1 at 15 years of age, and (C and F) individual 7 at 17 years of age.
CMTPedS assessment
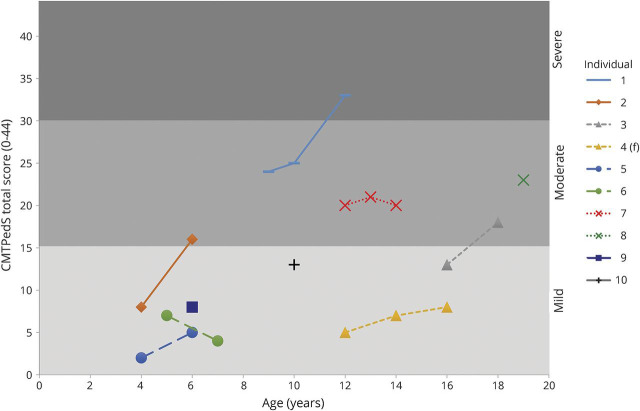
We reviewed 21 CMTPedS assessments in 10 children. CMTPedS scores ranged from 2 to 33 (figure 2). At the most recent visit, 5 boys had CMTPedS scores that placed their disability in the moderate (16–29) or severe (>29) range. We compared the baseline CMTPedS scores and rate of progression data to that of children and adolescents (age 3–20 years) in the Inherited Neuropathies Consortium.6 The baseline CMTPedS scores did not significantly differ among CMTX3 (12.3 ± 7.7, age 9.7 ± 5.1 years, n = 10), CMT1A (14.6 ± 7.1, age 9.9 ± 3.7 years, n = 111, p = 0.1), and CMTX1 (9.8 ± 8.2, age 9.8 ± 5.7 years, n = 4, p = 0.9). However, CMTX3 progressed significantly over 2 years (35% change from baseline, p = 0.03) and at a faster rate (4.3 ± 4.1 over 2 years, n = 7) compared with CMT1A (1.8 ± 4.2) and CMTX1 (2.5 ± 3.0).
Figure 2. CMTPedS scores for individual participants and progression over time.
Charcot-Marie-Tooth Pediatric Scale (CMTPedS) score on the y-axis has been demarcated into mild (CMTPedS score <16), moderate (16–29), and severe (>29) categories. Siblings are indicated with the same marker symbol (individuals 3 and 4, individuals 5 and 6, individuals 7 and 8).
Grip strength was measured during the most recent CMTPedS assessment (table e-1, links.lww.com/WNL/A426). Individuals ≤10 years of age had grip strength within a normal range, while 3 of the 4 boys above this age, in addition to severe distal lower limb weakness (0/5 power on ankle dorsiflexion), had a grip strength at least 2 SDs below normative reference values. This deterioration in upper limb strength can be quite rapid, as in the case of individual 7, who progressed from a power of 4/5 in the small muscles of the hand and a grip strength of 127 N at 14 years of age to a power of 0/5 in the small muscles of the hand and a grip strength of 49 N at 16 years of age. Individual 1 had a similar deterioration over a more gradual period of 5 years, between the ages of 7 and 12 years.
Neurophysiology
Nerve conduction studies were available for review from 9 of the 11 children in this study (table e-2, links.lww.com/WNL/A426). Eight children had a length-dependent sensorimotor demyelinating neuropathy with motor conduction velocities of ≤23 m/s in at least 1 nerve. Individuals 2 and 10 showed conduction block (>50% reduction in compound muscle action potential between distal and proximal stimulation sites) in 2 nerves each (median/tibial, ulnar/tibial), while individuals 6 and 7 showed conduction block in a single nerve each (tibial, median) (data not shown in the table). Two individuals in this cohort (individuals 3 and 4) had nonuniform slowing with normal or mildly slow upper limb motor conduction velocities. One child had a length-dependent sensorimotor axonal neuropathy. Nerve biopsy in 1 child (individual 1) at 6 years of age showed a mild reduction in myelinated large fibers, frequent thinly myelinated axons, small onion bulbs, and multiple regenerative clusters, consistent with a primary demyelinating neuropathy.
Discussion
CMTX3 is caused by a unique 78-kb insertion into chromosome Xq27.1 and cannot be detected with the single nucleotide polymorphism microarrays, multigene panels, or whole-exome sequencing currently used for the diagnosis of CMT. Instead, a multiplex PCR assay has been devised to detect the CMTX3 insertion. Currently, the CMTX3 interchromosomal insertion has been identified in only 2 large related families (CMT623 and CMT193-ext). An American family has also been linked to the CMTX3 locus, but samples are not available to test for the interchromosomal insertion.7 The exact mechanism of how this genetic abnormality causes disease has not yet been identified but is presumed to be a transcriptional dysregulation of 1 or more genes mapping within the CMTX3 locus.2 While other families with either the specific CMTX3 interchromosomal insertion or other processes causing dysregulation of the as-yet unidentified gene are likely to have a phenotype broadly similar to our cohort, the phenotype is likely to be influenced by the type of mutation and by genetic modifiers.
Compared to the most common forms of CMT, CMT1A and CMTX1, our cohort had early onset of symptoms and early and progressive hand weakness. Nine of the 11 children had onset of symptoms by the age of 2 years. In comparison, the median age at symptom onset in CMT1A is 6 years,8 while in a study of 41 male individuals with CMTX1, median age at onset was 15 years.9 The most common presentation in our cohort was foot deformity (6 children), with 5 children presenting with foot deformity within the first year of life. In a cohort of 8 children with CMTX1, symptom onset/presentation was predominantly gait abnormalities, and none had equinovarus foot deformity at presentation.4
In our cohort, because of the rapidly progressive neuropathy, hand function was affected early, and weakness progressed rapidly. In CMT1A, subtle hand weakness is present in early stages, followed by slow progression of impairment and disability. A study by Miller et al.10 in 68 individuals with CMT1A showed that the average onset of hand symptoms occurred 19 years after disease onset. In CMTX1, hand involvement is uncommon in young children but often noted in the early teens and can then progress to severe weakness.11
We have comprehensively evaluated a cohort of children with CMTX3 with the standardized and validated CMTPedS and have shown that neuropathy in children with CMTX3 progresses significantly at the average rate of 4.3 points (35% change from baseline) on the CMTPedS scale every 2 years. The early onset coupled with rapid progression means that many children with CMTX3 will have severe disability within the first 2 decades of life. One child (individual 1) in our cohort was in a wheelchair at 14 years of age. Early onset (within the first 2 years of life) is seen in other demyelinating neuropathies, including CMT with mutations in PMP22, MPZ, PRX, EGR2, FDG4, SH3TC2, MTMR2, and SBF2.12 Of the more common demyelinating neuropathies, CMT1E (PMP22 mutation) and CMT4C (SH3TC2 mutation) have been shown to progress rapidly in childhood.6 Similarly, the group previously classified as Dejerine-Sottas syndrome and now shown to be associated with mutations in PMP22, MPZ, PRX, EGR2, and FIG4 are also described to have an early onset, rapid progression, and hand involvement in the second decade of life.13
The phenotype of 11 affected boys from the United Kingdom and New Zealand family (CMT623),3 closely related to the family described in this report, including 4 boys between the ages of 8 and 15 years, and 2 affected boys from the American family, linked to the CMTX3 locus but not confirmed to have the CMTX3 insertion, has previously been reported. Onset in the UK/New Zealand family was in the first decade of life and slightly later (age 10–14 years) in the American family. Half of the affected boys in the UK/New Zealand family had prominent hand involvement with onset at a median age of 9.2 years. Nerve conduction studies showed motor conduction velocities in both the axonal and demyelinating range in different individuals. Carrier girls were asymptomatic with minimal clinical signs. A detailed study of the severity of neuropathy in adults with CMTX3 would provide further insights into the rate of progression and resulting disability in CMTX3.
Uniform slowing of motor conduction velocities is a feature of most demyelinating inherited neuropathies, especially CMT1A.14 Atypical features in some individuals in our cohort were nonuniform conduction slowing with different motor conduction velocities in the upper and lower limbs in 2 individuals, evidence of conduction block, and an axonal neuropathy in 1 individual. Nonuniform slowing of nerve conduction velocities, often associated with multifocal conduction slowing and conduction block, has been reported with hereditary neuropathy with liability to pressure palsy and CMTX1.14
Our comprehensive study of children with CMTX3 suggests that early diagnosis, frequent surveillance, and early institution of rehabilitative or surgical interventions are important in this rapidly progressive neuropathy. Hip dysplasia was identified in 2 children in our cohort, and we recommend that children with CMTX3 should have at least biennial x-ray surveillance for hip dysplasia. This unique phenotype of an X-linked condition presenting early in childhood with foot deformity, early hand weakness, and demyelinating peripheral neuropathy should prompt clinicians to consider the diagnosis of CMTX3 and request the CMTX3 insertion multiplex PCR assay.
Acknowledgment
The authors thank Prof. Robert Ouvrier for sharing clinical data on the participants and the participants and their families for their involvement in this project.
Glossary
- CMT
Charcot-Marie-Tooth disease
- CMTPedS
CMT Pediatric Scale
Author contributions
Manoj Kanhangad: study concept and design, acquisition of data, analysis and interpretation of data, drafting the manuscript, and critical revision of manuscript for intellectual content. Kayla Cornett: acquisition of data, analysis and interpretation of data, statistical analysis, critical revision of manuscript for intellectual content. Megan H. Brewer, Garth A. Nicholson, Monique M. Ryan, Robert L. Smith, Gopinath M. Subramanian, Helen K. Young, Stephan Züchner, and Marina L. Kennerson: acquisition of data, critical revision of manuscript for intellectual content. Joshua Burns: acquisition of data, analysis and interpretation of data, critical revision of manuscript for intellectual content. Manoj P. Menezes, study concept and design, study supervision, acquisition of data, analysis and interpretation of data, critical revision of manuscript for intellectual content.
Study funding
No targeted funding reported.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Braathen GJ. Genetic epidemiology of Charcot-Marie-Tooth disease. Acta Neurol Scand Suppl 2012;126:iv-22. [DOI] [PubMed] [Google Scholar]
- 2.Brewer MH, Chaudhry R, Qi J, et al. Whole genome sequencing identifies a 78 kb insertion from chromosome 8 as the cause of Charcot-Marie-Tooth neuropathy CMTX3. PLoS Genet 2016;12:e1006177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huttner IG, Kennerson ML, Reddel SW, Radovanovic D, Nicholson GA. Proof of genetic heterogeneity in X-linked Charcot-Marie-Tooth disease. Neurology 2006;67:2016–2021. [DOI] [PubMed] [Google Scholar]
- 4.Yiu EM, Geevasinga N, Nicholson GA, Fagan ER, Ryan MM, Ouvrier RA. A retrospective review of X-linked Charcot-Marie-Tooth disease in childhood. Neurology 2011;76:461–466. [DOI] [PubMed] [Google Scholar]
- 5.Burns J, Ouvrier R, Estilow T, et al. Validation of the Charcot-Marie-Tooth Disease Pediatric Scale as an outcome measure of disability. Ann Neurol 2012;71:642–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cornett KMD, Menezes MP, Shy RR, et al. Natural history of Charcot-Marie-Tooth disease during childhood. Ann Neurol 2017;82:353–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ionasescu VV, Trofatter J, Haines JL, Summers AM, Ionasescu R, Searby C. Heterogeneity in X-linked recessive Charcot-Marie-Tooth neuropathy. Am J Hum Genet 1991;48:1075–1083. [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas PK, Marques W Jr, Davis MB, et al. The phenotypic manifestations of chromosome 17p11.2 duplication. Brain 1997;120:465–478. [DOI] [PubMed] [Google Scholar]
- 9.Dubourg O, Tardieu S, Birouk N, et al. Clinical, electrophysiological and molecular genetic characteristics of 93 patients with X-linked Charcot-Marie-Tooth disease. Brain 2001;124:1958–1967. [DOI] [PubMed] [Google Scholar]
- 10.Miller MJ, Williams LL, Slack SL, Nappi JF. The hand in Charcot-Marie-Tooth disease. J Hand Surg Br 1991;16:191–196. [DOI] [PubMed] [Google Scholar]
- 11.Hahn AF, Bolton CF, White CM, et al. Genotype/phenotype correlations in X-linked dominant Charcot-Marie-Tooth disease. Ann NY Acad Sci 1999;883:366–382. [PubMed] [Google Scholar]
- 12.Baets J, Deconinck T, De Vriendt E, et al. Genetic spectrum of hereditary neuropathies with onset in the first year of life. Brain 2011;134:2664–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilmshurst JM, Ouvrier R. Hereditary peripheral neuropathies of childhood: an overview for clinicians. Neuromuscul Disord 2011;21:763–775. [DOI] [PubMed] [Google Scholar]
- 14.Lewis RA, Sumner AJ, Shy ME. Electrophysiological features of inherited demyelinating neuropathies: a reappraisal in the era of molecular diagnosis. Muscle Nerve 2000;23:1472–1487. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared on request by any qualified investigator.