Abstract
Objective:
To assess the design characteristics and reporting quality of published randomized controlled trials (RCTs) for treatments of chemotherapy-induced peripheral neuropathy (CIPN) initiated before or during chemotherapy.
Methods:
In this systematic review of RCTs of preventive or symptomatic pharmacologic treatments for CIPN initiated before or during chemotherapy treatment, articles were identified by updating the PubMed search utilized in the CIPN treatment guidelines published in the Journal of Clinical Oncology in 2014.
Results:
Thirty-eight articles were identified. The majority included only patients receiving platinum therapies (61%) and used a placebo control (79%). Common exclusion criteria were preexisting neuropathy (84%), diabetes (55%), and receiving treatments that could potentially improve neuropathy symptoms (45%). Ninety-five percent of studies initiated the experimental treatment before CIPN symptoms occurred. Although 58% of articles identified a primary outcome measure (POM), only 32% specified a primary analysis. Approximately half (54%) of the POMs were patient-reported outcome measures of symptoms and functional impairment. Other POMs included composite measures of symptoms and clinician-rated signs (23%) and vibration tests (14%). Only 32% of articles indicated how data from participants who prematurely discontinued chemotherapy were analyzed, and 21% and 29% reported the number of participants who discontinued chemotherapy due to neuropathy or other/unspecified reasons, respectively.
Conclusions:
These data identify reporting practices that could be improved in order to enhance readers' ability to critically evaluate RCTs of CIPN treatments and use the findings to inform the design of future studies and clinical practice. Reporting recommendations are provided.
Chemotherapy-induced peripheral neuropathy (CIPN) is a common and often dose-limiting side effect of certain types of cancer chemotherapy (e.g., platinum agents, taxanes, and vinca alkaloids). In some cases, CIPN can persist well after the termination of chemotherapy and impair cancer survivors' health-related quality of life (HR-QOL).1–3 A recently published treatment guideline concluded that insufficient evidence is available to recommend any treatments for CIPN prevention.4 The lack of effective evidence-based treatments for CIPN that occurs during chemotherapy highlights the need for high-quality clinical trials to identify interventions that can prevent or treat this condition.
Randomized controlled trials (RCTs) designed to investigate the efficacy of agents to prevent CIPN or treat it while patients are still receiving chemotherapy are methodologically challenging. Neurotoxic chemotherapy is administered in discrete doses over time and both the cumulative dosage of chemotherapy and the time since the last dose of chemotherapy affect neuropathy severity.5–7 Patients often experience dosage reductions, delays, or early terminations of their planned chemotherapy because of neuropathy, other chemotherapy-induced symptoms, or cancer progression. These alterations in planned dosing regimens can have a significant effect on the severity of CIPN if it is assessed at specific times after the initiation of chemotherapy. Therefore, alterations in chemotherapy regimens can lead to additional variability in the trial outcome that is not due to the experimental treatment, a phenomenon that the RCT is designed to minimize.8 These challenges can introduce bias, possibly leading to invalid results.
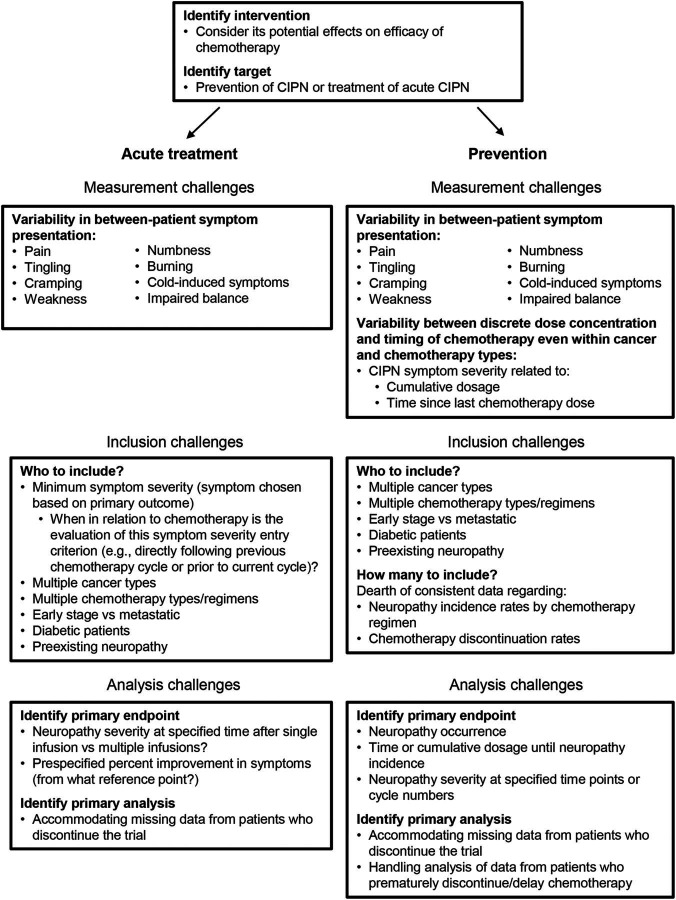
Another challenge of evaluating treatments for CIPN is the fact that it is a multisymptom condition with different presentations. Although there are common symptoms and signs that many patients experience (e.g., pain, tingling, balance difficulties, sensory loss), the relative severity of the symptoms and signs varies among patients. Some symptoms are more common with specific types of chemotherapy agents.7 Unless the experimental treatment is targeted toward a specific neuropathic symptom (e.g., pain), the trial outcomes must address multiple symptoms or signs. This can be achieved by including multiple outcome measures that assess individual symptoms or signs or a composite outcome measure that summarizes multiple symptoms or signs assessments in a single score. The former approach poses certain statistical challenges, whereas the latter can mask a specific treatment effect if only a subset of symptoms is affected by the treatment. These and other challenges confronted when designing RCTs to evaluate treatments for CIPN during chemotherapy are summarized in figure 1.
Figure 1. Methodologic challenges in designing randomized controlled trials to evaluate efficacy of preventive and acute symptomatic treatments for chemotherapy-induced peripheral neuropathy (CIPN).
The goals of this systematic review were to (1) summarize the important experimental design characteristics of RCTs evaluating interventions to prevent and treat CIPN during chemotherapy and (2) evaluate the clarity of reporting of these trials. By highlighting opportunities for standardization and improvement in future trial design and reporting, we hope to expedite discovery of new interventions to prevent or treat CIPN during chemotherapy.
METHODS
Article identification.
Reports of RCTs investigating pharmacologic treatments for CIPN administered before or during chemotherapy published before April 2013 were identified using the articles reviewed in the American Society of Clinical Oncology (ASCO) practice guidelines.4 To update the list of trials, the search reported in the guidelines was repeated in PubMed by a librarian (including January 2013 and November 2015) (appendix e-1 at Neurology.org). Two authors (J.S.G. and R.A.K.) screened the search results. Included articles reported an RCT investigating pharmacologic treatments for CIPN that were initiated before or during chemotherapy and included either a placebo or no treatment control group. Articles in which the main focus was not CIPN were excluded. Articles that included a mix of patients who were still receiving chemotherapy and individuals who were no longer receiving chemotherapy were excluded.
Data extraction.
A coding manual (appendix e-2) was developed to evaluate key design features and the adequacy of reporting of CIPN clinical trials conducted during chemotherapy, including outcome measures, eligibility criteria, timing of treatment and assessments, and analysis of data from participants who discontinued or altered the planned chemotherapy regimen during the study. The coding manual was pretested using reports of RCTs of chemotherapy-induced nausea. The manual was tested and modified for clarity in 3 coding rounds. The list of CIPN RCTs was randomized and each article was coded by 2 authors (J.S.G. and R.A.K.). After the articles were coded independently, J.S.G. adjudicated discrepancies (e.g., coding errors) in the data.
Descriptive statistics were used to summarize trial characteristics (e.g., publication year and sponsorship), design features (e.g., sample sizes, eligibility criteria, and timing of treatment initiation), and the adequacy of reporting of statistical details (e.g., specification and description of primary outcome measure [POM] [i.e., instruments used to assess neuropathy], primary endpoints [i.e., outcome variables derived from the instrument, for example, grade 2 neuropathy], and analyses, methods to accommodate missing data, and how participants who discontinued chemotherapy during the study were considered in the analyses). RCTs that initiated the experimental treatment prior to the development of neuropathy symptoms were considered preventive. RCTs that enrolled patients after CIPN symptoms developed were considered symptomatic treatment trials. Results for the prevention and symptomatic treatment trials are presented together unless otherwise noted. Trials were considered to have industry sponsorship if industry provided funds or supplied drugs for the studies.
RESULTS
Search results.
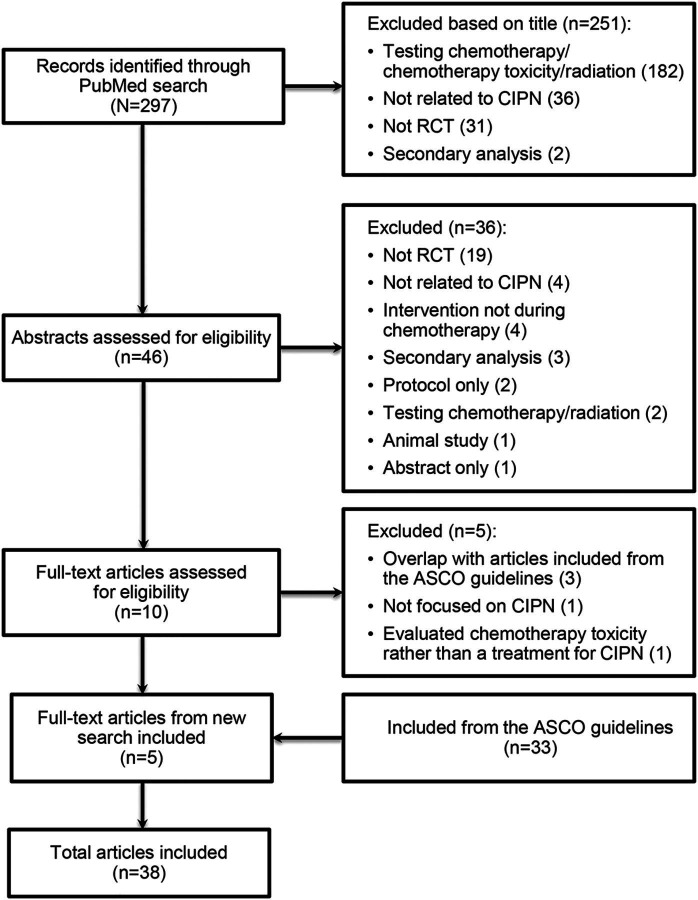
Of the 297 records identified in PubMed, 251 and 36 were excluded based on the title and abstract, respectively. An additional 5 were excluded after full text review: 3 were duplicates that were identified in the ASCO treatment guidelines,4 1 was not focused on CIPN, and 1 evaluated chemotherapy toxicity, not a CIPN intervention. An additional 33 articles were included from the ASCO treatment guidelines, resulting in 38 included articles (figure 2, appendix e-3).
Figure 2. Preferred Reporting Items for Systematic Reviews and Meta-Analyses diagram.
ASCO = American Society of Clinical Oncology; CIPN = chemotherapy-induced peripheral neuropathy; RCT = randomized clinical trial.
Coder discrepancies.
In total, 3,237 items were coded and 243 (7.5%) discrepancies occurred and were adjudicated by J.S.G. with consultation from M.P.M. when necessary. Of the 243 discrepancies, 220 (91%) were obvious oversights by one of the coders and 23 were due to differences in interpretation.
Trial characteristics.
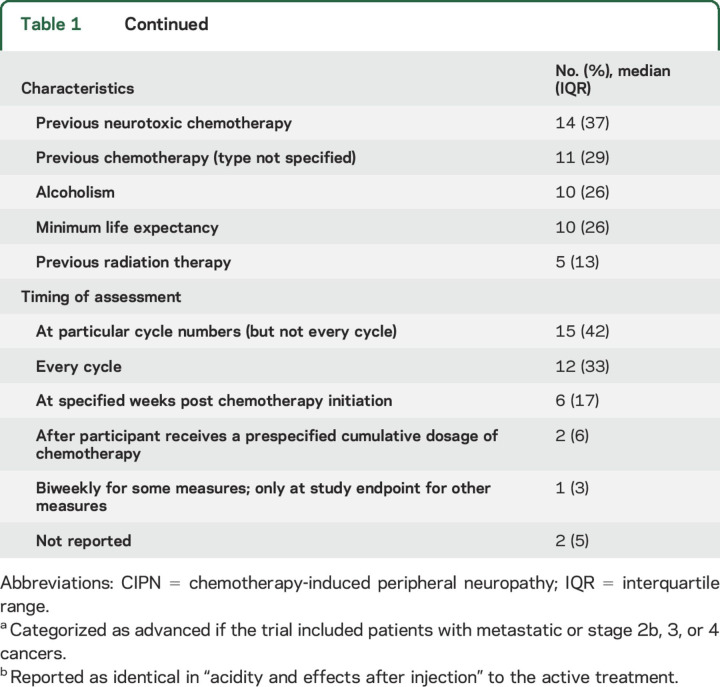
Thirty-six (95%) of the included studies initiated the experimental treatment before CIPN symptom onset (i.e., used a prevention design) (table 1). Twenty-eight (78%) of the prevention articles clearly reported when the experimental treatment was initiated in relation to chemotherapy initiation. Twenty-three (61%) of the articles specified that the experimental treatment was initiated prior to or on the same day as the initial chemotherapy infusion. The amount of time, when described, ranged from “immediately before” to 4 hours before (n = 11 [48%]); between 1 day and 1 week before (n = 5 [22%]); the same day as the chemotherapy dose (n = 5 [22%]); and “any time before the first chemotherapy dose” (n = 1 [4%]). Three trials allowed some flexibility in the timing of treatment initiation (e.g., “As close as possible to the beginning of chemotherapy”).
Table 1.
Trial characteristics
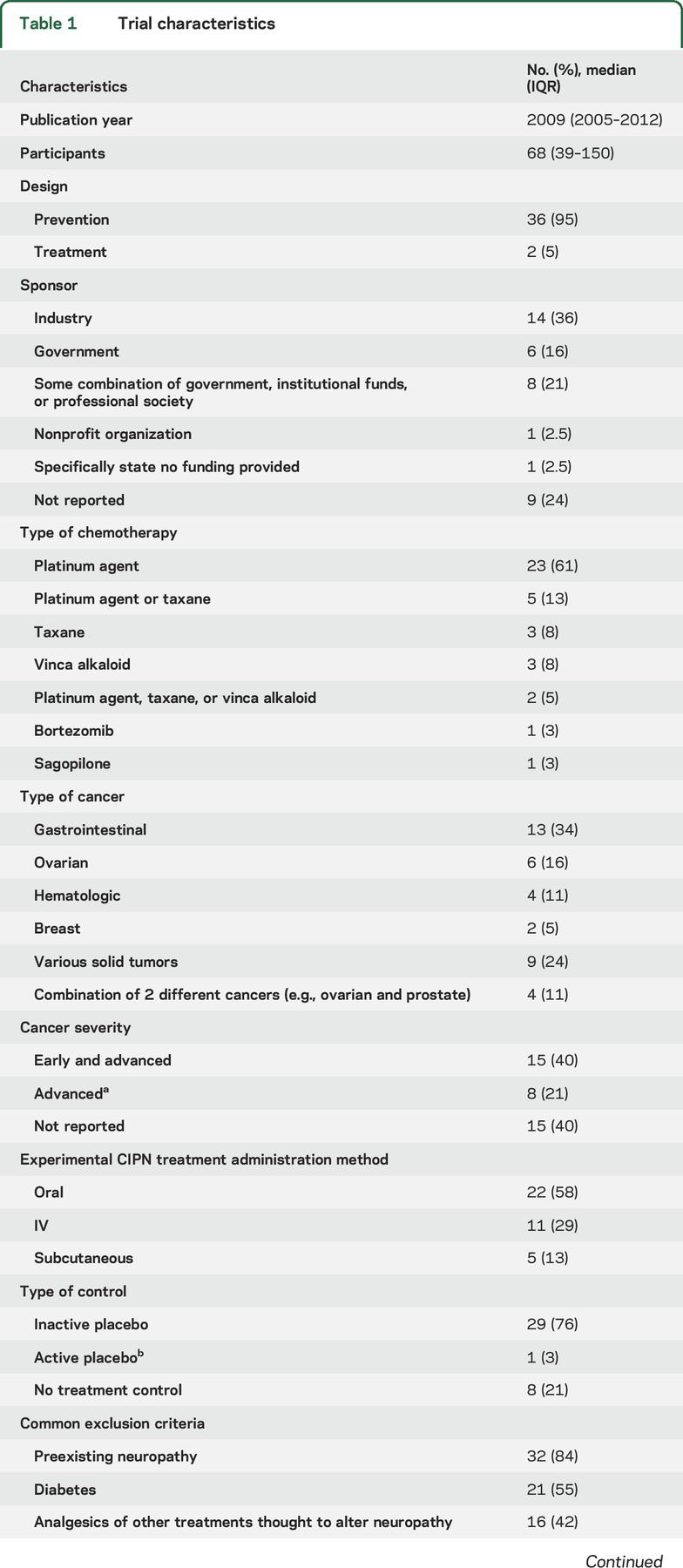
Almost half (42%) of the trials reported some industry sponsorship; 24% failed to indicate anything regarding funding source. CIPN from platinum agents was most commonly studied (table 1). Forty percent of articles stated that only patients receiving 1 planned chemotherapy regimen (i.e., 1 or a combination of agents administered using a single dosing protocol) were eligible. Approximately one-third of the trials included only gastrointestinal cancers and 16% included only ovarian cancer. Forty percent of the trials included both early and late-stage cancer patients, while 21% only included patients with advanced cancer. Seventy-nine percent of the trials included a placebo control; 21% included a control group that received no intervention (table 1). The most common exclusion criterion was preexisting neuropathy (84%). Other common exclusion criteria included diabetes (55%), use of agents thought to possibly improve neuropathy symptoms (45%), and previous neurotoxic chemotherapy (37%) (table 1).
Timing of assessments.
Table 1 outlines the treatment schedules used in the trials. Thirty-two (89%) of the prevention articles reported an assessment of neuropathy at baseline, which occurred before initiation of the experimental treatment and before or shortly after initiation of chemotherapy. Thirteen (34%) articles reported assessments of neuropathy after the cessation of chemotherapy (i.e., chronic CIPN). The longest time that elapsed between the end of chemotherapy and neuropathy assessments was 1 month (n = 2), 3 months (n = 6), 6 months (n = 2), and 24 months (n = 1). Eight trials terminated the experimental CIPN treatment prior to the final neuropathy assessment. Two of the remaining 30 articles provided a justification for not assessing neuropathy after cessation of chemotherapy and the experimental treatment. These justifications included rapid disease progression in the participants and early termination of the study. For the assessments made during chemotherapy, 10 (26%) articles reported some description of the timing of the neuropathy assessments in relation to when the chemotherapy doses were administered. Of those 10, 8 stated that the measurements were made prior to the chemotherapy cycle and 2 indicated the specific days after each chemotherapy dose at which the measurements were made (e.g., days 1 through 5 post chemotherapy infusion).
Outcome measures and analyses.
Twenty-two (58%) articles specified a POM, 20 (53%) identified a primary endpoint, and 12 (32%) identified the primary analysis or analyses (including the groups to be compared and statistical test used) (table 2). Only 10 articles reviewed identified a single primary analysis. Of the remaining 28 articles, only 1 article mentioned an adjustment for multiplicity in the efficacy analyses. This article identified 2 primary analyses and adjusted for those 2 comparisons using the Bonferroni method.
Table 2.
Primary outcome measures and endpoints
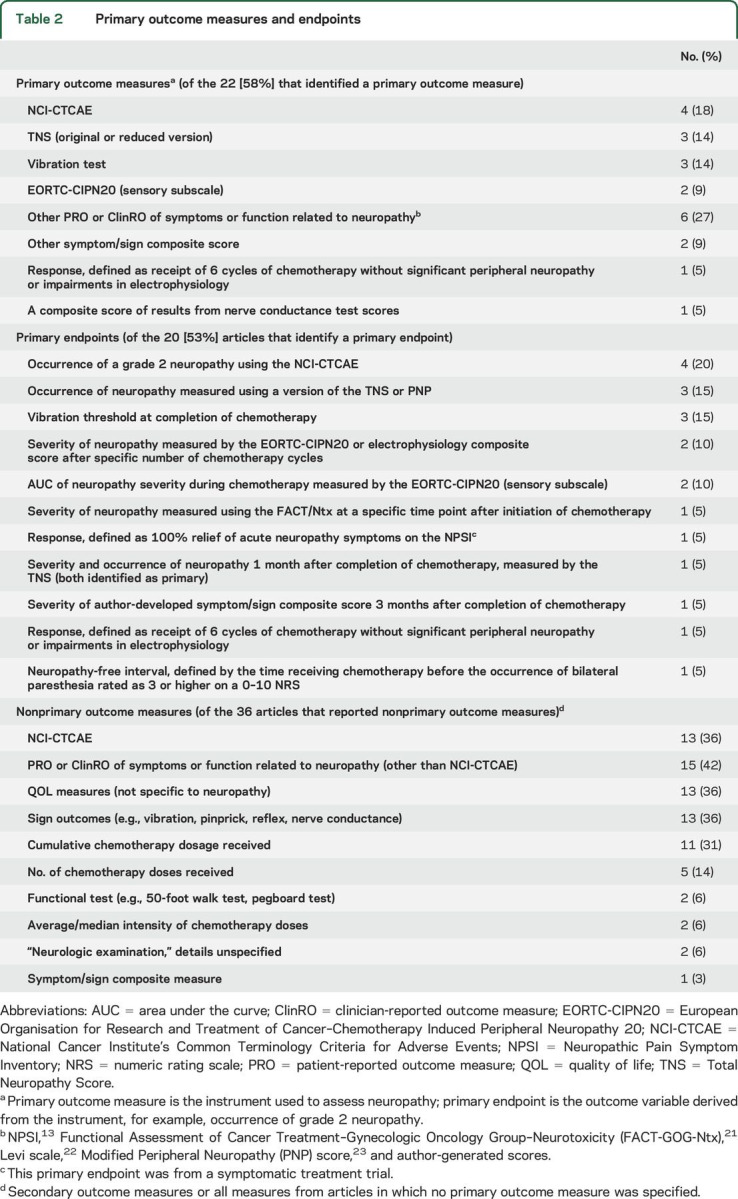
POMs that were used in more than 1 trial included the National Cancer Institute's Common Terminology Criteria for Adverse Events grading system (NCI-CTCAE)9 (n = 4 [18%]), a version of the Total Neuropathy Score10,11 (n = 3 [14%]), a vibration test (n = 3 [14%]), and the European Organisation for Research and Treatment of Cancer–Chemotherapy-Induced Peripheral Neuropathy 20 (EORTC-CIPN20) (sensory subscale)12 (n = 2 [9%]) (table 3). Only the single trial evaluating a treatment for acute CIPN symptoms used a primary outcome measure focused primarily on pain (i.e., the Neuropathic Pain Symptom Inventory13). The most common primary endpoints included the occurrence of grade 2 neuropathy as defined by the NCI-CTCAE (n = 4), the occurrence of neuropathy measured using composite measure of symptoms and signs (n = 3), and vibration threshold measured at the completion of chemotherapy (n = 3) (table 2).
Table 3.
Reporting of participant flow, chemotherapy completion, and accommodation of missing data
Thirty-six articles reported non-POMs (i.e., secondary outcome measures in articles that identified a POM and all outcome measures in articles that did not identify a POM). The most common non-POM was the NCI-CTCAE9 (n = 13 [36%]) (table 3). When considering all POMs and non-POMs, 15 (40%) articles reported only symptom measures, 6 (16%) reported both measures of symptoms and signs, 5 (13%) reported symptom measures and electrophysiology outcomes, 2 (5%) reported only sign outcomes, 2 (5%) reported symptom and objective function measures (e.g., pegboard test), 2 (5%) reported measures of symptoms and signs and electrophysiology outcomes, and 3 (8%) reported a symptom/sign composite measure with (1) sign measures, (2) electrophysiology outcomes, or (3) symptom and sign measures and electrophysiology outcomes. Pain was reported or analyzed separately from other symptoms in 10 articles. These pain assessments were frequently based on a single item from a composite outcome measure, grading system, or neurologic examination (n = 7).
Twenty-two (58%) articles mentioned some assessment of cancer progression in the trial. Ten stated that that no difference between groups in disease progression was detected. Twelve provided a measure of disease progression for each treatment group, 5 of which also stated that no difference was detected between the groups. Only one article provided tumor response rates by group, but acknowledged that due to the small sample size, the data could not support definitive conclusions regarding the effect of the experimental treatment on the efficacy of the chemotherapy.
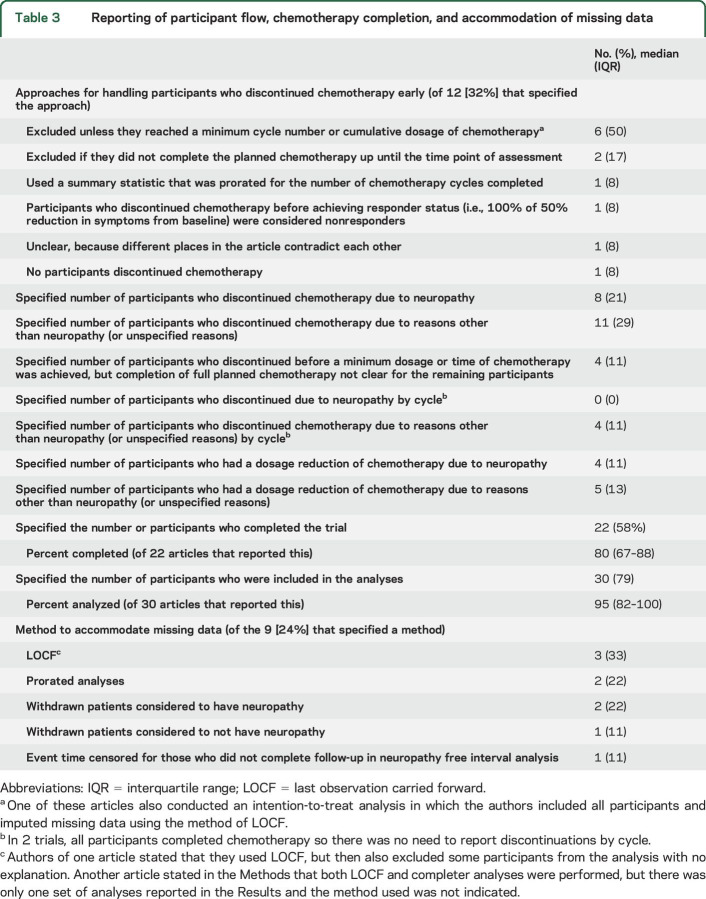
Only 12 (32%) articles indicated how data from participants who discontinued chemotherapy prematurely were handled in the analyses or that all participants completed the prescribed chemotherapy regimen. Excluding patients from the analyses unless they reached a minimum cumulative dosage of chemotherapy occurred most commonly (n = 6; table 3).
DISCUSSION
This systematic review identified common features among the research designs of trials evaluating treatments for CIPN administered during chemotherapy. For example, all but 2 of the trials began the experimental treatment before any neuropathy symptoms appeared, focusing on evaluation of the preventive efficacy of the intervention. A large majority of trials excluded patients with preexisting neuropathy. In contrast, two-thirds only assessed the ability of the treatment to prevent/treat CIPN symptoms occurring during chemotherapy while the experimental treatment was still being administered, a method that cannot distinguish preventive from symptomatic effects. Twenty percent assessed neuropathy symptoms following the cessation of chemotherapy and the experimental CIPN treatment, a method that allows evaluation of preventive effects if it can be assumed that symptomatic benefits have not persisted.
Only 10 of the articles provided any description of when the neuropathy assessments that occurred during chemotherapy were made in relation to chemotherapy treatments. Eight trials assessed the severity of neuropathy before the chemotherapy cycles, therefore evaluating subacute neuropathy symptoms that gradually increase with increasing cumulative dosages of neuropathy. Two trials measured neuropathy on days 1 through 5 after chemotherapy infusions, thus evaluating acute CIPN, occurring directly after each infusion. The remaining 28 articles did not indicate when in relation to chemotherapy the neuropathy assessments were made. This could simply reflect unclear reporting and not necessarily a lack of standardization of assessments. However, it does highlight the importance of standardizing the timing of assessments to ensure that subacute, acute, or both types of neuropathy symptoms are being measured consistently in clinical trials and that these symptoms, which are likely to have highly variable severity, are not analyzed together.
A variety of POMs and non-POMs were used to assess the severity of neuropathy. Patient-reported outcome (PRO) and clinician-reported outcome (ClinRO) measures for peripheral neuropathy that include both symptoms and signs have been developed for CIPN.14,15 However, no consensus exists regarding which of these measures is best to address a particular clinical trial objective. Moreover, few studies have assessed their reliability and validity or compared the performance of these measures.16,17 The lack of consensus regarding which measures of CIPN best represent the various neuropathy symptoms and signs is reflected in the results of this systematic review, which demonstrate little consistency across trials in the outcome measures used to assess neuropathy. Consistent inclusion of a specific CIPN measure in future RCTs and validation studies would help to standardize CIPN measurement and facilitate comparison across studies. Based on existing evidence,14 the current best candidate measure to include in all studies is the EORTC-CIPN20; however, future research to optimize this measure is necessary. Furthermore, inclusion of the EORTC-CIPN20 should not preclude the use of other primary or secondary outcome measures that may be useful for evaluating neuropathy from specific chemotherapies or in response to specific interventions. Finally, only 29% of articles identified a single primary analysis or adjusted for multiple analyses. Failure to prespecify a primary analysis can lead to multiple analyses, with only those that support the experimental treatment's efficacy being reported, increasing the chance of a false-positive conclusion.18–20
Approximately one-third of the trials included a general HR-QOL measure, highlighting the importance of evaluating the effects of treatments for CIPN on patients' health HR-QOL. Interestingly, only 2 trials included functional tests (e.g., pegboard or walking test). Furthermore, no studies included a measure that specifically aims to investigate the effects of CIPN symptoms on HR-QOL that does not also include items rating symptom severity. A measure targeted specifically at assessing the effects of CIPN on HR-QOL could improve our understanding of CIPN burden and improve evaluation of the meaningfulness of changes in the CIPN severity measured by the PROs and ClinROs often used in RCTs.
Endpoints were also highly variable across trials. They included neuropathy severity assessed at specific cycles, over the course of multiple cycles, or at specific time points after initiation of chemotherapy, as well as occurrence of neuropathy. Neuropathy severity following a specific cycle or time point after initiation of chemotherapy can be highly variable if multiple chemotherapy regimens with variable timing and magnitude of discrete doses are allowed in the study. This variability could be increased if a significant percentage of patients discontinue their chemotherapy earlier than planned because the severity of the neuropathy often subsides gradually with time after the last chemotherapy dose received.7 This phenomenon could lead to a decrease in neuropathy severity that is not attributable to the experimental treatment for participants who discontinue chemotherapy prematurely. If the RCT is evaluating an efficacious treatment, discontinuation of chemotherapy due to neuropathy may be more likely to occur in the placebo group than in the active group. Thus, when neuropathy is assessed at specific time points or cycles, the severity of neuropathy may be underestimated in the placebo group, leading to an estimated treatment effect that is biased toward the null. In contrast, endpoints such as the occurrence of neuropathy or the time or cumulative dosage to a specified severity of neuropathy accurately classify patients who discontinue chemotherapy because of neuropathy as having neuropathy.
The severity and occurrence of neuropathy are different endpoints. It would be advantageous to evaluate each separately, particularly because some treatments could decrease severity but not prevent CIPN altogether. However, due to the complicated nature of evaluating preventive and symptomatic treatments for CIPN during chemotherapy, obtaining an unbiased treatment effect estimate from an analysis of the neuropathy severity at a particular time point or after a particular number of chemotherapy cycles is challenging. Alternative endpoints could include composite measures that incorporate both the cumulative dosage of chemotherapy received and neuropathy severity. For example, the following endpoints could be considered: (1) response, defined as neuropathy severity that remains below various thresholds after receiving prespecified cumulative dosages of chemotherapy; or (2) discontinuation of chemotherapy due to neuropathy. Research is necessary to determine which endpoints would be most clinically meaningful to patients and have the highest assay sensitivity.
Regardless of which endpoints are used, it is important that the methodology and results are clearly reported so that readers understand the limitations of the analyses and conclusions. Only 22% of articles specified the number of participants who discontinued chemotherapy due to neuropathy and only one-third of the articles specified how the data from participants who prematurely terminated chemotherapy were accounted for in the analyses. This information is essential for proper interpretation of the study findings.
Eight articles reported excluding participants from the analyses if they did not reach a minimum cycle number or cumulative dosage of chemotherapy. Some may argue that removing data from participants who discontinue chemotherapy prior to a dose that is likely to cause neuropathy would be acceptable because these participants are not actually at risk of developing neuropathy. However, eliminating these participants can remove the benefits of randomization and lead to treatment groups that are not comparable with respect to important determinants of the outcome (known or unknown) and, hence, biased treatment effect estimates. Instead of excluding these participants from the primary analyses, the sample size of CIPN prevention trials could be increased to accommodate the percentage of patients anticipated to discontinue chemotherapy prior to reaching the minimum chemotherapy dosage likely to cause neuropathy. Sensitivity analyses that exclude participants who do not receive a protocol-defined minimum dosage of chemotherapy could be considered, with careful attention to adjustment for potential confounding that may result.
This review has limitations that should be acknowledged. We only assessed what was reported in publications, which may be different from the actual trial execution (e.g., some trials may have specified a primary endpoint in the protocol but not in the publication). In addition, we only examined RCTs of pharmacologic treatments; therefore, our results, including the types and severities of cancers, the chemotherapy treatments included, and the outcome measures used, may be different for studies of nonpharmacologic treatments. Finally, due to the lack of treatments for CIPN with established efficacy, we were not able to investigate whether any specific design or reporting characteristics were related to the treatment effect size reported in these RCTs.
The design of RCTs of treatments for CIPN prevention is highly variable, suggesting lack of consensus on the optimal designs for these studies. In addition, this systematic review identified many reporting deficiencies in publications of RCTs of treatments for both CIPN prevention and CIPN symptomatic treatment initiated during chemotherapy. Reporting recommendations are provided to enhance transparency and, in turn, better inform the design of future clinical trials (table 4). Improved clinical trial design will increase the probability that truly efficacious treatments can be identified for this important neurologic disorder with unmet clinical need.
Table 4.
Specific reporting recommendations for chemotherapy-induced peripheral neuropathy (CIPN) clinical trials initiated during chemotherapy
ACKNOWLEDGMENT
The authors thank Linda Hasman, MSLS, for conducting the literature search. This article was reviewed and approved by the Executive Committee of the Analgesic, Anesthetic, and Addiction Clinical Trial Translations, Innovations, Opportunities, and Networks public–private partnership with the US Food and Drug Administration.
GLOSSARY
- ASCO
American Society of Clinical Oncology
- CIPN
chemotherapy-induced peripheral neuropathy
- ClinRO
clinician-reported outcome
- EORTC-CIPN20
European Organisation for Research and Treatment of Cancer–Chemotherapy-Induced Peripheral Neuropathy 20
- HR-QOL
health-related quality of life
- NCI-CTCAE
National Cancer Institute's Common Terminology Criteria for Adverse Events grading system
- POM
primary outcome measure
- PRO
patient-reported outcome
- RCT
randomized controlled trial
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Jennifer S. Gewandter was involved in the design, analysis, and interpretation of the study. She coded the articles and drafted and revised the manuscript. Roy Freeman was involved in the design and interpretation of the study. He contributed intellectually to revisions of the manuscript. Rachel A. Kitt coded the articles and contributed intellectually to revisions of the manuscript. Guido Cavaletti contributed intellectually to revisions of the manuscript. Lynn R. Gauthier contributed intellectually to revisions of the manuscript. Michael P. McDermott was involved in the design and interpretation of the study. He provided statistical expertise when needed and contributed intellectually to revisions of the manuscript. Nimish A. Mohile contributed intellectually to revisions of the manuscript. Supriya G. Mohlie contributed intellectually to revisions of the manuscript. A. Gordon Smith contributed intellectually to revisions of the manuscript. Mohamedtaki A. Tejani contributed intellectually to revisions of the manuscript. Dennis C. Turk contributed intellectually to revisions of the manuscript. Robert H. Dworkin was involved in the design and interpretation of the study. He contributed intellectually to revisions of the manuscript.
STUDY FUNDING
Financial support for this project was provided by the Analgesic, Anesthetic, and Addiction Clinical Trial Translations, Innovations, Opportunities, and Networks (ACTTION) public–private partnership, which has received research contracts, grants, or other revenue from the Food and Drug Administration (FDA), multiple pharmaceutical and device companies, philanthropy, and other sources. The views expressed in this article are those of the authors and no official endorsement by the FDA or the pharmaceutical and device companies that provided unrestricted grants to support the activities of the ACTTION public–private partnership should be inferred. The preparation of this article was supported by the ACTTION public–private partnership, which has received research contracts, grants, or other revenue from the US FDA, multiple pharmaceutical and device companies, philanthropy, and other sources (list of current sponsors can be found at acttion.org/partners).
DISCLOSURE
J. Gewandter has received funds from Neurometrix for an investigator-initiated study of a TENs band for CIPN. R. Freeman, R. Kitt, G. Cavaletti, L. Gauthier, and M. McDermott report no disclosures relevant to the manuscript. N. Mohile has received funds from Neurometrix for an investigator-initiated study of a TENs band for CIPN. S. Mohlie, A. Smith, M. Tejani, and D. Turk report no disclosures relevant to the manuscript. R. Dworkin has received in the last 12 months research grants and contracts from US Food and Drug Administration and US NIH, and compensation from Abide, Aptinyx, Astellas, AstraZeneca, Biogen, Boston Scientific, Centrexion, Concert, Dong-A, Eli Lilly, Grace, Hope, Immune, Novartis, Olatec, Phosphagenics, Quark, Reckitt Benckiser, Regenacy, Relmada, Semnur, Trevena, and Vertex. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Seretny M, Currie GL, Sena ES, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain 2014;155:2461–2470. [DOI] [PubMed] [Google Scholar]
- 2.Ventzel L, Jensen AB, Jensen AR, Jensen TS, Finnerup NB. Chemotherapy-induced pain and neuropathy: a prospective study in patients treated with adjuvant oxaliplatin or docetaxel. Pain 2016;157:560–568. [DOI] [PubMed] [Google Scholar]
- 3.Mols F, Beijers T, Vreugdenhil G, van de Poll-Franse L. Chemotherapy-induced peripheral neuropathy and its association with quality of life: a systematic review. Support Care Cancer 2014;22:2261–2269. [DOI] [PubMed] [Google Scholar]
- 4.Hershman DL, Lacchetti C, Dworkin RH, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. JCO 2014;32:1941–1967. [DOI] [PubMed] [Google Scholar]
- 5.Loprinzi CL, Qin R, Dakhil SR, et al. Phase III randomized, placebo-controlled, double-blind study of intravenous calcium and magnesium to prevent oxaliplatin-induced sensory neurotoxicity (N08CB/Alliance). JCO 2014;32:997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reeves BN, Dakhil SR, Sloan JA, et al. Further data supporting that paclitaxel-associated acute pain syndrome is associated with development of peripheral neuropathy: North Central Cancer Treatment Group trial N08C1. Cancer 2012;118:5171–5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Argyriou AA, Bruna J, Marmiroli P, Cavaletti G. Chemotherapy-induced peripheral neurotoxicity (CIPN): an update. Crit Rev Oncol Hematol 2012;82:51–77. [DOI] [PubMed] [Google Scholar]
- 8.Friedman LM, Furberg CD, DeMets DL. Fundamentals of Clinical Trials. 4th ed. New York: Springer; 2010. [Google Scholar]
- 9.Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003;13:176–181. [DOI] [PubMed] [Google Scholar]
- 10.Cornblath DR, Chaudhry V, Carter K, et al. Total neuropathy score: validation and reliability study. Neurology 1999;53:1660–1664. [DOI] [PubMed] [Google Scholar]
- 11.Cavaletti G, Bogliun G, Marzorati L, et al. Grading of chemotherapy-induced peripheral neurotoxicity using the Total Neuropathy Scale. Neurology 2003;61:1297–1300. [DOI] [PubMed] [Google Scholar]
- 12.Postma TJ, Aaronson NK, Heimans JJ, et al. The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: the QLQ-CIPN20. Eur J Cancer 2005;41:1135–1139. [DOI] [PubMed] [Google Scholar]
- 13.Bouhassira D, Attal N, Fermanian J, et al. Development and validation of the neuropathic pain symptom inventory. Pain 2004;108:248–257. [DOI] [PubMed] [Google Scholar]
- 14.Gewandter JS, Burke L, Cavaletti G, et al. Content validity of symptom-based measures for diabetic, chemotherapy, and HIV peripheral neuropathy. Muscle Nerve 2017;55:366–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cavaletti G, Frigeni B, Lanzani F, et al. Chemotherapy-induced peripheral neurotoxicity assessment: a critical revision of the currently available tools. Eur J Cancer 2010;46:479–494. [DOI] [PubMed] [Google Scholar]
- 16.Cavaletti G, Cornblath DR, Merkies IS, et al. The chemotherapy-induced peripheral neuropathy outcome measures standardization study: from consensus to the first validity and reliability findings. Ann Oncol 2013;24:454–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alberti P, Rossi E, Cornblath DR, et al. Physician-assessed and patient-reported outcome measures in chemotherapy-induced sensory peripheral neurotoxicity: two sides of the same coin. Ann Oncol 2014;25:257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ioannidis JP. Why most published research findings are false. Plos Med 2005;2:e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gewandter JS, Smith SM, McKeown A, et al. Reporting of primary analyses and multiplicity adjustment in recent analgesic clinical trials: ACTTION systematic review and recommendations. Pain 2014;155:461–466. [DOI] [PubMed] [Google Scholar]
- 20.Turk DC, Dworkin RH, McDermott MP, et al. Analyzing multiple endpoints in clinical trials of pain treatments: IMMPACT recommendations: Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials. Pain 2008;139:485–493. [DOI] [PubMed] [Google Scholar]
- 21.Calhoun EA, Welshman EE, Chang CH, et al. Psychometric evaluation of the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group-neurotoxicity (Fact/GOG-Ntx) questionnaire for patients receiving systemic chemotherapy. Int J Gynecol Cancer 2003;13:741–748. [DOI] [PubMed] [Google Scholar]
- 22.Levi F, Misset JL, Brienza S, et al. A chronopharmacologic phase II clinical trial with 5-fluorouracil, folinic acid, and oxaliplatin using an ambulatory multichannel programmable pump: high antitumor effectiveness against metastatic colorectal cancer. Cancer 1992;69:893–900. [DOI] [PubMed] [Google Scholar]
- 23.Chaudhry V, Rowinsky EK, Sartorius SE, Donehower RC, Cornblath DR. Peripheral neuropathy from Taxol and cisplatin combination chemotherapy: clinical and electrophysiological studies. Ann Oncol 1994;35:304–311. [DOI] [PubMed] [Google Scholar]