Abstract
Brain metastases are an increasing global public health concern, even as survival rates improve for patients with metastatic disease. Both metastases and the sequelae of their treatment are key determinants of the inter-related priorities of patient survival, function, and quality of life, mandating a multidimensional approach to clinical care and research. At a virtual National Cancer Institute Workshop in September, 2022, key stakeholders convened to define research priorities to address the crucial areas of unmet need for patients with brain metastases to achieve meaningful advances in patient outcomes. This Policy Review outlines existing knowledge gaps, collaborative opportunities, and specific recommendations regarding consensus priorities and future directions in brain metastases research. Achieving major advances in research will require enhanced coordination between the ongoing efforts of individual organisations and consortia. Importantly, the continual and active engagement of patients and patient advocates will be necessary to ensure that the directionality of all efforts reflects what is most meaningful in the context of patient care.
Introduction
As survival rates for patients with cancer improve, brain metastases and the sequelae of their treatment are an increasing public health concern affecting growing numbers of patients with cancer. Management requires balancing inter-related priorities of patient survival, function, and quality of life, mandating a multidimensional approach to clinical care and research. Challenges specific to brain metastases stem from the unique and inadequately characterised brain tumour microenvironment, which modulates the initiation and progression of disease, and the subsequent adverse sequelae of therapy. Alteration of brain homoeostasis by the presence of brain metastases and the therapy needed to treat them directly contributes to patient survivorship and quality of life—more than in any other metastatic organ site.
Since the earliest seminal studies establishing the benefit of surgical resection in certain patients with limited intracranial disease,1 survival outcomes for particular subsets of patients have improved, but still remain suboptimal. Given the complexity of the metastatic continuum, the unique brain microenvironment, and the clinical challenges of obtaining tissue specimens, progress in the research and treatment of brain metastases has been slow. As outlined in the landmark US Food and Drug Administration (FDA) guidance in 2020,2 patients with brain metastases have historically been excluded from clinical trials given concerns around their shortened life expectancy and poor performance status with associated risks of toxicity. Moreover, few US federal funding resources and funding mechanisms have been specifically devoted to brain metastases research. This limited funding allocation might be related, in part, to the low number of funding applications focused on brain metastases; in the fiscal year 2022, new application submissions for brain metastases research comprised only 1·5% of all applications to the National Cancer Institute (NCI), reflecting a paucity of submissions despite the high incidence and impact of brain metastases. Among the NCI research awards reviewed between 2012 and 2023, the number of proposals submitted with a focus on brain metastases was approximately 60% lower than the number of proposals for glioblastoma, despite the substantially higher incidence of brain metastases. Although the success rate of funding appears to be similar on average between both groups (figure 1), why so few proposals are submitted for this area of unmet need is unclear.
Figure 1:
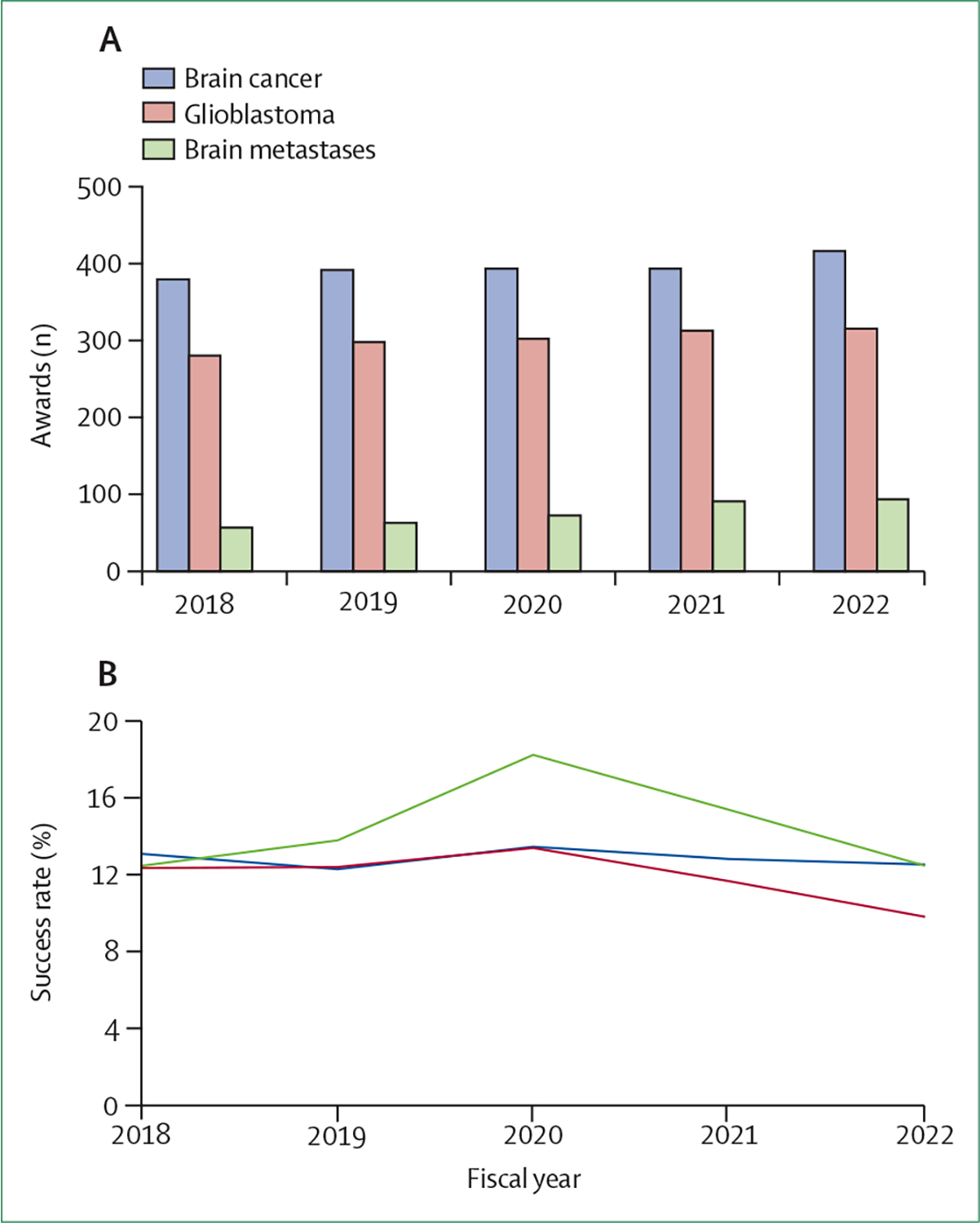
National Cancer Institute awards (A) and success rates (B) for brain cancer, including brain metastases, for the fiscal years 2018–22
At the virtual NCI Workshop on Shaping the Landscape of Brain Metastases Research (Sept 29–30, 2022), key stakeholders convened to define research priorities addressing crucial areas of unmet need in brain metastases research, and to propose new collaborations to address these priorities. These stakeholders included patients and patient advocates, clinicians and translational scientists, leaders from NCI-funded clinical trial networks, advocacy and foundation partners, academic societies, the FDA, and NCI leadership (panel 1). At the outset, in a discussion led by survivors of brain metastases, the often-used phrase of a meaningful advance was defined as “expedient discovery towards the cure or prevention of brain metastases that would enable patients to maintain maximal function, cognition and quality of life”. With this foundation, key challenges (panel 2) and recommended priorities for brain metastases research emerged.
Key challenges and knowledge gaps
Do research priorities align with patient-defined meaningful advances?
In a survey of patients with cancer conducted before the workshop by a participating patient advocate (CH), a key identified priority was gaining control of or curing brain metastases while maintaining a good quality of life. Among the phase 2 and 3 clinical trials conducted over the past decade that have defined new standard therapies for patients with brain metastases,3,4 only a small number have directly incorporated primary and secondary endpoints addressing durable disease control in conjunction with the maintenance of patient’s neurological function and quality of life. Given the rapid pace of drug development, whether common endpoints, such as radiographic response indicating activity in the CNS, are of substantial long-term or meaningful significance to patients is unclear. Moreover, how patient function and quality of life are affected by newer systemic agents with CNS activity in combination with, or in lieu of, surgery and radiotherapy is largely unknown. This knowledge gap is anticipated to grow with the rapid pace of drug development and the increasing number of trials without a backbone of standard-of-care local therapies.
As indicated by the patients and patient advocates represented at this workshop, novel approaches are needed not only for the treatment of established brain metastases, but also for their earlier detection and prevention.5,6 At present, the available guidelines7–14 for screening asymptomatic patients with cancer for brain metastases are not optimally tailored to the individual patient or to the subsets of patients who are at risk of developing brain metastases, whether on the basis of clinical or molecular predictors. Earlier detection is preferred over identifying advanced metastases after symptom onset, given the potentially higher toxicity of local therapies at later stages of CNS involvement.15,16 Yet neither a standard MRI protocol nor a clear identification of the subsets of patients who are at high risk for brain metastases has been established for each primary cancer subtype. This knowledge gap is partly related to limitations in the collection of epidemiological data and the absence of mandated reporting of brain metastases to local and federal registries such as the Central Brain Tumor Registry of the United States.17 As a result, the true population-based incidence of brain metastases and rate of neurological death due to intracranial disease by histological or molecular subtype are unknown. More importantly, the absence of these data limits our ability to construct models identifying patients who are at greatest risk of developing brain metastases.
Very few clinical trials18 have addressed the prevention of brain metastases for several possible reasons. Focused trials with key goals of primary and secondary prevention are rare, in part due to inadequate knowledge of which patients to recruit and to the anticipated lengthy duration, size, and cost of such trials. Insufficient emphasis on the development of agents and drug classes that affect the tumour microenvironment and modulate metastatic initiation is underscored by scarce funding for both preclinical and clinical investigations in this line of research. To our knowledge, no primary or secondary prevention studies for brain metastases have been conducted through the Experimental Therapeutics Clinical Trials Network or the National Clinical Trials Network of the United States. Many recent and developing trials over the past 5–7 years have been limited to available, industry-driven drug candidates primarily designed for systemic efficacy, with secondary evaluation of their activity in established brain metastases, and very little to no emphasis on the prevention of brain metastases. Many positive clinical trials of these therapies have produced only months of improvement in progression-free survival, with attendant adverse effects.
Can the spectrum of patient experience be adequately characterised to target areas for improvement?
Although instruments to assess patients’ brain tumour symptoms, neuropsychology, and quality of life are commonly used in clinical trials,19 no standardised toolkit has been established to measure these domains in patients with brain metastases.20 Among the practice-changing phase 2 and 3 trials in brain metastases treatment published over the past 5 years that evaluated neuroprotective strategies with radiation, or drug therapy regimens in lieu of radiation, substantial variation exists in the extent and type of the functional assessments undertaken.3,4 Approaches have ranged from no specific functional evaluation, to estimating global (non-brain-specific) health status, and implementing brain-specific batteries assessing patients’ neurocognition,21 symptoms, and quality of life. An even greater unmet need is the identification of instruments specific for brain metastases that could be feasibly implemented across organisations for integration into routine clinical care, outside of the rigour of prospective clinical trials. The unique trajectory of the disease course often includes repeated and combined methods of treatment over months to years, with the potential need for supportive medications (eg, steroids), and poorly understood interactions between therapies. Adequately profiling the longitudinal trajectory of patients with brain metastases both in clinical trials and in clinical practice is imperative.22–24 Moreover, understanding the extent and effect of interactions between newer systemic therapy agents and standard-of-care therapies (ie, radiotherapy) is crucial. These data are not captured in brain metastases trials designed to assess the benefit of systemic therapy regimens without a backbone of radiation treatment and must, therefore, be assessed with real-world data. A major limitation of this method of data collection includes the potential for bias (known and unknown), precluding definitive conclusions regarding the comparative efficacy and toxicity of single or combined methods of therapy.
Integrating patient-reported outcomes in the care of patients with metastatic cancer has been shown to reduce emergency room visits, increase patients’ adherence to treatment, and improve patients’ quality of life and overall survival.25 Although progress is being made in incorporating patient-reported and objective function assessments as primary and secondary objectives in NCI-funded clinical trials, optimising the large-scale integration of instruments specific to brain metastases into routine clinical practice will substantially increase the available data for analysis and action. Leveraging big data and technology platforms from non-medical industry stalwarts with artificial intelligence-powered informatics analyses could ultimately power predictive models that accurately estimate clinical outcomes. A focus on easy-to-use toolkits via online and electronic applications might enhance patient participation, potentially allowing patients to provide their own clinical data. Optimal integration across organisations’ electronic medical records and demonstration of the value and medical necessity of acquiring this data in routine care will be important in aiding widespread adoption in clinical practice as a communication tool and platform for shared decision making.
Can functional determinants be identified to mitigate risk factors for neurological injury?
Although neurological injury from cancer therapy is a multifactorial process with evidence for the centrality of dysregulated inflammation,26–28 the intraindividual and interindividual risk factors for clinically meaningful functional and cognitive impairments are poorly understood. Identification of modifiable and protective factors is crucially needed. Although individual patients have differing genetics, cofactors, and exposures that influence their susceptibility to toxicity, precisely how different patients experience greater injury (or impaired recovery) after exposure to therapies for brain metastases is unknown. A substantial number of patients still require whole-brain radiotherapy, and the consequences of repeated stereotactic radiosurgery and the under-studied effect of newer CNS-active systemic agents on neurological function underscore the importance of identifying modifiable risk factors for neurological injury. The potential benefits of enhancing patients’ resilience to injury through cognitive or physical exercise29 or of addressing concurrent mood related symptoms (eg, depression and anxiety) are yet to be fully explored.
At present, no validated screening tools exist to identify which patients are at highest risk for delayed, irreversible structural and functional neurological injury. Specific tools to assess mechanisms underlying neurological function (eg, neurotransmission, synaptic plasticity, glial homoeostasis, and neurogenesis) have not been translated to patient studies and should be developed and studied alongside the broader functional assessments currently in clinical use.
How therapy might most effectively be individualised to maximise safety, or whether patients who are at higher risk for neurological injury should be considered for de-intensified treatment strategies (without compromising disease control or survival), as opposed to offering intensified therapies to patients at lower neurological risk, is unknown. As a result, most therapies for brain metastases follow a one-size-fits-all approach. Understanding which specific impairments are most crucial to patients and understanding their dynamics in the treatment and survivorship timeline is imperative to improve the therapeutic ratio of standard and investigational therapies.
Which tumour and microenvironmental factors determine brain tropism, metastatic initiation, and progression?
The primary determinants of brain tropism, the initiation of micrometastases, and the progression to macrometastases are not well understood. Although a wide variety of primary tumour types metastasise to the brain, the unknown common denominator (if present) could involve a unique mutational30 and epigenetic evolutionary profile, promoting brain tropism and tumour cell survival through adaptation to the microenvironmental niche or construction of a new brain microenvironment. Better identification of the relationships between cells in the tumour microenvironment (eg, astrocytes, neurons, microglia, and immune cells) and tumour cells during the development of brain micrometastases31,32 is crucial for the design of new and innovative prevention trials. Further development of haematogenous or patient-derived xenograft preclinical models33 with an emphasis on understanding the relationship between novel therapies and standard-of-care treatments is needed. Preclinical models can efficiently facilitate the prioritisation of targets and therapies for clinical testing and validation. The development and testing of agents with blood–tumour barrier permeability that modulate the role of the tumour microenvironment in the formation and progression of metastases should be emphasised.
Biomarkers to predict the development, progression, and treatment resistance of brain metastases are scarce.34 Key unanswered questions, including which subsets of patients could safely defer radiation and surgery in lieu of newer CNS-active systemic agents, will require the identification of biomarkers to individualise care. Molecular, cellular, imaging, and even digital biomarkers should be explored through the prospective collection of multimodality datasets. Advances in mechanistic understanding of brain metastatic colonisation will lead to new, potentially brain-specific preventive therapies with subsequent improvements in disease control, quality of life, and potentially survival.
How should clinical trials be conducted to efficiently test promising therapies?
At present, the necessary coordination to support the efficient conduct of high-risk, high-impact brain metastases trials is poorly executed across organisations. The development and implementation of clinical trial concepts is opportunistic and focused on the priorities of an individual organisation, rather than on promoting a coordinated and unified series of trials on the basis of a well defined national vision and strategy. A collaborative and unified approach is required to move beyond incremental advances to major breakthroughs in the field.
Survival as an endpoint is an insufficient measure of a meaningful outcome in this patient population with a substantial competing risk of systemic disease progression. Endpoints that sufficiently capture elements of patient function should be routinely incorporated into trials specific for brain metastases. Novel and composite endpoints including survival, free of the development of initial or additional brain metastases, neuro progression-free survival (including neurological and neurocognitive function), intracranial progression-free survival (freedom from salvage therapies), freedom from neurological death, and survival with equal or less cognitive and functional decline should be implemented and tailored to the expected effect of the intervention under study.
Given the pace of drug discovery, novel trial designs should be encouraged that permit the rapid opening and closing of arms, with an emphasis on combinatorial drug–radiotherapy approaches based on sound biological rationale. Although novel trial designs are being implemented for rapid assessment of drug therapies, including in select patient populations with brain metastases, these streamlined trials should incorporate standard-of-care therapies (ie, radiotherapy and surgery) in rational combination or sequence with newer drug therapies. As most patients with brain metastases will require radiotherapy,4 prospective, comparative data characterising the potential synergy or toxicity of drug–radiotherapy combinations are crucial. Consideration of planned subset accrual for patients with known brain metastases in clinical trials evaluating systemic agents with CNS penetrance should be incorporated early in the clinical trial design process.35 Importantly, multidisciplinary team engagement including neurosurgeons, radiation oncologists, medical oncologists, and neuro-oncologists is necessary for patient recruitment and the successful conduct of high-impact clinical trials.
What barriers should be addressed to ensure adequate inclusion and representation in research?
As expressed by the patients and patient advocates at this workshop, involving patients early in the study development process will enhance study participation and enrolment in brain metastases trials. Patient input in trial design and the development of a dissemination plan and outreach materials is important for the successful conduct of clinical trials. Investigators should share data showing why the trial might be beneficial and help patients to understand why their responses and participation matter. During this process, investigators often discover patient willingness to engage in high-risk, high-reward research studies, especially when enabled by open communication regarding the valuable knowledge gained by their participation.
In the process of scientific research, ensuring adequate representation of diverse patient populations is crucial to ensure the generalisability of the results and to maximise the safety and efficacy of interventions.36 Challenges with travel, childcare, or access to electronic technologies might limit the participation of some patient populations. Disparities in access to advanced treatments, coordinated care at academic centres, and supportive therapies must be effectively addressed to mitigate persistent disparities in cancer outcomes.36
Major effort should be devoted to minimising delays and barriers in access to clinical trials for brain metastases.36–38 Lowering the barriers to entry includes reducing the minimum size criteria of measurable disease, optimising the classification of leptomeningeal disease (which might exclude patients from some trials), opening a separate trial group to include patients with leptomeningeal disease, and incorporating cohorts of patients with brain metastases in phase 1 or phase 2 trials when no major safety signals emerge in dose-finding studies.
Ultimately, the infrastructure of routine clinical care should be optimised through the development of multidisciplinary, patient-centric brain metastases clinics39–41 and CNS metastases care coordination programmes.42 Best practices from these programmes streamline multidisciplinary care and decrease time to initiation of CNS-directed therapies.43,44 Such multidisciplinary clinics can facilitate substantially higher enrolment onto clinical trials.39,41,42 As therapies continue to develop for molecular subgroups, agnostic to primary cancer type, greater coordination across disease sites and specialties will be increasingly paramount to ensure timely treatments and optimal patient accrual onto clinical trials.
How should common priorities be defined to enable collaborative national and international data sharing and accelerated discovery?
At present, numerous databanks incorporating clinical, treatment, and follow-up data of varying scope and magnitude have been established for patients with brain metastases across individual institutions, co-operative groups, and consortia. No common set of data elements has been established across these efforts, nor has standardised data entry been achieved to allow the required scale of analysis necessary to inform next-generation clinical trials.
Given the vast quantity of multidimensional, longitudinal data types required, public–private–academic partnerships with advanced artificial intelligence and machine learning algorithms will be needed to analyse digitalised real-world data. Multimodality data should include imaging; pathology; molecular, patient-reported, and objective function measures; social determinants of health; toxicity; and disease control and survival outcomes. Data collection must be modular, interoperable across vendors and organisations, and responsive to the rapidly changing landscape of therapies.
This NCI workshop was the first of its kind to convene the breadth of relevant stakeholders to reach a consensus on these key challenges and knowledge gaps and to define recommended priorities (figure 2) for future brain metastases research.
Figure 2:
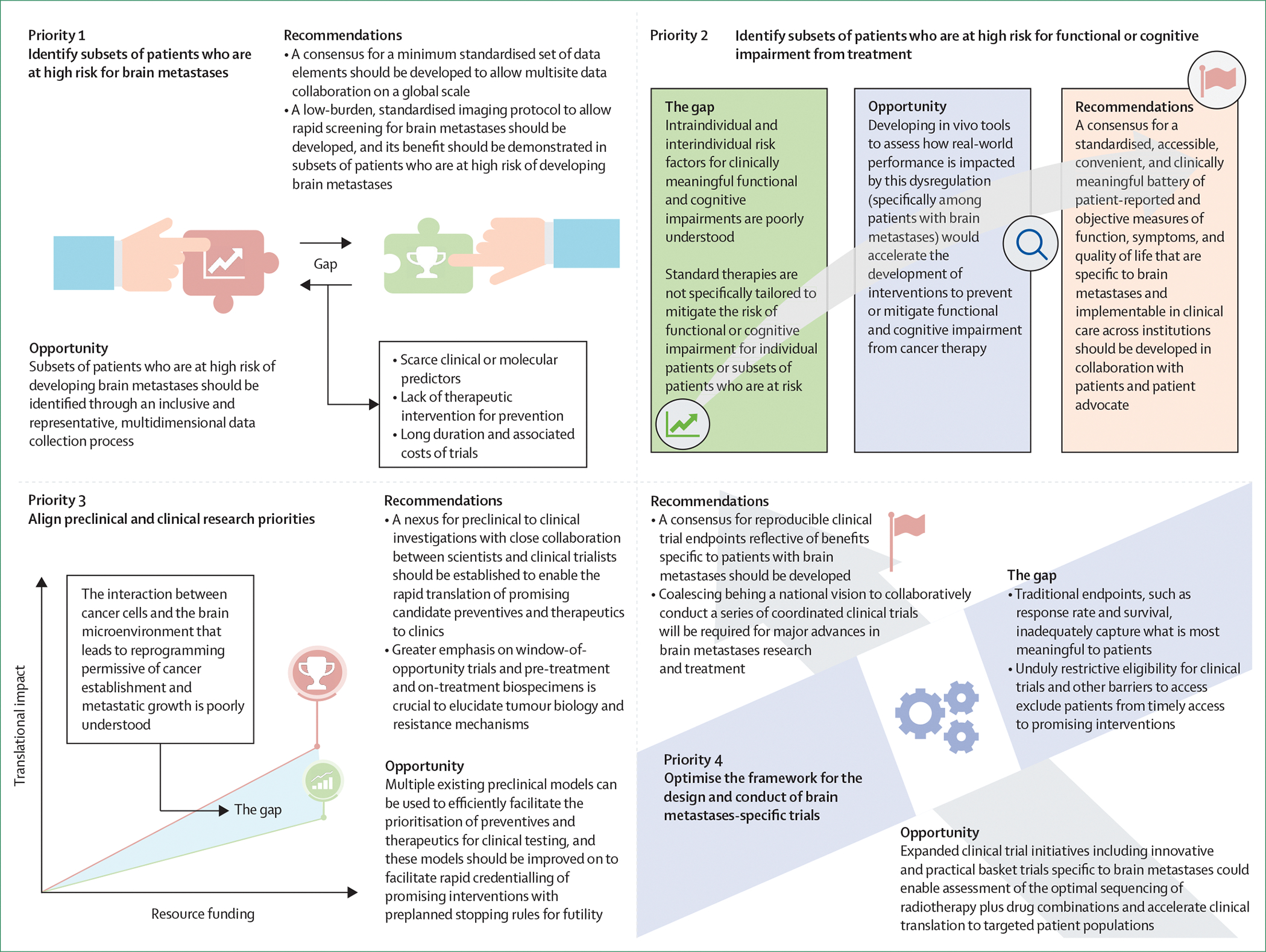
Recommended priorities for collaborative research on brain metastases
Recommended priorities for collaborative brain metastases research
Priority 1: identify subsets of patients who are at high risk for brain metastases
An unmet need is that patient populations who are at high risk of developing brain metastases are not well defined.
To advance the areas of enhanced screening and early intervention, and primary and secondary prevention of brain metastases, the specific subsets of patients who are at high risk for developing brain metastases should be identified through an inclusive and representative multidimensional data collection process. Understudied opportunities exist for screening before and disease monitoring after the development of brain metastases. These opportunities include low-burden, standardised MRI with the potential for rapid, automated assessment; liquid screening detection tools for molecular profiling of cerebrospinal fluid and blood; cognitive and symptom measures with online or app-based platforms for ease of patient and provider use; and wearable biometric technologies.
We recommend the development of a consensus for a minimum standardised set of data elements with the flexibility to incorporate advances in knowledge that permit multisite data collaboration on a global scale. A standardised imaging protocol to allow rapid screening for brain metastases in subsets of patients who are at high risk should also be developed, and the cost-effectiveness of its implementation across these patient subsets should be shown.
Priority 2: identify subsets of patients who are at high risk for functional or cognitive impairment from treatment
An unmet need is that standard therapies are not specifically tailored to mitigate the risk of functional or cognitive impairment for individual patients or subsets of patients who are at risk of these sequelae.
Numerous reporting and performance tools tailored to patients with primary brain tumours have been implemented across clinical trials. These tools measure multiple aspects of patients’ quality of life, including their neurocognition, ability to complete daily tasks, and psychosocial wellbeing, and present a unique opportunity to longitudinally characterise dysfunction and identify potential risk factors. Developing tools to best assess real-world performance among patients with brain metastases would help accelerate the development of interventions to prevent or mitigate functional and cognitive impairment from cancer therapy.
We recommend the development of a standardised, accessible, convenient, and clinically meaningful battery of patient-reported and objective measures of function, symptoms, and quality of life that are specific to brain metastases and implementable in routine clinical care across institutions. This standardised tool should be developed in collaboration with patients and patient advocates, to maximise patient participation and benefit.
Priority 3: align preclinical and clinical research priorities
An unmet need is that although many in vivo preclinical models for brain metastases evaluate the prevention of brain metastases, most clinical trials evaluate the treatment of established brain metastases. The interaction between cancer cells and the brain microenvironment that leads to reprogramming allowing for the establishment and initiation of metastatic growth might be distinct from the microenvironment of an established lesion. Thus, therapies developed in a preventive, preclinical setting might not be applicable in therapeutic clinical trials.
Expanding beyond the existing systemic therapy pipeline, an avenue is needed to develop and test preventive and therapeutic interventions that target the tumour microenvironment. Multiple existing haematogenous or patient-derived xenograft preclinical models can be used to efficiently prioritise preventives and therapeutics for clinical testing. These models should be improved on to facilitate the rapid credentialling of promising interventions with preplanned stopping rules for futility. Preclinical models should also include clinically relevant endpoints and explore the relationship between novel therapies and standard-of-care radiotherapy and surgery.
We recommend the establishment of a nexus for preclinical to clinical investigations with close collaboration between scientists and clinical trialists to enable the rapid translation of promising candidate preventives and therapeutics to clinical trials and patient care. In conjunction with preclinical models, greater emphasis on window-of-opportunity trials and obtaining both pre-treatment and on-treatment biospecimens is crucial to elucidate biological mechanisms and to develop biomarkers to predict the development of metastases and treatment resistance.
Priority 4: optimise the framework for the design and conduct of brain metastases-specific trials
An unmet need is that traditional endpoints for brain metastases clinical trials, such as response rate and survival, do not adequately capture what is most meaningful to patients. Moreover, unduly restrictive eligibility criteria hindering clinical trial enrolment, inefficiencies in the process to start protocol therapy, and barriers to access exclude patients from timely treatment with promising interventions. Inadequate intergroup collaborations underscore the absence of a pipeline of executable clinical trials framed to promote a well defined, overarching vision to advance the field.
Expanded clinical trial initiatives, including brain metastases-specific basket trials with innovative, practical design and targeted incorporation of informative endpoints, might enable assessment of the optimal sequencing of radiotherapy plus drug combinations and accelerate clinical translation to specific patient populations.
We recommend the development of a consensus for reproducible clinical trial endpoints that are reflective of benefits specific to patients with brain metastases, the phase of the trial, and the category of intervention. Ultimately, coalescing behind a national vision to collaboratively address areas of unmet need through a series of coordinated clinical trials will be required for major advances in brain metastases research and treatment.
Conclusion
Cancer research is typically organised around the cell of origin or anatomical site of the primary tumour. Therefore, a structural nexus to understand the brain metastases continuum that spans tropisms, unique brain-specific niche construction, points of treatment interventions, and quality of life is a persistent unmet need. Meaningful advances in treatment and outcomes will require an integrative, systems-level approach that builds on innovative programmes, such as the NCI Metastasis Research Network, aimed at characterising mechanisms across the metastatic continuum and therapeutic response.
Achieving meaningful large-scale advancement in brain metastases research will require enhanced collaboration and coordination between the ongoing efforts of individual groups and institutions. Convening the many varied stakeholders to coalesce as a community for the purpose of collectively enumerating the top priorities to shape future brain metastases research efforts was the first crucial step and intention of this workshop. Next, focused working groups with multidisciplinary expertise will be assembled from these stakeholder groups to specifically address the key challenges of each priority. Progress will be benchmarked and recalibrated moving forward with guidance from the NCI and, importantly, the working groups will continually and actively engage with patient advocates to ensure that the directionality of all efforts reflects what is most meaningful in the context of patient care. Successful output from these working groups will require continued collaboration with the advocacy groups, professional societies, and organisations represented at this workshop and others, whose ongoing efforts might now more effectively converge on these key priorities.
Translating promising treatment options to clinical practice requires multidisciplinary coordination. Partnerships with community providers and patient advocates to promote education and the dissemination of knowledge and to localise patient access to optimal care for brain metastases will help to ensure that the impact of these collective scientific endeavours will equitably and inclusively benefit all patients.
Panel 1: Organisations and societies represented by the experts at the NCI Collaborative Workshop on Shaping the Landscape of Brain Metastases Research.
Alliance for Clinical Trials in Oncology
American Association for Cancer Research
American Brain Tumor Association
American Society of Clinical Oncology
American Society for Radiation Oncology
Consortium for Intracranial Metastasis Academic Research
Department of Health and Human Services Food and Drug Administration
Guiding Researchers & Advocates to Scientific Partnerships (GRASP)
Living Beyond Breast Cancer
LUNGevity Foundation
Melanoma Research Foundation
Metastatic Breast Cancer Alliance
National Brain Tumor Society
National Cancer Institute Center for Cancer Research
National Cancer Institute Division of Cancer Biology
National Cancer Institute Division of Cancer Treatment and Diagnosis
National Surgical Adjuvant Breast and Bowel Project, Radiation Therapy Oncology Group, and the Gynecologic Oncology Group (NRG) Oncology
The Radiosurgery Society
Society for Immunotherapy of Cancer
The Society for Neuro-Oncology
Touch4Life
Panel 2: Key questions specific to research on and treatment for brain metastases and their associated knowledge gaps and challenges.
Do research priorities align with patient-defined meaningful advances?
Knowledge gap
Whether common endpoints in brain metastases clinical trials (eg, radiographic response) constitute meaningful advances for patients is unknown
With the rapid pace of drug discovery, how CNS-active systemic agents affect patient function, quality of life, and long-term disease control in combination with, or compared with, radiotherapy is largely unknown
Although earlier detection of brain metastases is a stated priority of patients and patient advocacy groups, subsets of patients who are at high risk of developing brain metastases have not been clearly defined, and no standardised screening tool exists
Although primary and secondary prevention of brain metastases remain an ultimate goal to improve patient outcomes, very few trials have been designed to answer this question
Challenges and barriers
Few brain metastases trials have emphasised functional or quality-of-life endpoints along with metrics of disease control and survival
Accurate population-based estimates of the incidence and outcomes of brain metastases in the USA are unavailable due to the absence of mandated reporting of cases to local and federal US registries and non-uniform data collection across institutions
Inadequate emphasis on prevention trials relates to poor knowledge of which patients to recruit, the prohibitive cost and duration of trials, and insufficient emphasis on the research and development of this therapeutic class
Can the spectrum of patient experience from CNS metastases treatment and survivorship be adequately characterised to target areas for improvement?
Knowledge gap
Patients with brain metastases have a unique disease trajectory, including combined methods of treatment for systemic and CNS disease; however, no standardised brain metastases-specific instruments have been developed and uniformly implemented to adequately measure patient symptoms, performance, toxicities, or quality of life and their dynamics over time
Most modern systemic therapy clinical trials do not capture the effect of delayed toxicities and interactions with standard-of-care treatment, such as radiotherapy
Real-world data are also scarce, as most centres do not incorporate patient-reported outcomes or functional assessments of patients with brain metastases into routine clinical practice
Challenges and barriers
The optimal integration of patient-reported and objective functional assessment tools into the electronic medical record across organisations must be established
The value of collecting patient-reported and objective performance measures as a clinical communication tool and platform for shared decision making should be shown
Simple and automated electronic data collection platforms supplemented with traditional methods of data collection might allow patients to provide their own clinical data and help overcome barriers to access for all patients
Can key determinants of function be identified to address modifiable risk factors for neurological injury from therapies for brain metastases?
Knowledge gap
The specific impairments that are most important to patients and their dynamics during the timeline of cancer treatment and survivorship are not well characterised
Intraindividual and interindividual risk factors for functional and cognitive impairments from therapies for brain metastases are poorly understood
The benefits of addressing psychological distress and enhancing patients’ resilience to neurological injury through exercise and rehabilitative interventions have not been well explored
Challenges and barriers
Validated screening tools must be developed to identify which patients are at the highest risk of delayed, irreversible structural and functional neurological injury
Specific tools assessing the factors underlying neurological impairment (eg, neurotransmission, synaptic plasticity, glial homeostasis, and neurogenesis) should be translated from the preclinical to the clinical arena
Standard and investigational therapies must be tailored to the neurological risk of the individual patient
Which tumour and microenvironmental factors determine brain tropism, metastatic initiation, and progression?
Knowledge gap
The primary determinants of brain tropism, the initiation of micrometastases, and their progression to macrometastases are unknown
Insufficient emphasis has been placed on elucidating the role of the tumour microenvironment in the metastatic cascade and developing agents that modulate the role of the tumour microenvironment
Biomarkers to predict brain metastases development, progression, and treatment resistance are scarce
Challenges and barriers
Further development of preclinical models with an emphasis on understanding the relationship of novel therapies to standard-of-care radiotherapy and surgery is needed
An avenue is needed to develop and test preventive and therapeutic interventions that target the tumour microenvironment and expand beyond the industry-driven systemic therapy pipeline
Streamlined workflows are needed to collect multidomain molecular, cellular, imaging, and digital data to identify and validate biomarkers for the development of brain metastases and treatment resistance
How should clinical trials for CNS metastases be conducted to efficiently test promising therapies?
Knowledge gap
Beyond survival and radiographic response, a standardised set of composite endpoints that capture meaningful elements of patient function, quality of life, freedom from new brain metastases, or salvage therapies should be routinely incorporated into therapeutic clinical trials for brain metastases
Novel trial designs for rapid assessment of newer drug therapies should incorporate standard-of-care treatment (eg, radiotherapy or surgery) in rational combination or sequencing with newer drug therapies
Challenges and barriers
Clinical trial concept development and implementation is opportunistic, and focused on the priorities of an individual organisation, rather than on promoting a coordinated and unified series of trials on the basis of a well defined national vision and strategy
A poor framework or familiarity with novel composite endpoints (disease control plus function) could hinder the development of beneficial trials for patients with brain metastases
Multidisciplinary team engagement including neurosurgeons, radiation oncologists, medical oncologists, and neuro-oncologists is crucial for patient recruitment and the successful conduct of high-impact clinical trials.
What barriers should be addressed to ensure adequate inclusion and representation of all patients in brain metastases research?
Knowledge gap
Insufficient early communication and feedback to patients regarding study rationale or output from research, participation could hinder patient willingness to engage in research activities
Inadequate peer education and partnership with community providers could limit patient access to clinical trials or timely, coordinated care
Overly restrictive entry criteria for brain metastases clinical trials limit available therapies and progress in areas of greatest need
Challenges and barriers
Inadequate representation of diverse patient populations limits the generalisability of results
Disparities in access to advanced treatment methods, coordinated care at academic centres, and supportive therapies must be effectively addressed to mitigate persistent disparities in cancer outcomes
Implementation of multidisciplinary, patient-centric clinics and care coordination programmes for brain metastases will be paramount to optimise clinical care and accrual onto clinical trials for this patient population
How should common priorities be defined to enable collaborative data sharing and accelerated discovery on a national and international scale?
Knowledge gap
No consensus regarding standardised, minimum data elements for brain metastases registries has been defined to allow for the national or international pooling of data
Public–private–academic partnerships to develop longitudinal, multimodality, real-world databases designed to advance discovery for brain metastases have not been realised on a large scale
Challenges and barriers
Individual efforts of institutions, consortia, research organisations, and professional societies should be coordinated to expand the impact of shared knowledge and research
Data collection must be modular, interoperable across vendors and organisations, and responsive to the rapidly changing landscape of cancer therapies
Digitalisation of a vast quantity of data types will be required to use artificial intelligence and machine learning platforms capable of addressing unanswered questions that will inform next-generation clinical trials
Search strategy and selection criteria.
No formal literature search was performed. Consensus guidelines for brain metastases management from 2018 to 2023 were reviewed, and additional literature was identified through a review of guideline bibliographies and reference lists cross-referenced by searching PubMed from database inception to April 1, 2023, with the terms “brain metastases” and “guidelines”. Only full-text articles published in English were reviewed. The final reference list was generated on the basis of the relevance of the papers to the scope of this Policy Review.
Acknowledgments
We sincerely thank Brunilde Gril and Bhadrasain Vikram for their support and collaboration as part of the core planning team, their participation in the workshop, and their contributions to this Policy Review. We thank Nyun Calvin Han and Elizabeth Duke from the US Food and Drug Administration (FDA) for their participation in the workshop and their valuable perspectives. MMK was supported by the NIH/NCI (grant number R50CA276015). MHG was supported by the NIH/NCI (grant number U54CA261717). MMK received salary support from the NIH/NCI (grant number R50CA276015). MHG received salary support from the NIH/NCI (grant number U54CA261717). The authors were not precluded from accessing data in the study, and they accept responsibility for submitting for publication. This Policy Review reflects the views of the individual authors and should not be construed to represent official views or policies of the NCI or the FDA.
Footnotes
Declaration of interests
TSA, DKS, PSS, JAH, MGE, JSB-S, PGP, CNC, and MMA are employees of the US National Institutes of Health. BME received consulting fees from Medicenna, MedQIA, Servier, Chimerix, Sumitomo Dainippon Pharma Oncology, ImmunoGenesis, Ellipses Pharma, Alpheus Medical, Curtana Pharma, Sagimet Biosciences, and Sapience Therapeutics; and other support from Siemens. SCF received grants from Siemens and Neosoma; consulting fees from Medicenna, MedQIA, Servier, Chimerix, Sumitomo Dainippon Pharma Oncology, ImmunoGenesis, Ellipses Pharma, Alpheus Medical, Curtana Pharma, Sagimet Biosciences, and Sapience Therapeutics; and other services from Siemens. AAA received grants from Varian and NH TheraAguix, and consulting fees from Novartis and Seagen. DMT received grants from Varian Medical Systems, Blue Earth Diagnostics, and NovoCure, and consulting fees from Boston Scientific. RK received grants from Medtronic, Blue Earth Diagnostics, NovoCure, GT Medical Technologies, AstraZeneca, Exelixis, Viewray, Brainlab, and Cantex Pharmaceuticals; consulting fees from Kazia Therapeutics, Elekta, Viewray, Castle Biosciences, and NovoCure; travel support from Elekta, Accuray, NovoCure, and Peerview Institute for Medical Education; other support from Elekta, Accuray, Novocure, and the Peerview Institute for Medical Education; and is on the Viewray Medical Advisory Board. CKA received grants from PUMA, Lilly, Merck, Seattle enetics, Nektar, Tesaro, G1-Therapeutics, ZION, Novartis, Pfizer, AstraZeneca, Elucida, and Caris; licences from UpToDate and Jones and Bartlett; other support from Genentech, Eisai, IPSEN, Seattle Genetics, AstraZeneca, Novartis, Immunomedics, Elucida, and Athenex; and is on the Genentech board. PWS received consulting fees from Varian. JH-G has funding from the National Institutes of Health (NIH)/National Cancer Institute (NCI) and received payment for a lecture from Aptitude Health. DNY has a Robert Wood Johnson Foundation Medical Grant and Brockman Foundation Medical Grant. ICGO received grants from Bristol Myers Squibb, Merck, and Pfizer; and consulting fees from Bristol Myers Squibb, Array, Novartis, Sintetica, and Leal Therapeutics. AM received grants from Eisai/H3B Pharmaceutical, Takeda Millenium Pharm, Lilly, Pfizer, MTEM, Merck, Roche, Zion, Norvatis, Dantari, and Genentech; payment from Taiho; research support from Tempus and PUMA; and was on the boards for Seagen and Eli Lilly. EG received grants from Celgene, Denovo Biopharma, MedImmune, and Servier Pharmaceuticals; and is on the boards for Karyopharm Therapeutics, Kiyatec, and Boston Scientific. VG received grants from ImmunoChem Therapeutics. PKB received grants from Mirati, Eli Lilly, Kinnate, Merck, NIH, the Breast Cancer Research Foundation, Damon Runyon, AACR, the Terry and Jean de Gunzburg MGH Research Scholar Fund, and the Demetra fund; consulting fees from Axiom Healthcare, Pfizer, Dantari, Advice Connect inspire, ElevateBio, Sintetica, SK Life Sciences, Voyager Therapeutics, Kazia, MPM Capital, Medscape, Eli Lilly, and Tesaro; other payments from Medscape and Pfizer; other support from GSK, Genentech-Roche, Eli Lilly, AstraZeneca, Kazia, Merck, Mirati, and Pfizer; and was the Chair of Society of Annual Neuro-Oncology Meetings. LC has grants from CDC/Johns Hopkins, DSI, Hological, and Myriad; US patent US7734496B1; stock from UNH; and a leadership role at Touch4Life and MD HBEB. MPM received consulting fees from Kazia, Novocure, Zap, Xoft, Karyopharm, and Sapience; has stocks at Oncoceutics and Chimerix; and is on the boards for Mevion, Oncoceutics, and Xcision. MK received grants from AbbVie, Bristol Myers Squibb, Daiichi Sankyo, BioNTech, CNS pharmaceuticals, Immorna Therapeutics, Celldex Therapeutics, and Astellas; consulting fees from Novocure and George Clinical; miscellaneous payment from Jax Lab, GSK, Voyager Therapeutics, and Johnson and Johnson; and is on the board for Berg Pharmaceuticals. MSA received grants from Seagen, AstraZeneca, Bristol Myers Squibb, Bayer, Incyte, Pharmacyclics, Novocure, Mimivax, and Merck; consulting fees from Bayer, Novocure, Kiyatec, Insightec, GSK, Xoft, Nuvation, Cellulartity, SDP Oncology, pollomics, Prelude, Janssen, Tocagen, Voyager Therapeutics, Viewray, Caris Lifesciences, Pyramid Biosciences, Anheart Therapeutics, Varian Medical Systems, Theraguix, and Menarini Ricerche; has stocks from Mimivax, Cytodyn, and Medlnnovate Advisors; and is on the boards for Cairn Therapeutics, Pyramid Biosciences, Modifi Biosciences, and Bugworks. MHG has grant number U54CA261717 from the NIH/NCI and received consulting fees from Midatech. JL has research funding from Bristol Myers Squibb. MMK has a grant (number R50CA276015) from the NIH/NCI and Blue Earth Diagnostics; and is on the boards for the NCCN CNS Cancers Guidelines Committee, International Journal of Radiation Oncology*Biology*Physics, Neuro-Oncology, and the External Advisory Board for Stanford U54 MetNet. JR received grants from Black Diamond Therapeutics, Blueprint Medicines, Hummingbird, Merck Sharp & Dohme, Vall d’Hebron Institute of Oncology/Cancer Core Europe, Yingli, AadiBioscience, Amgen, Bayer, Bicycle Therapeutics, BioAtla, BioMed Valley Discoveries, Cellestia, Curis, CytomX, Deciphera, ForeBio, GenMab, GlaxoSmithKline, Hummingbird, Hucthinson MediPharma, Ideaya, Kelun-Biotech, Linnaeus Therapeutics, Loxo Oncology, Merus, Mirati, Novartis, Nuvation, Pfizer, Roche Pharmaceuticals, Spectrum Pharmaceuticals, Symphogen, Taiho, Takeda-Millennium, and Tango Therapeutics; consulting fees from Alnylam Pharmaceuticals, Avoro Capital Advisors, Boxer Capital, the Chinese University of Hong Kong, Clarion Healthcare, Columbus Venture Partners, Cullgen, Debiopharm, Incyte, Macrogenics, Merus, Monte Rosa Therapeutics, Oncology One, Pfizer, Sardona Therapeutics, Tang Advisors, and the Vall d’Hebron Institute of Oncology/Ministero De Empleo Y Seguridad Social; travel support from European Society for Medical Oncology; is on boards for AadiBioscience, Ellipses Pharma, Envision Pharma Limited, Incyte, IONCTURA, Merus, and Monte Rosa Therapeutics; and is on the steering committee for the Vall d’Hebron Institute of Oncology/Ministero De Empleo Y Seguridad Social. AB received support from NIH grant number P30 CA008748, has four US patents, and is on the Evren Technologies Scientific Advisory Board. HT has grants from BMS Bristol Myers Squibb, Novartis, Merck, Genentech, Eisai, GSK, RAPT, and Dragonfly; and received consulting fees from BMS Bristol Myers Squibb, Novartis, Merck, Genentech, Eisai, Iovance, Pfizer, Karyopharm, Boxer Capital, Jazz Pharma, and Medicenna. All other authors declare no competing interests.
Contributor Information
Michelle M Kim, Department of Radiation Oncology, University of Michigan, Ann Arbor, MI, USA.
Minesh P Mehta, Department of Radiation Oncology, Miami Cancer Institute, Baptist Health South Florida, Miami, FL, USA.
DeeDee K Smart, Radiation Oncology Branch, Center for Cancer Research, National Cancer Institute, Bethesda, MD, USA.
Patricia S Steeg, Women’s Malignancies Branch, Center for Cancer Research, National Cancer Institute, Bethesda, MD, USA.
Julie A Hong, Radiation Research Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute, Rockville, MD, USA.
Michael G Espey, Radiation Research Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute, Rockville, MD, USA.
Pataje G Prasanna, Radiation Research Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute, Rockville, MD, USA.
Laura Crandon, Touch4Life, Clarksville, MD, USA.
Christine Hodgdon, GRASP, Baltimore, MD, USA.
Niki Kozak, Santa Maria, CA, USA.
Terri S Armstrong, Neuro-Oncology Branch, Center for Cancer Research, National Cancer Institute, Bethesda, MD, USA.
Aki Morikawa, Department of Internal Medicine, University of Michigan, Ann Arbor, MI, USA.
Nicole Willmarth, American Brain Tumor Association, Chicago, IL, USA.
Kirk Tanner, National Brain Tumor Society, Newton, MA, USA.
Adrienne Boire, Department of Neurology, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Melanie Hayden Gephart, Department of Neurosurgery, Stanford University, Stanford, CA, USA.
Kim A Margolin, St John’s Cancer Institute, Santa Monica, CA, USA.
Jona Hattangadi-Gluth, Department of Radiation Oncology, University of California San Diego Health, La Jolla, CA, USA.
Hussein Tawbi, Department of Melanoma Oncology, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Daniel M Trifiletti, Department of Radiation Oncology, Mayo Clinic Florida, Jacksonville, FL, USA.
Caroline Chung, Department of Radiation Oncology, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Upal Basu-Roy, LUNGevity Foundation, Chicago, IL, USA.
Robyn Burns, Melanoma Research Foundation, Washington, DC, USA.
Isabella C Glitza Oliva, Department of Melanoma Oncology, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Ayal A Aizer, Department of Radiation Oncology, Brigham and Women’s Hospital/Dana-Farber Cancer Institute, Boston, MA, USA.
Carey K Anders, Department of Medicine, Duke University Medical Center, Durham, NC, USA.
Joanne Davis, The Radiosurgery Society, San Jose, CA, USA.
Manmeet S Ahluwalia, Department of Medical Oncology, Miami Cancer Institute, Baptist Health South Florida, Miami, FL, USA.
Veronica Chiang, Department of Neurosurgery and Department of Therapeutic Radiology, Yale University School of Medicine, New Haven, CT, USA.
Jing Li, Department of Radiation Oncology, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Rupesh Kotecha, Department of Radiation Oncology, Miami Cancer Institute, Baptist Health South Florida, Miami, FL, USA.
Silvia C Formenti, Department of Radiation Oncology, Weill Cornell Medicine, New York, NY, USA.
Benjamin M Ellingson, UCLA Brain Tumor Imaging Laboratory, Department of Radiological Sciences, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA.
Vinai Gondi, Department of Radiation Oncology, Northwestern Medicine Cancer Center Warrenville and Proton Center, Warrenville, IL, USA.
Paul W Sperduto, Department of Radiation Oncology, Duke University Medical Center, Durham, NC, USA.
Jill S Barnholtz-Sloan, Informatics and Data Science Program, Center for Biomedical Informatics and Information Technology, Trans-Divisional Research Program, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD, USA.
Jordi Rodon, Department of Investigational Cancer Therapeutics, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Eudocia Q Lee, Center for Neuro-Oncology, ana-Farber Cancer Institute, Boston, MA, USA.
Mustafa Khasraw, Preston Robert Tisch Brain Tumor Center, Duke University Medical Center, Durham, NC, USA.
Debra Nana Yeboa, Department of Radiation Oncology, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Priscilla K Brastianos, Division of Hematology/Oncology and Division of Neuro-Oncology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Evanthia Galanis, Department of Oncology, Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, MN, USA.
C Norman Coleman, Radiation Research Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute, Rockville, MD, USA.
Mansoor M Ahmed, Radiation Research Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute, Rockville, MD, USA.
References
- 1.Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 1990; 322: 494–500. [DOI] [PubMed] [Google Scholar]
- 2.US Food and Drug Administration. Cancer clinical trial eligibility criteria: brain metastases. July, 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cancer-clinical-trial-eligibility-criteria-brain-metastases (accessed April 15, 2023).
- 3.Vogelbaum MA, Brown PD, Messersmith H, et al. Treatment for brain metastases: ASCO-SNO-ASTRO guideline. J Clin Oncol 2022; 40: 492–516. [DOI] [PubMed] [Google Scholar]
- 4.Gondi V, Bauman G, Bradfield L, et al. Radiation therapy for brain metastases: an ASTRO clinical practice guideline. Pract Radiat Oncol 2022; 12: 265–82. [DOI] [PubMed] [Google Scholar]
- 5.Steeg PS, Camphausen KA, Smith QR. Brain metastases as preventive and therapeutic targets. Nat Rev Cancer 2011; 11: 352–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soffietti R, Pellerino A, Rudà R. Strategies to prevent brain metastasis. Curr Opin Oncol 2019; 31: 493–500. [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network. Breast cancer (version 2.2023). NCCN Clinical Practice Guidelines in Oncology. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed Feb 1, 2023).
- 8.National Comprehensive Cancer Network. Melanoma: cutaneous (version 1.2023). NCCN Clinical Practice Guidelines in Oncology. https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf (accessed Jan 15, 2023).
- 9.National Comprehensive Cancer Network. Central nervous system cancers (version 2.2022). NCCN Clinical Practice Guidelines in Oncology. https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf (accessed Feb 1, 2023). [DOI] [PubMed]
- 10.National Comprehensive Cancer Network. Non-small cell lung cancer (version 2.2023). NCCN Clinical Practice Guidelines in Oncology. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (accessed Feb 1, 2023).
- 11.Ramakrishna N, Anders CK, Lin NU, et al. Management of advanced human epidermal growth factor receptor 2-positive breast cancer and brain metastases: ASCO guideline update. J Clin Oncol 2022; 40: 2636–55. [DOI] [PubMed] [Google Scholar]
- 12.Gennari A, André F, Barrios CH, et al. ESMO clinical practice guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol 2021; 32: 1475–95. [DOI] [PubMed] [Google Scholar]
- 13.Michielin O, van Akkooi ACJ, Ascierto PA, Dummer R, Keilholz U. Cutaneous melanoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2019; 30: 1884–901. [DOI] [PubMed] [Google Scholar]
- 14.Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018; 29 (suppl 4): iv192–237. [DOI] [PubMed] [Google Scholar]
- 15.Mills MN, Potluri TK, Kawahara Y, et al. The presentation of brain metastases in melanoma, non-small cell lung cancer, and breast cancer and potential implications for screening brain MRIs. Breast Cancer Res Treat 2022; 191: 209–17. [DOI] [PubMed] [Google Scholar]
- 16.Cagney DN, Martin AM, Catalano PJ, et al. Implications of screening for brain metastases in patients with breast cancer and non-small cell lung cancer. JAMA Oncol 2018; 4: 1001–03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aizer AA, Lamba N, Ahluwalia MS, et al. Brain metastases: a Society for Neuro-Oncology (SNO) consensus review on current management and future directions. Neuro-oncol 2022; 24: 1613–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jenkins S, Zhang W, Steinberg SM, et al. Phase I study and cell-free DNA analysis of T-DM1 and metronomic temozolomide for secondary prevention of HER2-positive breast cancer brain metastases. Clin Cancer Res 2023; 29: 1450–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armstrong TS, Dirven L, Arons D, et al. Glioma patient-reported outcome assessment in clinical care and research: a Response Assessment in Neuro-Oncology collaborative report. Lancet Oncol 2020; 21: e97–103. [DOI] [PubMed] [Google Scholar]
- 20.DiRisio AC, Harary M, van Westrhenen A, et al. Quality of reporting and assessment of patient-reported health-related quality of life in patients with brain metastases: a systematic review. Neurooncol Pract 2018; 5: 214–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin NU, Wefel JS, Lee EQ, et al. Challenges relating to solid tumour brain metastases in clinical trials, part 2: neurocognitive, neurological, and quality-of-life outcomes. A report from the RANO group. Lancet Oncol 2013; 14: e407–16. [DOI] [PubMed] [Google Scholar]
- 22.Le Rhun E, Guckenberger M, Smits M, et al. EANO-ESMO clinical practice guidelines for diagnosis, treatment and follow-up of patients with brain metastasis from solid tumours. Ann Oncol 2021; 32: 1332–47. [DOI] [PubMed] [Google Scholar]
- 23.Roth P, Pace A, Le Rhun E, et al. Neurological and vascular complications of primary and secondary brain tumours: EANO-ESMO clinical practice guidelines for prophylaxis, diagnosis, treatment and follow-up. Ann Oncol 2021; 32: 171–82. [DOI] [PubMed] [Google Scholar]
- 24.Walker MS, Wong W, Ravelo A, Miller PJE, Schwartzberg LS. Effect of brain metastasis on patient-reported outcomes in advanced NSCLC treated in real-world community oncology settings. Clin Lung Cancer 2018; 19: 139–47. [DOI] [PubMed] [Google Scholar]
- 25.Basch E, Deal AM, Dueck AC, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA 2017; 318: 197–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science 2003; 302: 1760–65. [DOI] [PubMed] [Google Scholar]
- 27.Gibson EM, Monje M. Microglia in cancer therapy-related cognitive impairment. Trends Neurosci 2021; 44: 441–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turnquist C, Beck JA, Horikawa I, et al. Radiation-induced astrocyte senescence is rescued by Δ133p53. Neuro-oncol 2019; 21: 474–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Firth J, Stubbs B, Vancampfort D, et al. Effect of aerobic exercise on hippocampal volume in humans: a systematic review and meta-analysis. Neuroimage 2018; 166: 230–38. [DOI] [PubMed] [Google Scholar]
- 30.Brastianos PK, Carter SL, Santagata S, et al. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov 2015; 5: 1164–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Priego N, Zhu L, Monteiro C, et al. STAT3 labels a subpopulation of reactive astrocytes required for brain metastasis. Nat Med 2018; 24: 1024–35. [DOI] [PubMed] [Google Scholar]
- 32.Venkataramani V, Tanev DI, Kuner T, Wick W, Winkler F. Synaptic input to brain tumors: clinical implications. Neuro-oncol 2021; 23: 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kabraji S, Ni J, Sammons S, et al. Preclinical and clinical efficacy of trastuzumab deruxtecan in breast cancer brain metastases. Clin Cancer Res 2023; 29: 174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rehman AU, Khan P, Maurya SK, et al. Liquid biopsies to occult brain metastasis. Mol Cancer 2022; 21: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Camidge DR, Lee EQ, Lin NU, et al. Clinical trial design for systemic agents in patients with brain metastases from solid tumours: a guideline by the Response Assessment in Neuro-Oncology Brain Metastases working group. Lancet Oncol 2018; 19: e20–32. [DOI] [PubMed] [Google Scholar]
- 36.Oyer RA, Hurley P, Boehmer L, et al. Increasing racial and ethnic diversity in cancer clinical trials: an American Society of Clinical Oncology and Association of Community Cancer Centers joint research statement. J Clin Oncol 2022; 40: 2163–71. [DOI] [PubMed] [Google Scholar]
- 37.Tan AC, Boggs DH, Lee EQ, Kim MM, Mehta MP, Khasraw M. Clinical trial eligibility criteria and recently approved cancer therapies for patients with brain metastases. Front Oncol 2022; 11: 780379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim AE, Wang GM, Waite KA, et al. Cross-sectional survey of patients, caregivers, and physicians on diagnosis and treatment of brain metastases. Neurooncol Pract 2021; 8: 662–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McKee MJ, Keith K, Deal AM, et al. A multidisciplinary breast cancer brain metastases clinic: the University of North Carolina experience. Oncologist 2016; 21: 16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rajpurohit A, Purandare N, Moiyadi A, et al. Multidisciplinary brain metastasis clinic: is it effective and worthwhile? Ecancermedicalscience 2020; 14: 1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Danielson B, Fairchild A. Beyond palliative radiotherapy: a pilot multidisciplinary brain metastases clinic. Support Care Cancer 2012; 20: 773–81. [DOI] [PubMed] [Google Scholar]
- 42.Fleege NMG, Pierce-Gjeldum D, Swartz LK, et al. IMPACT the Brain: a team-based approach to management of metastatic breast cancer with CNS metastases. JCO Oncol Pract 2023; 19: e67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moss NS, El Ahmadieh TY, Brown S, et al. Integrated multidisciplinary brain metastasis care reduces patient visits and shortens time to adjuvant irradiation. JCO Oncol Pract 2022; 18: e1732–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moss NS, Beal K, Tabar V. Brain metastasis—a distinct oncological disease best served by an integrated multidisciplinary team approach. JAMA Oncol 2022; 8: 1252–54. [DOI] [PMC free article] [PubMed] [Google Scholar]


