Abstract
Introduction:
Early initiation of chemotherapy after surgery for colon cancer has survival benefits. Immediate adjuvant chemotherapy (IAC) involves giving chemotherapy during surgical resection and immediately postoperatively. This novel approach has been shown to be safe, eliminating delays in adjuvant treatment that could increase the risk of micro-metastatic spread. The aim of this study was to assess the willingness of the general public to accept IAC.
Materials and methods:
Between March and April 2021, 800 telephone interviews were conducted with a sample of adult New York State residents. The Survey Research Institute of Cornell University conducted all surveys. Kruskal–Wallis, chi-squared, and Fisher’s tests were conducted using R 4.0.2.
Results:
Three scenarios were presented: (1) receiving IAC resulting in improved survival and quality of life, (2) finishing chemotherapy earlier without survival impact, and (3) finishing chemotherapy earlier but with possible side effects. Respondents with higher education were more likely to accept (1) & (2), males were more likely to accept (2) & (3), higher income respondents were more likely to accept (1) & (3), and those with more work hours were more likely to accept (2). Lastly, 16% responded they would be very or extremely likely, and 52% respondents would be somewhat likely or likely to accept intraoperative chemotherapy, even if it may not be necessary.
Conclusions:
Respondents were likely to accept IAC if offered. Given the known risk of delayed adjuvant chemotherapy (AC) in colon cancer, further research is warranted to determine the survival and quality of life (QOL) benefits of IAC.
Keywords: Adjuvant chemotherapy, Colon cancer, Colon resection, Early adjuvant chemotherapy, Perioperative phase of care
Introduction
For patients with nonmetastatic colon cancer, standard of care treatment involves surgical resection followed by adjuvant chemotherapy (AC) in high-risk patients.1 The intent of surgery is curative and to produce final pathologic staging that determines the need for adjuvant therapy. Multiple studies have demonstrated a decreased survival benefit with delays in AC.2–7 Linked to this information is the knowledge that treatment disparities, including delays in AC, are more likely to occur in minority populations.8,9 Therefore, a practice guideline to influence optimal initiation of AC could positively impact outcomes and reduce disparities. However, the National Comprehensive Cancer Network guidelines do not currently have recommendations regarding the timing of initiation of AC for patients with colon cancer.
The rate of recurrence of colon cancer remains high at 20% for stage II patients and 40% for stage III patients.10,11 Due to the high rate of recurrence and known risks of delaying AC, investigators have studied the perioperative period as a means to initiate treatment and improve outcomes for patients with colorectal cancer. The perioperative period can lead to systemic changes, including stress response and tissue integrity damage, which may ultimately cause immune suppression.12–14 These changes may allow for micro-metastatic spread during the perioperative period.15
A recent phase I trial evaluating immediate adjuvant chemotherapy (IAC) demonstrated the safety and feasibility of cytotoxic agent administration (intravenous 5-Flurouracin, 5FU) during colon resection and administration of standard AC as early as possible during the postoperative period.16 Secondary outcomes of this study also demonstrated favorable quality-of-life scores. Given the known risk of delayed AC in colon cancer and the feasibility demonstrated by the results of this recent phase I trial, further research into IAC is required. Specifically, will patients and providers be willing to initiate this novel treatment therapy? The aim of the current study was to assess the attitudes of the potential patients to treatment with IAC.
Materials and Methods
We developed four questions as part of the 2021 Empire State Poll. The Empire State Poll is an annual survey using dual frame random digit dial telephone sampling of New York State residents aged 18 y and older. The survey was administered by the Survey Research Institute at Cornell University in Ithaca, New York. The study was approved by the Cornell University Institutional Review Board, Ithaca, New York under protocol number 1902008602. Informed consent was waived.
Data were collected from March 5, 2021 to April 27, 2021. Eligible participants were called until 800 surveys were completed. All interviews were conducted in English using a web data collection instrument. Four hundred respondents were from upstate New York, while the remaining 400 were from downstate New York. Downstate New York was defined as all counties in: Manhattan, Brooklyn, Staten Island, Bronx, Queens, Long Island, Rockland, and Westchester. All other counties were defined as upstate New York. In order to ensure that every adult had an equal chance of being included in the poll, the most recent birthday method was employed once a household was sampled. This was done to ensure that the sample was generalizable to all New York State residents.
After collection of demographic data, respondents were asked to choose an answer on a 5-point Likert scale (1 = Not at all likely, 2 = Some what likely, 3 = Likely, 4 = Very Likely, and 5 = Extremely likely) for each of the four questions (Table 1). As the survey was administered by nonmedical personnel employed by the Survey Research Institute of Cornell, discussion regarding side effects beyond what was outlined in Table 1 was prohibited. All data were deidentified and respondents were given unique case-identification numbers. Numerical variables were analyzed using analysis of variance or Kruskal–Wallis test as appropriate. Normality check was done using Shapiro tests. Categorical variables were analyzed using chi-squared or Fisher’s exact test as appropriate. Statistical analysis was performed using R, version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
Table 1-.
Survey questions developed to determine attitudes toward receipt of a novel treatment: IAC for colon cancer.
| Question prompt | Some doctors have shown that giving the first chemotherapy dose while the patient is undergoing colon cancer surgery is safe, well- tolerated, and may improve survival. How likely would you be to accept chemotherapy during surgery if your doctor told you each of the following? |
| Question 1 | “It will give you a chance of improved survival and better quality of life.” |
| Question 2 | “You will finish cancer treatment much earlier than usual, with a better quality of life, but with no impact on survival.” |
| Question 3 | “You will finish cancer treatment much earlier than usual, but there is a possibility of more side effects like nausea, fatigue, and shortness ofbreath for the first month.” |
| Question 4 | “The tumor is small, and I am not certain if you need chemotherapy, but I want to offer it as an option.” |
The survey was administered via the Survey Research Institute of Cornell University’s 2021 Empire State Poll from March-April 2021. It was completed by 800 respondents across New York State. Responses were collected on a 5-point Likert scale (1 = Not at all likely, 2 = Somewhat likely, 3 = Likely, 4 = Very Likely, 5 = Extremely likely).
Results
Of the 5190 people contacted, 800 completed the survey. The median age of respondents was 53 y with an interquartile range of 37–66 y. There was an equal distribution of male and female respondents. 577 (74%) identified as White, 124 (16%) identified as Black, 93 (12%) of respondents identified as Hispanic, 32 (4%) as Native American, and 47 (6%) identified as Asian. 89 (11%) of respondents reported a personal history of cancer with skin cancer subtypes (17%) and breast cancer (12%) being the most common. Respondent demographics are summarized in Table 2.
Table 2 -.
Summary of demographics of the 800 survey respondents.
| Demographic characteristics | N (%) | Missing |
|---|---|---|
|
| ||
| Age | ||
| Median (IOR) | 53 (37–66) | 40 |
| Education | ||
| High school or below | 177 (22%) | 7 |
| College/some college/technical | 436 (55%) | |
| Postgraduate or professional schooling | 180 (23%) | |
| Ethnicity | ||
| Hispanic | 93 (12%) | 14 |
| Race | ||
| White | 577 (74%) | 21 |
| Black | 124 (16%) | 24 |
| American Indian | 32 (4%) | 27 |
| Asian | 47 (6%) | 26 |
| Other race | 59 (8%) | 35 |
| Gender | ||
| Female | 392 (50%) | 11 |
| Male | 395 (50%) | |
| Other | 2 (0%) | |
| Cancer history | 89 (11%) | 11 |
| Household income | ||
| <$50,000 | 203 (29%) | 91 |
| $50,000 - $150,000 | 349 (49%) | |
| >$150,000 | 157 (22%) | |
| Hours worked per week | ||
| Median (IQR) | 40.23 (15.58) | 457 |
| Marital status | ||
| Divorced/Separated/Widowed/Other | 136 (17%) | 21 |
| Married | 372 (48%) | |
| Single | 271 (35%) | |
The survey was developed to assess attitudes toward the receipt of IAC for colon cancer. It was administered via the Survey Research Institute of Cornell University’s 2021 Empire State Poll from March-April 2021. It was completed by 800 respondents across New York State.
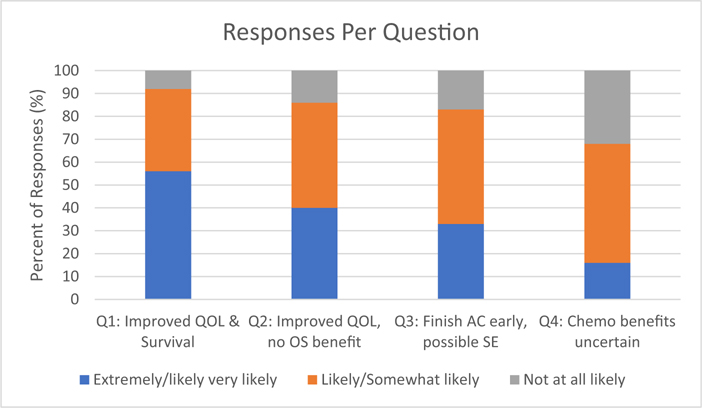
Stratified responses to questions are shown in Figure. When respondents were asked if they would be willing to accept IAC if they were to have an improved survival benefit and quality of life (QOL), 56% responded that they would be very or extremely likely to accept, 36% would be somewhat likely or likely to accept, and 8% reported that they would be not at all likely. Forty seven respondents did not complete this question. Those with higher levels of education (P < 0.001), White respondents (P < 0.001) and those with higher incomes (P < 0.001) were more likely to accept IAC (Table 3).
Fig. –
Survey respondents’ willingness to accept IAC in four different clinical scenarios. The survey was completed by 800 New York State residents via the 2021 Empire State Poll which was administered by the Survey Research Institute of Cornell from March–April 2021. QOL = quality of life; OS = overall survival; AC = adjuvant chemotherapy; SE = side effects.
Table 3 -.
Demographic and socioeconomic factors associated with willingness to accept IAC for colon cancer for four different scenarios.
| Demographic characteristics |
Question 1: Improved QOL & survival |
Question 2: Improved QOL, No OS benefit |
Question 3: Finish AC early, possible SE |
Question 4: AC benefits uncertain |
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Not at all likely | Somewhat likely/Likely | Very likely/Extremely likely | Missing | P-value | Not at all likely | Somewhat likely/Likely | Very likely/Extremely likely | Missing | P-value | Not at all likely | Somewhat likely/Likely | Very likely/Extremely likely | Missing | Not at all likely | P-value | Somewhat likely/Likely | Very likely/Extremely likely | Missing | P-value | Test | |
|
| |||||||||||||||||||||
| Education | 4 | <0.001 | 4 | 0.007 | 4 | 0.15 | 4 | 0.71 | Chi-squared | ||||||||||||
| High school or below | 16 (10%) | 68 (41%) | 81 (49%) | 23 (14%) | 86 (52%) | 56 (34%) | 30 (18%) | 86 (52%) | 49 (30%) | 56 (34%) | 83 (51%) | 24 (15%) | |||||||||
| College/some college/technical | 43 (10%) | 146 (35%) | 225 (54%) | 66 (16%) | 179 (44%) | 163 (40%) | 73 (18%) | 204 (50%) | 130 (32%) | 133 (33%) | 210 (51%) | 65 (16%) | |||||||||
| Postgraduate or professional schooling | 2 (1%) | 56 (33%) | 112 (66%) | 11 (7%) | 73 (45%) | 80 (49%) | 20 (12%) | 80 (48%) | 68 (40%) | 43 (27%) | 86 (55%) | 28 (18%) | |||||||||
| Gender | 8 | 0.24 | 8 | 0.007 | 8 | 0.004 | 8 | 0.14 | Fisher’s | ||||||||||||
| Female | 35 (9%) | 132 (36%) | 203 (55%) | 63 (17%) | 164 (45%) | 138 (38%) | 78 (21%) | 179 (48%) | 113 (31%) | 130 (36%) | 181 (50%) | 52 (14%) | |||||||||
| Male | 25 (7%) | 133 (36%) | 215 (58%) | 36 (10%) | 169 (46%) | 161 (44%) | 44 (12%) | 187 (51%) | 133 (37%) | 101 (28%) | 194 (54%) | 64 (18%) | |||||||||
| White | 42 (8%) | 175 (32%) | 330 (60%) | 14 | <0.001 | 73 (14%) | 230 (43%) | 230 (43%) | 14 | 0.058 | 80 (15%) | 267 (50%) | 191 (36%) | 13 | 0.072 | 152 (29%) | 283 (54%) | 91 (17%) | 15 | 0.03 | Chi-squared |
| Black | 10 (8 %) | 52 (44%) | 56 (47%) | 17 | 0.1 | 19 (16%) | 56 (47%) | 43 (36%) | 18 | 0.52 | 23 (19%) | 58 (49%) | 37 (31%) | 16 | 0.6 | 43 (37%) | 56 (48%) | 18 (15%) | 17 | 0.42 | Chi-squared |
| Asian | 3 (7%) | 22 (50%) | 19 (43%) | 20 | 0.14 | 4 (9%) | 24 (56%) | 15 (35%) | 19 | 0.36 | 7 (16%) | 22 (51%) | 14 (33%) | 18 | 0.98 | 16 (38%) | 19 (45%) | 7 (17%) | 20 | 0.59 | Chi-squared |
| American Indian | 3 (10%) | 15 (52%) | 11 (38%) | 21 | 0.098 | 8 (30%) | 12 (44%) | 7 (26%) | 20 | 0.045 | 7 (23%) | 16 (52%) | 8 (26%) | 19 | 0.51 | 7 (24%) | 12 (41%) | 10 (34%) | 21 | 0.041 | Fisher’s |
| Age, median (IRQ) | 51 (42–70) | 51 (34–66) | 53 (37– 66) | 35 | 0.3 | 54 (41.75– 67.75) | 52 (34–65) | 53 (37–66) | 31 | 0.059 | 48 (37.75–67) | 53 (36–67) | 53 (37–65) | 31 | 0.92 | 49 (34–66) | 52 (36–65.75) | 55 (43–67) | 32 | 0.059 | Kruskal —Wallis |
| Cancer history | 9 (10%) | 25 (29%) | 52 (60%) | 3 | 0.32 | 17 (20%) | 33 (39%) | 35 (41%) | 3 | 0.14 | 18 (21%) | 39 (45%) | 29 (34%) | 2 | 0.49 | 24 (29%) | 43 (52%) | 15 (18%) | 2 | 0.8 | Chi-squared |
| Household income | 78 | <0.001 | 74 | 0.085 | 74 | <0.001 | 74 | 0.084 | Chi-squared | ||||||||||||
| <$50,000 | 18 (9%) | 83 (44%) | 89 (47%) | 30 (16%) | 97 (51%) | 64 (34%) | 41 (21%) | 109 (52%) | 43 (22%) | 67 (35%) | 101 (53%) | 21 (11%) | |||||||||
| 50,000 - $150,000 | 23 (7%) | 125 (38%) | 183 (55%) | 35 (11%) | 151 (46%) | 141 (43%) | 41 (12%) | 160 (49%) | 125 (38%) | 100 (31%) | 166 (51%) | 60 (18%) | |||||||||
| >$150,000 | 7 (5%) | 33 (22%) | 112 (74%) | 20 (13%) | 57 (38%) | 72 (48%) | 23 (15%) | 66 (44%) | 62 (41%) | 36 (25%) | 85 (59%) | 22 (15%) | |||||||||
| Hours worked per week, median (IQR) | 40 (40–40.5) | 40 (37– 43) | 40 (35–50) | 428 | 0.24 | 40 (31.5– 40) | 40 (35–45) | 40 (40–50) | 416 | 0.004 | 40 (38.5–49) | 40 (35–47) | 40 (36–50) | 423 | 0.78 | 40 (35–46.25) | 40 (36.75– 45) | 40 (36–50) | 415 | 0.66 | Kruskal —Wallis |
Survey was completed by 800 New York State residents via the 2021 empire state poll which was administered by the survey research institute of Cornell from march–april 2021.
QOL = quality of life; OS = overall survival; AC = adjuvant chemotherapy; SE = side effects; IQR = interquartile range.
When respondents were asked if they would be willing to accept IAC if they were to finish treatment earlier, although there was no survival benefit, 40% responded that they would be very or extremely likely, 46% somewhat likely or likely, and 14% reported that they would be not at all likely to accept. 59 respondents did not complete this question. Those with higher education (P = 0.007), those who worked more hours per week (P = 0.004), and those who were males (P = 0.007) were more likely to accept (Table 3). Those of American-Indian race were less likely to accept compared to those who were not (Appendix 1).
When respondents were asked if they would be willing to accept IAC even if it may result in side effects, 33% responded that they would be very or extremely likely, 50% reported that they would be somewhat likely or likely, and 17% reported that they would be not at all likely to accept. Fifty six respondents did not complete this question. Those who were male (P = 0.004) and those with higher income (P < 0.001) were more likely to accept IAC despite potentially incurring side effects (Table 3).
Lastly, when respondents were asked if they would be willing to receive IAC even if it may not ultimately be needed as a part of their treatment plan, 16% reported that they would be very likely or extremely likely, 52% would be somewhat likely or likely, and 32% would be not at all likely to accept. Sixty eight respondents did not complete this question. Those who were White (P = 0.03) and those of Native American race (P = 0.041) were more likely to accept IAC compared to those who were not White or Native American in this scenario (Table 3).
Discussion
Our findings indicate that the majority of our survey respondents are willing to accept IAC if offered to them, regardless of survival benefits. Further, respondents were willing to accept IAC despite a potentially higher side effect profile. Lastly, the majority of respondents were also willing to accept a single intraoperative dose of chemotherapy, even if AC may ultimately not be necessary based on final pathologic staging. There were some socioeconomic differences noted. Those who identify as White, those with higher education levels, males, and those with higher income levels all being more likely to accept IAC. This is the first study to assess attitudes of patients toward the receipt of IAC. As this is a novel therapeutic strategy, literature on this topic is limited. The number of respondents indicating they would be somewhat likely, likely, very, or extremely likely to accept IAC exceeded the authors’ expectations in all four scenarios.
Racial and ethnic differences noted in this study maybe a reflection of the level of mistrust of physicians which is often seen among minorities. Traditionally, those who are White have a higher level of trust with their physicians and are more willing to participate in research studies compared to other minorities.17,18 A qualitative study of cancer patients shows that White and Hispanic patients valued optimism during treatment.19 While this may imply that these groups are more willing to participate in novel treatments or research studies to obtain a sense of optimism, our results showed no difference in willingness to receive IAC among Hispanic respondents. Socioeconomic differences which are noted, such as education and income level, may be a reflection of the medical literacy of the respondents and their willingness to participate in novel therapies. Interestingly, no difference was noted in the willingness of respondents with a cancer history to receive IAC compared to respondents without a cancer history. While we were unable to correlate this with a personal history of chemotherapy receipt, it is likely that this subgroup of patients with a cancer history likely have a higher level of medical literacy compared to respondents without a cancer history.
While National Comprehensive Cancer Network guidelines do not specify a timeline for the initiation of AC, the European society of medical oncologists recommends initiating chemotherapy as soon as possible following recovery from surgery and ideally within 8 wk.20 These guidelines are based on a meta-analysis which demonstrated worsened overall survival (odds ratio: 1.20; 95% confidence interval 1.15–1.26) for colorectal cancer patients who started adjuvant treatment after 8 wk.21 Despite these recommendations, there are often delays in patients starting adjuvant treatment which can include delayed surgical recovery and lack of social support.3 In the prior IAC phase I trial, the median time from surgery to standard AC (where AC was indicated), was just 14 d.16 The novel strategy of IAC helps to minimize delays in adjuvant treatment while potentially improving QOL by completing AC earlier. A second meta-analysis demonstrated a significant decrease in both overall survival and disease-free survival with each 4-wk increase in time to AC.4 IAC would theoretically eliminate this risk by reducing delays in AC. This is achieved via two mechanisms: 1) mandated multidisciplinary colon cancer care with preoperative engagement of medical oncology and 2) administering the first chemotherapy dose during surgical resection, thereby potentially minimizing micro-metastatic spread during the perioperative phase of care. Currently, mandated preoperative multidisciplinary care for colon cancer is not required, despite overwhelming evidence demonstrating the benefits of multidisciplinary care.22–30 While some colon cancer patients require multidisciplinary care postoperatively, this does not prevent delays in care and treatment disparities. The mandated preoperative multidisciplinary care necessitated by IAC will help to minimize known treatment disparities, with White respondents receiving adjuvant treatment for colon cancer earlier than those who are Black.8,9 It is well known that multidisciplinary care helps to increase compliance with treatment completion.22,31 In this case, preoperative medical oncology evaluation of underrepresented minorities will help to increase compliance with completion of AC. This is particularly relevant to the results seen here which demonstrate that White respondents are more likely, in general, to accept IAC compared to minority populations. Future work, including qualitative studies in order to elucidate the reasoning behind the socioeconomic differences observed herein is required. This is particularly important given the potential implications that this novel treatment could portend on eliminating treatment disparities and treatment delays.
The recent phase I trial demonstrated the safety of intraoperative chemotherapy followed by subsequent administration of AC when indicated by final pathologic staging as early as possible.16 The authors also pursued a corollary survey of surgeons, medical oncologists, and colon cancer patients regarding their willingness to give or receive IAC. This study showed that there was a significant disconnect, as patients were significantly more willing to receive IAC than providers were willing to give IAC.32 The results of this study were replicated herein, with participants surveyed being overwhelmingly likely to accept this novel treatment. The effect of chemotherapy on postoperative complications after colectomy has been debated, with some studies showing an association33,34 and other studies showing no association.35,36 The phase I IAC study showed one grade 3 adverse event, postoperative ileus resulting in readmission, the remainder of the adverse events were grade 1. No intraoperative complications were reported, and the postoperative complication of ileus was felt to be a result of surgery due to dense adhesions rather than the administration of IAC. Skepticism of IAC amongst medical providers must be balanced with the safety shown in the phase 1 trial and the results of survey data presented herein demonstrating the willingness of patients to receive this novel treatment.
Limitations to this study relate to those inherent to the cross-sectional design. Cancer treatment is a complex topic, and while the authors’ main objective was to assess the willingness of the general public to accept IAC for colon cancer, the study design did not allow for qualification of why or why not respondents were willing to accept or not willing to accept this treatment. Additionally, as the study was conducted by nonmedical personnel, side-effects of chemotherapy were listed but not explained. Further, while this study did include a portion of respondents who had a cancer history, it did not capture the responses of specifically colon cancer survivors or providers. Further research into the areas of provider willingness to administer IAC and colon cancer Survivors (rather than the general public) willingness to accept this treatment is required.
The data provided by the survey results presented here in, corollary survey by Jafari et al., and the phase 1 trial provide compelling evidence for further investigation into this novel treatment. Future directions for the authors include qualitative studies of patients and providers regarding attitudes and perceptions of IAC and a phase 2 trial in order to determine the efficacy of this treatment.
Conclusions
The majority of respondents are willing to accept IAC at the time of colon cancer surgery if offered to them. Given the willingness of patients to accept this treatment, the known decrease in survival with delayed AC, and recent phase I trial demonstrating safety and feasibility of IAC, further research into the survival, oncologic, and QOL benefits of IAC is warranted.
Supplementary Material
Disclosure
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Dr Alessio Pigazzi has received funds Intuitive, Ethicon, Covidien and AcelRx, has done consulting for Ethicon and Covidien, and has honoraria in Intuitive and Coloplast. Dr Jafari has received funds from Intuitive, Covidien, Erbe, Merz and AcelRx and does consulting for Covidien and Karl Storz. Dr Wenzel has done consulting work for Array BioPharma. Dr Carmichael has done consulting for Ethicon and Medical Device Business Services and received payments for services from Covidien, Coloplast, Cook Medical and Acell for food and education. The remainder of the authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
Footnotes
Meeting Presentation
This study was presented at the 2022 Academic Surgical Congress meeting.
Supplementary Materials
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jss.2022.11.024.
REFERENCES
- 1.Benson AB, Venook AP, Al-Hawary MM, et al. NCCN guidelines insights: colon cancer, version 2. J Natl Compr Canc Netw. 2018;16:359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim YW, Choi EH, Kim BR, Ko W-A, Do Y-M, Kim IY. The impact of delayed commencement of adjuvant chemotherapy (eight or more weeks) on survival in stage II and III colon cancer: a national population-based cohort study. Oncotarget. 2017;8:80061–80072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim IY, Kim BR, Kim YW. Factors affecting use and delay (≥8 Weeks) of adjuvant chemotherapy after colorectal cancer surgery and the impact of chemotherapy-use and delay on oncologic outcomes. PLoS One. 2015;10:e0138720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biagi JJ, Raphael MJ, Mackillop WJ, Kong W, King WD, Booth CM. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: a systematic review and meta-analysis. JAMA. 2011;305:2335–2342. [DOI] [PubMed] [Google Scholar]
- 5.Hershman D, Hall MJ, Wang X, et al. Timing of adjuvant chemotherapy initiation after surgery for stage III colon cancer. Cancer. 2006;107:2581–2588. [DOI] [PubMed] [Google Scholar]
- 6.Bos ACRK, van Erning FN, van Gestel YRBM, et al. Timing of adjuvant chemotherapy and its relation to survival among patients with stage III colon cancer. Eur J Cancer. 2015;51:2553–2561. [DOI] [PubMed] [Google Scholar]
- 7.Klein M, Azaquoun N, Jensen BV, Gögenur I. Improved survival with early adjuvant chemotherapy after colonic resection for stage III colonic cancer: a nationwide study. J Surg Oncol. 2015;112:538–543. [DOI] [PubMed] [Google Scholar]
- 8.Jones LA, Ferrans CE, Polite BN, et al. Examining racial disparities in colon cancer clinical delay in the Colon Cancer Patterns of Care in Chicago study. Ann Epidemiol. 2017;27:731–738.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Potosky AL, Harlan LC, Kaplan RS, Johnson KA, Lynch CF. Age, sex, and racial differences in the use of standard adjuvant therapy for colorectal cancer. J Clin Oncol. 2002;20:1192–1202. [DOI] [PubMed] [Google Scholar]
- 10.Sargent DJ, Wieand HS, Haller DG, et al. Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2005;23:8664–8670. [DOI] [PubMed] [Google Scholar]
- 11.Böckelman C, Engelmann BE, Kaprio T, Hansen TF, Glimelius B. Risk of recurrence in patients with colon cancer stage II and III: a systematic review and meta-analysis of recent literature. Acta Oncol. 2015;54:5–16. [DOI] [PubMed] [Google Scholar]
- 12.Lu H, Ouyang W, Huang C. Inflammation, a key event in cancer development. Mol Cancer Res. 2006;4:221–233. [DOI] [PubMed] [Google Scholar]
- 13.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. [DOI] [PubMed] [Google Scholar]
- 14.Inflammation Okada F. and free radicals in tumor development and progression. Redox Rep. 2002;7:357–368. [DOI] [PubMed] [Google Scholar]
- 15.van der Bij GJ, Oosterling SJ, Beelen RHJ, Meijer S, Coffey JC, van Egmond M. The perioperative period is an underutilized window of therapeutic opportunity in patients with colorectal cancer. Ann Surg. 2009;249:727–734. [DOI] [PubMed] [Google Scholar]
- 16.Jafari MD, Carmichael JC, Dayyani F, et al. Immediate adjuvant chemotherapy in non-metastatic colon cancer: phase I trial evaluating a novel treatment protocol. Clin Colorectal Cancer. 2021;21:114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armstrong K, Ravenell KL, McMurphy S, Putt M. Racial/ethnic differences in physician distrust in the United States. Am J Public Health. 2007;97:1283–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shavers VL, Lynch CF, Burmeister LF. Racial differences in factors that influence the willingness to participate in medical research studies. Ann Epidemiol. 2002;12:248–256. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell K-AR, Brassil KJ, Osborne ML, Lu Q, Brown RF. Understanding racial-ethnic differences in patient-centered care (PCC) in oncology through a critical race theory lens: a qualitative comparison of PCC among Black, Hispanic, and White cancer patients. Patient Educ Couns. 2022;105:2346–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Localised colon cancer | clinical practice guidelines. https://www.esmo.org/guidelines/gastrointestinal-cancers/localised-colon-cancer. Accessed February 16, 2022.
- 21.Des Guetz G, Nicolas P, Perret G-Y, Morere J-F, Uzzan B. Does delaying adjuvant chemotherapy after curative surgery for colorectal cancer impair survival? A meta-analysis. Eur J Cancer. 2010;46:1049–1055. [DOI] [PubMed] [Google Scholar]
- 22.Du C-Z. Effect of multidisciplinary team treatment on outcomes of patients with gastrointestinal malignancy. WJG. 2011;17:2013–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richardson B, Preskitt J, Lichliter W, et al. The effect of multidisciplinary teams for rectal cancer on delivery of care and patient outcome: has the use of multidisciplinary teams for rectal cancer affected the utilization of available resources, proportion of patients meeting the standard of care, and does this translate into changes in patient outcome? Am J Surg. 2016;211:46–52. [DOI] [PubMed] [Google Scholar]
- 24.Burton S, Brown G, Daniels IR, et al. MRI directed multidisciplinary team preoperative treatment strategy: the way to eliminate positive circumferential margins? Br J Cancer. 2006;94:351–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gabel M, Hilton NE, Nathanson SD. Multidisciplinary breast cancer clinics. Do they work? Cancer. 1997;79:2380–2384. [PubMed] [Google Scholar]
- 26.Chang JH, Vines E, Bertsch H, et al. The impact of a multidisciplinary breast cancer center on recommendations for patient management: the University of Pennsylvania experience. Cancer. 2001;91:1231–1237. [DOI] [PubMed] [Google Scholar]
- 27.Ortiz H, Wibe A, Ciga MA, et al. Impact of a multidisciplinary team training programme on rectal cancer outcomes in Spain. Colorectal Dis. 2013;15:544–551. [DOI] [PubMed] [Google Scholar]
- 28.Palmer G, Martling A, Cedermark B, Holm T. Preoperative tumour staging with multidisciplinary team assessment improves the outcome in locally advanced primary rectal cancer. Colorectal Dis. 2011;13:1361–1369. [DOI] [PubMed] [Google Scholar]
- 29.Stephens MR, Lewis WG, Brewster AE, et al. Multidisciplinary team management is associated with improved outcomes after surgery for esophageal cancer. Dis Esophagus. 2006;19:164–171. [DOI] [PubMed] [Google Scholar]
- 30.Coory M, Gkolia P, Yang IA, Bowman RV, Fong KM. Systematic review of multidisciplinary teams in the management of lung cancer. Lung Cancer. 2008;60:14–21. [DOI] [PubMed] [Google Scholar]
- 31.Is It Worth Reorganising Cancer Services On The Basis... -Google Scholar. [Google Scholar]
- 32.Jafari MD, Brouwer J, Mesiti A, et al. Attitudes of physicians and patients toward the timing of adjuvant treatment in colon cancer. J Clin Orthod. 2022;40(Suppl l):e15596. [Google Scholar]
- 33.Cheung WY, Neville BA, Earle CC. Etiology of delays in the initiation of adjuvant chemotherapy and their impact on outcomes for Stage II and III rectal cancer. Dis Colon Rectum. 2009;52:1054–1063. [DOI] [PubMed] [Google Scholar]
- 34.Midura EF, Hanseman D, Davis BR, et al. Risk factors and consequences of anastomotic leak after colectomy: a national analysis. Dis Colon Rectum. 2015;58:333–338. [DOI] [PubMed] [Google Scholar]
- 35.Kawada K, Hasegawa S, Hida K, et al. Risk factors for anastomotic leakage after laparoscopic low anterior resection with DST anastomosis. Surg Endosc. 2014;28:2988–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akiyoshi T, Ueno M, Fukunaga Y, et al. Incidence of and risk factors for anastomotic leakage after laparoscopic anterior resection with intracorporeal rectal transection and double-stapling technique anastomosis for rectal cancer. Am J Surg. 2011;202:259–264. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.