Abstract
Photorhabdus luminescens is a bacterium which is mutualistic with entomophagous nematodes and which secretes high-molecular-weight toxin complexes following its release into the insect hemocoel upon nematode invasion. Thus, unlike other protein toxins from Bacillus thuringiensis (δ-endotoxins and Vip’s), P. luminescens toxin (Pht) normally acts from within the insect hemocoel. Unexpectedly, therefore, the toxin complex has both oral and injectable activities against a wide range of insects. We have recently fractionated the protein toxin and shown it to consist of several native complexes, the most abundant of which we have termed Toxin complex a (Tca). This complex is highly active against the lepidopteran Manduca sexta. In view of the difference in the normal mode of delivery of P. luminescens toxin and the apparent communality in the histopathological effects of other gut-active toxins from B. thuringiensis, as well as cholesterol oxidase, we were interested in investigating the effects of purified Tca protein on larvae of M. sexta. Here we report that the histopathology of the M. sexta midgut is similar to that for other novel midgut-active toxins. Following oral ingestion of Tca by M. sexta, we observed an acceleration in the blebbing of the midgut epithelium into the gut lumen and eventual lysis of the epithelium. The midgut shows a similar histopathology following injection of Tca into the insect hemocoel. These results not only show that Tca is a highly active oral insecticide but also confirm the similar histopathologies of a range of very different gut-active toxins, despite presumed differences in modes of action and/or delivery. The implications for the mode of action of Tca are discussed.
The current and future widespread deployment of transgenic crops engineered to express δ-endotoxin genes from the bacterium Bacillus thuringiensis has generated concern about the rapid development of insect resistance (2, 14, 20). Given the limited number and activity spectra of B. thuringiensis δ-endotoxin cryotypes (9), one approach to resistance management is the deployment of alternative protein toxins, either via spatial or temporal alternations or via coexpression of different toxins in the same plant, termed “pyramiding” (see references 2 and 17 for discussions of alternative strategies). We have therefore recently focused our research on the purification of novel insecticidal proteins from the bacterium Photorhabdus luminescens and on cloning the genes underlying toxin production (3).
P. luminescens is a gram-negative bacterium belonging to the Enterobacteriaceae (15). This bacterium is mutualistic with entomophagous nematodes and is released into the insect hemocoel upon nematode invasion. The insect is then killed, presumably via a combination of toxin action and direct infection. The bacteria continue to replicate within the insect cadaver and the nematodes feed off the bacterial-insect medium within the dead or dying insect (6). Unexpectedly, despite the presumed normal delivery of the P. luminescens toxin directly into the insect hemocoel, several of the toxin complexes also show oral activity against insects (4). In order to examine the toxicity of the secreted toxin, we previously purified an orally active high-molecular-weight fraction which is secreted directly into growth media by P. luminescens during the stationary phase of bacterial growth (4). We subsequently determined that this high-molecular-weight fraction consists of several native toxin complexes, each of approximately 1 MDa, the most abundant of which we have termed Toxin complex a (Tca). The cloning of the toxin complex-encoding genes (tc) is described elsewhere (3).
We were therefore interested in determining the histopathological effects of one of the toxin complexes, Tca, on larvae of the tobacco hornworm Manduca sexta and in comparing these with symptoms associated with other novel orally active toxins such as the δ-endotoxins (7, 11, 18) and the vegetative insecticidal protein Vip3A (22) from B. thuringiensis and with those associated with cholesterol oxidase (16). Here we report that, despite its normal delivery into the insect hemocoel, purified Tca protein has profound effects on the insect midgut epithelium following either injection or oral delivery. Furthermore, the histopathology of the M. sexta midgut following oral Tca treatment is very similar to that described for both classes of B. thuringiensis toxins (7, 11, 18, 22) and for cholesterol oxidase (16). These observations highlight the utility of Tca as a lepidopteran-active insecticide with both oral and injectable activities and suggest interesting alternative hypotheses for its mode of action.
MATERIALS AND METHODS
Insect bioassays and modes of toxin delivery.
Eggs of M. sexta were obtained from Carolina Biological or as a kind gift of W. Goodman, University of Wisconsin—Madison. For oral bioassay, first-instar larvae were transferred to individual 1.3-cm2 discs of artificial diet and held in an incubator at 25°C. Diet was either treated with 100 μl of buffer (10 mM Tris-HCl, 250 mM KCl [pH 8.6]) alone as an untreated control or treated with 1 μg of Tca in the same volume of buffer. For injection, third-instar larvae were injected with 5 μl of either buffer alone (control) or the same volume containing 550 ng of Tca. Injections were performed directly into the insect hemocoel by using a 25-μl Hamilton syringe with a 30-gauge needle. Postinjection larvae were held individually with diet, and symptoms of toxicity were noted.
Tca purification.
The full purification of Tca is described elsewhere (3). Briefly, the P. luminescens culture broth was separated from the cells via centrifugation and concentrated by ultrafiltration with a 100,000-molecular-weight-cutoff membrane. Concentrated culture broth was batch mixed with DEAE Sephacel (185 ml) and poured into a 2.5- by 40-cm column; proteins were eluted stepwise with increasing concentrations of KCl. The 300 mM KCl fraction containing oral toxicity was further concentrated and applied to an S400HR Sephacryl gel filtration column (2.5 by 100 cm; Pharmacia) in potassium phosphate buffer (100 mM, pH 6.9). Toxic fractions were concentrated, equilibrated with 10 mM Tris-HCl, pH 8.6, and loaded onto a weak anion-exchange high-performance liquid chromatography (HPLC) column (301VHP575; Vydac). The proteins were eluted with a 0 to 250 mM KCl gradient (50 min) in 10 mM Tris-HCl (pH 8.6). This revealed several separate high-molecular-weight toxin complexes (Tc), the most abundant of which we termed Tca.
Sectioning and staining.
Larvae from both oral and injection bioassays were chilled on ice (20 min) and then fixed in Bouin’s fluid (24 h). First-instar larvae were fixed undissected. For third-instar larvae, several incisions were made in the cuticle to allow for penetration of the fixative. Following fixation, larvae were dehydrated in an ethanol-tetrahydrofuran-xylene series and embedded in Paraplast X-tra. Embedded larvae were sectioned (5 μm), and sections were then deparaffinized, rehydrated, and stained in Weigert’s iron hematoxylin (30 s) followed by Cason’s trichrome (30 s) (10). This staining protocol stains the columnar and goblet cells of the midgut blue and red, respectively. Following staining, sections were dehydrated, cleared in xylene, and mounted in Permount. Mounted sections were examined by light microscopy with a Nikon compound microscope equipped with Nomarski optics.
RESULTS
Characteristics of Tca and its encoding locus, tca.
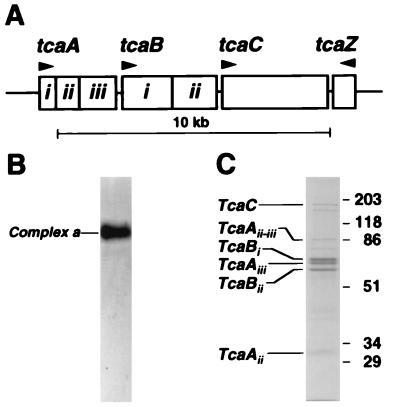
The high-molecular-weight complex Tca is encoded by the locus tca (Fig. 1A), whose cloning is described elsewhere (3). Briefly, HPLC-purified Tca migrates as a single complex on a native agarose gel (Fig. 1B) but segregates into each of its associated polypeptides on a denaturing sodium dodecyl sulfate-polyacrylamide gel (Fig. 1C). All of the experiments described here used the HPLC-purified complex a (Tca), previously termed Band 1 by Bowen and Ensign (4).
FIG. 1.
The Toxin complex a (tca) locus and the encoded Tca complex. (A) The tca locus consists of three open reading frames (tcaA, tcaB, and tcaC) transcribed in one direction and a shorter terminal open reading frame (tcaZ) transcribed in the opposite direction (3). Note that TcaA and TcaB are proteolytically cleaved (to TcaAi, TcaAii, and TcaAiii and to TcaBi and TcaBii, respectively) while TcaC is uncleaved. (B) On a native agarose gel, all of the components of Tca migrate as a single native complex (termed complex A or Band 1 [by Bowen and Ensign {4}]). (C) On a sodium dodecyl sulfate-polyacrylamide gel, the different polypeptides encoded by the tca locus can be resolved (except for the predicted TcaZ, which is not detectable).
Whole-animal toxicity symptoms.
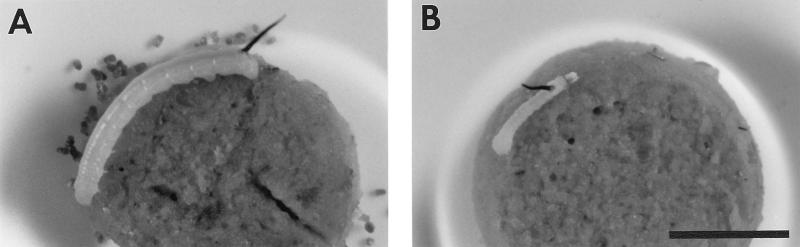
Following exposure of first-instar M. sexta larvae to diet treated with 1 μg of Tca, a dose corresponding to the 50% lethal concentration (LC50) (7 days), several repeatable responses were observed (Fig. 2). Firstly, within 24 h, insects would often feed briefly on the diet cube and then cease feeding. This feeding inhibition is readily apparent by the lack of frass production by treated larvae (Fig. 2B). After cessation of feeding, larvae can remain alive for several days; however, at the time of death they show little weight gain from their original weight as neonates (∼1.7 mg), while control insects which continue to feed have grown considerably (∼165 mg) by the end of the 7-day assessment period. At the lowest dose (40 ng) included in our LC50 experiments, described elsewhere (3), larval weight gain was only 14% of that of control animals, indicating potent sublethal effects on larval development.
FIG. 2.
Symptoms displayed by first-instar M. sexta larvae after 72 h of feeding on diet treated with a single component (Tca) of P. luminescens toxin. (A) Untreated control. Note normal frass production. (B) Larva exposed to treated diet. Note that the animal has not increased in size since hatching and that frass production is greatly reduced. Bar, 5 mm.
Following injection with Tca, third-instar larvae continue to feed normally for 2 to 3 days, often gaining weight at rates similar to those of control insects. However, by the third day postinjection, feeding ceases, body turgor is lost, and body color turns from green to yellow or black. Death occurs within 24 h of the cessation of feeding.
Histopathology of the insect midgut after oral delivery or injection.
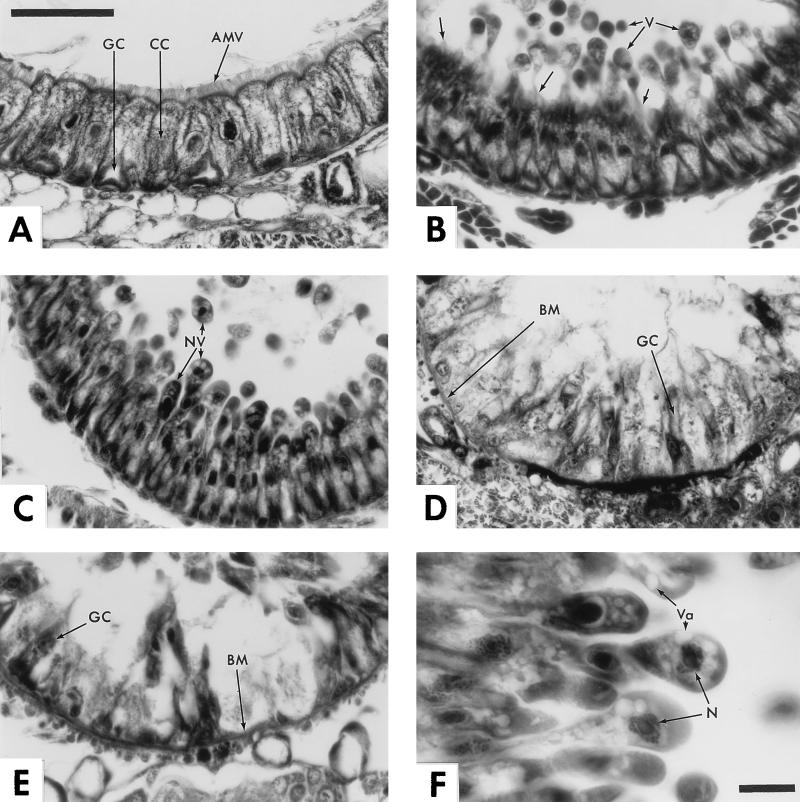
Initial observations of larvae spending only 1 h on Tca-treated diet revealed little reproducible signs of pathology; thus, although some insects displayed minor blebbing of the columnar cells, similar levels of blebbing were also noted in some controls fed untreated diet. The first reproducible histopathological symptoms appear at 3 h postexposure-postfeeding. At this initial stage, the columnar cells of the anterior midgut swell apically and begin to extrude large cytoplasmic vesicles into the gut lumen (Fig. 3B). At 6 h this pattern continues; however, the apically forming blebs now often contain nuclei as well as large vacuoles (Fig. 3C and F). Within the context of the rapidly deteriorating columnar cells, it is difficult to identify specific effects on the goblet cells, as their morphology is dependent on the supporting matrix of columnar cells. However, at 6 h, both blue (columnar cells) and red (goblet cells) vesicles can be identified in the gut lumen (Fig. 4A and B), indicating the presence of cellular debris from both cell types. At 12 h, destruction of the gut epithelium is essentially complete and only a disorganized layer of cell membranes and cell remnants lacking nuclei remains, along with a few isolated goblet cells (Fig. 3D). Finally, at 24 h, large gaps devoid of cellular material can be observed extending to the basal membrane itself (Fig. 3E). Note also that again, as at 12 h, a few isolated goblet cells persist.
FIG. 3.
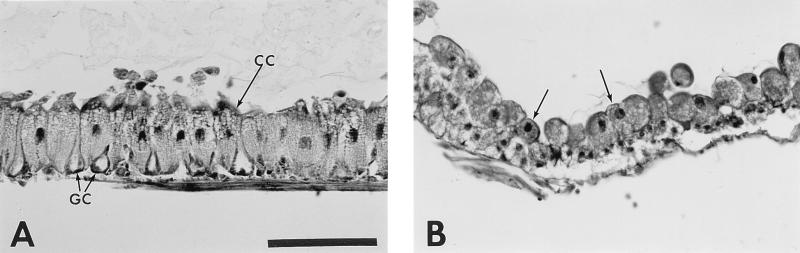
Time course of the histopathological effects of Tca on the anterior midgut of M. sexta larvae. (A) Control. Shown is the anterior midgut epithelium of a 24-h-old first-instar larva fed untreated diet. Note the columnar cells (CC) and goblet cells (GC) of the midgut epithelium and the apical microvilli (AMV) of the columnar cells which border the lumen. (B) Tca-treated larva sectioned after 3 h on diet. Arrows indicate the formation of apical vesicles (V) which are shed into the gut lumen. Note that at this early stage in poisoning, these vesicles appear to be shed through the apical microvilli (unlabelled arrows), which are still attached to the columnar cells. (C) Section after 6 h on treated diet. Vesicles continue to be shed into the gut lumen. Note the basal-apical elongation of the epithelial cells, the appearance of nuclei within the shedding vesicles (NV), and the absence of apical microvilli. (D) Section after 12 h on treated diet. By this time, the columnar cells are destroyed and all that remains of the anterior midgut epithelium is a disorganized matrix of cellular debris and a few isolated goblet cells. Note the clear view of the basal membrane (BM), which appears to thicken at this stage and is now largely devoid of intact attached cells. (E) At 24 h, cellular remains continue to be shed from the basal membrane (BM), often leaving it completely exposed to the gut lumen. Note that a few distorted goblet cells persist. (F) Detail of 6-h section. Note that the columnar cells extrude their contents into the gut lumen until the nucleus itself (N) is ejected, presumably resulting in cell death. Also note the large vacuoles (Va) seen in the apical regions of the budding cells. Bars, 50 μm in plate A (applies to plates A to E); 10 μm in plate F.
FIG. 4.
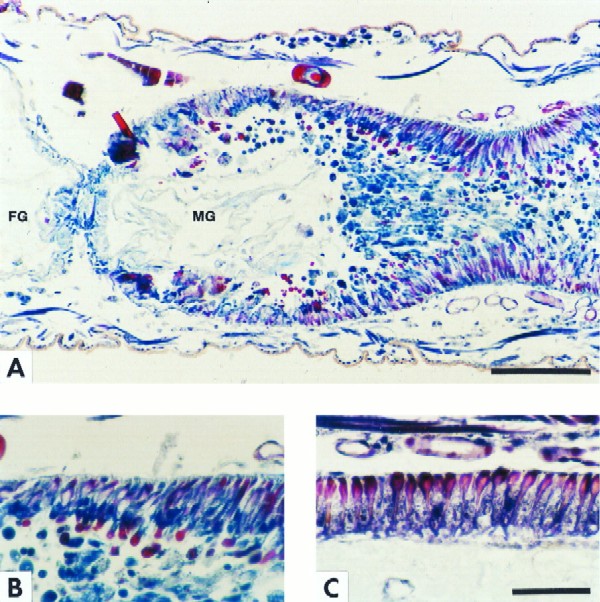
Color plate illustrating the fates of different cells in the midgut epithelium after Tca poisoning. Goblet cells are stained red, and columnar cells are stained blue. (A) Longitudinal section at 6 h after exposure to treated diet. Note the junction of the foregut (FG) and midgut (MG) and that the midgut lumen is occluded with extruded gut epithelial cells. The presence of red and blue vesicles in the lumen suggests blebbing of both the goblet cells (red) and the columnar cells (blue). The absence of vesicles from the anterior midgut is due to the presence of the esophageal diverticula, which can clearly be seen as nonstaining membranes in this region of the gut. (B) Detail of panel A. Note goblet cells (red) clearly forming apical cytoplasmic vesicles. (C) Control. Shown is a normal anterior midgut epithelium from a neonate fed untreated diet for 6 h. Bars, 100 μm in plate A; 50 μm in plate C (also applies to plate B).
The greatest damage to the gut epithelium occurs in the anterior region of the midgut. Although there is some anterior-to-posterior progression of pathology during the 24-h observation period, there is less cell disruption in the posterior midgut. Interestingly, even after 24 h of exposure, there seems to be little effect of Tca on undifferentiated regenerative cells. Thus, clusters of small round cells can still be observed intact adjacent to the basal membrane (data not shown).
Injection of Tca directly into the hemocoel of third-instar M. sexta larvae also results in extensive histopathological effects on the insect gut, although there are some differences from those observed following oral delivery. At 3 days postinjection, when whole-animal symptoms are most notable, effects in the anterior midgut are apparent. Thus, the columnar cells become rounded and the goblet cells are apparently destroyed or altered beyond recognition (Fig. 5B). Many of these rounded cells are again sloughed off into the gut lumen. Unlike the pathology after oral delivery, many of the cells still associated with the basal membrane appear nucleated. Also, although clearly affected and showing the apical swelling and blebbing of the columnar cells, the posterior midgut is less affected. Thus, in the latter region both the columnar and goblet cells are still readily identifiable. Finally, as with oral ingestion, stem cells persist in injected larvae without any overt pathology (data not shown).
FIG. 5.
Histopathology due to injected Tca in midgut of third-instar M. sexta larvae. (A) Control. Normal morphology of columnar cells (CC) and goblet cells (GC) is shown. (B) Injected larva. Anterior midgut at 72 h after injection of 550 ng of purified Tca is shown. Note the rounded appearance of epithelial cells (arrows) despite the fact that toxin delivery is from the hemocoel rather than from the gut lumen (see text). Bar, 50 μm.
DISCUSSION
The bacterium P. luminescens lives within the gut of entomophagous nematodes of the family Heterorhabditidae. Upon nematode invasion of the insect hemocoel, P. luminescens organisms are released, whereupon they kill the insect (6) probably via a combination of toxin secretion and sepsis. Presumably, in contrast to the oral activity of δ-endotoxins of B. thuringiensis, the normal mode of delivery of this toxin is directly into the insect hemocoel. Unexpectedly, however, this toxin has both injectable and oral activities against a wide range of insects (4). As this bacterium will also secrete the toxin directly into growth media, we have previously purified the Tca complex (3), allowing us to investigate the histopathological effects of a pure Tca preparation on the larvae of the lepidopteran M. sexta. Here we report that Tca can act on the insect midgut following either oral delivery or injection. Furthermore, these symptoms are very similar to those caused by other novel gut-active toxins such as the δ-endotoxins and Vip3A from B. thuringiensis (7, 11, 18, 22), as well as cholesterol oxidase (16).
Ingestion of Tca leads to apical swelling and blebbing of large cytoplasmic vesicles by the columnar cells, leading to the eventual extrusion of cell nuclei in vesicles into the gut lumen. Goblet cells are apparently affected in the same fashion, although we cannot determine if the toxin is the proximal cause. Furthermore, it is difficult to determine if the effects are cell specific. However, we do note two interesting cell-specific effects. Firstly, the observation that toxin action is greater in the anterior regions of the midgut has a number of possible explanations. Thus, this may either represent the failure of active toxin to actually reach the posterior midgut (possibly following proteolytic digestion as it traverses the gut) or be due to regional differences in cell sensitivity to Tca. Secondly, the failure of orally ingested Tca to attack the undifferentiated regenerative cells close to the basement membrane may suggest that only differentiated gut cells are targeted. This is also supported by our failure to detect pathological effects on any other tissues, following either oral ingestion or toxin injection. It is possible that survival of the stem cells may allow regrowth of the midgut epithelium following transient or sublethal exposure to the toxin.
One important difference in the histopathology of injected larvae is the apparent absence of goblet cells in the anterior midgut. This may simply be due to their close proximity to the basal membrane in this region, whereas in the posterior midgut the goblet cells are completely enveloped by the columnar cells and thus may be shielded from direct toxin exposure from the hemocoel. Generally, however, the observation of a broadly similar histopathology in the insect midgut, for both oral delivery and Tca injection, was unexpected alongside the initial observation of oral toxicity itself. Thus, it was not predicted that a toxin normally delivered into the insect hemocoel by the replicating bacteria would have oral toxicity or show similar effects on the insect midgut via delivery to either side of the gut (via the gut lumen or the insect hemocoel). This suggests two important working hypotheses for the mode of action of Tca. Firstly, unlike some of the δ-endotoxins produced by B. thuringiensis (8), proteolytic processing of the toxin complex components by the insect midgut itself may not be necessary for normal toxin activity. Secondly, the ability of Tca to affect the midgut from either side suggests that the factors governing the interaction of toxin with insect cells are either relatively nonspecific or that the receptors for Tca are found on both the apical and basal surfaces of the midgut epithelium. The observation that other tissues exposed to the hemocoel are unaffected by toxin action seems to preclude the possibility that the effects of Tca are nonspecific in relation to the type of tissue attacked.
Finally, although effects of Tca are seen on the midgut via both oral delivery and injection, we cannot exclude the possibility that the primary site of action of this toxin in vivo is in another part of the insect. For example, Tca secreted into the hemocoel by the bacterium may also be designed to destroy insect hemocytes and thus overcome insect bacterial immunity.
In conclusion, we have described here the effects of one of the purified toxin complexes, Tca, secreted by the bacterium P. luminescens on whole larvae and the histopathology of Tca action on the midgut. Tca, normally delivered into the insect hemocoel, shows similar histopathological effects on the insect midgut following either oral delivery or injection. These effects are similar to those seen with other novel orally active protein toxins (7, 11, 18, 22) and with cholesterol oxidase (16). This suggests that orally active toxins produce similar ranges of histopathological effects on the insect midgut despite different presumptive modes of action and, in the case of Tca, different modes of delivery. These results therefore confirm that Tca is a potent novel insecticide active against lepidoptera with toxicity similar to that of some B. thuringiensis δ-endotoxin cryotypes. Thus, following our recent cloning of the genes encoding these toxins (3), they may form useful alternatives to B. thuringiensis for expression in transgenic plants. Future studies will investigate the putative receptor for these toxins in work analogous to that currently being performed for B. thuringiensis δ-endotoxins (1, 5, 12, 13, 19, 21).
ACKNOWLEDGMENTS
This study was supported by grants to R.H.F.-C. from The Applied Research and Technology Fund and The Industrial and Economic Development Fund, both administered by the University of Wisconsin—Madison, and by Dow AgroSciences.
REFERENCES
- 1.Adang M J, Paskewitz S M, Garczynski S F, Sangadala S. Identification and functional characterization of the Bacillus thuringiensis CryIA(c) δ-endotoxin receptor in Manduca sexta. In: Clark J M, editor. Molecular action of insecticides on ion channels. Washington, D.C: American Chemical Society; 1995. pp. 320–329. [Google Scholar]
- 2.Bloomquist J R, Ferguson H J, Cox E D, Reddy M S, Cook J M. Mode of action of β-carboline convulsants on the insect nervous system and their potential as insecticides. Pestic Sci. 1997;51:1–6. [Google Scholar]
- 3.Bowen, D., T. A. Rocheleau, M. Blackburn, O. Andreev, E. Golubeva, R. Bhartia, and R. H. Ffrench-Constant. Novel insecticidal toxins from the bacterium Photorhabdus luminescens. Science, in press. [DOI] [PubMed]
- 4.Bowen D J, Ensign J C. Purification and characterization of a high-molecular-weight insecticidal protein complex produced by the entomopathogenic bacterium Photorhabdus luminescens. Appl Environ Microbiol. 1998;64:3029–3035. doi: 10.1128/aem.64.8.3029-3035.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denolf P, Jansens S, Peferoen M, Degheele D, Van Rie J. Two different Bacillus thuringiensis delta-endotoxin receptors in the midgut brush border membrane of the European corn borer, Ostrinia nubilalis (Hübner) (Lepidoptera: Pyralidae) Appl Environ Microbiol. 1993;59:1828–1837. doi: 10.1128/aem.59.6.1828-1837.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunphy G B, Webster J M. Virulence mechanisms of Heterorhabditis heliothidis and its bacterial associate, Xenorhabdus luminescens, in the non-immune larvae of the greater wax moth, Galleria mellonella. Int J Parasitol. 1988;18:729–737. [Google Scholar]
- 7.Endo Y, Nishiitsutsuji-Uwo J. Mode of action of Bacillus thuringiensis δ-endotoxin: histopathological changes in the silkworm midgut. J Invertebr Pathol. 1980;36:90–103. [Google Scholar]
- 8.Gill S S, Cowles E A, Pietrantonia P V. The mode of action of Bacillus thuringiensis endotoxins. Annu Rev Entomol. 1992;37:615–636. doi: 10.1146/annurev.en.37.010192.003151. [DOI] [PubMed] [Google Scholar]
- 9.Hofte H, Whitely H R. Insecticidal crystal protein of Bacillus thuringiensis. Microbiol Rev. 1989;53:242–255. doi: 10.1128/mr.53.2.242-255.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiernan J A. Histological and histochemical methods: theory and practice. New York, N.Y: Pergamon Press; 1990. [Google Scholar]
- 11.Kinsinger R A, McGaughey W H. Histopathological effects of Bacillus thuringiensis on larvae of the indianmeal moth and the almond moth. Ann Entomol Soc Am. 1979;72:787–790. [Google Scholar]
- 12.Lee M K, Aguda R M, Cohen M B, Gould F L, Dean D H. Determination of binding of Bacillus thuringiensis δ-endotoxin receptors to rice stem borer midguts. Appl Environ Microbiol. 1997;63:1453–1459. doi: 10.1128/aem.63.4.1453-1459.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo K, Lu Y-J, Adang M J. A 106 kDa form of aminopeptidase is a receptor for Bacillus thuringiensis CryIC δ-endotoxin in the brush border membrane of Manduca sexta. Insect Biochem Mol Biol. 1996;26:783–791. [Google Scholar]
- 14.McGaughey W H, Whalon M E. Managing insect resistance to Bacillus thuringiensis toxins. Science. 1992;258:1451–1455. doi: 10.1126/science.258.5087.1451. [DOI] [PubMed] [Google Scholar]
- 15.Poinar G O, Thomas G M, Hess R. Characteristics of the specific bacterium associated with Heterorhabditis bacteriophora (Heterorhabditidae: Rhabditida) Nematologica. 1977;23:97–102. [Google Scholar]
- 16.Purcell J P, Greenplate J T, Jennings M G, Ryerse J S, Pershing J C, Sims S R, Prinsen M J, Corbin D R, Tran M, Sammons R D, Stonard R J. Cholesterol oxidase: a potent insecticidal protein active against boll weevil larvae. Biochem Biophys Res Commun. 1993;196:1406–1413. doi: 10.1006/bbrc.1993.2409. [DOI] [PubMed] [Google Scholar]
- 17.Roush R T. Designing resistance management programs: how can you choose? Pestic Sci. 1989;26:423–441. [Google Scholar]
- 18.Sutter G R, Raun E S. Histopathology of European-corn-borer larvae treated with Bacillus thuringiensis. J Invertebr Pathol. 1967;9:90–103. [Google Scholar]
- 19.Valaitis A, Lee M K, Rajamohan F, Dean D H. Brush border membrane aminopeptidase-N in the midgut of the gypsy moth serves as the receptor for the CryIA(c) δ-endotoxin of Bacillus thuringiensis. Insect Biochem Mol Biol. 1995;25:1143–1151. doi: 10.1016/0965-1748(95)00050-x. [DOI] [PubMed] [Google Scholar]
- 20.van Rie J. Insect control with transgenic plant: resistance proof? Trends Biotechnol. 1991;9:177–179. [Google Scholar]
- 21.Wright D J, Iqbal M, Granero F, Ferré J. A change in a single midgut receptor in the diamondback moth (Plutella xylostella) is only in part responsible for field resistance to Bacillus thuringiensis subsp. kurstaki and B. thuringiensis subsp. aizawai. Appl Environ Microbiol. 1997;63:1814–1819. doi: 10.1128/aem.63.5.1814-1819.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu C-G, Mullins M A, Warren G W, Koziel M G, Estruch J J. The Bacillus thuringiensis vegetative insecticidal protein Vip3A lyses midgut epithelium cells of susceptible insects. Appl Environ Microbiol. 1997;63:532–536. doi: 10.1128/aem.63.2.532-536.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]