Abstract
Background
Both stereotactic radiosurgery (SRS) and microsurgical resection (SURGERY) are available as treatment options for sporadic vestibular schwannoma (VS). There are very few direct comparative studies comparing both treatment modalities in large cohorts allowing detailed subgroup analysis. This present study aimed to compare the nuances in the treatment of VS by SURGERY and SRS in 2 highly specialized neurosurgical centers.
Methods
This is a retrospective bicentric cohort study. Data from patients treated between 2005 and 2011 were collected retrospectively. Recurrence-free survival (RFS) was assessed radiographically by contrast-enhanced magnetic resonance imaging.
Results
The study population included N = 901 patients with a mean follow-up of 7 years. Overall, the incidence of recurrence was 7% after SURGERY, and 11% after SRS with superior tumor control in SURGERY in the Kaplan–Meier-analysis (P = 0.031). In small tumors (Koos I and II), tumor control was equivalent in both treatment arms. In large VS (Koos III and IV), however, RFS was superior in SURGERY. The extent of resection correlated with RFS (P < .001). Facial and hearing deterioration was similar in both treatment arms in small VS, but more pronounced in SURGERY of large VS. Tinnitus, vertigo, imbalance, and trigeminal symptoms were more often improved by SURGERY than SRS.
Conclusions
SRS can achieve similar tumor control compared to SURGERY in smaller VS (Koos I and II)—with similar postinterventional morbidities. In large VS (Koos III and IV), long-term tumor control of SRS is inferior to SURGERY. Based on these results, we suggest that if combination therapy is chosen, the residual tumor should not exceed the size of Koos II.
Keywords: acoustic neuroma, microsurgery, outcome, stereotactic radiosurgery, tumor recurrence, vestibular schwannoma
Key Points.
Tumor control is comparable in small VS (Koos I and II), but SURGERY is superior for large VS (Koos III and IV).
Tinnitus, vertigo, imbalance, and trigeminal symptoms are more likely to improve after SURGERY.
If combination therapy is chosen, the postsurgical residual tumor should not exceed the size of Koos II.
Importance of the Study.
The level of evidence to provide treatment recommendations for vestibular schwannoma (VS) is remarkably low. Both stereotactic radiosurgery (SRS) and microsurgical resection (SURGERY) are available as treatment options for VS. However, there are very few direct comparative studies comparing both treatment modalities in large cohorts allowing detailed subgroup analysis. More importantly, as the choice of treatment modality for VS has been regarded to be controversial, we have identified clear parameters for VS patients to benefit from SURGERY, like tumor size (Koos III and IV), tinnitus, trigeminal, and vertigo symptoms. In large tumors (Koos III and IV), SRS is not able to assure the same tumor control as SURGERY. This study adds valuable information to the current discussion on combination therapies: According to our data, residual tumor volume (TV) should not exceed Koos II after tumor decompression, if combination therapy should achieve similar tumor control as in SURGERY in large VS.
Vestibular schwannomas (VS) are benign nerve sheath tumors of the vestibular portion of the eight cranial nerves.1–3 Its incidence has been described to be approximately 1/100 000 per year.4 Serial observation, stereotactic radiosurgery (SRS) and microsurgical resection (SURGERY) are all available as contemporary treatment options for sporadic VS.5
Since the early days of neurosurgery, surgical resection has had a leading role in VS management. In the last decades, SRS has emerged as a viable and accepted alternative to SURGERY, also offered to young and healthy patients. However, the level of evidence to provide treatment recommendations for vestibular schwannoma (VS) is remarkably low and there are very few direct comparative studies regarding treatment modalities in large cohorts that allow detailed subgroup analysis.6 Decompressive surgery (DS) followed by adjuvant radiotherapy has also been discussed.7,8 However, the evidence for the rationale for choosing and recommending either SRS or SURGERY as a monotherapy is still lacking.6 Better clinical factors to stratify which treatment suits the patient best, and surrogate markers for therapy success need to be identified to assign VS patients to their best possible treatment.
The impact of VS as a disease on an individual patient is multifactorial, mainly constituting of quality of life, functional outcome, and tumor control (recurrence-free survival [RFS]). Studies focusing on questionnaires have shown that the modality of treatment (SRS, SURGERY, or observation) does not result in relevant differences in patients’ quality of life, even though it has been shown that SURGERY yielded worse hearing and facial nerve outcomes.5,9 Therefore, as primary VS outcomes like hearing and facial function have been investigated thoroughly, so-called secondary symptoms like tinnitus, trigeminal symptoms, vertigo, and imbalance ought to be assessed as well.
The present study aimed to compare the nuances of the treatment of primary VS with SURGERY and SRS in 2 specialized high-volume neurosurgical centers with more than 100 treated cases per year each. It investigated tumor control in RFS, primary (hearing and facial outcome) and secondary VS symptoms (tinnitus, vertigo/imbalance, and trigeminal function) as outcome parameters.
Methods
Study Design and Patient Cohort
This is a retrospective bicentric cohort study. Patients were identified by a prospectively kept registry by both senior authors (MT and GH) from 2 tertiary and specialized centers involved in the treatment of vestibular schwannomas. Clinical data were then retrospectively collected for patients with primary VS treated between 2005 and 2011 to enable long-term follow-up (FU).
Data Collection
Tumor size was classified by Koos Classification.10 Previously treated VS and VS associated with neurofibromatosis were systematically excluded. The clinical state of primary VS symptoms was reported by House and Brackmann (H&B)11 and Gardner–Robertson (G&R) scale (with H&B 1–2 and G&R 1–2 considered as good outcome).12 Peri-interventional complications were classified by Clavien–Dindo Classification (CDC).13 RFS was assessed radiographically by contrast-enhanced magnetic resonance (MR) imaging.14,15 The criteria for tumor recurrence/progression was progressive tumor growth in Gadolinium contrast-enhanced MR imaging (radiographic tumor control = RTC). To exclude the known phenomenon of pseudoprogression after SRS, patients with tumor volume (TV) increase 6 months after SRS with stable TV afterwards or TV decrease was not graded as VS recurrence/progression.16 The TV was measured using slice-by-slice manual contouring and supervised by multiple board-certified Gamma-Knife-Radiosurgery (GKR)-experts.
In the case of SURGERY, the extent of resection (EOR) was classified by first postoperative MRI (3 months postoperative): The residual contrast-enhancing tumor was defined as subtotal resection (STR), whereas gross total resection (GTR) was defined as lack of contrast-enhancement in Gadolinium-enhanced MR imaging. Secondary VS symptoms like trigeminal affection, tinnitus, and vertigo were also collected using subgroups as the following: grade 1 (intermittent symptomatic), grade 2 (persistent symptomatic), and grade 3 (invalidating symptomatic). A reduction of 1 grade was interpreted as symptom improvement and an increase in 1 grade was interpreted as symptom worsening.
The local ethics committee approved this analysis and was in accordance with the ethical standards laid down in the Declaration of Helsinki for research involving human subjects.
Treatment Modalities
Patients treated by SURGERY were all operated via retrosigmoid approach using intraoperative electrophysiological monitoring by the senior neurosurgeon (MT). Patients were either operated in a semisitting or supine position, depending on tumor size (supine position for Koos I and II, and semisitting position for Koos III and IV).17 All VS patients in the SRS cohort received GKR (Elekta AB, Stockholm, Sweden) with a prescription dose of 12–13 Gy to the 65% isodose line.
Statistical Analysis
Statistical analysis was performed in R Studio (Version 1.2) using descriptive statistics. To compare nonnumeric parameters of both groups, the chi-square test was applied. For small number sizes, Fisher-Exact t-Test was applied. For numeric parameters, Welch’s 2-sample t-test was used. Recurrence-free survival was estimated using the Kaplan–Meier method and compared between cases and controls using a log-rank test. The length of FU for recurrence-free survival was calculated from the date of surgical intervention to the date of either recurrence or the last clinical visit. Significance was defined as the probability of a 2-sided type 1 error being <5% (P <.05). Data are presented as mean ± SD if not indicated otherwise.
Results
Study Cohort
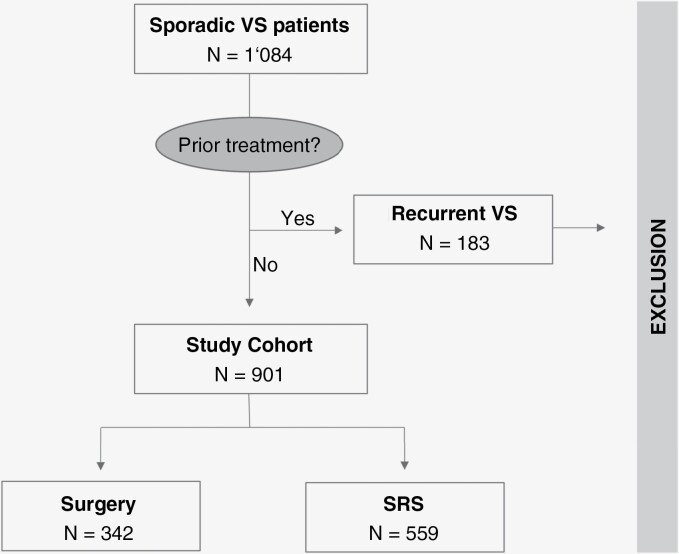
Overall, N = 1084 patients with VS were treated in both specialized centers within a 6-year period (2005–2011). One-hundred-eighty-three (17%) patients had received previous treatment or suffered from neurofibromatosis type II and were therefore excluded from the study. From the remaining N = 901 cases, N = 559/901 (62%) tumors were treated with SRS, while N = 342/901 (38%) tumors were treated with SURGERY. A patient flowchart of the cohort is shown in Figure 1.
Figure 1.
Patient cohort flowchart
Overall, the mean age was 54.47(±13.70) years with a significantly older patient cohort in SRS at 59.00 (±12.56) years compared to SURGERY at 47.45 (±12.48) years (P < .001). The SRS treatment arm consisted of significantly smaller tumors (Koos I and II) than its surgical counterpart (P <.001) (Table 1). Koos III tumors contributed to equal parts to both study cohorts (SRS: 36%, SURGERY: 38%) (P =.499). Of all treated VS tumors, 6% presented with MR-graphic cystic morphology. The proportion was significantly higher in SURGERY (9%) compared to SRS (4%) (P =.002). Demographics are shown in Table 1. Tumor isodose volume was in mean 1.64 (±2.11) cm3 with a mean Paddick Index of 0.81 (±0.11) in the SRS-treated cohort.
Table 1.
Patient Demographics, Tumor Characteristics, Clinical Parameters and Treatment Side Effects/ Perioperative Complication Rate and Their Severity. *P-Values Indicate Significant Differences. Values are Presented as the Number of Patients (%) and Mean ± SD, or Median and Interquartile Range Unless Indicated Otherwise. Significant P-Values (<.05) are Highlighted in Bold
| ALL (N = 901) |
SRS (N = 559) |
SURGERY (N = 342) | P-Value | |
|---|---|---|---|---|
| Age | 54.47(±13.70) | 59.00(±12.56) | 47.45(±12.48) | <.001 * |
| Female | 502 (56) | 319 (57) | 183 (54) | .297 |
| Tumor size | ||||
| Koos I | 114 (12) | 86 (15) | 28 (8) | .001 * |
| Koos II | 295 (33) | 213 (38) | 82 (24) | <.001 * |
| Koos III | 330 (37) | 200 (36) | 130 (38) | .499 |
| Koos IV | 162 (18) | 60 (11) | 102 (30) | <.001 * |
| Cystic morphology | 55 (6) | 24 (4) | 31 (9) | .002 * |
| Preoperative Clinical Status | ||||
| Functional hearing (G&R 1–2) | 466 (52) | 247 (44) | 219 (64) | <.001 * |
| Good facial function (HB1-2) | 886 (98) | 546 (98) | 340 (99) | .047 * |
| Facial spasm | 0 (0) | 0 (0) | 0 (0) | 1 |
| Tinnitus | 661 (73) | 417 (75) | 244 (71) | .284 |
| Trigeminus | 94 (10) | 46 (8) | 48 (14) | .006 * |
| Vertigo | 549 (61) | 343 (61) | 206 (60) | .737 |
| Postoperative Clinical Status | ||||
| Functional hearing (G&R 1–2) | 216 (24) | 134 (24) | 82 (24) | 1 |
| Good facial function (HB1-2) 1 y | 852 (95) | 543 (97) | 309 (90) | <.001 * |
| Facial spasm | 29 (3) | 29 (5) | 0 (0) | <.001 * |
| Tinnitus | 264 (29) | 200 (36) | 64 (19) | <.001 * |
| Trigeminus | 54 (6) | 41 (7) | 13 (4) | .030 * |
| Vertigo | 359 (40) | 268 (48) | 91 (27) | <.001 * |
| Treatment complications/side effects | 108 (12) | 62 (11) | 46 (13) | .290 |
| CDC | ||||
| 2 | 21 (2) | 11 (2) | 10 (3) | .356 |
| 3a | 29 (3) | 0 (0) | 29 (8) | <.001 * |
| 3b | 12 (1) | 2 (1) | 10 (3) | .001 * |
| >4 | 0 (0) | 0 (0) | 0 (0) | 1 |
Clinical Status and Outcome
Pre- and postoperative clinical status is reported in Table 1. The rate of patients with functional hearing at the time of treatment was significantly higher in SURGERY (P < .001) compared to SRS. Vertigo and tinnitus were comparably distinct in both subgroups.
At the last time of the last FU, from all patients, who had good hearing function preinterventionally (N = 466), only N = 216/466 (46%) had preserved hearing function. Posttreatment hearing preservation was significantly lower in the SURGERY group with N = 82/219 (37%) compared to N = 134/247 (54%) in the SRS group (P < .001).
Direct significant postoperative facial nerve deterioration (HB > 2) was observed in N = 97/342 (28%) in SURGERY. However, after 1 year the majority of these patients had improved in facial function, yielding a long-term favorable facial function outcome (H&B 1–2) of N = 309/342 (90%). In SRS, only N = 3 (0.5%) patients suffered from relevant postinterventional facial paresis HB > 2, but N = 29 patients suffered from facial spasm, yielding a favorable facial nerve outcome of 95% N = 527/559 (95%) if we considered facial spasm as relevant postinterventional facial deterioration. Thus, SRS was superior to SURGERY in facial function preservation (P = .027) in general.
Shunt dependency was indifferent in both treatment groups (SRS: N = 13/559 (2%) and SURGERY: N = 6/342 (2%); P = .562). N = 8 patients suffered from postoperative new trigeminal hypesthesia (P < .001) in SURGERY. From all patients suffering from trigeminal symptoms, N=72 patients reported improvement or resolution of symptoms after treatment, This rate was significantly higher in the SURGERY group with N=46/48 (95%), compared to N = 26/46 (56%) in SRS (P < .001).
The incidence of peri-interventional complications/adverse effects is listed in Table 1 including its CDC Classification. The most common SRS-related side effects were symptomatic brain edema or hydrocephalus, while SURGERY-related complications were CSF fistula (N = 30), hemorrhage (N = 5), hydrocephalus (N = 5), sinus thrombosis (N = 3), symptomatic pneumocephalus (N = 2), hygroma (N = 2), and infection (N = 1). The perioperative complication rate was independent of tumor size (P = .623).
When tinnitus was present, patients significantly benefited from surgical resection (P < .001); the same was shown in vertigo (P < .001). This study reported a higher incidence of worsening tinnitus when treated with SRS (P < .001) (Table 2).
Table 2.
Postoperative Secondary Clinical Parameters. *P-Values Indicate Significant Differences. Values are Presented as the Number of Patients (%). Significant P-Values (<.05) are Highlighted in Bold
| TINNITUS | VERTIGO | |||
|---|---|---|---|---|
| Worsening (N = 83) | Improvement (N = 268) | Worsening (N = 100) | Improvement (N = 276) | |
|
SRS
(N = 559) |
69 (12) | 77 (14) | 69 (12) | 130 (23) |
| Microsurgery (N = 342) | 14 (4) | 191 (56) | 31 (31) | 146 (53) |
| P-value | <.001 * | <.001 * | .128 | <.001 * |
Tumor Control
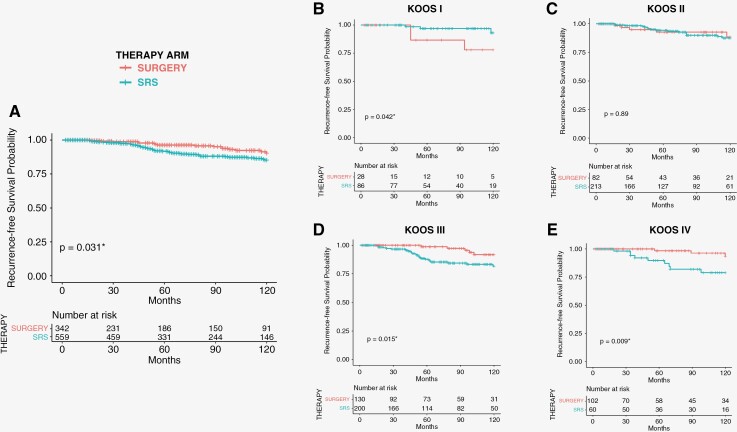
Overall mean postoperative FU was 78.38 (±52.48) months with 82.21 (±52.00) months after SRS and 72.12 (±52.72) months after SURGERY. The incidence of pseudoprogression was N = 177/559 (32%) in the SRS cohort. Incidence of recurrence (RTC) was reported to be N = 84/901 (9%) in the overall study cohort, the incidence of RTC was significantly lower in SURGERY (N = 25/342; 7%) compared to SRS (N = 59/559; 11%) (P = .032). N = 37/59 (62%) of SRS recurrences required tumor-specific clinical intervention (Clinical Tumor Control = CTC) (second SRS, or SURGERY within 1 year). This rate was significantly lower at N = 4/25 (16%) in the SURGERY cohort (P < .001). The mean time to recurrence in VS patients treated with SURGERY was 91.07(±40.79) months with 86.84(±44.77) months, when GTR was achieved, and 119.43(±25.98) months in STR. The mean time to recurrence in SRS was 64.07 (±38.97) months. Kaplan–Meier-analysis and risk tables are shown in Figure 2.
Figure 2:
Overall 10-years Kaplan–Meier-analysis shows significantly higher probability of recurrence-free-survival (RFS) in patients treated microsurgically (P = .031*) in A. 10 Years-recurrence-free-survival (RFS) according to B. Koos I, C. Koos II, D. Koos III and E. Koos IV. There is a highly significant higher probability for RFS with microsurgical treatment in Koos III and Koos IV VS patients (P = .015 and P = .009 respectively).
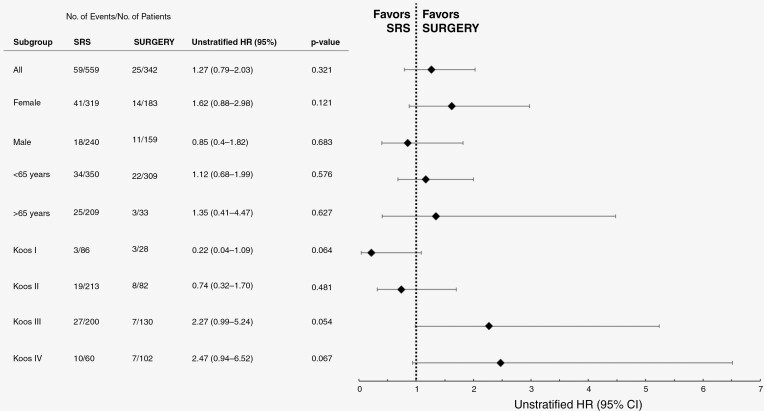
In a subgroup analysis of tumor size according to Koos-Classification, RFS was not significantly different in Koos I and Koos II comparing both treatment arms. However, there was a significant benefit of SURGERY in regard to RFS for larger tumors (Koos III and IV) (Figure 2). Unstratified hazard ratios for the incidence of radiographic recurrence (radiographic tumor control = RTC) in patient subgroups are shown in Figure 3.
Figure 3.
Unstratified hazard ratios for incidence of recurrence in patient subgroups.
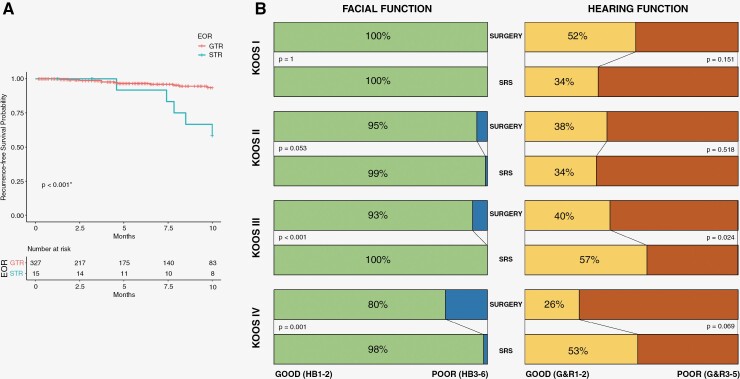
Functional outcomes of hearing and facial function according to tumor size are shown in Figure 4. RFS in the SURGERY-group was significantly associated with EOR. STR yielded in earlier tumor recurrences (P = .001). However, achievable EOR was significantly impacted by preoperative tumor size (P = .013) with N = 28/28 (100%) GTR in Koos I, N = 81/82 (99%) in Koos II, N = 124/130 (95%) in Koos III, and N = 94/102 (92%) in Koos IV (Figure 4).
Figure 4.
A. Shows 10 years-recurrence-free-survival (RFS) according to the extent of resection, B. Shows postinterventional functional outcome depending on tumor size (Koos I–IV) classified in good (H&B1–2 and G&R1–2) and poor outcome (H&B3–6 and G&R3–5).
Discussion
The present study aimed to compare the nuances in the treatment of VS with SURGERY and SRS in 2 specialized neurosurgical centers. To our knowledge, this is the largest controlled study comparing SRS and SURGERY as monotherapy in solitary and primary VS. The patient cohort treated with SRS was significantly older and consisted of smaller tumors, compared to SURGERY. This study reported an incidence of recurrence in SURGERY of 7%, and in SRS of 11% with a mean FU of 7 years. When comparing both treatment arms, RFS—therefore tumor control—was similar for smaller tumors (Koos I and II). In general, treatment-related functional deterioration (e.g. hearing and facial deterioration) was more common in patients treated with SURGERY. What is more, even though the events of treatment-related complications were not significantly different in both groups, SURGERY-related complications were classified to be more severe according to CDC (compared to SRS). Incidence of recurrence was associated with subtotal resection when treated with SURGERY alone. Secondary symptoms like tinnitus, vertigo, imbalance, and trigeminal symptoms were significantly improved by SURGERY, but not by SRS.
Primary VS Functional Outcome: Hearing and Facial Function
In either treatment arm, disease-specific mortality was 0% and VS remained a benign tumor with no disease-specific limited life expectancy. Furthermore, clinical outcomes remained satisfactory in both treatment arms.5 The rate of patients with functional hearing at the time of treatment (preinterventional) was significantly higher in SURGERY (P < .001) compared to SRS, which may be a result of a significantly younger cohort of the microsurgically treated patients. Long-term postinterventional hearing loss (if the pretreatment hearing function was G&R1–2) was 54% overall, with the significantly highest rate of hearing loss in SURGERY (63%), compared to SRS (46%). The hearing outcome was comparable in both treatment arms for smaller tumors (Koos I and II). For larger tumors (Koos III and IV), SRS was superior to SURGERY. Past long-term studies have reported hearing preservation rates of 63–68% at the last follow-up in studies with similar follow-up lengths in SRS.18,19 However, it remains undocumented, whether even longer follow-up might uncover more delayed cases of hearing loss after SRS or even SURGERY. Future studies should aim to investigate this aspect of long-term functional outcomes.
SRS was superior to SURGERY in facial function preservation, especially in large VS, which is concordant to a prospective comparative study by Myrsethet al.20 Our data showed a direct postinterventional facial paresis was approx. 30% of Koos III and Koos IV VS tumors right after SURGERY. However, notably, as reported in the past by Samii et al. in 1997,21 the majority recovered after 1 year in our cohort—yielding a permanent facial paresis HB > II rate of 9.6%. Compared with other available rates of facial function deterioration in the literature from 20% to 46% by retrosigmoid approach, this number reported in this study represents a rather low number (9.6% in the general cohort and 12.5% in large VS Koos III and IV).19,20,22–24 The numbers of postoperative facial function outcomes vary largely in the literature, most likely due to the different VS-specific expertise levels and different caseloads between the centers, that have published their data.19–21,23–25 In conclusion, surgical and radiosurgical treatment of VS should be carried out in specialized centers with VS-specific expertise, where facial preservation rates posttreatment are the highest. If we regard facial spasm also as a relevant facial affection, SRS still yielded a smaller rate of unfavorable facial outcomes at 5.7%.
Several meta-analyses have reported excellent facial function preservation in SRS, measured predominantly in HB. Even though facial spasm has been described to be a possible side-effect of SRS, this is the first study to report its incidence in comparison with SURGERY: Facial spasm is a radiosurgery-specific therapy-related-side effect, with an incidence of 5% in SRS, whereas no patient treated with SURGERY suffered from facial spasm. Until now, recent literature has focused on facial motor function as a clinical parameter, neglecting facial spasm as a significant compound of SRS-induced facial neuropathy.9,26 It is clearly to be debated, whether quality of life is similarly restricted for patients with facial spasm as a facial motor function HB > 2. The true difference or similarity of impact on the everyday life of facial spasm compared to facial paralysis can only be evaluated in a prospective study determining the quality of life in a comparative setting.
Secondary VS Functional Outcome: Trigeminal, Tinnitus, and Balance
Pre- and posttreatment tinnitus and vestibular function (vertigo, imbalance) are recognized to be fundamental factors that can influence one’s decision in VS management, but are seldom compared in both SRS and SURGERY treatment arms.5,9 When tinnitus was present, patients significantly benefited from surgical resection, which has also been shown by Wang et al. in a small prospective study evaluating Tinnitus Handicap Inventory in N = 41 with the best prognosis in low-frequency tinnitus.27 Our study also reported a high incidence of 12% in worsening tinnitus, when treated with SRS. Compared to more recent studies, this data showed an improvement of tinnitus of more than 50% in patients treated with SURGERY, this incidence is higher than described by Trakolis et al. in 202128 in a similar setting—however, our study uses a more detailed method (grading from grade 1-3 according to symptom severity) to more sensitively describe postinterventional tinnitus dynamic in both SRS and SURGERY group.
While many studies have focused on hearing, facial function, or quality of life assessment, our data demonstrate that if secondary symptoms (tinnitus, vertigo, etc.) are the main problematic points in the patient’s disease status, SURGERY can be evaluated even in smaller tumors. Therefore, physicians have to closely examine, whether these symptoms are present, assess the gravity of these symptoms and discuss the possibility of improvement in case of SURGERY during patient consultation.
Tumor Control
An increased incidence of recurrence was associated with treatment arm SRS and in the case of SURGERY with lower rates of EOR. The incidence of recurrence, RFS, and mean-time-to-recurrence was significantly worse in SRS, showing that SRS is inferior to SURGERY considering tumor control generally—but especially in Koos III and IV tumors. In small tumors, RFS did not reach statistical significance, therefore from a purely statistical point of view, both treatments (SRS and SURGERY) are comparable considering tumor control as an outcome parameter. Within the SURGERY group, time to recurrence was significantly associated with greater EOR (i.e. GTR), suggesting that residual TV is associated with tumor recurrences. If VS is treated with SURGERY alone, safe GTR should be the intended treatment to assure tumor control. Our results are concordant with several series, where a lower extent of resection grades has been shown to be associated with a higher risk for tumor recurrence.29–31
Tumor control following SRS in large VS is significantly worse compared to smaller VS and microsurgically treated VS of comparable size, which reflects the disease progression rates published in smaller cohorts in the past.18,32,33 Accordingly, Hasegawa et al. reported in 2005 that worse tumor control was achieved in large VS treated with GKR with a mean-time-to-recurrence below 3 years.18 However, the same group later described that tumor regression after 3 years (over 5 years) postinterventionally can be observed.26 In conclusion, our data suggest that postinterventional FU investigating tumor control in VS should exceed 5 years even in the SRS cohort, as mean-time-to-recurrence was 5.3 years in SRS and even longer in SURGERY.18 Patients treated with SURGERY were significantly younger and had larger tumors, which is a phenomenon often described by current literature.5 What is more, tumor control was significantly better in young patients and large tumors in subgroup analyses, suggesting that in patients < 65 years of age with large VS, SURGERY in a center with VS-specific expertise should be the treatment of choice.
This study did not include patients treated with combination therapy (decompressive surgery followed by adjuvant radiotherapy). However, our dataset shows that SRS may not achieve a similar tumor control outcome as SURGERY in VS larger than Koos II. In the context of the ongoing discussion about combination therapy (STR or decompressive surgery plus adjuvant SRS) in VS, our data add value to the tumor state/size that should be intended to be achieved during surgical decompression.6 If we assume that the growth pattern of VS is indifferent after surgical STR, the residual TV should not exceed the tumor category of Koos II, as our data show that tumor control drastically decreases, when VS reaches Koos III (mean TV of 1.57 (±0.87) ccm3) when treated with SRS. In Conclusion, if combination therapy is chosen—as recommended by the most recent VS management guidelines as Good Clinical Practice Point and other smaller series6–8, residual TV after surgical resection should not exceed Koos II, otherwise the patient will be at significantly higher risk for recurrence after adjuvant SRS compared to SURGERY alone.
The incidence of recurrence of 11% is comparable to the current literature with available comparable incidence rates of 12–15% when the incidence/progression definition was equivalent. Evaluating the effect and tumor control efficacy in 2 modalities so different as SRS and SURGERY poses a challenge. Residual TV can be higher in noninvasive SRS treatment compared to STR, and the difference in size reduction is massive compared to GTR. Detecting tumor recurrence by MR imaging—therefore is more sensitive in SURGERY compared to SRS. Moreover, the phenomenon of pseudoprogression is only SRS-related and can complicate direct comparative efforts immensely.34 This phenomenon is observed as studies report a wide range of MR-based recurrence incidence.19,20,26,35,36
Although not a primary outcome of this study, the incidence of pseudoprogression of ca. 30% is higher compared to the 23%, which Hayhurst et al. reported in the past in a cohort of N = 200 between 2005 and 2009 with a median FU of 29 months (2.42 years).16 Indeed, the numbers on pseudoprogression in GKR vary from 10% to 71%.19,26,34,37,38 This variability most likely results from different FU times (range 29–65 months) and is vividly discussed in the context of the definition of true VS recurrence in SRS therapy.19,37–39 With a median FU time of 75 months (6.5 years), and retrospective design of this study, no pseudoprogressions are included in the incidence of recurrence as treatment failure, which was defined by sequential growth in serial imaging.
In general, when comparing values in incidence or recurrence, CTC is often used in SRS, which is defined by the necessity for direct clinical intervention, while RTC is more often used in SURGERY.35,40,41 Therefore, when the comparison is drawn between tumor control rates inter-modally (between SRS and SURGERY), this has to be taken into account when interpreting any numbers of recurrence. Our study presented an incidence of recurrence in SRS of 11%. If the definition is switched to CTC (“necessity for clinical intervention”), 7% (N = 37/559) is calculated. Indeed, reported CTC vary from 2% to 12% in GKR studies.18,35,42,43 In case of recurrence, the necessity for clinical intervention (within 1 year) was significantly lower in SURGERY compared to SRS, which is most likely a manifestation of lower residual tumor volume after SURGERY.
It is noteworthy that additional studies have shown that the quality of life between treatment groups was not significantly different and Carlson et al. described that SURGERY may confer an advantage with regard to patient anxiety, presumably relating to the psychological benefit of “cure” from having the tumor removed.2,44 Therefore, even though the incidence of recurrence and functional outcome are important statistical statements, the psychological impact of both tumor “cure” and facial paresis must be investigated more thoroughly in the future.
Limitations of This Study
Although we are providing very detailed clinical information and data in a large study cohort of N = 901 identified by prospective registries, the data have been collected in a retrospective manner. Most recently, there was a strong shift in the management of VS away from primary surgery and radiation and toward a “wait-and-scan” approach.45 The aspect of the “wait-and-scan”-approach was not included in the study design.
Conclusions
Our data show that SRS can achieve similar long-term tumor control and similar functional results for hearing and facial function to SURGERY in smaller VS (Koos I and II)—in combination with less severe postinterventional morbidities. If secondary VS symptoms (e.g l tinnitus, vertigo/imbalance, and trigeminal symptoms) are severe—even in small VS—SURGERY can be recommended, as SURGERY may more likely improve these symptoms, but SRS may not. In Koos III and IV VS, functional results of hearing and facial function are superior in SRS, still, the effect of SRS on tumor control is inferior compared to SURGERY. Therefore, especially in the young population and large VS, SURGERY should be favored to SRS. If combination therapy is chosen, the residual tumor should not exceed the size of Koos II.
Contributor Information
Marcos Tatagiba, Department of Neurosurgery, Eberhard Karls University, Tubingen, Germany.
Sophie S Wang, Department of Neurosurgery, Eberhard Karls University, Tubingen, Germany.
Ahmed Rizk, Department of Neurosurgery, Eberhard Karls University, Tubingen, Germany.
Florian Ebner, Department of Neurosurgery, Eberhard Karls University, Tubingen, Germany.
Albertus T C J van Eck, Gamma Knife Center, Krefeld, Germany.
Georgios Naros, Department of Neurosurgery, Eberhard Karls University, Tubingen, Germany.
Gerhard Horstmann, Gamma Knife Center, Krefeld, Germany.
Conflict of interest statement
The authors report no conflict of interest.
Author contributions
Conception and design: M.T. and G.H.; acquisition of data: S.S.W. and A.R.; analysis and interpretation of data: S.S.W., M.T., and G.H.; statistical analysis: S.S.W. and G.N.; drafting the article: S.S.W. and M.T.; critically revising the article: G.N., F.E., A.T.C.J.V.E., and G.H.; reviewed submitted version of the manuscript: M.T.
Data availability
All data and materials are available and can be provided upon reasonable request.
References
- 1. McClelland S, 3rd, Guo H, Okuyemi KS.. Morbidity and mortality following acoustic neuroma excision in the United States: Analysis of racial disparities during a decade in the radiosurgery era. Neuro Oncol. 2011;13(11):1252–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carlson ML, Barnes JH, Nassiri A, et al. Prospective study of disease-specific Quality-of-life in sporadic vestibular schwannoma comparing observation, radiosurgery, and microsurgery. Otol Neurotol. 2021;42(2):e199–e208. [DOI] [PubMed] [Google Scholar]
- 3. Carlson ML, Link MJ.. Vestibular schwannomas. N Engl J Med. 2021;384(14):1335–1348. [DOI] [PubMed] [Google Scholar]
- 4. Hoffman S, Propp JM, McCarthy BJ.. Temporal trends in incidence of primary brain tumors in the United States, 1985–1999. Neuro Oncol. 2006;8(1):27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carlson ML, Tveiten OV, Driscoll CL, et al. Long-term quality of life in patients with vestibular schwannoma: An international multicenter cross-sectional study comparing microsurgery, stereotactic radiosurgery, observation, and nontumor controls. J Neurosurg. 2015;122(4):833–842. [DOI] [PubMed] [Google Scholar]
- 6. Goldbrunner R, Weller M, Regis J, et al. EANO guideline on the diagnosis and treatment of vestibular schwannoma. Neuro Oncol. 2020;22(1):31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Suero Molina E, van Eck A, Sauerland C, et al. Local tumor control and clinical symptoms after gamma knife radiosurgery for residual and recurrent vestibular schwannomas. World Neurosurg. 2019;122:e1240–e1246. [DOI] [PubMed] [Google Scholar]
- 8. van de Langenberg R, Hanssens PE, van Overbeeke JJ, et al. Management of large vestibular schwannoma. Part I. Planned subtotal resection followed by gamma knife surgery: Radiological and clinical aspects. J Neurosurg. 2011;115(5):875–884. [DOI] [PubMed] [Google Scholar]
- 9. Maniakas A, Saliba I.. Microsurgery versus stereotactic radiation for small vestibular schwannomas: A meta-analysis of patients with more than 5 years follow-up. Otol Neurotol. 2012;33(9):1611–1620. [DOI] [PubMed] [Google Scholar]
- 10. Erickson NJ, Schmalz PGR, Agee BS, et al. Koos classification of vestibular schwannomas: A reliability study. Neurosurgery. 2019;85(3):409–414. [DOI] [PubMed] [Google Scholar]
- 11. Yen TL, Driscoll CL, Lalwani AK.. Significance of House–Brackmann facial nerve grading global score in the setting of differential facial nerve function. Otol Neurotol. 2003;24(1):118–122. [DOI] [PubMed] [Google Scholar]
- 12. Mohr G, Sade B, Dufour JJ, Rappaport JM.. Preservation of hearing in patients undergoing microsurgery for vestibular schwannoma: degree of metal filling. J Neurosurg. 2005;102(1):1–5. [DOI] [PubMed] [Google Scholar]
- 13. Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien–Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–196. [DOI] [PubMed] [Google Scholar]
- 14. Gardner G, Robertson JH.. Hearing preservation in unilateral acoustic neuroma surgery. Ann Otol Rhinol Laryngol. 1988;97(1):55–66. [DOI] [PubMed] [Google Scholar]
- 15. Pollock BE, Lunsford LD, Kondziolka D, et al. Outcome analysis of acoustic neuroma management: A comparison of microsurgery and stereotactic radiosurgery. Neurosurgery. 1995;36(1):215–224; discussion 224. discussion 224-9. [DOI] [PubMed] [Google Scholar]
- 16. Hayhurst C, Zadeh G.. Tumor pseudoprogression following radiosurgery for vestibular schwannoma. Neuro Oncol. 2012;14(1):87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tatagiba M, Ebner FH, Nakamura T, Naros G.. Evolution in surgical treatment of vestibular schwannomas. Curr Otorhinolaryngol Rep. 2021;9(4):467–476. [Google Scholar]
- 18. Hasegawa T, Fujitani S, Katsumata S, et al. Stereotactic radiosurgery for vestibular schwannomas: Analysis of 317 patients followed more than 5 years. Neurosurgery. 2005;57(2):257–265; discussion 257. discussion 257-65. [DOI] [PubMed] [Google Scholar]
- 19. Pollock BE, Driscoll CL, Foote RL, et al. Patient outcomes after vestibular schwannoma management: A prospective comparison of microsurgical resection and stereotactic radiosurgery. Neurosurgery. 2006;59:77–185; discussion 77. discussion 77-85. [DOI] [PubMed] [Google Scholar]
- 20. Myrseth E, Moller P, Pedersen PH, Lund-Johansen M.. Vestibular schwannoma: surgery or gamma knife radiosurgery? A prospective, nonrandomized study. Neurosurgery. Apr 2009;64(4):654–661; discussion 661. discussion 661-3. [DOI] [PubMed] [Google Scholar]
- 21. Samii M, Matthies C.. Management of 1000 vestibular schwannomas (acoustic neuromas): the facial nerve—preservation and restitution of function. Neurosurgery. Apr 1997;40(4):684–694; discussion 694. discussion 694-5. [DOI] [PubMed] [Google Scholar]
- 22. Arthurs BJ, Fairbanks RK, Demakas JJ, et al. A review of treatment modalities for vestibular schwannoma. Neurosurg Rev. Jul 2011;34(3):265–277; discussion 277. discussion 277-9. [DOI] [PubMed] [Google Scholar]
- 23. Anderson DE, Leonetti J, Wind JJ, Cribari D, Fahey K.. Resection of large vestibular schwannomas: Facial nerve preservation in the context of surgical approach and patient-assessed outcome. J Neurosurg. Apr 2005;102(4):643–649. [DOI] [PubMed] [Google Scholar]
- 24. Sughrue ME, Yang I, Rutkowski MJ, Aranda D, Parsa AT.. Preservation of facial nerve function after resection of vestibular schwannoma. Br J Neurosurg. 2010;24(6):666–671. [DOI] [PubMed] [Google Scholar]
- 25. Samii M, Gerganov V, Samii A.. Improved preservation of hearing and facial nerve function in vestibular schwannoma surgery via the retrosigmoid approach in a series of 200 patients. J Neurosurg. 2006;105(4):527–535. [DOI] [PubMed] [Google Scholar]
- 26. Hasegawa T, Kida Y, Yoshimoto M, Koike J, Goto K.. Evaluation of tumor expansion after stereotactic radiosurgery in patients harboring vestibular schwannomas. Neurosurgery. 2006;58(6):1119–28; discussion 1119. discussion 1119-28. [DOI] [PubMed] [Google Scholar]
- 27. Wang JJ, Feng YM, Wang H, et al. Changes in tinnitus after vestibular schwannoma surgery. Sci Rep. 2019;9(1):1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Trakolis L, Bender B, Ebner FH, et al. Cortical and subcortical gray matter changes in patients with chronic tinnitus sustaining after vestibular schwannoma surgery. Sci Rep. 2021;11(1):8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Park CK, Jung HW, Kim JE, et al. Therapeutic strategy for large vestibular schwannomas. J Neurooncol. 2006;77(2):167–171. [DOI] [PubMed] [Google Scholar]
- 30. Bloch DC, Oghalai JS, Jackler RK, Osofsky M, Pitts LH.. The fate of the tumor remnant after less-than-complete acoustic neuroma resection. Otolaryngol Head Neck Surg. 2004;130(1):104–112. [DOI] [PubMed] [Google Scholar]
- 31. Seol HJ, Kim CH, Park CK, et al. Optimal extent of resection in vestibular schwannoma surgery: Relationship to recurrence and facial nerve preservation. Neurol Med Chir (Tokyo). 2006;46(4):176–80; discussion 180. discussion 180-1. [DOI] [PubMed] [Google Scholar]
- 32. Milligan BD, Pollock BE, Foote RL, Link MJ.. Long-term tumor control and cranial nerve outcomes following gamma knife surgery for larger-volume vestibular schwannomas. J Neurosurg. 2012;116(3):598–604. [DOI] [PubMed] [Google Scholar]
- 33. Johnson S, Kano H, Faramand A, et al. Long term results of primary radiosurgery for vestibular schwannomas. J Neurooncol. 2019;145(2):247–255. [DOI] [PubMed] [Google Scholar]
- 34. Nagano O, Higuchi Y, Serizawa T, et al. Transient expansion of vestibular schwannoma following stereotactic radiosurgery. J Neurosurg. 2008;109(5):811–816. [DOI] [PubMed] [Google Scholar]
- 35. Hasegawa T, Kida Y, Kato T, et al. Long-term safety and efficacy of stereotactic radiosurgery for vestibular schwannomas: Evaluation of 440 patients more than 10 years after treatment with gamma knife surgery. J Neurosurg. 2013;118(3):557–565. [DOI] [PubMed] [Google Scholar]
- 36. Lobato-Polo J, Kondziolka D, Zorro O, et al. Gamma knife radiosurgery in younger patients with vestibular schwannomas. Neurosurgery. 2009;65(2):294–300; discussion 300. discussion 300-1. [DOI] [PubMed] [Google Scholar]
- 37. Nakamura H, Jokura H, Takahashi K, et al. Serial follow-up MR imaging after gamma knife radiosurgery for vestibular schwannoma. AJNR Am J Neuroradiol. 2000;21(8):1540–1546. [PMC free article] [PubMed] [Google Scholar]
- 38. Delsanti C, Roche PH, Thomassin JM, Regis J.. Morphological changes of vestibular schwannomas after radiosurgical treatment: pitfalls and diagnosis of failure. Prog Neurol Surg. 2008;21:93–97. [DOI] [PubMed] [Google Scholar]
- 39. Yu CP, Cheung JY, Leung S, Ho R.. Sequential volume mapping for confirmation of negative growth in vestibular schwannomas treated by gamma knife radiosurgery. J Neurosurg. 2000;93(Suppl 3):82–89. [DOI] [PubMed] [Google Scholar]
- 40. Carlson ML, Jacob JT, Pollock BE, et al. Long-term hearing outcomes following stereotactic radiosurgery for vestibular schwannoma: Patterns of hearing loss and variables influencing audiometric decline. J Neurosurg. 2013;118(3):579–587. [DOI] [PubMed] [Google Scholar]
- 41. Watanabe S, Yamamoto M, Kawabe T, et al. Stereotactic radiosurgery for vestibular schwannomas: average 10-year follow-up results focusing on long-term hearing preservation. J Neurosurg. 2016;125(Suppl 1):64–72. [DOI] [PubMed] [Google Scholar]
- 42. Ruess D, Pohlmann L, Hellerbach A, et al. Acoustic neuroma treated with stereotactic radiosurgery: Follow-up of 335 patients. World Neurosurg. 2018;116:e194–e202. [DOI] [PubMed] [Google Scholar]
- 43. Regis J, Pellet W, Delsanti C, et al. Functional outcome after gamma knife surgery or microsurgery for vestibular schwannomas. J Neurosurg. 2002;97(5):1091–1100. [DOI] [PubMed] [Google Scholar]
- 44. Miller LE, Brant JA, Naples JG, et al. Quality of life in vestibular schwannoma patients: A longitudinal study. Otol Neurotol. 2020;41(2):e256–e261. [DOI] [PubMed] [Google Scholar]
- 45. Torres Maldonado S, Naples JG, Fathy R, et al. Recent trends in vestibular schwannoma management: An 11-year analysis of the National Cancer Database. Otolaryngol Head Neck Surg. 2019;161(1):137–143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and materials are available and can be provided upon reasonable request.