Abstract
Précis:
This study demonstrates the efficacy and safety of once-daily 0.002% omidenepag isopropyl (OMDI) in patients with primary open angle glaucoma (POAG) or ocular hypertension (OHT) who do not respond or respond poorly to latanoprost.
Purpose:
The purpose of this study was to evaluate the intraocular pressure (IOP)-lowering efficacy and safety of OMDI in latanoprost low/nonresponders with POAG or OHT.
Materials and Methods:
Phase 3, nonrandomized, 2-phase, open-label, multicenter study (NCT03697811) in the United States. Key inclusion criteria included individuals aged 18 years or above, POAG or OHT diagnosis in both eyes, IOP ≥22 mm Hg in ≥1 eye, and ≤34 mm Hg in both eyes at all time points. Overall, 107 patients were enrolled; 104 completed treatment. Included a screening period (≤35-day washout period and 8-week latanoprost run-in period) and a 3-month treatment period comprising one drop of OMDI 0.002% once daily in both eyes. The primary study endpoint was changed from baseline in the mean diurnal (MD) IOP at month 3. Safety endpoints included incidence of adverse events, serious adverse events, and adverse drug reactions.
Results:
At baseline (visit 4), 75 (70.1%) patients had POAG, 32 (29.9%) had OHT, and 68 (63.6%) had prior use of prostaglandin/prostaglandin analogs (37.4% of whom used latanoprost). The mean (SD) baseline MD IOP was 23.34 mm Hg (2.12). The mean (SD) 3-month (visit 7) MD IOP change from baseline (following latanoprost run-in period and OMDI treatment period) was an additional decrease of 2.96 mm Hg (2.83) (P<0.0001). No significant safety issues were reported during OMDI treatment.
Conclusions:
These data demonstrate OMDI efficacy and safety in latanoprost low/nonresponders with POAG or OHT, suggesting OMDI is a treatment option in the patient population in this study.
Key Words: glaucoma, intraocular pressure, omidenepag isopropyl
Glaucoma refers to a group of related diseases involving optic neuropathy frequently associated with elevated intraocular pressure (IOP).1,2 There are several nonmodifiable risk factors for glaucoma, such as family history, ethnicity, and age; the only known modifiable risk factor for glaucoma is elevated IOP.3 Results from multiple studies demonstrated that treating elevated IOP with ocular hypotensive medications, such as prostaglandin F (FP) receptor agonists (FP agonists, often referred to as prostaglandin analogs), can delay or even prevent glaucoma disease progression and glaucomatous visual field loss.2,4,5
FP agonists are the current first-line treatment for reducing IOP in patients with glaucoma and ocular hypertension (OHT).6 While adequate IOP lowering can be achieved by FP agonists (such as latanoprost) in many individuals, certain patients do not respond or respond poorly to FP agonists (nonresponders are generally defined as those experiencing IOP reductions of <10% or <15%6), and others prefer to avoid potential appearance-altering adverse events (AEs).6,7 Appearance-altering AEs include prostaglandin-associated periorbitopathy (PAP), hyperpigmentation in the iris and around the eyelids, eyelash growth, and deepening of the upper eyelid sulcus.6,7 Therefore, alternative glaucoma therapies are required for these patients.
Omidenepag isopropyl (OMDI) was approved for treating patients with primary open angle glaucoma (POAG) or OHT in the United States in 2022, in Japan in 2018, and in several other Asian countries.8 Unlike FP agonists that lower IOP predominantly by enhancing uveoscleral outflow,9 OMDI—the prodrug of omidenepag, a selective prostanoid EP2 receptor agonist—reduces IOP using a mechanism of action that increases aqueous humor outflow via both the conventional (trabecular) and uveoscleral pathways.6,7
The phase 3, open-label, multicenter FUJI study in Japan10 showed that OMDI 0.002% resulted in a clinically significant reduction from baseline in the mean diurnal (MD) IOP in a small cohort of patients with POAG or OHT who were low/nonresponders to latanoprost (P<0.0001).10 We report results from a phase 3, open-label, multicenter study in the United States that investigated the efficacy and safety of OMDI once daily for 3 months in latanoprost low/nonresponders diagnosed with POAG or OHT. The intention of this study was not to challenge the well-established efficacy of latanoprost nor compare it with that of OMDI, but rather, to investigate OMDI as an efficacious alternative to adding a second agent in latanoprost low/nonresponders.
MATERIALS AND METHODS
Study Design
A phase 3, open-label, single-arm, multicenter study (clinicaltrials.gov identifier: NCT03697811) was conducted at 27 sites in the United States. The study was prospectively approved by an appropriate institutional review board and conducted in compliance with regulatory requirements and in accordance with the protocol, Good Clinical Practice, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use international guidelines, and the Declaration of Helsinki. All patients provided written informed consent before participation.
Participants and Procedures
The study included a screening period involving washout and run-in periods and an open-label treatment period. An overview of the study design is presented in Supplemental Digital Content Figure 1, Supplemental Digital Content 1, http://links.lww.com/IJG/A847. Key exclusion criteria, applied from visits 1–4, include patients exposed to OMDI before visit 1 (screening) or use of >2 active ingredients to lower IOP before the washout period; use of contact lenses within 2–3 weeks before visit 2 until end of treatment in either eye; and presence of advanced glaucoma (visual field mean deviation worse than −12 dB in either eye). Key inclusion criteria for entering the washout period were as follows: age 18 years or above and a diagnosis of POAG or OHT in both eyes (or 1 eye with POAG and the other with OHT). Full exclusion and inclusion criteria are presented in the Supplemental Digital Content, Supplemental Digital Content 1, http://links.lww.com/IJG/A847.
FIGURE 1.

Patient disposition. aThree full analysis set patients did not meet the eligibility criteria at baseline but were still included in the analyses. bStudy period is from the start of screening (visit 1) to the end of the open-label treatment period (visit 7, month 3).
At visit 1, patients were screened for eligibility and entered a washout period of ≤35 days. Eligible patients were instructed to discontinue the use of all IOP-lowering medications during the washout period, according to the schedule in the Supplemental Digital Content, Supplemental Digital Content 1, http://links.lww.com/IJG/A847.
Following the washout period, eligibility to enter an 8-week run-in period with latanoprost was determined at visit 2 (week −8). Eligible patients entered the run-in period and were dosed with one drop of latanoprost ophthalmic solution 0.005% every evening at 8 pm (±60 minutes) in both eyes. Visit 3 occurred at week −4 (midpoint of the run-in period) to check whether the following IOP criteria were met: a ≤25% decrease in IOP from visit 2 (start of run-in period) at all measurement time points and IOP of ≤34 mm Hg in both eyes at all measurement time points. If met, patients continued the remainder of the 8-week run-in period.
Visit 4 (day 1) occurred at the end of the run-in period and was considered the baseline of the OMDI open-label treatment phase. All baseline characteristics were collected at this visit. Patients began the 3-month treatment period with 1 drop of OMDI 0.002% every evening at 8 pm (±60 minutes) in both eyes if the following criteria were met: a ≤15% decrease in IOP from visit 2 (start of run-in period) at all measurement time points and IOP of ≤34 mm Hg in both eyes at all measurement time points.
IOP was measured at 8 am, 12 pm, and 4 pm (±60 minutes) at all visits except at visit 1 (screening) and an optional, mid-washout visit, when one measurement was taken at any time. Further details on efficacy analyses are included in the Supplemental Digital Content, Supplemental Digital Content 1, http://links.lww.com/IJG/A847.
Safety Analyses
At all scheduled visits, patients were queried regarding potential AEs. Investigators asked patients whether there had been a change in their general health. Direct questioning and examination were then performed as appropriate. At screening and all scheduled visits, best-corrected visual acuity and slit lamp biomicroscopy were performed before the first IOP measurement (8 am). At visit 1, visit 4 (baseline), and visit 7 (month 3), ophthalmoscopy (fundus examination) was performed after the last IOP measurement for the day (4 pm). Six photographs of the iris, eyelids, and eyelashes of each eye were taken at visit 4 (baseline, day 1), visit 7 (month 3), and study exit. The photographs taken at visit 4 were used to help the investigator assess iris color and any changes from baseline in iris color, eyelashes, and eyelids at visit 7.
Outcomes
The primary end point was the change from baseline (day 1, visit 4; after 8-week run-in with latanoprost) in MD IOP at month 3 (visit 7). IOP was measured at 8 am, 12 pm, and 4 pm (±60 minutes). The secondary endpoints included percent change from baseline in MD IOP at month 3 (visit 7); change and percent change from baseline in MD IOP at week 2 (visit 5) and week 6 (visit 6); change and percent change from baseline in IOP for each postbaseline visit/time point; proportion of study eyes with MD IOP reduction of ≥10%, ≥15%, ≥20%, ≥25%, or ≥30% from baseline at month 3; and proportion of study eyes with MD IOP of ≤18 mm Hg at month 3. Safety end points included ocular assessments and incidence of AEs, serious AEs, and adverse drug reactions.
Statistical Methods
A target sample size was calculated using a one-sample t test, with a significance level of 5%. A sample size of 100 would have 75% power to detect an MD IOP reduction of 1.0 mm Hg from baseline with an SD of 3.5 mm Hg after accounting for ≤12% of patients not completing the study. The safety population included all patients who provided informed consent and received ≥1 dose of OMDI 0.002%. All safety analyses were performed on the safety population. The full analysis set (FAS) included all patients in the safety population who had ≥1 postbaseline IOP measurement. The per-protocol set was a subset of the FAS, restricted to patients who fulfilled the protocol in terms of eligibility, interventions, and other assessments. Efficacy analyses were performed using the FAS or a subset of the FAS, and the primary efficacy analysis was based on observed cases in the FAS. A paired t test determined whether the change from baseline in MD IOP at month 3 (the primary endpoint) differed from 0.
The continuous secondary endpoints of change and percent change from baseline in IOP and MD IOP at week 2, week 6, and month 3 were summarized descriptively. For the binary secondary endpoint of MD IOP reduction of ≥10%, ≥15%, ≥20%, ≥25%, or ≥30%, and the secondary end point of IOP ≤18 mm Hg, response rates were tabulated using frequencies and percentages.
RESULTS
Patient Disposition
Between December 11, 2018, and October 21, 2021, 173 patients entered the latanoprost run-in period; 107 of these patients were included in the safety population and FAS (and were treated with OMDI); 104 patients met the IOP eligibility criteria and completed study treatment (and were considered low responders/nonresponders). The per-protocol set comprised 102 (95.3%) patients who exited the study without any significant protocol deviation that could impact efficacy outcomes (Fig. 1).
The mean (SD) age was 63.1 years (10.2), 54 (50.5%) patients were aged ≥65 years, and 63 (58.9%) were female. Overall, 64 (59.8%) patients were White, 42 (39.3%) were Black or African American, and 16 (15.0%) were Hispanic or Latino. For the primary diagnosis of the study eye, 75 (70.1%) patients had POAG, and 32 (29.9%) had OHT at baseline. Most patients had prior use of FP agonists (68 [63.6%]; 37.4% of these patients used latanoprost) and topical carbonic anhydrase inhibitors [CAIs; 34 (31.8%)]. For patients previously using a single FP agonist, the mean length of time using this agonist was 791.4 days. At baseline, MD IOP (SD) was 23.34 mm Hg (2.12), the mean (SD) percent change in MD IOP at the end of the latanoprost run-in period/baseline was −6.7% (4.5), and the mean best-corrected visual acuity was 0.051 [logarithm of the minimum angle of resolution (logMAR), 0.0999]. Demographics and baseline characteristics are summarized in Table 1.
TABLE 1.
Patient Demographics and Baseline Characteristics (Full Analysis Set)
| Characteristic | OMDI 0.002% (N=107) |
|---|---|
| Age, y, mean (SD) | 63.1 (10.2) |
| Aged ≥65 y, n (%) | 54 (50.5) |
| Female, n (%) | 63 (58.9) |
| Race and ethnicity, n (%) | |
| White | 64 (59.8) |
| Black or African American | 42 (39.3) |
| Hispanic or Latino | 16 (15.0) |
| Primary diagnosis, study eye, n (%) | |
| POAG | 75 (70.1) |
| OHT | 32 (29.9) |
| Previous use of IOP-lowering medications, study eye, n (%) | |
| None | 16 (15.0) |
| Oral/topical carbonic anhydrase inhibitors | 34 (31.8) |
| Alpha agonists | 5 (4.7) |
| Beta blockers | 15 (14.0) |
| FP agonists | 68 (63.6) |
| Latanoprost | 40 (37.4) |
| Bimatoprost | 21 (19.6) |
| Travoprost | 11 (10.3) |
| Lens status, study eye, n (%) | |
| Phakic | 87 (81.3) |
| Pseudophakic | 20 (18.7) |
| MD IOP, study eye; mm Hg, mean (SD) | 23.34 (2.12) |
| BCVA (logMAR) | 0.051 (0.0999) |
BCVA indicates best-corrected visual acuity; FP, prostaglandin F; IOP, intraocular pressure; logMAR, logarithm of the minimum angle of resolution; MD, mean diurnal; OHT, ocular hypertension; OMDI, omidenepag isopropyl; POAG, primary open angle glaucoma.
Efficacy
At the end of the washout period and before the latanoprost run-in period (week −8), the mean (SD) of MD IOP was 25.03 mm Hg (2.06). At the end of the run-in period with latanoprost (baseline, day 1), the mean (SD) of MD IOP had decreased to 23.34 mm Hg (2.12)—a change of −6.7%—and at the end of the OMDI treatment period (month 3), had decreased further to 20.48 mm Hg (3.31) (Fig. 2 and Supplemental Digital Content Table 1, Supplemental Digital Content 1, http://links.lww.com/IJG/A847), which is a change of −4.55 mm Hg (−18.16%) from the start of the run-in period to the end of the treatment period. The mean (SD) of MD IOP change from baseline following the latanoprost run-in period was −2.96 mm Hg (2.83), which was statistically significant (P<0.0001) and, as baseline was after the latanoprost run-in period, this IOP change with OMDI is in addition to the IOP decreases that occurred with latanoprost.
FIGURE 2.
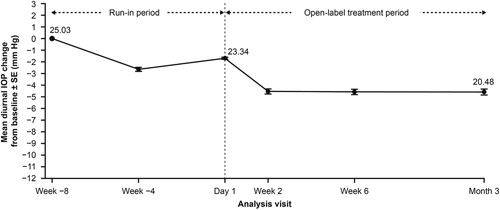
Mean diurnal IOP change from the start of run-in period (week −8) to month 3 by analysis visit. Efficacy analyses were performed on study eyes of the full analysis set. IOP indicates intraocular pressure.
At the first postbaseline visit (week 2), OMDI showed early-onset reductions in IOP, and a stable IOP-lowering effect was observed throughout the study. IOP changes from baseline at each postbaseline visit/time point were consistent with the findings from the changes in MD IOP from baseline. The mean (SD) of MD IOP changes from baseline were an additional decrease of 2.87 mm Hg (2.53), 2.89 mm Hg (2.58), and 2.96 mm Hg (2.83) in the study eye at week 2, week 6, and month 3, respectively (Fig. 3 and Supplemental Digital Content Table 2, Supplemental Digital Content 1, http://links.lww.com/IJG/A847). The mean (SD) percent changes from baseline were −12.21% (10.86), −12.40% (11.22), and −12.57% (11.91) in the study eye at week 2, week 6, and month 3, respectively (Supplemental Digital Content Table 2, Supplemental Digital Content 1, http://links.lww.com/IJG/A847). The proportion of patients with no reduction from baseline in MD IOP, those attaining response rates of between 0 and 10%, ≥10%, ≥15%, ≥20%, ≥25%, and ≥30% reduction from baseline in MD IOP, and the response rate of MD IOP reduced to ≤18 mm Hg at month 3 are shown in Table 2. Most patients [63 (61.8%)] achieved a ≥10% reduction in MD IOP during the 3-month treatment period (from the end of the run-in period to the end of the treatment period), and 14.7% of patients had no reduction in MD IOP from baseline.
FIGURE 3.

Mean IOP change from BL at each post-BL visit/time point (full analysis set). BL indicates baseline; IOP, intraocular pressure; M, month; W, week.
TABLE 2.
Mean Diurnal Intraocular Pressure: Response Rate at Month 3 (Full Analysis Set)
| Omidenepag isopropyl 0.002% (N=107) | |||
|---|---|---|---|
| Response | n | Response rate, n (%) | 95% CI |
| No reduction | 102 | 15 (14.7) | 7.8, 21.6 |
| 0%–10% reduction | 102 | 24 (23.5) | 15.3, 31.8 |
| ≥10% reduction | 102 | 63 (61.8) | 52.3, 71.2 |
| ≥15% reduction | 102 | 40 (39.2) | 29.7, 48.7 |
| ≥20% reduction | 102 | 22 (21.6) | 13.6, 29.6 |
| ≥25% reduction | 102 | 15 (14.7) | 7.8, 21.6 |
| ≥30% reduction | 102 | 8 (7.8) | 2.6, 13.1 |
| Reduced to ≤18 mm Hg | 102 | 22 (21.6) | 13.6, 29.6 |
A subgroup analysis of the mean (SD) of MD IOP changes from baseline by prior IOP-lowering medication showed that a trend of greater reductions in IOP was observed at month 3 in patients who received alpha agonists, beta blockers, and no medication before the study compared with those who received FP agonists (Supplemental Digital Content Table 3, Supplemental Digital Content 1, http://links.lww.com/IJG/A847).
Safety
A summary of AEs occurring during the OMDI treatment period is shown in Table 3. Of all 107 patients in the safety population during the treatment period, 27 (25.2%) reported AEs; no serious AEs were reported, and no patients discontinued the study due to AEs. One patient (0.9%) developed an appearance-altering AE (increase in eyelash pigmentation) that was reported on day 92 of the treatment period. The most frequently reported AE was conjunctival hyperemia, occurring in 9 (8.4%) patients; 8 were mild in severity, with 1 moderate case. Other AEs included punctate keratitis [3 (2.8%)] and eye pain [2 (1.9%)]. One patient (0.9%), who had intraocular lens implants in both eyes, developed macular edema (ME) and ocular inflammation in both eyes; these AEs occurred on day 92 and were later resolved in both eyes. Both AEs reported for this patient were classified as moderate. AEs occurring during the latanoprost run-in period are summarized in Supplemental Digital Content Table 4, Supplemental Digital Content 1, http://links.lww.com/IJG/A847.
TABLE 3.
Summary of AEs Occurring During the Open-Label Treatment Period (Safety Population)
| Patients, n (%) | OMDI 0.002% (N=107) |
|---|---|
| AEs | 27 (25.2) |
| SAEs | 0 (0.0) |
| ADRs | 17 (15.9) |
| Ocular AEs | 19 (17.8) |
| Ocular ADRs | 17 (15.9) |
| Nonocular AEs | 9 (8.4) |
| AEs leading to study discontinuation | 0 (0.0) |
| Deaths | 0 (0.0) |
| Eyes that developed appearance-altering effects* during treatment | 1 (0.9) |
| Eyes that developed ocular inflammation during treatment | 1 (0.9) |
| Eye disorders | 15 (14.0) |
| Conjunctival hyperemia | 9 (8.4) |
| Punctate keratitis | 3 (2.8) |
| Eye pain | 2 (1.9) |
| Anterior chamber cell | 1 (0.9) |
| Conjunctival edema | 1 (0.9) |
| Eye irritation | 1 (0.9) |
| Eyelash hyperpigmentation | 1 (0.9) |
| Macular edema† | 1 (0.9) |
| Photophobia | 1 (0.9) |
| Vision blurred | 1 (0.9) |
| Investigations | 1 (0.9) |
| Vital dye staining cornea present | 1 (0.9) |
| General disorders and administration site conditions | 3 (2.8) |
| Cyst | 1 (0.9) |
| Instillation site foreign body sensation | 1 (0.9) |
| Instillation site pain | 1 (0.9) |
| Injury, poisoning, and procedural complications | 3 (2.8) |
| Contusion | 1 (0.9) |
| Muscle strain | 1 (0.9) |
| Suture-related complication | 1 (0.9) |
| Nervous system disorders | 1 (0.9) |
| Dizziness | 1 (0.9) |
| Headache | 1 (0.9) |
An increase in eyelash pigmentation.
Reported in both eyes of 1 male patient; both lenses were pseudophakic and the AE resolved in both eyes. Macular edema includes adverse events under the preferred terms macular edema and cystoid macular edema. Any patient who experienced multiple events within a system organ class or preferred term was counted only once for that system organ class or preferred term. AEs were coded using the Medical Dictionary for Regulatory Activities, version 21.1.
ADR indicates adverse drug reaction; AE, adverse event; OMDI, omidenepag isopropyl; SAE, serious adverse event.
DISCUSSION
Prostaglandin analogs remain the first-line treatment for the medical management of glaucoma.6 However, some patients treated with prostaglandin analogs experience insufficient IOP reduction or prefer to avoid the appearance-altering AEs that can occur with these medications. These patients have several other medical options or can consider laser trabeculoplasty. However, options such as beta blockers, alpha agonists, and CAIs, while effective, typically require more than once-daily dosing and can have systemic side effects.11,12 Findings from this study suggest that once-daily dosing of OMDI 0.002% is another viable option with a favorable side-effect profile in the patient population in this study (in latanoprost low/nonresponders with POAG or OHT). Switching to OMDI following a latanoprost run-in period resulted in statistically significant reductions from baseline in MD IOP at all time points through month 3.
The IOP-lowering efficacy from baseline seen with OMDI in this study is similar to findings from previous studies with FP agonists, such as latanoprost,13 and a switching study with bimatoprost.14 The IOP reductions with OMDI, additional to those from latanoprost at baseline observed in this study, are similar to those seen in Japanese patients in the phase 3 FUJI study.10
The novel mechanism of action of OMDI may explain the additional IOP-lowering efficacy seen from latanoprost baseline in this study. Omidenepag, the active metabolite of OMDI, selectively binds to the prostanoid EP2 receptor found in several ocular tissues associated with aqueous humor dynamics. This leads to an increase in intracellular cyclic adenosine monophosphate and smooth muscle relaxation, thereby reducing IOP by improving both the conventional (trabecular) and uveoscleral outflow,6,7 unlike FP agonists that lower IOP by predominantly enhancing uveoscleral outflow.9 Remodeling of the extracellular matrix following bimatoprost treatment has been shown to increase uveoscleral outflow;9 similarly, this remodeling (which involves Schlemm canal endothelial cells inhibition of transforming growth-factor 2 beta-induced mRNA and protein expression and downregulation of COL12A1 and COL13A1 mRNA in trabecular meshwork cells), has also been theorized to occur following OMDI, leading to increases in trabecular outflow.15,16
OMDI 0.002% was well tolerated in this patient population; no patients discontinued from the study due to AEs, and no significant safety concerns were reported. Almost all (8/9) cases of conjunctival hyperemia (physician-reported) were mild in severity. One case of an appearance-altering AE (an increase in eyelash pigmentation) was reported on day 92 during the OMDI 3-month treatment period. Although 1 patient experienced both ME and ocular inflammation during OMDI treatment, both AEs were classified as moderate and were resolved during the study. Similar to the case following OMDI treatment in this study, one case of bilateral ME was reported recently in a patient with POAG following bimatoprost implant injections.17 In another study of OMDI, all cases of treatment-related ME occurred in eyes with intraocular lens implants.18 PAPs, such as eyelid pigmentation, eyelash growth, and deepening of the upper eyelid sulcus have been reported with FP agonists, and OMDI is associated with a lower frequency (0%–2%) of these PAPs compared with FP agonists.6,19 This further emphasizes the benefits of switching to OMDI in patients who are latanoprost low responders/nonresponders rather than continuing with another FP agonist. An additional advantage of OMDI compared with other IOP-lowering medications, including alpha agonists, beta blockers, and CAIs, is the once-daily dosing regimen, which may be preferred by patients, thus potentially improving patient compliance and adherence.12
Studies evaluating nonresponders to treatments are often difficult to design and can present challenges with recruitment. Notably, many of the patients in this study were previously on FP agonists for a substantial period, which is surprising given that they were found to be nonresponders after the latanoprost run-in period and that IOP reductions in those previously on FP agonists were lower than in patients on some other IOP-lowering medications prior with the study. A potential reason for this is that these treatments may have been used because of an unfavorable AE profile of, or allergy to, other medications. As nonresponder patients were not washed out after the 8-week latanoprost run-in period, it is unclear whether this influenced the early IOP reductions. A high proportion of the patients in this study was latanoprost nonresponders, which may indicate a selection bias, given that patients who are aware that they are nonresponders may be more likely to seek out studies, such as this one, in search of alternative therapies.
The open-label design and lack of both randomization and a separate control group in this study may be considered a limitation; however, the patients served as their own controls due to the nature of the study design. As the main objective of the study was to investigate the IOP-lowering efficacy of a medication with a different mechanism of action to existing medications (OMDI compared with latanoprost), a realistic clinical practice treatment plan was followed. Since patient response was limited when treated with latanoprost for 2 months during the run-in period, continuing with latanoprost for an additional 3 months was avoided; therefore, the switching design was used. As a result, this study did not focus on direct comparisons of efficacy and safety with latanoprost. In studies investigating OMDI in those with POAG and OHT but with randomized, masked, and control group study designs, a similar IOP-lowering efficacy to this study was observed.20,21 Another potential study limitation includes the possibility of regression to the mean. There can be a tendency for patients who were low responders/nonresponders to previous medications to respond better to the next therapy, although the washout period means that it is unlikely that previous use of FP agonists still had an effect by the time the patients entered the OMDI treatment period. The IOP eligibility criteria for entering each study visit to ensure only latanoprost nonresponders remained in the study may have led to selection bias. The study was also limited by the relatively low proportion of patients of Hispanic or Latino ethnicity included (15.0%) and the moderately short OMDI treatment period (3 months). Future studies with longer treatment periods and a larger, more diverse population will provide valuable additions to the evidence supporting the IOP-lowering efficacy of OMDI.
Collectively, these data demonstrate the efficacy and safety of once-daily OMDI in latanoprost low/nonresponders with POAG or OHT, suggesting OMDI is a potential treatment option for the patient population in this study.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the patients, the families, and all investigators involved in this study, and Santen Inc., who were involved in the study implementation.
Footnotes
Supported by Santen Inc. Medical writing support was provided by India Wright, MSc, and editorial support was provided by Jess Galbraith, BSc, and Sharmin Saleque, MSc, all of Core Medica, London, UK, supported by Santen, Inc., according to Good Publication Practice guidelines (https://www.acpjournals.org/doi/10.7326/M22-1460). The sponsor was involved in the study design, collection, analysis, and interpretation of data, as well as data checking of information provided in the manuscript. Ultimate responsibility for opinions, conclusions, and data interpretation lies with the authors.
Data underlying the findings described in this manuscript may be requested in accordance with Santen's data sharing policy.
J.F.P. and E.C.B. contributed equally as co-first authors.
Disclosure: E.C.B. has served as an advisor for Santen Inc., and has received financial support from CorneaGen Inc. E.B.M. has conducted research funded by AbbVie, Aerie Inc., Allergan, Nicox Ophthalmics Inc., Ocular Therapeutix, ReGenTree, LLC, Perrigo, and Santen Inc. N.O.-K., Z.P., and A.R. are employees of Santen. J.F.P. has been a consultant/advisor for Aerie Pharmaceuticals, Allergan, Glaukos, New World Medical, and Santen Inc., and has received grant support from CorneaGen Inc. M.E.T. has conducted research with Allergan, Bausch + Lomb, Biorasi, Eyenovia, Insite, Kala, Kowa, Oculos, Ocuphire, Osmotica, Nicox, Novartis, Santen Inc., Senju, Sylentis, Tear Solutions, and Valeant.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.glaucomajournal.com.
Contributor Information
Joseph F. Panarelli, Email: Joseph.Panarelli@nyulangone.org.
Eileen C. Bowden, Email: eileen.bowden@austin.utexas.edu.
Michael E. Tepedino, Email: mtepedino@wakehealth.edu.
Noriko Odani-Kawabata, Email: noriko.odani@santen.com.
Zifan Pei, Email: Zifan.Pei@santen.com.
Eugene B. McLaurin, Email: gene@emclaurin.us.
Auli Ropo, Email: auli.ropo@santen.com.
REFERENCES
- 1.Quigley H. Glaucoma. Lancet. 2011;377:1367–1377. [DOI] [PubMed] [Google Scholar]
- 2.Garway-Heath DF, Crabb DP, Bunce C, et al. Latanoprost for open-angle glaucoma (UKGTS): a randomised, multicentre, placebo-controlled trial. Lancet. 2015;385:1295–1304. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan TM, Sit AJ. Emerging drugs for the treatment of glaucoma: a review of phase II & III trials. Expert Opin Emerg Drugs. 2022;27:321–331. [DOI] [PubMed] [Google Scholar]
- 4.Kass MA, Heuer DK, Higginbotham EJ, et al. The ocular hypertension treatment study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–713. [DOI] [PubMed] [Google Scholar]
- 5.The AGIS Investigators . The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130:429–440. [DOI] [PubMed] [Google Scholar]
- 6.Matsuo M, Matsuoka Y, Tanito M. Efficacy and patient tolerability of omidenepag isopropyl in the treatment of glaucoma and ocular hypertension. Clin Ophthalmol. 2022;16:1261–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuwa M, Toris CB, Fan S, et al. Effects of a novel selective EP2 receptor agonist, omidenepag isopropyl, on aqueous humor dynamics in laser-induced ocular hypertensive monkeys. J Ocul Pharmacol Ther. 2018;34:531–537. [DOI] [PubMed] [Google Scholar]
- 8.Santen. Santen and UBE Received FDA Approval for OMLONTI® (Omidenepag Isopropyl Ophthalmic Solution) 0.002% for the Reduction of Elevated Intraocular Pressure in Patients with Primary Open-Angle Glaucoma or Ocular Hypertension. 2022. Accessed November 2, 2022. https://www.santen.com/en/news/20220926-1.pdf
- 9.Weinreb RN, Toris CB, Gabelt BT, et al. Effects of prostaglandins on the aqueous humor outflow pathways. Surv Ophthalmol. 2002;47:S53–S64. [DOI] [PubMed] [Google Scholar]
- 10.Aihara M, Ropo A, Lu F, et al. Intraocular pressure-lowering effect of omidenepag isopropyl in latanoprost non-/low-responder patients with primary open-angle glaucoma or ocular hypertension: the FUJI study. Jpn J Ophthalmol. 2020;64:398–406. [DOI] [PubMed] [Google Scholar]
- 11.Inoue K. Managing adverse effects of glaucoma medications. Clin Ophthalmol. 2014;8:903–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robin AL, Muir KW. Medication adherence in patients with ocular hypertension or glaucoma. Expert Rev Ophthalmol. 2019;14:199–210. [Google Scholar]
- 13.Aihara M, Lu F, Kawata H, et al. Phase 2, randomized, dose-finding studies of omidenepag isopropyl, a selective EP2 agonist, in patients with primary open-angle glaucoma or ocular hypertension. J Glaucoma. 2019;28:375–385. [DOI] [PubMed] [Google Scholar]
- 14.Myers JS, Vold S, Zaman F, et al. Bimatoprost 0.01% or 0.03% in patients with glaucoma or ocular hypertension previously treated with latanoprost: two randomized 12-week trials. Clin Ophthalmol. 2014;8:643–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura N, Honjo M, Yamagishi R, et al. Effects of selective EP2 receptor agonist, omidenepag, on trabecular meshwork cells, Schlemm’s canal endothelial cells and ciliary muscle contraction. Sci Rep. 2021;11:16257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumon M, Fuwa M, Shimazaki A, et al. Downregulation of COL12A1 and COL13A1 by a selective EP2 receptor agonist, omidenepag, in human trabecular meshwork cells. PLoS One. 2023;18:e0280331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel SH, Badar A, Bakhsh S, et al. Bilateral cystoid macular edema following bimatoprost implants. Retin Cases Brief Rep. 2022. doi: 10.1097/ICB.0000000000001346. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 18.Aihara M, Lu F, Kawata H, et al. Twelve-month efficacy and safety of omidenepag isopropyl, a selective EP2 agonist, in open-angle glaucoma and ocular hypertension: the RENGE study. Jpn J Ophthalmol. 2021;65:810–819. [DOI] [PubMed] [Google Scholar]
- 19.Inoue K, Shiokawa M, Katakura S, et al. Periocular adverse reactions to omidenepag isopropyl. Am J Ophthalmol. 2022;237:114–121. [DOI] [PubMed] [Google Scholar]
- 20.Aihara M, Lu F, Kawata H, et al. Omidenepag isopropyl versus latanoprost in primary open-angle glaucoma and ocular hypertension: the phase 3 AYAME study. Am J Ophthalmol. 2020;220:53–63. [DOI] [PubMed] [Google Scholar]
- 21.Olander KW, Sato MA, Abrams MA, et al. A randomized phase 2 trial comparing omidenepag isopropyl 0.002% once and twice daily in subjects with primary open-angle glaucoma or ocular hypertension (SPECTRUM-6). J Glaucoma. 2021;30:473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]


