Abstract
Aldosterone is a steroid hormone regulating fluid and electrolyte homeostasis and is known to increase the risk of atherosclerosis. In this study, we examined the associations of serum aldosterone concentrations with subclinical atherosclerosis and all-cause mortality. This study included 948 adults aged 46–88 years from the Multi-Ethnic Study of Atherosclerosis (MESA) with measurements of serum aldosterone and plasma renin activity (PRA) and not taking antihypertensive medications. Coronary calcification was longitudinally assessed using Agatston coronary artery calcium (CAC) score from computed tomography scans. All-cause mortality was ascertained from the medical record. The average age (SD) was 62.3 (9.4) years, and 53% were male. Among 700 subjects who had follow-up CAC score (median follow-up of 6.4 years), higher aldosterone levels (per 100 pg/ml) were associated with higher CAC (relative ratio, 1.17; 95% confidence interval [CI], 1.04–1.32), with the association being most prominent in males with suppressed PRA (≤0.5 μg/L). Systolic or diastolic blood pressure mediated around 45% of the total effect of aldosterone on CAC. Over a median follow-up of 12.5 years (120 deaths identified among 948 subjects), aldosterone was associated with the increased risk of all-cause mortality when PRA was suppressed; hazard ratio per 100 pg/ml, 1.70; 95% CI, 1.10–2.63. In this study, we found that higher aldosterone levels were associated with the increased risk of subclinical coronary atherosclerosis and all-cause mortality particularly when renin was suppressed. Our findings indicate the importance of aldosterone levels (even within the reference range) with respect to the cardiovascular system and overall health.
Keywords: Aldosterone, Coronary artery calcium, Blood pressure, Mediation analysis, Mortality, Multi-ethnic atherosclerosis study
Introduction
Aldosterone is a steroid hormone that contributes to the regulation of sodium reabsorption, water retention, and blood pressure control.1 Primary aldosteronism, characterized by elevated aldosterone concentrations and suppressed renin activity, is a major cause of secondary hypertension.2 The associations of aldosterone with long-term adverse health outcomes including cardiovascular events and mortality have been demonstrated in high-risk populations such as patients with congestive heart failure,3–7 acute myocardial infarction,7–10 and coronary artery disease.11 The association between aldosterone and atherosclerosis has also been widely shown on animal models,12,13 but is still controversial in humans.11,14,15 Moreover, since aldosterone levels are usually only measured when its excess (i.e. primary aldosteronism) is suspected, much less is known about the long-term health outcomes of increasing aldosterone concentrations among the general population and even less is known about the consequences of higher aldosterone levels within the reference range.
Coronary artery calcium (CAC) is a marker of subclinical atherosclerosis and strongly predicts atherosclerotic cardiovascular disease events.16 While previous studies have evaluated the association of aldosterone with subclinical atherosclerosis makers such as carotid intima-media thickness (IMT) and ankle-brachial index (ABI),17 its association with CAC is incompletely explored. Given the strong association between aldosterone and blood pressure, it is important to clarify the extent to which blood pressure mediates the relationship between aldosterone and cardiovascular disease.
In the present study, using data from the Multi-Ethnic Study of Atherosclerosis (MESA), we investigated the longitudinal association between serum aldosterone concentrations and CAC. Given that plasma renin activity (PRA) could be one of the indicators of autonomous aldosterone secretion and mineralocorticoid receptor activity,18–20 we also investigated the association stratified by PRA levels. Then, using causal mediation analysis, we examined the extent to which an increase in blood pressure mediates the association between aldosterone and CAC. Finally, we investigated the association between serum aldosterone concentrations and all-cause mortality.
Methods
Data Sources and Study Population
The data of this study can be accessed at https://www.mesa-nhlbi.org. MESA is a multicenter longitudinal cohort study of 6,814 community-dwelling adults aged 45–84 years free from overt CV disease at baseline from six communities (Baltimore, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles, California; New York, New York; and St. Paul, Minnesota).21 Its main goal was to investigate risk factors related to the development and progression of cardiovascular disease and incident cardiovascular events. Participants were recruited between July 2000 and August 2002 with a longitudinal follow-up through July 2015. The Institutional Review Boards of all participating institutions approved the study protocols and consent procedures. More detail about MESA study design and recruitment is described elsewhere.21
As part of an ancillary study investigating renal artery calcification, a random sample of 1,960 participants had aldosterone and PRA levels measured at either visit 2 (between September 2002 and February 2004) or 3 (between March 2004 and September 2005).18,22 Among the 1,960 participants, we excluded participants if either aldosterone or PRA was missing (n=185), or if the CAC score was missing at the visit when aldosterone and PRA were measured (i.e. visit 2 or 3) (n=52). We further excluded participants with antihypertensive medications (n=775) as these medications impact aldosterone and PRA levels. Among the final analytical cohort who had mortality follow-up data (n=948), 700 subjects (74%) had CAC score measured at either visit 4 or 5.
Aldosterone levels and renin activity
Aldosterone and PRA were measured two times using competition-based radioimmunoassay (DiaSorin) and radioimmunoassay (DiaSorin), respectively.22 PRA was defined as nanograms of angiotensin I generated per milliliter of sample per hour (ng/mL/h = μg/L/h).22 Aldosterone-renin ratio (ARR) was calculated by dividing aldosterone by PRA. In addition, we dichotomized participants based on PRA; i.e. suppressed renin phenotype, PRA ≤0.5 μg/L/h; intermediate to unsuppressed renin phenotype, PRA >0.5 μg/L/h.18 We combined intermediate and unsuppressed phenotype to keep statistical power in our main analysis.
Blood pressure
Blood pressure was measured 3 times with the participant in a seated position using a Dinamap Pro 1000 automated oscillometric sphygmomanometer (Critikon, Tampa, FL). The mean of the last 2 measurements were used in our analysis as previous studies did.18,22 We divided both the systolic and diastolic blood pressure by 10 to facilitate result interpretation as per 10 mmHg increase in systolic or diastolic blood pressure.
Outcomes
The primary outcome was CAC score at either visit 4 (between September 2005 and June 2007) or visit 5 (between April 2010 and December 2011). Each participant underwent two coronary artery computed tomography (CT) scans, and all CT scans were read at the Harbor-UCLA Research and Education Institute in Torrance, California.21 The Agatson score was calculated for each scan (phantom adjusted) and the mean of the two CAC scores was employed in the analysis.23,24 The same approach was used to measure CAC at visit 2 and 3. The secondary outcome was all-cause mortality ascertained from the medical record. Two physicians from the MESA study events committee independently reviewed all the medical records for endpoint classification using prespecified criteria for all-cause death. In this study, we did not assess cardiovascular events due to the small number of the outcome. A detailed description of the follow-up methods is available on the MESA website (www.mesa-nhlbi.org).
Covariates
At each visit, participants completed self-administered questionnaires which include age, sex (male, female), race/ethnicity (White, Chinese American, African American, Hispanic), education status (≤11 grade, high school to college, associate’s or bachelor’s or professional degree), health insurance (yes, no), annual income (<$30,000, $30,000 to $75,000, >$75,000), smoking status (never, former, current), alcohol intake (yes, no), physical activity levels (MET-min/week), and medication intake. Body mass index (BMI) was measured by trained staff. Fasting peripheral blood samples were obtained after 12-hour overnight fasting and at least 5 minutes of the seated posture. Specimens were immediately flash-frozen, processed, and stored at −80 °C.22 Serum and urinary sodium and potassium were measured at visit 1 as previously described.18,25 Estimated glomerular filtration rate (eGFR) was calculated from serum creatinine measurements employing the Chronic Kidney Disease Epidemiology Collaboration equation.26
Statistical analyses
First, we described baseline characteristics among the total study sample, suppressed renin phenotype group, and intermediate to unsuppressed renin phenotype group. Second, we examined the cross-sectional association between serum aldosterone concentrations and systolic or diastolic blood pressure (at baseline; i.e. the same visit at which aldosterone was measured [visit 2 or 3]) using ordinary least squares (OLS) regression after we confirmed that blood pressures were almost normally distributed. Third, we examined the association between serum aldosterone concentrations (at visit 2 or 3) and CAC score (at visit 4 or 5) using multivariable negative binomial regression models to account for a right-skewed distribution of CAC score. The analysis was conducted for the total population and each renin phenotype subgroup. We first adjusted for age, sex, CAC score at visit 2 or 3, and time from aldosterone measurement to coronary CT scan at visit 4 or 5 (Model 1). As the main model, we further adjusted for race, education, insurance, annual income, smoking status, alcohol use, physical activity, statin prescription, BMI, low-density lipoprotein (LDL), serum sodium and potassium levels, urinary sodium and potassium levels, and plasma renin activity in addition to covariates in Model 1 (Model 2: main model). Missing covariate values were replaced using a multiple imputation algorithm that included all of the abovementioned covariates in the model.27
Then, we examined the dose-response association between serum aldosterone concentrations and the relative ratio of CAC by fitting a restricted cubic spline model with three knots at 10th, 50th, and 90th percentile of aldosterone concentrations.28 Given the difference in the CVD risks according to sex and age,29,30 stratum-specific analyses were conducted to estimate the effects of aldosterone on subclinical atherosclerosis by sex (male, female), age (≤60 years, >60 years), and both (i.e. 2×2=4) categories. In addition, using the causal mediation analysis,31,32 we aimed to quantify the degree to which systolic and diastolic blood pressure mediates the association between aldosterone and subclinical atherosclerosis. We employed a marginal structural approach within the counterfactual framework and the mediated proportion was computed as the pure natural indirect effect divided by the total effect (IE/TE).31,32 Bias-corrected 95% confidence intervals (CIs) were estimated by repeating the analysis on 200 bootstrapped samples.
Finally, multivariable Cox proportional hazards regression models were employed adjusting for potential confounders to generate hazard ratios (HRs) of all-cause mortality for 100 pg/ml increase in serum aldosterone among the total population and subgroups based on renin phenotypes. All analyses were performed using Stata, version 15 (Stata-Corp). The study was exempted from human subjects review by the institutional review board at the University of California, Los Angeles.
Sensitivity analyses
We conducted a series of sensitivity analyses. First, to evaluate whether our findings can generalize to people without high aldosterone concentrations, we restricted participants to those with aldosterone <200 pg/ml.2 Second, to assess the robustness of our findings, we additionally adjusted for fasting glucose levels and eGFR (Model 3) as well as smoking status and alcohol use at visit 4 or 5 (Model 4) assuming that these risk factors of atherosclerosis might not be affected by aldosterone concentrations at baseline. We also estimated the effect of aldosterone on the absolute change in CAC score from baseline (i.e. visit 2 or 3) to visit 4 or 5. Lastly, we analyzed the data using logistic regression for the following two dichotomized outcomes; i) CAC>0 vs CAC=0 and ii) CAC>300 vs CAC≤300.16
Additional analyses
To further understand our findings from the context of the renin-angiotensin-aldosterone system (RAAS), we dichotomized subjects based on both ARR and aldosterone levels (ARR>200 pg/ml per μg/L/h and aldosterone >150 pg/ml) according to the previous literature about primary aldosteronism.2,33 Then, we run the negative binomial regression models to estimate the relative ratio of CAC and Cox proportional hazard models to estimate HR of all-cause mortality among these categories. We also analyzed the data using the same approach by categorizing subjects into 4 groups based on PRA and serum aldosterone concentrations (i.e. suppressed PRA & low aldosterone, suppressed PRA & high aldosterone, unsuppressed PRA & low aldosterone, and unsuppressed PRA & high aldosterone). In this analysis, we defined high aldosterone when they showed >150 pg/ml.2,33
Results
The mean ± standard deviation (SD) age of participants was 62.3 ± 9.4 years, and 53% were male. Baseline characteristics are shown in Table 1. The median [interquartile range] aldosterone concentrations and PRA were 127.1 [90.6–173.3] pg/ml and 0.5 [0.3–0.9] μg/L/h, respectively. Among 948 participants, 218 (23%) had both aldosterone concentrations >150 pg/ml and ARR>200 pg/ml per ng/ml/h, and 129 (14%) showed systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg at baseline. Individuals with suppressed renin phenotype were more likely to be older, female, African American, and less physically active compared to intermediate or unsuppressed renin phenotype.
Table 1.
Baseline Characteristics of Study Participants *
| Variables | Total | Suppressed Renin Phenotype (PRA ≤ 0.5) | Unsuppressed Renin Phenotype (PRA > 0.5) |
|---|---|---|---|
| N of participants | 948 | 467 | 481 |
| Age | 62.3 ± 9.4 | 64.3 ± 9.6 | 60.4 ± 8.8 |
| Male, N (%) | 498 (52.5) | 198 (41.2) | 283 (58.8) |
| Race/ethnicity, N (%) | |||
| White | 395 (41.7) | 187 (40.0) | 208 (43.2) |
| Chinese American | 151 (15.9) | 74 (15.9) | 77 (16.0) |
| African American | 136 (14.4) | 101 (21.6) | 35 (7.3) |
| Hispanic | 266 (28.1) | 105 (22.5) | 161 (33.5) |
| Education status, N (%) | |||
| ≤11 grade | 160 (16.9) | 79 (16.9) | 81 (16.9) |
| High school to college | 361 (38.0) | 182 (39.0) | 179 (37.3) |
| Associate’s, bachelor’s, or professional degree | 426 (45.0) | 206 (44.1) | 220 (45.8) |
| Annual income, N (%) | |||
| <$30,000 | 290 (31.6) | 149 (33.3) | 141 (31.6) |
| $30,000 to $75,000 | 388 (42.2) | 183 (40.9) | 205 (42.2) |
| >$75,000 | 241 (26.2) | 115 (25.7) | 126 (26.2) |
| Health insurance, N (%) | 683 (72.1) | 322 (69.1) | 361 (75.1) |
| Smoking status, N (%) | |||
| Never | 483 (51.2) | 249 (53.4) | 234 (49.0) |
| Former | 339 (35.9) | 164 (35.2) | 175 (36.6) |
| Current | 122 (12.9) | 53 (11.4) | 69 (14.4) |
| Alcohol use, N (%) | 521 (55.0) | 244 (52.4) | 277 (57.6) |
| Physical activity, MET-min/week, Median [Interquartile range] | 3885 [2040–7003] | 3630 [2145–6675] | 4080 [2015–7230] |
| BMI, kg/m2 | 27.2 ± 4.9 | 27.0 ± 4.8 | 27.3 ± 5.0 |
| Systolic blood pressure, mmHg | 118.6 ± 18.5 | 122.9 ± 19.3 | 114.4 ± 16.8 |
| Diastolic blood pressure, mmHg | 69.4 ± 9.5 | 70.2 ± 9.8 | 68.7 ± 9.1 |
| Serum sodium, mEq/l | 147.1 ± 3.4 | 147.0 ± 3.4 | 147.1 ± 3.3 |
| Serum potassium, mEq/l | 4.3 ± 0.3 | 4.4 ± 0.4 | 4.3 ± 0.3 |
| Fasting glucose, mg/dl | 95.5 ± 26.9 | 93.7 ± 23.5 | 97.2 ± 29.8 |
| eGFR, mL/min/1.73 m2 | 75.3 ± 14.0 | 74.5 ± 13.8 | 76.1 ± 14.1 |
| LDL, mg/dl | 116.1 ± 29.9 | 115.5 ± 30.3 | 116.6 ± 29.4 |
| Statin prescription | 132 (13.9) | 50 (10.7) | 82 (17.1) |
| Urinary sodium, mmol/l | 104.9 ± 53.9 | 103.6 ± 53.7 | 106.1 ± 54.1 |
| Urinary potassium, mmol/l | 56.2 ± 30.8 | 54.1 ± 31.3 | 58.3 ± 30.1 |
| Aldosterone concentration, pg/ml | |||
| Mean ± Standard deviation | 139.7 ± 70.9 | 118.3 ± 52.8 | 160.5 ± 79.6 |
| Median [Interquartile range] | 127.1 [90.6–173.3] | 109.3 [80.1–147.6] | 147.3 [109.0–194.9] |
| PRA, μg/L per h | |||
| Mean ± Standard deviation | 0.7 ± 0.7 | 0.3 ± 0.1 | 1.1 ± 0.7 |
| Median [Interquartile range] | 0.5 [0.3–0.9] | 0.3 [0.2–0.4] | 0.9 [0.7–1.3] |
| ARR | |||
| Mean ± Standard deviation | 518 ± 1597 | 874 ± 2219 | 173 ± 98 |
| Median [Interquartile range] | 243 [148–408] | 395 [267–710] | 157 [104–217] |
BMI, body mass index; eGFR, estimated glomerular filtration rate; PRA, plasma renin activity; ARR, aldosterone-renin ratio (pmol/L)/(μg/L per h); CAC, coronary artery calcium score.
Mean ± standard deviation was described for continuous variables, otherwise indicated.
Aldosterone, Blood pressure, and subclinical atherosclerosis
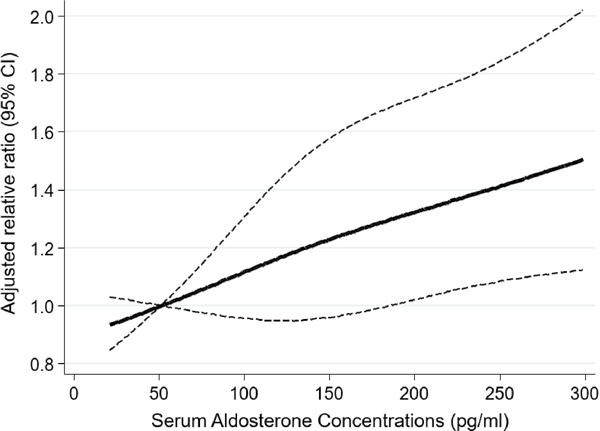
Among 700 subjects with coronary CT scans, the median duration of follow-up for CAC ascertainment was 6.4 (interquartile range, 6.1–7.6) years. After adjusting for potential confounders, we found a positive linear relationship of serum aldosterone concentrations (per 100 pg/ml) with systolic blood pressure (coefficient, 2.46; 95% CI, 0.81–4.12; p-value=0.004) and diastolic blood pressure (coefficient, 1.91; 95% CI, 1.06–2.78; p-value<0.001) (Figure S1). We also found a significant association between higher aldosterone concentrations and higher CAC score (relative ratio, 1.17; 95% CI, 1.04–1.32). The association between aldosterone (per 100 pg/ml increase) and CAC was prominent in subjects with suppressed renin phenotype (relative ratio, 1.41; 95% CI, 1.11–1.81; p for interaction, 0.05) (Table 2). Moreover, we found the dose-response association between increasing aldosterone and CAC (Figure 1).
Table 2.
Association between serum aldosterone concentrations and CAC.
| Estimates | Adjusted relative ratio [95% CI] *, †, ‡ | ||
|---|---|---|---|
| N | Model 1§ | Model 2‖ | |
| Total | 700 | 1.25 [1.12–1.40] | 1.17 [1.04–1.32] |
| Renin phenotype | |||
| PRA ≤ 0.5 | 338 | 1.58 [1.30–1.93] | 1.41 [1.11–1.81] |
| PRA > 0.5 | 362 | 1.14 [1.01–1.29] | 1.14 [1.00–1.30] |
| P for interaction | 0.001 | 0.05 | |
CAC, coronary artery calcium score; CI, confidence interval.
Relative ratio of CAC at exam 4 or 5 (follow-up) is calculated per 100 pg/ml increase in serum aldosterone concentrations.
Negative binomial regression model was employed to estimate relative ratio accounting for a right-skewed distribution of CAC at follow-up.
All models were adjusted for time from aldosterone measurement to CAC follow-up.
Adjusted for age, sex, and CAC at exam 2 or 3 (baseline).
Adjusted for race, education, insurance status, income, smoking status, alcohol intake, physical activity, statin prescription, BMI, LDL, serum sodium, serum potassium, urinary sodium, urinary potassium, and plasma renin activity in addition to covariates in Model 1.
Figure 1.
Dose-response association between serum aldosterone concentrations and CAC
Association between aldosterone and CAC using a restricted cubic spline regression model with three knots at 10th, 50th, and 90th percentile of serum aldosterone concentrations. Negative binomial regression model was employed adjusting for age, sex, CAC at exam 2 or 3, race, education, insurance status, income, smoking status, alcohol intake, physical activity, statin prescription, BMI, LDL, serum sodium, serum potassium, urinary sodium, urinary potassium, plasma renin activity, and time from aldosterone measurement to CAC follow-up. The dashed lines represent the 95% CIs for the spline model (reference is 50 pg/ml). We restricted the range of aldosterone to below 300 pg/ml because predictions >300pg/ml are based on too few data points.
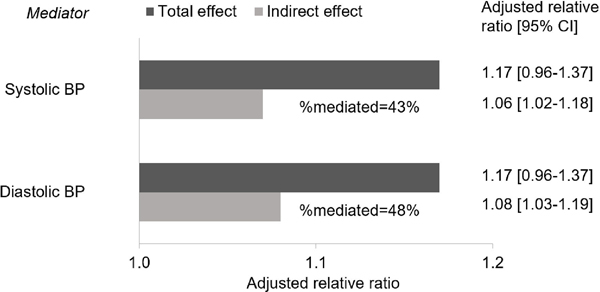
In stratified analyses, we found a modestly larger magnitude of the association between aldosterone and CAC score among males as compared to females with suppressed renin phenotype (male: relative ratio, 1.67; 95% CI, 1.20–2.32; female: relative ratio, 1.21; 95% CI, 0.68–2.12) (p for interaction = 0.002) (Table 3). The sex difference was observed among subjects aged ≤60 years when we further stratified by age (Table S1). In mediation analyses, we estimated that systolic or diastolic blood pressure mediated 43% or 48% of the association between increasing aldosterone concentrations and subclinical atherosclerosis, respectively (Figure 2).
Table 3.
Association between serum aldosterone concentrations and CAC stratified by sex or age.
| Estimates | Adjusted relative ratio [95% CI] *, †, ‡ | P for interaction | |
|---|---|---|---|
| Male | Female | ||
| Total | 1.23 [1.04–1.45] | 1.00 [0.84–1.20] | 0.06 |
| PRA ≤ 0.5 | 1.67 [1.20–2.32] | 1.21 [0.68–2.12] | 0.002 |
| PRA > 0.5 | 1.10 [0.90–1.35] | 1.05 [0.82–1.34] | 0.77 |
| Age ≤60 years | Age >60 years | ||
| Total | 1.25 [1.04–1.51] | 1.19 [1.00–1.42] | 0.66 |
| PRA ≤ 0.5 | 0.62 [0.36–1.04] | 1.67 [1.15–2.43] | 0.11 |
| PRA > 0.5 | 1.38 [1.10–1.74] | 1.08 [0.86–1.35] | 0.21 |
CAC, coronary artery calcium score; CI, confidence interval.
Relative ratio of CAC at follow-up is calculated per 100 pg/ml increase in serum aldosterone concentrations.
Negative binomial regression model was employed to estimate relative ratio accounting for a right-skewed distribution of CAC.
Adjusted for age, sex, CAC at exam 2 or 3, race, education, insurance status, income, smoking status, alcohol intake, physical activity, statin prescription, BMI, LDL, serum sodium, serum potassium, urinary sodium, urinary potassium, plasma renin activity, and time from aldosterone measurement to CAC follow-up.
Figure 2.
Direct and Indirect effects of serum aldosterone concentrations on coronary artery calcium score via 10mmHg increase in blood pressure
CAC, coronary artery calcium score; CI, confidence interval.
Adjusted for age, sex, CAC at exam 2 or 3, race, education, insurance status, income, smoking status, alcohol intake, physical activity, statin prescription, BMI, LDL, serum sodium, serum potassium, urinary sodium, urinary potassium, plasma renin activity, and time from aldosterone measurement to CAC follow-up. Relative ratio of CAC at follow-up is calculated per 100 pg/ml increase in serum aldosterone concentrations. 200 iterations were performed for bootstrapping to estimate 95% CI.
Our findings were not qualitatively affected by restricting participants to those with serum aldosterone concentrations <200 pg/ml (Table S2). The results were also consistent when we additionally adjusted for covariates which can be both mediators and confounders (Table S3) or when we used a change in CAC score as an outcome (Table S4). In the logistic regression analysis, while we did not find the association between increasing aldosterone and the presence of CAC (i.e. CAC >0), the association was found between increasing aldosterone and the presence of high CAC score (i.e. CAC>300) (Table S5). This association was also prominent among subjects with suppressed renin phenotype, but 95% CI was wide.
Aldosterone and mortality
Among 948 subjects, the median duration of follow-up for mortality ascertainment was 12.5 (interquartile range, 11.9–13.3) years, during which 120 deaths from all causes were identified (13%). After adjusting for potential confounders, we did not find the association between serum aldosterone concentrations and all-cause mortality (Table S6). However, when we stratified by renin phenotype, serum aldosterone concentrations among subjects with suppressed renin phenotype were associated with risk for all-cause mortality (HR, 1.70; 95% CI, 1.10–2.63). In contrast, we found a decreased risk of all-cause mortality in individuals with elevated aldosterone concentrations and unsuppressed renin phenotype.
Additional analyses
We found the consistent results when we analyzed the data with categorical exposures: i.e. subjects with high ARR and high aldosterone showed the significant association with higher CAC and increased risk of mortality (only among PRA suppressed group for mortality) compared to others (Table S7), and subjects with low PRA and high aldosterone showed the highest relative ratio of CAC and hazard ratio of all-cause mortality (Table S8).
Discussion
Using a prospective longitudinal cohort from the multiethnic population, we found the association between higher serum aldosterone concentrations and higher levels of CAC measured 6–7 years later. The association was most prominent in participants with suppressed renin phenotype, particularly among males. We also found a dose-response relationship between aldosterone and CAC, supporting a possible causal relationship between aldosterone and subclinical atherosclerosis. Blood pressure mediated around 45% of the association. These findings highlight the potential health burden of elevated aldosterone concentrations particularly with renin suppression phenotype and the importance of the management targeting blood pressure control to prevent cardiovascular disease due to aldosterone.
To the best of our knowledge, this is the first study to investigate the association of serum aldosterone concentration with CAC scores among an ethnically diverse population without antihypertensive medication and free of cardiovascular disease at baseline. A recent meta-analysis revealed that patients with primary aldosteronism showed the increased risk of stroke, coronary artery disease, atrial fibrillation, and heart failure compared to patients with essential hypertension.7 Moreover, aldosterone has been associated with subclinical atherosclerosis makers including IMT and ABI among patients with primary aldosteronism or essential hypertension.17,34,35 However, little is known about the association between aldosterone and CAC. Given the important role of CAC as a non-invasive marker of subclinical atherosclerosis which strongly predicts the risk of cardiovascular events,13 our findings advance the current state of knowledge about the potential effect of aldosterone on subclinical atherosclerosis. Moreover, aldosterone concentrations were associated with the increased risk of all-cause mortality only among renin suppression phenotype. The inconsistent results between suppressed and unsuppressed renin phenotype could be due to the possibility that some individuals with suppressed renin phenotype might have subclinical primary aldosteronism.18,20 Previous studies have shown the associations of aldosterone with mortality among individuals with prevalent CVD.3–6,8,9 A recent study using the Jackson Heart Study showed that aldosterone was associated with CVD and mortality among African Americans without prevalent CVD. Our findings from the multiethnic population free of CVD allow us to generalize the idea that high aldosterone concentrations might increase the long-term adverse health outcomes even among individuals with low-risk of CVD.
We found a larger association between aldosterone and subclinical atherosclerosis among males as compared to females with renin suppression phenotype. This sex heterogeneity remained in the younger group but not the older group when we additionally stratified by age. These findings may be due to dynamic changes in sex hormones in females before and after menopause, as premenopausal females are relatively protected from CVD as compared to males of the same age while postmenopausal females are not.29 In addition, a recent study found that endothelial cell mineralocorticoid receptor deletion significantly reduced atherosclerotic vascular inflammation only in male mice.36 Taken together, we may need to consider the potential cardiovascular burden of aldosterone differently based on sex and age. Although we could not perform a stratified analysis by race due to insufficient statistical power, future research is needed to entangle the race-specific association given the reported difference in the association between aldosterone and blood pressure by race/ethnicity.22
It has been well known that aldosterone increases the risk of acute ischaemic events both directly and indirectly (mostly mediated by hypertension),11 but few epidemiological studies have sufficiently quantified these two pathways separately. Previous research has shown an increased risk of hypertension and cardiovascular disease in patients with primary aldosteronism mainly due to the excessive stimulation of the mineralocorticoid receptor.37–42 Our findings from the mediation analysis support that the management targeting blood pressure might be effective to prevent subclinical atherosclerosis due to the activation of aldosterone system. Meanwhile, it is also noteworthy that not all effects of aldosterone could be explained by elevated blood pressure. Based on the previous biological studies, aldosterone induces vascular calcification mediated through genomic responses (e.g., activation of osteoinductive signaling, oxidative stress, inflammation, and vascular smooth muscle cells apoptosis) and non-genomic responses (e.g. activation of mitogen-activated protein kinase signaling and protein kinase C signaling).43,44 Given these mechanisms, our findings highlight the importance of considering the potential burden of elevated aldosterone levels on cardiovascular system not through hypertension.
Our study has several limitations. First, we have only a single measurement of aldosterone, and therefore, it was not possible to consider any trends or changes in aldosterone levels over follow-up. In this context, the present study does not provide information as to the clinical effectiveness of reducing aldosterone levels among the study sample. Second, in our mediation analysis, the exposure-mediator association is not well defined temporarily, and therefore, we need to consider the possibility of reverse causation (i.e. blood pressure levels might affect aldosterone concentrations). However, given the negative feedback system of RAAS, this temporality is likely to induce bias towards the null. Third, we assumed that there were no other unmeasured confounders and no confounding between the mediator and outcome as affected by exposure.32 Given the reported associations of aldosterone with obesity, glucose metabolism, adipokines, and renal function,38,45,46 such metabolic factors can be confounders between blood pressure and subclinical atherosclerosis affected by aldosterone. More advanced mediation analysis with multiple mediators would be helpful to disentangle the causal pathway from aldosterone to cardiovascular disease in future research.47
Perspectives
Higher aldosterone concentrations are associated with the increased risk of subclinical coronary atherosclerosis and all-cause mortality particularly among individuals with renin suppression. Increase in blood pressure substantially, but not entirely, mediated the effect of increasing aldosterone on subclinical atherosclerosis. Our findings indicate the importance of aldosterone levels (even within the reference range) with respect to the cardiovascular system and overall health. Future studies with a larger sample and longitudinal measures of aldosterone concentrations are warranted to overcome the limitations of our studies, to replicate and validate our findings, and establish causality including sequential mediating effects of metabolic disorders and blood pressure levels on the pathway from serum aldosterone concentrations to long-term adverse health outcomes.
Supplementary Material
Novelty and Significance.
1. What Is New?
This study firstly showed that aldosterone was associated with higher CAC among an ethnically diverse population without antihypertensive medication and free of cardiovascular disease at baseline.
The association was most prominent in males with suppressed renin phenotype.
Our mediation analysis revealed that elevated blood pressure mediated around 45% of this association.
2. What Is Relevant?
Our findings highlight the potential health burden of elevated aldosterone concentrations particularly with renin suppression phenotype and the importance of the management targeting blood pressure control to prevent cardiovascular disease due to aldosterone.
3. Summary
Higher aldosterone levels are associated with the increased risk of subclinical coronary atherosclerosis and all-cause mortality particularly when renin was suppressed.
Future investigation regarding the potential benefit of controlling aldosterone levels (even within the reference range) with respect to the cardiovascular system and overall health would be warranted.
Acknowledgement:
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Funding/Support:
KI was supported by the Summer Research Fellowship Award from the Endocrine Society, the Burroughs Wellcome Fund Interschool Training Program in Chronic Diseases (BWF-CHIP), and a Graduate Student Fellowship from the Department of Epidemiology at the UCLA Fielding School of Public Health. The MESA study was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from NCATS. Study sponsors were not involved in study design, data interpretation, writing, or the decision to submit the article for publication. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Disclosures: None.
References
- 1.Struthers AD, MacDonald TM. Review of aldosterone- and angiotensin II-induced target organ damage and prevention. Cardiovasc Res. 2004;61(4):663–670. doi: 10.1016/j.cardiores.2003.11.037 [DOI] [PubMed] [Google Scholar]
- 2.Funder JW, Carey RM, Mantero F, et al. The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016;101(5):1889–1916. doi: 10.1210/jc.2015-4061 [DOI] [PubMed] [Google Scholar]
- 3.Güder G, Bauersachs J, Frantz S, et al. Complementary and incremental mortality risk prediction by cortisol and aldosterone in chronic heart failure. Circulation. 2007;115(13):1754–1761. doi: 10.1161/CIRCULATIONAHA.106.653964 [DOI] [PubMed] [Google Scholar]
- 4.Latini R, Masson S, Anand I, et al. The comparative prognostic value of plasma neurohormones at baseline in patients with heart failure enrolled in Val-HeFT. Eur Heart J. 2004;25(4):292–299. doi: 10.1016/j.ehj.2003.10.030 [DOI] [PubMed] [Google Scholar]
- 5.Swedberg K, Eneroth P, Kjekshus J, Wilhelmsen L. Hormones regulating cardiovascular function in patients with severe congestive heart failure and their relation to mortality. CONSENSUS Trial Study Group. Circulation. 1990;82(5):1730–1736. doi: 10.1161/01.cir.82.5.1730 [DOI] [PubMed] [Google Scholar]
- 6.Vantrimpont P, Rouleau JL, Ciampi A, et al. Two-year time course and significance of neurohumoral activation in the Survival and Ventricular Enlargement (SAVE) Study. Eur Heart J. 1998;19(10):1552–1563. doi: 10.1053/euhj.1998.1093 [DOI] [PubMed] [Google Scholar]
- 7.Monticone S, D’Ascenzo F, Moretti C, et al. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2018;6(1):41–50. doi: 10.1016/S2213-8587(17)30319-4 [DOI] [PubMed] [Google Scholar]
- 8.Beygui F, Montalescot G, Vicaut E, et al. Aldosterone and long-term outcome after myocardial infarction: A substudy of the french nationwide Observatoire sur la Prise en charge hospitalière, l’Evolution à un an et les caRactéristiques de patients présentant un infArctus du myocarde avec ou sans onde Q (OPERA) study. Am Heart J. 2009;157(4):680–687. doi: 10.1016/j.ahj.2008.12.013 [DOI] [PubMed] [Google Scholar]
- 9.Beygui F, Collet J-P, Benoliel J-J, et al. High plasma aldosterone levels on admission are associated with death in patients presenting with acute ST-elevation myocardial infarction. Circulation. 2006;114(24):2604–2610. doi: 10.1161/CIRCULATIONAHA.106.634626 [DOI] [PubMed] [Google Scholar]
- 10.Palmer BR, Pilbrow AP, Frampton CM, et al. Plasma aldosterone levels during hospitalization are predictive of survival post-myocardial infarction. Eur Heart J. 2008;29(20):2489–2496. doi: 10.1093/eurheartj/ehn383 [DOI] [PubMed] [Google Scholar]
- 11.Ivanes F, Susen S, Mouquet F, et al. Aldosterone, mortality, and acute ischaemic events in coronary artery disease patients outside the setting of acute myocardial infarction or heart failure. Eur Heart J. 2012;33(2):191–202. doi: 10.1093/eurheartj/ehr176 [DOI] [PubMed] [Google Scholar]
- 12.Shlomo Keidar, Marielle Kaplan, Elsa Pavlotzky, et al. Aldosterone Administration to Mice Stimulates Macrophage NADPH Oxidase and Increases Atherosclerosis Development. Circulation. 2004;109(18):2213–2220. doi: 10.1161/01.CIR.0000127949.05756.9D [DOI] [PubMed] [Google Scholar]
- 13.McGraw AP, Bagley J, Chen W-S, et al. Aldosterone increases early atherosclerosis and promotes plaque inflammation through a placental growth factor-dependent mechanism. J Am Heart Assoc. 2013;2(1):e000018. doi: 10.1161/JAHA.112.000018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomaschitz A, Pilz S, Ritz E, Meinitzer A, Boehm BO, März W. Plasma aldosterone levels are associated with increased cardiovascular mortality: the Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Eur Heart J. 2010;31(10):1237–1247. doi: 10.1093/eurheartj/ehq019 [DOI] [PubMed] [Google Scholar]
- 15.Hillaert MA, Lentjes EG, Kemperman H, et al. Aldosterone, atherosclerosis and vascular events in patients with stable coronary artery disease. Int J Cardiol. 2013;167(5):1929–1935. doi: 10.1016/j.ijcard.2012.05.034 [DOI] [PubMed] [Google Scholar]
- 16.Budoff MJ, Young R, Burke G, et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multi-ethnic study of atherosclerosis (MESA). Eur Heart J. 2018;39(25):2401–2408. doi: 10.1093/eurheartj/ehy217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Concistrè A, Petramala L, Bisogni V, et al. Subclinical atherosclerosis due to increase of plasma aldosterone concentrations in essential hypertensive individuals. J Hypertens. June 2019. doi: 10.1097/HJH.0000000000002170 [DOI] [PubMed] [Google Scholar]
- 18.Brown JM, Robinson-Cohen C, Luque-Fernandez MA, et al. The Spectrum of Subclinical Primary Aldosteronism and Incident Hypertension: A Cohort Study. Ann Intern Med. 2017;167(9):630–641. doi: 10.7326/M17-0882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newton-Cheh C, Guo C-Y, Gona P, et al. Clinical and genetic correlates of aldosterone-to-renin ratio and relations to blood pressure in a community sample. Hypertension. 2007;49(4):846–856. doi: 10.1161/01.HYP.0000258554.87444.91 [DOI] [PubMed] [Google Scholar]
- 20.Hundemer GL, Baudrand R, Brown JM, Curhan G, Williams GH, Vaidya A. Renin Phenotypes Characterize Vascular Disease, Autonomous Aldosteronism, and Mineralocorticoid Receptor Activity. J Clin Endocrinol Metab. 2017;102(6):1835–1843. doi: 10.1210/jc.2016-3867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. doi: 10.1093/aje/kwf113 [DOI] [PubMed] [Google Scholar]
- 22.Rifkin DE, Khaki AR, Jenny NS, et al. Association of renin and aldosterone with ethnicity and blood pressure: the Multi-Ethnic Study of Atherosclerosis. Am J Hypertens. 2014;27(6):801–810. doi: 10.1093/ajh/hpt276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–832. doi: 10.1016/0735-1097(90)90282-t [DOI] [PubMed] [Google Scholar]
- 24.Alluri K, Joshi PH, Henry TS, Blumenthal RS, Nasir K, Blaha MJ. Scoring of coronary artery calcium scans: history, assumptions, current limitations, and future directions. Atherosclerosis. 2015;239(1):109–117. doi: 10.1016/j.atherosclerosis.2014.12.040 [DOI] [PubMed] [Google Scholar]
- 25.Chatterjee R, Zelnick L, Mukamal KJ, et al. Potassium Measures and Their Associations with Glucose and Diabetes Risk: The Multi-Ethnic Study of Atherosclerosis (MESA). PLoS ONE. 2016;11(6):e0157252. doi: 10.1371/journal.pone.0157252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jha HC, Divya A, Prasad J, Mittal A. Plasma circulatory markers in male and female patients with coronary artery disease. Heart Lung. 2010;39(4):296–303. doi: 10.1016/j.hrtlng.2009.10.005 [DOI] [PubMed] [Google Scholar]
- 27.Rubin DB, Schenker N. Multiple imputation in health-care databases: an overview and some applications. Stat Med. 1991;10(4):585–598. [DOI] [PubMed] [Google Scholar]
- 28.Greenland S Avoiding power loss associated with categorization and ordinal scores in dose-response and trend analysis. Epidemiology. 1995;6(4):450–454. doi: 10.1097/00001648-199507000-00025 [DOI] [PubMed] [Google Scholar]
- 29.Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139(10):e56–e528. doi: 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- 30.Wong ND, Kouwabunpat D, Vo AN, et al. Coronary calcium and atherosclerosis by ultrafast computed tomography in asymptomatic men and women: relation to age and risk factors. Am Heart J. 1994;127(2):422–430. doi: 10.1016/0002-8703(94)90133-3 [DOI] [PubMed] [Google Scholar]
- 31.Pearl J The causal mediation formula--a guide to the assessment of pathways and mechanisms. Prev Sci. 2012;13(4):426–436. doi: 10.1007/s11121-011-0270-1 [DOI] [PubMed] [Google Scholar]
- 32.VanderWeele TJ. Mediation Analysis: A Practitioner’s Guide. Annu Rev Public Health. 2016;37:17–32. doi: 10.1146/annurev-publhealth-032315-021402 [DOI] [PubMed] [Google Scholar]
- 33.Young WF. Primary aldosteronism: renaissance of a syndrome. Clinical Endocrinology. 2007;66(5):607–618. doi: 10.1111/j.1365-2265.2007.02775.x [DOI] [PubMed] [Google Scholar]
- 34.Bernini G, Galetta F, Franzoni F, et al. Arterial stiffness, intima-media thickness and carotid artery fibrosis in patients with primary aldosteronism. J Hypertens. 2008;26(12):2399–2405. doi: 10.1097/HJH.0b013e32831286fd [DOI] [PubMed] [Google Scholar]
- 35.Ambrosino P, Lupoli R, Tortora A, et al. Cardiovascular risk markers in patients with primary aldosteronism: A systematic review and meta-analysis of literature studies. Int J Cardiol. 2016;208:46–55. doi: 10.1016/j.ijcard.2016.01.200 [DOI] [PubMed] [Google Scholar]
- 36.Moss ME, Lu Q, Iyer SL, et al. Endothelial Mineralocorticoid Receptors Contribute to Vascular Inflammation in Atherosclerosis in a Sex-Specific Manner. Arterioscler Thromb Vasc Biol. 2019;39(8):1588–1601. doi: 10.1161/ATVBAHA.119.312954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Catena C, Colussi G, Nadalini E, et al. Cardiovascular outcomes in patients with primary aldosteronism after treatment. Arch Intern Med. 2008;168(1):80–85. doi: 10.1001/archinternmed.2007.33 [DOI] [PubMed] [Google Scholar]
- 38.Sechi LA, Novello M, Lapenna R, et al. Long-term renal outcomes in patients with primary aldosteronism. JAMA. 2006;295(22):2638–2645. doi: 10.1001/jama.295.22.2638 [DOI] [PubMed] [Google Scholar]
- 39.Milliez P, Girerd X, Plouin P-F, Blacher J, Safar ME, Mourad J-J. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45(8):1243–1248. doi: 10.1016/j.jacc.2005.01.015 [DOI] [PubMed] [Google Scholar]
- 40.Mulatero P, Monticone S, Bertello C, et al. Long-term cardio- and cerebrovascular events in patients with primary aldosteronism. J Clin Endocrinol Metab. 2013;98(12):4826–4833. doi: 10.1210/jc.2013-2805 [DOI] [PubMed] [Google Scholar]
- 41.Reincke M, Fischer E, Gerum S, et al. Observational study mortality in treated primary aldosteronism: the German Conn’s registry. Hypertension. 2012;60(3):618–624. doi: 10.1161/HYPERTENSIONAHA.112.197111 [DOI] [PubMed] [Google Scholar]
- 42.Rossi G-P, Sechi LA, Giacchetti G, Ronconi V, Strazzullo P, Funder JW. Primary aldosteronism: cardiovascular, renal and metabolic implications. Trends Endocrinol Metab. 2008;19(3):88–90. doi: 10.1016/j.tem.2008.01.006 [DOI] [PubMed] [Google Scholar]
- 43.Gao J, Zhang K, Chen J, et al. Roles of aldosterone in vascular calcification: An update. Eur J Pharmacol. 2016;786:186–193. doi: 10.1016/j.ejphar.2016.05.030 [DOI] [PubMed] [Google Scholar]
- 44.Jaffe IZ, Mendelsohn ME. Angiotensin II and aldosterone regulate gene transcription via functional mineralocortocoid receptors in human coronary artery smooth muscle cells. Circ Res. 2005;96(6):643–650. doi: 10.1161/01.RES.0000159937.05502.d1 [DOI] [PubMed] [Google Scholar]
- 45.Krug Alexander W, Ehrhart-Bornstein Monika. Aldosterone and Metabolic Syndrome. Hypertension. 2008;51(5):1252–1258. doi: 10.1161/HYPERTENSIONAHA.107.109439 [DOI] [PubMed] [Google Scholar]
- 46.Allison MA, Jenny NS, McClelland RL, Cushman M, Rifkin D. The associations of adipokines with selected markers of the renin-angiotensinogen-aldosterone system: the multi-ethnic study of atherosclerosis. J Hum Hypertens. 2015;29(2):127–133. doi: 10.1038/jhh.2014.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steen J, Loeys T, Moerkerke B, Vansteelandt S. Flexible Mediation Analysis With Multiple Mediators. Am J Epidemiol. 2017;186(2):184–193. doi: 10.1093/aje/kwx051 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.