Abstract
The mechanism by which planktonic marine cyanobacteria of the genus Trichodesmium fix N2 aerobically during photosynthesis without heterocysts is unknown. As an aid in understanding how these species protect nitrogenase, we have developed an immunofluorescence technique coupled to light microscopy (IF-LM) with which intact cyanobacteria can be immunolabeled and the distribution patterns of nitrogenase and other proteins can be described and semiquantified. Chilled ethanol was used to fix the cells, which were subsequently made permeable to antibodies by using dimethyl sulfoxide. Use of this technique demonstrated that about 3 to 20 cells (mean ± standard deviation, 9 ± 4) consecutively arranged in a Trichodesmium trichome were labeled with the nitrogenase antibody. The nitrogenase-containing cells were distributed more frequently around the center of the trichome and were rarely found at the ends. On average 15% of over 300 randomly encountered cells examined contained nitrogenase. The percentage of nitrogenase-containing cells (nitrogenase index [NI]) in an exponential culture was higher early in the light period than during the rest of the light-dark cycle, while that for a stationary culture was somewhat constant at a lower level throughout the light-dark cycle. The NI was not affected by treatment of the cultures with the photosynthetic inhibitor dichloro 1,3′-dimethyl urea or with low concentrations of ammonium (NH4Cl). However, incubation of cultures with 0.5 μM NH4Cl over 2 days reduced the NI. The IF technique combined with 14C autoradiography showed that the CO2 fixation rate was lower in nitrogenase-containing cells. The results of the present study suggest that (i) the IF-LM technique may be a useful tool for in situ protein localization in cyanobacteria, (ii) cell differentiation occurs in Trichodesmium and only a small fraction of cells in a colony have the potential to fix nitrogen, (iii) the photosynthetic activity (CO2 uptake) is reduced if not absent in N2-fixing cells, and (iv) variation in the NI may be a modulator of nitrogen-fixing activity.
While nitrogen fixation by cyanobacteria of the genus Trichodesmium is the most important biological source of new nitrogen in the tropical and subtropical oceans (4, 8), there remain unsolved questions regarding the regulation of nitrogen-fixing activity. One of the most intriguing issues is how these nonheterocystous species manage to fix N2 during daylight hours when photosynthesis is active (3, 21, 25, 29), while nitrogenase, the key enzyme responsible for N2 fixation, is extremely sensitive to oxygen deactivation. The cells have to tackle not only the problem of ambient O2 but that of endogenously generated O2 as well. Respiratory consumption of O2 may be a partial response (7) but is not likely to be the sole approach (2). Temporal differentiation between N2 and CO2 fixation, as found for other nonheterocystous diazotrophic cyanobacteria (13, 19, 20), is not present in Trichodesmium. The hypothesis of spatial segregation, which proposed that cells fixing N2 and lacking O2 evolution are located in the center of the colony where external oxygen was low (6), has been challenged with conflicting discoveries. Some studies have shown no differentiation between the central trichomes and surface ones, in terms of photosystem I and II activities (5) and presence of nitrogenase (dinitrogen reductase, Fe protein) (23). Using transmission electron microscopy (TEM) coupled with immunogold (IMG) labeling (TEM-IMG) with cross sections of colonies, Bergman and Carpenter (1) demonstrated that nitrogenase in Trichodesmium thiebautii was restricted to some trichomes randomly distributed in the colony. In Trichodesmium contortum, TEM-IMG labeling with longitudinal sections showed that only about 10% of cells arranged consecutively in the central region of the trichome contained nitrogenase (14). For three other species of Trichodesmium, a study using immunofluorescence of cross-sectional specimens showed that on average about 14% of cells randomly located across the colony contained this enzyme (11). More recently, a two-way section (transactional and longitudinal) was performed which provided a partial reconstruction of a three-dimensional view (12). To observe in situ localization, however, immunolabeling of intact (unsectioned) colonies is highly desirable.
In addition to providing an in situ view of protein localization in the colony, whole-cell immunolabeling would also facilitate rapid examination of a large number of cells for presence of the protein (18). Large numbers of cells are often required for statistical analysis of variations. To date, variations in nitrogenase abundance or activity have been determined for light-dark cycles (3), photosynthetic inhibition, and ammonium addition (3, 21) but only at bulk levels. It would be of great interest to determine whether such variations are reflected in changes in enzyme abundance in each cell or in the fraction of cells containing the enzyme. Finally, the whole-cell immunofluorescence technique can be combined with autoradiography to determine how the nitrogenase-harboring cells perform other activities (e.g., CO2 uptake). In the present study, we developed a whole-cell immunofluorescence protocol that has proven to be simple and effective. With this protocol, we attempted to revisit the following questions. (i) How is the nitrogenase distributed in the colony? (ii) Is there specialization of nitrogen-fixing and carbon-fixing cells? (iii) How is N2-fixing activity modulated in terms of nitrogenase localization under different growth conditions?
MATERIALS AND METHODS
Algal cultures.
A culture of Trichodesmium sp. (strain IMS 101) was provided by Hans Paerl and grown in an amended seawater medium (24). This strain is believed to be most closely related to Trichodesmium erythraeum based on the HetR (13a) and nifH (30) sequences. The cultures were maintained at room temperature (about 20 to 25°C) with a 12-h light-12-h dark photocycle. The illumination was provided with a cool white fluorescent light bank, with a photon flux of about 50 microeinsteins m−2 · s−1. Anabaena sp. strain PCC 7120, provided by P. Falkowski, was grown in modified Jaworski’s medium (15) for freshwater algae with the same temperature and illumination as described above. The modification included replacement of Ca(NO3)2 and Na2CO3 with CaCl2 and NaHCO3, respectively, omission of silicate, and supplementation with 0.03 mM Na2HPO4 and 0.034 mM NaCl.
Growth stage and diel cycle experiments.
A 1.5-liter culture growing in the exponential stage was split into two, and half was amended with fresh medium every 4 to 5 days by removing half of the subculture and adding the same volume of fresh medium. When the other subculture reached the stationary stage (over 1 month after inoculation), a 50-ml sample was collected from each of the two cultures every 2 h for a 24-h period.
DCMU inhibition.
A 500-ml exponential culture was divided into three equal parts. Dichloro 1,3′-dimethyl urea (DCMU) was added to one of them to a final concentration of 10 μM. Since the DCMU stock solution (1 mM) was prepared in ethanol, the same amount of ethanol as in DCMU was added to the second subculture for a control, and the third subculture was used as a second control without any addition. Samples of 15 ml were collected from each culture at 0 h (right before DCMU addition) and at 2, 6, 12, and 24 h after addition. For an additional exponential culture treated with DCMU in the same way, [14C]NaHCO3 was added at 24 h at a final specific activity of 0.15 μCi · ml−1, and samples were collected for both immunolabeling and autoradiography.
Ammonium addition experiments.
Two experiments were performed with the addition of various concentrations of ammonium chloride. For the short-term experiment (experiment 1), four subcultures were set up from a 2-liter exponential culture and NH4Cl was added to final concentrations of 0, 0.001, 0.01, and 0.5 mM, respectively. Samples were collected at 2, 6, 12, 24, and 48 h after the addition of ammonium. For the long-term experiment (experiment 2), four subcultures were set up in the same way but were maintained longer and samples were collected on day 2 (48 h) and days 5 and 10.
Immunofluorescence labeling.
A 15-ml sample was withdrawn from cultures after gentle mixing. The sample was filtered, by using a manifold setup with low vacuum pressure (ca. 15 cm of Hg), onto a 25-mm-diameter and 5- or 8-μm-pore-size Nuclepore membrane. Care was taken to keep the vacuum pressure low and to prevent the membrane from being dried out. The membrane bearing the sample was removed from the filtration setup and placed into a 15-ml plastic Falcon tube containing 5 ml of chilled (−20°C) ethanol (100%). For field samples, collected 500 m offshore of Zanzibar, Tanzania, individual colonies were isolated from the net-towed samples with a plastic loop. The colonies were transferred into a clean petri dish containing filtered seawater. After a brief rinse, the colonies were transferred (with a plastic loop) into a 15-ml plastic Falcon tube and fixed as described above. Care was taken not to disrupt the colonies. The sample in ethanol was stored at −20°C from overnight to 1 week. Preliminary experiments showed that when stored this way the antigenicity of the samples remained essentially unchanged for up to 2 weeks.
In preliminary experiments, paraformaldehyde (3 and 4% [wt/vol] dissolved in phosphate-buffered saline [PBS] containing 137 mM NaCl, 2.7 mM KCl, and 10 mM phosphate buffer) and glutaraldehyde (2.5% [wt/vol] dissolved in filtered seawater) were also used to fix samples before they were extracted in chilled (−20°C) methanol or ethanol. They all failed to produce any staining.
About 2 to 3 ml of the fixed sample was removed and filtered onto a clean membrane as described above. The sample on the membrane was rinsed three times with 4 ml of PBS. The membrane was then removed from the filtration apparatus and laid onto a poly-l-lysine coated slide (Sigma) with the sample-bearing side contacting the slide. A hydrophobic boundary was drawn with a PAP Pen (Energy Beam Science, Agawam, Mass.), surrounding the membrane to restrict buffers inside during subsequent incubations. The slide was assembled into a slide holder that fit into a rotor and was centrifuged for 1 min at 890 × g at 4°C. Preliminary experiments showed that this centrifugation was sufficient to transfer the majority of cells from the membrane to the slide and to retain most cells on the slides after the subsequent procedures.
The slides bearing Trichodesmium samples were immersed in 0.5% dimethyl sulfoxide (DMSO), diluted in PBS (vol/vol), and incubated at 4°C for 15 min to make the cell walls and membrane permeable for antibody penetration. Triton X-100 (0.1% [vol/vol]) and Nonidet P-40 were also used in preliminary experiments and were found to be less effective than DMSO. Subsequently, immunolabeling was carried out according to a previously reported protocol (18). The primary antibodies used were rabbit anti-Rhodospirillum rubrum nitrogenase (Fe protein) (gift of S. Nordlund) and anti-spinach ribulose, 1,5-bisphosphate carboxylase-oxygenase (Rubisco) (gift of B. Ranty). Both antibodies were diluted in PBS at a 1:100 dilution and were incubated with the samples for 4 h. Anti-rabbit immunoglobulin G conjugated with AMCA (Molecular Probes, Eugene, Oreg.) was used as the secondary antibody and was incubated with the samples for 1 h. The only variation in the immunolabeling procedure, when working with field-collected colonies, was the omission of the centrifugation step. Instead, samples were air dried on the slides for approximately 15 min. After the final washing with PBS, the samples were mounted with a coverslip by using Gel/Mount (Biomeda, Foster City, Calif.).
Autoradiography of 14C.
Fifty milliliters was transferred from a 1-liter culture to a 125-ml flask and spiked with [14C]NaHCO3 at the final concentration of 0.15 μCi/ml. After incubation for 0.5, 1, and 1.5 h under the same conditions described above, a 15-ml sample was collected and fixed as described above. Preliminary results showed that incubation periods of 1 and 1.5 h yielded a reasonable number of silver grains and thus were used for the subsequent experiments. After remaining overnight in cold ethanol, the samples were processed for immunostaining as aforementioned. After the final washing, the slide was air dried for 15 min before further processing for autoradiography (6). The dried slides were dipped into prewarmed (43°C) and dissolved NTB-2 nuclear track emulsion (Kodak). The slides, coated with a thin layer of emulsion, were held vertically in a rack for 30 min for the emulsion to dry. Next, the slides were stored at 4°C in a light-proof storage box with some drying gel at the bottom. Various exposure times, i.e., 2, 5, and 7 days, were compared, and 5 days proved to be optimal. After the exposure step, the slides were processed in film developer (D19; Kodak) for 3 min and distilled water for 5 min, followed by fixer (Kodak) for 10 min. After a final washing in distilled water for a few minutes, the slides were mounted with a coverslip.
Sample examination and quantification.
Immunolabeled and radiolabeled cells were examined with a Zeiss Axioskop epifluorescence microscope. The immunolabeling was examined with UV light excitation under the following filter settings: excitation, 365 nm (band pass); dichroic splitter, 395 nm; and emission (long pass), 397 nm, while the radiolabeled nuclear track was examined with tungsten light. Micrographs were taken with a Minolta camera with 400X Kodak color film and an auto exposure setting.
To semiquantify the labeling, the percentage of cells containing Rubisco (Rubisco index [RI]) or nitrogenase (nitrogenase index [NI]) immunolabeling was determined by examining over 300 randomly encountered cells (from randomly encountered trichomes). The percentage was calculated as the number of positive cells divided by the total number of cells examined (see Fig. 1 for the distinction between positive and negative cells). The silver grains in the cell areas were counted separately for nitrogenase-containing (i.e., positively stained) and non-nitrogenase-containing cells. Background silver grains were also counted for cell-free areas on the slides and the count was normalized to the silver grain number per cell area and subtracted from the silver grain count for the cell areas. Counts from different trichomes or from colonies from the same experiment were pooled to obtain the percentage for each experiment.
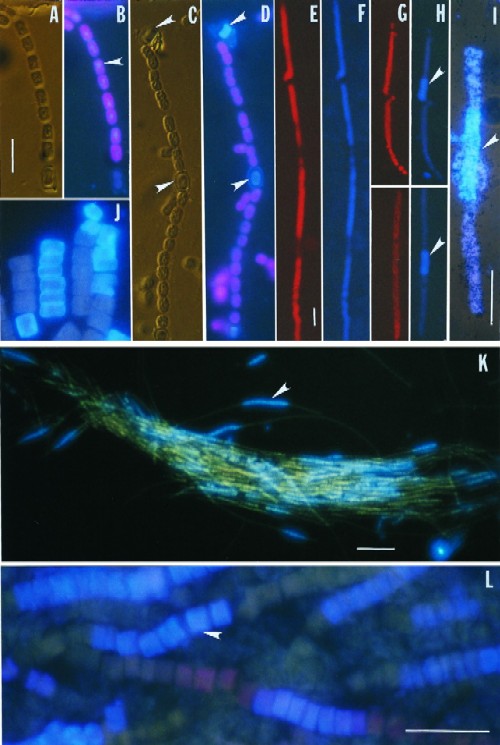
FIG. 1.
Micrographs of whole-cell immunofluorescent labeling of nitrogenase and Rubisco. (A to D) Anabaena sp. strain PCC7120. (A) Differential interference contrast (DIC) image of a trichome. (B) Rubisco immunolabeling (blue, imposed on red fluorescence of chlorophyll a) of the same trichome as in panel A. Note that staining appears fairly even for the vegetative cells (arrow) and weaker for heterocysts (the lower end of the trichome shown). (C) DIC image. Arrows indicate heterocysts. (D) Nitrogenase immunolabeling of the same trichome as in panel C. Note that only heterocysts are stained (arrows); vegetative cells displayed only autofluorescence of chlorophyll a. (E to L) Trichodesmium, strain IMS 101 (E to J and L) and field samples (K). (E) Autofluorescence of phycoerythrin under blue light excitation. (F) Rubisco immunolabeling of the same trichome as in panel E. Note that the staining is even throughout the trichome except for cells having no phycoerythrin. (G) Autofluorescence of phycoerythrin. (H) Nitrogenase immunolabeling of the same trichomes as in panel G. Note that staining is restricted to several consecutively located cells at the center of the trichomes (arrows). (I) Nitrogenase immunolabeling combined with 14C autoradiography. The panel shows an IF image imposed on a dim transmission light image showing silver grains (black dots). The arrow indicates immunofluorescent cells. (J) A nitrogenase immunolabeling of a trichome that shows peripheral localization of this enzyme on night-collected samples. (K) A whole-colony view of nitrogenase immunolabeling of field-collected samples. The arrow indicates a cluster of immunofluorescent cells. (L) A close-up of a nitrogenase-immunolabeled colony. The arrow indicates an immunofluorescent cell. Scale bars, 10 μm for panels A to D, 25 μm for panels E to J and L, and 100 μm for panel K.
RESULTS
Before being used in this study the nitrogenase antibody was tested for its monospecificity by Western blotting following a procedure described previously (17). The antibody specifically recognized a protein of 38 kDa in Trichodesmium sp. strain IMS 101 (not shown), presumably the Fe protein of the nitrogenase as reported previously (1). With this antibody, the immunofluorescence protocol resulted in a clear labeling signal (Fig. 1), with no background of nonspecific cross-reaction (not shown). Rubisco labeling was observed in all vegetative cells of Anabaena with a hardly detectable level in heterocysts (Fig. 1A and B). As expected, nitrogenase was only labeled in heterocysts (Fig. 1C and D). These results validated this protocol as being effective for protein preservation and cell wall and membrane permeabilization. Following this protocol, Rubisco staining was homogeneous in all Trichodesmium cells except those lacking phycoerythrin (Fig. 1E and F). It is not clear whether the cells without phycoerythrin were dead and lost the pigment. In contrast, nitrogenase localization was heterogeneous along the trichome (Fig. 1G and H). Positively stained cells were arranged consecutively in a trichome (Fig. 1H to L), more frequently around the center of the trichome (Fig. 1H, I, K, and L) and occasionally toward the ends (Fig. 1J). Both cultured and field cells were labeled with the same pattern (Fig. 1K and L), although colonies from the field appeared to be tighter than those from cultured cells and hence easier to handle in immunolabeling. The positively stained cells were clearly distinguishable from the negative cells. Apparently, all trichomes examined contained some nitrogenase-harboring cells. The labeling normally appeared to be homogeneous throughout the cell but was sometimes more concentrated at the peripheral regions (Fig. 1J).
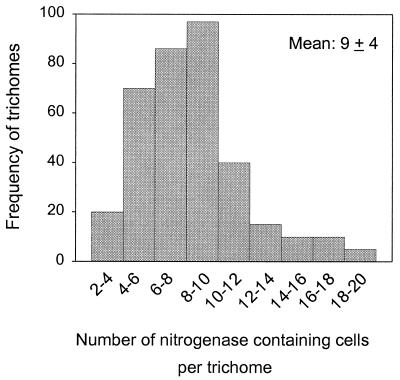
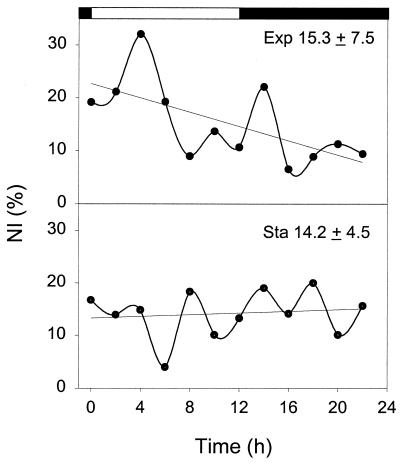
In individual trichomes, the number of nitrogenase-containing cells ranged from 3 to 20, with 8 to 10 being most common (Fig. 2). From a total of 350 trichomes examined, an average of 9 cells per trichome contained nitrogenase (standard deviation, 4). To quantify the nitrogen-fixing potential within each sample, an NI, or the percentage of nitrogenase-containing cells, was obtained by examining over 300 randomly selected cells (from randomly selected trichomes). Typical NI diel patterns in actively growing and in nongrowing cultures are shown in Fig. 3. In the exponential culture, the NI appeared to be higher during the first half of the light period and remained at a lower and somewhat constant level for the rest of the light-dark cycle. For a stationary culture, in contrast, no obvious diel variation was noticed and the NI was close to the lower level of the exponential culture. Surprisingly, the means (± standard deviations) of the NIs (15.3 ± 7.5 versus 14.2 ± 4.5) for the two cultures over the diel cycle were very close (t test; P > 0.05).
FIG. 2.
Frequency distribution of trichomes containing different numbers of nitrogenase-harboring cells.
FIG. 3.
Diel variation of NIs for exponential (Exp) and stationary cultures (Sta). Straight lines indicate linear regression. Means ± standard deviations are specified.
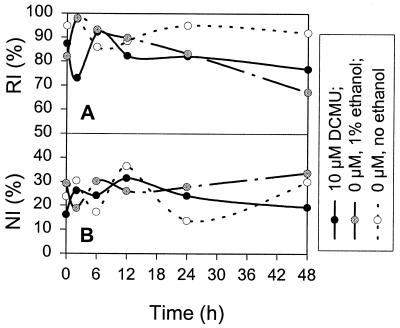
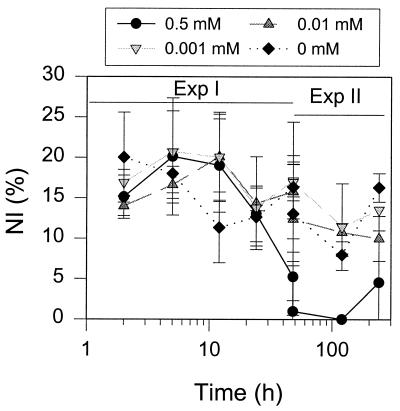
No significant effect on the NI was observed for treatment by DCMU, the photosynthetic inhibitor, for 2 days. The treatment with DCMU or its solvent ethanol caused loss of Rubisco in some cells, indicating detrimental effects of a low concentration of ethanol on Rubisco (Fig. 4). Short-term NH4Cl addition (<48 h) at concentrations from 0.001 to 0.5 μM also had no effect on the NI (Fig. 5). However, a dramatic decrease in the NI was noticed 48 h after the addition of 0.5 mM NH4Cl.
FIG. 4.
Effects of DCMU on the RI (A) and the NI (B). Note that the RI was decreased slightly by DCMU with ethanol or ethanol alone while the NI was not affected.
FIG. 5.
Effects of ammonium on the NI. Exp. I, short-term experiment; Exp. II, long-term experiment. Vertical bars indicate standard deviations from triplicates.
The light-dark alternation, as well as DCMU and NH4Cl addition, did not affect the mode of localization of nitrogenase-containing cells (not shown). Furthermore, the localization mode did not change noticeably from the exponential to the stationary growth phase of the cultures (not shown).
The autoradiography results demonstrated that there were slight but statistically significant differences in 14C uptake between the nitrogenase-containing and the non-nitrogenase-containing cells (Fig. 1; Table 1). Overall, the nitrogenase-containing cells displayed fewer silver grains than the non-nitrogenase-containing cells, and the ratios of the former to the latter were about 0.70 for the two experiments performed (Table 1). When the culture was treated with DCMU for 24 h, the 14C uptake was drastically reduced, the silver grain number per cell was low, and no significant differences between nitrogenase-containing and non-nitrogenase-containing cells were seen.
TABLE 1.
Quantitation of 14C-derived silver grains in nitrogenase-containing and non-nitrogenase-containing cells
| Culture | No. of Ag grains per cell in:
|
Ratioa | No. of cells | |
|---|---|---|---|---|
| Nitrogenasecontaining cells | Non-nitrogenasecontaining cells | |||
| Expt. 1 | 16.76 ± 5.46 | 23.85 ± 8.18 | 0.70 | 310 |
| Expt. 2 | 17.73 ± 5.31 | 24.90 ± 11.25 | 0.71 | 130 |
| 10 μM DCMU | 1.58 ± 2.26 | 1 ± 1.23 | 444 | |
Number of Ag grains per cell in nitrogenase-containing cells/number in non-nitrogenase-containing cells. Results were significantly different from a value of 1 (t test, P < 0.05).
DISCUSSION
Immunogold electron microscopy is a powerful technique for localizing proteins inside cells and quantifying the abundance of the proteins by counting gold particles (e.g., see reference 14). However, one disadvantage of this technique is that it is fairly difficult, if not impossible, to examine the large numbers of cells and samples that are often required for statistical analysis. Furthermore, it is rather a challenge to gain a three-dimensional view of enzyme localization for an intact cyanobacterial colony. The whole-cell immunofluorescence (IF) protocol presented in this study seems to be a useful technique for overcoming these difficulties, although it has its own disadvantages, e.g., difficulty in quantifying enzyme abundance in each cell without a sophisticated image analysis system. The staining patterns of Rubisco and nitrogenase in Anabaena demonstrated that sample fixation and cell permeabilization, the two most critical steps in this technique, are both sufficient. This technique also allows storage of samples for up to 1 week, which will facilitate field studies where immediate processing of samples may not be feasible. In comparison to previous methods (9), an additional advantage of the present protocol is that it does not require digestion of the cell wall with lysozyme, which may not be easy to control for optimal permeabilization. Ethanol has been shown to be an effective fixative for some other phytoplankton species (22, 27). Furthermore, ethanol may permeabilize the cell wall and membrane to some degree as well (10). In developing the IF protocol for eukaryotic phytoplankton (18), ethanol was found to cause major cell breakage for several phytoplankton species. In contrast, Trichodesmium seems to possess a cell wall and membranes that are amenable to ethanol treatment.
Heterogeneous localization of nitrogenase.
The nitrogenase localization pattern in the Trichodesmium trichomes and colonies demonstrated with the IF technique confirms previous results and provides an explanation for some of the contradictions in those studies. As found in this study, a varying but small fraction of cells in the trichome contained nitrogenase and the nitrogenase-containing trichomes were arranged randomly in the colony. This confirms previous observations made with cross-sectional samples that nitrogenase-containing cells are randomly distributed within the colony (1, 11). It also agrees with more recent results obtained with longitudinally sectioned samples that nitrogenase-containing cells tend to be arranged consecutively and around the center of the trichome (12, 14). With the IF technique applied to intact colonies, we are now able to conclude that virtually all trichomes in a colony contain a cluster of nitrogenase-bearing cells. Occasional short trichomes lacked nitrogenase-containing cells; these are suspected to be either a part of a trichome that broke or the result of recent trichome division. Although one earlier study with TEM showed that all cells in a Trichodesmium colony contained nitrogenase (23), the patchy localization of nitrogenase-containing cells observed in the present study agrees well with the more recent reports mentioned above. The uniform distribution of nitrogenase found in the earlier study may be due to artifacts of the TEM-IMG process.
The average NI found in this study (15%) was very close to that found by using IF on thick sections of natural samples (14%) (11). The slightly lower NI reported for T. contortum (14) may be attributable to differences in N2-fixing potential among different species, or to the relatively small number of trichomes examined and may depend on what time of day the trichomes were fixed. The NI values in all cases are rather low, however. To meet the N nutrition requirement, either these species have to utilize other nitrogen sources or the nitrogenase-containing cells fix nitrogen very efficiently. The number of heterocysts in Anabaena and other heterocystous species is also low and is similar to the number of nitrogenase-containing cells in Trichodesmium; they all perform aerobic nitrogen fixation during light hours. On the contrary, for Oscillatoria limosa, which fixes nitrogen at night and uses a temporal segregation scheme to separate nitrogen fixation from photosynthesis, almost 100% of its cells contain nitrogenase at night (26). It is tempting to speculate that the low percentage of nitrogenase-containing cells (heterocyst or nonheterocyst) may be associated with the spatial segregation scheme. Further comparative studies are warranted to verify this speculation.
Specialization of N2- and CO2-fixing cells and oxygen protection.
No thickening of the cell wall and loss of photosystem II, characteristic of heterocyst development (28), are found in Trichodesmium (12). However, cells seem to differentiate into two groups in terms of the potential to fix nitrogen. The distinction between nitrogenase-containing and non-nitrogenase-containing cells was unambiguous, thus demonstrating a clear specialization of these two groups of cells. The nitrogenase-containing cells are arranged in a cluster, as opposed to the single heterocyst occurring at regular intervals along the filament in heterocystous species. Recent TEM studies showed that the nitrogenase-containing cells in Trichodesmium spp. are distinguishable from the surrounding cells by their having more cytochrome oxidase (2); a denser thylakoid network, dividing vacuole-like spaces into smaller units; less extensive gas vacuoles; and smaller cyanophycin granules (12). Furthermore, at least in T. contortum, nitrogenase-containing cells are shorter than adjacent cells (14). These findings suggest that for Trichodesmium, there occurs a different type of cell specialization which is less visible than that of a heterocyst.
Oxygen protection may involve depressed O2 evolution, elevated photorespiration and dark respiration, and scavenging of oxygen radicals in the nitrogenase-containing cells (16). Our results provide the first direct evidence that potential N2-fixing cells have lower CO2 uptake activities and, to some extent, support one of the earlier hypotheses that Trichodesmium cells are differentiated into CO2-fixing and N2-fixing units (6). The reduction in CO2 uptake in the nitrogenase-containing cells suggests a reduced photosynthetic light reaction and therefore a reduced O2 evolution, which would offer a relief for the nitrogenase from O2 inactivation.
The small magnitude of reduction (30%) in CO2 uptake in nitrogenase-containing cells found in the present study may be due to import of fixed carbon from non-nitrogenase-containing cells. If this is true, a gradual increase of 14C label should be seen in the nitrogenase-containing cells over time. In our autoradiography experiments in which cultures were incubated with 0.15 μCi per ml for 0.5, 1, and 1.5 h, hardly any 14C uptake was detected at 0.5 h and no difference in the level of uptake was found between that at 1 h and that at 1.5 h (not shown). The possibility cannot be excluded, however, that a higher [14C] and a shorter incubation time may be required to see the time-sequential increase. Alternatively, the small reduction may suggest that photosynthesis occurred, albeit at a reduced rate, in the nitrogenase-containing cells. This possibility is supported by an earlier study which showed no zonation in the Trichodesmium colony in regard to photosystem I and photosystem II activities (5). The IF technique coupled with autoradiography, developed in this study, will provide the tool for further investigation of this issue.
On the other hand, reduction in intracellular oxygen production does not seem to be a sufficient condition for inducing more cells to contain nitrogenase. DCMU treatment in this study remarkably reduced CO2 uptake, and therefore probably O2 evolution as well, for both nitrogenase-containing and non-nitrogenase-containing cells (Table 1) but failed to increase the NI (Fig. 5). Apparently, interruption of photosynthesis could block the continuous supply of newly generated ATP and reductants which are required for nitrogenase synthesis in existing or newly born cells. Nevertheless, DCMU inhibition of photosynthesis may have increased the synthesis of nitrogenase in the cells that already had this enzyme or may have enhanced the activity of existing nitrogenase.
Whether the nitrogenase localization at the central region of the trichome would help to lessen the impact of environmental oxygen on nitrogenase inactivation is still unclear. In some trichomes, nitrogenase-containing cells were distributed toward the end of the trichome, which could be random or could be a result of trichome breakage. It is noteworthy, however, that there does not seem to be more nitrogenase-containing cells in the center of the colony compared with the number at the colony surface. It is thus suggested that if there is any advantage associated with nitrogenase-containing cells being localized at the central regions of the trichome and the colony, it may lie in elevated abundance or activity of nitrogenase rather than in the number of cells containing this enzyme.
Modulation of NI under different growth conditions.
A recent study based on cross sections of Trichodesmium colonies showed that the NI was higher during the light period and lower during the dark period (11). The same trend was found for exponential cultures by the IF technique based on unsectioned samples. This diel pattern is in accordance with that of acetylene reduction rates which peak at mid-day and decline markedly at night (3). However, the magnitude of the changes in the NI is remarkably smaller than that in nitrogenase activity. In addition to the possibility that the abundance of this enzyme in each cell may vary, modification status and hence the activity of the enzyme may change in the light-dark cycle (31). Interestingly, the diel pattern was not observed for the stationary culture where the NI fluctuated slightly around a low level. This suggests that in the stationary culture, the nitrogen-fixing activity may be low and no modulation of the NI was required. Under such circumstances, probably only modification of nitrogenase (inactivation) is involved.
Ammonium addition to Trichodesmium cultures has been found to inhibit acetylene reduction only over a long-term incubation, although it inhibited growth during short-term treatment (21). Similarly, the present IF analysis showed that the NI was reduced only at a concentration of 0.5 mM NH4Cl and an incubation period of over 24 h.
Based on these results, it can be proposed that the variation in the number of nitrogenase-containing cells may constitute an additional modulator of nitrogen fixation activity besides modification and abundance of nitrogenase (11). A small variation of nitrogenase activity may involve only enzyme modification (31) or changes of enzyme abundance (3, 11), and the NI probably changes only when growth conditions induce a considerable change in nitrogen fixation.
ACKNOWLEDGMENTS
We thank S. Nordlund and B. Ranty for providing antibodies against nitrogenase and Rubisco. H. Paerl and P. Falkowski provided Trichodesmium and Anabaena cultures, respectively. Thanks are also due to A. Kustka, for assistance in autoradiography, and to S. Janson, for comments on the manuscript in the early stages.
This research was supported by U.S. National Science Foundation grant OCE9633744 (E.J.C.) and by The Swedish Foundation for International Cooperation in Research and Higher Education (STINT), SIDA/SAREC, and the Swedish National Science Research Council (B.B.).
Footnotes
Contribution no. 1115 of the Marine Sciences Research Center.
REFERENCES
- 1.Bergman B, Carpenter E J. Nitrogenase confined to randomly distributed trichomes in the marine cyanobacterium Trichodesmium thiebautii. J Phycol. 1991;27:158–165. [Google Scholar]
- 2.Bergman B, Siddiqui P J A, Carpenter E J, Peschek G A. Cytochrome oxidase: subcellular distribution and relationship to nitrogenase expression in the nonheterocystous marine cyanobacterium Trichodesmium thiebautii. Appl Environ Microbiol. 1993;59:3239–3244. doi: 10.1128/aem.59.10.3239-3244.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capone D G, O’Neil J M, Zehr J, Carpenter E J. Basis for diel variation in nitrogenase activity in the marine planktonic cyanobacterium Trichodesmium thiebautii. Appl Environ Microbiol. 1990;56:3532–3536. doi: 10.1128/aem.56.11.3532-3536.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capone D G, Zehr J P, Paerl H W, Bergman B, Carpenter E J. Trichodesmium, a globally significant marine cyanobacterium. Science. 1997;276:1221–1229. [Google Scholar]
- 5.Carpenter E J, Chang J, Cottrell M, Schubauer J, Paerl H W, Bebout B M, Capone D G. Re-evaluation of nitrogenase oxygenase-protective mechanisms in the planktonic marine cyanobacterium Trichodesmium. Mar Ecol Prog Ser. 1990;65:151–158. [Google Scholar]
- 6.Carpenter E J, Price C C. Marine Oscillatoria (Trichodesmium): explanation for aerobic nitrogen fixation without heterocysts. Science. 1976;191:1278–1280. doi: 10.1126/science.1257749. [DOI] [PubMed] [Google Scholar]
- 7.Carpenter E J, Roenneberg T. The marine planktonic cyanobacteria Trichodesmium spp.: photosynthetic rate measurements in the SW Atlantic Ocean. Mar Ecol Prog Ser. 1995;118:267–273. [Google Scholar]
- 8.Carpenter E J, Romans K. Major role of the cyanobacterium Trichodesmium in nutrient cycling in the North Atlantic Ocean. Science. 1991;254:1356–1358. doi: 10.1126/science.254.5036.1356. [DOI] [PubMed] [Google Scholar]
- 9.Currin C A, Paerl H W, Suba G K, Alberte R S. Immunofluorescence detection and characterization of N2-fixing microorganisms from aquatic environments. Limnol Oceanogr. 1990;35:59–71. [Google Scholar]
- 10.Elias J M. Immunohistopathology: a practical approach to diagnosis. Chicago, Ill: ASCP Press; 1990. [Google Scholar]
- 11.Fredriksson C, Bergman B. Nitrogenase quantity varies diurnally in a subset of cells within colonies of the non-heterocystous cyanobacteria Trichodesmium spp. Microbiology. 1995;141:2471–2478. [Google Scholar]
- 12.Fredriksson C, Bergman B. Ultrastructural characterization of cells specialized for nitrogen fixation in a non-heterocystous cyanobacterium, Trichodesmium spp. Protoplasma. 1997;197:76–85. [Google Scholar]
- 13.Grobbelar N, Huang T C, Lin J Y, Chow T J. Dinitrogen-fixing endogenous rhythm in Synechococcus RF-1. FEMS Microbiol Lett. 1986;37:173–177. [Google Scholar]
- 13a.Janson, S. Personal communication.
- 14.Janson S, Carpenter E J, Bergman B. Compartmentalization of nitrogenase in a non-heterocystous cyanobacterium: Trichodesmium contortum. FEMS Microbiol Lett. 1994;118:9–14. [Google Scholar]
- 15.Jaworski G H M, Talling J F, Heaney S I. The influence of carbon dioxide depletion on growth and sinking rate of two planktonic diatoms in culture. Br Phycol J. 1981;16:395–410. [Google Scholar]
- 16.Kana T M. Oxygen cyclin in cyanobacteria with specific reference to oxygen protection in Trichodesmium spp. In: Carpenter E J, Capone D G, Rueter J G, editors. Marine pelagic cyanobacteria: trichodesmium and other diazotrophs. Boston, Mass: Kluwer Academic Publishers; 1992. pp. 29–41. [Google Scholar]
- 17.Lin S, Change J, Carpenter E J. Detection of proliferating cell nuclear antigen analog in four species of marine phytoplankton. J Phycol. 1994;30:449–456. [Google Scholar]
- 18.Lin S, Carpenter E J. An empirical protocol for whole-cell immunofluorescence of marine phytoplankton. J Phycol. 1996;32:1083–1094. [Google Scholar]
- 19.Millineaux P M, Gallon J R, Chaplin A E. Acetylene reduction (nitrogen fixation) by cyanobacteria grown under alternating light-dark cycles. FEMS Microbiol Lett. 1981;10:245–247. [Google Scholar]
- 20.Mitsui A, Kumsui S, Takahashi S, Ikemoto H, Cao C, Aria T. Strategy by which nitrogen fixing unicellular cyanobacteria grow photoautotrophically. Nature (London) 1986;323:720–722. [Google Scholar]
- 21.Ohki K, Zehr J, Falkowski P G, Fujita Y. Regulation of nitrogen fixation by different nitrogen sources in the marine non-heterocystous cyanobacterium Trichodesmium sp. NIBB1067. Arch Microbiol. 1991;156:335–337. [Google Scholar]
- 22.Orellana M V, Perry M J. Optimization of an immunofluorescent assay of internal enzyme ribulose-1,5-bisphosphate carboxylase (Rubisco) in single phytoplankton cells. J Phycol. 1995;31:785–794. [Google Scholar]
- 23.Paerl H W, Priscu J C, Brawner D L. Immunochemical localization of nitrogenase in marine Trichodesmium aggregates: relationship to N2 fixation potential. Appl Environ Microbiol. 1989;55:2965–2975. doi: 10.1128/aem.55.11.2965-2975.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prufert-Bebout L, Paerl H, Lassen C. Growth, nitrogen fixation, and spectral attenuation in cultivated Trichodesmium species. Appl Environ Microbiol. 1993;59:1367–1375. doi: 10.1128/aem.59.5.1367-1375.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saino T, Hattori A. Diel variation in nitrogen fixation by a marine blue-green alga, Trichodesmium thiebautii. Deep-Sea Res. 1978;25:1259–1263. [Google Scholar]
- 26.Stal L J, Bergman B. Immunological characterization of nitrogenase in the filamentous non-heterocystous cyanobacterium Oscillatoria limosa. Planta. 1990;182:287–291. doi: 10.1007/BF00197123. [DOI] [PubMed] [Google Scholar]
- 27.Vladimirova M G, Markelova A G, Semenendo V E. Use of the cytoimmunofluorescent method to clarify localization of ribulose bisphosphate carboxylase in pyrenoids of unicellular algae. Russ Plant Physiol. 1982;29:941–950. [Google Scholar]
- 28.Wolk P, Ernst A, Elhai J. Heterocyst metabolism and development. In: Bryant D A, editor. The molecular biology of cyanobacteria. Dordrecht, The Netherlands: Kluwer; 1994. pp. 769–823. [Google Scholar]
- 29.Wyman M, Zehr J P, Capone D G. Temporal variability in nitrogenase gene expression in natural populations of the marine cyanobacterium Trichodesmium thiebautii. Appl Environ Microbiol. 1996;62:1073–1075. doi: 10.1128/aem.62.3.1073-1075.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zehr J P, Harris D, Dominic B, Salerno J. Structural analysis of the Trichodesmium nitrogenase iron protein: implications for aerobic nitrogen fixation activity. FEMS Microbiol Lett. 1997;153:303–309. doi: 10.1111/j.1574-6968.1997.tb12589.x. [DOI] [PubMed] [Google Scholar]
- 31.Zehr J P, Wyman M, Miller V, Duguay L, Capone D G. Modification of the Fe protein of nitrogenase in natural populations of Trichodesmium thiebautii. Appl Environ Microbiol. 1993;59:669–676. doi: 10.1128/aem.59.3.669-676.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]