Abstract
PURPOSE
RET rearrangements and RET activating point mutations represent targetable genomic alterations in advanced solid tumors. However, the frequency and clinicopathologic characteristics of wild-type RET amplification in cancer and its potential role as a targetable oncogenic driver are not well-characterized.
METHODS
In two institutional cohorts of patients with solid cancers from the Dana-Farber Cancer Institute (DFCI) and Memorial Sloan Kettering Cancer Center (MSKCC) whose tumors underwent next-generation sequencing (NGS), the frequency and clinicopathologic features of wild-type RET amplification in the absence of RET rearrangements or activating mutations was assessed. The findings were validated using merged data from The Cancer Genome Atlas (TCGA), Genomics Evidence Neoplasia Information Exchange (GENIE), and China Pan-Cancer data sets.
RESULTS
The frequency of wild-type RET amplification across all solid cancers was 0.08% (26 of 32,505) in the DFCI cohort, 0.05% (26 of 53,152) in the MSKCC cohort, and 0.25% (71 of 28,623) in the cohort from TCGA, GENIE, and China Pan-Cancer. Cancer types with RET amplification included non–small-cell lung cancer (NSCLC), hepatobiliary cancer, prostate cancer, breast cancer, and others. The median RET copy number in RET-amplified cases was 7.5 (range, 6-36) in the DFCI cohort and 5.7 (range, 4-27.7) in the MSKCC cohort. Among 11 RET-amplified NSCLCs, eight had no other concurrent driver mutations. Finally, we report on a 69-year-old man with recurrent NSCLC harboring high-level wild-type RET amplification (22-28 copies) as the only identified putative genomic driver who experienced both a systemic and intracranial confirmed response to the RET inhibitor selpercatinib.
CONCLUSION
Amplification of wild-type RET represents a novel, targetable molecular subset of cancer.
This study establishes wild-type RET amplification as a novel, actionable genomic subset of cancer.
INTRODUCTION
Genomic alterations in the rearranged during transfection (RET) receptor tyrosine kinase gene are targetable oncogenic drivers across multiple cancer types.1,2 RET activating point mutations are common in medullary thyroid cancer while RET fusions are found in papillary thyroid cancer (approximately 20%), non–small-cell lung cancer (NSCLC, 1%-2%), and other solid tumors (<1%).2 Earlier studies with multikinase inhibitors in RET-altered cancers were limited by modest efficacy and unfavorable side effect profiles.3,4 Recently, the selective RET inhibitors selpercatinib and pralsetinib demonstrated higher response rates, more durable efficacy, and fewer toxicities than multikinase RET inhibitors, leading to their approval for cancers with RET fusions or activating point mutations.5-10
CONTEXT
Key Objective
Although RET fusions and activating mutations are well-studied as actionable genomic alterations in multiple solid tumor types, wild-type RET amplification remains poorly characterized in cancer. This study examined the frequency and clinicopathologic features of wild-type RET-amplified cancer in three pan-cancer cohorts.
Knowledge Generated
Across all solid cancers, the frequency of wild-type RET amplification was 0.08%, 0.05%, and 0.25% in the three cohorts, respectively, and was observed in cancer types including non–small-cell lung cancer (NSCLC), hepatobiliary cancer, prostate cancer, and breast cancer. Among RET-amplified NSCLCs, 73% had no other concurrent drivers. Finally, we present the first reported case of a response to the RET inhibitor selpercatinib in a patient with RET-amplified NSCLC without RET fusion or other oncogenic drivers.
Relevance
Wild-type RET amplification represents a novel genomic subtype of cancer with susceptibility to targeted therapy, a finding with important implications for targeted treatment strategies in multiple cancer types.
Despite robust characterization of RET rearrangements and activating point mutations as oncogenic drivers, whether wild-type RET amplification acts as a targetable oncogenic driver is not well-understood. This study investigates the frequency, clinicopathologic characteristics, and genomic features of wild-type RET amplification across solid tumor types. We report the first known case of clinical response to selpercatinib in a patient with metastatic NSCLC with high-level, focal amplification of RET without other known RET alterations or oncogenic drivers.
METHODS
The frequency of wild-type RET amplification in solid tumors was evaluated in three independent pan-cancer cohorts (Data Supplement [Fig A1]). Hematologic malignancies were excluded. For all cohorts, RET-amplified cases with a concurrent oncogenic/likely oncogenic RET point mutation (per OncoKB11) or a concurrent RET fusion reported on DNA next-generation sequencing (NGS) were not considered wild-type RET-amplified. RET-amplified cases identified with sequencing platforms that did not assess for fusions/structural variants (n = 1 case) were also not considered wild-type amplified.
The first pan-cancer cohort consisted of consecutive patients with solid cancers at the Dana-Farber Cancer Institute (DFCI) between 2013 and 2022 whose tumors underwent targeted NGS using the OncoPanel platform,12 which assesses 277 (version 1, April 2013-July 2014), 302 (version 2, July 2014-September 2016), and 447 (version 3, September 2016-present) cancer-associated genes. RET amplification was defined as ≥6 copies. This cutoff is based on an established threshold used for determining amplifications in the OncoPanel platform. Cases with amplification of the entire chromosome 10 (n = 2) rather than having focal RET amplification were excluded. Chromosome 10 polysomy was determined by manual visualization of the chromosome 10 copy plots derived from calculated log2 ratios for all DFCI RET-amplified cases (by a dedicated pathologist, M.S.G.),12 which allowed for easy discernment between focal RET amplification versus polysomy (Data Supplement [Fig A2]).
A second institutional analysis was performed in a pan-cancer cohort of patients with solid cancers at the Memorial Sloan Kettering Cancer Center (MSKCC) whose tumors underwent NGS using the MSK-IMPACT platform, which assesses 341 (version 1), 410 (version 2), and 468 (version 3) cancer-associated genes.13 RET-amplified cases from MSKCC were initially identified as those with a RET fold change >2, an established threshold used for clinical reporting of amplifications detected using MSK-IMPACT.13 These identified cases were further evaluated using fraction and allele-specific copy number estimates (FACETS) segmentation methodology, allowing for allele-specific, tumor purity-adjusted determinations of RET total copy number and segment size.14 To ensure high-quality copy number information, samples with an estimated tumor purity <20% or that otherwise failed FACETS quality control were excluded from this further analysis. Ploidy-corrected total copy number was subsequently calculated for samples meeting this requirement; this adjustment allowed for more accurate gene amplification calling since it accounts for the presence of chromosomal aneuploidy or whole-genome doubling. Cases with a ploidy-corrected total copy number ≥4 (an empirical cutoff identified in previous research on detection of amplifications with FACETS)15 were defined as RET-amplified in the MSKCC cohort. For the DFCI and MSKCC cohorts, the thresholds for RET amplification were copy number cutoffs specific to the respective unique platforms, which were felt to be more appropriate than arbitrarily introducing a new single threshold across the two cohorts. For both institutional cohorts, patients provided written informed consent to institutional review board–approved protocols at each site.
Finally, analyses using merged data from The Cancer Genome Atlas (TCGA) Pan-Cancer Atlas,16 Project Genomics Evidence Neoplasia Information Exchange (GENIE) version 13.017 (excluding cases from DFCI and MSKCC), and China Pan-Cancer18 data sets (all accessed via the cBioPortal for Cancer Genomics19,20) were performed to identify the frequency of wild-type RET amplification (according to annotations in each data set) in a third pan-cancer cohort.
For all three cohorts, multiple samples from the same patient were only considered as separate cases if they were of different cancer types; for the DFCI cohort, cases from the same patient in which it was ambiguous whether they were of different cancer types were resolved by chart review. Clinicopathologic characteristics of RET-amplified cases were abstracted from the medical record or cBioPortal, where available. Given the varied methodologies to evaluate copy count in each cohort, we determined cohort-specific frequencies of RET amplification, rather than presenting a single pooled RET amplification frequency. For clinicopathologic and genomic features of RET-amplified cases, we evaluated both pooled data (especially to allow for exploratory analyses within cancer types, where sample sizes were small) and cohort-specific data.
In the patient case, DNA NGS was performed by OncoPanel.12 Targeted RNA sequencing was performed by Solid Fusion Assay (Massachusetts General Hospital) using Anchored Multiplex PCR.21 RET fluorescence in situ hybridization (FISH) was performed using a RET break-apart probe (Kreatech RET, 10q11 Dual Color). Lesions were assessed by computed tomography (CT) and magnetic resonance imaging (MRI) and reviewed by a dedicated radiologist (M.N.) and radiation oncologist (A.A.) according to the RECIST and response assessment in neuro-oncology brain metastases (RANO-BM) criteria, respectively. Consent from the patient for publication of this case was obtained.
RESULTS
RET Amplification Frequency and Clinicopathologic Features Across Cancer Types
To assess RET amplification frequency across cancer types and explore associated clinicopathologic and genomic characteristics, two pan-cancer institutional cohorts of solid tumors from DFCI (N = 32,505) and MSKCC (N = 53,152) were assessed. Additionally, merged data of solid cancer cases from the TCGA, GENIE, and China Pan-Cancer data sets (N = 28,623) were evaluated (excluding cases in GENIE from DFCI and MSKCC). Nine cases of RET amplification occurring concurrently with RET fusion or activating RET point mutations were identified (detailed in Data Supplement [Table A1]); these cases were excluded from subsequent analyses to focus on wild-type RET amplification. The frequency of wild-type RET amplification across all solid cancers was 0.08% (26 of 32,505) in the DFCI cohort, 0.05% in the MSKCC cohort (26 of 53,152), and 0.25% (71 of 28,623) in the cohort from TCGA, GENIE, and China Pan-Cancer (Figs 1A-1C, Data Supplement [Fig A1]). In NSCLC, the frequency of RET amplification was 0.08% (4 of 4,778) in the DFCI cohort, 0.04% (3 of 7,139) in the MSKCC cohort, and 0.09% (4 of 4,293) in the TCGA, GENIE, and China Pan-Cancer cohort (Figs 1A-1C). Of the 11 RET-amplified NSCLC cases, five were adenocarcinoma, four were squamous cell carcinoma, one was NSCLC favor adenocarcinoma, and one was adenoid cystic carcinoma. Other cancer types with RET amplification included breast cancer, hepatobiliary cancer, and prostate cancer, among others. Detailed frequencies of RET amplification across cancer types are presented in the Data Supplement (Table A2).
FIG 1.
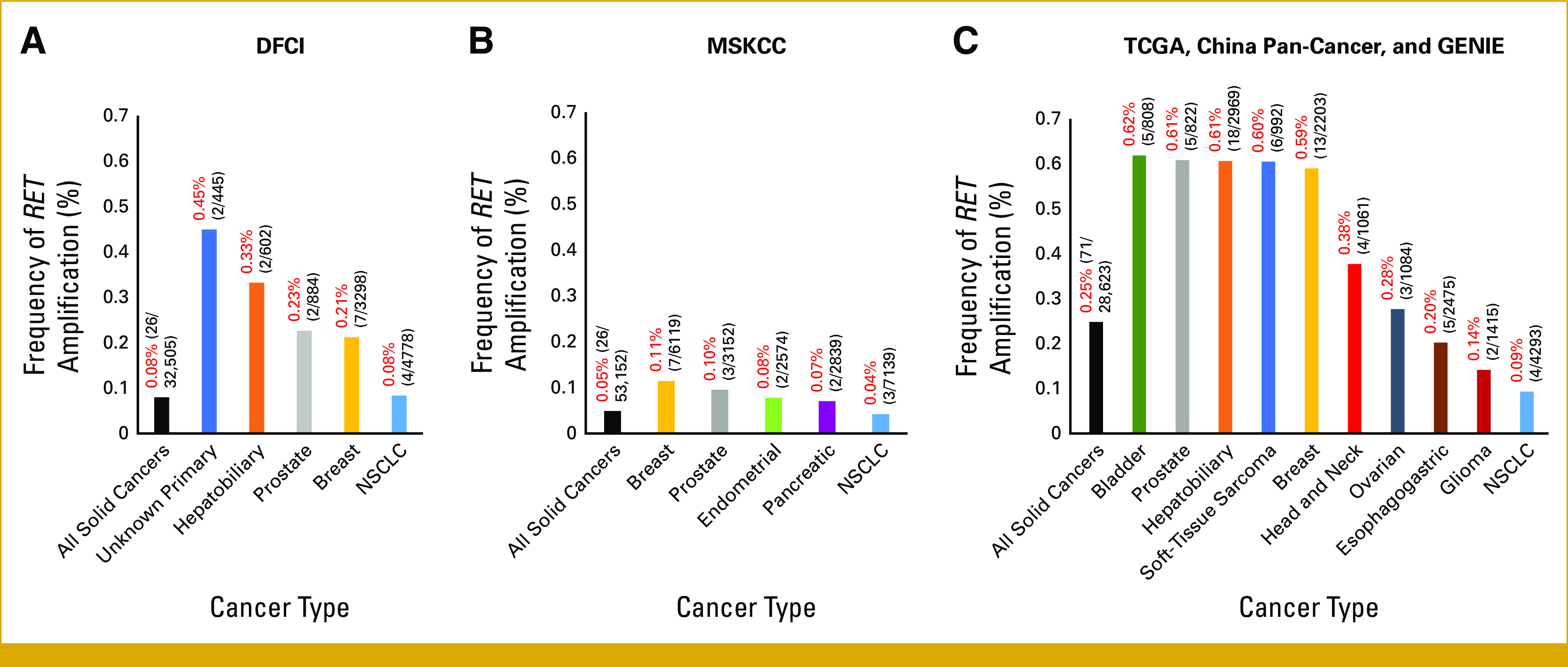
(A) Frequency of wild-type RET amplification overall and by individual cancer type in a pan-cancer cohort of 32,505 sequenced solid tumor cases from DFCI. (B) Frequency of wild-type RET amplification overall and by individual cancer type in a pan-cancer cohort of 53,152 solid tumor cases from MSKCC. (C) Frequency of wild-type RET amplification overall and by individual cancer type in 28,623 solid tumor cases from the TCGA Pan-Cancer Atlas, GENIE (excluding cases from DFCI and MSKCC), and the China Pan-Cancer data sets. For all three bar graphs (A-C), cancer types displayed are those with a frequency of >1% of the cohort and those in which >1 RET-amplified case was identified. Data Supplement (Table A2) summarizes frequencies across all observed cancer types. DFCI, Dana-Farber Cancer Institute; GENIE, Genomics Evidence Neoplasia Information Exchange; MSKCC, Memorial Sloan Kettering Cancer Center; NSCLC, non–small-cell lung cancer; TCGA, The Cancer Genome Atlas.
In the DFCI cohort, the median RET gene copy number among amplified cases was 7.5 (range, 6-36) while in the MSKCC cohort, the median RET gene copy number was 5.7 (range, 4-27.7) (Data Supplement [Fig A3A]). Regarding clinicopathologic and genomic features of RET-amplified cases, among the 11 RET-amplified NSCLCs, eight had no other codriver alterations while three had concurrent driver alterations (MET amplification, KRAS G12C, and EGFR exon 20 insertion) (Data Supplement [Fig A3B]). The median age of patients with RET-amplified NSCLC was 69 years and 36.4% were female. In eight RET-amplified NSCLC cases with available smoking status, five had a history of tobacco use, two of which had concurrent drivers (KRAS G12C and MET amplification). Of the 24 RET-amplified breast cancer cases, four had concurrent PIK3CA mutations, and of the 15 RET-amplified breast cancers with immunohistochemistry available, seven were triple-negative, two were triple-positive, three were estrogen receptor–negative (ER–)/progesterone receptor–negative (PR–)/human epidermal growth factor receptor 2–positive (HER2+), two were ER+/PR+/HER2–, and one was ER+/PR–/HER2– (Data Supplement [Fig A3C]). Detailed clinicopathologic and genomic characteristics for the three cohorts are summarized in the Data Supplement (Tables A3-A5).
Case
We present a case of response to selpercatinib in a patient with wild-type RET-amplified NSCLC without RET fusion or other oncogenic alterations. The patient is a 69-year-old man with a 40 pack-year history of tobacco use who presented with hoarseness and was found to have unresectable stage III NSCLC favor adenocarcinoma, with a 5.6-cm left upper lobe (LUL) lung mass and involvement of 4R and 4L lymph nodes (Data Supplement [Fig A4]). Pathologic analysis showed weak positivity for TTF-1 and INSM1, positive (retained) RB1 expression, and negative p40 and PD-L1 expression (Data Supplement [Fig A5A-C]). Positron emission tomography-CT imaging showed no distant metastases, and a brain MRI was negative for metastatic disease. NGS showed focal RET amplification (estimated 22 copies, Data Supplement [Fig A6]) without RET fusion or RET point mutation and was negative for other oncogenic driver mutations in KRAS, EGFR, ALK, ROS1, BRAF, MET, NTRK, HER2, and NRG1. Targeted RNA sequencing demonstrated no fusions but a high number of RET transcripts compared with historical NSCLC controls.
The patient was initially treated with cisplatin plus pemetrexed and concurrent radiation followed by durvalumab consolidation, with a decrease in the LUL mass. Subsequent imaging approximately 3 months after durvalumab initiation showed increased right axillary lymphadenopathy, and a biopsy of this lymph node revealed recurrent NSCLC with similar morphology and immunohistochemical phenotype to the prior (Data Supplement [Fig A5D-F]). Additionally, a brain MRI demonstrated a new enhancing right frontal lobe metastasis. Genomic sequencing of the recurrent axillary lymph node redemonstrated RET amplification (28 copies), without other oncogenic alterations (Fig 2A). Additionally, RET FISH on the axillary recurrence showed no split signals or isolated 3′ signals to indicate a RET rearrangement but did show marked 5′ RET signal amplification (5′ signals with >25 copies per cell and an intact number of normal fused 5′ and 3′ signals, Fig 2B), supportive of focal RET amplification rather than chromosome 10 polysomy. Repeat targeted RNA sequencing again showed no oncogenic fusions but increased RET transcript levels compared with historical NSCLC controls, similar to the previous analysis (Fig 2C).
FIG 2.
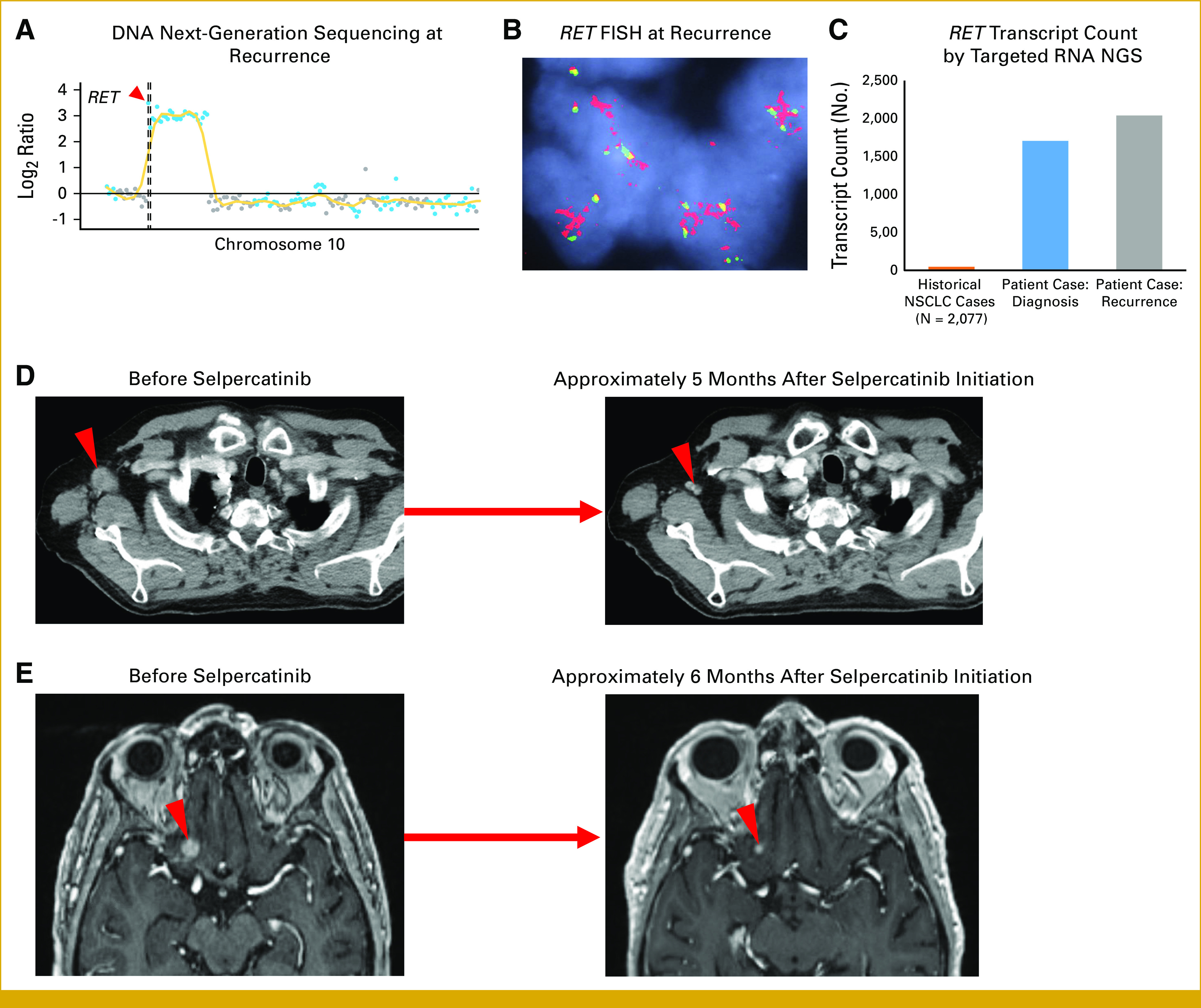
(A) Copy number plot from NGS of the right axillary lymph node recurrence showing high-level RET amplification. The red arrow denotes the position of the RET gene along chromosome 10. The vertical dashed line represents the centromere position. (B) FISH of formalin-fixed paraffin-embedded tissue from the right axillary lymph node recurrence. The red 5′ probe covers most of the RET gene and sequences upstream of RET while the green 3′ probe is outside and downstream to RET. (C) Quantification of total RET intragenic transcript count (ie, all splice variants) using a targeted RNA NGS fusion assay by anchored multiplexed PCR in the patient case at diagnosis and recurrence compared with the mean intragenic transcript count from a historical cohort of NSCLC controls between 2015 and 2022. (D) CT imaging demonstrates a response to selpercatinib in the right axillary lymph node. (E) Contrast-enhanced brain MRI shows a response to selpercatinib in a right inferior frontal lobe metastasis. CT, computed tomography; FISH, fluorescence in situ hybridization; MRI, magnetic resonance imaging; NGS, next-generation sequencing; NSCLC, non–small-cell lung cancer; PCR, polymerase chain reaction.
Given the progression after recent platinum-doublet chemotherapy and PD-L1 immunotherapy, along with the finding of high-level RET amplification, the patient was started on off-label selpercatinib at the standard dose of 160 mg twice daily. Stereotactic radiosurgery to the brain metastasis was considered in multidisciplinary discussion with radiation oncology but deferred given the lesion's close proximity to the optic nerve. Subsequent serial imaging while on selpercatinib demonstrated an ongoing confirmed objective response, with a complete response in the right axillary lymph node achieved at approximately 6 weeks (ongoing at approximately 5 months; Fig 2D, Data Supplement [Fig A7]) and a partial response in the brain metastasis achieved at 3 months (ongoing at approximately 6 months; Fig 2E, Data Supplement [Fig A7]). Selpercatinib was tolerated with an adverse effect profile consistent with that previously published.8 The patient experienced transient grade 1 transaminase elevation that subsequently resolved as well as intermittent abdominal pain, indeterminate for casual relationship to selpercatinib, or baseline chronic diverticulitis.
DISCUSSION
Selective targeting of RET has become the standard of care for patients with RET fusion–positive cancer or RET activating point mutations. Here, we demonstrate that amplification of wild-type RET represents a novel, targetable, albeit rare molecular subtype across cancer types, and to our knowledge, we report the first case of clinical response to the RET inhibitor selpercatinib in a patient with advanced NSCLC with RET amplification and no other known oncogenic alterations.
RET amplification was first described in thyroid cancer and has subsequently been identified in various cancer types. In a previous pan-cancer cohort (N = 4,871), 0.5% had RET amplification (with an incidence of 0.48% in NSCLC)22; in a separate breast cancer cohort (N = 9,693), the frequency was 0.84%.23 To our knowledge, our analyses provide the largest pan-cancer assessment of RET amplification to date. We found that RET amplification frequency across all solid cancer types was 0.08% in the DFCI cohort, 0.05% in the MSKCC cohort, and 0.25% in the TCGA, GENIE, and China Pan-Cancer cohort and that the frequency in NSCLC was 0.08%, 0.04%, and 0.09% in these three cohorts, respectively. Our observed frequencies are lower than previously reported, which may, in part, be due to the larger cohort size in our study. Additionally, in the DFCI cohort, cases with amplification of the entire chromosome 10 rather than focal RET amplification were excluded while in the MSKCC cohort, FACETS was used to correct for tumor ploidy as previously described14; these measures may contribute to a lower, more accurate representation of the frequency of focal RET amplification (information on ploidy was not available in the TCGA, GENIE, and China Pan-Cancer cohort). Differing amplification thresholds may additionally account for some of the differences in frequency estimates between our cohorts. Given the unique methodologies in determining copy number count and distinct amplification thresholds in the DFCI and MSKCC platforms, we used these cohort-specific cutoffs, rather than impose a new single arbitrary cutoff across cohorts. Varied methodologies between institutions in evaluating copy count pose inherent limitations, an area that merits future work.
Our analyses also shed light on the clinicopathologic and genomic features of RET-amplified tumors. A disproportionate number of RET-amplified breast cancer cases were triple-negative (7 of 15 cases with available immunohistochemistry); although the sample size is small, this finding is intriguing and is in line with prior evidence that RET-amplified breast cancer tends to be ER-negative and ERBB2 nonamplified.23 This observation must be interpreted in the context of the fact that breast cancer in general, and TP53-mutant triple-negative breast cancer in particular, tends to be characterized by genomic instability with multiple amplification events.24 Regarding NSCLC, compared with previous real-world reports on clinical characteristics of NSCLC harboring a RET fusion, the 11 RET-amplified NSCLC cases in this study were slightly older (median age 69 years, compared with a median age of 56-65 years in previous studies of RET-fused NSCLC) and more male-predominant (36.4% female, compared with 45%-56% female in previous studies of RET-fused NSCLC).25-28 A history of smoking was present in 62.5% of RET-amplified NSCLC cases, with available smoking status slightly higher than the historical rate of tobacco use (31%-49.1%) observed among RET-fused NSCLC in previous studies.25-28 Additionally, 36.4% of RET-amplified NSCLC cases were squamous cell carcinoma, higher than the frequency (0%-1.7%) seen previously in RET-fused NSCLC.25-28 These conclusions are limited by the small sample size of our RET-amplified NSCLC subset. There was not sufficient data on PD-L1 and tumor mutational burden (TMB) in our RET-amplified NSCLC cases to allow for meaningful comparisons. Finally, a key finding from our exploration of genomic features of RET-amplified tumors is that 8 of the 11 RET-amplified NSCLC cases did not have a concurrent driver alteration, suggesting that RET amplification may be the sole potential oncogenic driver in a subset of lung cancers.
The ability of RET amplification to serve as a potential oncogenic alteration vulnerable to targeted therapy is supported by our case of a patient with RET-amplified NSCLC without other known drivers who responded to selpercatinib. Three previous case reports observed efficacy of multikinase inhibitors in RET-amplified tumors: one case described response to sunitinib in treatment-refractory RET-amplified germ cell tumor,29 the second documented response to cabozantinib + nivolumab in a patient with hepatocellular carcinoma harboring RET amplification, high TMB, and positive PD-L1 expression (although whether the kinase inhibitor or the immunotherapy drove this response is unclear),30 and the third reported stable disease in a patient with RET-amplified adenocarcinoma of the tongue (thought to have originated in a minor salivary gland) treated with sunitinib, with 22% shrinkage of lung metastases.31 Importantly, the first two cases did not pursue RNA NGS or FISH testing and thus may have overlooked the presence of RET fusion since RET fusion and amplification can cooccur. In contrast to the activity of multikinase inhibitors in these cases, another study observed no difference in response rate among 24 patients with RET-amplified NSCLC who received vandetanib versus a comparator arm in four phase III trials of vandetanib, although conclusions from this study are limited by the small sample size and the modest impact of multikinase inhibitors even in RET fusion-positive cases.32 With the advent of selective RET inhibitors, whether these therapies are effective against RET-amplified tumors is of interest. One report documented response to pralsetinib in a patient with NSCLC with a novel intergenic RET fusion and RET amplification, although whether the fusion or amplification accounted for the response is unclear.33 Notably, a recent case report observed a response to selpercatinib in RET-amplified glioblastoma.34 This report together with our case of response to selpercatinib in wild-type RET-amplified NSCLC provide compelling motivation for further investigation of RET amplification as a possible novel targetable oncogenic driver across cancer types. Indeed, the potential oncogenicity and targetability of RET amplification is supported by preclinical studies showing that RET amplification promotes transformation of nontumorigenic mammary cells in vitro and that overexpression or amplification of wild-type RET in mice induces formation of mammary tumors that are susceptible to RET inhibitors.23,35 The possibility of RET amplification serving as a targetable oncogenic driver is further supported by analogous situations that exist for HER2 and MET amplification in NSCLC.36-38 Together, the body of preclinical data supporting wild-type RET amplification as an oncogenic driver, our case and prior cases of response of wild-type RET-amplified cancer to selective RET inhibition, and the parallels with HER2 and MET amplification offer important evidence that wild-type RET amplification may serve as a novel driver alteration.
Another intriguing question is whether RET amplification can serve as a mechanism of acquired resistance to targeted therapies for other driver alterations. Our RET-amplified cases with available treatment course information either already had RET amplification at baseline or did not have paired pretreatment and post-treatment genomics available to examine this question. However, RET amplification was previously reported as a potential mechanism of acquired resistance to HER2-targeted therapy in a patient with breast cancer.23 Further research is warranted to probe this question in greater depth.
Overall, this study provides evidence that amplification of wild-type RET represents a novel, actionable, rare genomic subset of NSCLC and other cancers. This finding underscores the importance of broad next-generation sequencing to identify rare but actionable alterations that can profoundly influence patients' lives and motivates future work in larger RET-amplified cohorts to help inform targeted treatment strategies across cancer types.
Biagio Ricciuti
Consulting or Advisory Role: Regeneron, AstraZeneca, Amgen
Travel, Accommodations, Expenses: Bristol Myers Squibb
Honoraria: Targeted Oncology
Guilherme Harada
Speakers' Bureau: MSD, AstraZeneca, Pfizer, Lilly, Merck
Matteo Repetto
Travel, Accommodations, Expenses: Sanofi
Ankit Singh
Employment: Massachusetts General Hospital
Travel, Accommodations, Expenses: Massachusetts General Hospital
Yvonne Y. Li
Stock and Other Ownership Interests: g.Root Biomedical Services
Ayal Aizer
Consulting or Advisory Role: Novartis, NH TherAGUIX, seagen
Research Funding: Varian Medical Systems, NH TherAGUIX
Mizuki Nishino
Consulting or Advisory Role: AstraZeneca
Research Funding: AstraZeneca (Inst), Daiichi Sankyo (Inst), Canon Medical System (Inst), Konica Minolta (Inst)
Alexander Drilon
Stock and Other Ownership Interests: Treeline Biosciences, mBrace
Honoraria: Pfizer, Loxo/Bayer/Lilly, IASLC, Helsinn Therapeutics, BeiGene, Remedica, Remedica, TP Therapeutics, Verastem, Ignyta/Genentech/Roche, AstraZeneca, Liberum, Lungevity, NIH, PER, OncLive/MJH Life Sciences, Clinical Care Options/NCCN, Lung Cancer Research Foundation, Associazione Italiana Oncologia Toracica (AIOT), Chugai Pharma, Sirio Libanes Hospital, Answers in CME, Research to Practice, i3 Health, RV Mais
Consulting or Advisory Role: Ignyta, Loxo, AstraZeneca, Pfizer, Blueprint Medicines, Genentech/Roche, BeiGene, Hengrui Therapeutics, Exelixis, Bayer, Tyra Biosciences, Takeda/Millennium, BerGenBio, MORE Health, Lilly, AbbVie, 14ner Oncology/Elevation Oncology, Monopteros Therapeutics, Novartis, EMD Serono/Merck, Repare Therapeutics, Melendi, Archer, Nuvalent, Inc, Janssen, Amgen, Merus, Axis Pharma, Medscape, Liberum, Med Learning, PeerView, EPG Health, Journal of the National Comprehensive Cancer Network, Ology Medical Education, Ology Medical Education, Clinical Care Options, Clinical Care Options, touchIME, Entos, Prelude Therapeutics, Applied Pharmaceutical Science, Treeline Biosciences, Monte Rosa Therapeutics, EcoR1 Capital
Research Funding: Foundation Medicine
Patents, Royalties, Other Intellectual Property: Wolters Kluwer (Royalties for Pocket Oncology), Osimertinib Selpercatinib
Other Relationship: Merck, GlaxoSmithKline, Teva, Taiho Pharmaceutical, Pfizer, PharmaMar, Puma Biotechnology, Pfizer, Merus, Boehringer Ingelheim
Lynette Sholl
Stock and Other Ownership Interests: Moderna Therapeutics
Consulting or Advisory Role: Genentech (Inst), Lilly (Inst), AstraZeneca
Research Funding: Roche/Genentech (Inst), Bristol Myers Squibb (Inst)
Mark M. Awad
Consulting or Advisory Role: Merck, Pfizer, Bristol Myers Squibb, Foundation Medicine, Novartis, Gritstone Bio, Mirati Therapeutics, EMD Serono, AstraZeneca, Instil Bio, AstraZeneca, Regeneron, Janssen, Affini-T Therapeutics, Affini-T Therapeutics
Research Funding: Genentech/Roche (Inst), Lilly (Inst), AstraZeneca (Inst), Bristol Myers Squibb (Inst), Amgen (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb Foundation
Open Payments Link: https://openpaymentsdata.cms.gov/physician/1127368
Julia Rotow
Honoraria: AstraZeneca
Consulting or Advisory Role: AstraZeneca, Gritstone Bio, AbbVie, Lilly, Takeda, Guardant Health, Sanofi/Regeneron, Genentech, Janssen, Bioatla, Bioatla, G1 Therapeutics, Jazz Pharmaceuticals, Amgen, Amgen
Travel, Accommodations, Expenses: AstraZeneca
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at ASCO annual meeting, Chicago, IL, June 2-6, 2023.
SUPPORT
Supported by institutional funding to M.M.A. from Bristol Myers Squibb, Lilly, Genentech, AstraZeneca, and Amgen; institutional funding to J.R. from AbbVie, AstraZeneca, Bicycle Therapeutics, BioAtla, Blueprint, Daiichi Sankyo, Enliven, EpimAb, Loxo Oncology, ORIC, and Redcloud; institutional funding to M.N. from Canon Medical Systems, AstraZeneca, and Daiichi Sankyo; support to A.D. by the National Cancer Institute/National Institutes of Health P30CA008748, 1RO1CA251591001A1, and 1RO1CA273224-01, and Lungevity grant; and institutional funding to L.S. from Genentech and Bristol Myers Squibb.
M.M.A. and J.R. are senior authors and contributed equally to this work.
AUTHOR CONTRIBUTIONS
Conception and design: Malini M. Gandhi, Biagio Ricciuti, Matteo Repetto, Mark M. Awad, Julia Rotow
Provision of study materials or patients: Ankit Singh, Kelly Fitzgerald, Joao Alessi
Collection and assembly of data: Malini M. Gandhi, Biagio Ricciuti, Guilherme Harada, Matteo Repetto, Melissa S. Gildenberg, Ankit Singh, Yvonne Y. Li, Mizuki Nishino, Joao Alessi, Federica Pecci, Adam Fisch, Alexander Drilon, Lynette Sholl, Mark M. Awad
Data analysis and interpretation: Malini M. Gandhi, Biagio Ricciuti, Guilherme Harada, Matteo Repetto, Melissa S. Gildenberg, Ankit Singh, Yvonne Y. Li, Andréanne Gagné, Xinan Wang, Ayal Aizer, Kelly Fitzgerald, Mizuki Nishino, Alessandro Di Federico, Adam Fisch, Alexander Drilon, Valentina Nardi, Lynette Sholl, Mark M. Awad, Julia Rotow
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Biagio Ricciuti
Consulting or Advisory Role: Regeneron, AstraZeneca, Amgen
Travel, Accommodations, Expenses: Bristol Myers Squibb
Honoraria: Targeted Oncology
Guilherme Harada
Speakers' Bureau: MSD, AstraZeneca, Pfizer, Lilly, Merck
Matteo Repetto
Travel, Accommodations, Expenses: Sanofi
Ankit Singh
Employment: Massachusetts General Hospital
Travel, Accommodations, Expenses: Massachusetts General Hospital
Yvonne Y. Li
Stock and Other Ownership Interests: g.Root Biomedical Services
Ayal Aizer
Consulting or Advisory Role: Novartis, NH TherAGUIX, seagen
Research Funding: Varian Medical Systems, NH TherAGUIX
Mizuki Nishino
Consulting or Advisory Role: AstraZeneca
Research Funding: AstraZeneca (Inst), Daiichi Sankyo (Inst), Canon Medical System (Inst), Konica Minolta (Inst)
Alexander Drilon
Stock and Other Ownership Interests: Treeline Biosciences, mBrace
Honoraria: Pfizer, Loxo/Bayer/Lilly, IASLC, Helsinn Therapeutics, BeiGene, Remedica, Remedica, TP Therapeutics, Verastem, Ignyta/Genentech/Roche, AstraZeneca, Liberum, Lungevity, NIH, PER, OncLive/MJH Life Sciences, Clinical Care Options/NCCN, Lung Cancer Research Foundation, Associazione Italiana Oncologia Toracica (AIOT), Chugai Pharma, Sirio Libanes Hospital, Answers in CME, Research to Practice, i3 Health, RV Mais
Consulting or Advisory Role: Ignyta, Loxo, AstraZeneca, Pfizer, Blueprint Medicines, Genentech/Roche, BeiGene, Hengrui Therapeutics, Exelixis, Bayer, Tyra Biosciences, Takeda/Millennium, BerGenBio, MORE Health, Lilly, AbbVie, 14ner Oncology/Elevation Oncology, Monopteros Therapeutics, Novartis, EMD Serono/Merck, Repare Therapeutics, Melendi, Archer, Nuvalent, Inc, Janssen, Amgen, Merus, Axis Pharma, Medscape, Liberum, Med Learning, PeerView, EPG Health, Journal of the National Comprehensive Cancer Network, Ology Medical Education, Ology Medical Education, Clinical Care Options, Clinical Care Options, touchIME, Entos, Prelude Therapeutics, Applied Pharmaceutical Science, Treeline Biosciences, Monte Rosa Therapeutics, EcoR1 Capital
Research Funding: Foundation Medicine
Patents, Royalties, Other Intellectual Property: Wolters Kluwer (Royalties for Pocket Oncology), Osimertinib Selpercatinib
Other Relationship: Merck, GlaxoSmithKline, Teva, Taiho Pharmaceutical, Pfizer, PharmaMar, Puma Biotechnology, Pfizer, Merus, Boehringer Ingelheim
Lynette Sholl
Stock and Other Ownership Interests: Moderna Therapeutics
Consulting or Advisory Role: Genentech (Inst), Lilly (Inst), AstraZeneca
Research Funding: Roche/Genentech (Inst), Bristol Myers Squibb (Inst)
Mark M. Awad
Consulting or Advisory Role: Merck, Pfizer, Bristol Myers Squibb, Foundation Medicine, Novartis, Gritstone Bio, Mirati Therapeutics, EMD Serono, AstraZeneca, Instil Bio, AstraZeneca, Regeneron, Janssen, Affini-T Therapeutics, Affini-T Therapeutics
Research Funding: Genentech/Roche (Inst), Lilly (Inst), AstraZeneca (Inst), Bristol Myers Squibb (Inst), Amgen (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb Foundation
Open Payments Link: https://openpaymentsdata.cms.gov/physician/1127368
Julia Rotow
Honoraria: AstraZeneca
Consulting or Advisory Role: AstraZeneca, Gritstone Bio, AbbVie, Lilly, Takeda, Guardant Health, Sanofi/Regeneron, Genentech, Janssen, Bioatla, Bioatla, G1 Therapeutics, Jazz Pharmaceuticals, Amgen, Amgen
Travel, Accommodations, Expenses: AstraZeneca
No other potential conflicts of interest were reported.
REFERENCES
- 1.Shabbir A Kojadinovic A Shafiq T, et al. : Targeting RET alterations in cancer: Recent progress and future directions. Crit Rev Oncol Hematol 181:103882, 2023 [DOI] [PubMed] [Google Scholar]
- 2.Subbiah V, Cote GJ: Advances in targeting RET-dependent cancers. Cancer Discov 10:498-505, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drilon A Rekhtman N Arcila M, et al. : Cabozantinib in patients with advanced RET-rearranged non-small-cell lung cancer: An open-label, single-centre, phase 2, single-arm trial. Lancet Oncol 17:1653-1660, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gautschi O Milia J Filleron T, et al. : Targeting RET in patients with RET-rearranged lung cancers: Results from the Global, Multicenter RET Registry. J Clin Oncol 35:1403-1410, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wirth LJ Sherman E Robinson B, et al. : Efficacy of selpercatinib in RET-altered thyroid cancers. N Engl J Med 383:825-835, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Subbiah V Hu MI Wirth LJ, et al. : Pralsetinib for patients with advanced or metastatic RET-altered thyroid cancer (ARROW): A multi-cohort, open-label, registrational, phase 1/2 study. Lancet Diabetes Endocrinol 9:491-501, 2021 [DOI] [PubMed] [Google Scholar]
- 7.Griesinger F Curigliano G Thomas M, et al. : Safety and efficacy of pralsetinib in RET fusion-positive non-small-cell lung cancer including as first-line therapy: Update from the ARROW trial. Ann Oncol 33:1168-1178, 2022 [DOI] [PubMed] [Google Scholar]
- 8.Drilon A Oxnard GR Tan DSW, et al. : Efficacy of selpercatinib in RET fusion-positive non-small-cell lung cancer. N Engl J Med 383:813-824, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Subbiah V Cassier PA Siena S, et al. : Pan-cancer efficacy of pralsetinib in patients with RET fusion-positive solid tumors from the phase 1/2 ARROW trial. Nat Med 28:1640-1645, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Subbiah V Wolf J Konda B, et al. : Tumour-agnostic efficacy and safety of selpercatinib in patients with RET fusion-positive solid tumours other than lung or thyroid tumours (LIBRETTO-001): A phase 1/2, open-label, basket trial. Lancet Oncol 23:1261-1273, 2022 [DOI] [PubMed] [Google Scholar]
- 11.Chakravarty D Gao J Phillips SM, et al. : OncoKB: A precision oncology knowledge base. JCO Precis Oncol 10.1200/PO.17.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia EP Minkovsky A Jia Y, et al. : Validation of OncoPanel: A targeted next-generation sequencing assay for the detection of somatic variants in cancer. Arch Pathol Lab Med 141:751-758, 2017 [DOI] [PubMed] [Google Scholar]
- 13.Cheng DT Mitchell TN Zehir A, et al. : Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn 17:251-264, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen R, Seshan VE: FACETS: Allele-specific copy number and clonal heterogeneity analysis tool for high-throughput DNA sequencing. Nucleic Acids Res 44:e131, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Repetto M Richards AL Chen MF, et al. : MYC amplification segment-size mutual exclusivity analysis with other oncogenic events in a pan-cancer sequencing cohort. J Clin Oncol 41, 2023. (suppl 16; abstr 3073) [Google Scholar]
- 16.Weinstein JN Collisson EA Mills GB, et al. : The Cancer Genome Atlas Pan-cancer analysis project. Nat Genet 45:1113-1120, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.André F Arnedos M Baras AS, et al. : AACR Project GENIE: Powering precision medicine through an International Consortium. Cancer Discov 7:818-831, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu L Yao H Chen H, et al. : Landscape of somatic alterations in large-scale solid tumors from an Asian population. Nat Commun 13:4264, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cerami E Gao J Dogrusoz U, et al. : The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov 2:401-404, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao J Aksoy BA Dogrusoz U, et al. : Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 6:pl1, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng Z Liebers M Zhelyazkova B, et al. : Anchored multiplex PCR for targeted next-generation sequencing. Nat Med 20:1479-1484, 2014 [DOI] [PubMed] [Google Scholar]
- 22.Kato S Subbiah V Marchlik E, et al. : RET aberrations in diverse cancers: Next-generation sequencing of 4,871 patients. Clin Cancer Res 23:1988-1997, 2017 [DOI] [PubMed] [Google Scholar]
- 23.Paratala BS Chung JH Williams CB, et al. : RET rearrangements are actionable alterations in breast cancer. Nat Commun 9:4821, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duijf PHG Nanayakkara D Nones K, et al. : Mechanisms of genomic instability in breast cancer. Trends Mol Med 25:595-611, 2019 [DOI] [PubMed] [Google Scholar]
- 25.Aldea M Marinello A Duruisseaux M, et al. : RET-MAP: An International multicenter study on clinicobiologic features and treatment response in patients with lung cancer harboring a RET fusion. J Thorac Oncol 18:576-586, 2023 [DOI] [PubMed] [Google Scholar]
- 26.Lee J Ku BM Shim JH, et al. : Characteristics and outcomes of RET-rearranged Korean non-small cell lung cancer patients in real-world practice. Jpn J Clin Oncol 50:594-601, 2020 [DOI] [PubMed] [Google Scholar]
- 27.Hess LM Han Y Zhu YE, et al. : Characteristics and outcomes of patients with RET-fusion positive non-small lung cancer in real-world practice in the United States. BMC Cancer 21:28, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drilon A Lin JJ Filleron T, et al. : Frequency of brain metastases and multikinase inhibitor outcomes in patients with RET-rearranged lung cancers. J Thorac Oncol 13:1595-1601, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Subbiah V Meric-Bernstam F Mills GB, et al. : Next generation sequencing analysis of platinum refractory advanced germ cell tumor sensitive to Sunitinib (Sutent®) a VEGFR2/PDGFRβ/c-kit/FLT3/RET/CSF1R inhibitor in a phase II trial. J Hematol Oncol 7:52, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang X Shi J Chen X, et al. : Efficacy of cabozantinib and nivolumab in treating hepatocellular carcinoma with RET amplification, high tumor mutational burden, and PD-L1 expression. Oncologist 25:470-474, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones SJ Laskin J Li YY, et al. : Evolution of an adenocarcinoma in response to selection by targeted kinase inhibitors. Genome Biol 11:R82, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Platt A Morten J Ji Q, et al. : A retrospective analysis of RET translocation, gene copy number gain and expression in NSCLC patients treated with vandetanib in four randomized Phase III studies. BMC Cancer 15:171, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang SS Wang F Zeng Z, et al. : Case report: A novel intergenic MIR4299/MIR8070-RET fusion with RET amplification and clinical response to pralsetinib in a lung adenocarcinoma patient. Front Oncol 12:929763, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Czech C Chen A Morgan KP, et al. : Response to selpercatinib in a patient with recurrent glioblastoma and RET amplification. J Natl Compr Canc Netw 20:966-971, 2022 [DOI] [PubMed] [Google Scholar]
- 35.Gattelli A Garcia Sola ME Roloff TC, et al. : Chronic expression of wild-type Ret receptor in the mammary gland induces luminal tumors that are sensitive to Ret inhibition. Oncogene 37:4046-4054, 2018 [DOI] [PubMed] [Google Scholar]
- 36.Remon J Hendriks LEL Mountzios G, et al. : MET alterations in NSCLC-current perspectives and future challenges. J Thorac Oncol 18:419-435, 2023 [DOI] [PubMed] [Google Scholar]
- 37.Noonan SA Berry L Lu X, et al. : Identifying the appropriate FISH criteria for defining MET copy number-driven lung adenocarcinoma through oncogene overlap analysis. J Thorac Oncol 11:1293-1304, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riudavets M Sullivan I Abdayem P, et al. : Targeting HER2 in non-small-cell lung cancer (NSCLC): A glimpse of hope? An updated review on therapeutic strategies in NSCLC harbouring HER2 alterations. ESMO Open 6:100260, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]


