Abstract
This study aimed to develop atorvastatin‐loaded emulgel and nano‐emulgel dosage forms and investigate their efficiency on surgical wound healing and reducing post‐operative pain. This double‐blind randomized clinical trial was conducted in a surgical ward of a tertiary care hospital affiliated with university of medical sciences. The eligible patients were adults aged 18 years or older who were undergoing laparotomy. The participants were randomized in a 1:1:1 ratio to one of three following groups of atorvastatin‐loaded emulgel 1% (n = 20), atorvastatin‐loaded nano‐emulgel 1% (n = 20), and placebo emulgel (n = 20) twice a day for 14 days. The primary outcome was the Redness, Edema, Ecchymosis, Discharge, and Approximation (REEDA) scores to determine the rate of wound healing. The Visual Analogue Scale (VAS) and quality of life were the secondary outcomes of this study. A total of 241 patients assessed for eligibility; of them, 60 patients completed the study and considered for final evaluation. A significant decrease in REEDA score was observed on Days 7 (63%) and 14 (93%) of treatment with atorvastatin nano‐emulgel (p‐value < 0.001). A significant decrease of 57% and 89% in REEDA score was reported at Days 7 and 14, respectively, in atorvastatin the emulgel group (p‐value < 0.001). Reduction in pain VAS in the atorvastatin nano‐emulgel was also recorded at Days 7 and 14 of the intervention. The results of the present study suggested that both topical atorvastatin‐loaded emulgel and nano‐emulgel 1% were effective in acceleration of wound healing and alleviation of pain of laparotomy surgical wounds, without causing intolerable side effects.
Keywords: atorvastatin, emulgel, nano‐emulgel, post‐laparotomy pain, surgical wounds
1. INTRODUCTION
Wounds are caused by the damage to the integrity of biological tissues, such as skin and mucous membranes. 1 There are several types of wounds, including diabetic ulcers, burns, osteomyelitis, pressure ulcers and surgical wounds. 2 , 3 , 4 , 5 Wounds should be properly managed to prevent possible complications, such as infection and scars. In general, the wound‐healing process consists of the following steps: inflammatory phase, which takes place immediately after the formation of wound, proliferation phase, in which the wound initiates to rebuild itself, and the final step of the remodelling phase, in which the wound acquires its tensile strength. 6
Patients undergoing surgery are potentially vulnerable and usually require to be hospitalized after the operation. Therefore, post‐operative wounds necessitate special care and proper management. These wounds can get easily infected and cause substantial complications. In this regard, various approaches have been recommended, the key of which is the use of anti‐inflammatory and immunomodulating compounds to promote the process of wound healing. 7 Laparotomy surgical incisions are onerous to heal, since the incision passes through various layers of the skin. 8 Notably, the quality of post‐operative wound healing has a significant impact on the patient's recovery and rehabilitation. Furthermore, the complications of surgical wounds affect the mobility and mortality of these patients and all of these highlights the importance of acceleration in healing of these wounds. 9
Atorvastatin belongs to a group of anti‐hyperlipidemia medicines called statins and are prescribed to prevent cardiovascular diseases. 10 In addition to its cholesterol‐lowering effects, it possesses antioxidant, analgesic, immunomodulatory, and anti‐inflammatory properties, 11 , 12 , 13 making it a feasible wound healing candidate. Systemic consumption of this drug is associated with several side effects, such as headache, muscle pain, gastrointestinal symptoms, myopathy, and liver toxicity. 14 In order to prevent possible side effects following systemic drug use, topical drug delivery systems can be applied to directly release the drug into the wounded site and prevent systemic spread of the drug. 15 Development of novel nano delivery systems for this purpose is a notable advantage, as these systems possess a high surface‐to‐volume ratio and regulate the drug release. 16
Nano‐emulgels are considered an optimal system for topical drug delivery due to the high specificity of the site of action, targeted delivery of the precise medicine, maintenance of the physical stability of the drug and the dual control system for the release of the drug. 17 The efficacy of statin emulgel and nano‐emulgel systems in acceleration of healing process of post‐operative wounds and amelioration of post‐hemorrhoidectomy pain has been investigated in several in vivo studies with promising effects. 18 , 19
Therefore, this clinical trial was designed to evaluate the efficiency of topical atorvastatin‐loaded emulgel and nano‐emulgel 1% on wound‐healing rate, pain relief and quality of life in patients undergoing laparotomy surgeries.
2. MATERIALS AND METHODS
2.1. Materials
Atorvastatin was obtained from Sobhan Daru (Iran). Polysorbate 80, glycerol, methylparaben and propylparaben were purchased from Merck (Germany). Sesame oil was obtained from Shabli (Iran). Propylene glycol and carbomer were purchased from Rose Shimi Company (Iran).
2.2. Preparation of emulgels and nano‐emulgels (1%)
Primary nano emulsion was formulated by oil titration method. For this purpose, 500 mg of atorvastatin was dissolved in 2 mL of preheated sesame oil at 70°C for 20 min. Then, mixtures of surfactant and co‐surfactant contained 1.4 mL of polysorbate 80, 1.3 mL of propylene glycol and 12.5 mL glycerol were added to form the oil phase. In order to prepare the aqueous phase, 20 mL water was heated to 70°C. Thereafter, 90 mg methylparaben, 10 mg propylparaben and 20 mg Carbomer were added and fully dissolved in the water. Oily phase was added dropwise to aqueous phase under the effect of magnetic stirring for 10 min and nanoemulsion was formed after extra sonication for 10 min. The total nanoemulsion volume was reached to 50 mL with distilled water. Nanoemulgel was formed by increasing the pH of the formulation via addition of triethanolamine. The final Gel pH was fixed to 7 using a digital pH meter. The placebo was formulated as mentioned above without addition of atorvastatin as the active ingredient. The formulated gels were filled in similar lacquered aluminium collapsible tubes.
2.3. Physical characterization of nano‐emulgel
The formed nanoemulsion showed a uniform oil in water dispersion with particle size of 224.20 ± 3.6 and zeta potential of −12.9 mv. The pH of final formulations was adjusted to 7 and the viscosity of formulation were in the range of 1400–1500 cp at 25°C.
Microbial contamination of both formulations was evaluated after culture on Mueller Hinton agar, soybean casein digest agar and sabouraud dextrose agar. No microbiological contamination was detected.
2.4. Particle size analysis
The droplet sizes and zeta potential of nano emulsion were measured by photon correlation spectroscopy. For this purpose, the nano emulsion was diluted with deionized water with the ratio of 1:10 before the analysis. Measurement of size as well as zeta potential were performed on a Malvern Zetasizer 3000 (Malvern Instruments, Worcestershire, UK).
2.5. Gel viscosity analysis
Viscosities of gels were determined using Brookfield Viscometer RV. The measurements were performed on 25°C.
2.6. Study design and population
This prospective double‐blind placebo‐controlled, randomized clinical trial was registered at Iranian Registry of Clinical Trials (IRCT20190810044500N3). Written informed consent was provided for each participant before administration of any study intervention. No one received compensation or was offered any incentive for participating in this study. Eligible patients were adults aged 18 years or older who were undergoing laparotomy from February 2020 to May 2021. The trial was conducted in two university affiliated hospitals in Yazd, Iran. Patients who were consuming any systemic statin, corticosteroids, non‐steroidal anti‐inflammatory drugs (NSAIDs), and cytotoxic/ immunosuppressive drugs were considered ineligible to enrol in the study.
Patients were randomized in a 1:1:1 ratio to atorvastatin‐loaded emulgel 1% (n = 20), atorvastatin‐loaded nano‐emulgel 1% (n = 20), and placebo emulgel (n = 20) groups by applying the Random allocation software (version 1). After confirming the eligibility of participants, patients were given the option for enrolment. Interested patients were guided to receive either intervention. The ward nurse who arranged the preoperative clinic visits recruited the patients. The surgeon, patients, and data collector were all blinded to the allocated arms of the study.
The first dose of the emulgel was administered by the ward nurse within 6 h of surgery. All patients were self‐instructed to apply the topical emulgel in their study arm twice a day for a 14‐day period. The dose of the emulgel in patients was approximately 2.0 ± 0.5 g (w/w), equal to 20‐mg atorvastatin, twice a day. During hospitalization, patients were visited 24 and 48 h after surgery. The surgeon visited the patients on the 7th and 14th post‐operative days to evaluate the wound site. Drug safety and compliance were assessed by patient reports, clinical visits and physical examination.
2.7. Primary and secondary outcomes
Before the surgical procedure, demographic and clinical characteristics of the patients were collected. The primary outcome was the REEDA score to investigate the wound healing process. This score consists of five major domains including redness, oedema, ecchymosis/bruising, discharge, and approximation. Each domain is given a score of 0–3. A lower score favours more advanced healing.
The secondary outcomes were unexpected adverse effects, quality of life (wound‐QoL) and visual analogue scale (VAS), which is ranging from 0 (illustrating no pain) to 10 (illustrating the worst) for quantitative assessment of the post‐operative pain. Assessment of quality of life was based on wound‐QoL questionnaire at baseline and Days 7 and 14. The questionnaire contains 17 questions, ranging from 0 (not at all) to 4 (very much). 20
2.8. Sample size
Data from a previous randomized prospective study were used for sample size calculation. 21 An overall sample size of 51 patients (17 in each group) achieved 80.0% power at a 0.05 significance level and REEDA score standard deviation of 0.41 to detect a mean difference (reduction in total clinical score) of 0.4 using the sample size equation
2.9. Statistical analysis
The quantitative and qualitative variables in the groups were presented as means with standard deviation (SDs), medians with Interquartile range (IQRs), or as number (%). Normality of data was assessed using the Kolmogorov–Smirnov test. The distributed quantitative variables were compared between the groups by using the unpaired t‐test and Mann–Whitney tests depending on the variable and its distribution. Moreover, within‐group changes from baseline were assessed using Wilcoxon signed rank tests, paired t tests, and Fisher exact tests and repeated measurement to compare the changes of variables over time. The Chi‐square test was utilized for the comparison of the qualitative data between the groups. Statistically significant differences between the groups were detected through one‐way analysis of variance (ANOVA) followed by Tukey's post‐analysis test for group comparisons. Statistical analyses were performed using Statistical Package for the Social Sciences (SPSS), version 24. Two‐tailed p‐values <0.05 were considered statistically significant.
3. RESULTS
3.1. Patients and treatment
Between May 18, 2021 and February 10, 2020, 241 patients were screened for eligibility. Among them, 181 patients patients were not included in the study due to lack of inclusion criteria. Of the remaining 60 eligible patients, all were randomized to each of the interventions (20 per group). Eight patients were subsequently excluded from the study, because of the improper use of the administration. Finally, 52 patients completed the study. Half of the participants (50.0%) were male, and the mean (SD) age was 45.44 (8.55) years (Figure 1). The baseline demographic and disease characteristics were well balanced between the groups (Table 1).
FIGURE 1.
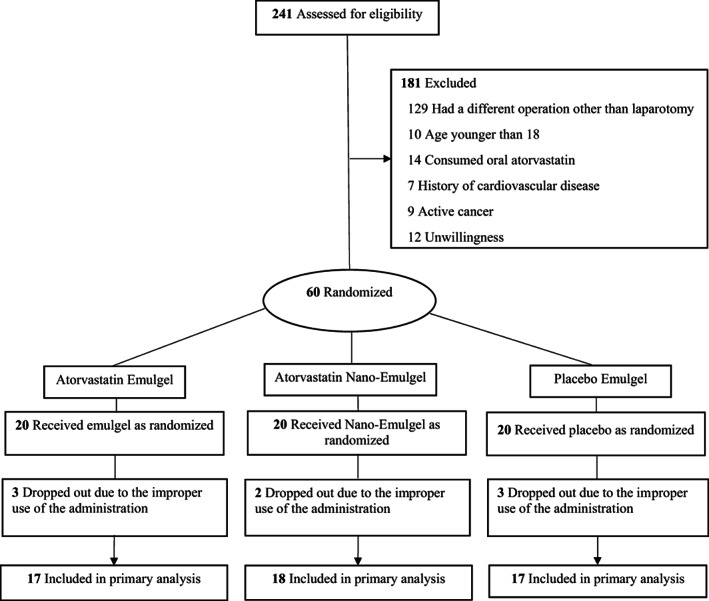
Outline of the protocol for double‐blind, controlled and clinical trial of topical atorvastatin‐loaded emulgel and nano‐emulgel 1% on post‐laparotomy pain and wound healing during study follow‐up.
TABLE 1.
Patient demographics profile and baseline disease characteristics.
| Characteristic | Atorvastatin nano‐emulgel 1% w/w | Atorvastatin emulgel 1% w/w | Placebo emulgel | p‐value |
|---|---|---|---|---|
| Age, mean (SD) | 42.4 (9.7) | 47.6 (7.9) | 46.5 (7.2) | 0.14 |
| Sex, N (%) | ||||
| Men | 7.0 (38.9) | 10.0 (58.8) | 9.0 (52.9) | 0.38 |
| Concurrent comorbidities, N (%) | ||||
| Diabetes mellitus | 4.0 (28.5) | 2 (11.7) | 5.0 (29.4) | 0.89 |
| Hypertension | 6.0 (33.3) | 5 (29.4) | 7.0 (41.2) | |
| Hypothyroidism | 1.0 (5.5) | 1 (5.8) | 1.0 (5.8) | |
| Current smoking, N (%) | ||||
| Yes | 11.0 (61.1) | 8.0 (47.1) | 9.0 (52.9) | 0.79 |
Abbreviations: HTN, hypertension; N, number; y, year; w/w, weight/weight.
3.2. Efficacy and safety
In this study, REEDA scale was applied to represent the changes in wound healing among the three arms of the study. The responses to each of the REEDA scale parameters between groups are presented in Table 2. REEDA satisfaction scores in the atorvastatin nano‐emulgel and atorvastatin emulgel arm ranked low across all REEDA scale domains on Day 7, and the changes of redness and ecchymosis were also significant on Day 14. A significant decrease in REEDA score from a mean (SDs) of 1.48 (0.33) to 0.54 (0.19) and 0.12 (0.04) was observed on Day 7 (63%) and 14 (93%) of treatment with atorvastatin nano‐emulgel, 1% w/w (p‐value < 0.001). A significant decrease of 57% and 89% in REEDA score was reported on Days 7 and 14 in the atorvastatin emulgel, 1% w/w group (p‐value < 0.001).
TABLE 2.
Primary and secondary outcomes at baseline and Days 7 and 14.
| Variable | Groups | p‐value | ||
|---|---|---|---|---|
| Atorvastatin nano‐emulgel 1% w/w | Atorvastatin emulgel 1% w/w | Placebo emulgel | ||
| Mean (SD) | ||||
| Redness | ||||
| Baseline | 2.44 (0.7) | 2.40 (0.6) | 2.29 (0.68) | 0.955 |
| Day 7 | 0.8 (0.4) | 1.0 (0.89) | 1.53 (0.51) | 0.003 * |
| Day 14 | 0.15 (0.06) | 0.2 (0.44) | 0.82 (0.52) | 0.001 * |
| Oedema | ||||
| Baseline | 1.63 (0.7) | 1.5 (0.63) | 1.76 (0.66) | 0.804 |
| Day 7 | 0.72 (0.66) | 0.56 (0.51) | 1.00 (0.50) | 0.001 * |
| Day 14 | 0.11 (0.32) | 0.19 (0.40) | 0.24 (0.43) | 0.630 |
| Ecchymosis | ||||
| Baseline | 2.2 (0.4) | 2.00 (0.3) | 2.10 (0.28) | 0.859 |
| Day 7 | 0.8 (0.24) | 0.95 (0.36) | 1.35 (0.06) | 0.001 * |
| Day 14 | 0.2 (0.08) | 0.31 (0.1) | 0.59 (0.15) | 0.001 * |
| Discharge | ||||
| Baseline | 0.78 (0.42) | 0.65 (0.15) | 0.67 (0.10) | 0.844 |
| Day 7 | 0.17 (0.06) | 0.15 (0.07) | 0.3 (0.06) | 0.001 * |
| Day 14 | 0.00 (0.0) | 0.00 (0.0) | 0.00 (0.0) | 1.000 |
| Approximation | ||||
| Baseline | 0.61 (0.11) | 0.55 (0.09) | 0.6 (0.1) | 0.754 |
| Day 7 | 0.25 (0.07) | 0.20 (0.20) | 0.30 (0.06) | 0.021 * |
| Day 14 | 0.08 (0.01) | 0.06 (0.02) | 0.1 (0.02) | 0.853 |
| REEDA | ||||
| Baseline | 1.48 (0.33) | 1.40 (0.30) | 1.41 (0.28) | 0.665 |
| Day 7 | 0.54 (0.19) | 0.60 (0.19) | 0.90 (0.12) | 0.022 * |
| Day 14 | 0.12 (0.04) | 0.15 (0.05) | 0.35 (0.06) | 0.019 * |
| VAS | ||||
| Baseline | 9.22 (0.8) | 9.00 (0.8) | 9.26 (0.77) | 0.551 |
| Day 1 | 6.72 (0.89) | 7.50 (1.3) | 7.06 (0.74) | 0.010 |
| Day 2 | 5.86 (0.9) | 6.38 (0.95) | 6.82 (0.88) | 0.015 |
| Day 7 | 3.56 (1.9) | 4.06 (1.43) | 5.35 (1.11) | 0.001 * |
| Day 14 | 2.22 (1.16) | 2.87 (1.54) | 3.7 (0.15) | 0.006 * |
Statistically significant (p‐value < 0.05); REEDA: Redness, Edema, Ecchymosis/bruising, Discharge, Approximation; VAS: visual analogue scale; w/w: weight/weight; SD: standard deviation.
Reduction in pain VAS in the atorvastatin nano‐emulgel group was also observed, as the mean (SD) scores of VAS improved from 9.2 (0.8) mm at baseline to 3.56 (1.9) and 2.22 (1.16) mm on Days 7 and 14, respectively. The mean reduction from baseline to Days 7 and 14 in the atorvastatin emulgel group was 55% and 68%, respectively. Pain VAS score improved progressively with continued use of interventions from baseline to Days 7 and 14 (Table 2).
Tukey HSD test was applied to compare between‐group REEDA score differences. Post hoc analysis comparing REEDA score reduction on Days 7 and 14 from baseline indicated no statistically significance between the atorvastatin emulgel vs atorvastatin nano‐emulgel groups (p‐value = 0.702, and 0.932, respectively), but a significant reduction in either the atorvastatin emulgel or atorvastatin nano‐emulgel groups was reported on days 7 (p‐value = 0.043 and 0.039, respectively) and 14 compared with the placebo group (p‐value = 0.028 and 0.022, respectively) (Figure 2).
FIGURE 2.
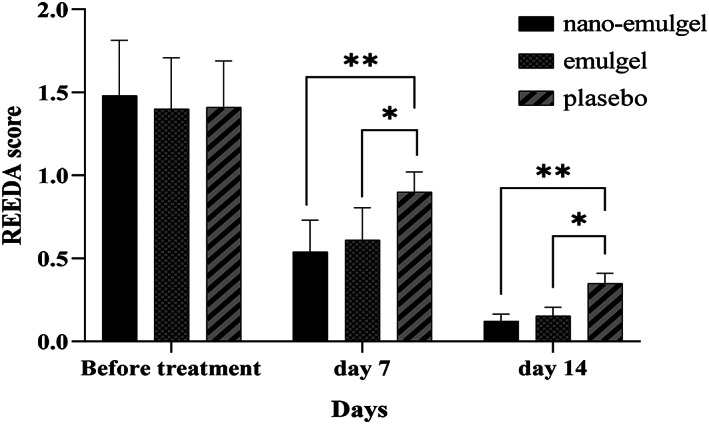
The reduction in the REEDA score during the study period.
Regarding the quality of life of the patients, about 20% improvement in Wound‐QoL was observed after 7 days of each of interventions compared with baseline. Although wound‐QoL showed no improvement between groups from baseline to day 7 (p‐value = 0.132), the difference was significant on day 14 (p‐value = 0.05).
Side effects experienced by patients were tolerable. Mild side effects, including erythema, itching and irritation were reported both with atorvastatin and placebo emulgels. However, none of the participants discontinued the formulation because of the side effects.
4. DISCUSSION
The results of the current randomized controlled clinical trial demonstrated that both topical atorvastatin‐loaded emulgel and nano‐emulgel 1% significantly lead to healing of the laparotomy surgical wounds, pain relief and quality of life compared to placebo.
Patients undergoing surgery are vulnerable and post‐surgical wounds require prompt and particular care, since they easily get infected and are associated with patient’ worth outcomes. 22 Notably, the wound caused by a laparotomy surgical incision is deep and passes through several layers of the skin. Therefore, the management of surgical wounds is of great importance and any measure that is taken to expenditure the healing of these wounds is associated with acceleration of the recovery process of the patients and hospital discharge. 9 , 23 The participants of the present study were selected from the patients undergoing laparotomy surgeries, since these wounds are difficult to heal and their complications affect the mortality and morbidity rate. 9
In this respect, adopting measures such as topical administration of drugs to accelerate wound healing process is a viable option. Incorporation of drugs into the nanostructures has the advantage of controlling the release of the product and consequent increase in drug concentration in the target sites. 24
It has been previously shown that the use of atorvastatin has diminished the pain and accelerated healing in some wounds. Topical atorvastatin ointment 1% resulted in the expenditure of healing of pressure ulcers. 25 Atorvastatin appears to play a role in accelerating wound healing and tissue repair through expressions of proteins and cytokines associated with cell‐growth pathways, such as apoptosis, proliferation, and migration. 26 The anti‐inflammatory properties of atorvastatin are independent of its cholesterol‐lowering effects. 27 This medication can also promote tissue recovery by enhancing the production of growth factors such as vascular endothelial growth factor (VEGF) and fibroblast growth factors (FGFs). 28
Generally, the wound healing process is evaluated by REEDA score. At baseline, patients were not significantly different from each other in terms of this score. However, at the end of the study period, the intervention groups had significantly lower mean scores of redness, oedema, ecchymosis, discharge, approximation, and total REEDA score, which was in favour of wound healing. Previously, topical administration of 1% simvastatin ointment has been associated with similar results. 29 Nevertheless, we did not find a significant difference between the atorvastatin‐loaded emulgel and nano‐emulgel in terms of wound healing. A possible explanation could be due to the presence of polysorbate 80 in the emulsion formulations, which is a non‐ionic surfactant and interferes with skin lipids. This interaction leads to an increase in fluidity of the skin membranes and a subsequent increase in the rate of drug diffusion across the skin layers. 18
As mentioned, in addition to accelerated wound healing, patients in the intervention group also experienced a significantly lower VAS score only within 24 h of drug administration. Alleviation of the inflammation and irritation of the skin in the wound area results in pain reduction, which is another beneficial effect of administration of this medication. 30 The nanoemulsion formulation of simvastatin was shown to reduce the VAS score within 7 days of surgery. 31 It has been demonstrated that atorvastatin emulgel (2%) successfully diminished the post‐operative pain. 19
With the continued use of these formulations for 14 days, the quality of life of the patients who were in the intervention group was eventually superior to the placebo group, which could be demonstrative of the accelerated recovery of this group of patients. Nevertheless, it should be noted that these topical formulations cannot be the sole pain killer. These products are considered as adjuvants, and surgical patients may require other pain relief procedures such as narcotics.
Although the findings of this pilot clinical trial were interesting, care should be taken in interpreting the results. The sample size of the study was small. Although 241 patients were screened for eligibility, due to the COVID‐19 pandemic, we could only include 60 patients in the study. The COVID‐19 pandemic also affected the compliance of the patients to attend the follow‐up visits. In future studies, it is suggested to evaluate the efficacy of different doses of these formulations with extended duration of treatment and follow‐ups in wound healing process.
5. CONCLUSION
The results of the current randomized controlled clinical trial demonstrated that both topical atorvastatin‐loaded emulgel and nano‐emulgel 1% are associated with a significant acceleration in healing of the laparotomy surgical wounds, pain relief and quality of life compared to placebo, without causing intolerable side effects.
FUNDING INFORMATION
The manuscript was financially supported by a grant from the Research and Technology Department of Shahid Sadoughi University of Medical Sciences (grant no. 6668), Yazd, Iran.
CONFLICT OF INTEREST STATEMENT
The authors of present study declare that they have no conflict of interest.
ETHICS STATEMENT
The study was approved by the Research Ethics Committee of Shahid Sadoughi University of Medical Sciences with the registration number of IR.SSU.MEDICINE.REC.1398.196. The obtained data remained confidential, and Informed consent and permission were obtained from participants. All procedures performed in this study involving human participants were in accordance with declaration of Helsinki.
ACKNOWLEDGEMENTS
This article is derived from the thesis ‘Evaluation of therapeutic effects of topical atorvastatin in post‐operative ulcers: A randomized double‐blind placebo‐controlled trial’ supervised by Assistant Professor Dr. Fatemeh Saghafi and submitted by Dr. Adeleh Sahebnasagh to the Faculty of Pharmacy of Shahid Sadoughi University of Medical Sciences, Yazd, Iran, in partial fulfilment of the requirements for the Degree of Pharm‐D of Javad Zarekamali.
Saghafi F, Ramezani V, Jafari‐Nedooshan J, et al. Efficacy of topical atorvastatin‐loaded emulgel and nano‐emulgel 1% on post‐laparotomy pain and wound healing: A randomized double‐blind placebo‐controlled clinical trial. Int Wound J. 2023;20(10):4006‐4014. doi: 10.1111/iwj.14289
DATA AVAILABILITY STATEMENT
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.The raw SPSS file of this study before analysis is available upon your request.
REFERENCES
- 1. Wilkins RG, Unverdorben M. Wound cleaning and wound healing: a concise review. Adv Skin Wound Care. 2013;26(4):160‐163. [DOI] [PubMed] [Google Scholar]
- 2. Davani F, Alishahi M, Sabzi M, Khorram M, Arastehfar A, Zomorodian K. Dual drug delivery of vancomycin and imipenem/cilastatin by coaxial nanofibers for treatment of diabetic foot ulcer infections. Mater Sci Eng C: C. 2021;123:111975. [DOI] [PubMed] [Google Scholar]
- 3. Alishahi M, Khorram M, Asgari Q, et al. Glucantime‐loaded electrospun core‐shell nanofibers composed of poly(ethylene oxide)/gelatin‐poly(vinyl alcohol)/chitosan as dressing for cutaneous leishmaniasis. Int J Biol Macromol. 2020;163:288‐297. [DOI] [PubMed] [Google Scholar]
- 4. Vu LT, Nobuhara KK, Lee H, Farmer DL. Conflicts in wound classification of neonatal operations. J Pediatr Surg. 2009;44(6):1206‐1211. [DOI] [PubMed] [Google Scholar]
- 5. Onyekwelu I, Yakkanti R, Protzer L, Pinkston CM, Tucker C, Seligson D. Surgical wound classification and surgical site infections in the Orthopaedic patient. J Am Acad Orthop Surg Glob Res Rev. 2017;1(3):e022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yao K, Bae L, Yew W. Post‐operative wound management. Aust Fam Physician. 2013;42:867‐870. [PubMed] [Google Scholar]
- 7. Busti AJ, Hooper JS, Amaya CJ, Kazi S. Effects of perioperative antiinflammatory and immunomodulating therapy on surgical wound healing. Pharmacotherapy. 2005;25(11):1566‐1591. [DOI] [PubMed] [Google Scholar]
- 8. Ramneesh G, Sheerin S, Surinder S, Bir S. A prospective study of predictors for post laparotomy abdominal wound dehiscence. J Clin Diagn Res. 2014;8(1):80‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Amarini R, Chien S, Kotwal GJ. Facilitation of wound healing following laparoscopic and conventional abdominal surgery with dressings, patches, antibiotics, etc. Wound Healing‐Current Perspectives. IntechOpen; 2019. [Google Scholar]
- 10. Mooradian AD. Evidence‐based cardiovascular risk management in diabetes. Am J Cardiovasc Drugs. 2019;19(5):439‐448. [DOI] [PubMed] [Google Scholar]
- 11. Khattri S, Zandman‐Goddard G. Statins and autoimmunity. Immunol Res. 2013;56(2–3):348‐357. [DOI] [PubMed] [Google Scholar]
- 12. Pathak NN, Balaganur V, Lingaraju MC, et al. Antihyperalgesic and anti‐inflammatory effects of atorvastatin in chronic constriction injury‐induced neuropathic pain in rats. Inflammation. 2013;36(6):1468‐1478. [DOI] [PubMed] [Google Scholar]
- 13. Lobna A, Soliman MH, Attwa EM, Nana A. Role of atorvastatin in treatment of chronic spontaneous urticaria patients: a controlled clinical trial. Egypt J Immunol. 2018;25(2):133‐139. [PubMed] [Google Scholar]
- 14. Thompson PD, Panza G, Zaleski A, Taylor B. Statin‐associated side effects. J Am Coll Cardiol. 2016;67(20):2395‐2410. [DOI] [PubMed] [Google Scholar]
- 15. Asgari Q, Alishahi M, Davani F, et al. Fabrication of amphotericin B‐loaded electrospun core‐shell nanofibers as a novel dressing for superficial mycoses and cutaneous leishmaniasis. Int J Pharm. 2021;120911:120911. [DOI] [PubMed] [Google Scholar]
- 16. Bibi N, Ahmed N, Khan GM. Nanostructures for drug delivery systems. Nanostruct Drug Deliv: Elsevier. 2017;639‐668. [Google Scholar]
- 17. Choudhury H, Gorain B, Pandey M, et al. Recent update on nanoemulgel as topical drug delivery system. J Pharm Sci. 2017;106(7):1736‐1751. [DOI] [PubMed] [Google Scholar]
- 18. Morsy MA, Abdel‐Latif RG, Nair AB, et al. Preparation and evaluation of atorvastatin‐loaded nanoemulgel on wound‐healing efficacy. Pharmaceutics. 2019;11(11):609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ala S, Alvandipour M, Saeedi M, et al. Effects of topical atorvastatin (2%) on posthemorrhoidectomy pain and wound healing: a randomized double‐blind placebo‐controlled clinical trial. World J Surg. 2017;41(2):596‐602. [DOI] [PubMed] [Google Scholar]
- 20. Brandenburg VM, Sinha S, Torregrosa J‐V, et al. Improvement in wound healing, pain, and quality of life after 12 weeks of SNF472 treatment: a phase 2 open‐label study of patients with calciphylaxis. J Nephrol. 2019;32(5):811‐821. [DOI] [PubMed] [Google Scholar]
- 21. Molazem Z, Mohseni F, Younesi M, Keshavarzi S. Aloe vera gel and cesarean wound healing; a randomized controlled clinical trial. Global J Health Sci. 2015;7(1):203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Judson RT. Management of surgical wounds. Textbook of Surgery, Fourth Edition. Wiley; 2019:45‐48. [Google Scholar]
- 23. Ray M. Management of surgical wounds, wounds healing and burst abdomen. Multidisciplinary Approach to Surgical Oncology Patients. Springer; 2021:181‐189. [Google Scholar]
- 24. Hejazi M, Zareshahrabadi Z, Ashayeri S, et al. Characterization and physical and biological properties of tissue conditioner incorporated with Carum copticum L . Biomed Res Int. 2021;2021:5577760 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Farsaei S, Khalili H, Farboud ES, Karimzadeh I, Beigmohammadi MT. Efficacy of topical atorvastatin for the treatment of pressure ulcers: a randomized clinical trial. Pharmacotherapy. 2014;34(1):19‐27. [DOI] [PubMed] [Google Scholar]
- 26. Suzuki‐Banhesse VF, Azevedo FF, Araujo EP, et al. Effect of atorvastatin on wound healing in rats. Biol Res Nurs. 2015;17(2):159‐168. [DOI] [PubMed] [Google Scholar]
- 27. Bracht L, Caparroz‐Assef SM, Magon TFS, Ritter AMV, Cuman RKN, Bersani‐Amado CA. Topical anti‐inflammatory effect of hypocholesterolaemic drugs. J Pharm Pharmacol. 2011;63(7):971‐975. [DOI] [PubMed] [Google Scholar]
- 28. Bitto A, Minutoli L, Altavilla D, et al. Simvastatin enhances VEGF production and ameliorates impaired wound healing in experimental diabetes. Pharmacol Res. 2008;57(2):159‐169. [DOI] [PubMed] [Google Scholar]
- 29. Adami M, da Silveira Prudente A, Mendes DAGB, da Silva Horinouchi CD, Cabrini DA, Otuki MF. Simvastatin ointment, a new treatment for skin inflammatory conditions. J Dermatol Sci. 2012;66(2):127‐135. [DOI] [PubMed] [Google Scholar]
- 30. Ghaisas MM, Dandawate PR, Zawar SA, Ahire YS, Gandhi SP. Antioxidant, antinociceptive and anti‐inflammatory activities of atorvastatin and rosuvastatin in various experimental models. Inflammopharmacology. 2010;18(4):169‐177. [DOI] [PubMed] [Google Scholar]
- 31. Madi M, Kassem A. Topical simvastatin gel as a novel therapeutic modality for palatal donor site wound healing following free gingival graft procedure. Acta Odontol Scand. 2018;76(3):212‐219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.The raw SPSS file of this study before analysis is available upon your request.


