Abstract
Pleural mesothelioma is an aggressive disease that is enriched for inactivating alterations in tumor suppressor genes. Systemic therapeutic strategies for pleural mesothelioma generally involve chemotherapies and immunotherapies that are chosen without consideration of the tumor's molecular profile. As this generalized approach to treatment rarely yields durable responses, alternative therapeutic regimens are urgently indicated. Preclinical studies have identified synthetic lethal protein and metabolic interactions, recurrently overexpressed proteins, and frequent pathway perturbations that may be therapeutically exploited in mesothelioma. This review discusses the mechanism of action of emerging investigational therapies and summarizes findings from phase I-II clinical trials exploring selective, biomarker-driven therapeutic strategies for mesothelioma, with a focus on five common targets. Finally, using lessons learned from these clinical trials, imperatives for successful implementation of targeted therapy in mesothelioma are discussed.
This timely review discusses therapies targeting mesothelin, MTAP, BAP1, NF2/Hippo pathway, and ASS1 in Meso.
INTRODUCTION
Pleural mesothelioma is an aggressive disease that claims the lives of 30,000 people annually worldwide, with fewer than 15% of patients surviving 5 years.1 Most patients with this disease are treated with systemic therapy because of the extent of tumor involvement at diagnosis and lack of consensus regarding the benefit of cytoreductive surgery.2,3 For two decades, the doublet of platinum and pemetrexed was the cornerstone of systemic therapy for pleural mesothelioma.4 Recently, however, immunotherapy—namely ipilimumab and nivolumab—has challenged the standard, particularly for patients with unfavorable histologic types (eg, sarcomatoid and biphasic) where the immunotherapy doublet is significantly more efficacious than platinum-pemetrexed.5 Despite these advances, progress has been incremental rather than seismic, as nearly 80% of patients treated with immunotherapy will succumb to mesothelioma within 3 years of diagnosis.5 Given the limitations of molecularly agnostic therapies in mesothelioma, there is glaring need for selective regimens.
The molecular landscape of pleural mesothelioma is characterized by a preponderance of inactivated tumor suppressor genes.6-8 This molecular topography has thwarted the repurposing of the oral kinase inhibitors that have proven efficacious for oncogene-driven malignancies.9 However, the encouraging activity of synthetic lethal approaches in other malignancies, such as BRCA-mutant cancers, highlights the potential utility of exploring alternative paths to personalized therapy.10,11 Furthermore, the recent success of selectively targeting cancer-associated proteins using antibody-drug conjugates (ADCs) and early success with chimeric antigen receptor-T (CAR-T) cells and bispecific T-cell engagers in solid tumors have redefined the boundaries of precision medicine,12-14 paving the way for similar strategies in diseases traditionally treated with a chemotherapy-centric lens.
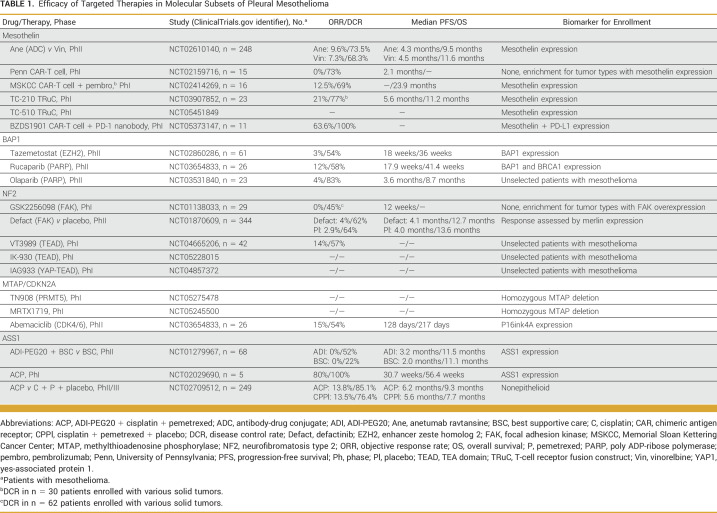
This review will provide updates regarding selective therapeutic approaches to treatment of pleural mesothelioma (Table 1), with a focus on five targets: mesothelin, BRCA1-associated protein 1 (BAP1), neurofibromatosis type 2 (NF2), methylthioadenosine phosphorylase (MTAP), and argininosuccinate synthetase 1 (ASS1). As the emphasis of the review is on efficacy, data pertaining to safety and tolerability are intentionally sparse.
TABLE 1.
Efficacy of Targeted Therapies in Molecular Subsets of Pleural Mesothelioma
MESOTHELIN
Mesothelin is a cell-surface glycoprotein and tumor differentiation antigen that is expressed in low levels on benign mesothelial cells and overexpressed in some solid tumors, including epithelioid mesothelioma.15 Because of the lack of expression of mesothelin in essential tissues, there has been considerable interest in developing mesothelin-directed therapies for mesothelioma. Indeed, mesothelin is perhaps the most intuitive target for this disease. Thus far, the reception of antibody- and antitoxin-based strategies has been tepid. Specifically, the antimesothelin antibody amatuximab did not significantly improve outcomes when combined with platinum-pemetrexed versus platinum-pemetrexed alone, and the toxicity of antimesothelin immunotoxins (SS1P and LMB-100) has been prohibitive.16-18 Furthermore, despite promising phase I results,19 the activity of anetumab ravtansine, an ADC pairing a fully human IgG1 antimesothelin antibody with the maytansinoid tubulin inhibitor DM4, was not superior to vinorelbine (4.3 v 4.5 months) in follow-up studies.20
CAR-T Cells
In recent years, the cache of mesothelin-centered therapies has grown to include CAR-T cells. Initial CAR-T cell development strategies focused on serial infusions of a transiently expressed mRNA-based product in an effort to circumvent on-target, off-tumor toxicities (eg, pleuritis, peritonitis, and pericarditis) that had been described with earlier antimesothelin immunotoxins.17,18,21 In a report summarizing outcomes of two patients who received the mesothelin-targeting mRNA CAR-T cells, one patient experienced a partial response lasting 6 months. The remaining patient had an anaphylactic reaction during the third infusion.22 Notably, pleuritis, peritonitis, and pericarditis were not observed in either of the two patients or among patients with other solid tumors treated on the study.21
The same group subsequently conducted a phase I study (ClinicalTrials.gov identifier: NCT02159716) to evaluate a second-generation lentiviral-based product with stable expression of a mesothelin-redirected CAR.23 Among 15 patients with mesothelin-expressing tumors enrolled in the study, five of whom had pleural mesothelioma, no partial responses were observed. Rather, the best response was stabilization of disease in 11 patients.23 Despite the sustained CAR expression, persistence of the T cells was poor and compounded by sparse infiltration of tumors, suggesting impaired CAR-T cell trafficking.23 To overcome issues with CAR-T cell trafficking to the tumor, regional administration of CAR-T cells has been compared with systemic delivery in preclinical models.24 On the basis of superior results with locally delivered CAR-T cells in laboratory studies, investigators at Memorial Sloan Kettering Cancer Center (MSKCC) launched a single-institution phase I study of intrapleural mesothelin-targeted CAR-T cell therapy in patients with mesothelin-expressing solid tumors.25 The trial largely enrolled patients with pleural mesothelioma. Preliminary results from the trial have been encouraging, with CAR-T cells detected in peripheral blood beyond 100 days for nearly 40% of patients.25 Although bona fide partial responses were rare with intrapleural administration, durable disease control translated to a median overall survival (OS) of approximately 18 months from the date of T-cell infusion.25
T-Cell Receptor Fusion Constructs
T-cell receptor fusion constructs (TRuCs) represent the next innovation in cellular therapies for mesothelioma. TRuCs merge an antibody-based binding domain with the CD3ε subunit of the T-cell receptor complex. In contrast to CAR-T cells, TRuCs leverage all subunits of the T-cell receptor for activation. Specifically, the fusion protein is incorporated into the native T-cell receptor complex, effectively reconfiguring the receptor to recognize tumor antigens in an HLA-independent manner.26 In preclinical models, TRuCs exert more potent antitumor activity and accumulate in tumors to a greater degree than CAR-T cells.26,27 Published data supporting the clinical efficacy of TRuC therapy in mesothelioma are limited but promising. For example, TC-210 (gavocabtagene autoleucel or gavo-cel), a mesothelin-directed TRuC therapy, has been evaluated in mesothelin-expressing solid tumors in an early-phase study (ClinicalTrials.gov identifier: NCT03907852). In a preliminary report of the efficacy in 23 patients with heavily pretreated pleural or peritoneal mesothelioma enrolled in the study, gavo-cel led to objective responses in 21% of patients with a median progression-free survival (PFS) and OS of 5.6 months and 11.2 months, respectively.28
Early clinical experience with mesothelin-directed TRuCs has simultaneously confirmed and negated preclinical assumptions. For example, preclinical studies suggested that cytokine release might be attenuated with TRuCs relative to CAR-T cells.26,27 However, grade ≥3 cytokine release syndrome (CRS) occurred in 15% of patients treated with gavo-cel at the recommended phase II dose, whereas grade 3 CRS was not seen in the MSKCC CAR-T cell study.25,28 Thus, preclinical models may have underestimated clinical toxicities. By contrast, conclusions regarding efficacy of TRuC therapy derived from preclinical studies have been preliminarily substantiated, as the early efficacy of TRuC therapy, namely the ability to induce tumor shrinkage, surpasses what has previously been seen with CAR-T cell therapy. However, given the absence of clinical trials comparing the two approaches and considering the small number of patients treated with the therapies, further data are needed to fully validate this hypothesis. Furthermore, the fact that only a subset of patients has had an objective response when treated with mesothelin-directed CAR-T cells and TRuCs suggests that biomarkers besides mesothelin expression may be needed to predict efficacy. Indeed, an analysis of pretreatment and on-treatment biopsies from five patients who received gavo-cel proposed robust lymphocytic infiltration as a predictor of sensitivity and upregulation of PD-L1 as a deterrent to deep responses.28
Checkpoint Inhibitor Combinations and Next-Generation T-Cell Products
The realization that the immunosuppressive interaction between PD-L1 on tumor cells and PD-1 on CAR-T cells and TRuCs undermines the effector function of T-cell–based therapies has inspired combination regimens featuring checkpoint inhibitors and either mesothelin-targeting CAR-T cells or TRuCs. These studies build upon trials combining other mesothelin-directed therapies (eg, immunotoxins and ADCs) with checkpoint inhibitors.29 In the study conducted by the MSKCC investigators referenced above, 18 patients received pembrolizumab with the intrapleural mesothelin-directed CAR-T cells. Sustained clinical benefit was observed with this strategy, with eight patients experiencing stable disease lasting at least 6 months.25 Among the 18 patients who received concurrent pembrolizumab, median OS from T-cell infusion was 23.9 months.25 The phase II component of the gavo-cel TRuC study (ClinicalTrials.gov identifier: NCT03907852) is adopting a similar strategy whereby gavo-cel will be combined with either nivolumab or both nivolumab and ipilimumab.
To prevent T-cell exhaustion and counteract the immunosuppressive milieu, studies are also exploring next-generation mesothelin-targeting T-cell therapies that engage inhibitory immune checkpoints.30 For example, BZDS1901, an armored mesothelin-targeting CAR-T cell equipped with a secreted PD-1 nanobody, is the focus of a first-in-human study (ClinicalTrials.gov identifier: NCT05373147). In preliminary reports from a cohort of 11 patients with PD-L1 expressing pleural mesothelioma, an objective response rate of 63.6% was observed, including one complete response and a response lasting more than 2 years.31 With respect to TRuCs, a phase I study of TC-510 (ClinicalTrials.gov identifier: NCT05451849), a novel mesothelin-targeting TRuC that co-expresses a PD1xCD28 switch converting PD-L1 to a costimulatory signal, is underway.
BAP1
BAP1 is a tumor suppressor gene located on chromosome 3p21 that encodes a nuclear deubiquitinase enzyme.32 BAP1 is the most commonly altered gene in pleural mesothelioma, with more than 60% of tumors harboring inactivating BAP1 alterations.8,33 Although most BAP1 alterations are somatic, approximately 3%-6% of pleural mesotheliomas arise in the context of a germline BAP1 mutation.34-36 Prognosis of patients with germline BAP1 mutations is favorable compared with all-comers with mesothelioma, with median survival measured in years.35,37 There is controversy surrounding the prognostic implications of somatic BAP1 mutations, as some studies suggest improved prognosis, while others do not support this conclusion.38-40
Enhancer Zeste Homolog 2 Inhibition
BAP1 inactivation increases the expression of the polycomb repressive complex 2 enzyme enhancer zeste homolog 2 (EZH2).41,42 EZH2 facilitates chromatin remodeling through trimethylation of histone H3 lysine 27 (H3K27me3). Silencing EZH2 using short hairpin-RNAs selectively induces apoptosis and reduces tumor burden in BAP1-mutant mesothelioma cell lines and xenografts, with minimal activity in BAP1 wild-type models.42 To follow-up on this preclinical lead, a multicenter, international, single-arm, phase II clinical trial evaluated the efficacy of the EZH2 inhibitor tazemetostat in patients with BAP1-mutant (as determined by immunohistochemistry) pleural mesothelioma who had previously received pemetrexed. Among 61 patients enrolled, the disease control rates (DCRs) at 12 and 24 weeks were 54% and 33%, respectively, including two patients with partial responses.43 Median PFS and OS with tazemetostat were 18 weeks and 36 weeks, respectively. Analysis of longitudinal biopsies from 10 patients demonstrated reduction in H3K27me3 expression in biopsies from eight patients for whom disease control was observed. By contrast, H3K26me3 expression was increased in biopsies from two patients with progression at 12 weeks.43 The evidence of durable disease control despite a modest response rate highlights an opportunity to delve into key molecular determinants of sensitivity to EZH2 inhibition, as BAP1 inactivation appears to be an incomplete predictive biomarker.
Poly ADP-Ribose Polymerase Inhibition
BAP1 engages with BARD1 to form a BRCA1-BARD1-BAP1 complex involved in homologous recombination–mediated repair of double-strand DNA breaks.44 BAP1 loss causes defective homologous recombination repair and impairs the cell's ability to correct double-stranded breaks.45 Poly ADP-ribose polymerase (PARP) enzymes are essential for repair of single-strand DNA breaks. In cells with homologous recombination repair deficiency, PARP inhibition fosters accumulation of single-strand breaks that eventually become double-strand breaks.44 Exploiting this synthetic lethal interaction has proven fruitful and led to regulatory approvals of PARP inhibitors for BRCA-mutated breast, ovarian, prostate, and pancreatic cancer. Although inactivating BRCA1 mutations occur infrequently in pleural mesothelioma, reduced BRCA1 expression is observed in approximately 40% of tumors.46,47 Additionally, depleting BAP1 in mesothelioma cells concordantly reduced BRCA1 expression in laboratory studies, suggesting that there is interplay between the two proteins leading to overlap of BRCA1-deficient and BAP1-deficient mesothelioma.46 However, it is noteworthy that analysis of clinical mesothelioma specimens fails to identify a consistent relationship between reduced BAP1 expression and reduced BRCA1 expression.46,48
A handful of reports have observed antiproliferative effect of PARP inhibitor–based combinations on mesothelioma cell lines harboring BAP1 alterations.49,50 On the basis of this preclinical rationale and efficacy in other tumors with homologous recombination repair defects, two single-center clinical trials have evaluated PARP inhibitors in patients with mesothelioma. The European, single-arm, investigator-initiated, phase IIa MiST1 study assessed rucaparib in 26 patients with BAP1-deficient or BRCA1-deficient pleural or peritoneal mesothelioma who had previously received chemotherapy.48 Notably, 25 (96%) of the enrolled patients had pleural mesothelioma, and BRCA1 and BAP1 status was defined by immunohistochemistry. With rucaparib, disease control was observed in 58% of patients at 12 weeks with ongoing disease control at 24 weeks in 23% of cases.48 Three (12%) partial responses were observed, all of which occurred in patients with tumors harboring BRCA1 loss. Median PFS and OS with rucaparib were 17.9 weeks and 41.4 weeks, respectively.48 Neither BAP1 nor BRCA1 expression correlated with objective response to rucaparib.
The National Cancer Institute group conducted a separate single-center phase II study of olaparib in 23 chemotherapy-pretreated patients with peritoneal or pleural mesothelioma.51 Patients were enrolled irrespective of BAP1 or BRCA1 status, as defined by whole-exome sequencing. With olaparib, 78% of patients experienced stable disease at 6 weeks; median PFS and OS were 3.6 months and 8.7 months, respectively.51 No objective responses to olaparib were seen in the four patients with germline BAP1 mutations. Rather, a single partial response lasting 6.9 months occurred in a patient with germline MRE11A mutation and concurrent somatic BAP1 mutation.51 The efficacy in patients with tumors with somatic BAP1 mutations was comparable to that of patients with BAP1 wildtype tumors (PFS, 3.3 v 3.6 months; P = .28 and OS, 8.8 v 9.6 months; P = .98).51 The outcome of this trial recapitulates preclinical observations that BAP1 inactivation does not correlate with sensitivity to either olaparib or talazoparib.52
Although the small size of the two clinical trials and heterogeneity of the cohorts enrolled may explain inconsistencies across studies, the absence of robust efficacy in either study challenges the utility of BAP1 mutation as a predictive biomarker for PARP inhibitor therapy. Rather, it is possible that other indicators of homologous recombination deficiency, including BRCA1 status and presence of alterations in other genes such as MRE11A, may be more informative.53 It remains to be determined whether PARP1-selective inhibitors (eg, AZD5305) that are associated with less myelosuppression can improve efficacy by allowing for higher exposures.54
NF2
The Hippo pathway is dysregulated in approximately 60% of pleural mesotheliomas.55 Inactivation of the Hippo pathway results from inactivating alterations in the tumor suppressors NF2 and LATS1/2 and overexpression of RASSF genes; these alterations drive activation of the downstream transcriptional cofactors yes-associated protein 1 (YAP1) and transcriptional coactivator with PDZ-binding motif (TAZ), allowing YAP1 and TAZ to bypass cytoplasm sequestration and proteasomal degradation, translocate to the nucleus, and engage with TEA domain (TEAD) transcription factors to promote transcription of cancer-promoting genes (Fig 1).55-57 With more than one third of pleural mesotheliomas harboring loss-of-function mutations, focal 22q deletions, and/or chromosome 22 loss, biallelic inactivation of NF2 is the most common Hippo pathway alteration in mesothelioma.6,7 Alterations in core Hippo pathway genes (including NF2 and LATS1/2 mutations) are more prevalent in nonepithelioid tumors.58 Decreased expression of merlin, the protein encoded by NF2, is associated with poor prognosis in unresectable mesothelioma and also portends early relapse after cytoreductive surgery.59,60
FIG 1.
Hippo pathway signaling and mechanism of action of investigational YAP and TEAD inhibitors. The figure illustrates normal and abnormal Hippo pathway signaling, the latter in the setting of inactivating alterations such as NF2 mutations, and depicts the mechanism of action of IAG933, IK-930, and VT3989. Figure created with BioRender.com. TEAD, TEA domain; YAP1, yes-associated protein 1.
YAP1/TEAD Inhibition
Given the pervasive nature of Hippo pathway perturbation in mesothelioma, there is interest in developing therapies that blunt YAP1 activation. In mesothelioma cell lines and xenografts, knockdown of YAP1 inhibits tumor growth, induces apoptosis, and downregulates expression of YAP1-TEAD target genes.58 Furthermore, introducing an allosteric inhibitor of TEAD palmitoylation, K-975, led to significant tumor regression in two mesothelioma xenograft models, including a model harboring an NF2 deletion.58,61 Findings from a phase I clinical trial of VT3989 (ClinicalTrials.gov identifier: NCT04665206), a first-in-class inhibitor of TEAD palmitoylation, were presented at AACR 2023. Among 43 patients with mesothelioma treated across various dose levels, six (14%) partial responses were observed; the clinical benefit rate at 8 weeks was 57%. Partial responses to VT3989 were seen in patients with NF2-mutant and NF2 wild-type mesothelioma. These findings support the actionability of this pathway in mesothelioma. There are ongoing studies of other inhibitors of YAP1 signaling (Fig 1), including another inhibitor of TEAD palmitoylation (IK-930, ClinicalTrials.gov identifier: NCT05228015) and a YAP1-TEAD protein-protein interface inhibitor (IAG933, ClinicalTrials.gov identifier: NCT04857372).
Focal Adhesion Kinase Inhibition
Focal adhesion kinase (FAK) is a cytoplasmic protein tyrosine kinase that integrates signals from extracellular matrix proteins to influence cell adhesion, invasion, and migration.62 Merlin regulates cell-cell adhesion by maintaining adherens junction integrity.63 Thus, inactivation of NF2 augments the migratory and invasive capacity of mesothelioma.64 This enhanced ability to migrate and invade tissue relies on compensatory cell-extracellular matrix interactions mediated by FAK signaling.63 In a pharmacologic screen, merlin-negative mesothelioma cells demonstrated greater sensitivity to the FAK inhibitor VS-4718.63 These findings were replicated in vivo using mesothelioma xenograft models.63 On the basis of the synthetic lethal interaction, several studies have evaluated FAK inhibitors in mesothelioma.
In a first-in-human study of the FAK inhibitor GSK2256098, median PFS among 29 patients with pleural mesothelioma was 12 weeks.65 A retrospective exploratory analysis suggested that PFS on GSK2256098 was more durable in merlin-negative mesothelioma (23.4 weeks) compared with merlin-positive mesothelioma (11.4 weeks).65 The global phase II COMMAND trial assessed switch maintenance therapy with the FAK inhibitor defactinib (Defact) versus placebo in patients with unresectable pleural mesothelioma who had response to or disease stability on platinum + pemetrexed.60 The median PFS on maintenance Defact was identical to placebo in patients with merlin-low (defined as a H-score of 0-150 by immunohistochemistry, 4.5 months in both groups) and merlin-high tumors (defined as a H-score of 150-300, 2.8 months in both groups).60 Similarly, OS was not improved with Defact (median, 12.7 months v 13.6 months; hazard ratio [HR], 1.0), even among patients with merlin-low tumors (9.0 v 9.5 months).60 Partial response (objective response rate, 4.0% with Defact v 2.9% with placebo) and stable disease (58.4% with Defact v 60.8% with placebo) rates were also comparable.60 Because of these findings, the study was terminated early for futility. Early study closure precluded definitive correlation of merlin expression with Defact efficacy. Given the underwhelming clinical findings and the absence of a convincing predictive biomarker, enthusiasm for FAK inhibitors in mesothelioma has waned.
MTAP AND CDKN2A
Homozygous deletion of the MTAP gene occurs in one third of pleural mesothelioma.6 MTAP deletion is enriched in nonepithelioid mesothelioma where it is detected in approximately half of the cases.6 MTAP deletion is an adverse prognostic feature that is associated with shorter survival.8 Even among patients with the most favorable disease burden, MTAP deletion defines a subgroup with reduced survival after pleurectomy decortication.66
PRMT5 Inhibition
MTAP plays an essential role in the methionine salvage pathway where it catalyzes the metabolism of methylthioadenosine (MTA) to regenerate adenine and methionine. The MTAP-deficient state fosters accumulation of its intermediate substrate MTA, an endogenous inhibitor of the enzymatic activity of protein arginine methyltransferase 5 (PRMT5; Fig 2).67,68 MTA accumulation renders tumors with MTAP loss vulnerable to additional depletion of PRMT5.66,67,69 This unique dependency is being exploited in ongoing first-in-human studies of investigational MTA-cooperative PRMT5 inhibitors, such as TNG908 (ClinicalTrials.gov identifier: NCT05275478) and MRTX1719 (ClinicalTrials.gov identifier: NCT05245500), which are recruiting patients with mesothelioma and other MTAP-deleted tumors (Fig 2). The MTA-cooperative mechanism of action of both drugs is purported to enhance selectivity and minimize toxicity. A recent report confirmed clinical activity of MRTX1719 in mesothelioma.70
FIG 2.
Prevalence and consequences of MTAP deletion in mesothelioma. The bar graphs illustrate the prevalence of MTAP gene loss (or deletion) in epithelioid, nonepithelioid, and all histologies of mesothelioma. The figure on the right demonstrates accumulation of MTA in the setting of MTAP loss leading to endogenous inhibition of PRMT5 and enhancing preclinical sensitivity to further PRMT5 inhibition with the investigational drugs TNG908 and MRTX1719. Figure created with BioRender.com. MTA, methylthioadenosine; MTAP, methylthioadenosine phosphorylase; PRMT5, protein arginine methyltransferase 5.
CDK4/6 Inhibition
Because of the proximity of the genes on chromosome 9p21, >90% of pleural mesotheliomas with MTAP loss also harbor deletion of CDKN2A.6 Deletion of the 9p21 locus results in loss of the tumor suppressor p16ink4a, a CDK4 and CDK6 inhibitor. CDKN2A loss and CDK4/CDK6 overexpression are associated with poor prognosis in pleural mesothelioma.71,72 In preclinical studies, the CDK4/6 inhibitors abemaciclib and palbociclib increase cell senescence and exert antiproliferative activity in mesothelioma lines and xenograft models.71 This therapeutic approach has been evaluated in MiST2 (ClinicalTrials.gov identifier: NCT03654833), a single-arm, phase II trial conducted at two centers in the United Kingdom, through which 26 patients with pleural or peritoneal mesothelioma who had progressed on platinum-based chemotherapy received abemaciclib.73 Enrollment was limited to patients whose mesothelioma was negative for p16ink4A expression by immunohistochemistry.73 With abemaciclib, the DCRs at 12 weeks and 24 weeks were 54% and 23%, meeting the target threshold for efficacy.73 Four (15%) patients experienced partial responses. Median PFS and OS with abemaciclib were 128 days and 217 days, respectively. In a post hoc exploratory analysis, concurrent MTAP loss and p16ink4a loss, as identified in 44% of tumors, correlated with greater regression.73 Findings from this small study substantiate CDK4/6 inhibition as a viable therapeutic strategy for a subset of the mesothelioma that harbor 9p21 deletion.
ASS1
The urea cycle enzyme ASS1 is frequently downregulated in pleural mesothelioma, primarily because of epigenetic inactivation.74 ASS1 loss, which confers poor prognosis and fuels tumorigenesis, is found in 48%-63% of pleural mesothelioma and enriched in the nonepithelioid histologic types.75,76 As ASS1 functions as a rate-limiting enzyme that is crucial for biosynthesis of arginine from citrulline, mesotheliomas lacking ASS-1 are unable to synthesize arginine de novo and, thus, have an auxotrophic dependence on exogenous arginine for survival (Fig 3). Consequently, these tumors are ill-prepared to withstand deprivation of arginine.74,77 The enzyme arginine deiminase (ADI) degrades external arginine to generate citrulline and ammonia. The arginine-depleted state resulting from introduction of a modified form of this enzyme that is pegylated with a 20,000-molecular-weight polyethylene glycol (ADI-PEG 20) suppresses proliferation of ASS1-deficient cancer cells in vivo and in vitro.78
FIG 3.
Arginine synthesis via the urea cycle and extracellular arginine metabolism via ADI. The figure captures the intracellular urea cycle and demonstrates the result of deficiencies in pathway enzymes, specifically ASS1. Extracellular metabolism of arginine to generate citrulline via the ADI enzyme (and the drug ADI-PEG20) is also depicted. Figure created with BioRender.com. ADI, arginine deiminase; ARG, arginase; ASL, argininosuccinate lyase; ASS1, argininosuccinate synthetase 1.
ADI-PEG20
The potential benefits of arginine deprivation via ADI-PEG20 have been evaluated in patients with ASS1-deficent mesothelioma. The multicenter phase II ADAM study randomly assigned 68 patients who had advanced ASS-1 deficient pleural mesothelioma (defined as loss of ASS1 expression in >50% of tumor cells) to receive intramuscular ADI-PEG20 and best supportive care (BSC) versus BSC alone.76 No partial or complete responses were observed with ADI-PEG20. However, 52% of patients treated with ADI-PEG20 who had evaluable disease at 4 months experienced stable disease compared with 22% of patients in the BSC arm. The median PFS with ADI-PEG20 was 3.2 months versus 2.0 months (HR, 0.56; P = .03) in the comparator group. OS was not substantially different in the ADI-PEG20 group compared with BSC (11.5 v 11.1 months; P = .08). Notably, patients with tumors with a high degree of ASS1 loss, as defined by cutoff of 75%, derived the greatest benefit from treatment.76
The ADAM study described above enrolled patients who were chemotherapy-pretreated and chemotherapy-naïve. Interestingly, comparison of longitudinal biopsies obtained from chemotherapy-exposed patients demonstrated a decrease in ASS1 expression in four (44%) of nine patients after chemotherapy.76 Furthermore, in preclinical studies, ADI-PEG20 downregulates the folate-dependent nucleotide synthesis enzyme thymidylate synthase and imparts enhanced sensitivity to combining ADI-PEG20 with the antifolate pemetrexed.79 In support of this finding, the combination of cisplatin + pemetrexed + ADI-PEG20 induced partial responses in seven (78%) of nine patients with pleural mesothelioma or nonsquamous non–small-cell lung cancer treated in the multicenter phase I TRAP study, including four (80%) of five patients with mesothelioma.80 In a larger dose-expansion cohort that included 32 patients with ASS1-deficient pleural mesothelioma,75 the DCR was 93.5% with a partial response rate of 35.5%. Median PFS and OS were 5.6 months and 10.1 months, respectively. Notably, ASS1 levels increased in postprogression biopsies from two (33%) of six patients who underwent longitudinal sampling, consistent with preclinical descriptions of adaptive re-expression of ASS1 as a potential resistance mechanism to ADI-PEG20.75,77 The efficacy of platinum + pemetrexed + ADI-PEG20 has been confirmed in the randomized phase II/III ATOMIC-meso study (ClinicalTrials.gov identifier: NCT02709512), in which the combination prolonged PFS (median, 6.2 v 5.6 months; P = .019) and OS (median, 9.3 v 7.7 months; P = .023) compared with platinum + pemetrexed + placebo in patients with nonepithelioid mesothelioma.81 The poor performance of platinum + pemetrexed in nonepithelioid mesothelioma echoes findings from Checkmate743 where ipilimumab + nivolumab demonstrated clear superiority over platinum + pemetrexed.5 Acknowledging the limitations of cross-trial comparisons, the more impressive outcomes with immunotherapy in the first-line setting and practical considerations (more frequent administration of ADI-PEG20 compared with immunotherapy) suggest that platinum + pemetrexed + ADI-PEG20 may be better suited for the postimmunotherapy setting.
CONCLUSIONS
In conclusion, the pleural mesothelioma therapeutic landscape has been dominated by nonselective approaches. Indeed, treatment stratification has mostly been influenced by tumor histology. This rudimentary approach to personalization of treatment of a heterogeneous disease has not translated into significant survival gains. The inability of standard systemic therapies to significantly arrest the disease is compounded by a biology that makes the screening strategies that have facilitated early detection of other malignancies intangible and impractical for most patients. Thus, given the critical role of systemic therapies for management of mesothelioma and the sluggish pace of progress with molecularly agnostic approaches, there is an urgency for developing more precise strategies for treating this disease.
The review summarized the activity of investigational therapies for five mesothelioma targets. Although the strategies for engaging the targets vary, common lessons have emerged from clinical trials that can inform implementation of precision medicine in pleural mesothelioma (Fig 4). It is apparent that factors beyond the genetic constitution of mesothelioma modulate efficacy of targeted therapies. Thus, integrated biomarkers (eg, gene and protein signatures) may be necessary to refine patient selection.53 Furthermore, it is important to optimize existing protein biomarkers to better define thresholds most predictive of efficacy. Success in this endeavor relies on incorporating correlative analyses and mandatory biopsies into clinical trials. As several targets are enriched in the most aggressive subsets, future confirmatory studies will need designs that permit the assessment of activity in distinct histologic cohorts. Moreover, with a prevailing treatment paradigm that gives primacy to immunotherapy for nonepithelioid tumors, future trials will need to reconsider the comparator arms and the optimal backbone for combination therapies for nonepithelioid tumors.
FIG 4.
Mandates for successful implementation of precision medicine in pleural mesothelioma. The figure lists some necessary best practice steps for future precision medicine approaches in pleural mesothelioma. aBeyond genomic sequencing.
Studies to date have embraced the conservative end point of disease control. Although understandable, given the refractoriness of mesothelioma and the shortcomings of later-line chemotherapies, true progress will be accelerated by frequent sustained dramatic responses. As assessment of response is particularly challenging in mesothelioma because of the dominant rind-like pattern of growth, small size of dispersed pleural nodules, impact of respiratory motion on dimension of nodules, and obscuration of lesions by pleural fluid, investment in automated technologies (eg, artificial intelligence) and alternative response assessment tools (eg, modified RECIST and volumetric methods) is essential to evaluate benefit from treatment and identify meaningful efficacy end points.82,83 Furthermore, given the rarity of dramatic and durable shrinkage with monotherapy with the drugs referenced above, the impending era of precision medicine should investigate combinations that originate from promising preclinical observations and clinical correlative studies. Advancing precision medicine cannot depend on industry-sponsored studies conducted at a few academic centers. Rather, given the overall prevalence of mesothelioma, implementing precision approaches for molecular subsets of this relatively rare disease will require broad adoption of molecular profiling,84 engagement of cooperative groups, partnering with patient groups and foundations, creation of international registries,85 and support from regulatory agencies to enable innovative trial designs (eg, external control arms) that efficiently establish efficacy and expedite clinical access to promising therapies.86
Ibiayi Dagogo-Jack
Honoraria: Foundation Medicine, Aptitude Health, Creative Educational Concepts, OncLive, American Society of Clinical Oncology, DAVA Oncology, Medscape, Total Health, Triptych Health Partners, Research to Practice, American Lung Association
Consulting or Advisory Role: Boehringer Ingelheim, AstraZeneca, Xcovery, Catalyst Pharmaceuticals, Pfizer, Syros Pharmaceuticals, BostonGene, Bayer, Genentech, Sanofi, Janssen, Bristol Myers Squibb Foundation, Novocure
Research Funding: Pfizer (Inst), Array BioPharma (Inst), Novartis (Inst), Genentech (Inst), Calithera Biosciences (Inst), Vivace Therapeutics (Inst), Guardant Health, BostonGene, Tango Therapeutics (Inst)
No other potential conflicts of interest were reported.
SUPPORT
Supported by DF/HCC Lung SPORE Developmental Research Program Sub-Award (1P50CA265826) to I.D.-J.
AUTHOR'S DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by the author of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Ibiayi Dagogo-Jack
Honoraria: Foundation Medicine, Aptitude Health, Creative Educational Concepts, OncLive, American Society of Clinical Oncology, DAVA Oncology, Medscape, Total Health, Triptych Health Partners, Research to Practice, American Lung Association
Consulting or Advisory Role: Boehringer Ingelheim, AstraZeneca, Xcovery, Catalyst Pharmaceuticals, Pfizer, Syros Pharmaceuticals, BostonGene, Bayer, Genentech, Sanofi, Janssen, Bristol Myers Squibb Foundation, Novocure
Research Funding: Pfizer (Inst), Array BioPharma (Inst), Novartis (Inst), Genentech (Inst), Calithera Biosciences (Inst), Vivace Therapeutics (Inst), Guardant Health, BostonGene, Tango Therapeutics (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Zhai Z Ruan J Zheng Y, et al. : Assessment of global trends in the diagnosis of mesothelioma from 1990 to 2017. JAMA Netw Open 4:e2120360, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kindler HL Ismaila N Armato SG, et al. : Treatment of malignant pleural mesothelioma: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 36:1343-1373, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim E Darlison L Edwards J, et al. : Mesothelioma and Radical Surgery 2 (MARS 2): Protocol for a multicentre randomised trial comparing (extended) pleurectomy decortication versus no (extended) pleurectomy decortication for patients with malignant pleural mesothelioma. BMJ Open 10:e038892, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vogelzang NJ Rusthoven JJ Symanowski J, et al. : Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 21:2636-2644, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Peters S Scherpereel A Cornelissen R, et al. : First-line nivolumab plus ipilimumab versus chemotherapy in patients with unresectable malignant pleural mesothelioma: 3-year outcomes from CheckMate 743. Ann Oncol 33:488-499, 2022 [DOI] [PubMed] [Google Scholar]
- 6.Dagogo-Jack I Madison RW Lennerz JK, et al. : Molecular characterization of mesothelioma: Impact of histologic type and site of origin on molecular landscape. JCO Precis Oncol 6:e2100422, 2022 [DOI] [PubMed] [Google Scholar]
- 7.Bueno R Stawiski EW Goldstein LD, et al. : Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat Genet 48:407-416, 2016 [DOI] [PubMed] [Google Scholar]
- 8.Hmeljak J Sanchez-Vega F Hoadley KA, et al. : Integrative molecular characterization of malignant pleural mesothelioma. Cancer Discov 8:1548-1565, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garland LL Rankin C Gandara DR, et al. : Phase II study of erlotinib in patients with malignant pleural mesothelioma: A Southwest Oncology Group Study. J Clin Oncol 25:2406-2413, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Golan T Hammel P Reni M, et al. : Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med 381:317-327, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geyer CE Garber JE Gelber RD, et al. : Overall survival in the OlympiA phase III trial of adjuvant olaparib in patients with germline pathogenic variants in BRCA1/2 and high-risk, early breast cancer. Ann Oncol 33:1250-1268, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qi C Gong J Li J, et al. : Claudin18.2-specific CAR T cells in gastrointestinal cancers: Phase 1 trial interim results. Nat Med 28:1189-1198, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Powles T Rosenberg JE Sonpavde GP, et al. : Enfortumab vedotin in previously treated advanced urothelial carcinoma. N Engl J Med 384:1125-1135, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nathan P Hassel JC Rutkowski P, et al. : Overall survival benefit with tebentafusp in metastatic uveal melanoma. N Engl J Med 385:1196-1206, 2021 [DOI] [PubMed] [Google Scholar]
- 15.Pastan I, Hassan R: Discovery of mesothelin and exploiting it as a target for immunotherapy. Cancer Res 74:2907-2912, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hassan R Kindler HL Jahan T, et al. : Phase II clinical trial of amatuximab, a chimeric antimesothelin antibody with pemetrexed and cisplatin in advanced unresectable pleural mesothelioma. Clin Cancer Res 20:5927-5936, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hassan R Sharon E Thomas A, et al. : Phase 1 study of the antimesothelin immunotoxin SS1P in combination with pemetrexed and cisplatin for front-line therapy of pleural mesothelioma and correlation of tumor response with serum mesothelin, megakaryocyte potentiating factor, and cancer antigen: SS1P plus chemotherapy for mesothelioma. Cancer 120:3311-3319, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hassan R Alewine C Mian I, et al. : Phase 1 study of the immunotoxin LMB-100 in patients with mesothelioma and other solid tumors expressing mesothelin. Cancer 126:4936-4947, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hassan R Blumenschein GR Moore KN, et al. : First-in-human, multicenter, phase I dose-escalation and expansion study of anti-mesothelin antibody-drug conjugate anetumab ravtansine in advanced or metastatic solid tumors. J Clin Oncol 38:1824-1835, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kindler HL Novello S Bearz A, et al. : Anetumab ravtansine versus vinorelbine in patients with relapsed, mesothelin-positive malignant pleural mesothelioma (ARCS-M): A randomised, open-label phase 2 trial. Lancet Oncol 23:540-552, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beatty GL Haas AR Maus MV, et al. : Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol Res 2:112-120, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maus MV Haas AR Beatty GL, et al. : T cells expressing chimeric antigen receptors can cause anaphylaxis in humans. Cancer Immunol Res 1:26-31, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haas AR Tanyi JL O'Hara MH, et al. : Phase I study of lentiviral-transduced chimeric antigen receptor-modified T cells recognizing mesothelin in advanced solid cancers. Mol Ther 27:1919-1929, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adusumilli PS Cherkassky L Villena-Vargas J, et al. : Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci Transl Med 6:261ra151, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adusumilli PS Zauderer MG Rivière I, et al. : A phase I trial of regional mesothelin-targeted CAR T-cell therapy in patients with malignant pleural disease, in combination with the anti-PD-1 agent pembrolizumab. Cancer Discov 11:2748-2763, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baeuerle PA Ding J Patel E, et al. : Synthetic TRuC receptors engaging the complete T cell receptor for potent anti-tumor response. Nat Commun 10:2087, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding J Guyette S Schrand B, et al. : Mesothelin-targeting T cells bearing a novel T cell receptor fusion construct (TRuC) exhibit potent antitumor efficacy against solid tumors. Oncoimmunology 12:2182058, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smalley M Ross M Fowler SF, et al. : Abstract CT197: Profiling of tumor infiltrating T cells in malignant pleural/peritoneal mesothelioma (MPM) and ovarian cancer patients as part of a Phase 1 clinical trial of gavo-cel (TC-210). Cancer Res 83, 2023. (suppl 8; abstr CT197) [Google Scholar]
- 29.Jiang Q Ghafoor A Mian I, et al. : Enhanced efficacy of mesothelin-targeted immunotoxin LMB-100 and anti-PD-1 antibody in patients with mesothelioma and mouse tumor models. Sci Transl Med 12:eaaz7252, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lesch S Nottebrock A Rataj F, et al. : PD-1-CD28 fusion protein strengthens mesothelin-specific TRuC T cells in preclinical solid tumor models. Cell Oncol 46:227-235, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Z Xia Y Li L, et al. : Abstract CT134: Non-viral mesothelin-targeted CAR-T cells armored with IFNg-induced secretion of PD-1 nanobody in treatment of malignant mesothelioma in phase I clinical trial. Cancer Res 83, 2023. (suppl 8; abstr CT134) [Google Scholar]
- 32.Carbone M Harbour JW Brugarolas J, et al. : Biological mechanisms and clinical significance of BAP1 mutations in human cancer. Cancer Discov 10:1103-1120, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nasu M Emi M Pastorino S, et al. : High Incidence of Somatic BAP1 alterations in sporadic malignant mesothelioma. J Thorac Oncol 10:565-576, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Panou V Gadiraju M Wolin A, et al. : Frequency of germline mutations in cancer susceptibility genes in malignant mesothelioma. J Clin Oncol 36:2863-2871, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hassan R Morrow B Thomas A, et al. : Inherited predisposition to malignant mesothelioma and overall survival following platinum chemotherapy. Proc Natl Acad Sci U S A 116:9008-9013, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo R DuBoff M Jayakumaran G, et al. : Novel germline mutations in DNA damage repair in patients with malignant pleural mesotheliomas. J Thorac Oncol 15:655-660, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baumann F Flores E Napolitano A, et al. : Mesothelioma patients with germline BAP1 mutations have 7-fold improved long-term survival. Carcinogenesis 36:76-81, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luchini C Veronese N Yachida S, et al. : Different prognostic roles of tumor suppressor gene BAP1 in cancer: A systematic review with meta-analysis. Genes Chromosomes Cancer 55:741-749, 2016 [DOI] [PubMed] [Google Scholar]
- 39.Forest F Patoir A Dal Col P, et al. : Nuclear grading, BAP1, mesothelin and PD-L1 expression in malignant pleural mesothelioma: Prognostic implications. Pathology 50:635-641, 2018 [DOI] [PubMed] [Google Scholar]
- 40.Louw A Panou V Szejniuk WM, et al. : BAP1 loss by immunohistochemistry predicts improved survival to first-line platinum and pemetrexed chemotherapy for patients with pleural mesothelioma: A validation study. J Thorac Oncol 17:921-930, 2022 [DOI] [PubMed] [Google Scholar]
- 41.Kuznetsov JN Aguero TH Owens DA, et al. : BAP1 regulates epigenetic switch from pluripotency to differentiation in developmental lineages giving rise to BAP1-mutant cancers. Sci Adv 5:eaax1738, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.LaFave LM Béguelin W Koche R, et al. : Loss of BAP1 function leads to EZH2-dependent transformation. Nat Med 21:1344-1349, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zauderer MG Szlosarek PW Le Moulec S, et al. : EZH2 inhibitor tazemetostat in patients with relapsed or refractory, BAP1-inactivated malignant pleural mesothelioma: A multicentre, open-label, phase 2 study. Lancet Oncol 23:758-767, 2022 [DOI] [PubMed] [Google Scholar]
- 44.Nishikawa H Wu W Koike A, et al. : BRCA1-associated protein 1 interferes with BRCA1/BARD1 RING heterodimer activity. Cancer Res 69:111-119, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Yu H Pak H Hammond-Martel I, et al. : Tumor suppressor and deubiquitinase BAP1 promotes DNA double-strand break repair. Proc Natl Acad Sci U S A 111:285-290, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh A Busacca S Gaba A, et al. : BAP1 loss induces mitotic defects in mesothelioma cells through BRCA1-dependent and independent mechanisms. Oncogene 42:572-585, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Busacca S Sheaff M Arthur K, et al. : BRCA1 is an essential mediator of vinorelbine-induced apoptosis in mesothelioma. J Pathol 227:200-208, 2012 [DOI] [PubMed] [Google Scholar]
- 48.Fennell DA King A Mohammed S, et al. : Rucaparib in patients with BAP1-deficient or BRCA1-deficient mesothelioma (MiST1): An open-label, single-arm, phase 2a clinical trial. Lancet Respir Med 9:593-600, 2021 [DOI] [PubMed] [Google Scholar]
- 49.Parrotta R Okonska A Ronner M, et al. : A novel BRCA1-associated protein-1 isoform affects response of mesothelioma cells to drugs impairing BRCA1-mediated DNA repair. J Thorac Oncol 12:1309-1319, 2017 [DOI] [PubMed] [Google Scholar]
- 50.Borchert S Wessolly M Schmeller J, et al. : Gene expression profiling of homologous recombination repair pathway indicates susceptibility for olaparib treatment in malignant pleural mesothelioma in vitro. BMC Cancer 19:108, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ghafoor A Mian I Wagner C, et al. : Phase 2 study of olaparib in malignant mesothelioma and correlation of efficacy with germline or somatic mutations in BAP1 gene. JTO Clin Res Rep 2:100231, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rathkey D Khanal M Murai J, et al. : Sensitivity of mesothelioma cells to PARP inhibitors is not dependent on BAP1 but is enhanced by temozolomide in cells with high-schlafen 11 and low-O6-methylguanine-DNA methyltransferase expression. J Thorac Oncol 15:843-859, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mangiante L Alcala N Sexton-Oates A, et al. : Multiomic analysis of malignant pleural mesothelioma identifies molecular axes and specialized tumor profiles driving intertumor heterogeneity. Nat Genet 55:607-618, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yap T Im SA Schram AM, et al. : Abstract CT007: PETRA: First in class, first in human trial of the next generation PARP1-selective inhibitor AZD5305 in patients (pts) with BRCA1/2, PALB2 or RAD51C/D mutations. Cancer Res 82, 2022. (suppl 12; abstr CT007) [Google Scholar]
- 55.Nastase A Mandal A Lu SK, et al. : Integrated genomics point to immune vulnerabilities in pleural mesothelioma. Sci Rep 11:19138, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tranchant R Quetel L Tallet A, et al. : Co-occurring mutations of tumor suppressor genes, LATS2 and NF2, in malignant pleural mesothelioma. Clin Cancer Res 23:3191-3202, 2017 [DOI] [PubMed] [Google Scholar]
- 57.Totaro A, Panciera T, Piccolo S: YAP/TAZ upstream signals and downstream responses. Nat Cell Biol 20:888-899, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Calvet L Dos-Santos O Spanakis E, et al. : YAP1 is essential for malignant mesothelioma tumor maintenance. BMC Cancer 22:639, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meerang M Bérard K Friess M, et al. : Low Merlin expression and high Survivin labeling index are indicators for poor prognosis in patients with malignant pleural mesothelioma. Mol Oncol 10:1255-1265, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fennell DA Baas P Taylor P, et al. : Maintenance defactinib versus placebo after first-line chemotherapy in patients with merlin-stratified pleural mesothelioma: COMMAND-A double-blind, randomized, phase II study. J Clin Oncol 37:790-798, 2019 [DOI] [PubMed] [Google Scholar]
- 61.Kaneda A Seike T Danjo T, et al. : The novel potent TEAD inhibitor, K-975, inhibits YAP1/TAZ-TEAD protein-protein interactions and exerts an anti-tumor effect on malignant pleural mesothelioma. Am J Cancer Res 10:4399-4415, 2020 [PMC free article] [PubMed] [Google Scholar]
- 62.Mitra SK, Hanson DA, Schlaepfer DD: Focal adhesion kinase: In command and control of cell motility. Nat Rev Mol Cell Biol 6:56-68, 2005 [DOI] [PubMed] [Google Scholar]
- 63.Shapiro IM Kolev VN Vidal CM, et al. : Merlin deficiency predicts FAK inhibitor sensitivity: A synthetic lethal relationship. Sci Transl Med 6:237ra68, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Poulikakos PI Xiao GH Gallagher R, et al. : Re-expression of the tumor suppressor NF2/merlin inhibits invasiveness in mesothelioma cells and negatively regulates FAK. Oncogene 25:5960-5968, 2006 [DOI] [PubMed] [Google Scholar]
- 65.Soria JC Gan HK Blagden SP, et al. : A phase I, pharmacokinetic and pharmacodynamic study of GSK2256098, a focal adhesion kinase inhibitor, in patients with advanced solid tumors. Ann Oncol 27:2268-2274, 2016 [DOI] [PubMed] [Google Scholar]
- 66.Busacca S Zhang Q Sharkey A, et al. : Transcriptional perturbation of protein arginine methyltransferase-5 exhibits MTAP-selective oncosuppression. Sci Rep 11:7434, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kryukov GV Wilson FH Ruth JR, et al. : MTAP deletion confers enhanced dependency on the PRMT5 arginine methyltransferase in cancer cells. Science 351:1214-1218, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guccione E, Richard S: The regulation, functions and clinical relevance of arginine methylation. Nat Rev Mol Cell Biol 20:642-657, 2019 [DOI] [PubMed] [Google Scholar]
- 69.Mavrakis KJ McDonald ER Schlabach MR, et al. : Disordered methionine metabolism in MTAP/CDKN2A-deleted cancers leads to dependence on PRMT5. Science 351:1208-1213, 2016 [DOI] [PubMed] [Google Scholar]
- 70.Engstrom LD Aranda R Waters L, et al. : MRTX1719 is an MTA-cooperative PRMT5 inhibitor that exhibits synthetic lethality in preclinical models and patients with MTAP deleted cancer. Cancer Discov 10.1158/2159-8290.CD-23-0669 [epub ahead of print on August 8, 2023] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aliagas E Alay A Martínez-Iniesta M, et al. : Efficacy of CDK4/6 inhibitors in preclinical models of malignant pleural mesothelioma. Br J Cancer 125:1365-1376, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.López-Ríos F Chuai S Flores R, et al. : Global gene expression profiling of pleural mesotheliomas: Overexpression of aurora kinases and P16/CDKN2A deletion as prognostic factors and critical evaluation of microarray-based prognostic prediction. Cancer Res 66:2970-2979, 2006 [DOI] [PubMed] [Google Scholar]
- 73.Fennell DA King A Mohammed S, et al. : Abemaciclib in patients with p16ink4A-deficient mesothelioma (MiST2): A single-arm, open-label, phase 2 trial. Lancet Oncol 23:374-381, 2022 [DOI] [PubMed] [Google Scholar]
- 74.Szlosarek PW Klabatsa A Pallaska A, et al. : In vivo loss of expression of argininosuccinate synthetase in malignant pleural mesothelioma is a biomarker for susceptibility to arginine depletion. Clin Cancer Res 12:7126-7131, 2006 [DOI] [PubMed] [Google Scholar]
- 75.Szlosarek PW Phillips MM Pavlyk I, et al. : Expansion phase 1 study of pegargiminase plus pemetrexed and cisplatin in patients with argininosuccinate synthetase 1-deficient mesothelioma: Safety, efficacy, and resistance mechanisms. JTO Clin Res Rep 1:100093, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Szlosarek PW Steele JP Nolan L, et al. : Arginine deprivation with pegylated arginine deiminase in patients with argininosuccinate synthetase 1-deficient malignant pleural mesothelioma: A randomized clinical trial. JAMA Oncol 3:58-66, 2017 [DOI] [PubMed] [Google Scholar]
- 77.Locke M Ghazaly E Freitas MO, et al. : Inhibition of the polyamine synthesis pathway is synthetically lethal with loss of argininosuccinate synthase 1. Cell Rep 16:1604-1613, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ensor CM Holtsberg FW Bomalaski JS, et al. : Pegylated arginine deiminase (ADI-SS PEG20,000 mw) inhibits human melanomas and hepatocellular carcinomas in vitro and in vivo. Cancer Res 62:5443-5450, 2002 [PubMed] [Google Scholar]
- 79.Allen MD Luong P Hudson C, et al. : Prognostic and therapeutic impact of argininosuccinate synthetase 1 control in bladder cancer as monitored longitudinally by PET imaging. Cancer Res 74:896-907, 2014 [DOI] [PubMed] [Google Scholar]
- 80.Beddowes E Spicer J Chan PY, et al. : Phase 1 dose-escalation study of pegylated arginine deiminase, cisplatin, and pemetrexed in patients with argininosuccinate synthetase 1-deficient thoracic cancers. J Clin Oncol 35:1778-1785, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Szlosarek PW Creelan B Sarkodie T, et al. : Abstract CT007: Phase 2-3 trial of pegargiminase plus chemotherapy versus placebo plus chemotherapy in patients with non-epithelioid pleural mesothelioma. Cancer Res 83, 2023. (suppl 8; abstr CT007) [Google Scholar]
- 82.Armato SG, Nowak AK: Revised modified response evaluation criteria in solid tumors for assessment of response in malignant pleural mesothelioma (version 1.1). J Thorac Oncol 13:1012-1021, 2018 [DOI] [PubMed] [Google Scholar]
- 83.Katz SI Straus CM Roshkovan L, et al. : Considerations for imaging of malignant pleural mesothelioma: A consensus statement from the International Mesothelioma Interest Group. J Thorac Oncol 18:278-298, 2023 [DOI] [PubMed] [Google Scholar]
- 84.Flores-Toro JA Jagu S Armstrong GT, et al. : The childhood cancer data initiative: Using the power of data to learn from and improve outcomes for every child and young adult with pediatric cancer. J Clin Oncol 41:4045-4053, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.French CA Cheng ML Hanna GJ, et al. : Report of the first international symposium on NUT carcinoma. Clin Cancer Res 28:2493-2505, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mishra-Kalyani PS Amiri Kordestani L Rivera DR, et al. : External control arms in oncology: Current use and future directions. Ann Oncol 33:376-383, 2022 [DOI] [PubMed] [Google Scholar]