Abstract
Objective:
Patients with mental health disorders are at risk for receiving inequitable cancer treatment, likely resulting from various structural, social, and health-related factors. This study aims to assess the relationship between mental health disorders and the use of definitive treatment in a population-based cohort of those with localized, clinically significant prostate cancer.
Methods:
We conducted a cohort study analysis in SEER-Medicare (2004–2015). History of a mental health disorder was defined as presence of specific ICD-9 or ICD-10 diagnostic codes in the 2 years preceding cancer diagnosis. Descriptive statistics were performed using Wilcoxon-rank sum and chi-square testing. Multivariable logistic regression was used to evaluate the relationship between mental health disorders and definitive treatment utilization (defined as surgery or radiation).
Results:
Of 101,042 individuals with prostate cancer, 7,945 (7.8%) had a diagnosis of a mental health disorder. They were more likely to be unpartnered, have a lower socioeconomic status (SES), and were less likely to receive definitive treatment (61.8% vs 68.2%, respectively, p<0.001). Definitive treatment rates were >66%, 62.8%, 60.3%, 58.2%, 54.3%, and 48.1% for PTSD, depressive disorder, bipolar disorder, anxiety disorder, substance abuse disorder and schizophrenia, respectively. After adjusting for age, race and ethnicity, marital status and SES, history of a mental health disorder was associated with decreased odds of receiving definitive treatment (OR 0.74, 95% CI 0.66–0.83).
Conclusions:
Individuals with mental health disorders and prostate cancer represent a vulnerable population; careful attention to clinical and social needs is required to support appropriate use of beneficial treatments.
Keywords: prostate cancer, racial disparities, health services, mental health
INTRODUCTION
Mental health disorders are common, associated with bias and stigma, and may contribute to the inequitable receipt of health services.1 It is estimated that approximately half of US adults would meet the criteria for a DSM-IV disorder at some point in their lifetime.2 Severe mental health disorders are associated with adverse social factors such as houselessness, unemployment, and lack of social support.3 The interaction between mental health and social determinants of health (i.e. economic stability, neighborhood/physical environment, education, food sources, community/social support, and healthcare systems) exacerbates health inequity, especially among individuals with severe mental health disorders.4–6 Studies have demonstrated an increased risk of death from any cancer among patients with mental health disorders when compared with the general population, despite a similar incidence of cancer between the two populations.7–9 Previous work evaluating the relationship between mental health disorders and prostate cancer outcomes have focused on the mental health of patients post cancer diagnosis, with studies demonstrating the increased burden of mental health disorders in individuals following a prostate cancer diagnosis.10–11 Despite being the most common non-skin cancer among men, there is limited data currently on the associations between mental health, social factors (i.e., race), and prostate cancer-related treatment and outcomes.12 We hypothesized that those with a history of a mental health disorder were less likely to receive definitive therapies for localized prostate cancer when compared with those without a history of a mental health disorder. This study aims (1) to evaluate the relationship between mental health disorders and types of prostate cancer treatment received and (2) to determine the association between definitive treatment of prostate cancer and mental health disorders in a cohort of individuals with localized prostate cancer.
METHODS
This is a retrospective, observational cohort study using data from the Surveillance Epidemiology, End Result cancer registry linked to Medicare claims data (SEER-Medicare) of patients diagnosed with localized prostate cancer between 2004 and 2015. The SEER-Medicare database links patient demographics and cancer-related variables from 13 state cancer registries with patient health utilization claims. The Medicare program provides medical insurance for more than 95% of US adults aged ≥ 65 years. As more than 99% of Medicare beneficiaries subscribe to Medicare Part A benefits and 95% subscribe to Medicare Part B benefits, the Medicare data capture most health care services delivered to Medicare beneficiaries, including inpatient hospitalizations, outpatient hospital services, physician services, durable medical equipment, home care, and hospice care. 13 To facilitate the identification of individuals with a diagnosis of one or more of our pre-specified mental health disorders, patients had to have Medicare claims coverage data with no managed care coverage in the 24 months prior to diagnosis. This study was deemed exempt by the Institutional Review Board at the University of Washington.
We limited this cohort to individuals with localized, clinically-significant prostate cancer. This was defined as Grade Group 2–5, cT1–4, cN0/NX, and cM0/MX disease at diagnosis. We excluded those with PSA levels of > 50 ng/mL. Demographic data obtained from the SEER-Medicare database included age, partner status, race and ethnicity, and census tract-level socioeconomic status. The National Cancer Institute (NCI) comorbidity index was calculated using Medicare claims for the year period prior to diagnosis14 Pre-diagnosis PSA testing was determined from 2 years to 3 months prior to diagnosis. The 3-month period prior to diagnosis was excluded to omit diagnostic PSA.
The primary exposure for this analysis was a history of a mental health disorder in the two years preceding cancer diagnosis. Using Medicare claims data, patients with mental health disorders were identified using the International Classification of Disease, Ninth and Tenth Revision codes (ICD-9 and ICD-10). First, we identified the most commonly coded primary diagnoses: depressive disorders, bipolar disorder, schizophrenia, and substance abuse disorders. We also added anxiety-related disorders, which were the most common secondary diagnosis for mental health disorders. Lastly, we included post-traumatic stress disorder (PTSD), which is less common, yet can contribute significantly to functional impairment.15
The primary outcome of this analysis was definitive treatment, which was defined as radical prostatectomy or radiation therapy with or without androgen deprivation therapy (ADT). The first primary treatment from the time of diagnosis was used to classify definitive treatment groups as: radical prostatectomy, radiation therapy alone, and radiation therapy with ADT. Radiation therapy with ADT was defined as the initiation of both radiation therapy and ADT initiation within 90 days of each other. Primary ADT and no treatment (i.e., observation) were coded as non-definitive treatments.
Statistical Analysis
Baseline demographic and clinical data were assessed using descriptive statistics. Continuous variables were compared using non-parametric testing with Wilcoxon rank-sum test, and categorical variables were compared using chi-square tests. Multivariable logistic regression analyses were performed to assess the association of various clinical and demographic variables with the (1) receipt of any definitive treatment and (2) the receipt of radical prostatectomy. As a potential marker of disease severity, we identified cases for which a mental health diagnosis was reported in both out and inpatient claims. In the final model we adjusted for the clinical characteristics. This history of inpatient admission with a concurrent mental health diagnosis was included in the multivariable variable risk models. The models were constructed by the sequential addition of variables, which were selected a priori based on their known association with treatment utilization. Variables were kept in the final model (Table 2) if they demonstrated a greater than 20% effect on the model, or if they were considered to be clinically relevant. All statistical tests were two-sided with significance defined as p < 0.05. Statistical analyses were performed using SAS 9.4 (SAS, Cary, NC).
Table 2:
In patients with prostate cancer, adjusted OR of receiving definitive treatment and, of those receiving definitive treatment, adjusted OR of receiving radical prostatectomy
| Odds of Receiving Definitive Treatment | Of those Receiving Definitive Treatment, Odds of Receiving Radical Prostatectomy | |
|---|---|---|
|
| ||
| OR (95% CI) | OR (95% CI) | |
|
| ||
| Mental Health Disorder | ||
| No | ref | ref |
| Yes | 0.74 (0.66–0.83) | 0.81 (0.7–0.95) |
| Admission for a Mental Health Disorder | ||
| No | ref | ref |
| Yes | 0.67 (0.6–0.75) | 1.03 (0.88–1.21) |
| Age | 0.88 (0.88–0.88) | 0.82 (0.81–0.82) |
| Married | ||
| Yes | ref | ref |
| No | 0.75 (0.72–0.77) | 0.73 (0.7–0.77) |
| Urban | ||
| No | ref | ref |
| Yes | 0.88 (0.85–0.91) | 1.02 (0.97–1.06) |
| Race/Ethnicity | ||
| White | ref | ref |
| Black | 0.74 (0.71–0.78) | 0.57 (0.54–0.62) |
| Latino | 0.89 (0.83–0.95) | 1.25 (1.16–1.36) |
| Other | 0.89 (0.84–0.95) | 0.99 (0.91–1.07) |
| SES | ||
| 0%-<5% poverty | ref | ref |
| 5%-<10% poverty | 0.91 (0.87–0.95) | 1.03 (0.98–1.08) |
| 10%-<20% poverty | 0.77 (0.73–0.8) | 0.92 (0.88–0.97) |
| 20%-100% poverty | 0.73 (0.7–0.77) | 0.86 (0.81–0.92) |
| National Cancer Institute Index | ||
| 0 | ref | ref |
| <2 | 0.97 (0.93–1) | 0.7 (0.67–0.73) |
| 2+ | 0.73 (0.7–0.76) | 0.48 (0.45–0.51) |
| Screened | ||
| No | ref | ref |
| Yes | 2.29 (2.21–2.38) | 1.19 (1.14–1.25) |
| Year of Diagnosis of Prostate Cancer | ||
| 2004–2006 | ref | ref |
| 2007–2009 | 1.31 (1.26–1.37) | 1.15 (1.09–1.21) |
| 2010–2012 | 1.61 (1.54–1.67) | 1.11 (1.06–1.17) |
| 2013–2015 | 1.7 (1.62–1.77) | 1.28 (1.21–1.35) |
| T-stage | ||
| T1 | ref | ref |
| T2 | 1.04 (1–1.07) | 1.11 (1.07–1.15) |
| T3 | 1.29 (1.18–1.41) | 1.18 (1.07–1.3) |
| T4 | 0.5 (0.41–0.61) | 0.7 (0.47–1.04) |
| Gleason Grade | ||
| 7 | ref | ref |
| 8–10 | 0.94 (0.91–0.97) | 0.96 (0.92–1) |
| PSA | ||
| <10 | ref | ref |
| 10.1–20.0 | 0.81 (0.78–0.84) | 0.77 (0.73–0.81) |
| 20.1–50.0 | 0.55 (0.52–0.58) | 0.51 (0.47–0.56) |
| Unknown | 0.38 (0.37–0.4) | 2.07 (1.95–2.2) |
RESULTS
Baseline clinical and socio-demographic data are presented in Table 1. In total, 101,042 individuals met our inclusion criteria for localized, clinically significant prostate cancer. Of those, 7,945 (7.8%) had a mental health diagnosis prior to the cancer diagnosis. The most common mental health diagnosis was depression (86.2%) followed by bipolar disorder (9.3%). The least common mental health diagnosis was PTSD (0.22%). This subgroup was repressed from comparative analyses (due to their small sample size) to comply with SEER patient confidentiality rules of reporting specific data in groups over 11. The mean patient age was 74 years. The cohort was predominantly married/partnered (66.5%), non-Hispanic White (78.0%), and lived in urban settings (73.4%). The majority of the cohort (71.6%) had at least 1 claim for a PSA test prior to their prostate cancer diagnosis. The majority of the cohort (59.31%) was healthy based on having an NCI comorbidity index score of 0. The majority of the cohort had clinical stage T1 (53.8%), Gleason score 7 (68.0%) and had a PSA ranging from 0.1–10.0 (58.7%).
Table 1:
Baseline clinical and demographics of patients with grade group 2–5, N0, M0 prostate cancer in SEER-Medicare from 2004–2015
| No mental health disorder (N=93097) | Mental health disorder* (N=7945) | Schizophrenia (N=337) | Bipolar (N=740) | Anxiety (N=376) | PTSD (N=18) | Substance Abuse (N=898) | Depression (N=6846) | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Age, Mean (STD) | 74.12 (5.96) | 74.44 (6.21) | 72.52 (5.45) |
73.14 (5.65) | 74.13 (6.27) | 69.56 (4.66) | 73.88 (6.13) | 74.57 (6.23) |
| Year of Diagnosis of Prostate Cancer | ||||||||
| 2004–2006 | 28.26% | 23.49% | 25.82% | 22.30% | 12.77% | 0% | 25.72% | 22.98% |
| 2007–2009 | 27.91% | 25.21% | 29.08% | 23.51% | 15.69% | 11.11% | 27.06% | 25.24% |
| 2010–2012 | 23.73% | 24.77% | 23.15% | 24.59% | 22.87% | 27.78% | 22.38% | 25.04% |
| 2013–2015 | 20.10% | 26.53% | 21.96% | 29.59% | 48.67% | 61.11% | 24.83% | 26.75% |
| T-Stage | ||||||||
| T1 | 53.8% | 54.4% | 56.7% | 55.0% | 58.2% | >60% | 53.7% | 54.5% |
| T2 | 41.6% | 40.6% | 40.1% | 40.4% | 36.4% | <40% | 41.1% | 40.5% |
| T3 | 3.5% | 3.2% | <5% | 2.7 | <5% | 0% | 3.2% | 3.2% |
| T4 | 0.5% | 0.9% | <5% | <2% | <5% | 0% | <2% | 0.9% |
| unknown | 0.7% | 0.9% | <5% | <2% | <5% | 0% | <2% | 0.9% |
| Gleason Grade | ||||||||
| 7 | 68.2% | 65.5% | 67.7% | 64.7% | 63.8% | >60% | 62.9% | 65.6% |
| 8–10 | 31.8% | 34.5% | 32.3% | 35.3% | 36.2% | <40% | 37.1% | 34.4% |
| PSA | ||||||||
| 0.1–10.0 | 59.1% | 54.0% | 46.9% | 53.7% | 49.2% | >70% | 43.4% | 54.8% |
| 10.1–20.0 | 17.9% | 18.0% | 19.9% | 18.8% | 20.7% | <25% | 19.8% | 17.7% |
| 20.1–50.0 | 8.1% | 9.5% | 12.5% | 9.5% | 8.8% | . | 15.5% | 9.1% |
| unknown | 14.9% | 18.6% | 20.8% | 18.1% | 21.3% | <25% | 21.3% | 18.4% |
| Married | ||||||||
| Yes | 67.2% | 57.7% | 24.3% | 55.5% | 52.4% | 22.2% | 46.8% | 58.8% |
| No | 19.1% | 28.0% | 59.9% | 30.1% | 32.5% | 61.1% | 38.4% | 26.9% |
| Unknown | 13.7% | 14.3% | 15.7% | 14.3% | 15.2% | 16.7% | 14.8% | 14.3% |
| Race and Ethnicity | ||||||||
| White | 77.71% | 81.17% | 59.64% | 83.65% | 81.91% | 66.67% | 74.39% | 82.33% |
| Latino | 5.37% | 5.37% | 4.15% | 4.59% | 5.05% | 11.11% | 6.68% | 5.32% |
| Black | 10.60% | 9.48% | 31.45% | 8.51% | 6.12% | 5.56% | 15.81% | 8.31% |
| Other | 6.32% | 3.98% | 4.75% | 3.24% | 6.91% | 16.67% | 3.12% | 4.05% |
| Urban | ||||||||
| Yes | 73.52% | 71.81% | 78.64% | 73.65% | 59.84% | 44.44% | 72.38% | 71.75% |
| Socioeconomic status (SES) | ||||||||
| 0%-<5% poverty | 26.75% | 23.41% | 14.54% | 23.78% | 17.82% | 5.56% | 20.71% | 23.72% |
| 5%-<10% poverty | 27.73% | 26.21% | 17.21% | 23.92% | 22.61% | 27.78% | 25.39% | 26.54% |
| 10%-<20% poverty | 27.24% | 28.24% | 26.71% | 28.24% | 34.04% | 33.33% | 27.06% | 28.43% |
| 20%-100% poverty | 17.48% | 21.35% | 39.76% | 23.38% | 23.67% | 27.78% | 26.28% | 20.48% |
| NCI Index | ||||||||
| 0 | 60.86% | 41.21% | 31.16% | 41.22% | 41.22% | 50% | 35.30% | 41.15% |
| <2 | 25.57% | 27.84% | 24.33% | 25.81% | 29.26% | 27.78% | 23.83% | 27.99% |
| 2+ | 13.57% | 30.95% | 44.51% | 32.97% | 29.52% | 22.22% | 40.87% | 30.86% |
| PSA performed in 2 years prior to diagnostic PSA | ||||||||
| Yes | 77.64% | 82.52% | 74.48% | 82.43% | 85.37% | 66.67% | 77.84% | 83.17% |
Socioeconomic status (SES) - % of individuals in census tract data below or at poverty level
National Cancer Institute Index - Comorbidity Index
Univaraite comparison of all variables by MH status (no versus any) show significant differences at p<0.0001.
Individuals with a diagnosis of any mental health disorder were more likely to be unpartnered, to reside in a census tract with a higher proportion of individuals in poverty, and to have severe comorbidities (p<0.001, see Table 1) compared with those without mental health disorder diagnoses. Those with schizophrenia were significantly more likely to be unpartnered, Black, reside in an urban census tract with a higher proportion of poverty, and have a severe comorbidity (p<0.001, see Table 1).
Within the entire cohort, 90.3% received any type of prostate cancer treatment and 67.7% received definitive treatment. Median time to first treatment was 61 (IQR: 40–94) days and 65 (IQR: 44–96) days, respectively. Time to treat did not differ between those with a mental health disorder diagnosis versus no diagnosis (p=0.52). Within those who did not receive definitive treatment, individuals with a diagnosis of a mental health disorder had a higher proportion of deaths by the end of study follow-up period (57.4% versus 45.4%, p<0.001). These individuals also had a higher proportion of dying within 6 months of diagnosis (13.1% versus 7.6%, p<0.001).
Distribution of Treatments by Mental Health Status
Figure 1 shows the distribution of treatment among the cohort and subgroups. Those without a history of a mental health disorder were most likely to receive radiation with or without ADT (46.1%), followed by radical prostatectomy (22.1%). Among individuals with a diagnosis of a mental health disorder most patients received ADT alone (23.6%). Those diagnosed with schizophrenia were most likely to receive ADT alone (30.3%) and least likely to receive radical prostatectomy (11.3%). Similar treatment trends were observed among individuals with substance use disorders (see Figure 1). Lastly, those diagnosed with PTSD were the most likely to receive no treatment (>27%).
Figure 1:
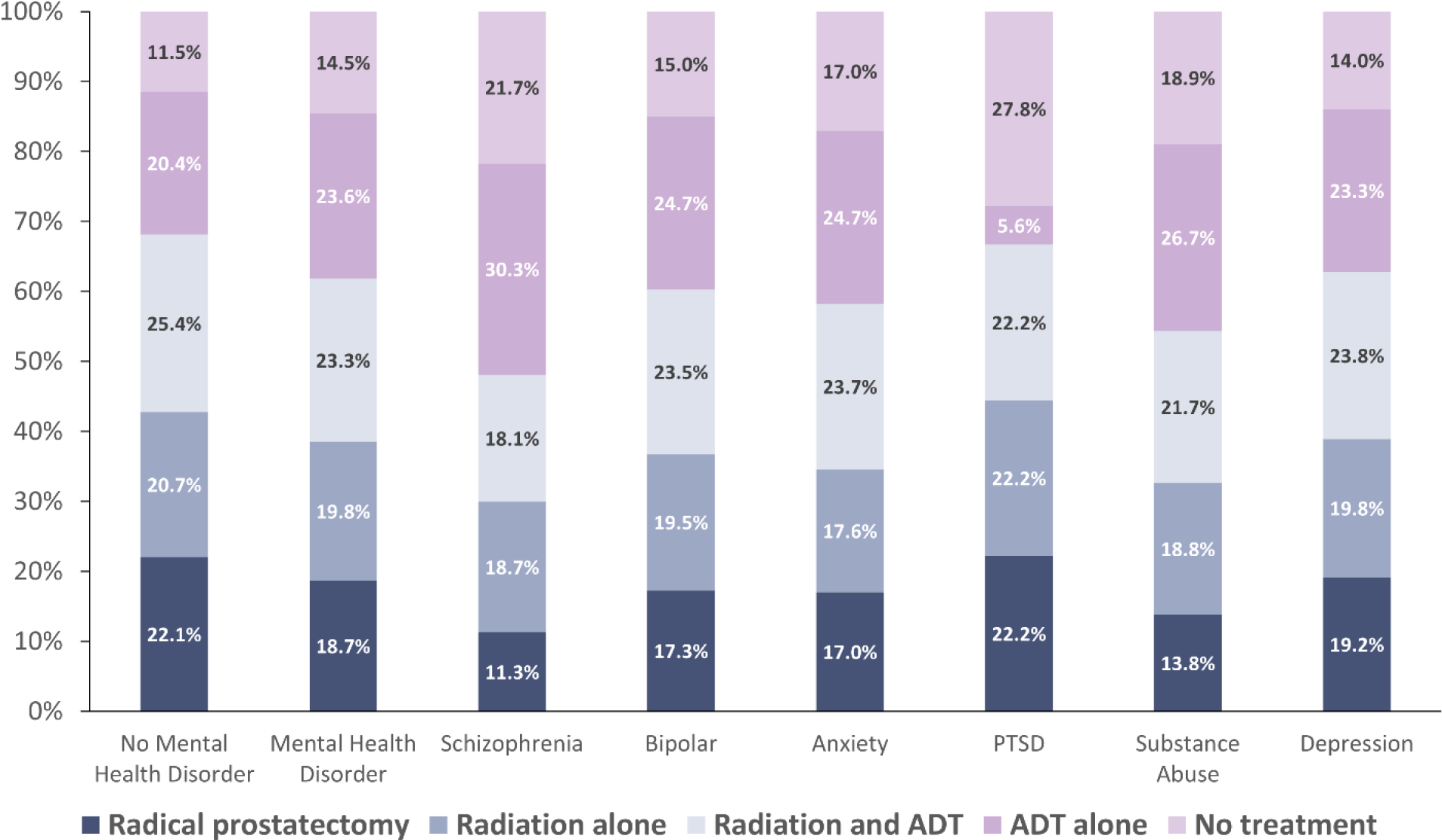
The distribution of treatments by mental health diagnosis among men in SEER-Medicare (2004–2015) with localized, clinically significant prostate cancer
Receipt of Definitive Treatment
In total, 67.7% (95% CI 67.4–67.9) of the cohort received definitive treatment. Individuals without a history of a mental health disorder more commonly received definitive treatment compared with those with a history of a mental health disorder (68.2%, 95% CI 67.9–68.5 vs 61.8%, 95% CI 60.8–62.9; p < 0.001). When stratified by specific mental health disorder, the majority of the cohort with PTSD (>66%, 95% CI 42.5–90.8), depressive disorder (62.8%, 95% CI 61.6–63-9), bipolar disorder (60.3%, 95% CI 56.7–63.8), anxiety disorder (58.2%, 95% CI 53.2–63.3), and substance abuse disorder (54.5%, 95% CI 51.1–57.6) received definitive treatment compared with those with schizophrenia (48.1%, 95% CI 42.7–53.4). Among patients with a mental health diagnosis during an admission claim, 51.7% (95% CI 49.6–53.8) received definitive treatment whereas of those with a mental health diagnosis in the outpatient setting 65.6% (95% CI 64.3–66.8) received definitive treatment.
On multivariable analysis, patients were less likely to receive definitive treatment if diagnosed with a mental health disorder (OR 0.74, 95% CI 0.66–0.83), adjusting for inpatient admission, marital status, urban/rural location, race and ethnicity, socioeconomic status, and NCI comorbid status (Table 2). A history of admission associated with a mental health diagnosis is also an independent predictor of receiving definitive treatment (OR 0.67, 95% CI 0.6–0.75). In this model Black individuals independently had a reduced likelihood of receiving definitive treatment (OR 0.74, 95% CI 0.71–0.78). There was no significant interaction between Black race and mental health disorder in the association with receipt of definitive treatment. Among patients who did receive definitive treatment, patients were less likely to have undergone a radical prostatectomy if they were diagnosed with a mental health disorder (OR 0.81, 95% CI 0.7–0.95), unpartnered (OR 0.73, 95% CI 0.7–0.77), Black (OR 0.57, 95% CI 0.54–0.62), or had a moderate or severe comorbidity based on their NCI comorbidity index score (OR 0.48, 95% CI 0.45–0.51). Individuals were more likely to receive definitive treatment later in the study period (OR 1.7, 95% CI 1.62–1.77) when compared with earlier on (OR 1.31, 95% CI 1.26–1.37).
DISCUSSION:
We found that those with a history of a mental health disorder had 26% lower odds of receiving definitive therapy for clinically localized prostate cancer, after adjusting for clinical, social, and demographic factors associated with treatment utilization. We also found that non-definitive treatment such as primary ADT and observation were more common amongst those with specific mental health disorders associated with severe functional impairment such as schizophrenia, PTSD, and substance use disorder. Furthermore, our analysis showed that individuals with an inpatient admission associated with a mental health diagnosis were less likely to receive definitive treatment. These findings all suggest that a relationship may exist between the severity of a mental health disorder and the type of prostate cancer treatment received and may be exacerbated in those with more severe disease.
Treatment type (i.e., radiation, surgery) may vary between individuals with and without a history of a mental health disorder, reflecting potential differences in structural, social, and health-related factors. These may also reflect biases (both implicit and explicit) against individuals with mental health disorders, especially those with severe conditions such as schizophrenia and substance abuse disorder. Structural, social, and health related factors may also drive disparities in other comorbid conditions, thereby contributing to the perception that those with mental health disorders may be less fit for certain prostate cancer therapies. Data from the cardiac literature has shown that when compared with the general population, patients with mental health disorders were less likely to receive procedures such as cardiac catheterization in both the inpatient (RR 0.41, 95% CI 0.26 – 0.65) and outpatient setting (RR 0.56 95% CI 0.44 – 0.70) despite higher mortality rates from coronary artery disease when compared with the general population. This study highlighted how for various reasons mental health status had an significant impact on patient survival with and access to procedures for circulatory disease in a universal health care system. 16 In our analysis, we found that treatment utilization differed by diagnosis of mental health disorders. Specifically, individuals with a diagnosis of a severe mental health disorder were far more likely to receive non-curative treatments such as primary ADT and observation for their cancers compared with all the other groups we analyzed. These findings are similar to previous studies done which demonstrated that individuals with clinically significant depression were less likely to utilize definitive therapy. 17 Given the substantial side effects of ADT, including cognitive impairment, consideration should be given to the potential harms of primary ADT in this vulnerable population.18, 19 This is especially important given the lack of a survival benefit from primary ADT in the management of localized prostate cancer.20 It is important to note that use of primary ADT decreased from 36.0% to 15.5% from 2005 to 2015 among individuals with mental health disorders, which is a positive trend in its utilization in this vulnerable population.
Overall, we found that individuals with a history of a mental health disorder were less likely to receive definitive treatment for localized, clinically significant prostate cancers when compared with those without a history of mental health disorder (61.8% vs 68.2%). No prior studies have been published assessing patterns of localized therapies for prostate cancer in patients with a diagnosis of one of several mental health disorders. However, studies have shown higher rates of cancer-related death among individuals with mental health disorders. This likely reflects inequities in early diagnosis, access to care, and implementation of curative treatment—especially among those with long life expectancies prior to diagnosis. Stigma associated with mental health disorders can create barriers to treatment as well as the underestimation of life expectancy. In the case of prostate cancer providers’ perception of factors such as patients’ poor health, houselessness, or perceived inability to provide self-care (i.e. catheter care) may bias against offering radical prostatectomy. Similarly, a perceived inability to comply with daily treatments for a period of 5–6 weeks may bias a provider against offering external beam radiation therapy. Lastly, perceived biases may cause clinicians to underestimate life expectancy among those with severe mental health diagnoses who may otherwise be good candidates for local therapy. It is important to note that patients with mental health disorders are less likely to undergo prostate cancer screening, which likely exacerbates inequities in this group.
The Institute of Medicine defines a healthcare disparity as a difference in healthcare quality not due to differences in health care needs or preferences. Healthcare disparities arise from differences in access and implementation of care attributable to race and ethnicity, socioeconomic status, lack of insurance coverage, or healthcare provider bias.21 Stigmas associated with people with mental health disorders can lead to inequitable receipt of health services, resulting from delays in help-seeking, treatment discontinuation, suboptimal therapeutic relationships, and more.1 Thus, it is essential to acknowledge and investigate how care differs for people with mental health disorders. It is also important to note the intersectionality that exists between mental health and other social strata such as race, income, and social support. Individuals with a history of a mental health disorder in our cohort were more likely to be unpartnered, live in an urban setting, self-report non-White race, and reside in an area with a lower socioeconomic standing. This is consistent with the literature that demonstrates that those with mental health disorders are more likely to experience social drift towards lower socioeconomic status.22 These findings suggest that greater care and attention is needed to assess and address the social needs of individuals with mental health disorders who are diagnosed with localized prostate cancer.
Limitations of this study include its retrospective design, which is susceptible to selection bias, and the reliance on the ICD and Current Procedural Terminology codes, which are subject to coding errors. This study looked at the two years preceding a diagnosis of prostate cancer; thus, only men aged 67 and older were included in the cohort. The data also does not provide granularity on disease severity for mental health diagnosis, and there are limitations on social determinants of health data in the SEER-Medicare registry. We attempted to maximize the accuracy of mental health diagnoses in the cohort by requiring individuals to have a code associated with a mental health condition at two different visits within two years of diagnosis of prostate cancer. These restrictions in defining mental health disorders in the claims data may have excluded individuals with mental health disorders—especially those with less severe mental health conditions. Previous studies in Medicare report a prevalence of mental health disorders in 30.2% of Medicare beneficiaries, which is higher that reported in this select cohort of prostate cancer patients (7.8%).23 In addition, we do not have information on how well that mental health disorder disease was controlled, a factor to consider in the impact on prostate cancer care. Misclassification bias may also exist in terms of treatment exposure as we do not have information on intent to treat, only actual treatment. In some scenarios individuals die before they receive treatment. In those classified as receiving “no treatment”, those with a mental health disorder did have a higher rate of death in the 6-month period following diagnosis, lending credence to the idea that more of these individuals had intent to treat but never received that treatment. Omitting these patients via a sensitivity analysis did not, however, change the findings. The exclusion of Medicare 15, which was necessary to avoid issues related to care outside of traditional Medicare, may confound our results as these patients are more likely to be older and from minoritized population.24 Another limitation of this analysis is the use of census-tract estimates of social and economic factors such as poverty, which may not capture individual risk in our cohort and sometimes oversimplify complex relationships between social, political, and geographic factors. Lastly, this sample is restricted to those over the age of 65 who were insured, and the associations we describe may not be generalizable.
Conclusion:
Those with a history of a mental health disorder demonstrated a disproportionately lower rate of receiving definitive treatment and a higher rate of primary ADT when compared with individuals without a history of a mental health disorder. These disparities remained even after adjusting for comorbidities. Individuals with mental health disorders and prostate cancer are at increased risk of death and undertreatment. It is important to address their specific clinical and social needs to better support their receipt of beneficial prostate cancer treatments, when appropriate.
Supplementary Material
Funding:
This material is based upon work supported by the U.S. Department of Defense, Office of the Congressionally Directed Medical Research Programs under Grant Number W81XWH2110531 and made possible in part by the Andy Hill Cancer Research Endowment (CARE) Fund (award number 2021-DR-01)
Footnotes
Conflict of Interest: The authors report no relevant financial disclosures pertaining to this review article
References:
- 1.Knaak Stephanie, Mantler Ed, and Szeto Andrew. “Mental Illness-Related Stigma in Healthcare: Barriers to Access and Care and Evidence-Based Solutions.” Healthcare Management Forum 30, no. 2 (March 2017): 111–16. 10.1177/0840470416679413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kessler Ronald C., Berglund Patricia, Demler Olga, Jin Robert, Merikangas Kathleen R., and Walters Ellen E.. “Lifetime Prevalence and Age-of-Onset Distributions of DSM-IV Disorders in the National Comorbidity Survey Replication.” Archives of General Psychiatry 62, no. 6 (June 1, 2005): 593. 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 3.Alegría Margarita, NeMoyer Amanda, Bagué Irene Falgàs, Wang Ye, and Alvarez Kiara. “Social Determinants of Mental Health: Where We Are and Where We Need to Go.” Current Psychiatry Reports 20, no. 11 (November 2018): 95. 10.1007/s11920-018-0969-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hennekens Charles H., Hennekens Alissa R., Hollar Danielle, and Casey Daniel E.. “Schizophrenia and Increased Risks of Cardiovascular Disease.” American Heart Journal 150, no. 6 (December 2005): 1115–21. 10.1016/j.ahj.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Laursen Thomas Munk, Munk-Olsen Trine, Nordentoft Merete, and Mortensen Preben Bo. “Increased Mortality Among Patients Admitted With Major Psychiatric Disorders: A Register-Based Study Comparing Mortality in Unipolar Depressive Disorder, Bipolar Affective Disorder, Schizoaffective Disorder, and Schizophrenia.” The Journal of Clinical Psychiatry 68, no. 06 (June 15, 2007): 899–907. 10.4088/JCP.v68n0612. [DOI] [PubMed] [Google Scholar]
- 6.Piatt Elizabeth E., Munetz Mark R., and Ritter Christian. “An Examination of Premature Mortality Among Decedents With Serious Mental Illness and Those in the General Population.” Psychiatric Services 61, no. 7 (July 2010): 663–68. 10.1176/ps.2010.61.7.663. [DOI] [PubMed] [Google Scholar]
- 7.Lawrence D, D’Arcy C, Holman J, Jablensky AV, Threfall TJ, and Fuller SA. “Excess Cancer Mortality in Western Australian Psychiatric Patients Due to Higher Case Fatality Rates: Cancer Mortality in Psychiatric Patients.” Acta Psychiatrica Scandinavica 101, no. 5 (May 2000): 382–88. 10.1034/j.1600-0447.2000.101005382.x. [DOI] [PubMed] [Google Scholar]
- 8.Kisely Stephen, Crowe Elizabeth, and Lawrence David. “Cancer-Related Mortality in People With Mental Illness.” JAMA Psychiatry 70, no. 2 (February 1, 2013): 209. 10.1001/jamapsychiatry.2013.278. [DOI] [PubMed] [Google Scholar]
- 9.Guan Ng Chong, Termorshuizen Fabian, Laan Wijnand, Smeets Hugo M., Zuraida Zainal Nor, Kahn René S., De Wit Niek J., and Boks Marco P. M.. “Cancer Mortality in Patients with Psychiatric Diagnoses: A Higher Hazard of Cancer Death Does Not Lead to a Higher Cumulative Risk of Dying from Cancer.” Social Psychiatry and Psychiatric Epidemiology 48, no. 8 (August 2013): 1289–95. 10.1007/s00127-012-0612-8. [DOI] [PubMed] [Google Scholar]
- 10.Korfage Ida J., de Koning Harry J., Roobol Monique, Schröder Fritz H., and Essink-Bot Marie-Louise. “Prostate Cancer Diagnosis: The Impact on Patients’ Mental Health.” European Journal of Cancer 42, no. 2 (January 2006): 165–70. 10.1016/j.ejca.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 11.Ravi Praful, Karakiewicz Pierre I., Roghmann Florian, Gandaglia Giorgio, Choueiri Toni K., Menon Mani, McKay Rana R., et al. “Mental Health Outcomes in Elderly Men with Prostate Cancer1Equal Contribution.” Urologic Oncology: Seminars and Original Investigations 32, no. 8 (November 2014): 1333–40. 10.1016/j.urolonc.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Washington Samuel L., Nyame Yaw A., and Moses Kelvin A.. “What Is the Impact of Racial Disparities on Diagnosis and Receipt of Appropriate Mental Health Care Among Urology Patients?” European Urology Focus 6, no. 6 (November 2020): 1155–57. 10.1016/j.euf.2019.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warren Joan, Klabunde Carrie, Schrag Deborah, Bach Peter, and Riley Gerald. “Overview of the SEER-Medicare Data: Content, Research Applications, and Generalizability to the United States Elderly Population.” Medical Care 40, no. 8 (August 2002): IV3–18. [DOI] [PubMed] [Google Scholar]
- 14.Klabunde Carrie N., Legler Julie M., Warren Joan L., Baldwin Laura-Mae, and Schrag Deborah. “A Refined Comorbidity Measurement Algorithm for Claims-Based Studies of Breast, Prostate, Colorectal, and Lung Cancer Patients.” Annals of Epidemiology 17, no. 8 (August 2007): 584–90. 10.1016/j.annepidem.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 15.Jellestad Lena, Vital Nicolà A., Malamud Jolanda, Taeymans Jan, and Mueller-Pfeiffer Christoph. “Functional Impairment in Posttraumatic Stress Disorder: A Systematic Review and Meta-Analysis.” Journal of Psychiatric Research 136 (April 2021): 14–22. 10.1016/j.jpsychires.2021.01.039. [DOI] [PubMed] [Google Scholar]
- 16.Kisely S, Smith M, Lawrence D, Cox M, Campbell LA, and Maaten S. “Inequitable Access for Mentally Ill Patients to Some Medically Necessary Procedures.” Canadian Medical Association Journal 176, no. 6 (March 13, 2007): 779–84. 10.1503/cmaj.060482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prasad Sandip M., Eggener Scott E., Lipsitz Stuart R., Irwin Michael R., Ganz Patricia A., and Hu Jim C.. “Effect of Depression on Diagnosis, Treatment, and Mortality of Men With Clinically Localized Prostate Cancer.” Journal of Clinical Oncology 32, no. 23 (August 10, 2014): 2471–78. 10.1200/JCO.2013.51.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Lisa M., Diefenbach Michael A., Gordon Wayne A., Cantor Joshua B., and Cherrier Monique M.. “Cognitive Problems in Patients on Androgen Deprivation Therapy: A Qualitative Pilot Study.” Urologic Oncology: Seminars and Original Investigations 31, no. 8 (November 2013): 1533–38. 10.1016/j.urolonc.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McHugh Deaglan J., Root James C., Nelson Christian J., and Morris Michael J.. “Androgen-Deprivation Therapy, Dementia, and Cognitive Dysfunction in Men with Prostate Cancer: How Much Smoke and How Much Fire?: Dementia Risk With ADT in Prostate Ca.” Cancer 124, no. 7 (April 1, 2018): 1326–34. 10.1002/cncr.31153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu-Yao Grace L. “Survival Following Primary Androgen Deprivation Therapy Among Men With Localized Prostate Cancer.” JAMA 300, no. 2 (July 9, 2008): 173. 10.1001/jama.300.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care (with CD). Washington, D.C.: National Academies Press, 2003. 10.17226/12875. [DOI] [PubMed] [Google Scholar]
- 22.Lee Sze Chim, DelPozo-Banos Marcos, Lloyd Keith, Jones Ian, Walters James T.R., Owen Michael J., O’Donovan Michael, and John Ann. “Area Deprivation, Urbanicity, Severe Mental Illness and Social Drift — A Population-Based Linkage Study Using Routinely Collected Primary and Secondary Care Data.” Schizophrenia Research 220 (June 2020): 130–40. 10.1016/j.schres.2020.03.044 [DOI] [PubMed] [Google Scholar]
- 23.Figueroa JF, Phelan J, Orav EJ, Patel V, Jha AK. Association of Mental Health Disorders With Health Care Spending in the Medicare Population. JAMA Netw Open. 2020. Mar 2;3(3):e201210. doi: 10.1001/jamanetworkopen.2020.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beckman AL, Frakt AB, Duggan C, Zheng J, Orav EJ, Tsai TC, & Figueroa JF (2023). Evaluation of potentially avoidable acute care utilization among patients insured by Medicare Advantage vs Traditional Medicare. JAMA Health Forum, 4(2). 10.1001/jamahealthforum.2022.5530 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


