Abstract
OBJECTIVES:
Acute liver failure (ALF) is an orphan disease often complicated by acute kidney injury (AKI). We assessed the impact of transient versus persistent AKI on survival in patients with ALF.
DESIGN:
International multicenter retrospective cohort.
SETTING:
U.S. ALF Study Group prospective registry.
PATIENTS:
Patients with greater than or equal to 18 years and ALF in the registry from 1998 to 2016 were included. Patients with less than 3 days of follow-up, without kidney function evaluation on day 3, or with cirrhosis were excluded.
INTERVENTIONS:
AKI was defined by Kidney Disease Improving Global Outcomes guidelines on day 1. Kidney recovery was defined on day 3 as transient AKI, by a return to no-AKI within 48 hours or persistent AKI if no such recovery or renal replacement therapy (RRT) was observed. Primary outcome was transplant-free survival (TFS) at 21 days.
MEASUREMENTS AND MAIN RESULTS:
Among 1,071 patients with ALF, 339 (31.7%) were males, and median (interquartile range) age was 39 years (29–51 yr). Acetaminophen-related ALF was found in 497 patients (46.4%). On day 1, 485 of 1,071 patients (45.3%) had grade 3–4 hepatic encephalopathy (HE), 500 of 1,070 (46.7%) required invasive mechanical ventilation (IMV), 197 of 1,070 (18.4%) were on vasopressors, and 221 of 1,071 (20.6%) received RRT. On day 1, 673 of 1,071 patients (62.8%) had AKI. On day 3, 72 of 1,071 patients (6.7%) had transient AKI, 601 of 1,071 (56.1%) had persistent AKI, 71 of 1,071 (6.6%) had late onset AKI, and 327 of 1,071 (30.5%) remained without AKI. Following adjustment for confounders (age, sex, race, etiology, HE grade, use of IMV and vasopressors, international normalized ratio, and year), although persistent acute kidney injury (adjusted odds ratio [aOR] [95% CI] 0.62 [0.44–0.88]) or late onset AKI (aOR [95% CI] 0.48 [0.26–0.89]) was associated with lower TFS, transient AKI was not (aOR [95% CI] 1.89 [0.99–3.64]).
CONCLUSIONS:
In a multicenter cohort of patients with ALF, persistent but not transient AKI was independently associated with lower short-term TFS.
Keywords: hepatitis, kidney function, liver failure, outcomes, renal failure, survival
Acute kidney injury (AKI) is highly prevalent and has been associated with worse survival in patients with acute liver failure (ALF) (1). Time to kidney recovery following AKI has been shown to differentially impact patients’ outcomes (2). Complete and sustained reversal of AKI within 48–72 hours of its onset has been associated with better outcomes than longer durations of AKI (3–7).
A report from the Acute Disease Quality Initiative (ADQI) has defined two types of kidney recovery within 7 days following AKI: persistent AKI is characterized by continuing AKI, as per Kidney Disease Improving Global Outcomes (KDIGO) criteria, greater than 48 hours from AKI onset; or transient AKI if there is complete reversal of AKI within 48 hours of AKI onset (8, 9).
This stratification of AKI recovery during the first week of its course may help clinicians to implement strategies to prevent AKI prolongation (e.g. resuscitation or treatment of infection), improve prognostic assessment (e.g. risk of acute or chronic kidney disease [CKD] or survival), and contribute to further research on this topic (2, 10).
The differential impact of persistent versus transient AKI on outcomes has been studied in general critically ill patients, but there is a paucity of data for patients with ALF. In this context, we hypothesized that persistent AKI would more negatively impact these patients’ outcomes than transient AKI. Therefore, we aimed to determine among patients with ALF: the prevalence of AKI by KDIGO criteria, the prevalence of persistent and transient AKI by ADQI criteria, and the association between the type of kidney recovery following the initial episode of AKI and outcomes.
MATERIALS AND METHODS
Design, Setting, and Participants
This was a multicenter retrospective cohort study that included all ALF patients who were prospectively enrolled in the U.S. ALF Study Group (U.S.-ALFSG) registry between January 1998 and August 2016. Patients with less than 3 days of follow-up, without kidney function evaluation on day 3 post study inclusion, or with cirrhosis were excluded. The study was approved by the Health Research Ethics Board (HREB) at the University of Alberta (Approval number Pro00041365) and has received institutional review board (IRB) approval from all enrolling sites within the U.S.-ALFSG (see Acknowledgments section). All protocols were approved by the IRBs/HREBs at participating sites (tertiary liver transplant [LT] referral centers). Consent/assent was obtained from each participant/next of kin at the time of enrollment. All research procedures were conducted according to the Declaration of Helsinki (11). The reporting of this study followed the Strengthening the Reporting of Observational Studies in Epidemiology statement (12).
Operational Definitions
ALF was defined according to following criteria: international normalized ratio (INR) greater than or equal to 1.5, any degree of hepatic encephalopathy (HE) (West Haven criteria), acute illness onset less than 26 weeks, and no cirrhosis (13). AKI definition and staging on day 1 post study inclusion were based on KDIGO criteria. Kidney recovery was assessed on day 3 to allow for a 48-hour window of kidney function evolution, as per ADQI criteria (8, 9). As urine output was not available, AKI definition relied on serum creatinine dynamics. Baseline creatinine was estimated using the modification of diet in renal disease (MDRD) equation as per ADQI criteria (average glomerular filtration rate of 75 mL/min/1.73 m2) (14, 15). The use of renal replacement therapy (RRT) was not protocolized; therefore, indications, modalities, replacement fluids, anticoagulation, treatment doses, and timings for initiation and discontinuation of treatment were based on clinical judgment at each enrolling site. Any patient receiving RRT was considered to have AKI even if started for a nonkidney indication (e.g. clearance of ammonia).
Exposures and Endpoints
Data on the following characteristics of patients with ALF were retrieved from the U.S.-ALFSG registry (University of Texas Southwestern Medical Center): age, sex, race, etiology, HE (West Haven criteria), organ support requirements (invasive mechanical ventilation [IMV], vasopressors, and RRT), serum profile (platelet count [PLT], INR, bilirubin, alanine aminotransferase [ALT], ammonia (NH3), creatinine, potassium, pH, and lactate), biochemical Model for End-Stage Liver Disease (MELD) score, year of enrollment, and outcomes (21-d LT] transplant-free survival [TFS], and overall survival) (16–19). Our primary endpoint was 21-day TFS. This has been used in previous literature because it better reflects the impact of ALF course on patients’ survival, without the confounding effect of LT. The secondary outcome was receipt of LT by day 21.
Statistical Analysis
Categorical variables were presented as proportions and continuous variables as median and interquartile ranges (IQRs). Univariable comparisons between independent samples used chi-square or Kruskal-Wallis tests, where appropriate. Missing data across all values were 4.4%, and no imputation was performed.
Associations with outcomes were studied with logistic regression. For the multivariable analyses, the following covariates were initially chosen based on clinical relevance or p < 0.10 on univariable comparisons: age, sex, race (White vs other), and etiology (acetaminophen-related vs other); HE (grade 3–4 vs grade 1–2), IMV, vasopressors, and RRT; PLT, INR, bilirubin, ALT, NH3, potassium, pH, and lactate; MELD; type of kidney recovery on day 3; and year of enrollment (≥2008 vs < 2008). The following variables were evaluated accounting for the potential for collinearity: AKI staging, RRT, creatinine, pH, or potassium with renal recovery on day 3; bilirubin, ALT, NH3, or lactate with INR; and MELD with both renal recovery on day 3 and INR.
The final models were derived using a stepwise backward elimination process based on the lowest p value. Furthermore, the models’ discriminative ability was assessed by C-statistic (95% CI), and internal validation was studied with bootstrapping (1,000 samples). The threshold for statistical significance was α equals to 0.05 (two-tailed). Statistical analysis used IBM SPSS Statistics, Version 27 (IBM Corp, North Castle, NY).
RESULTS
Baseline Characteristics
The original cohort within the U.S.-ALFSG registry comprised 2,050 patients with ALF. Following exclusion of patients with less than 3 days of follow-up or without kidney function evaluation on day 3, the final cohort included 1,071 patients. There were no substantial differences in the baseline characteristics between these cohorts (Table S1, Supplementary Digital Content, http://links.lww.com/CCM/H125).
Among 1,071 patients with ALF, median (IQR) age was 39 years (29–51 yr), and 339 (31.7%) were males. Overall, 497 of 1,070 patients (46.4%) had acetaminophen. All etiologies of ALF are depicted in Figure S1 (Supplementary Digital Content, http://links.lww.com/CCM/H125). On day 1 post study inclusion, 485 of 1,071 (45.3%) had grade 3–4 HE, 500 of 1,070 (46.7%) required IMV, 197 of 1,070 (18.4%) were on vasopressors, and 221 of 1,071 (20.6%) were receiving RRT. Overall, median (IQR) baseline and day 1 creatinine were 0.96 mg/dL (0.88–1.10 mg/dL) and 1.70 mg/dL (0.90–3.00 mg/dL), respectively (Table 1).
TABLE 1.
Baseline Characteristics and 21-Day Outcomes Stratified by the Type of Recovery on Day 3 Post Study Inclusion
| Characteristics, n (%) or Median (IQR) | Total (N = 1,071) | Transient AKI (N = 72) | Persistent AKI (N = 601) | Late Onset AKI (N = 71) | No AKI (N = 327) | p a |
|---|---|---|---|---|---|---|
| Age (yr) | 39 (29–51) | 41 (29–51) | 42 (31–54) | 35 (26–47) | 35 (26–47) | < 0.001 |
|
| ||||||
| Sex (male) | 339 (31.7) | 30/72 (41.7) | 191/601 (31.8) | 28/71 (39.4) | 90/327 (27.5) | 0.048 |
|
| ||||||
| Race | 0.003 | |||||
| White | 797 (74.4) | 58/72 (80.6) | 472/601 (78.5) | 44/71 (62.0) | 223/327 (68.2) | |
| African-American | 162 (15.1) | 8/72 (11.1) | 78/601 (13.0) | 17/71 (23.9) | 59/327 (18.0) | |
| Others | 112 (10.5) | 6/72 (8.3) | 51/601 (8.5) | 10/71 (14.1) | 45/327 (13.8) | |
|
| ||||||
| Etiology (N = 1,070) | < 0.001 | |||||
| Acetaminophen paracetamol overdose | 497 (46.4) | 42/72 (58.3) | 306/600 (51.0) | 32/71 (45.1) | 117/327 (35.8) | |
| Others | 573 (53.6) | 30/72 (41.7) | 294/600 (49.0) | 39/71 (54.9) | 210/327 (64.2) | |
|
| ||||||
| Organ failures and support | ||||||
| Hepatic encephalopathy Grade 3–4 D1 | 485 (45.3) | 34/72 (47.2) | 315/601 (52.4) | 27/71 (38.0) | 109/327 (33.3) | < 0.001 |
| Invasive mechanical ventilation D1 (N = 1,070) | 500 (46.7) | 34/72 (47.2) | 345/601 (57.4) | 29/71 (40.8) | 92/326 (28.2) | < 0.001 |
| Vasopressors D1 (N = 1,070) | 197 (18.4) | 5/72 (6.9) | 168/601 (28.0) | 8/71 (11.3) | 16/326 (4.9) | < 0.001 |
| RRT D1 | 221 (20.6) | 0/72 (0) | 221/601 (36.8) | 0/71 (0) | 0/327 (0) | < 0.001 |
| RRT D1–D3 | 337 (31.5) | 0/72 (0) | 316/601 (52.6) | 21/71 (29.6) | 0/327 (0) | < 0.001 |
|
| ||||||
| Laboratory (serum) | ||||||
|
| ||||||
| Baseline serum creatinine (mg/dL) | 0.96 (0.88–1.10) | 0.98 (0.89–1.14) | 0.95 (0.87–1.10) | 1.04 0.90–1.19) | 0.97 (0.89–1.11) | 0.007 |
| Serum creatinine D1 (mg/dL) | 1.70 (0.90–3.00) | 1.70 (1.40–2.08) | 2.80 (1.90–4.09) | 1.00 (0.80–1.15) | 0.80 (0.60–1.00) | < 0.001 |
| Serum creatinine D3 (mg/dL) | 1.60 (0.80–3.10) | 1.00 (0.80–1.20) | 2.70 (1.78–4.30) | 1.90 (1.50––2.70) | 0.70 (0.60–0.90) | < 0.001 |
| Platelet count D1 (109/L) (N = 1,065) | 127 (82–188) | 121 (80–187) | 111 (63–164) | 139 (76–204) | 161 (110–231) | < 0.001 |
| International normalized ratio D1 | 2.9 (2.2–4.4) | 2.7 (2.0–4.3) | 3.0 (2.2–4.4) | 3.2 (2.2–6.4) | 2.9 (2.2–4.2) | 0.17 |
| Bilirubin D1 (mg/dL) (N = 1,063) | 7.5 (4.0–19.8) | 6.1 (3.9–12.0) | 6.6 (4.0–16.9) | 11.2 (3.1–24.2) | 12.5 (4.6–21.8) | < 0.001 |
| Alanine transferase D1 (U/L) N = 1,058) | 2109 (674–4780) | 2646 (863–5747) | 2511 (756–5126) | 2409 (804–5522) | 1435 (531–3377) | < 0.001 |
| Serum ammonia D1 (μmol/L) (N = 608) | 99 (65–161) | 101 (68–152) | 107 (65–172) | 95 (55–124) | 93 (67–130) | 0.15 |
| pH D1 (N = 818) | 7.42 (7.36–7.48) | 7.45 (7.38–7.50) | 7.40 (7.34–7.46) | 7.44 (7.39–7.48) | 7.46 (7.41–7.50) | < 0.001 |
| Potassium D1 (mmol/L) (N = 1,069) | 3.8 (3.4–4.3) | 3.8 (3.1–4.3) | 3.9 (3.5–4.5) | 3.9 (3.3–4.4) | 3.6 (3.3–4.0) | < 0.001 |
| Lactate D1 (mmol/L) (n = 586) | 4.2 (2.6–7.7) | 4.4 (2.8–5.6) | 4.9 (2.9–8.9) | 5.3 (3.4–9.3) | 3.2 (2.2–4.8) | < 0.001 |
| MELD D1 (N = 1,063) | 35 (29–40) | 32 (28–36) | 39 (34–43) | 33 (27–45) | 28 (24–32) | < 0.001 |
| MELD D3 (N = 1,045) | 33 (26–38) | 23 (20–27) | 36 (32–41) | 37 (31–41) | 26 (21–31) | < 0.001 |
| Maximum MELD D1–D7 | 38 (31–43) | 32 (28–37) | 40 (36–46) | 41 (35–48) | 31 (26–36) | < 0.001 |
|
| ||||||
| Outcomes day 21 post inclusion | ||||||
| Liver transplant (N = 1,065) | 213/1065 (20.0) | 5/71 (7.0) | 91/597 (15.2) | 19/70 (27.1) | 98/327 (30.0) | < 0.001 |
| Transplant-free survival (N = 992) | 475/992 (47.9) | 44/62 (71.0) | 252/565 (44.6) | 24/67 (35.8) | 155/298 (52.0) | < 0.001 |
| Overall survival (N = 966) | 642/966 (66.5) | 49/62 (79.0) | 322/553 58.2) | 37/64 (57.8) | 234/287 (81.5) | < 0.001 |
AKI = acute kidney injury, D1 = day 1 post inclusion, D3 = day 3 post inclusion, D7 = day 7 post inclusion, IQR = interquartile range, MELD = model for end-stage liver disease, RRT = renal replacement therapy.
p for comparisons between all subgroups of patients (transient AKI vs persistent AKI vs late onset AKI vs no AKI). α = 0.05.
On day 1, AKI was diagnosed in 673 of 1,071 patients (62.8%). AKI staging as per KDIGO guidelines was as follows: stage 1 in 150 of 673 patients (22.3%), stage 2 in 122/673 (18.1%), and stage 3 in 401 of 673 patients (59.6%). Only 29 of 673 patients (4.3%) were classified as having AKI based on the receipt of RRT on day 1. All baseline characteristics are depicted in Table 1.
Outcomes
At the end of study (day 21 post study inclusion), 213 of 1,065 (20.0%) underwent emergent LT. At this time point, TFS and overall survival rates were 47.9% (475/992 patients) and 66.5% (642/966 patients), respectively. All causes of death are depicted in Figure S2 (Supplementary Digital Content, http://links.lww.com/CCM/H125).
Renal Recovery on Day 3 and Its Association With 21-Day TFS
On day 3, kidney recovery was as follows: 72 of 1,071 patients (6.7%) had transient AKI, 601 of 1,071 (56.1%) had persistent AKI, 71 of 1,071 (6.6%) had late-onset AKI (not present on day 1), and 327 of 1,071 (30.5%) remained without AKI (Table 1).
In comparison with the three other patient groups (Table 1), patients with transient AKI were more frequently male (41.7% vs ≤ 39.4%) and White (80.6% ≤ 78.5%) and had more acetaminophen (58.3% vs ≤ 51.0%) than others. In comparison with the three other patient groups, patients with persistent AKI had more often Grade 3–4 HE (52.4% vs ≤ 47.2%), IMV (57.4% vs ≤ 47.2%), and vasopressors (28.0% vs ≤ 11.3%) on day 1. From days 1 to 3, 316 of 601 patients (52.6%) with persistent AKI and 21 of 71 (29.6%) with late-onset AKI received RRT (Table 1). Among patients with persistent AKI, differences between those who received or not RRT are depicted in Table S2 (Supplementary Digital Content, http://links.lww.com/CCM/H125).
Patients with persistent AKI had higher median creatinine than others both on days 1 and 3 (2.80 and 2.70 mg/dL, respectively). Although patients with persistent AKI had more frequently stage 3 AKI on day 1 (66.1%), patients with transient AKI had more often stage 1 AKI at this time point (72.2%) (Fig. 1) (p < 0.001).
Figure 1.
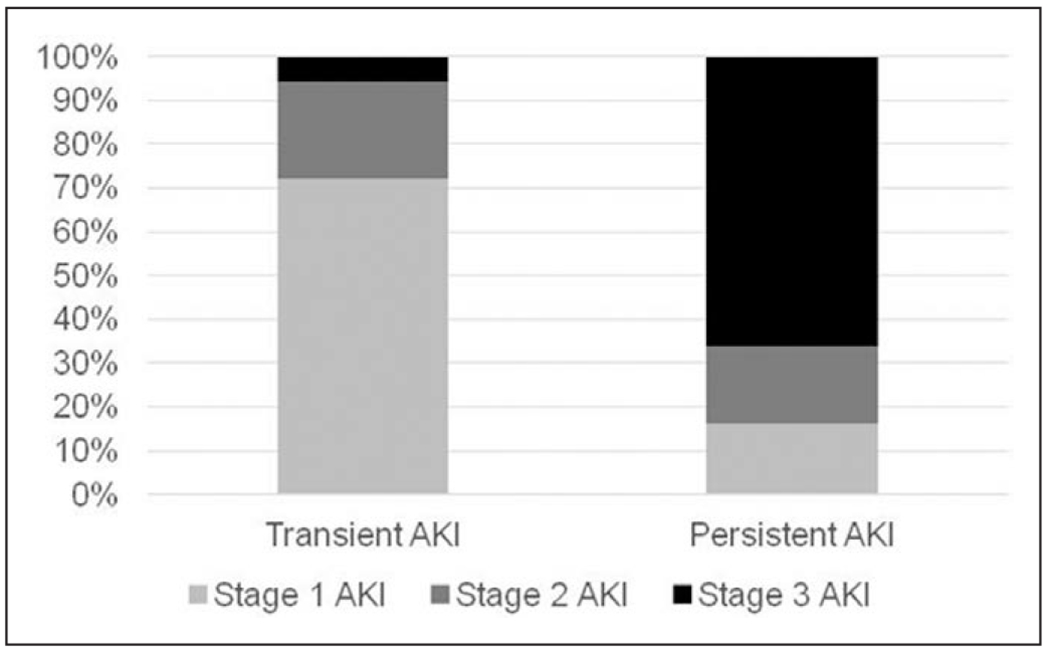
The association between the stage of acute kidney injury (AKI) on day 1 post inclusion and kidney recovery on day 3 post inclusion.
Patients with late-onset AKI had higher median INR (3.2 vs ≤ 3.0) and lactate (5.3 vs ≤ 4.9 mmol/L) than others on day 1. Patients with late-onset AKI had the following KDIGO staging: Stage 1 in 28 of 71 patients (39.4%), Stage 2 in 11 of 71 (15.5%), and Stage 3 in 32 of 71 (45.1%).
At the end of study (day 21), patients with no AKI received more often LT (30.0% vs ≤ 27.1%) than others. At this time point, patients with late-onset AKI had lower rates of TFS (35.8% vs ≥ 44.6%) and overall survival (57.8% vs ≥ 58.2%) than others. Noticeably, patients with transient AKI had the lowest rate of LT (7.0%) and the highest rate of TFS (71.0%) (Table 1).
Patients alive without LT at 21 days were younger (37 vs 41 yr), more frequently female (71.4% vs 65.2%), and White (79.2% vs 67.9%) and had more often acetaminophen (62.1% vs 27.7%) than others. Furthermore, the former less frequently required vasopressors on day 1 (14.7% vs 22.5%) than the latter (Table 2).
TABLE 2.
Baseline Characteristics Stratified by 21-Day Outcomes
| Characteristics, n (%) or Median (IQR) | 21-d Transplant-Free Survival (N = 475) | 21-d Liver Transplant or Death (N = 517) | p |
|---|---|---|---|
| Age (yr) | 37 (29–49) | 41 (29–53) | 0.003 |
|
| |||
| Sex (male) | 136/475 (28.6) | 180/517 (34.8) | 0.037 |
|
| |||
| Race | < 0.001 | ||
| White | 376/475 (79.2) | 351/517 (67.9) | |
| African-American | 61/475 (12.8) | 96/517 (18.6) | |
| Others | 38/475 (8.0) | 70/517 (13.5) | |
|
| |||
| Etiology (N = 991) | < 0.001 | ||
| Acetaminophen paracetamol overdose | 295/475 (62.1) | 143/516 (27.7) | |
| Others | 180/475 (37.9) | 373/516 (72.3) | |
|
| |||
| Organ failures and support | |||
| Hepatic encephalopathy Grade 3–4 D1 | 211/475 (44.4) | 244/517 (47.2) | 0.70 |
| Invasive mechanical ventilation D1 (N = 991) | 212/475 (44.6) | 258/516 (50.0) | 0.09 |
| Vasopressors D1 (N = 991) | 70/475 (14.7) | 116/516 (22.5) | 0.002 |
| RRT D1 | 96/475 (20.2) | 111/517 (21.5) | 0.63 |
| RRT D1–D3 | 138/475 (29.1) | 179/517 (34.6) | 0.06 |
|
| |||
| Laboratory (serum) | |||
| Baseline serum creatinine (mg/dL) | 0.94 (0.88–1.10) | 0.99 (0.88–1.12) | 0.18 |
| Serum creatinine D1 (mg/dL) | 1.70 (0.90–3.10) | 1.74 (0.90–3.00) | 0.88 |
| Serum creatinine D3 (mg/dL) | 1.40 (0.80–3.10) | 1.90 (1.00–3.10) | < 0.001 |
| Platelet count D1 (109/L) (N = 986) | 127 (86–188) | 124 (76–189) | 0.37 |
| International normalized ratio D1 | 2.9 (2.1–4.2) | 3.1 (2.3–4.9) | 0.002 |
| Bilirubin D1 (mg/dL) (n = 986) | 5.2 (3.2–9.0) | 16.2 (6.1–26.0) | < 0.001 |
| Alanine transferase D1 (U/L) (N = 981) | 3,239 (1,427–5,660) | 1,063 (340–3,280) | < 0.001 |
| Serum ammonia D1 (μmol/L) (N = 562) | 94 (58–144) | 107 (71–170) | 0.003 |
| pH D1 (n = 753) | 7.42 (7.36–7.48) | 7.42 (7.35–7.48) | 0.79 |
| Potassium D1 (mmol/L) (N = 991) | 3.8 (3.4–4.2) | 3.9 (3.5–4.5) | < 0.001 |
| Lactate D1 (mmol/L) (N = 543) | 3.5 (2.3–5.5) | 5.4 (3.5–9.5) | < 0.001 |
| MELD D1 (N = 986) | 32 (27–38) | 37 (32–43) | < 0.001 |
| MELD D3 (N = 970) | 29 (23–35) | 36 (31–42) | < 0.001 |
| Maximum MELD D1–D7 | 34 (28–40) | 41 (36–47) | < 0.001 |
| AKI D1 | 296/475 (62.3) | 331/517 (64.0) | 0.58 |
| Kidney recovery D3 | < 0.001 | ||
| Transient AKI | 44/475 (9.3) | 18/517 (3.5) | |
| Persistent AKI | 252/475 (53.1) | 313/517 (60.5) | |
| Late-onset AKI | 24/475 (5.1) | 43/517 (8.3) | |
| No AKI | 155/475 (32.6) | 143/517 (27.0) | |
| Year of enrollment (≥ 2008) | 194/475 (40.8) | 177/517 (34.2) | 0.032 |
AKI = acute kidney injury, D1 = day 1 post inclusion, D3 = day 3 post inclusion, D7 = day 7 post inclusion, IQR = interquartile range, MELD = model for end-stage liver disease, RRT = renal replacement therapy.
α = 0.05.
Although median creatinine on day 1 was similar between survivors without LT and others (1.70 vs 1.74 mg/dL), the former had lower median serum creatinine on day 3 than the latter (1.40 vs 1.90 mg/dL). On day 1, AKI was diagnosed in similar rates between patients alive without LT at 21 days and others (62.3% vs 64.0%). However, on day 3, survivors without LT had lower rates of persistent (53.1% vs 60.5%) or late-onset AKI (5.1% vs 8.3%) and a higher rate of transient AKI (9.3% vs 3.5%) than others (Table 2). All baseline characteristics stratified by 21-day TFS status are depicted in Table 2.
Following adjustment for clinically and statistically significant confounders, six variables were independently associated with 21-day TFS (Table 3; and Table S3, Supplementary Digital Content, http://links.lww.com/CCM/H125): acetaminophen (adjusted odds ratio [aOR] [95% CI] 5.55 [4.03–7.65]) and enrollment greater than or equal to 2008 (aOR [95% CI] 1.48 [1.11–1.98]) were associated with higher odds of TFS; conversely, vasopressors (aOR [95% CI] 0.61 [0.41–0.90]), INR (aOR [95% CI] 0.85 [0.79–0.91]), persistent AKI (aOR [95% CI] 0.62 [0.44–0.88]), and late-onset AKI (aOR [95% CI] 0.48 [0.26–0.89]) were associated with lower odds of TFS. Noticeably, transient AKI was not associated with 21-day TFS (aOR [95% CI] 1.89 [0.99–3.64]). The discriminative ability of this model was good (C-statistic [95% CI] 0.75 [0.72–0.78]).
TABLE 3.
Variables Associated With 21-Day Transplant-Free Survival
| Variables | OR (950/0 CI) | Adjusted OR (95% CI) | p a |
|---|---|---|---|
| Age (yr) | 0.99 (0.98–1.00) | 0.99 (0.98–1.00) | 0.11 |
|
| |||
| Sex (male) | 0.75 (0.57–0.98) | 0.94 (0.69–1.28) | 0.69 |
|
| |||
| Race (White vs other) | 1.80 (1.35–2.40) | 1.37 (0.99–1.89) | 0.06 |
|
| |||
| Etiology (acetaminophen paracetamol overdose vs other) | 4.28 (3.27–5.59) | 5.55 (4.03–7.65) | < 0.001 |
|
| |||
| Hepatic encephalopathy Grade 3–4 D1 | 0.89 (0.70–1.15) | 0.88 (0.59–1.29) | 0.51 |
|
| |||
| Invasive mechanical ventilation D1 | 0.81 (0.63–1.04) | 0.74 (0.49–1.12) | 0.16 |
|
| |||
| Vasopressors D1 | 0.60 (0.43–0.83) | 0.61 (0.41–0.90) | 0.014 |
|
| |||
| International normalized ratio D1 | 0.92 (0.87–0.97) | 0.85 (0.79–0.91) | < 0.001 |
|
| |||
| Kidney recovery D3 | |||
| Transient AKI | 2.26 (1.25–4.08) | 1.89 (0.99–3.64) | 0.06 |
| Persistent AKI | 0.74 (0.56–0.98) | 0.62 (0.44–0.88) | 0.007 |
| Late-onset AKI | 0.52 (0.30–0.89) | 0.48 (0.26–0.89) | 0.020 |
|
| |||
| Year of enrollment (≥ 2008) | 1.33 (1.03–1.72) | 1.48 (1.10–1.98) | 0.008 |
AKI = acute kidney injury, D1 = day 1 post inclusion, D3 = day 3 post inclusion, OR = odds ratio.
p following bootstrapping (1,000 samples).
Patients included n = 990; 21 -d spontaneous survival events n = 475; p < 0.001; C-statistic (95% CI) = 0.75 (0.72–0.78); α = 0.05.
Sensitivity Analysis: Variables Associated With 21-Day TFS Following Exclusion of Patients With Acetaminophen
Given the known impact of etiology on ALF course and following an interaction analysis with modeling that yielded similar overall correct classification of cases (70% vs 72%), we performed a sensitivity analysis. Therefore, following exclusion of 497 patients with acetaminophen (the most common etiology), the multivariable analysis was performed including the same covariates, except for etiology (Table S4, Supplementary Digital Content, http://links.lww.com/CCM/H125).This analysis yielded that only INR was independently associated with lower odds of 21-day TFS (aOR [95% CI] 0.82 [0.74–0.92]). Therefore, among patients with ALF caused by etiologies different than acetaminophen, kidney recovery on day 3 was not associated with 21-day TFS. The discriminative ability for this model was worse than the previous one (C-statistic [95% CI] 0.64 [0.59–0.69]).
Variables Associated With Receipt of LT Within First 21 Days
Both AKI on days 1 (45.1% vs 67.1%) and 3 (persistent or late-onset: 51.6% vs 65.4%) were less frequent in patients who received LT by day 21 (end of follow-up of ALFSG study) compared with those who did not (Table S5, Supplementary Digital Content, http://links.lww.com/CCM/H125).
Following adjustment for clinically and statistically significant confounders, six variables were independently associated with receipt of LT by day 21 (Table S6, Supplementary Digital Content, http://links.lww.com/CCM/H125): age (aOR [95% CI] 0.98 [0.97–0.99]), acetaminophen (aOR [95% CI] 0.13 [0.08–0.20]), transient AKI (aOR [95% CI] 0.26 [0.10–0.68]), persistent AKI (aOR [95% CI] 0.63 [0.43–0.92]), and enrollment greater than or equal to 2008 (aOR [95% CI] 0.64 [0.45–0.92]) were associated with lower odds of receipt of LT by day 21; conversely, INR (aOR [95% CI] 1.15 [1.07–1.22]) was associated with higher odds of receipt of LT by day 21. Noticeably, late-onset AKI was not associated with 21-day LT (aOR [95% CI] 0.97 [0.51–1.85]). The discriminative ability of this model was good (C-statistic [95% CI] 0.79 [0.75–0.82]).
DISCUSSION
Key Results and Comparisons With Previous Literature
Previous studies have reported on AKI in patients with ALF. In a multicenter study from the U.S.-ALFSG (n = 1604), AKI was diagnosed in 69.8% of patients with ALF at any time point during follow-up (1). However, baseline creatinine and AKI criteria considered were different, which may preclude direct comparisons. In a single-center study from Germany (n = 134), AKI was diagnosed in 40.3% of patients with ALF upon ICU admission, with stage 3 in 72.2% of those (20). Although AKI definitions used were updated as ours, this cohort had only 9.7% of patients with acetaminophen against 46.4% in our cohort. This is relevant because it has been established that AKI is more prevalent among patients with acetaminophen than with other etiologies (1, 21). This is due to acetaminophen hyperacute course, characterized by a strong systemic inflammatory response syndrome. Consequently, there is higher risk of shock, which often leads to AKI. Furthermore, acetaminophen exerts direct toxicity to kidneys’ cells (22, 23).
Studies on kidney recovery following an episode of AKI in patients with ALF are lacking. In a single-center study from Portugal (n = 51), AKI was diagnosed in 66.7% of patients with ALF during the ICU stay (24). Of those patients, 70.6% had persistent AKI, and 29.4% had transient AKI as per ADQI criteria. In our cohort, among patients with AKI diagnosed on day 1, 89.3% had persistent AKI and 11.7% had transient AKI on day 3. Both the smaller sample size and the timeframe used to define AKI in the Portuguese cohort in comparison to ours may explain such differences.
In our cohort, patients with ALF and persistent or late-onset AKI had lower odds of 21-day TFS than those without AKI on day 3, contrary to those with transient AKI. In the Portuguese cohort previously mentioned, there was also an association between persistent AKI and in-hospital survival (23). However, due to the smaller size of this cohort, this association was poorly studied. Furthermore, we were able to ascertain that both persistent and late-onset AKI negatively impacted survival. To the best of our knowledge, this association has been reported in an international cohort of patients with septic shock (n = 5443), but not in patients with ALF (25). Additionally, although survivors at 21 days without LT had a similar rate of AKI on day 1 (62.3% vs 64.0%) than others, they actually differed on the type of kidney recovery on day 3. This suggests that the duration of AKI may have had greater impact on 21-day TFS than its initial diagnosis per se.
In our cohort, although the type of kidney recovery was associated with 21-day TFS for the whole set of patients with ALF, following exclusion of those with acetaminophen, this association did not stand. Some specific characteristics of acetaminophen may have contributed to this: the most frequent single etiology (46.4%), the greatest rate of transient AKI (58.3% vs < 51%), the lowest rate of 21-day LT (15.6% vs 54.0%), and the greatest rate of 21-day TFS (62.1% vs 27.7%) (18, 20, 21).
Overall, the following remarks about our findings should be noted. First, AKI on day 1 was highly prevalent among patients with ALF. Second, the duration of AKI had a differential impact on short-term survival. Therefore, its consideration may be important to initiate timely preventive and treatment strategies for AKI and better establish these patients’ prognosis (9).
Limitations
The following limitations warrant consideration. First, this retrospective cohort may have been prone to selection bias. However, the large sample size, multicenter character, and the use of data from prospectively enrolled patients meeting entry criteria in the U.S.-ALFSG registry may have minimized the risk of such bias. Second, the study included patients for 18 years; therefore, advances in the standard of care provided may have affected the evolution of patients’ outcomes overtime. To minimize this limitation, the multivariable analysis was adjusted for time of inclusion. Third, we were unable to capture kidney function variables prior to study inclusion, whether there was evolving kidney dysfunction or CKD, which may have influenced the classification of kidney recovery following AKI. However, in a previous study, the prevalence of CKD in patients with ALF was only 5.5%, a rate presumably also low in our sample, therefore unlikely to substantially change the definitions of AKI and kidney recovery we have used (1). Fourth, the use of the MDRD formula to estimate baseline creatinine may have overestimated AKI diagnosis. However, any estimation of baseline creatinine has limitations and ADQI suggests that MDRD is the most sensitive for detecting AKI especially in patients with low risk of CKD (9). Fifthly, we lacked data on urine output to potentially improve the diagnostic accuracy of AKI (9, 14, 15). However, in the absence of urine output, serum creatinine continues to be a widely used surrogate to assess kidney function in critically ill patients. Furthermore, a previous study on ALF did not find added value by incorporating urine output for AKI diagnosis (24).
Despite these limitations, we believe that our findings add to the literature. To the best of our knowledge, this is the largest multicenter study to evaluate kidney recovery following an episode of AKI in patients with ALF. To further study AKI in ALF, studies should potentially address the following issues: 1) the pathophysiology of AKI and kidney function work up in different etiologies of ALF, 2) the impact of AKI in ALF for LT selection, 3) and optimized strategies for the management of AKI in ALF, including extracorporeal circulatory support.
CONCLUSIONS
In a large multicenter cohort of patients with ALF, persistent but not transient AKI was independently associated with lower short-term TFS. This may help to improve early management and prognostic assessment of these patients.
Supplementary Material
ACKNOWLEDGMENTS
Members and institutions participating in the Acute Liver Failure Study Group (1998–2016) are as follows: W. M. Lee, MD (Principal Investigator); Anne M. Larson, MD, Iris Liou, MD, University of Washington, Seattle, WA; Oren Fix, MD, Swedish Medical Center, Seattle, WA; Michael Schilsky, MD, Yale University, New Haven, CT; Timothy McCashland, MD, University of Nebraska, Omaha, NE; J. Eileen Hay, MBBS, Mayo Clinic, Rochester, MN; Natalie Murray, MD, Baylor University Medical Center, Dallas, TX; A. Obaid S. Shaikh, MD, University of Pittsburgh, Pittsburgh, PA; Andres Blei, MD, Northwestern University, Chicago, IL (deceased), Daniel Ganger, MD, Northwestern University, Chicago, IL; Atif Zaman, MD, University of Oregon, Portland, OR; Steven H. B. Han, MD, University of California, Los Angeles, CA; Robert Fontana, MD, University of Michigan, Ann Arbor, MI; Brendan McGuire, MD, University of Alabama, Birmingham, AL; Raymond T. Chung, MD, Massachusetts General Hospital, Boston, MA; Alastair Smith, MB, ChB, Duke University Medical Center, Durham, NC; Robert Brown, MD, Cornell/Columbia University, New York, NY; Jeffrey Crippin, MD, Washington University, St Louis, MO; Edwin Harrison, Mayo Clinic, Scottsdale, AZ; Adrian Reuben, MBBS, Medical University of South Carolina, Charleston, SC; Santiago Munoz, MD, Albert Einstein Medical Center, Philadelphia, PA; Rajender Reddy, MD, University of Pennsylvania, Philadelphia, PA; R. Todd Stravitz, MD, Virginia Commonwealth University, Richmond, VA; Lorenzo Rossaro, MD, University of California Davis, Sacramento, CA; Raj Satyanarayana, MD, Mayo Clinic, Jacksonville, FL; and Tarek Hassanein, MD, University of California, San Diego, CA; Constantine J. Karvellas, MD, University of Alberta, Edmonton, AB, Canada; Jodi Olson, MD, University of Kansas, Kansas City, KS; Ram Subramanian, MD, Emory, Atlanta, GA; James Hanje, MD, Ohio State University, Columbus, OH; Bilal Hameed, MD, University of California San Francisco, CA. The University of Texas Southwestern Administrative Group included: Grace Samuel, Ezmina Lalani, Carla Pezzia, and Corron Sanders, PhD, Nahid Attar, Linda S. Hynan, PhD, and the Medical University of South Carolina Data Coordination Unit included Valerie Durkalski, PhD, Wenle Zhao, PhD, Jaime Speiser, Catherine Dillon, Holly Battenhouse, and Michelle Gottfried. The Canadian Association for the Study of the Liver. The Liver Failure Study Group of the Portuguese Society of Intensive Care Medicine.
Supported, in part, by the National Institute of Health grant U-01 58369 (from the National Institute of Diabetes and Digestive and Kidney Diseases).
Drs. Cardoso, Lee, and Karvellas received support for article research from the National Institutes of Health. Dr. Bagshaw received funding from Baxter and BioPorto. Dr. Olson received funding from Mallinckrodt Pharmaceuticals. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
REFERENCES
- 1.Tujios SR, Hynan LS, Vazquez MA, et al. ; Acute Liver Failure Study Group: Risk factors and outcomes of acute kidney injury in patients with acute liver failure. Clin Gastroenterol Hepatol 2015; 13:352–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duff S, Murray PT: Defining early recovery of acute kidney injury. Clin J Am Soc Nephrol 2020; 15:1358–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uchino S, Bellomo R, Bagshaw SM, et al. : Transient azotaemia is associated with a high risk of death in hospitalized patients. Nephrol Dial Transplant 2010; 25:1833–1839 [DOI] [PubMed] [Google Scholar]
- 4.Perinel S, Vincent F, Lautrette A, et al. : Transient and persistent acute kidney injury and the risk of hospital mortality in critically ill patients: Results of a multicenter cohort study. Crit Care Med 2015; 43:e269–e275 [DOI] [PubMed] [Google Scholar]
- 5.Bhatraju PK, Zelnick LR, Chinchilli VM, et al. : Association between early recovery of kidney function after acute kidney injury and long-term clinical outcomes. JAMA Netw Open 2020; 3:e202682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kellum JA, Sileanu FE, Bihorac A, et al. : Recovery after acute kidney injury. Am J Respir Crit Care Med 2017; 195:784–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basu RK, Hackbarth R, Gillespie S, et al. Clinical phenotypes of acute kidney injury are associated with unique outcomes in critically ill septic children. Pediatr Res 2021; 2:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group: KDIGO clinical practice guideline for acute kidney injury. Kidney Int 2012; 2(suppl):1–138 [Google Scholar]
- 9.Chawla LS, Bellomo R, Bihorac A, et al. ; Acute Disease Quality Initiative Workgroup 16: Acute kidney disease and renal recovery: Consensus report of the acute disease quality initiative (ADQI) 16 workgroup. Nat Rev Nephrol 2017; 13:241–257 [DOI] [PubMed] [Google Scholar]
- 10.Uchino S: The meaning of transient azotemia. Contrib Nephrol 2010; 165:337–344 [DOI] [PubMed] [Google Scholar]
- 11.World Medical Association: World medical association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013; 310:2191–2194 [DOI] [PubMed] [Google Scholar]
- 12.von Elm E, Altman DG, Egger M, et al. ; STROBE Initiative: The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007; 370:1453–1457 [DOI] [PubMed] [Google Scholar]
- 13.Lee W, Larson AM, Stravitz RT: AASLD position paper: The management of acute liver failure: Update 2011, page 5. Available at: https://www.aasld.org/sites/default/files/2019-06/AcuteLiverFailureUpdate201journalformat1.pdf. Accessed April 18, 2022
- 14.Bellomo R, Ronco C, Kellum JA, et al. ; Acute Dialysis Quality Initiative workgroup: Acute renal failure - Definition, outcome measures, animal models, fluid therapy and information technology needs: The second international consensus conference of the Acute Dialysis Quality Initiative (ADQI) group. Crit Care 2004; 8:R204–R212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 2002; 39(2 Suppl 1):S1–S266 [PubMed] [Google Scholar]
- 16.McPhail MJ, Farne H, Senvar N, et al. : Ability of King’s College criteria and model for end-stage liver disease scores to predict mortality of patients with acute liver failure: A meta-analysis. Clin Gastroenterol Hepatol 2016; 14:516–525.e5; quiz e43 [DOI] [PubMed] [Google Scholar]
- 17.Bernal W, Hyyrylainen A, Gera A, et al. : Lessons from lookback in acute liver failure? A single centre experience of 3300 patients. J Hepatol 2013; 59:74–80 [DOI] [PubMed] [Google Scholar]
- 18.Lee WM, Hynan LS, Rossaro L, et al. ; Acute Liver Failure Study Group: Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology 2009; 137:856–64, 864.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacDonald AJ, Speiser JL, Ganger DR, et al. ; US Acute Liver Failure Study Group: Clinical and neurologic outcomes in acetaminophen-induced acute liver failure: A 21-year multicenter cohort study. Clin Gastroenterol Hepatol 2021; 19:2615–2625.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hadem J, Kielstein JT, Manns MP, et al. : Outcomes of renal dysfunction in patients with acute liver failure. United European Gastroenterol J 2019; 7:388–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cardoso FS, Marcelino P, Bagulho L, et al. : Acute liver failure: An up-to-date approach. J Crit Care 2017; 39:25–30 [DOI] [PubMed] [Google Scholar]
- 22.Moore JK, Love E, Craig DG, et al. : Acute kidney injury in acute liver failure: A review. Expert Rev Gastroenterol Hepatol 2013; 7:701–712 [DOI] [PubMed] [Google Scholar]
- 23.Bernal W, Lee WM, Wendon J, et al. : Acute liver failure: A curable disease by 2024? J Hepatol 2015; 62:S112–S120 [DOI] [PubMed] [Google Scholar]
- 24.Coelho S, Fonseca JN, Gameiro J, et al. : Transient and persistent acute kidney injury in acute liver failure. J Nephrol 2019; 32:289–296 [DOI] [PubMed] [Google Scholar]
- 25.Sood MM, Shafer LA, Ho J, et al. ; Cooperative Antimicrobial Therapy in Septic Shock (CATSS) Database Research Group: Early reversible acute kidney injury is associated with improved survival in septic shock. J Crit Care 2014; 29:711–717 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


