Abstract
Introduction
The anterior pituitary gland (PG) is a potential locus of hypothalamic–pituitary–adrenal (HPA) axis responsivity to early life stress, with documented associations between dehydroepiandrosterone (DHEA) levels and anterior PG volumes. In adults, elevated anxiety/depressive symptoms are related to diminished DHEA levels, and studies have shown a positive relationship between DHEA and anterior pituitary volumes. However, specific links between responses to stress, DHEA levels, and anterior pituitary volume have not been established in developmental samples.
Methods
High‐resolution T1‐weighted MRI scans were collected from 137 healthy youth (9–17 years; M age = 12.99 (SD = 1.87); 49% female; 85% White, 4% Indigenous, 1% Asian, 4% Black, 4% multiracial, 2% not reported). The anterior and posterior PGs were manually traced by trained raters. We examined the mediating effects of salivary DHEA on trauma‐related symptoms (i.e., anxiety, depression, and posttraumatic) and PG volumes as well as an alternative model examining mediating effects of PG volume on DHEA and trauma‐related symptoms.
Results
DHEA mediated the association between anxiety symptoms and anterior PG volume. Specifically, higher anxiety symptoms related to lower DHEA levels, which in turn were related to smaller anterior PG.
Conclusions
These results shed light on the neurobiological sequelae of elevated anxiety in youth and are consistent with adult findings showing suppressed levels of DHEA in those with greater comorbid anxiety and depression. Specifically, adolescents with greater subclinical anxiety may exhibit diminished levels of DHEA during the pubertal window, which may be associated with disruptions in anterior PG growth.
Keywords: brain structure, DHEA, MRI, pituitary, stress
In a sample of typically‐developing youth (9–17 years), mediating effects of DHEA levels on the relationship between trauma‐related anxiety symptoms and anterior pituitary volume were uncovered. Specifically, higher anxiety symptoms related to lower DHEA levels in adolescents, which in turn related to smaller anterior pituitary gland volume. This study highlights the utility of manually tracing the anterior and posterior pituitary glands separately, and that the anterior pituitary may be a litmus for neurobiological responses to early life stress.
1. INTRODUCTION
A fundamental component of the human neuroendocrine and stress response system is the hypothalamic–pituitary–adrenal (HPA) axis, which instigates and regulates a series of intricate mechanisms critical to stress reactivity, hormone regulation, and immune functioning (Bateman et al., 1989; Handa et al., 1994; Herman et al., 2016; Tsigos & Chrousos, 2002). Briefly, the HPA axis contains the paraventricularis nucleus of the hypothalamus, which releases arginine vasopressin and corticotrophin‐releasing hormone, particularly in the context of stress. These factors act upon the pituitary gland (PG), a neuroendocrine structure at the base of the brain with two primary components: the anterior and posterior lobes. When the PG senses arginine vasopressin and corticotrophin‐releasing hormone, it releases adrenocorticotropic hormone into circulation to act upon the adrenal gland cortex. The adrenal gland is responsible for secreting glucocorticoids, such as cortisol and dehydroepiandrosterone sulfate (DHEA‐S). Importantly, the HPA axis has a critical negative feedback loop insofar as glucocorticoids can regulate corticotrophin‐releasing hormone‐producing neurons in the hypothalamus, thereby influencing the full extent of the HPA axis (Gjerstad et al., 2018). In other words, the pituitary maintains a primary role in neuroendocrine regulation and stress responsivity. Thus, it is unsurprising that the most well‐documented effects involving PG structure are specifically related to stress responsivity and mental health disorders, particularly during development (Ganella et al., 2015; MacMaster et al., 2008; MacMaster & Kusumakar, 2004; Whittle et al., 2012; Zipursky et al., 2011).
The PG undergoes a protracted developmental trajectory, with volumetric increases well into adolescence and pubertal development (Sari et al., 2014; Wong et al., 2014). Given the known role of the PG in stress functions, a burgeoning literature provides some evidence for correspondence between PG volume and mental health disorders, including internalizing symptoms during adolescence. Though mixed (Díaz‐Arteche et al., 2021; Farrow et al., 2020; Ganella et al., 2015), some findings suggest that larger whole PG volume may be associated with elevated or clinical levels of mental health symptoms. For instance, larger PG volume has been shown to prospectively predict an increase in internalizing symptoms during early to mid‐adolescence (Zipursky et al., 2011). Furthermore, others have observed that adolescents with bipolar and unipolar depression had larger PG volume compared to controls; however, the participants with mood disorders did not significantly differ from each other in PG volume (MacMaster et al., 2008). An earlier study showed that adolescents with major depressive disorders had a 25% increase in PG volume compared to control participants, and that age was only correlated with PG volume in controls (MacMaster & Kusumakar, 2004). Larger PG volume has also been found to prospectively mediate the relation between higher depressive symptoms and early pubertal onset for both males and females (Whittle et al., 2012). Finally, a limited amount of work has shown that stressful childhood experiences (e.g., child maltreatment) may accelerate PG growth in females, although in that study PG volume did not portend psychopathology symptoms (Ganella et al., 2015). Given that prior work has largely focused on whole PG volume, those findings do not address the extent to which the anterior lobe may specifically relate to emerging symptomology in youth, given its role in stress reactivity and hormonal output (Le Tissier et al., 2012; Martí & Armario, 1998). Further, stress‐related variations in anterior versus posterior volumes may contribute to the mixed findings noted above and decrease sensitivity to real effects.
An emerging literature has begun to examine how pubertal hormone levels relate to PG volume during the transition from childhood to adolescence and how this may impact mental health symptoms. In a longitudinal study of 8–13 year olds in which PG volumes were estimated, and salivary testosterone, DHEA, and DHEA‐S were collected, it was found that above and beyond age, each pubertal hormone predicted PG volume development (Whittle et al., 2020). This relation differed in sex such that female participants consistently showed a stronger association compared to males (Whittle et al., 2020). Moreover, relatively high DHEA and DHEA‐S levels have been associated with larger PG volume, with PG volume mediating the relationship between high DHEA/DHEA‐S levels and overall anxiety symptoms, which did not differ by sex (Murray et al., 2016). Taken together, this growing body of literature suggests that greater levels of DHEA may represent a risk factor for larger PG volume and psychopathology symptoms.
While these findings provide key insights into the complex associations among components of the neuroendocrine system and emergent psychopathology during development, studies to date have tended to examine whole PG volume, as noted above, rather than separating the anterior and posterior lobes. This is important because the anterior and posterior PG are anatomically and functionally distinct (Kaiser & Ho, 2016; Melmed & Kleinberg, 2016). The anterior pituitary controls endocrine activity of the pituitary, whereas the posterior pituitary is primarily comprised of axon terminals ascending from the hypothalamus. Considering the distinct but complementary roles of the anterior and posterior lobes of the PG, it is crucial to examine them both separately and conjunctively. Importantly, only the anterior pituitary secretes hormones involved in the HPA axis. Indeed, decades‐old animal work has specifically highlighted the anterior pituitary's sensitivity to stressful experiences (Armario, Lopez‐Calderon, Jolin, & Balasch, et al., 1986; Armario, Lopez‐Calderon, Jolin, & Castellanos, et al., 1986; Dorshkind & Horseman, 2001; Martí & Armario, 1998), which could provide insights into vulnerability to psychopathology. Along this line, one recent study in 6‐ to 8 year‐old children, revealed that the anterior pituitary may be particularly sensitive to the effects of childhood neglect (Farrow et al., 2020), with greater levels of neglect being associated with larger anterior PG volume at baseline and 18 months later. These results suggest that early life stress may alter HPA functioning and ultimately lead to enlargement of the anterior PG. Interestingly, this study did not find associations with psychopathology symptoms, which may be due to the younger age range of the sample.
The present study sought to build upon existing literature by examining specific links between stress response symptoms (i.e., anxiety/depression), salivary DHEA levels, and anterior PG volume in a unique developmental dataset. To our knowledge, this investigation represents one of the first to integrate stress responses, pubertal hormones secreted by both the hypothalamic–pituitary–gonadal axis and the HPA axis, and PG volume in a sample of youth. Critically, we used manual tracing to separate the anterior and posterior PG volume, which is considered the gold standard for estimating volumes of small brain structures (Murray et al., 2016; Pariante et al., 2004) and provided us with the precision needed to look at these functionally and structurally divergent components of the pituitary. Because the age range of our sample (i.e., 9–17 years), observable symptomatology of stress has likely already emerged. Moreover, recent calls in the literature have urged researchers to consider responsivity to stress (Smith & Pollak, 2020, 2021) as a key individual differences factor in predicting neurodevelopmental patterns following early life stress. For this reason and because DHEA levels are predictive of PG size during development (Whittle et al., 2020), we examined whether DHEA concentration mediated associations between stress‐related symptoms and anterior and posterior pituitary volume. Based upon prior literature showing that elevated stress and DHEA levels, respectively, relate to larger whole PG volumes, we hypothesized that greater symptoms would be related to elevated DHEA, which in turn would be associated with larger anterior PG volume. We also tested a plausible, alternative model in which anterior and posterior PG volumes mediate the association between DHEA levels and stress symptoms. This alternative model was tested, as it is comparable with previously proposed brain‐mediated models in the literature examining associations between early life stress and PG volume (Murray et al., 2016; Whittle et al., 2012). Support for this alternative model would suggest that stress symptoms, per se, may not be an influencing factor in alterations to potential markers of HPA axis functioning (i.e., DHEA levels and anterior PG volume, in the current study) and that sensitization of the system may confer risk for elevated symptomology. Finally, in order to address replicability of prior study approaches (e.g., Murray et al., 2016; Whittle et al., 2012, 2020) and whether there are general, and not lobe specific, effects of PG volume in our sample, we have tested both models using whole PG volume as well.
2. MATERIALS AND METHODS
2.1. Participants
A sample of 153 healthy children and adolescents ages 9–17 years old completed a structural magnetic resonance imaging (MRI) scan and hormonal assay as part of the Developmental Chronnecto‐Genomics study (meanage = 13.06 years, SD = 1.92; 74 females; Stephen et al., 2021). The study was multisite, with 74 participants recruited at the University of Nebraska Medical Center (UNMC) and 79 participants from the Mind Research Network (MRN) for the initial study assessment (https://devcog.mrn.org/). Participants were invited back to participate annually for 3 years. Note that only a single time point of data was examined in the present, cross‐sectional investigation as a first step in this line of inquiry and to maximize statistical power. That is, the current study evaluated only those participants with a high‐resolution MRI scan and a valid DHEA hormone sample from the same time point (i.e., the MRI scan and hormone assessment were completed at the same visit). After excluding 16 participants (~12%) due to excessive motion and/or artifacts in their MRI data, the final sample included 137 participants. Inclusion criteria included English as a primary language, age, and participant and parent willingness to assent/consent, respectively. Exclusion criteria determined via parent report were as follows: history of developmental delays and/or diagnosed psychiatric disorders, history of neurological disorders (e.g., epilepsy), history of concussion or head injury, pregnancy, prenatal exposure to drugs, use of medications known to affect brain function, and MRI contraindications (e.g., orthodontia, metallic foreign bodies). All parents and youth provided written consent or assent, respectively, prior to participating in the study. The appropriate institutional review boards for both study sites approved all study procedures.
2.2. Structural Neuroimaging Acquisition and Processing
Participants underwent a structural T1‐weighted MRI with either a Siemens 3 T Skyra scanner at the UNMC study site (N = 64) or a Siemens 3 T TIM Trio at the MRN site (N = 73). MR images at both data collection sites were acquired with a 32‐channel head coil and an isotropic MPRAGE sequence with the following parameters: repetition time = 2400 ms; echo time = 1.94 ms; flip angle = 8°; field of view = 256 mm; slice thickness = 1 mm (no gap); base resolution = 256; 192 slices; voxel size = 1 × 1 × 1 mm. The images of all participants were processed using FreeSurfer version 6 (http://surfer.nmr.mgh.harvard.edu) to extract total intracranial volume (ICV) estimates. We followed the ENIGMA protocol for quality assurance, including performing visual checks on all images (http://enigma.usc.edu/protocols/imaging-protocols) and checking for motion artifacts.
2.3. Pituitary tracing
The anterior and posterior lobes of the PG were manually traced using the 3D Slicer software (v4.11, www.slicer.org; Fedorov et al., 2012). Prior work has demonstrated that the pituitary is optimally visualized in the coronal and sagittal planes of T1‐weighted MR images (Díaz‐Arteche et al., 2021; Farrow et al., 2020; Lorenzetti et al., 2009; Whittle et al., 2012). The pituitary was defined based upon previously published tracing techniques (Farrow et al., 2020). The infundibular stalk of the PG was excluded from tracing, as prior work has done (Díaz‐Arteche et al., 2021; Farrow et al., 2020; Lorenzetti et al., 2009; Whittle et al., 2012). Several boundaries and structures were visually inspected by the tracers to determine the boundaries of the PG (Figure 1). Specifically, in the coronal view, the diaphragm sellae and lateral ventricles were located superiorly, the sphenoid sinus was located inferiorly and bilaterally, and the internal carotid arteries were located bilaterally. Contrast differences in the sagittal view were used to determine the boundary between the anterior and posterior lobes of the PG, which appear darker and lighter, respectively (Figure 1). Estimates for the anterior and posterior lobe volumes were computed by summing all the voxels selected by tracers across each slice. The full sample was split in half and traced by two pairs of trained tracers (i.e., Lauren Ott ‐ Nathan Petro, Giorgia Picci ‐ Chloe Casagrande). Each tracer traced both the anterior and posterior lobes. Intraclass coefficients (ICCs) with absolute agreement were calculated for anterior and posterior traces, and were .94–.97 and .93–.94, respectively.
FIGURE 1.
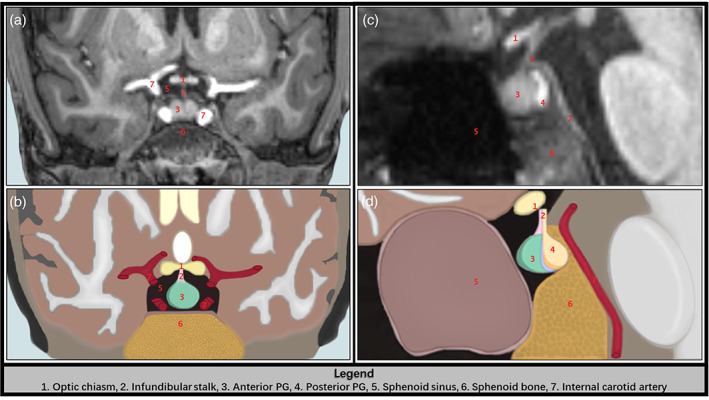
The anatomical boundaries used to trace the PG. (a) and (c) are coronal and sagittal slices from a T1‐weighted scan from a representative participant, while (b) and (d) are illustrated depictions of the representative T1 slices in (a) and (c). The red numbers correspond to the anterior and posterior lobes of the pituitary gland, as well as the anatomical boundaries used to manually trace the anterior and posterior PG. Refer to the legend (bottom of figure) for the anatomical structures corresponding to each red number in panels a–d.
2.4. Hormonal assays
At least 2.0 mL of whole unstimulated saliva was collected from each participant. Specifically, children were asked to passively drool into an Oragene DISCOVER (OGR‐500; www.dnagenotek.com) collection tube until liquid saliva (not bubbles) exceeded the fill line indicated on the tube. Participants were instructed to refrain from consuming any food, liquids, or chewing gum for at least an hour before providing the saliva sample, and generally completed the study in the afternoon. Prior to the release of the protease inhibitors for long‐term storage, a single‐channel pipette was used to extract 0.5 mL from the collection tube, which was immediately transferred into a labeled micro‐centrifuge tube and placed in a −20°C freezer for storage. All samples were assayed together with duplicate testing using a commercially available assay kit for salivary DHEA. The assay kit had a sensitivity of 5 pg/mL, with a range of 10.2–1000 pg/mL. The intra‐ and inter‐assay coefficients of variation were 7.95% and 10.17%, respectively. The averages of duplicate tests were used for analyses in the present study. A log transform was applied to DHEA measurements to account for skewness of the raw data (before transform: skewness = 3.22, kurtosis = 12.29; after transform: skewness = 0.36, kurtosis = −0.05).
2.5. Trauma symptoms
Participants completed the self‐report Trauma Symptom Checklist for Children (TSCC; Briere, 1996), which measures the frequency of symptoms on a scale of 0 to 3 across a number of psychopathology scales. Higher scores indicate greater symptom severity. Items load onto six symptom subscales that are summed, including depression, anxiety, and posttraumatic stress, which were examined here. The depression symptoms subscale focuses on feelings of loneliness and sadness. The anxiety symptoms subscale assesses specific phobias as well as generalized worry. The posttraumatic stress symptoms subscale probes intrusive thoughts, avoidance, and nightmares. Two items assessing suicidality were excluded due to Institutional Review Board concerns. A log transform was applied to raw scores of all three scales to account for skewness in the data (before transform: anxiety skew = −3.32, kurtosis = 9.15; after transform: anxiety skew = −0.41, kurtosis = −0.21; before: depression skew = −3.32, kurtosis = 9.15; after: depression skew = −0.21, kurtosis = −0.29; before: posttraumatic stress skew = −3.31, kurtosis = 9.14; after: posttraumatic stress skew = −0.32, kurtosis = −0.30). These transformed raw scores were used in all analyses.
2.6. Data analytic plan
We began by running descriptive statistics on demographics and all variables of interest. Variables entered into subsequent models were examined for violations of normality (i.e., skewness and kurtosis) and were transformed according to their distribution type (e.g., positive vs. negative skew). Next, we fit structural equation models to estimate (1) whether DHEA levels mediated the association between concurrent anxiety, depression, and posttraumatic stress symptoms and anterior and/or posterior PG volumes or (2) whether anterior and posterior PG volumes mediated associations between DHEA levels and concurrent psychopathology symptoms (see Figure 2 for model examples). In addition to these main analyses, we also examined these same models with total PG volume instead of separate anterior and posterior PG volumes.
FIGURE 2.
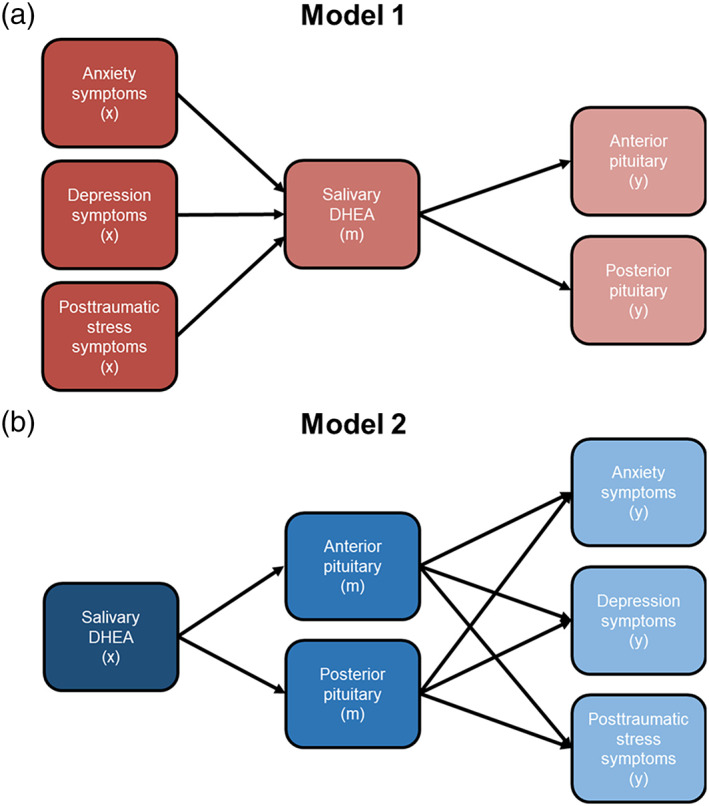
Competing models testing mediating effects of PG volume and DHEA levels. (a) Model 1 depicts salivary DHEA levels mediating associations between trauma‐related anxiety, depression, and posttraumatic stress symptoms and anterior and posterior PG volume. (b) Model 2 shows an alternative model whereby anterior and posterior PG volume mediate associations between salivary DHEA levels and anxiety, depression, and posttraumatic stress symptoms. For simplicity, the direct effects from x to y are not shown in (a) or (b) but were tested. In addition, covariates included age, sex, and study site for all variables. Intracranial volume estimates were also a covariate for anterior and posterior PG volume only. x = predictor, m = mediator, y = outcome variable.
Base models were fit to examine the effect of independent variables on the dependent variables. Next, covariates of no interest were introduced into the model, which included sex, age, study site, and total ICV. Anterior and posterior PG volume, or whole PG volume, were regressed on ICV. After introducing the covariates, models were fit to examine the mediating effect of either DHEA levels on the association between symptomology and anterior and posterior PG volumes (i.e., indirect effects of symptomology via DHEA on anterior and posterior PG volume estimates) or the mediating effect of anterior and posterior PG volume on the association between DHEA levels and symptomology levels (i.e., indirect effects of PG volume via DHEA on symptomology). Note that identical models were also run with whole PG volume as well. In addition, in order to examine patterns of effects in each sex, we also ran both sets of models separately by sex, which we report in Tables S6, S7 of Data S1, Supporting Information. All mediation models were bootstrapped with 1000 iterations with bias‐corrected bootstrapping to test for significance of the indirect associations based upon the 95% confidence intervals (MacKinnon et al., 2004). Unstandardized estimates of individual paths were examined for the directionality of relations. Finally, all symptomology scales (i.e., anxiety, depression, and posttraumatic stress symptoms derived from the TSCC) and PG volume estimates (i.e., anterior and posterior lobe volumes) were permitted to freely correlate and all parameters were freely estimated. All models were tested in Mplus (v7.4).
We examined the goodness of fit for both models using standard criteria (Hu & Bentler, 1999). Specifically, we evaluated models for the root mean square error of approximation (RMSEA) <0.06, standardized root mean square residual (SRMR) <0.08, and comparative fit index (CFI) >0.95. We also examined the χ 2 test of model fit, where a nonsignificant result indicates good model fit.
3. RESULTS
3.1. Demographic statistics
Descriptive statistics and demographic variables for the final sample (N = 137) are reported in Table 1. Correlations among study variables of interest are reported in Table S1. There were study site differences in ethnicity whereby the MRN site had more Latinx participants compared to the UNMC site (all p < .05, Table 1). There were no other site differences in participant distributions of age, sex, race, and symptomology. Importantly, study site was included in all models as a covariate to account for these initial differences.
TABLE 1.
Descriptive statistics and study site comparisons.
| Variable | Full sample (n = 137) | |
|---|---|---|
| n | % | |
| Sex, female | 67 | 49 |
| Race | ||
| I/AN | 5 | 4 |
| Asian | 1 | 1 |
| B/AA | 5 | 4 |
| White | 117 | 85 |
| More than 1 race | 6 | 4 |
| Not reported | 3 | 2 |
| Ethnicity | ||
| Latinx | 28 | 20 |
| Not Latinx | 109 | 80 |
| Not reported | 0 | 0 |
| Mean (SD) | Range | |
|---|---|---|
| Age | 12.99 (1.87) | 9–17 |
| DHEA | 54.08 (64.60) | 5.15–418 |
| Anxiety symptoms | 5.20 (3.91) | 0–18 |
| Depression symptoms | 3.39 (2.98) | 0–19 |
| Posttraumatic stress symptoms | 6.06 (4.98) | 0–22 |
| Anterior pituitary volume | 406.91 (153.48) | 87–851 |
| Posterior pituitary volume | 91.82 (35.53) | 29–215 |
| Whole pituitary volume | 498.73 (163.24) | 162–936 |
Note: Brain measures are reported in volume mm3. Age is reported in years, at the time of the scan. DHEA is reported in picograms per milliliter (pg/mL). Anxiety, depression, and posttraumatic stress symptoms are all raw scores from the Trauma Symptom Checklist for Children.
Abbreviations: AI/AN = American Indian/Alaska Native, B/AA = Black, African American; L, left; R, right; T, time.
3.2. Mediation results: DHEA as mediator
The DHEA mediation model had excellent fit (χ 2(4) = 4.44, p = .35; RMSEA = 0.03; SRMR = 0.02; CFI = 1.00). Table 2 reports all direct and indirect effects and all path estimates are reported in Table S2. In terms of the mediation results, DHEA mediated the association between anxiety symptoms and anterior PG volume (b = −0.14; 95% CI [−0.32, −0.03]; Figure 3). There was no direct path between anxiety symptoms and anterior PG volume (b = 0.05; 95% CI [−0.38, 0.52]). Participants with greater anxiety symptoms had lower levels of DHEA (b = −0.03, p = .03), which were, in turn, related to smaller anterior PG volume (b = 4.42, p = .001). DHEA did not have a mediating effect on associations between depressive (b = 0.07; 95% CI [−0.06, 0.21]) or posttraumatic stress symptoms (b = 0.07; 95% CI [−0.05, 0.23]) and anterior PG volume, nor did depressive (b = −0.06; 95% CI [−0.53, 0.36]) or posttraumatic stress symptoms (b = 0.05; 95% CI [−0.42, 0.46]) have direct effects on anterior PG volume.
TABLE 2.
Direct and indirect effect estimates for mediation model 1.
| Estimates | 95% CIs | ||||
|---|---|---|---|---|---|
| Mediator | Outcome | β | b | Lower | Upper |
| Indirect effects for anxiety | |||||
| DHEA | aPG | −0.06 | −0.14 | −0.32 | −0.03 |
| pPG | 0.01 | 0.004 | −0.01 | 0.04 | |
| Indirect effects for depression | |||||
| DHEA | aPG | 0.03 | 0.07 | −0.06 | 0.21 |
| pPG | −0.004 | −0.002 | −0.03 | 0.01 | |
| Indirect effects for PTS | |||||
| DHEA | aPG | 0.03 | 0.07 | −0.05 | 0.23 |
| pPG | −0.004 | −0.002 | −0.03 | 0.01 | |
| Estimates | 95% CIs | |||
|---|---|---|---|---|
| β | b | Lower | Upper | |
| Direct effects | ||||
| Anxiety ➔ aPG | 0.02 | 0.05 | −0.38 | 0.52 |
| Anxiety ➔ pPG | 0.27 | 0.14 | 0.03 | 0.22 |
| Depression ➔ aPG | −0.02 | −0.06 | −0.53 | 0.36 |
| Depression ➔ pPG | −0.06 | −0.03 | −0.13 | 0.06 |
| PTS ➔ aPG | 0.02 | 0.05 | −0.42 | 0.46 |
| PTS ➔ pPG | −0.19 | −0.09 | −0.18 | 0.01 |
Note: Significant results are bolded based upon bootstrapped 95% confidence intervals for indirect effects and p < .05 for direct effects.
Abbreviations: aPG, anterior pituitary volume; pPG, posterior pituitary volume; PTS, posttraumatic stress.
FIGURE 3.
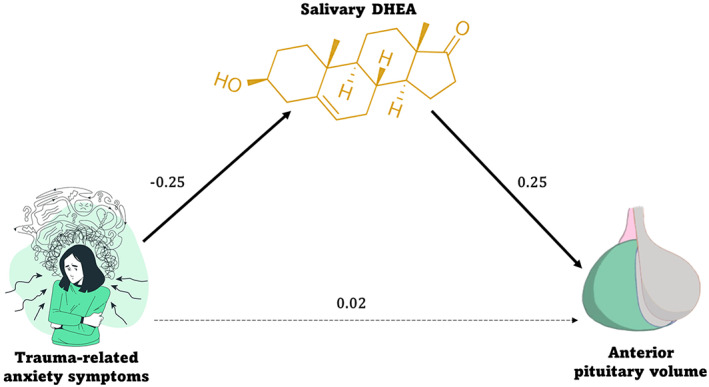
Mediation results from model 1 with DHEA as the mediator. Higher trauma‐related anxiety symptoms (x) related to lower levels of salivary DHEA (mediator), which in turn related to smaller anterior pituitary volume (y). There was no direct association between anxiety symptoms and anterior PG volume. Standardized estimates are shown in the figure, with significant paths indicated with bold black lines (p < .05) and nonsignificant paths indicated with black dotted lines.
For the posterior PG volume, there was not a mediating effect of DHEA levels on the association between anxiety symptoms and posterior PG volume (b = .004; 95% CI [−0.01, 0.04]), but there was a significant direct effect of anxiety symptoms on posterior PG volume, such that greater symptom severity related to larger posterior PG volume (b = 0.14; 95% CI [0.03, 0.22]). There were no mediation effects of DHEA for depression (b = −0.002; 95% CI [−0.03, 0.01]) or posttraumatic stress symptoms (b = −0.002; 95% CI [−0.03, 0.01]). Likewise, there were no significant direct effects of depression symptoms or posttraumatic stress symptoms on posterior PG volume (depression: b = −0.03; 95% CI [−0.13, 0.06]; posttraumatic stress: b = −0.09; 95% CI [0.18, 0.01]).
3.3. Mediation results: Anterior and posterior pituitary gland volume as mediator
There were no significant mediating effects of anterior or posterior PG volume on the association between DHEA levels and anxiety, depression, or posttraumatic stress symptom severity. Statistical results are fully reported in Tables S3, S4. Note that model fit comparisons between models 1 and 2 were done and the model fits were not statistically different based upon a χ 2 difference test (χ 2 model1 = 4.44, df = 4; χ 2 model2 = 4.65, df = 4; χ 2 diff = 0.21, p = .99). In addition, the AIC/BIC value differences were negligible (model 1 AIC = 824.85, BIC = 960.70; model 2 AIC = 825.06, BIC = 960.90), suggesting that the model fits were comparable.
3.4. Mediation results: Whole pituitary volume models
Neither mediation model (i.e., with DHEA as the mediator or PG volume as the mediator) had significant mediating effects. Specifically, there was not a significant indirect effect in the model with DHEA mediating the association between stress symptom severity and PG volume. Likewise, there was not a significant indirect effect in the model with PG volume as the mediator between DHEA levels and stress symptom severity. Statistical results are fully reported in Table S5.
3.5. Mediation results: Modeled separately by sex
None of the mediation models (i.e., with DHEA as the mediator or PG volume as the mediator in males or females) showed significant mediating effects. Specifically, there was not a significant indirect effect in the model with DHEA mediating the association between stress symptom severity and PG volume in neither males nor females. Likewise, there was not a significant indirect effect in the model with PG volume as the mediator between DHEA levels and stress symptom severity in neither males nor females. Statistical results are fully reported in Tables S6, S7.
4. DISCUSSION
The present study represents one of the first to establish key associations between understudied indicators of HPA axis functioning (i.e., DHEA levels and pituitary volume) and symptoms related to traumatic experiences in a developmental sample. We report three key findings. First, we uncovered a robust mediation effect such that greater trauma‐related anxiety symptoms related to lower DHEA levels, which was associated with smaller anterior pituitary volume. Critically, this pattern of results was not found with respect to posterior PG volume, which is consistent with extensive animal literature documenting sensitivity to stress specifically in the anterior PG (Armario, Lopez‐Calderon, Jolin, & Balasch, 1986; Armario, Lopez‐Calderon, Jolin, & Castellanos, 1986; Dorshkind & Horseman, 2001; Martí & Armario, 1998). Second, we found no evidence to support an alternative model whereby pituitary volume (anterior or posterior) mediated the association between DHEA levels and trauma‐related symptoms. Third, there was a direct effect in which greater trauma‐related anxiety symptoms were related to larger posterior pituitary volume. This finding was largely unexpected; however, most prior literature has focused on whole pituitary volumes, making it unclear to what extent posterior pituitary may be sensitive to stress effects and symptomology. Notably, we tested models incorporating whole pituitary volumes, which did not reveal mediating effects of either DHEA or whole PG volume. This stands in contrast to prior literature reporting effects with whole pituitary wholes (e.g., Murray et al., 2016; Whittle et al., 2012; Zipursky et al., 2011), and suggests that the greater specificity afforded by separating the anterior and posterior PG lobes has added value in linking pubertal hormones and stress symptomology during development. We also tested all models separately by sex, which did not yield any sex‐specific mediating effects in our sample. Critically, this study is among the first to integrate hormone levels (i.e., DHEA), pituitary volume separately by lobe, and trauma‐related symptomology in a sample of adolescents, with implications for understanding the cascade of neurobiological effects of trauma responsivity on the HPA axis during development. Taken together, these findings add to a small, but emerging literature in developmental samples that have identified the anterior pituitary as an exquisitely sensitive region to early life stress (Díaz‐Arteche et al., 2021; Farrow et al., 2020).
Our primary mediation finding indicated that greater trauma‐related anxiety symptoms corresponded with lower DHEA levels and smaller anterior pituitary volume, which contradicts some existing literature in high‐risk developmental samples. The few extant studies that have examined early life stress have shown that greater levels of trauma (e.g., child maltreatment) are associated with higher levels of DHEA and larger anterior pituitary volumes (Díaz‐Arteche et al., 2021; Farrow et al., 2020; Murray et al., 2016). Several methodological differences between the current study and those prior studies may explain this discrepancy. Specifically, not all prior studies have controlled for total intracranial volume, which would significantly affect overall estimates of pituitary volume. Moreover, although studies to date have generally taken great care to incorporate relevant control variables (e.g., age, sex, hormonal levels), they have generally not utilized structural equation modeling, as in the current study. Structural equation modeling offers sophisticated statistical techniques for examining associations between variables while simultaneously accounting for relations between other variables in the model, and it allows for specific assignment of control variables (e.g., only ICV on neural measures, as opposed to all variables). This stands in contrast to more traditional regression or ANOVA approaches that have been used in previous studies of pituitary volume in developmental cohorts. In addition, it is worth noting that most of the extant developmental literature seems to have been conducted in only three cohorts (Díaz‐Arteche et al., 2021; Farrow et al., 2020; Ganella et al., 2015; Murray et al., 2016; Zipursky et al., 2011).
Another notable difference with the current study is that the sample is a healthy developing cohort that was not oversampled for early life stress, which differs from prior work showing accelerated PG growth in samples with greater variability in exposure to early adversity than the current study (Díaz‐Arteche et al., 2021; Farrow et al., 2020; Ganella et al., 2015). Interestingly, findings are largely mixed with respect to whether larger pituitary volume is a neurobiological risk factor for emergent psychopathology, per se. That is, a number of studies with more variability in early adversity have not documented associations between trauma‐related symptomology and pituitary volume as the current study does (Díaz‐Arteche et al., 2021; Farrow et al., 2020; Ganella et al., 2015). Yet, two existing studies suggest that having a larger baseline pituitary volume may prospectively predict internalizing symptoms in adolescents (Whittle et al., 2020; Zipursky et al., 2011). Of note, these studies focused their analyses on whole pituitary volume and not anterior versus posterior pituitary volumes, which limits comparability with the current study. Relatedly, a whole volume analysis would not have yielded the unexpected finding in the current sample that higher anxiety levels were related to larger posterior pituitary volume. Our findings suggest that there are distinct links between trauma‐related anxiety and the lobes of the pituitary that necessitate further exploration in subsequent studies. Moreover, the separability of effects in the anterior and posterior pituitary lobes shown here suggest that collapsing across the whole structure could lead to spurious results and potential difficulties in replication. The mixed findings in prior work make it difficult to determine the specific roles of anterior and posterior pituitary volumes in promoting risk for psychopathology. Indeed, a previous study comparing healthy adults and adults diagnosed with major depressive disorder (MDD) did not uncover differences in whole pituitary volume, nor was whole pituitary volume associated with clinical features of the MDD diagnosis (Lorenzetti et al., 2009). This, along with previously mentioned studies in adolescent samples, suggests that although experiencing stress may confer larger PG volume, it may not necessarily explain emergent psychopathology.
The current investigation highlights more mechanistically that responses to stress (i.e., anxiety symptomology) may suppress DHEA output, which relates to smaller anterior pituitary volume. These findings and interpretation are corroborated by findings demonstrating neuroprotective effects of elevated DHEA levels against internalizing symptomology (Farooqi et al., 2018). Conversely, systematically lower levels of DHEA have been found in patients with internalizing disorders (Mocking et al., 2015). Mechanistically, DHEA has been shown to be an antagonist of glucocorticoid receptors, suggesting that greater levels of DHEA may protect against release of glucocorticoid in response to stressful experiences (Chen et al., 2015; Jiang et al., 2017; Kalimi et al., 1994; Kamin & Kertes, 2017). Taken together, results presented here are consistent with this prior literature on neuroprotective effects of DHEA, which has generally been separate from the developmental literature examining early life stress and pituitary volume. In other words, although inconsistent with prior developmental findings, our results support the notion that stress symptomology (i.e., anxiety symptoms) may correspond with lower levels of DHEA, which may have targeted attenuation effects on anterior pituitary volume during adolescence. These interpretations will need to be systematically followed‐up with a longitudinal investigation probing such effects.
Although the current study has many strengths, there are several limitations that must be acknowledged. Specifically, the present study was cross‐sectional in nature, which limits any causal interpretations regarding developmental changes among the variables examined (i.e., pituitary volume, symptomology, hormone levels). Documenting changes in any of these variables and their correspondence across time would add substantially to our understanding of the neurobiological cascade of stress effects on the developing brain. It should also be noted that the present sample was not entirely representative of the demographics of the general US population, particularly with respect to the racial composition of our sample, which is predominantly white and lacking in Black/African American representation. As has been recently noted by others (Garcini et al., 2022), it is paramount that greater efforts be made in future studies to have more accurate population‐level representation by including historically underrecognized racial and ethnic communities in research. In addition, participants in the present study were healthy youth and did not have any diagnosed mental health disorders upon entry into the study, which limits generalizability to samples at heightened risk for developmental psychopathology. Thus, future work would benefit from examining the links established here in a higher‐risk sample with greater variability in clinical‐levels of psychopathology. However, we think that there is utility in capturing subclinical, or even preclinical, processes that may underlie neurobiological responses to trauma; establishing possible prodromal biomarkers is essential to understanding the emergence, progression, and eventual targeted treatment of psychopathology that may follow exposure to early life stress. Moreover, given the known diurnal fluctuations of DHEA (Matchock et al., 2007), it should be acknowledged that, despite being collected at comparable times of day across participants, time of hormone collection was not obtained in the current sample. Finally, other environmental and neurobiological factors (e.g., parenting and epigenetic factors, respectively) cannot be ruled out from influencing or better explaining the pattern of results reported here. As such, subsequent studies should evaluate the moderating and mediating effects of such factors in order to better capture the highly complex interplay of environmental and neurobiological mechanisms that influence the cascade of responses to early life stress.
5. CONCLUSIONS
The current study aimed to investigate the mediating effects of DHEA levels on associations between trauma‐related symptoms and PG volume in a sample of healthy children and adolescents. We uncovered a robust mediation effect whereby greater levels of trauma‐related anxiety were linked to lower DHEA levels, which in turn related to decreased anterior PG volume. These findings corroborate prior work suggesting that DHEA suppression may be a pertinent risk factor for subclinical and clinical symptomatology. This work underscores the complex interplay among hormonal levels and stress‐sensitivity of the anterior PG, which is a pivotal structure in the HPA axis. Characterizing biological sequalae of stress is essential to understanding subclinical and even pre‐clinical biomarkers, including the dynamics of hormonal and neurobiological processes, which may serve as potential targets of preventative intervention aimed at protecting against emergent psychopathology.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare.
Supporting information
Data S1: Supporting information.
ACKNOWLEDGEMENTS
This work was supported by the National Science Foundation (#1539067 to Tony W. Wilson, Yu‐Ping Wang, Julia M. Stephen, and Vince D. Calhoun), the National Institutes of Health (R01‐MH121101, R01‐MH116782, R01‐MH118013, and P20‐GM144641 to Tony W. Wilson; R01‐EB020407 and R01‐MH118695 to Vince D. Calhoun), and At Ease, USA. Funding agencies had no part in the study design or the writing of this report. All salivary assays were performed at the University of Nebraska‐Lincoln Salivary Bioscience Laboratory. The authors thank Dr. Jessica L. Calvi for her insight and assistance with this aspect of the study.
Picci, G. , Casagrande, C. C. , Ott, L. R. , Petro, N. M. , Christopher‐Hayes, N. J. , Johnson, H. J. , Willett, M. P. , Okelberry, H. J. , Wang, Y.‐P. , Stephen, J. M. , Calhoun, V. D. , & Wilson, T. W. (2023). Dehydroepiandrosterone mediates associations between trauma‐related symptoms and anterior pituitary volume in children and adolescents. Human Brain Mapping, 44(18), 6388–6398. 10.1002/hbm.26516
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Armario, A. , Lopez‐Calderon, A. , Jolin, T. , & Balasch, J. (1986). Response of anterior pituitary hormones to chronic stress. The specificity of adaptation. Neuroscience & Biobehavioral Reviews, 10(3), 245–250. 10.1016/0149-7634(86)90011-4 [DOI] [PubMed] [Google Scholar]
- Armario, A. , Lopez‐Calderón, A. , Jolin, T. , & Castellanos, J. M. (1986). Sensitivity of anterior pituitary hormones to graded levels of psychological stress. Life Sciences, 39(5), 471–475. 10.1016/0024-3205(86)90527-8 [DOI] [PubMed] [Google Scholar]
- Bateman, A. , Singh, A. , Kral, T. , & Solomon, S. (1989). The immune‐hypothalamic‐pituitary‐adrenal axis. Endocrine Reviews, 10(1), 92–112. 10.1210/edrv-10-1-92 [DOI] [PubMed] [Google Scholar]
- Briere, J. (1996). Trauma symptom checklist for children (TSCC). Assessment of Family Violence: A Handbook for Researchers and Practitioners. Odessa, FL: Psychological Assessment Resources. 10.1037/t06631-000 [DOI] [Google Scholar]
- Chen, F. R. , Raine, A. , & Granger, D. A. (2015). Tactics for modeling multiple salivary analyte data in relation to behavior problems: Additive, ratio, and interaction effects. Psychoneuroendocrinology, 51, 188–200. 10.1016/j.psyneuen.2014.09.027 [DOI] [PubMed] [Google Scholar]
- Díaz‐Arteche, C. , Simmons, J. G. , Ganella, D. E. , Schwartz, O. , Kim, J. H. , Farrow, P. , & Whittle, S. (2021). Associations between early life stress and anterior pituitary gland volume development—A novel index of long‐term hypothalamic‐pituitary‐adrenal axis functioning. Developmental Psychobiology, 63(4), 808–816. 10.1002/dev.22047 [DOI] [PubMed] [Google Scholar]
- Dorshkind, K. , & Horseman, N. D. (2001). Anterior pituitary hormones, stress, and immune system homeostasis. BioEssays, 23(3), 288–294. [DOI] [PubMed] [Google Scholar]
- Farooqi, N. A. I. , Scotti, M. , Lew, J. M. , Botteron, K. N. , Karama, S. , McCracken, J. T. , & Nguyen, T.‐V. (2018). Role of DHEA and cortisol in prefrontal‐amygdalar development and working memory. Psychoneuroendocrinology, 98, 86–94. 10.1016/j.psyneuen.2018.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrow, P. , Simmons, J. G. , Pozzi, E. , Díaz‐Arteche, C. , Richmond, S. , Bray, K. , Schwartz, O. , & Whittle, S. (2020). Associations between early life stress and anterior pituitary gland volume development during late childhood. Psychoneuroendocrinology, 122, 104868. 10.1016/j.psyneuen.2020.104868 [DOI] [PubMed] [Google Scholar]
- Fedorov, A. , Beichel, R. , Kalpathy‐Cramer, J. , Finet, J. , Fillion‐Robin, J.‐C. , Pujol, S. , Bauer, C. , Jennings, D. , Fennessy, F. , Sonka, M. , Buatti, J. , Aylward, S. , Miller, J. V. , Pieper, S. , & Kikinis, R. (2012). 3D slicer as an image computing platform for the quantitative imaging network. Magnetic Resonance Imaging, 30(9), 1323–1341. 10.1016/j.mri.2012.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganella, D. E. , Allen, N. B. , Simmons, J. G. , Schwartz, O. , Kim, J. H. , Sheeber, L. , & Whittle, S. (2015). Early life stress alters pituitary growth during adolescence—A longitudinal study. Psychoneuroendocrinology, 53, 185–194. 10.1016/j.psyneuen.2015.01.005 [DOI] [PubMed] [Google Scholar]
- Garcini, L. M. , Arredondo, M. M. , Berry, O. , Church, J. A. , Fryberg, S. , Thomason, M. E. , & McLaughlin, K. A. (2022). Increasing diversity in developmental cognitive neuroscience: A roadmap for increasing representation in pediatric neuroimaging research. Developmental Cognitive Neuroscience, 58, 101167. 10.1016/j.dcn.2022.101167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjerstad, J. K. , Lightman, S. L. , & Spiga, F. (2018). Role of glucocorticoid negative feedback in the regulation of HPA axis pulsatility. Stress, 21(5), 403–416. 10.1080/10253890.2018.1470238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa, R. J. , Burgess, L. H. , Kerr, J. E. , & O'Keefe, J. A. (1994). Gonadal steroid hormone receptors and sex differences in the hypothalamo‐pituitary‐adrenal axis. Hormones and Behavior, 28(4), 464–476. 10.1006/hbeh.1994.1044 [DOI] [PubMed] [Google Scholar]
- Herman, J. P. , McKlveen, J. M. , Ghosal, S. , Kopp, B. , Wulsin, A. , Makinson, R. , Scheimann, J. , & Myers, B. (2016). Regulation of the hypothalamic‐pituitary‐adrenocortical stress response. Comprehensive Physiology, 6(2), 603–621. 10.1002/cphy.c150015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, L. , & Bentler, P. M. (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal, 6(1), 1–55. 10.1080/10705519909540118 [DOI] [Google Scholar]
- Jiang, X. , Zhong, W. , An, H. , Fu, M. , Chen, Y. , Zhang, Z. , & Xiao, Z. (2017). Attenuated DHEA and DHEA‐S response to acute psychosocial stress in individuals with depressive disorders. Journal of Affective Disorders, 215, 118–124. 10.1016/j.jad.2017.03.013 [DOI] [PubMed] [Google Scholar]
- Kaiser, U. , & Ho, K. K. Y. (2016). Chapter 8—Pituitary physiology and diagnostic evaluation. In Melmed S., Polonsky K. S., Larsen P. R., & Kronenberg H. M. (Eds.), Williams textbook of endocrinology (Thirteenth edition) (pp. 176–231). Elsevier. 10.1016/B978-0-323-29738-7.00008-3 [DOI] [Google Scholar]
- Kalimi, M. , Shafagoj, Y. , Loria, R. , Padgett, D. , & Regelson, W. (1994). Anti‐glucocorticoid effects of dehydroepiandrosterone (DHEA). Molecular and Cellular Biochemistry, 131(2), 99–104. 10.1007/BF00925945 [DOI] [PubMed] [Google Scholar]
- Kamin, H. S. , & Kertes, D. A. (2017). Cortisol and DHEA in development and psychopathology. Hormones and Behavior, 89, 69–85. 10.1016/j.yhbeh.2016.11.018 [DOI] [PubMed] [Google Scholar]
- Le Tissier, P. R. , Hodson, D. J. , Lafont, C. , Fontanaud, P. , Schaeffer, M. , & Mollard, P. (2012). Anterior pituitary cell networks. Frontiers in Neuroendocrinology, 33(3), 252–266. 10.1016/j.yfrne.2012.08.002 [DOI] [PubMed] [Google Scholar]
- Lorenzetti, V. , Allen, N. B. , Fornito, A. , Pantelis, C. , De Plato, G. , Ang, A. , & Yücel, M. (2009). Pituitary gland volume in currently depressed and remitted depressed patients. Psychiatry Research: Neuroimaging, 172(1), 55–60. 10.1016/j.pscychresns.2008.06.006 [DOI] [PubMed] [Google Scholar]
- MacKinnon, D. P. , Lockwood, C. M. , & Williams, J. (2004). Confidence limits for the indirect effect: Distribution of the product and resampling methods. Multivariate Behavioral Research, 39(1), 99–128. 10.1207/s15327906mbr3901_4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMaster, F. P. , & Kusumakar, V. (2004). MRI study of the pituitary gland in adolescent depression. Journal of Psychiatric Research, 38(3), 231–236. 10.1016/j.jpsychires.2003.11.001 [DOI] [PubMed] [Google Scholar]
- MacMaster, F. P. , Leslie, R. , Rosenberg, D. R. , & Kusumakar, V. (2008). Pituitary gland volume in adolescent and young adult bipolar and unipolar depression: Pituitary gland in adolescent mood disorders. Bipolar Disorders, 10(1), 101–104. 10.1111/j.1399-5618.2008.00476.x [DOI] [PubMed] [Google Scholar]
- Martí, O. , & Armario, A. (1998). Anterior pituitary response to stress: Time‐related changes and adaptation. International Journal of Developmental Neuroscience, 16(3), 241–260. 10.1016/S0736-5748(98)00030-6 [DOI] [PubMed] [Google Scholar]
- Matchock, R. L. , Dorn, L. D. , & Susman, E. J. (2007). Diurnal and seasonal cortisol, testosterone, and DHEA rhythms in boys and girls during puberty. Chronobiology International, 24(5), 969–990. 10.1080/07420520701649471 [DOI] [PubMed] [Google Scholar]
- Melmed, S. , & Kleinberg, D. (2016). Chapter 9—Pituitary masses and tumors. In Melmed S., Polonsky K. S., Larsen P. R., & Kronenberg H. M. (Eds.), Williams textbook of endocrinology (Thirteenth edition) (pp. 232–299). Elsevier. 10.1016/B978-0-323-29738-7.00009-5 [DOI] [Google Scholar]
- Mocking, R. J. T. , Pellikaan, C. M. , Lok, A. , Assies, J. , Ruhé, H. G. , Koeter, M. W. , Visser, I. , Bockting, C. L. , Olff, M. , & Schene, A. H. (2015). DHEAS and cortisol/DHEAS‐ratio in recurrent depression: State, or trait predicting 10‐year recurrence? Psychoneuroendocrinology, 59, 91–101. 10.1016/j.psyneuen.2015.05.006 [DOI] [PubMed] [Google Scholar]
- Murray, C. R. , Simmons, J. G. , Allen, N. B. , Byrne, M. L. , Mundy, L. K. , Seal, M. L. , Patton, G. C. , Olsson, C. A. , & Whittle, S. (2016). Associations between dehydroepiandrosterone (DHEA) levels, pituitary volume, and social anxiety in children. Psychoneuroendocrinology, 64, 31–39. 10.1016/j.psyneuen.2015.11.004 [DOI] [PubMed] [Google Scholar]
- Pariante, C. M. , Vassilopoulou, K. , Velakoulis, D. , Phillips, L. , Soulsby, B. , Wood, S. J. , Brewer, W. , Smith, D. , Dazzan, P. , Yung, A. R. , Zervas, I. M. , Christodoulou, G. N. , Murray, R. , McGorry, P. D. , & Pantelis, C. (2004). Pituitary volume in psychosis. The British Journal of Psychiatry, 185(1), 5–10. 10.1192/bjp.185.1.5 [DOI] [PubMed] [Google Scholar]
- Sari, S. , Sari, E. , Akgun, V. , Ozcan, E. , Ince, S. , Saldir, M. , Babacan, O. , Acikel, C. , Basbozkurt, G. , Ozenc, S. , Yesilkaya, S. , Kilic, C. , Kara, K. , Vurucu, S. , Kocaoglu, M. , & Yesilkaya, E. (2014). Measures of pituitary gland and stalk: From neonate to adolescence. Journal of Pediatric Endocrinology and Metabolism, 27(11‐12), 1071–1076. 10.1515/jpem-2014-0054 [DOI] [PubMed] [Google Scholar]
- Smith, K. E. , & Pollak, S. D. (2020). Early life stress and development: Potential mechanisms for adverse outcomes. Journal of Neurodevelopmental Disorders, 12(1), 34. 10.1186/s11689-020-09337-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, K. E. , & Pollak, S. D. (2021). Rethinking concepts and categories for understanding the neurodevelopmental effects of childhood adversity. Perspectives on Psychological Science, 16(1), 67–93. 10.1177/1745691620920725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen, J. M. , Solis, I. , Janowich, J. , Stern, M. , Frenzel, M. R. , Eastman, J. A. , Mills, M. S. , Embury, C. M. , Coolidge, N. M. , Heinrichs‐Graham, E. , Mayer, A. , Liu, J. , Wang, Y. P. , Wilson, T. W. , & Calhoun, V. D. (2021). The developmental chronnecto‐genomics (Dev‐CoG) study: A multimodal study on the developing brain. NeuroImage, 225, 117438. 10.1016/j.neuroimage.2020.117438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsigos, C. , & Chrousos, G. P. (2002). Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. Journal of Psychosomatic Research, 53(4), 865–871. 10.1016/S0022-3999(02)00429-4 [DOI] [PubMed] [Google Scholar]
- Whittle, S. , Barendse, M. , Pozzi, E. , Vijayakumar, N. , & Simmons, J. G. (2020). Pubertal hormones predict sex‐specific trajectories of pituitary gland volume during the transition from childhood to adolescence. NeuroImage, 204, 116256. 10.1016/j.neuroimage.2019.116256 [DOI] [PubMed] [Google Scholar]
- Whittle, S. , Yücel, M. , Lorenzetti, V. , Byrne, M. L. , Simmons, J. G. , Wood, S. J. , Pantelis, C. , & Allen, N. B. (2012). Pituitary volume mediates the relationship between pubertal timing and depressive symptoms during adolescence. Psychoneuroendocrinology, 37(7), 881–891. 10.1016/j.psyneuen.2011.10.004 [DOI] [PubMed] [Google Scholar]
- Wong, A. P.‐Y. , Pipitone, J. , Park, M. T. M. , Dickie, E. W. , Leonard, G. , Perron, M. , Pike, B. G. , Richer, L. , Veillette, S. , Chakravarty, M. M. , Pausova, Z. , & Paus, T. (2014). Estimating volumes of the pituitary gland from T1‐weighted magnetic‐resonance images: Effects of age, puberty, testosterone, and estradiol. NeuroImage, 94, 216–221. 10.1016/j.neuroimage.2014.02.030 [DOI] [PubMed] [Google Scholar]
- Zipursky, A. R. , Whittle, S. , Yücel, M. , Lorenzetti, V. , Wood, S. J. , Lubman, D. I. , Simmons, J. G. , & Allen, N. B. (2011). Pituitary volume prospectively predicts internalizing symptoms in adolescence: Pituitary volume prospectively predicts internalizing symptoms in adolescence. Journal of Child Psychology and Psychiatry, 52(3), 315–323. 10.1111/j.1469-7610.2010.02337.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1: Supporting information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


