Abstract
Background
Human T-lymphotropic virus type 1 (HTLV-1)-associated myelopathy/tropical spastic paraparesis (HAM/TSP) is a chronic neurological condition characterized by progressive myelopathic symptoms including spasticity, pain, weakness, and urinary symptoms, without proven treatments. Mogamulizumab (MOG) is a monoclonal antibody that binds CCR4 and leads to the clearance of HTLV-1-infected CCR4+ cells. A phase 1-2a study in Japan evaluated MOG for the treatment of HAM/TSP and reported decreases in HTLV-1 proviral load and neuroinflammatory markers, with clinical improvement in some participants.
Methods
We administered MOG 0.1 mg/kg every 8 weeks to individuals with HAM/TSP as a compassionate and palliative treatment. Patients who received MOG had (1) a positive peripheral HTLV-1 antibody, (2) progressive myelopathic symptoms, and (3) a diagnosis of HAM/TSP.
Results
Four female patients, ages 45–68, received MOG (range, 2–6 infusions) between 1 November 2019 and 30 November 2022. Two patients with <3 years of symptoms had milder disease, with Osame scores <4. The other 2, with >7 years of symptoms, had Osame scores >5. One patient, with 6 total treatments, received dose-reduced MOG after she developed a rash at the initial dose. The 2 patients with milder baseline disease reported symptomatic improvement and saw reductions in Osame and/or modified Ashworth scale scores during follow-up. The other 2 patients showed no improvement. All 4 developed rashes after receiving MOG—a treatment-limiting event in some cases.
Conclusions
Clinical trials are needed including diverse patient populations to assess the potential role of MOG for HAM/TSP. Our findings may help inform the development of these trials.
Keywords: mogamulizumab, HAM/TSP, HTLV-1, myelopathy, rash
Four women received mogamulizumab for human T-lymphotropic virus type 1 (HTLV-1) associated myelopathy/tropical spastic paraparesis (HAM/TSP) at a single US center. Two individuals with milder baseline disease showed clinical improvement. All 4 developed rashes, which were treatment limiting.
Graphical abstract
This graphical abstract is also available at Tidbit: https://tidbitapp.io/tidbits/challenges-in-the-long-term-management-of-patients-with-coccidioidal-meningitis-a-retrospective-analysis-of-treatment-and-outcomes
Around 1% of people with human T-lymphotropic virus type 1 (HTLV-1) infection develop HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) [1], a chronic neurological condition characterized by progressive lower extremity spasticity, weakness, bladder and bowel dysfunction, and pain [2]. Consensus recommendations note pulse dose methylprednisolone and low dose maintenance prednisone may be associated with temporary improvements in pain and mobility, with insufficient evidence for other therapies [3].
HTLV-1 infects and activates CCR4+ T-lymphocytes, driving aberrant inflammation that causes neurologic damage [1]. Mogamulizumab (MOG) is a humanized monoclonal antibody against CCR4 that leads to antibody-dependent clearance of CCR4+ cells [4]. Currently, the primary clinical role for MOG is in treating T-cell lymphomas, including relapsed cutaneous T-cell lymphoma (CTCL), for which it received approval from the US Food and Drug Administration (FDA), and adult T-cell leukemia/lymphoma (ATLL), another severe complication of HTLV-1 infection, for which it is approved in Japan [5, 6].
Although there has been great interest in the possibility of using MOG for HAM/TSP, published data remain extremely limited. A preclinical study showed MOG decreases HTLV-1 proviral load and proinflammatory cytokines in peripheral blood mononuclear cells (PBMCs) from people with HAM/TSP and those with asymptomatic HTLV-1 infection. A phase 1-2a dose escalation study of MOG in 21 participants with HAM/TSP found a dose-dependent reduction in PBMC HTLV-1 proviral load and cerebrospinal fluid (CSF) inflammatory markers, including neopterin and CXCL10 [4]. We identified no published reports of MOG for HAM/TSP in the United States.
In the phase 1-2a study, the lowest dose (0.003 mg/kg) showed a reduction in PBMC HTLV-1 proviral load by day 15 after first infusion that returned to baseline by 3 months. The highest doses tested (0.1 mg/kg and 0.3 mg/kg) showed greater reductions in proviral load that persisted for at least 3 months. Clinical improvement in muscle tone, myelopathic symptoms, and reduction in disability scores was observed in some participants [4]. Modified Ashworth [7] score improved in 15/19 (79%) and Osame [8] Motor Disability Score in 6/19 (32%), with 24 weeks of study follow-up. A half-life of 14.13 ± 6.49 days was calculated for the 0.1 mg/kg dose.
In the absence of approved treatments in the United States, we hypothesized that administration of MOG may mitigate the symptoms and progression of HAM/TSP. Here we report the compassionate use of MOG in 4 patients with painful and progressive HAM/TSP.
METHODS
We administered MOG for HAM/TSP to 4 people at a single center in the United States from 1 November 2019 through 30 November 2022. We chose a 0.1 mg/kg dose with an 8-week interval as the initial regimen based on published trial results, anticipating a sustained reduction in proviral load between treatments, with fewer adverse events than 0.3 mg/kg [4]. MOG was offered to patients who met the following criteria: (1) peripheral HTLV-1 antibody positive, (2) progressive myelopathic symptoms, (3) HAM/TSP diagnosis, and (4) ability to understand and consent to this off-label use. A multi-disciplinary team including neurologists, infectious diseases specialists, and dermatologists cared for these patients.
This off-label use of MOG was assessed to be exempt from relevant federal regulations by the Massachusetts General Brigham Human Research Committee (MGB IRB). Patients were informed that no specific treatments for HAM/TSP were available, and that MOG was not FDA-approved for HAM/TSP. Patients were given detailed information about the limited data to support its off-label use and potential side effects and risks of MOG. Clinical response was measured by neurologic assessments, including Osame and modified Ashworth scores.
RESULTS
Four female patients ages 45–68 years received 2–6 intravenous MOG treatments for HAM/TSP at a dose of 0.1 mg/kg administered every 8 weeks (Table 1). After 4 treatments, 1 patient received dose-reduced MOG (0.025 mg/kg) for 2 additional infusions after developing a rash at the higher dose. Two patients with milder baseline disease reported symptomatic improvement in mobility with reductions in Osame and/or modified Ashworth scores during follow-up. The other 2, with more advanced baseline disease, showed no improvement.
Table 1.
Baseline Features, Outcomes, and Adverse Events
| Case 1 | Case 2 | Case 3 | Case 4 | |
|---|---|---|---|---|
| Age at first MOG infusion (years) | 60 | 49 | 68 | 45 |
| Years of symptoms at baseline | 8 | 2 | 13 | 2 |
| CSF WBC (% lymphocytes) at Baseline (cells/mL3) | 4 (91%) | 14 (91%) | 2 (98%) | 5 (93%) |
| Peripheral HTLV-1 Ab | Pos | Pos | Pos | Pos |
| Qualitative Peripheral HTLV-1 DNA | Detected | N/A | Detected | N/A |
| CSF HTLV-1 Ab | N/A | Pos | Posa | Pos |
| Symptom profile | ||||
|
X | X | X | X |
|
X | X | X | X |
|
X | … | … | X |
|
X | X | X | X |
| Treatments prior to MOG | ||||
|
X | … | … | … |
|
X | … | … | … |
|
… | … | … | X |
|
… | … | X | … |
| Baseline neurologic evaluation | ||||
| Modified Ashworth score | 3 | 1 | 2 | 2 |
| Osame motor disability score | 8 | 3.5 | 5 | 3 |
| Outcome | ||||
| Number of infusions received | 4 | 6 | 2 | 3 |
| Modified Ashworth after most recent infusion | 3 | 1 | 2 | 1 |
| Osame score after most recent infusion | 8 | 3 | 7 | 2 |
| Reported symptom improvement after 2 infusions | No | Yes | No | Yes |
| Adverse events | ||||
| Timing of rash onset after first infusion (wks) | 3 | 11 | 1 | 12 |
| Liver test elevations | Yes | Yes | No | No |
HTLV-1 antibody testing was performed using the immunoassay “human T-cell lymphotropic virus types I and II antibody screen with confirmation” (Mayo Clinic Laboratories, Rochester, Minnesota, USA). HTLV-1 DNA was performed with a qualitative assay called “HTLV I/II DNA, Qualitative, Real-Time PCR” (Quest Diagnostics, San Juan Capistrano, California, USA).
= Note Case 3 had additional HTLV-1 testing at the National Institutes of Health (NIH) that included a positive CSF HTLV-1 proviral load. IV = intravenous. For details about modified Ashworth and Osame scores, please see Supplementary Table 1.
Abbreviations: Ab, antibody; CSF, cerebrospinal fluid; HTLV-1, human T-lymphotropic virus type 1; IV, intravenous; MOG, mogamulizumab; N/A, not available or not tested; Pos, positive.
Case 1:
A woman, born in Haiti with the onset of myelopathic symptoms at age 52, was diagnosed with HAM/TSP at 59 and treated with MOG at 60. Her predominant baseline symptoms were urinary incontinence, bladder spasms, constipation, and lower extremity spasticity, weakness, and pain. At the time of initial diagnosis, she received IV methylprednisolone (250 mg daily for 3 days, then 500 mg weekly for 4 weeks) followed by methotrexate (15 mg weekly) as a steroid-sparing agent. She noted transient improvement in pain and mobility after methylprednisolone.
At her first MOG treatment, she had advanced disease with an Osame score of 8 and a modified Ashworth score of 3 (Supplementary Table 1). Her last doses of methylprednisolone and methotrexate were at 18 and 1 month prior to MOG, respectively.
A CSF examination 18 months before MOG showed 7 nucleated cells/μL (95% lymphocytes) (reference range: 0–5 cells/μL), glucose 58 mg/dL (reference range: 50–75 mg/dL), and protein 52 mg/dL (reference range: 3–55 mg/dL), without neopterin assessment. A repeat CSF exam 2 weeks before MOG showed 4 nucleated cells/μL (91% lymphocytes), glucose 63 mg/dL, protein 33 mg/dL, and neopterin 281 nmol/L (reference range: 8–28 nmol/mL).
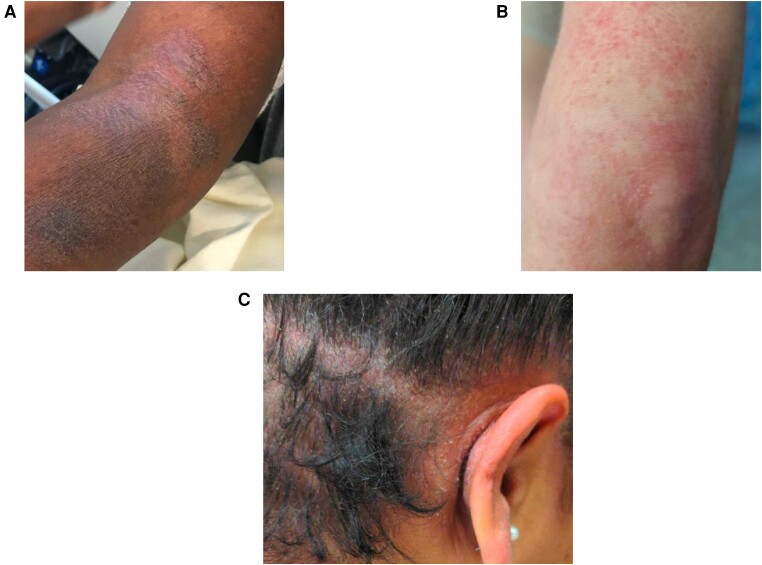
She received 4 total 0.1 mg/kg infusions, the first 2 separated by 8 weeks. The third was delayed nearly 6 months because of a rash (Figure 1A) and a local COVID-19 wave.
Figure 1.
Cutaneous adverse events observed in patients treated with MOG for HAM/TSP. A, Left arm of patient 1 shows erythematous and hyperpigmented patches and plaques with scale. This photo was taken 6 wks after first infusion B, Area around left elbow of patient 2 showing a morbilliform rash. This photo was taken 4 wks after second infusion, prior to development of DRESS-like rash. C, The scalp shows erythematous patches and papules with scale of patient 4. This photo was taken 6 wks after third infusion. Abbreviations: HAM/TSP, HTLV-1-associated myelopathy/tropical spastic paraparesis; MOG, mogamulizumab.
A rash developed 3 weeks after the first infusion and flared 3 weeks after each subsequent infusion. The rash was notable for pruritic, scaly plaques, particularly on flexural surfaces, without mucous membrane involvement. She was treated with topical steroids and, 8 weeks after her second infusion, prednisone (40 mg daily for 10 days), with improvement.
Transaminase elevations were observed following the first MOG infusion. Aspartate transaminase (AST) and alanine aminotransaminase (ALT) levels peaked at approximately 5 times the upper limit of normal 11 days after first MOG and then subsequently normalized by day 63 (Supplementary Table 2). Notably, she did not have transaminase elevations following subsequent infusions. MOG was discontinued after 4 infusions because of the rash and the lack of perceived clinical benefit. Her Osame score remained at 8 and modified Ashworth at 3, with no discernable change in functional status or pain.
Case 2:
A woman, born in the United States with onset of myelopathic symptoms at age 47, was diagnosed with HAM/TSP at 48 and treated with MOG at 49. She had presumed sexual acquisition of HTLV-1 from a male partner who died from ATLL 15 years before the onset of her own symptoms. Her predominant baseline symptoms were urinary incontinence, right lower extremity weakness, and spasms. She had a baseline Osame score of 3.5 and modified Ashworth of 1. She had no prior HAM/TSP-directed therapies.
A CSF examination 7 weeks before MOG showed 14 nucleated cells/μL (91% lymphocytes), glucose 59 mg/dL, protein 34 mg/dL, and neopterin 137 nmol/L.
Her first 4 infusions were dosed at 0.1 mg/kg at 8-week intervals. She developed a generalized erythematous papular rash 3 weeks after her second infusion (Figure 1B ), which improved with topical triamcinolone. The rash significantly worsened 2 weeks after her third infusion, with a pruritic, morbilliform eruption covering 70% of her body surface area (BSA). Around 4 weeks after the third infusion she developed mild perioral and facial swelling and small post-auricular and axillary lymphadenopathy (noted by patient and clinicians). She was treated with topical corticosteroids and oral fexofenadine (60 mg daily), with a prednisone taper 6 weeks after her third infusion (60 mg for 2 days, 40 mg for 2 days, 20 mg for 2 days, 10 mg for 2 days), with improved but not resolved cutaneous symptoms.
Her rash worsened again a week after her fourth infusion, with erythema and overlying desquamation covering 70% BSA. She had transaminase elevations, with ALT peaking 3 times the upper limit of normal 24 days after her fourth infusion. Mild eosinophilia peaked at 350 K/μL (reference range: 50–250 K/μL) 9 days after infusion. Transaminases and eosinophil count normalized by day 50 (Supplementary Table 3). Taken together there was concern for a drug reaction with eosinophilia and systemic symptoms (DRESS)-like reaction. She received prednisone (40 mg for 2 weeks, 30 mg for 2 weeks, 20 mg for 2 weeks, 10 mg for 3 weeks) and methotrexate (10 mg daily). She transitioned to mycophenolate mofetil (MMF) 500 mg orally twice daily 6 weeks after her fourth infusion, with supplemental topical corticosteroids.
Five months after the fourth infusion, a reduced dose of 0.025 mg/kg was chosen to reintroduce MOG with concurrent MMF in hopes of inducing tolerance to allow for continued MOG administration. She tolerated lower dose MOG well, with no rash, eosinophilia, or transaminase elevation. A sixth infusion of 0.05 mg/kg was given 8 weeks after the fifth. She plans to continue off-label MOG with possible continued dose escalation while maintaining MMF to prevent further DRESS-like sequelae. MMF has been used as a steroid-sparing agent for the treatment of severe cutaneous adverse reactions [9] and reintroduction with slow dose escalation of offending agents may allow for continued treatment, a strategy that has been employed for people experiencing DRESS-like reactions during tuberculosis treatment [10].
The patient feels her neurologic symptoms have improved. Whereas she required intermittent assistance with mobilization previously, she now walks independently. Her Osame score improved from 3.5 to 3, her modified Ashworth score is stable at 1, and her 4-meter walk and 3-meter up and go tests remain stable at around 3 and 6–7 seconds, respectively, for over a year.
Case 3:
A woman, born in Jamaica with onset of myelopathic symptoms at age 55, was diagnosed with HAM/TSP at 65, and treated with MOG at 68. Her predominant baseline symptoms were bladder dysfunction requiring self-catheterization for more than 10 years, severe low back and lower extremity pain, and difficulty walking, requiring a cane. Her baseline Osame and modified Ashworth scores were 5 and 2, respectively. She received prior treatment for HAM/TSP with intermittent spinal corticosteroid injections (dosage unknown, last >1 year prior to first MOG treatment), without improvement.
She previously enrolled in a study of HTLV-1 infection at the National Institutes of Health (NIH), where she was found to have an HTLV-1 proviral load of 40% in the CSF and 12% in the peripheral blood. A CSF examination 2 weeks prior to MOG showed two nucleated cells/μL (98% lymphocytes), glucose 61 mg/dL, and protein 30/dL. Neopterin was not obtained.
She underwent 2 MOG infusions (0.1 mg/kg separated by 8 weeks). She noted a mild rash 1 week after her first infusion with significant worsening within days of her second infusion, with widespread pruritic scaly plaques on her trunk, extremities, and scalp, and associated alopecia. She used topical steroids, with eventual resolution by 4 months after her second infusion. She had no associated transaminase abnormalities and no eosinophilia on labs collected 2 weeks after her second infusion, when her rash was present. Due to the rash and no improvement in urinary symptoms, pain, or gait after 2 infusions, the patient declined further treatments. In the year since her last infusion, she has experienced progression of her myelopathic symptoms, with an Osame score of 7 (using a walker rather than a cane for very short rather than longer distances) and a modified Ashworth of 2.
Case 4:
A woman, born in Cape Verde with onset of myelopathic symptoms at age 43, was diagnosed with HAM/TSP at 44 and treated with MOG at 45. Her predominant baseline symptoms were difficulty walking, dragging her feet, and left greater than right leg stiffness and weakness, leading to falls. She walked 10–15 feet independently with bilateral knee braces. Additionally, she developed intermittent paresthesias in her feet and ankles with painful nocturnal leg spasms. She experienced urinary urgency, bladder spasms, and constipation, occasionally requiring manual disimpaction. Baseline Osame and modified Ashworth scores were 3 and 2, respectively.
She reported several weeks of improved stiffness and spasms following a prednisone taper (60 mg for 7 days, 40 mg for 7 days, 20 mg for 7 days, 10 mg for 7 days) completed 4 months prior to MOG. She took long-term secukinumab 150 mg subcutaneously every 28 days for ankylosing spondylitis. She previously received etanercept and adalimumab, stopped 4 and 2 years prior to MOG, respectively.
A CSF examination 2 months prior to MOG (and 2 months after prednisone) showed 5 nucleated cells/μL (93% lymphocytes), glucose 62 mg/dL, protein 25 mg/dL, and neopterin >300 nmol/L.
She received 3 MOG infusions (0.1 mg/kg at 8-week intervals). Starting 4 weeks after her second infusion she developed hyperpigmented, scaly patches under her breast and axilla, treated with selenium sulfide with some improvement. A week after her third infusion, she developed a diffuse erythematous and pruritic rash (Figure 1C ), without systemic symptoms or associated transaminase abnormalities. At week 5 after the infusion, she was started on a prednisone taper (40 mg for 7 days, 30 mg for 5 days, 20 mg for 5 days, 10 mg for 5 days), with resolution of her rash over several weeks. Her absolute eosinophil count (AEC) ranged between 210 and 370 K/μL while on treatment. Notably her AEC was 80–440 K/μL during the 2 years prior to MOG. Besides rash, she noted 2 days of headache, fatigue, and diarrhea after each infusion that resolved spontaneously.
She reported significant improvement in neurologic symptoms within weeks after the second infusion, characterized by improved stability of walking, which she accomplished without braces. Her Osame and modified Ashworth scores improved to 2 and 1, respectively, within 4 weeks of her third infusion. She reported faster walking, confirmed on a 10-meter walk test (16 vs 24 seconds following third and first infusions, respectively). Though the patient had significant subjective and objective neurologic improvement, MOG was discontinued because of the rash. In the 6 months after her final infusion, she noted worsening of lower extremity pain as well as bladder and lower extremity spasms. Her Osame score remained at 2 and her modified Ashworth increased to 2. She reported symptomatic improvement on chronic low dose prednisone of 10 mg daily, started 3 months after her final MOG treatment, which she continues at this time.
DISCUSSION
We present our experience with off-label MOG for treatment of 4 women with HAM/TSP. To our knowledge this is the first report of MOG for HAM/TSP outside of Japan. Two individuals, with shorter duration of baseline symptoms (2 vs 8–13 years) and lower baseline Osame and modified Ashworth scores (3–3.5 vs 5–8 and 1–2 vs 2–3, respectively) had subjective improvement in myelopathic symptoms accompanied by objective improvements in disability scores. The other 2 individuals showed no improvement.
Rash was a treatment-limiting adverse event, with heterogeneous and evolving presentations and onset 1–12 weeks after first MOG infusion. The delayed and varied onset and diverse clinical presentations are consistent with prior descriptions of MOG-associated rash. For instance, in a series of 19 patients with MOG-associated rashes (treating mycosis fungoides or Sezary syndrome), median time to rash onset was 199 days (range 56 days to 3.8 years) and 4 major distinct cutaneous presentations were described [11]. In the same review, 4/19 patients were continued on MOG and no life-threatening or severe cutaneous reactions occurred.
The occurrence of MOG-associated rashes in all our patients was unexpected. In the Japanese trial of MOG for HAM/TSP, 10/21 (48%) developed a rash [4]. A randomized controlled trial of MOG for treatment of cutaneous T-cell leukemia (CTCL), a non-HTLV-1 driven condition, found 44/184 (24%) developed a rash, the most common adverse event leading to MOG discontinuation (13/184, 7%) [12]. The dose of MOG used for CTCL of 1.0 mg/kg/week for 28 days and every other week thereafter is far higher than the dose used for HAM/TSP. One patient (Case 2) with concern for a DRESS-like reaction managed to remain on MOG with dose reduction/escalation and MMF, with close monitoring by an experienced multi-disciplinary team. The prevalence and management of MOG-associated cutaneous reactions during treatment for HAM/TSP need to be examined closely in future studies.
Besides rash, MOG was well tolerated. We noted idiosyncratic mild to moderate elevations in serum transaminase levels ranging from 3 to 5 times the upper limit of normal in 2 patients (Cases 1 and 2), peaking 1–2 weeks and normalizing by 50–60 days after infusion. Notably, transaminase elevations did not recur with future infusions. Another patient (Case 4) reported limited headache, diarrhea, and fatigue following each treatment. No other adverse events were observed. Although we did not see varicella zoster or herpes simplex virus reactivation, both were reported in the Japanese trial. We recommended the recombinant, adjuvanted herpes zoster vaccine for eligible patients prior to MOG.
Although we cannot evaluate MOG efficacy in our exploratory series, our results are hypothesis-generating. Specifically, we believe that patients with milder baseline neurological disease and fewer total years of symptoms may be more likely to respond favorably to MOG. This is consistent with the results from the Japanese trial, where improvement in motor skills was seen in individuals with earlier disease (Osame score <5) [4]. Future trials could target patients with fewer total years of symptoms and milder baseline neurologic impairments or could stratify randomization on these factors. Randomized clinical trials should also compare different doses and intervals.
Although the investigators from the Japanese trial found a dose-dependent reduction in HTLV-1 proviral load in PBMCs, they also reported improved HTLV-1 virologic, CSF inflammatory, and clinical parameters in at least 1 participant who received repeated low dose (0.03 mg/kg) MOG. We did not measure quantitative HTLV-1 proviral load, as currently there is no available commercial assay. We found markedly elevated baseline CSF neopterin levels (5 to >10 times the upper limit of normal) in 3 of our patients with testing, although we did not obtain follow-up measurements. Future trials should assess whether neuroinflammatory and HTLV-1 virologic markers can help identify patients most likely to benefit and serve as surrogate markers for response to treatment.
HAM/TSP remains a neglected condition that causes severe pain and disability. Without proven or approved treatments HAM/TSP providers currently have little to offer patients. We hope our findings generate further interest in this condition and spark funding for the desperately needed well-designed trials to assess the potential impact of MOG on this condition.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Eric A Meyerowitz, Division of Infectious Diseases, Montefiore Medical Center, Bronx, New York, USA; Albert Einstein College of Medicine, Bronx, New York, USA.
Shibani S Mukerji, Division of Neuroimmunology and Neuro-Infectious Diseases, Massachusetts General Hospital, Boston, Massachusetts, USA; Harvard Medical School, Boston, Massachusetts, USA.
G Kyle Harrold, Division of Neuroimmunology and Neuro-Infectious Diseases, Massachusetts General Hospital, Boston, Massachusetts, USA; Harvard Medical School, Boston, Massachusetts, USA.
Rachel M Erdil, Division of Geographic Medicine and Infectious Diseases, Tufts Medical Center, Boston, Massachusetts, USA.
Steven T Chen, Harvard Medical School, Boston, Massachusetts, USA; Department of Dermatology, Massachusetts General Hospital, Boston, Massachusetts, USA.
Emily A Rudmann, Division of Neuroimmunology and Neuro-Infectious Diseases, Massachusetts General Hospital, Boston, Massachusetts, USA.
Athe Tsibris, Harvard Medical School, Boston, Massachusetts, USA; Division of Infectious Diseases, Brigham and Women's Hospital, Boston, Massachusetts, USA; Division of Infectious Diseases, Massachusetts General Hospital, Boston, Massachusetts, USA.
Nagagopal Venna, Division of Neuroimmunology and Neuro-Infectious Diseases, Massachusetts General Hospital, Boston, Massachusetts, USA; Harvard Medical School, Boston, Massachusetts, USA.
Gregory K Robbins, Harvard Medical School, Boston, Massachusetts, USA; Division of Infectious Diseases, Massachusetts General Hospital, Boston, Massachusetts, USA.
References
- 1. Bangham CR, Araujo A, Yamano Y, Taylor GP. HTLV-1-associated myelopathy/tropical spastic paraparesis. Nat Rev Dis Primers 2015; 1:15012. [DOI] [PubMed] [Google Scholar]
- 2. Olindo S, Cabre P, Lézin A, et al. Natural history of human T-lymphotropic virus 1-associated myelopathy: a 14-year follow-up study. Arch Neurol 2006; 63:1560–6. [DOI] [PubMed] [Google Scholar]
- 3. Araujo A, Bangham CRM, Casseb J, et al. Management of HAM/TSP: systematic review and consensus-based recommendations 2019. Neurol Clin Pract 2021; 11:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sato T, Coler-Reilly ALG, Yagishita N, et al. Mogamulizumab (Anti-CCR4) in HTLV-1-associated myelopathy. N Engl J Med 2018; 378:529–38. [DOI] [PubMed] [Google Scholar]
- 5. Mehta-Shah N, Ratner L, Horwitz SM. Adult T-cell leukemia/lymphoma. J Oncol Pract 2017; 13:487–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moore DC, Elmes JB, Shibu PA, Larck C, Park SI. Mogamulizumab: an anti-CC chemokine receptor 4 antibody for T-cell lymphomas. Ann Pharmacother 2020; 54:371–9. [DOI] [PubMed] [Google Scholar]
- 7. Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther 1987; 67:206–7. [DOI] [PubMed] [Google Scholar]
- 8. Muniz AL, Rodrigues W Jr, Santos SB, et al. Association of cytokines, neurological disability, and disease duration in HAM/TSP patients. Arquivos de neuro-psiquiatria 2006; 64:217–21. [DOI] [PubMed] [Google Scholar]
- 9. Nadelmann ER, Yeh JE, Chen ST. Management of cutaneous immune-related adverse events in patients with cancer treated with immune checkpoint inhibitors: a systematic review. JAMA Oncol 2022; 8:130–8. [DOI] [PubMed] [Google Scholar]
- 10. Oh JH, Yun J, Yang MS, et al. Reintroduction of antituberculous drugs in patients with antituberculous drug-related drug reaction with eosinophilia and systemic symptoms. J Allergy Clin Immunol Pract 2021; 9:3442–3449.e3. [DOI] [PubMed] [Google Scholar]
- 11. Hirotsu KE, Neal TM, Khodadoust MS, et al. Clinical characterization of mogamulizumab-associated rash during treatment of mycosis fungoides or sézary syndrome. JAMA Dermatol 2021; 157:700–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim YH, Bagot M, Pinter-Brown L, et al. Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): an international, open-label, randomised, controlled phase 3 trial. Lancet Oncol 2018; 19:1192–204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.