Diabetes-related foot infection (DFI) can devastate patient’s mobility and quality of life, with many patients fearing lower limb amputation more than death. This disease disproportionately affects marginalized communities and social barriers frequently complicate treatment. Multidisciplinary DFI teams can reduce major (above-ankle) amputations, yet inconsistent and unstructured collaboration between specialists remains common. In our full review (available online, summarized here), we overview a comprehensive approach to DFI and offer best practices for clinician-clinician and patient-clinician shared decision-making.
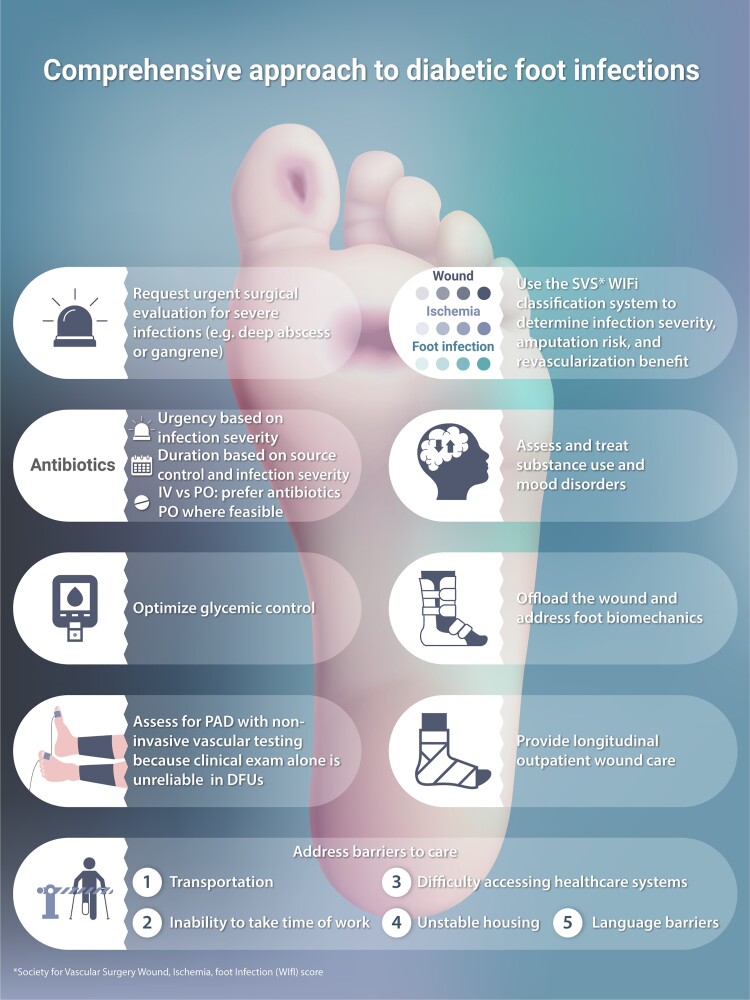
A common language is key to multidisciplinary collaborations. We advocate infectious disease (ID) clinicians familiarize themselves with the Society for Vascular Surgery’s WIfI (Wound, Ischemia, Foot infection) classification system, which iterates on the 2012 IDSA DFI criteria with wound and ischemia staging. WIfI, a validated predictor of both a patient’s risk of amputation and potential to benefit from revascularization, incorporates key factors informing our surgical colleagues’ practice.
Shared decision-making about antimicrobial and surgical therapies for DFI and diabetes-related foot osteomyelitis (DFO) should acknowledge that these are just two components of a comprehensive strategy (Table 1). While reports of cure are modestly higher with surgery for DFO versus nonoperative strategies, published data indicate most patients can achieve cure nonoperatively. Key caveats are that a nonoperative strategy is inappropriate for life-threatening infection (eg, necrotizing fasciitis), substantial gangrene or undrained purulence, or clear worsening on antimicrobial therapy. Surgical delay in such cases increase risks of proximal amputation and death. Clinical trials indicate that well-selected oral antimicrobials are as effective as IV therapy, and emerging trial data suggests shorter-than-traditional durations are often adequate for multiple forms of DFI.
Table 1.
“To-do” list for a comprehensive approach to diabetes-related foot infection care
| Surgical debridement: if present, drain deep purulence and excise necrotic tissue. Assess risk of amputation using clinically validated criteria (WIfI) and if elevated request surgical evaluation and risk-benefit/shared decision-making |
| Peripheral artery disease: Obtain relevant vascular studies (eg toe pressure measurements); request vascular surgery evaluation if patient is likely to benefit from revascularization (ie, by WIfI classification) |
Antibiotic therapy: Once patient is stabilized and has responded to initial antimicrobial therapy, select an appropriate oral (or IV) definitive antimicrobial regimen, with considerations including:
|
| Offloading: Provide the patient with either a non-removable device (eg, total contact cast) or removable device (ie, surgical boot) to provide mechanical off-loading of the diabetic foot wound; consider surgical off-loading referral for select patients who do not heal with mechanical devices. |
| Wound care: Secure longitudinal outpatient followup with a wound care specialist who can provide serial assessment, debridement, and appropriate dressings or negative pressure wound therapy. Ensure the patient has access to adequate wound care supplies upon discharge and at each followup visit. |
Glycemic control: Initiate or intensify diabetes treatment to achieve goal HbA1c to optimize wound healing
|
Concurrent foot pathology: Identify and address other conditions that provide a bacterial portal of entry into the foot or otherwise predispose to infection:
|
Other key comorbidities:
|
Barriers to care: Mitigate social factors likely to impede patient’s adherence to treatment (see table 4 of full online text for specific potential solutions)
|
Conservative surgical management of DFI can preserve independent mobility. The decision to proceed with debridement and/or amputation should depend not only on the extent of infection but on the degree of mobility at risk, which in turn depends on the patient’s baseline functional status and the foot territory under threat (eg, a heel DFO for which a nonhealing surgical wound would require BKA, versus a distal phalanx DFO that can be cured surgically with little impact on mobility). All patients with DFI should undergo vascular studies and revascularization if indicated, because concurrent PAD substantially increases risk of amputation.
When conflict arises out of shared decision-making, simple miscommunication, conflicting prognostications, and genuine conflicts in values (eg, a surgical colleagues’ emphasis on preservation of mobility, or a patient’s emphasis on avoidance of amputation, versus an ID clinician’s emphasis on eradication of the infection with minimal necessary antimicrobial exposure) are common sources. Specifically identifying and addressing these sources often facilitates reaching consensus. In some cases of conflicting values where surgery is not strictly indicated, the ID clinician may most benefit the patient’s care by framing antimicrobials as a “therapeutic trial” to be reevaluated at early clinic follow-up, and by ensuring that antimicrobials are being given as part of a comprehensive treatment strategy to preserve the patient’s functional status via limb salvage. Multidisciplinary DFI teams can mitigate these conflicts by fostering mutual trust between clinicians based on frequent communication and longitudinal relationships and can centralize patient’s care and optimize outcomes.
Contributor Information
Nicolas W Cortes-Penfield, Division of Infectious Diseases, University of Nebraska Medical Center, Omaha, Nebraska, USA.
David G Armstrong, Department of Surgery, University of Southern California, Los Angeles, California, USA.
Meghan B Brennan, Division of Infectious Diseases, University of Wisconsin-Madison Medical Center, Madison, Wisconsin, USA.
Maya Fayfman, Division of Endocrinology & Metabolism, Emory University, Atlanta, Georgia, USA; Department of Medicine, Grady Memorial Hospital, Atlanta, Georgia, USA.
Jonathan H Ryder, Division of Infectious Diseases, University of Nebraska Medical Center, Omaha, Nebraska, USA.
Tze-Woei Tan, Department of Surgery, University of Southern California, Los Angeles, California, USA.
Marcos C Schechter, Department of Medicine, Grady Memorial Hospital, Atlanta, Georgia, USA; Division of Infectious Diseases, Emory University, Atlanta, Georgia, USA.
Notes
Financial support . This work is partially supported by the National Institute of Diabetes and Digestive and Kidney Diseases Award Numbers 1R01124789-01A1 (D. G. A.), R01-DK132569-01 (M. B. B.), and 1K23DK122126 (T. W. T.), in addition to the Vascular Cures Collaborative Patient-Centered Research grant (T. W. T.), Society of Vascular Surgery Foundation award (1K23DK122126 supplement to T. W. T.), National Science Foundation Center to Stream Healthcare in Place (#C2SHiP) (CNS Award Number 2052578 to D. G. A.), and the National Institute on Minority Health and Health Disparities (R21-MD017943-01 to M. C. S.