Abstract
Background
Tuberculosis infection (TBI) and TB disease (TBD) incidence remains poorly described following household contact (HHC) rifampin-/multidrug-resistant TB exposure. We sought to characterize TBI and TBD incidence at 1 year in HHCs and to evaluate TB preventive treatment (TPT) use in high-risk groups.
Methods
We previously conducted a cross-sectional study of HHCs with rifampin-/multidrug-resistant TB in 8 high-burden countries and reassessed TBI (interferon-gamma release assay, HHCs aged ≥5 years) and TBD (HHCs all ages) at 1 year. Incidence was estimated across age and risk groups (<5 years; ≥5 years, diagnosed with human immunodeficiency virus [HIV]; ≥5 years, not diagnosed with HIV/unknown, baseline TBI-positive) by logistic or log-binomial regression fitted using generalized estimating equations.
Results
Of 1016 HHCs, 850 (83.7%) from 247 households were assessed (median, 51.4 weeks). Among 242 HHCs, 52 tested interferon-gamma release assay–positive, yielding a 1-year 21.6% (95% confidence interval [CI], 16.7–27.4) TBI cumulative incidence. Sixteen of 742 HHCs developed confirmed (n = 5), probable (n = 3), or possible (n = 8) TBD, yielding a 2.3% (95% CI, 1.4–3.8) 1-year cumulative incidence (1.1%; 95% CI, .5–2.2 for confirmed/probable TBD). TBD relative risk was 11.5-fold (95% CI, 1.7–78.7), 10.4-fold (95% CI, 2.4–45.6), and 2.9-fold (95% CI, .5–17.8) higher in age <5 years, diagnosed with HIV, and baseline TBI high-risk groups, respectively, vs the not high-risk group (P = .0015). By 1 year, 4% (21 of 553) of high-risk HHCs had received TPT.
Conclusions
TBI and TBD incidence continued through 1 year in rifampin-/multidrug-resistant TB HHCs. Low TPT coverage emphasizes the need for evidence-based prevention and scale-up, particularly among high-risk groups.
Keywords: household contacts, multidrug-resistant tuberculosis, tuberculosis infection, tuberculosis disease, tuberculosis preventive treatment
To our knowledge, this is among the largest multinational studies to provide a 1-year incidence estimate of tuberculosis infection (TBI) and disease in both adult and child household contacts of rifampin-/multidrug-resistant TB. We add to the existing literature by providing a 1-year TBI and disease cumulative incidence in key at-risk populations (people living with human immunodeficiency virus, children aged <5 years, and baseline TBI) and detail TB preventive treatment practice patterns. These findings suggest that high-risk household contacts of multidrug-resistant TB cases may benefit from reassessment following initial contact and underscore the need for expansion of coverage, providing programmatic evidence to guide which key high-risk groups to target.
Despite being preventable, 10.6 million individuals develop tuberculosis disease (TBD) yearly [1]. Breaking the cycle of transmission and identifying those at risk of TBD, including multidrug-resistant TB (MDR TB), are both critical to ending the global TB epidemic. Unfortunately, the prevalence of MDR TB infection is increasing, particularly among children and young adults [2]. Prevention of TB in household contacts (HHCs) of people with MDR TB, defined as resistance to rifampin and isoniazid, is a particular challenge.
Tuberculosis preventive treatment (TPT) seeks to preempt TBD development through targeted preventive chemotherapy. Standard TPT for HHCs of drug-sensitive TB, consisting of isoniazid-based and/or rifamycin-based regimens, reduces progression to TBD [3]. The optimal regimen for MDR TB HHCs is not well defined given the lack of randomized controlled trials; therefore, the World Health Organization has provided only a conditional recommendation for TPT in high-risk MDR TB HHCs, based largely on observational data [3, 4]. High-risk HHC populations include children aged <5 years, people with human immunodeficiency virus (HIV; PWH), individuals on immunosuppressive therapy, and individuals with TBI [3, 5–7]. Furthermore, TPT uptake in low- and middle-income countries (LMICs) has been limited due to required infrastructure, costs, and side effects of available regimens [8–10].
Given existing clinical equipoise and challenges with implementation of widespread TPT, high-quality data on TBI and TBD incidence among HHCs of MDR TB are needed to inform epidemiological models, randomized clinical trials of TPT and candidate vaccines, and national TB programs. Our Protecting Households On Exposure To Newly Diagnosed Index Multidrug-Resistant Tuberculosis Patients (PHOENIx) Feasibility Study team previously reported a high baseline TBI prevalence of 72% (by interferon-gamma release assay [IGRA] or tuberculin skin test [TST]) and a 12% TBD prevalence among child and adult HHCs of recently diagnosed adult rifampin-resistant (RR)/MDR TB index cases [11]. Here, we report 1-year outcomes, namely, the 1-year TBI and TBD incidence following initial baseline evaluation.
METHODS
Study Design and Population
Between October 2015 and March 2016, recently routinely diagnosed and treated adults with RR/MDR TB and their child and adult HHCs were enrolled in the PHOENIx Feasibility Study at 16 National Institutes of Health–funded AIDS Clinical Trials Group/International Maternal Pediatric Adolescent AIDS Clinical Trials Network study sites in Botswana, Brazil, Haiti, India, Kenya, Peru, South Africa, and Thailand, as previously detailed [11]. Additional information on index case and HHC eligibility is provided in the Supplementary Material. HHC participants were reenrolled to a single 1-year follow-up visit to estimate the 1-year incidence of TBI and TBD; analyses included those who died prior to the 1-year visit. There was no follow-up of participants in between the baseline and 1-year visit.
Study Procedures
Tuberculosis Screening Among Household Contacts
Sociodemographic and clinical data were collected at baseline study enrollment. At baseline and 1 year, all HHCs were evaluated using a symptom questionnaire and chest radiography (CXR) and underwent respiratory sample collection for acid-fast bacilli (AFB) smear, GeneXpert MTB/RIF (Cepheid, Sunnyvale, CA), and Mycobacterium tuberculosis culture (gastric aspirate from children) as clinically indicated. HHCs with a negative/indeterminate baseline IGRA (by QuantiFERON Gold or Gold-In-Tube) underwent QuantiFERON Gold or Gold-In-Tube (Qiagen, Venlo, The Netherlands) testing at 1 year.
Outcomes and Definitions
The primary outcomes were the 1-year incidence of TBI and TBD. Incident TBI was defined as IGRA conversion among HHCs aged ≥5 years with a baseline negative or indeterminate IGRA result. Children aged <5 years were excluded from the analysis of TBI (but not TBD), as current guidelines in high-burden settings recommend TPT regardless of TBI status and the IGRA was not yet approved for use in those aged <5 years. The positive IGRA threshold was set per manufacturer instructions [12]. Incident TBD was defined as any confirmed, probable, or possible event (as per expert review described in the Supplementary Material) between enrollment and the 1-year evaluation among HHCs with a negative baseline TBD evaluation. Confirmed TBD was defined as bacteriologically confirmed via liquid or solid culture or rapid molecular tests and either symptoms of TBD or CXR suggestive of TB (for children aged <15 years). Probable TBD was defined as symptoms compatible with TB and either AFB smear positivity or CXR suggestive of TB. Possible TBD in children aged <15 years was defined as symptoms compatible with TBD, being AFB-positive, or having a CXR suggestive of TBD. HHCs were classified into the following mutually exclusive high-risk groups at enrollment: age <5 years (regardless of baseline TBI or HIV status); age ≥5 years and diagnosed with HIV (regardless of baseline TBI status); and age ≥5 years, not diagnosed with HIV or unknown, and baseline TBI-positive (by IGRA or TST). All others were considered not high-risk.
Statistical Analyses
Descriptive statistics were used to describe study population characteristics, and differences were assessed by a score test using the generalized estimating equations (GEE) approach. Proportions with 95% confidence intervals (CIs) were estimated using logistic regression, fitted using the GEE approach to account for clustering among HHCs within the same household [13]. Using a 4-level categorical variable to define 3 high-risk and not high-risk groups, we used log-binomial regression fitted using a GEE approach to estimate relative risks with HHC who were not high-risk as the reference group. Wald 95% CIs were estimated using empirical standard errors. Testing was 2-sided and considered significant at the 5% level based on score tests (SAS, Version 9.4, Cary, NC).
Ethics Statement
Written informed consent or assent was obtained for 1-year TB evaluations from all HHCs and their parents/caregivers for children aged <18 years. The study was approved by all local institutional review boards/ethics committees and partnering US institutions. Two study sites (in Brazil and Kenya) did not receive approval to enroll HHCs aged <18 years.
RESULTS
Characteristics of Household Contacts at 1 Year
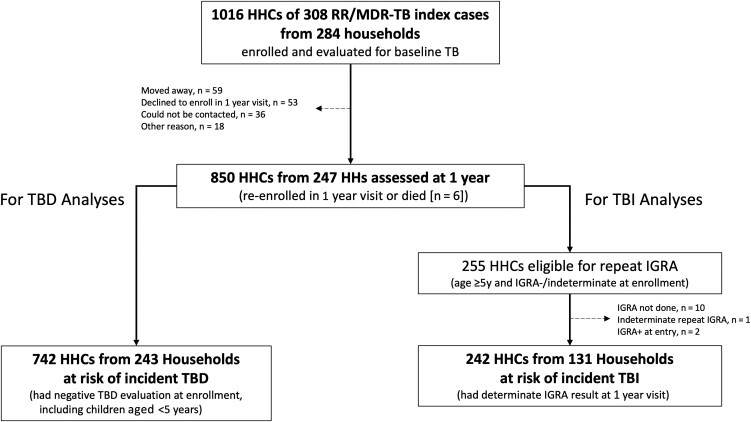
Of 1016 HHCs enrolled at baseline from 308 RR/MDR TB index cases, 850 (83.7%) participants from 247 households were assessed at the 1-year visit, including 6 individuals who had died (n = 2 TB-related; Figure 1). Of the 166 individuals not included in the 1-year follow-up, the majority had moved (n = 59), declined to participate (n = 53), or could not be contacted (n = 36). Follow-up TB evaluations occurred at a median of 51.4 weeks (interquartile range [IQR], 43.3–67.7) after initial study entry. The median baseline age of HHCs among those included in the 1-year visit was 25 years (IQR, 11–43); 59% were assigned female sex at birth; and the majority were from South Africa (41%), India (22%), and Peru (21%; Table 1). Seventy-five percent (641 of 850) were high-risk (age <5 years, diagnosed with HIV, or diagnosed with TBI). HHCs at the 1-year follow-up were comparable to HHCs at initial study entry by age, sex, and risk group. Those with self-reported diabetes, daily alcohol consumption, or substance use at baseline were less likely to be assessed at 1 year. There was a significant difference by country, with those in Haiti and South Africa less likely to be evaluated at 1 year (P ≤ .01). Supplementary Table 1 shows that the proportions assessed at 1 year were similar by age, HIV status, and risk group. The proportion of HHCs assessed by site was heterogeneous (40.7% to 100%; Supplementary Table 2).
Figure 1.
Flow from baseline enrollment of the index MDR TB cases and HHCs to HHCs reassessed at 1 year (from 247 households) who were either at risk of TBD or TBI. TBD and TBI analyses were completed separately, as all HHCs at risk of incident TBD were not necessarily at risk of incident TBI. Abbreviations: HHC, household contact; IGRA, interferon-gamma release assay; MDR, multidrug-resistant; RR, rifampin-resistant; TB, tuberculosis; TBD, tuberculosis disease; TBI, tuberculosis infection.
Table 1.
Comparison of Baseline Characteristics of Rifampin-Resistant Multidrug-Resistant Tuberculosis Household Contacts at Baseline and 1-Year Visits
| Baseline Characteristic | Baseline Visit (n = 1016) | 1-Year Visita (n = 850) | P Valueb |
|---|---|---|---|
| Median age, (interquartile range), y | 25 (12, 43) | 25 (11, 43) | .48 |
| Sexc | … | … | .94 |
| Female | 598 (59%) | 501 (59%) | … |
| Male | 418 (41%) | 349 (41%) | … |
| Age group, y | … | … | .52 |
| <5 | 103 (10%) | 89 (10%) | … |
| 5 to <15 | 201 (20%) | 171 (20%) | … |
| 15 to <45 | 477 (47%) | 387 (46%) | … |
| ≥45 | 235 (23%) | 203 (24%) | … |
| HIV status | … | … | .40 |
| Diagnosed with HIV | 65 (6%) | 50 (6%) | … |
| Not diagnosed with HIV or unknown | 951 (94%) | 800 (94%) | … |
| TB riskd | … | … | .66 |
| Not high-risk | 232 (23%) | 200 (24%) | … |
| High-riske | 775 (76%) | 641 (75%) | … |
| Age <5 y | 102 (13%) | 88 (14%) | … |
| Diagnosed with HIV | 63 (8%) | 49 (8%) | … |
| TB infection-positive | 610 (79%) | 504 (79%) | … |
| Country | … | … | <.01 |
| Botswana | 38 (4%) | 37 (4%) | … |
| Brazil | 17 (2%) | 17 (2%) | … |
| Haiti | 52 (5%) | 39 (5%) | … |
| India | 206 (20%) | 190 (22%) | … |
| Kenya | 12 (1%) | 10 (1%) | … |
| Peru | 203 (20%) | 181 (21%) | … |
| South Africa | 458 (45%) | 348 (41%) | … |
| Thailand | 30 (3%) | 28 (3%) | … |
| Employed/in school | 504 (50%) | 412 (48%) | .07 |
| Highest education attained | … | … | .17 |
| None | 246 (24%) | 213 (25%) | … |
| Primary | 328 (32%) | 283 (33%) | … |
| Secondary | 351 (35%) | 275 (32%) | … |
| College/university or higher | 83 (8%) | 71 (8%) | … |
| Unknown | 8 (1%) | 8 (1%) | … |
| History of incarceration | 21 (2%) | 15 (2%) | .79 |
| Medical history | … | … | … |
| Current or former smoker | 212 (21%) | 165 (19%) | .28 |
| Self-reported diabetes | 26 (3%) | 24 (3%) | .02 |
| Daily alcohol consumptionf | 60 (6%) | 39 (5%) | .01 |
| History of substance usef | 59 (6%) | 43 (5%) | .05 |
| Prior history of TB treatment | 89 (9%) | 73 (9%) | .56 |
Abbreviations: HIV, human immunodeficiency virus; TB, tuberculosis.
Includes n = 6 individuals who died between baseline and 1-year visit. Individuals who were TB infection (TBI)-positive at baseline were not tested again for TBI, but were assessed for tuberculosis disease.
Score test from generalized estimating equations models.
Information on gender was not collected, which might differ from sex assigned at birth.
Excludes 9 household contacts diagnosed with TB before initial study entry.
High-risk defined as age <5 years (regardless of HIV and TBI status); age ≥5 years and diagnosed with HIV (regardless of TBI status); or age ≥5 years, not diagnosed with HIV/unknown and TBI-positive at entry via tuberculin skin test or interferon-gamma release assay.
Alcohol and substance use were self-reported with a 12-month recall period. Alcohol use was defined as a time or times drinking alcoholic beverages almost every day.
1-Year Incidence of Tuberculosis Infection
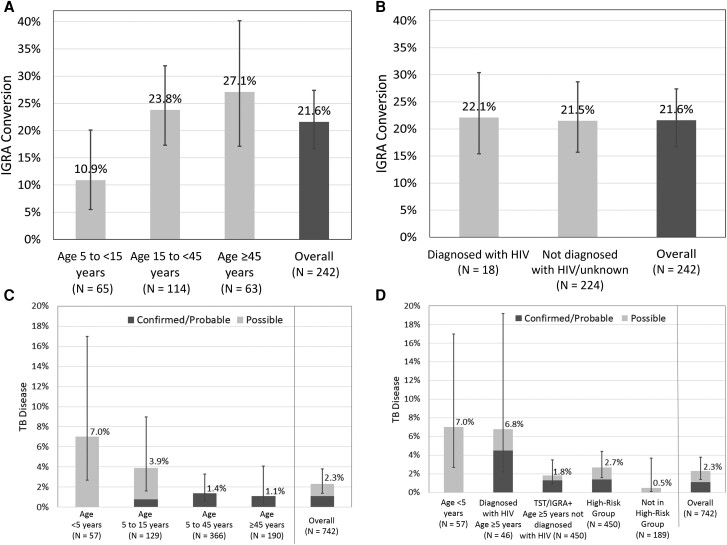
Overall, 242 HHCs from 131 households were at risk for incident TBI (negative/indeterminate baseline IGRA, excluding children aged <5 years; Figure 1). Of these, 52 had IGRA conversion at 1 year, yielding a 1-year TBI cumulative incidence of 21.6% (95% CI, 16.7–27.4; Figure 2A, 2B ). Incident TBI was significantly higher among adolescents and adults (≥15 years) compared with children aged 5 to <15 years (25.5%; 95% CI, 19.3–32.8 vs 10.9%; 95% CI, 5.5–20.1; P = .007). TBI incidence increased with age group, with 10.9% in children aged 5 to <15 years (95% CI, 5.5–20.1), 23.8% in those aged 15 to <45 years (95% CI, 17.3–31.9; P = .021), and 27.1% in those aged ≥45 years (95% CI, 17.1–40.2; P = .024). There was no significant difference by HIV status (PWH, 22.1%; 95% CI, 11.9–37.3 vs not diagnosed with HIV/unknown, 21.5%; 95% CI, 16.4–27.7; P = .095; Figure 2B ). We observed no sex-specific differences in TBI incidence (Supplementary Table 3).
Figure 2.
One-year cumulative incidence of TB infection (TBI) and TB disease (TBD) in household contacts of rifampin- or multidrug-resistant TB index cases. One-year cumulative incidence of TBI defined by IGRA conversion with comparisons made between age groups (A) and HIV status (B). One-year cumulative incidence of TBD stratified by confirmed/probable TB and possible disease with comparisons made between age groups (C) and risk group (D). High-risk group includes age <5 (regardless of HIV and TBI status); age ≥5 years diagnosed with HIV (regardless of TBI status); and age ≥5, and not diagnosed with HIV, baseline TST/IGRA positive. Cumulative incidence estimated using generalized estimated equations to account for household clustering. Longitudinal bars represent 95% confidence intervals. Abbreviations: HIV, human immunodeficiency virus; IGRA, interferon-gamma release assay; TB, tuberculosis; TST, tuberculin skin test.
1-Year Incidence of Tuberculosis Disease
Overall, 742 HHCs from 243 households were at risk for incident TBD at 1 year (negative baseline TBD evaluation; Figure 1), including 553 high-risk contacts (n = 57 aged <5 years, n = 46 PWH, and n = 450 TBI-positive at baseline). We identified 16 incident TBD events (8 diagnosed through routine care before the 1-year visit and 8 during the 1-year evaluations), yielding an overall TBD cumulative incidence of 2.3% (95% CI, 1.4–3.8; Figure 2C, 2D ). The TBD cumulative incidence was significantly higher in children (aged <15 years) compared with adolescents/adults (4.9%; 95% CI, 2.6–9.2 vs 1.3%; 95% CI, .6–2.6; P = .023). There were 5 cases of confirmed, 3 cases of probable, and 8 cases of possible TBD. All 8 possible TBD cases were classified based on symptoms, and none had a positive smear or suggestive CXR. Excluding possible TBD, the confirmed/probable TBD cumulative incidence was 1.1% (95% CI, .5–2.2). High-risk HHCs contributed 15 of 16 TBD events, with confirmed TBD accounting for 5 of 7 events in adolescents/adults (aged ≥15 years) and possible TB accounting for 8 of 9 events among children aged <15 years. Among the 5 confirmed TBD cases, 3 had drug-susceptibility data reported, with all resistant to isoniazid and rifampin. The TBD 1-year cumulative incidence was significantly higher in high-risk contacts compared with not high-risk (2.7%; 95% CI, 1.6–4.4 vs 0.5%; 95% CI, .1–3.7; P = .006), particularly among children aged <5 years (7.0%; 95% CI, 2.7–17.0; P = .012) and PWH (6.8%; 95% CI, 2.2–19.2; P = .002; Figure 2D ). Among those with TBI, 1.8% (8 of 442; 95% CI, .9–3.5; P = .25) progressed to active disease by 1 year. HHCs with HIV had a higher estimated cumulative incidence of TBD than those not living with HIV/unknown, but the difference was not statistically significant (6.6%; 95% CI, 2.1–18.8 vs 1.9%; 95% CI, 1.1–3.2; P = .21). The relative risk of TBD was 11.5-fold (95% CI, 1.7–78.7) and 10.4-fold (95% CI, 2.4–45.6) higher in children aged <5 years and HIV + high-risk groups, respectively, compared with not high-risk HHCs (Table 2). The estimated relative risk of TBD in the high-risk TBI group compared with not high-risk HHCs was increased at 2.9 but was not statistically significant (95% CI, .5–17.8). Overall, there was a significantly higher relative risk of TBD in the high-risk groups vs not high-risk HHCs (P = .0015).
Table 2.
Relative Risk of Incident Tuberculosis Disease in Rifampin-Resistant Multidrug-Resistant Tuberculosis Household Contacts at 1-Year Follow-up
| Household Contact Risk Groupa | Relative Risk of Incident TB Disease (95% Confidence Interval)b,c,d | Overall P Value |
|---|---|---|
| Age <5 y | 11.5 (1.7–78.7) | .0015 |
| Diagnosed with human immunodeficiency virus | 10.4 (2.4–45.6) | |
| TB infection-positive | 2.9 (.5, 17.8) | |
| Not high-risk | Reference group |
Abbreviation: TB, tuberculosis.
High-risk defined as age <5 years (regardless of human immunodeficiency virus (HIV) and TB infection (TBI) status); age ≥5 years and diagnosed with HIV (regardless of TBI status); or age ≥5 years, not diagnosed with HIV/unknown and TBI-positive at entry via tuberculin skin test or interferon-gamma release assay.
Log-binominal regression fitted using generalized estimating equations to account for clustering within households. Relative risks use household contacts who were categorized as not high-risk as the reference group.
TB disease includes confirmed, possible, and probable TB disease.
Wald 95% confidence interval, estimated using empirical standard errors.
Use of Tuberculosis Preventive Treatment
Among 553 high-risk contacts at risk for incident TBD at follow-up (no TBD identified during baseline review), 21 (4%) reported receiving TPT at or between enrollment and the 1-year visit (19 of these had discontinued TPT before the 1-year visit), 528 never received TPT, and 4 had unknown TPT status (Table 3). Within the high-risk groups, 11 of 55 (20%) aged <5 years, 6 of 45 (13%) PWH, and 4 of 449 (1%) with TBI received TPT. TPT regimens included isoniazid monotherapy (n = 12), isoniazid/levofloxacin/ethambutol (n = 5), isoniazid/moxifloxacin/ethionamide (n = 2), and unknown (n = 2). TPT use varied by country.
Table 3.
Summary of Tuberculosis Preventive Treatment Use in High-Risk Rifampin-Resistant Multidrug-Resistant Tuberculosis Household Contacts by 1 Year
| Characteristic | No TPT, n (%) | Receipt of TPT, n (%) |
|---|---|---|
| Overalla | 528 (96) | 21 (4) |
| High-risk groupb | … | … |
| Age <5 y | 44 (80) | 11 (20) |
| Living with human immunodeficiency virus | 39 (87) | 6 (13) |
| Tuberculosis infection-positive | 445 (99) | 4 (1) |
| Regimen | ||
| INH/LVX/EMB | … | 5 |
| INH | … | 12 |
| INH/MOX/ETH | … | 2 |
| Unknown | … | 2 |
| Country | ||
| Botswana | 18 (100) | 0 (0) |
| Brazil | 9 (90) | 1 (10) |
| Haiti | 29 (100) | 0 (0) |
| India | 129 (98) | 3 (2) |
| Kenya | 5 (83) | 1 (17) |
| Peru | 94 (100) | 0 (0) |
| South Africa | 228 (93) | 16 (7) |
| Thailand | 16 (100) | 0 (0) |
Abbreviations: EMB, ethambutol; ETH, ethionamide; INH, isoniazid; LVX, levofloxacin; MOX, moxifloxacin; TPT, tuberculosis preventive treatment.
Excluding n = 4 individuals for whom receipt of TPT was unknown.
High-risk defined as age <5 years (regardless of human immunodeficiency virus (HIV) and tuberculosis infection (TBI) status); age ≥5 years and diagnosed with HIV (regardless of TBI status); or age ≥5 years, not diagnosed with HIV/unknown and TBI-positive at entry via tuberculin skin test or interferon-gamma release assay.
DISCUSSION
This study of RR/MDR TB HHCs from multiple high-burden countries demonstrates the importance of ongoing HHC evaluation after index case exposure and provides key evidence of incident TBI and TBD at 1 year. Despite index case treatment and comprehensive HHC TBI and TBD evaluation at baseline, 1 year later, we identified a 21.6% TBI cumulative incidence by IGRA conversion in HHCs aged ≥5 years, a 2.3% TBD cumulative incidence in HHCs of all ages, and a 1.1% cumulative incidence of confirmed/probable TBD, highlighting the importance of longer-term follow-up of MDR TB HHCs. We observed age-specific differences, with incident TBI being significantly higher in adolescents/adults aged ≥15 years compared with children aged 5 to <15 years (25.5% vs 10.9%). Importantly, our high-risk population was especially vulnerable to TBD development (2.7% cumulative incidence high-risk vs 0.5% not high-risk), with the highest incidence among children aged <5 years (7.0%) and PWH (6.8%). We observed a significantly higher relative risk of TBD in children <5. Although this group was high-risk for TBD development, only 4% received TPT. Our data from one of the largest multinational studies add to the evidence that describes the ongoing risk of progressing to TBI and TBD after household RR/MDR TB exposure. Low TPT coverage of high-risk HHCs despite this risk underscores the urgent need to identify and scale-up effective TPT regimens in this vulnerable population.
After reporting a 72% baseline TBI prevalence (by IGRA or TST) among recently exposed HHCs [11], we observed a 21.6% 1-year TBI cumulative incidence in HHCs aged ≥5 years by IGRA. In comparison to our 22.1% PWH TBI cumulative incidence, Kenyan PWH had a 12.5% IGRA conversion rate 1 year postpartum [14] vs 9% in PWH in a low-incidence region [15]. We found cumulative incident TBI was substantially higher among adolescents/adults (aged ≥15 years, 25.5%) compared with children (aged 5 to <15 years, 10.9%), mirroring age-specific TBI prevalence trends [7, 16]. Our pediatric TBI cumulative incidence was similar to the 14% IGRA conversion rate of a South African cohort (aged 12–18 years) [17]. We notably did not IGRA test children aged <5 years at 1 year. A TBI incidence of 11.8%–56% has been reported in HHCs in high-burden TB regions (by IGRA and TST) [18, 19]. While TBI prevalence studies in HHCs of drug-resistant TB have been conducted (by TST screening) [16, 20], to our knowledge, a large multicountry study from high-burden TB regions has not provided a 1-year TBI incidence estimate from RR/MDR TB HHCs measuring IGRA conversion. Some of our high TBI incidences may be attributed to delayed IGRA conversion following household infection or could reflect infection originating from the community. This is supported by our higher TBI incidence in adults/adolescents vs children, emphasizing the need to prioritize TPT and follow-up in this population. Nevertheless, the incidence of infection is much higher than the annual risk of infection in the general population [21], suggesting that many new cases of infection originated from household exposure and highlighting that our RR/MDR TB HHC population is especially vulnerable to incident TBI.
Our highest TBD incidence proportions and relative risk occurred in high-risk groups, specifically PWH and children. Our 6.6% TBD incidence in PWH (all ages) is similar to that from a prospective South African HHC cohort study of TBD incidence rate (PWH, 5.4/100 person-years vs not diagnosed with HIV, 0.7/100 person-years) [22]. While our TBD incidence between PWH and not diagnosed with HIV/unknown (6.6% vs 1.9%) was not statistically significant, this was likely due to a small PWH sample size. We observed a significantly higher TBD cumulative incidence among children, including a 7% TBD cumulative incidence in children aged <5 years, consistent with reports of a 2-year TBD risk of 19% among IGRA/TST + children (aged 2–5 years) [5] vs a South African TBD incidence of 2.9/100 child-years [23]. While our incidence in children is particularly high, it could reflect the more rapid progression from exposure to TBD in children, the prolonged infectious periods in MDR TB index cases, or the relative immunocompromised state of young children [24]. Notably, this cumulative incidence occurred despite 20% (11 of 55) of children aged <5 years receiving TPT. Common to many cohort studies, inclusion of possible TB in children aged <15 years could also account for this high incidence, as many events were not confirmed microbiologically. Similar to our 2.3% TBD cumulative incidence, a meta-analysis of contacts of drug-sensitive and drug-resistant TB cases in LMICs showed the highest TBD incidence in the first year (1.5/100 person-years) and declining to 0.5/100 person-years at 3 years [7]. Specific to MDR TB index cases, the HHC TBD incidence in high- and middle-TB burden countries has ranged from 0.2 to 4.0 per 100 person-years [25–30]. Overall, our TBD incidence findings highlight that easy-to-measure risk factors in HHCs, such as age and HIV status, could be used to target those at risk for TBD progression.
Finally, overall low TPT coverage in our study mirrors the slow progress to expand TPT among HHCs globally [31] and reflects the limited-quality data to guide TPT for MDR TB contacts. Despite a reported willingness to take part in an MDR TB TPT regimen [32], only 4% of high-risk HHCs received TPT by 1 year. Children aged <5 years were the most common high-risk group to receive TPT, and the most common regimen was isoniazid monotherapy, followed by isoniazid/fluoroquinolone paired with ethambutol or ethionamide, based on local practice. This supports literature describing the heterogeneity of TPT regimens used in RR/MDR TB contacts [4], highlighting the need for ongoing randomized, controlled trials to evaluate fluoroquinolone-based regimens vs newer alternatives such as delamanid.
Our study has some limitations. There is no gold standard for TBI diagnosis, and IGRA can have reduced sensitivity in PWH and poor reproducibility on repeat testing [33]. While we estimated TBI incidence, we lacked data on the precise timing of IGRA conversions, qualitative IGRA results, and reversion rates. Next, we could not determine whether incident TBD represented community or household transmission, as many events were not bacteriologically confirmed. Similarly, TBI could be due to household exposure or from an alternative source. While we estimated the relative risk of TBD in high-risk groups, the CIs were large, reflecting in part low event numbers. Our TBI and TBD cumulative incidence could be an underestimate, given our inability to follow 16.3% of participants; conversely, our TBD cumulative incidence could be an overestimate, given our inclusion of possible TBD. Finally, we were less likely to reenroll those with diabetes, daily alcohol consumption, and substance use, all known to be associated with adverse TBD outcomes, highlighting the need to better determine strategies for reidentifying these vulnerable populations.
In summary, our baseline and 1-year analyses of HHCs of RR/MDR TB in multiple high-burden countries suggest that while the highest risk of TBI and TBD acquisition occurs soon after household RR/MDR TB exposure, the risk continues through the first year. High-risk HHCs (children aged <5 years, PLWH, and those with TBI) contributed most of the TBD events (94%) and had a significantly higher relative risk of TBD. Furthermore, by 1 year, 21.6% HHCs aged≥5 years had developed TBI. Given the costs associated with contact tracing and TPT administration, these data could inform programmatic strategies, including targeted TPT provision in resource-limited settings. The development of incident TBI and TBD following initial assessment suggests that reassessment, particularly in high-risk HHCs, may be beneficial. Last, low TPT use among this high-risk population underscores the need for effective strategies to expand HHC coverage in high-burden regions and provides evidence for key groups to target in programmatic settings.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Sonya Krishnan, Department of Medicine, Division of Infectious Diseases, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Xingye Wu, Harvard T. H. Chan School of Public Health, Boston, Massachusetts, USA.
Soyeon Kim, Frontier Science Foundation, Brookline, Massachusetts, USA.
Katie McIntire, Department of Medicine, Division of Infectious Diseases, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Linda Naini, Social & Scientific Systems, Silver Spring, Maryland, USA.
Michael D Hughes, Harvard T. H. Chan School of Public Health, Boston, Massachusetts, USA.
Rodney Dawson, University of Cape Town Lung Institute and Department of Medicine, Cape Town, South Africa.
Vidya Mave, Department of Medicine, Division of Infectious Diseases, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA; Byramjee Jeejeebhoy Government Medical College, Pune, India.
Sanjay Gaikwad, Byramjee Jeejeebhoy Government Medical College, Pune, India.
Jorge Sanchez, Centro de Investigaciones Biomedicas y Medioambientales (CITBM), Universidad Nacional Mayor de San Marcos, Lima, Peru.
Alberto Mendoza-Ticona, TASK Applied Science Clinical Research Site, Bellville, South Africa.
Pedro Gonzales, Asociació n Civil Impacta Salud y Educació n, Lima, Peru.
Kyla Comins, TASK Applied Science Clinical Research Site, Bellville, South Africa.
Justin Shenje, South African Tuberculosis Vaccine Initiative, Cape Town, South Africa.
Sandy Nerette Fontain, GHESKIO Centers Institute of Infectious Diseases and Reproductive Health, Port-au-Prince, Haiti.
Ayotunde Omozoarhe, Botswana Harvard AIDS Institute Partnership CTU, Gaborone Clinical Research Site, Gaborone, Botswana.
Lerato Mohapi, Soweto Clinical Research Site, University of the Witwatersrand, Johannesburg, South Africa.
Umesh G Lalloo, Durban International Clinical Research Site, Durban University of Technology, Durban, South Africa.
Ana Cristina Garcia Ferreira, Instituto Nacional de Infectologia—INI/Fiocruz, Rio de Janiero, Brazil.
Christopher Mugah, Kenya Medical Research Institute, Kisumu, Kenya.
Mark Harrington, Treatment Action Group, New York, New York, USA.
N Sarita Shah, Emory Rollins School of Public Health, Atlanta, Georgia, USA.
Anneke C Hesseling, Desmond Tutu TB Centre, Department of Paediatrics and Child Health, Stellenbosch University, Cape Town, South Africa.
Gavin Churchyard, Aurum Institute, Parktown, South Africa; University of the Witwatersrand, School of Public Health, Johannesburg, South Africa; Advancing Care and Treatment, South African Medical Research Council, Johannesburg, South Africa.
Susan Swindells, Department of Medicine, Division of Infectious Diseases, University of Nebraska Medical Center, Omaha, Nebraska, USA.
Amita Gupta, Department of Medicine, Division of Infectious Diseases, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA; Byramjee Jeejeebhoy Government Medical College, Pune, India.
Notes
Author Contributions. A. G., A. C. H., G. C., S. S., N. S. S., M. D. H., and M. H. designed the study. L. N., R. D., V. M., S. G., J. S., A. M. T., P. G., K. C., J. S., S. N. F., A. O., L. M., U. G. L., A. C. G. F., and C. M. coordinated the individual study sites. X. W. and S. Ki. accessed and verified all data and completed data analyses. S. Kr., X. W., S. Ki., K. M., and A. G. contributed to manuscript drafting. All authors had full access to study data, contributed to the interpretation of study data and manuscript revision, approved the final manuscript version, and accept responsibility for the decision to submit for publication.
Acknowledgments. The authors thank the study participants and their families, as well as the site community advisory boards. They also thank the following contributors: investigator: Richard E. Chaisson; clinical representatives: Dan Johnson, George K. Siberry, and Elizabeth Smith; microbiologist: Anne-Marie Demers; field representatives: Savita Kanade, Janet Nicotera; laboratory technologists: Patricia Anthony and Christopher Lane; Community Scientific Subcommittee representatives: Ujwala A. Kadam and Ronald Ssenyonga; international site specialist: Akbar Shahkolahi; data managers: Lynne Jones and Barbara Heckman; and laboratory data manager: Adam Manzella.
Disclaimer. The content presented here is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support . Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health, all components of the NIH, under awards UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC), and UM1AI106716 (IMPAACT LC), and by NICHD contract HHSN275201800001I. Overall support for the AIDS Clinical Trials Group (ACTG) was provided by the NIAID of the NIH under awards UM1AI068634 (ACTG SDMC), UM1AI068636 (ACTG LOC), and UM1AI106701 (ACTG LC). Additional support was provided by UM1AI069465 (Johns Hopkins University Baltimore-India Clinical Trials Unit) to S. Kr. and A. G. Author S. K. was supported by the NIH T32 AI007291-27 and the JHU Clinician Scientist Award.
Data sharing statement. Data are available to interested researchers for approved concept sheets upon request to the Statistical and Data Analysis Center of the AIDS Clinical Trials Group (e-mail: sdac.data@sdac.harvard.edu) and the Statistical and Data Management Center Data Access Committee of the IMPAACT network (email address: sdac.data@fstrf.org) with the written agreement of both networks. Refer to https://actgnetwork.org/submit-a-proposal/for additional details.
References
- 1. World Health Organization . Global tuberculosis report 2022. Geneva, Switzerland: WHO, 2022. Available at: https://www.who.int/teams/global-tuberculosis-programme/tb-reports. Accessed 10 February 2023
- 2. Knight GM, McQuaid CF, Dodd PJ, Houben R. Global burden of latent multidrug-resistant tuberculosis: trends and estimates based on mathematical modelling. Lancet Infect Dis 2019; 19:903–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization . Latent tuberculosis infection: updated and consolidated guidelines for programmatic management. Geneva, Switzerland: WHO, 2018. Report No.: 9789241550239. Available at: https://apps.who.int/iris/handle/10665/260233. Accessed 3 October 2022. [PubMed]
- 4. Marks SM, Mase SR, Morris SB. Systematic review, meta-analysis, and cost-effectiveness of treatment of latent tuberculosis to reduce progression to multidrug-resistant tuberculosis. Clin Infect Dis 2017; 64:1670–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martinez L, Cords O, Horsburgh CR, Andrews JR, Pediatric TBCSC. The risk of tuberculosis in children after close exposure: a systematic review and individual-participant meta-analysis. Lancet 2020; 395:973–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morrison J, Pai M, Hopewell PC. Tuberculosis and latent tuberculosis infection in close contacts of people with pulmonary tuberculosis in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Infect Dis 2008; 8:359–68. [DOI] [PubMed] [Google Scholar]
- 7. Fox GJ, Barry SE, Britton WJ, Marks GB. Contact investigation for tuberculosis: a systematic review and meta-analysis. Eur Respir J 2013; 41:140–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wingfield T, Tovar MA, Huff D, et al. A randomized controlled study of socioeconomic support to enhance tuberculosis prevention and treatment, Peru. Bull World Health Organ 2017; 95:270–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rocha C, Montoya R, Zevallos K, et al. The Innovative Socio-economic Interventions Against Tuberculosis (ISIAT) project: an operational assessment. Int J Tuberc Lung Dis 2011; 15(Suppl 2):50–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sterling TR, Villarino ME, Borisov AS, et al. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med 2011; 365:2155–66. [DOI] [PubMed] [Google Scholar]
- 11. Gupta A, Swindells S, Kim S, et al. Feasibility of identifying household contacts of rifampin-and multidrug-resistant tuberculosis cases at high risk of progression to tuberculosis disease. Clin Infect Dis 2020; 70:425–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Qiagen . QuantiFERON-TB Gold Plus (QFT-Plus) [package insert], 2017.
- 13. Hanley JA, Negassa A, Edwardes MD, Forrester JE. Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol 2003; 157:364–75. [DOI] [PubMed] [Google Scholar]
- 14. Jonnalagadda S, LaCourse SM, Otieno P, et al. Incidence and correlates of tuberculosis IGRA conversion among HIV-infected postpartum women. Int J Tuberc Lung Dis 2015; 19:792–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aichelburg MC, Reiberger T, Breitenecker F, Mandorfer M, Makristathis A, Rieger A. Reversion and conversion of interferon-gamma release assay results in HIV-1-infected individuals. J Infect Dis 2014; 209:729–33. [DOI] [PubMed] [Google Scholar]
- 16. Shah NS, Yuen CM, Heo M, Tolman AW, Becerra MC. Yield of contact investigations in households of patients with drug-resistant tuberculosis: systematic review and meta-analysis. Clin Infect Dis 2014; 58:381–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Andrews JR, Hatherill M, Mahomed H, et al. The dynamics of QuantiFERON-TB Gold in-tube conversion and reversion in a cohort of South African adolescents. Am J Respir Crit Care Med 2015; 191:584–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pai M, Joshi R, Dogra S, et al. T-cell assay conversions and reversions among household contacts of tuberculosis patients in rural India. Int J Tuberc Lung Dis 2009; 13:84–92. [PMC free article] [PubMed] [Google Scholar]
- 19. Stein CM, Zalwango S, Malone LL, et al. Resistance and susceptibility to Mycobacterium tuberculosis infection and disease in tuberculosis households in Kampala, Uganda. Am J Epidemiol 2018; 187:1477–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fox GJ, Nhung NV, Sy DN, et al. Household-contact investigation for detection of tuberculosis in Vietnam. N Engl J Med 2018; 378:221–9. [DOI] [PubMed] [Google Scholar]
- 21. Dowdy DW, Behr MA. Are we underestimating the annual risk of infection with Mycobacterium tuberculosis in high-burden settings? Lancet Infect Dis 2022;22:e271–8. [DOI] [PubMed] [Google Scholar]
- 22. van Schalkwyk C, Variava E, Shapiro AE, et al. Incidence of TB and HIV in prospectively followed household contacts of TB index patients in South Africa. PLoS One 2014; 9:e95372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martinez L, le Roux DM, Barnett W, Stadler A, Nicol MP, Zar HJ. Tuberculin skin test conversion and primary progressive tuberculosis disease in the first 5 years of life: a birth cohort study from Cape Town, South Africa. Lancet Child Adolesc Health 2018; 2:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marais BJ, Amanullah F, Gupta A, et al. Tuberculosis in children, adolescents, and women. Lancet Respir Med 2020; 8:335–7. [DOI] [PubMed] [Google Scholar]
- 25. Vella V, Racalbuto V, Guerra R, et al. Household contact investigation of multidrug-resistant and extensively drug-resistant tuberculosis in a high HIV prevalence setting. Int J Tuberc Lung Dis 2011; 15:1170–5. [DOI] [PubMed] [Google Scholar]
- 26. Grandjean L, Crossa A, Gilman RH, et al. Tuberculosis in household contacts of multidrug-resistant tuberculosis patients. Int J Tuberc Lung Dis 2011; 15:1164–9. [DOI] [PubMed] [Google Scholar]
- 27. Becerra MC, Franke MF, Appleton SC, et al. Tuberculosis in children exposed at home to multidrug-resistant tuberculosis. Pediatr Infect Dis J 2013; 32:115–9. [DOI] [PubMed] [Google Scholar]
- 28. Becerra MC, Appleton SC, Franke MF, et al. Tuberculosis burden in households of patients with multidrug-resistant and extensively drug-resistant tuberculosis: a retrospective cohort study. Lancet 2011; 377:147–52. [DOI] [PubMed] [Google Scholar]
- 29. Leung EC, Leung CC, Kam KM, et al. Transmission of multidrug-resistant and extensively drug-resistant tuberculosis in a metropolitan city. Eur Respir J 2013; 41:901–8. [DOI] [PubMed] [Google Scholar]
- 30. Singla N, Singla R, Jain G, Habib L, Behera D. Tuberculosis among household contacts of multidrug-resistant tuberculosis patients in Delhi, India. Int J Tuberc Lung Dis 2011; 15:1326–30. [DOI] [PubMed] [Google Scholar]
- 31. Harries AD, Kumar AMV, Satyanarayana S, et al. The growing importance of tuberculosis preventive therapy and how research and innovation can enhance its implementation on the ground. Trop Med Infect Dis 2020; 5:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Suryavanshi N, Murrill M, Gupta A, et al. Willingness to take multidrug-resistant tuberculosis (MDR-TB) preventive therapy among adult and adolescent household contacts of MDR-TB index cases: an international multisite cross-sectional study. Clin Infect Dis 2020; 70:436–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Oh CE, Ortiz-Brizuela E, Bastos ML, Menzies D. Comparing the diagnostic performance of QuantiFERON-TB Gold Plus to other tests of latent tuberculosis infection: a systematic review and meta-analysis. Clin Infect Dis 2021; 73:e1116–e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.