Abstract
A fine balance between uptake, storage, and the use of high energy fuels, like lipids, is crucial in the homeostasis of different metabolic tissues. Nowhere is this balance more important and more precarious than in the heart. This highly energy-demanding muscle normally oxidizes almost all the available substrates to generate energy, with fatty acids being the preferred source under physiological conditions.
In patients with cardiomyopathies and heart failure, changes in the main energetic substrate are observed; these hearts often prefer to utilize glucose rather than oxidizing fatty acids. An imbalance between uptake and oxidation of fatty acid can result in cellular lipid accumulation and cytotoxicity. In this review, we will focus on the sources and uptake pathways used to direct fatty acids to cardiomyocytes. We will then discuss the intracellular machinery used to either store or oxidize these lipids and explain how disruptions in homeostasis can lead to mitochondrial dysfunction and heart failure. Moreover, we will also discuss the role of cholesterol accumulation in cardiomyocytes. Our discussion will attempt to weave in vitro experiments and in vivo data from mice and humans and use several human diseases to illustrate metabolism gone haywire as a cause of or accomplice to cardiac dysfunction.
Keywords: Lipids, Lipoprotein, Cholesterol, Heart failure
1. Introduction
Heart failure (HF) is a clinical syndrome characterized by structural, functional, and metabolic alterations of the heart that it is not able to pump enough blood to prevent lung congestion and to support oxygen and nutrient demand of peripheral tissues. In some situations, the heart increases ventricular wall thickness to compensate for the reduced contractility and this initially maintains heart ejection fraction (EF). With time, anatomical adaptations cannot compensate for the impaired function finally affecting EF. Several factors contribute to HF worsening; among these are myocardial infarction, arrhythmia, pneumonia, hypertension, and likely ventricular fibrosis.
Depending on the reduction or preservation of EF, HF patients are clustered in different classes from reduced (HFrEF with HF <40%), to mildly reduced (HFmrEF with HF 40–49%), and to preserved EF (HFpEF ≥50%).1 All three HF groups have comparable morbidity and mortality. HFpEF is often observed in patients with diabetes,2 in contrast to HFrEF, which often develops as a consequence of ischaemic heart disease, hypertension, or valvular disease. At the molecular level, impaired mitochondrial function was suggested to contribute to HFpEF development.3 Why should diabetic hearts have a mitochondrial defect? The heart is one of the most metabolic tissues in the body and mainly uses fatty acids to fuel oxidative phosphorylation and energy production.4 During HF, the heart has the capacity to switch its substrate preference and can utilize more glucose as a source of energy. Although fatty acid oxidation (FAO) remains the most important source of adenosine triphosphate (ATP) even in HF, a mismatch between lipid uptake and lipid utilization within cardiomyocytes may develop resulting in increased intracellular lipid accumulation, mainly of triglycerides (TGs), diacylglycerols (DAGs), and ceramides as well as cholesterol and its derivatives.5,6 Ceramides and DAGs are proposed as cardiotoxic mediators involved in insulin resistance and mitochondrial dysfunction, a process that could lead to cardiac fibrosis and cardiomyocyte death.7 Also, the accumulation of cholesterol and particularly free cholesterol within cellular membranes associates with cardiomyocyte cytotoxicity as it increases both cell and organelle rigidity, and also affects membrane permeability to antioxidant agents, proton flux, and mitochondrial dynamism.8 Cytoplasmic and membrane content of these lipids is a balance involving their sequestration within lipid droplets (LDs), a haven to allow non-toxic storage of hydrophobic lipids such as TGs and cholesteryl esters.
The aim of this review is to discuss how lipid metabolism within cardiomyocytes is tightly regulated to avoid lipotoxicity and mitochondrial dysfunction. Alterations in this balance compromise heart lipid homeostasis and likely explain the relationships of obesity and diabetes with human HF. Readers are referred to other reviews that have focused on the role of endothelial cells in cardiac metabolism.9,10
2. Cardiac metabolism
The human heart consumes kilograms of ATP per day to sustain basal metabolism and normal contraction that is essential to support systemic and pulmonary blood pressure. Oxidative metabolism in mitochondria accounts for 95%11 of the total energy produced in the heart, while 5% comes from anaerobic glycolysis. Changes in substrate availability and uptake, such as is the case in diabetes and other metabolic disorders, impact cardiac energy production and mitochondrial function, including calcium homeostasis and flux,12 ROS production,13 and the initiation of a pro-apoptotic cascade. Notably, increased glucose metabolism is sometimes followed by reduced FAO. This is the consequence of increased production of malonyl CoA that acts as an allosteric inhibitor of carnitine palmitoyl transferase in several cells14 including cardiomyocytes,15 thus limiting the transfer of fatty acids into mitochondria.
The heart is characterized by a high metabolic flexibility that allows it to alter its energetic substrates. In pathological conditions like HF, the heart lose its metabolic flexibility and ability to optimally produce energy.16–18 This metabolic deficiency, in turn, impacts cardiomyocyte biology and mitochondrial function. Indeed, mitochondrial dynamics change with cardiomyopathies and specifically the balance between fusion and fission is altered.19 Inhibition of inner mitochondrial membrane fusion20 promotes apoptosis and the development of HF, while increased mitochondrial fusion protects from the development of pressure-induced HF.21 Mitochondrial dynamics are associated not only with metabolic modelling of cardiac tissue but also with a major modification in oxidative pathways. Overexpression of inner membrane fusion protein can modulate ROS production reducing cardiac oxidative stress.22 Heart substrate utilization modulates cardiac lipid homeostasis, the regulation of fatty acid uptake, storage, and oxidation23 and as discussed below can affect heart function exclusive of changes in ATP production.
HFrEF is characterized by a marked increase in anaerobic glucose metabolism that is associated with an increased glycolytic flux with lactate and pyruvate accumulation.24 Proton accumulation in the cytosol is therefore associated with increased acidosis that contributes to worsening cardiac contraction via inhibiting contractile proteins and intracellular Ca2+ flux.24 This alteration in ionic homeostasis further aggravates the energy deficient status of HF and the reduced ATP production. A major metabolic derangement in HFpEF, which is mainly associated with obesity, is insulin resistance.25,26
Both HFrEF and HFpEF have a significant reduction in aerobic glycolysis. However, the lack of an appropriate animal model of HFpEF has hindered metabolic studies of this disease.24,25 In addition to FAO, anaerobic glycolysis contributes 5–30% of the energy generated by the healthy heart.27 As with changes in FAO, changes in glucose uptake and oxidation can occur in a failing heart.28 Indeed, studies have shown that increased glycolytic uptake observed in some models of HF leads to an increased pentose phosphate pathway flux, which is a modulator of both cardiac redox state and cell proliferation.29 This metabolic imbalance between glucose uptake and glucose oxidation in the failing heart is out of the scope of the review.
3. Lipid uptake in the heart
TGs, cholesterol, phospholipids, and a number of other lipids primarily circulate as components of lipoproteins. The basic lipoprotein structure with hydrophobic lipids such as TGs and cholesteryl esters in the core and amphipathic molecules such as phospholipids and proteins in the surface coat allows these particles to interact with cell surface receptors and to modulate enzymes involved in their metabolism. Chylomicrons, lipoproteins created from dietary lipids, mediate transport of fatty acids and fat-soluble vitamins from the gut. Very low-density lipoproteins (VLDL) and low-density lipoproteins (LDL) transfer lipids from the liver to peripheral tissues such as heart, skeletal muscle and adipose.
A second lipid delivery system operates to allow inter-organ movement of fatty acids and fat-soluble vitamins. Blood fatty acid levels are highly regulated by insulin, which inhibits the actions of intracellular lipases within adipocytes. During fasting and with defective insulin actions, as occurs with diabetes, adipocytes release non-esterified fatty acids (NEFAs) that associate with albumin. In a parallel process, retinoids (vitamin A) and tocopherols (vitamin E) associate with specific proteins that allow their movement from the liver to peripheral tissues.
Oxidation of fatty acids produces approximately 10-fold more ATP per molecule compared to glucose. Thus, not surprisingly high energy-requiring organs such as the heart and kidney primarily utilize circulating lipids for energy. The heart obtains fatty acids from three sources: NEFAs, chylomicrons, and VLDL (Figure 1). As early as the 1960s, studies of arterial venous differences across the human heart showed the importance of uptake of esterified fatty acids, i.e. lipoprotein-derived fatty acids,5 as a primary fuel for heart energy. The heart lipolyzes and acquires lipids from both chylomicrons and VLDL. A more recent and comprehensive analysis of metabolites in the heart of fasting humans undergoing cardiac catheterization confirmed the uptake and almost total utilization of fatty acids. In some HF patients, fatty acid uptake decreases while the use of ketones and lactate increases.30 Surprisingly, this study found little heart consumption of glucose in hearts from normal subject or patients with HF and did not identify greater glucose uptake with HF following 2-deoxy glucose administration.
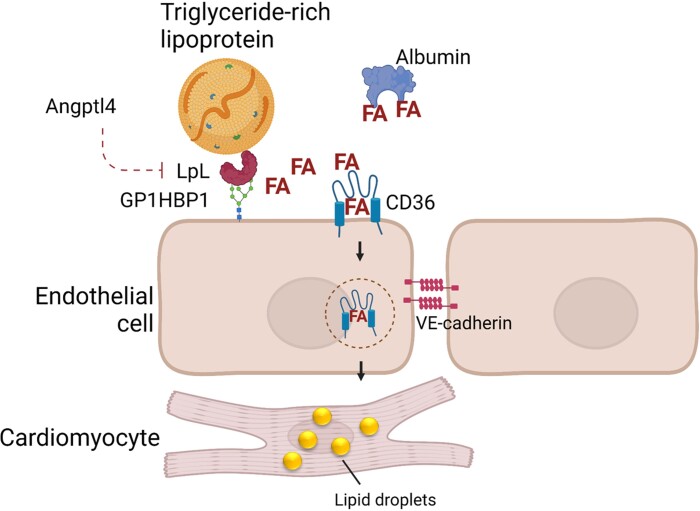
Figure 1.
Uptake of circulating lipids. The heart obtains fatty acids (FAs) from circulating triglyceride-rich lipoproteins (chylomicrons and VLDL) and albumin-bound non-esterified FAs. Lipoprotein lipase (LpL) catalyses hydrolysis of triglyceride-rich lipoproteins at the endothelial cell surface. FA uptake by the heart requires transport across the endothelial cell barrier, which is mediated by the scavenger receptor CD36. Genetic deletion of CD36 or LpL or overexpression of the LpL inhibitor angiopoietin-like protein 4 (Angptl4) leads to defective lipid uptake and heart dysfunction.
The heart is one of the most active sites of uptake of NEFAs. Non-specific transfer of NEFAs across cell membranes would lead to NEFA uptake into all tissues as a function of their size or blood supply. Rather the high rates of uptake into heart and brown adipose must reflect a receptor-mediated process (discussed in more detail in Abumrad et al.10). NEFA uptake in the heart requires several steps: uptake and movement across the endothelial barrier, transfer from endothelial to subendothelial cells, and lipid uptake into cardiomyocytes. In vivo, both processes are mediated by the fatty acid transporter CD36.31,32 Genetic deletion of CD36 reduces intramyocellular lipid accumulation in LDs33 and CD36 overexpression increases heart lipid uptake and oxidation.34 Although studies failed to show defective heart NEFA uptake by cardiomyocyte-specific CD36 knockout mice under acute experimental design,31 others have noted a reduction in FAO in ex vivo working heart preparations from cardiomyocyte-specific inducible CD36 knockout mice.35 Thus, it is likely that cardiomyocyte and endothelial cell CD36 mediate parallel or different stages of a single NEFA uptake pathway. CD36 is a peroxisomal proliferator-activated receptor (PPAR) downstream target; marked overexpression of either PPARα36 or PPARγ37 increased CD36 expression, which likely creates lipotoxicity due to more NEFA uptake. Indeed, knockout of CD36 protected PPARα transgenic mice from HF.38
In agreement with the studies of substrate extraction across the heart, loss of lipoprotein lipase (LpL) in the heart reduces heart uptake of both chylomicron and VLDL TGs.39 LpL is primarily synthesized by cardiomyocytes but functions on the luminal surface of capillary endothelial cells where it is anchored to glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1. As reviewed elsewhere,40,41 LpL activity is regulated by the size and apoprotein composition of TG-rich lipoproteins and its inhibitory proteins, angiopoietin-like proteins (Angptls) 3, 4, and 8. How LpL leaves the cardiomyocyte to begin its journey to the endothelial cells is unclear. One option is that LpL release from cardiomyocyte or matrix heparan sulphate proteoglycans requires the actions of a heparinase, which is regulated by hyperglycaemia and blood flow.42,43
Loss of LpL and reduced lipid uptake is, under non-stressed conditions, balanced by a greater uptake of glucose. When greater energy uptake is required during stress, LpL knockout hearts develop greater HF with transaortic coarctation44 and hypertension.45 Overexpression of the LpL inhibitor angiopoietin-like protein 4 (Angptl4) leads to a similar phenotype.46 Cardiomyocyte transgenic expression of GLUT1, which allows greater uptake of glucose, corrects many of the defects found in heart LpL knockout mice.47 These findings suggest that heart LpL knockout causes heart impairment due to defective substrate for ATP production. It should be noted that humans with LpL deficiency do not have heart dysfunction, perhaps because the energetic needs of a mouse heart, which beats ∼8–10 times more frequently than that of humans, are greater,48 thus unmasking a defect in substrate delivery.
An indication of the central role of lipid use for heart function is illustrated by obesity, a condition associated with greater FAO.49 With high fat diets, the heart rapidly becomes insulin-resistant,50 but knockout of insulin signalling leads to a relatively minor phenotype.51 Cardiac-specific GLUT1 knockout mice have a 50% reduction in glucose uptake and use. By increasing FAO, these mice do not have an increased propensity to HF.52 GLUT1 transgenic mice also have normal heart function on chow, although they develop HF when fed high fat diets.53 Thus, factors that augment both fatty acid and glucose metabolic pathways are not benign. This hypothesis that increasing glucose uptake into the heart in the setting of greater fatty acid uptake was proposed as a cause of diabetic cardiomyopathy and led to a correct prediction that unlike insulin sensitizers, sodium glucose co-transporter inhibitors would reduce HF in patients with diabetes.54
4. Cardiomyocyte lipid oxidation
PPARs are the central regulators of heart fatty acid metabolism. Of this family of transcription factors, PPARα is most highly expressed in cardiomyocytes and controls the expression of downstream genes associated with FAO and lipid uptake. Notably, PPARα knockout mice have normal heart function,55 likely reflecting the ability of the heart to adjust to different substrates. In contrast, transgenic expression of PPARα leads to HF associated with cardiac LD accumulation despite greater FAO.36 This implies that the transcription factor causes a mismatch between lipid uptake and oxidation. A similar mismatch occurs when aryl hydrocarbon nuclear translocator (HIF1β) is knocked out in the heart.56 PPARγ overexpression also leads to greater heart LDs and HF.56
The transcription activation of PPARs is mediated by different factors and among them Kruppel-like factor (KLF) 5, which binds to the PPARα promoter.57 Another member of this family of transcription factors, KLF4 regulates mitochondrial biogenesis,58 while KLF15 directly associates with PPARα.59 Genetic deletion of each of these KLFs leads to defective FAO and some degree of heart dysfunction.
Humans with severe defects in lipid oxidation display a clinical phenotype during childhood while less severe defects become clinically relevant during adulthood. Very long chain fatty acid dehydrogenase (VLCAD) deficiency impairs mitochondrial lipid oxidation, followed by altered muscle energy metabolism and eventually cardiomyopathy. VLCAD defects not associated with neonatal lethality as a consequence of severe hypoglycaemia often affect skeletal muscle more than cardiac muscle with marked increases in circulating creatine kinase at baseline and after exercise.60 As discussed below, this and other defects in mitochondrial lipid uptake should also lead to intracellular lipid accumulation and, as expected, this was reported in mouse models of long chain61 and very long chain acyl-CoA dehydrogenase deficiency.62
Although many studies show reduced FAO in the failing heart, recent data have reported that in HFpEF, heart FAO can increase while glucose use decreases63 allowing greater ROS generation.63–65
5. Cardiac lipid accumulation
All mammalian cells generate LDs under the appropriate conditions. Although circulating lipids are the main energy source for the heart, myocardial TG storage within LDs is vital for cardiac lipid homeostasis. LDs constitute an accessible TG reserve that allows the heart to meet varying energy demands and compensate for the fluctuating availability of circulating fatty acids.66 Hearts of wild-type mice on a chow diet develop LDs after an overnight fast,67 as fatty acids are redistributed from adipose tissue. Increased LDs have been reported in pathological samples from humans with aortic stenosis and metabolic syndrome.68 Also, a number of genetic modifications lead to pathological lipid accumulation; the most dramatic of which is the loss of the intracellular TG degrading enzyme adipose TG lipase (ATGL) that leads to marked heart lipid accumulation and early death.69 In humans, ATGL deficiency produces skeletal muscle and cardiac myopathy, i.e. neutral lipid storage disease. ATGL deficiency markedly down-regulates PPARα and reduces heart FAO; this defect in FAO can be corrected by pharmacologic activators.70 Thus, ATGL lipolysis liberates PPAR agonists, but fatty acids do not necessarily need to traffic through LDs prior to their oxidation.71 Similarly, cardiac-specific ATGL also decreased expression of PPARα downstream gene expression.72 Surprisingly, deletion of neither LpL nor CD36 to block lipid uptake reduced ATGL-mediated cardiac toxicity.73 In vitro, LD accumulation occurred in ATGL-deficient cardiomyocytes grown in lipid-free media due to induction of de novo lipogenesis.73 These data suggest that several metabolic pathways might contribute to intracellular LD formation.
LD accumulation in the heart depends on three factors: the synthesis of core lipids, i.e. TG synthesis, the synthesis of amphipathic surface molecules, and TG hydrolysis. Because the normal heart performs limited de novo synthesis of fatty acids from carbohydrates, most LD TGs are derived from lipoproteins and NEFAs. The final step in TG synthesis is catalysed by diacylglycerol acyl transferases (DGATs), which esterify the third fatty acid chain onto the DAG background. There are two forms of DGAT, and both enzymes contribute to LD formation; a knockout of both enzymes is required to block LD production in adipocytes.74 In the heart, loss of DGAT1 reduces LD production and increases heart levels of its substrate DAG.75 DGAT1 knockout mice were reported to develop cardiac dysfunction75; a finding that was not reproduced in another mouse model with partial or inducible DGAT1 deletion.76 DGAT1 deficiency also occurs in heart tissues from humans with advanced HF.77 DGAT1 overexpression increases heart TG levels and reduces heart DAGs but does not lead to cardiac dysfunction.78 Moreover, DGAT1 transgenic hearts have improved response to ischaemia/reperfusion,79 presumably because cardiomyocytes have easier access to stored TG. Transgenic DGAT1 expression, which reduces both DAG and ceramides, also improves cardiac lipotoxicity in several experimental conditions. In contrast, inhibition of DGAT2 using a pharmacological approach prevented high fat diet-induced heart lipid accumulation and was well tolerated.76 DAGs are therefore a principal TG metabolic intermediate that is crucially involved in the lipotoxicity of unused fatty acids that flux into the LDs.
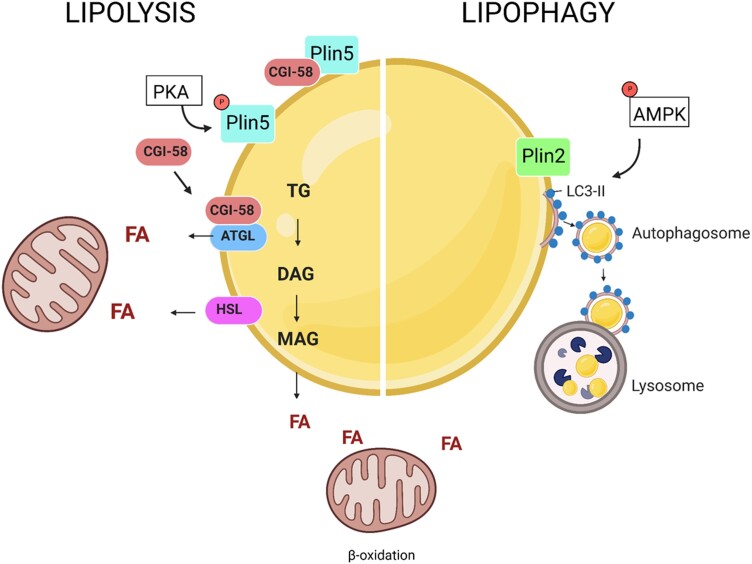
The surface of LDs contains phospholipids and proteins. Smaller droplets have a greater surface to core ratio. Using an unbiased screen to assess LD characteristics in a drosophila cell line, the Farese/Walther laboratory found that enzymes mediating phospholipid synthesis regulated the size of LDs.80 The major structural proteins of LDs are the members of the perilipin (Plin) family. Plins target to the LD surface and regulate TG storage and hydrolysis (Figure 2). Heart LD catabolism is largely under the regulation of Plin5, which is highly expressed in cardiac as well as other oxidative tissues. Plin5 interacts with central regulators of intracellular lipolysis, including hormone sensitive lipase (HSL), ATGL, and its co-activator CGI-58.81 Plin5 binding to CGI-58 inhibits lipolysis by sequestering CGI-58 and precluding ATGL activation. PKA-mediated phosphorylation of Plin5 at serine 155 triggers the release of CGI-58 and its subsequent binding to and activation of ATGL.82 Global knockout of Plin5 in mice results in heart LD depletion,83 allowing cardiac lipids to directly route to oxidation rather than storage. Notably, Plin5 deficiency exacerbated myocardial infarct size, heart dysfunction, and mitochondria damage following ischaemia/reperfusion injury, likely due to increased oxidative stress.84 Conversely, Plin5 overexpression in cardiomyocytes leads to severe TG accumulation akin to that found in ATGL-deficient mice. However, in contrast to ATGL-deficient mice, cardiac Plin5 overexpression does not negatively affect heart function or life span.85
Figure 2.
Cardiac lipid droplet hydrolysis. Cardiac lipid droplets provide a pool of fatty acids (FAs) to meet the varying energy requirements of the heart. Their hydrolysis is mediated by cytosolic triglyceride (TG) lipases—adipose TG lipase (ATGL) and hormone sensitive lipase (HSL) (left)—or acidic lysosomal lipases (lipophagy, right). Both processes are tightly regulated by members of the perilipin (Plin) family of lipid droplet proteins. Plin5 inhibits lipolysis by binding and sequestering ATGL co-activator CGI-58. Plin5 phosphorylation by PKA results in the release of CGI-58 and recruitment of ATGL, which catalyses the rate-limiting step of lipid droplet TG hydrolysis. Knockout of Plin5 results in lipid droplet depletion, whereas its overexpression induces severe lipid accumulation similar to that found in ATGL-deficient hearts. In addition, Plin2 has been reported to regulate cardiac lipophagy. Plin2 knockout results in heart lipid accumulation and reduced LC3-II, phosphorylated AMPK, and lipid droplet co-localization with lysosomes, all indicative of impaired lipophagy.
A recent study by Kolleritsch et al.86 aimed to understand the mechanisms protecting Plin5 transgenic mice from the development of heart dysfunction. Cardiomyocyte-specific overexpression of wild-type Plin5 or a Plin5-S155A mutant that lacks the PKA phosphorylation site led to cardiac steatosis with a marked increase in TG (22-fold), total cholesterol (6-fold), ceramides, and DAG, all of which can lead to cardiac stress and lipotoxicity.87 However, both Plin5 and Plin5-S155A transgenic mice exhibited normal heart function and ATP production. Plin5 overexpression is associated with increased mitochondrial size,85,88 and the authors observed the same in mitochondria from cardiomyocyte-specific Plin5-S155A transgenic mice. Cardiac overexpression of Plin5 markedly decreased the phosphorylation of mitochondrial fission factor, which is required for the recruitment of dynamin-related protein-1 (Drp1) to mitochondria and subsequent induction of mitochondrial fission. Mitochondria prepared from Plin5-S155A and Plin5 transgenic mice showed markedly reduced Drp1 protein levels compared to wild type, consistent with reduced mitochondrial fission. Elongated mitochondria are considered to be less vulnerable to oxidative stress and are metabolically more efficient.89 Therefore, reduced mitochondrial fission may protect from the development of lipotoxic heart dysfunction.86
Another member of the Plin family, Plin2, has also been reported to participate in the regulation of heart LD metabolism. In mouse hearts, Plin2 was up-regulated during fasting-induced lipid accumulation,90 and its cardiac overexpression induced severe LD accumulation, likely by preventing access of ATGL and HSL to the hydrophobic core. In this model, LD accumulation was resolved by overexpression of HSL.91,92 Unlike global Plin5 knockout mice, Plin2-deficient mice also exhibited increased heart LD accumulation. Absence of Plin2 reduced levels of LC3B-II, phosphorylated AMPK, and co-localization of LDs with lysosomes, all indicative of impaired autophagy-mediated LD hydrolysis (lipophagy).92 In contrast, Plin2 deficiency in the liver has been shown to reduce LD accumulation and stimulate lipophagy.93,94 These tissue-specific effects highlight the importance of further research to elucidate the nature and role of cardiac LDs in heart disease.
6. Mechanisms of fatty acid induced lipotoxicity
Lipid excess within the heart is detrimental for cardiomyocyte metabolism and function, and this is particularly relevant when some specific classes of lipids are accumulated.95–98 Oleic acid, for example, is not deleterious and can counteract palmitate-induced cellular dysfunction.99 On the contrary, palmitic acid is more cytotoxic99; perhaps due to a defect in its incorporation into TGs.100 A number of possible reasons have been proposed to explain palmitic acid toxicity: (i) altered membrane flexibility, (ii) increased ceramide production, (iii) greater ROS generation,101 and (iv) more palmitoylation of cellular proteins or nucleic acids.102 One mechanism downstream of palmitic acid accumulation is increased expression of Plin2 that promotes the phosphorylation of the stress-activated-protein-kinase/Jun-amino-terminal-kinase, which is involved in cardiomyocyte apoptosis.103
Sphingolipids, including ceramides, and DAGs also act as lipotoxic mediators and contribute to cardiac lipotoxicity via several mechanisms.103 Sphingolipids and ceramides modify cellular membrane organization and thus directly promote cardiomyocyte apoptosis—contributing to HFrEF—and also promote insulin resistance via inducing the dephosphorylation of Akt downstream of the insulin receptor.104 These lipids also cause PKC and MAPK activation and ER and mitochondrial stress, contributing to both HFpEF and HFrEF.105 A beneficial action of adiponectin, which is reduced in type 2 diabetes, is to induce ceramidase and alleviate ceramide-induced insulin resistance and cardiac toxicity.106
The activation of PKCα and the development of insulin resistance is also a common consequence of the accumulation of DAGs.107 Sterols, as well as DAGs and ceramides, have been proposed as crucial player in cardiac lipotoxicity (see below).
7. Cholesterol, oxysterols, and mitochondrial function in the context of heart failure
Cholesterol is a crucial component of cellular membranes but can also modulate physical properties and membrane receptor activity. Cellular cholesterol needs can be fulfilled in two different ways: (i) through de novo synthesis or (ii) via uptake from circulating lipoproteins. De novo cholesterol synthesis via the mevalonate pathway is an energy-consuming pathway, which takes place part in the cytoplasm and part in the endoplasmic reticulum and requires elevated oxygen concentration for sterol branching where squalene is converted to cholesterol. Cholesterol biosynthesis occurs mainly in the liver and steroid hormone-synthesizing organs, i.e. adrenals and gonads. Cardiomyocyte cholesterol requirement is mainly fulfilled via uptake from lipoproteins. Cardiomyocyte-anchored LpL increases LDL uptake and cholesterol accumulation in the heart and promotes HF.108 Whether HF is the direct consequence of increased fatty acid or LDL cholesterol accumulation is unclear. As the LDL receptor (LDLR) is expressed at very low levels in the heart, cholesterol uptake appears to be driven in an LDLR-independent manner. Notably early studies suggested that chylomicrons contribute to heart cholesterol delivery.109 Another option is that cholesterol enters cardiomyocytes via either CD36 or the VLDL receptor (VLDLR), which can internalize remnant lipoproteins.110 VLDLR was shown to be up-regulated under ischaemic conditions and to contribute to heart lipoprotein uptake and cardiac lipotoxicity.110 Similarly, increased cardiac expression of CD36, as a consequence of PCSK9 deficiency, was associated with increased heart cholesterol content.98
Whether the increased flux of cholesterol into cardiomyocytes directly translates into cellular cytotoxicity is unknown, but it is reasonable to speculate that it could worsen lipotoxicity.23 Once in the cell, cholesterol is distributed between cellular compartments through vesicular or non-vesicular transport.111 While vesicular transport involves the direct transfer of cholesterol between membranes of different cellular compartments, non-vesicular transport is mediated by the steroidogenic acute regulatory protein (StAR), the prototype for the StAR-related lipid transfer family of transport proteins, which include also the ceramide transfer protein.112 Within the cell, cholesterol can be found as free form, mainly localized in the membrane, or esterified within LDs. Cholesterol is highly toxic for cells, and excessive levels of free cholesterol can promote cell lipotoxicity.8,113 To limit this possibility, cholesterol excess could be esterified by the action of acyl-CoA cholesterol acyltransferase.114 Cardiomyocytes can esterify sterols within the cytoplasm to limit their cytotoxicity.114
Cellular cholesterol can also be oxidized. Oxysterols can be produced by both enzymatic processing and non-enzymatic oxidation via the addition of a second oxygen atom as a carbonyl, a hydroxyl, or an epoxide group.115 Oxysterols generated by enzymatic reaction are 24α-hydroxycholesterol (24α-OH), 25-hydroxycholesterol (25-OH), and 27-hydroxycholesterol (27-OH). Oxysterols generated by non-enzymatic oxidation includes 7β-hydroxycholesterol (7β-OH) and 7-ketocholesterol (7-KC). Oxysterols are 1000-fold less abundant compared to cholesterol; nonetheless, they play an important role in cellular metabolism.116 Cholesterol supplementation is associated with a worsening of cardiac functionality associated with an increased amount of enzymatic oxysterols like 25-OH and 27-OH as well as 7β-OH.117 Oxysterols can be found as a main component of oxidized circulating LDL (Ox-LDL) as well as within the cells. Cellular oxysterol levels increase following cholesterol accumulation and are extremely cytotoxic. To prevent this, increased cellular oxysterol concentrations activate feedback mechanisms to dampen intracellular cholesterol biosynthesis via the inhibition of sterol-regulatory element binding proteins and also to promote cellular cholesterol efflux via the activation of liver X receptor-dependent pathways.
Oxysterols and in particular 7-KC have a direct effect on mitochondria promoting morphological changes leading to mitochondrial membrane rupture, increased cytochrome c release, altered membrane phospholipid content, and activation of autophagic processes.118,119 Notably, 7KC seems to be able to promote cardiomyocyte ROS production through an ATF4-dependent pathway.120 Further analyses show that oxysterols can rewire cardiomyocyte lipid metabolism.114 In vitro, Ox-LDL increases the expression of brain natriuretic peptide in cardiomyocytes121 and induces apoptosis via the modulation of calcium-sensing receptors.122 Moreover, Ox-LDL stimulates PCSK9 in cardiomyocytes, reduces contractile capacity of cardiomyocytes, and increases cell death.123
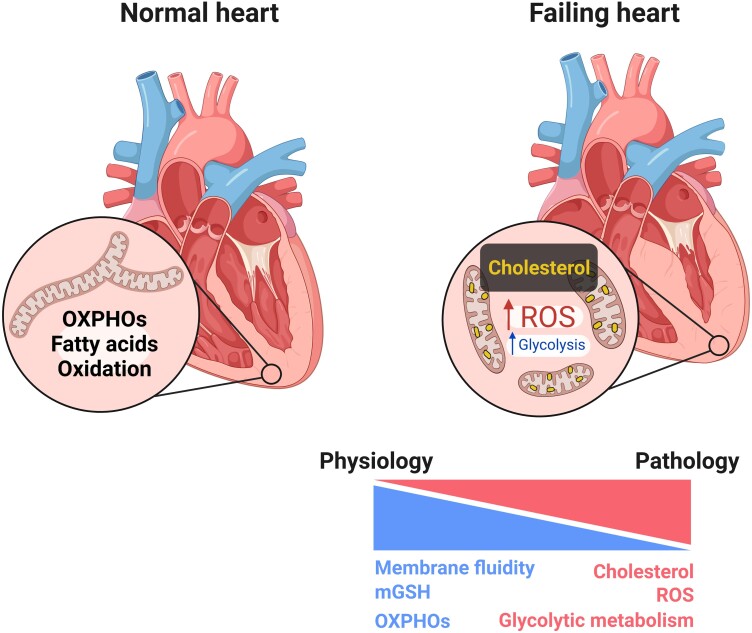
Within the cell, mitochondria, although known to be cholesterol poor organelles (around 3–5% of the total cellular pool), are crucially involved in steroidogenesis and bile acids synthesis. In cardiomyocytes, mitochondrial cholesterol enrichment alters membrane organization, which negatively impacts the fluidity by altering the balance between lipid-ordered and disordered membrane phases.124,125 Moreover, cholesterol impacts the spatial organization of membrane carriers, including that of the GSH transport system. Increased mitochondrial cholesterol levels negatively affect the mitochondrial pool of GSH (mGSH) and result in increased mitochondrial ROS production (Figure 3), which in turns could favour ceramide generation, cardiolipin peroxidation, and mitochondrial pore opening. These factors, together, contribute to the development of the fibrotic phenotype that is characteristic of hypertrophic hearts and promote cardiomyocyte metabolic reprogramming,126,127 which can further promote mitochondrial ROS generation and oxidative stress.128 Factors that increase the expression of lipid and lipoprotein receptors on cardiac tissue can promote cholesterol accumulation and LD generation. This in turn affects mitochondrial respiration leading to the development of the metabolic changes typical of HFpEF.98 Indeed, mitochondrial function is altered during HF; this includes mitochondrial biogenesis, which is reduced as a consequence of decreased PGC1a expression,129,130 and mitochondrial dynamism, which was also shown to be altered during HF with increased mitochondrial fission and reduced fusion.20
Figure 3.
Metabolic impact of cardiac sterols. A normal/healthy heart mainly relies on fatty acid oxidation and mitochondrial oxidative phosphorylation (OXPHOs) for energy production. Failing heart has reduced OXPHOs and mitochondrial antioxidant pathways (mGSH) and greater reliance on glycolysis for ATP production. If lipid uptake is not reduced, sterols and other potentially toxic lipids can accumulate and trigger greater reactive oxygen species (ROS) production.
An open question is whether these intracellular mechanisms are affected by changes in systemic cholesterol levels. Notably, low circulating cholesterol levels are neutral or even deleterious in patients with HF leading to a worst outcome. Whether this is the consequence of the reduced availability of cholesterol-rich substrates to the heart is debated.131 Alternatively, low cholesterol levels might reflect an underlying cachexia in very sick HF patients.
8. Cardiac lipoprotein biosynthesis to overcome lipotoxicity
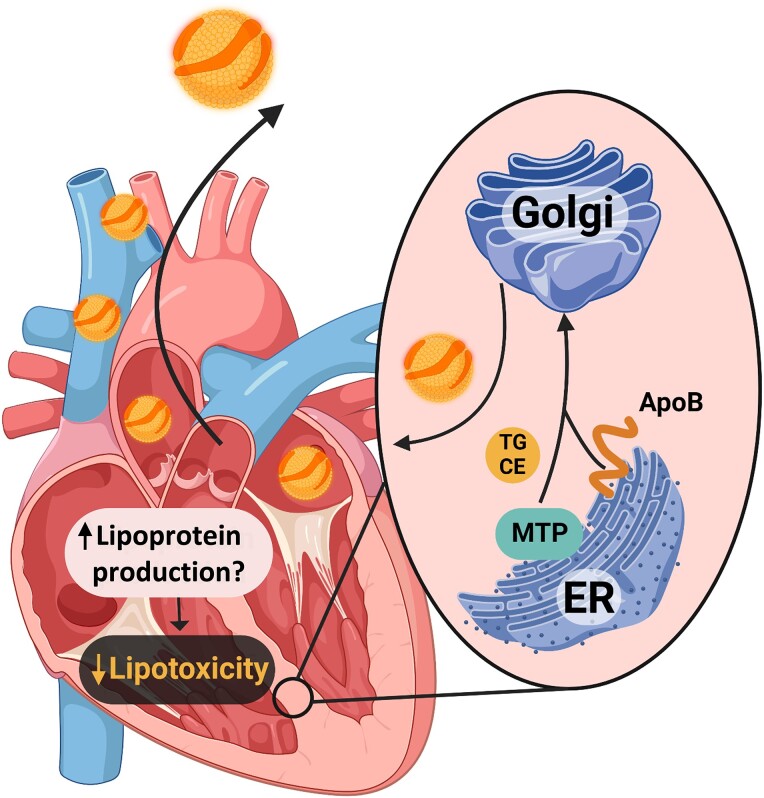
A poorly investigated aspect of physiology is the fact that cardiomyocytes, in addition to hepatocytes and enterocytes, have the possibility to synthesize lipoproteins. The importance of this pathway in normal cardiac physiology and in response to heart lipid overload is unclear. Cardiomyocytes synthesize apolipoprotein B (ApoB), the major structural protein of VLDL and LDL, and mitochondrial TG transfer protein (MTTP) is required for lipidation of the ApoB. While the intestine synthesizes lipoproteins (chylomicrons) to route exogenous lipids to the blood stream and the liver produces VLDL to distribute lipids to different tissues, the biological reason for the heart to produce lipoproteins remains a mystery. The finding that the ApoB and MTP genes are expressed in both human and mouse hearts,132 two species separated by more than 80 million years of mammalian evolution, suggests that the expression of these genes is important and might be an evolutionarily conserved mechanism involved in heart lipid metabolism. Notably, analysis of lipoprotein fractions has shown that the lipoproteins produced by the heart are LDL-like particles.133 However, the relative contribution of this pathway in unloading lipids from the heart and therefore limiting lipotoxicity as compared to the mechanisms described above is still a matter of investigation (Figure 4).
Figure 4.
Cardiac lipoprotein production. The heart has the capacity to alleviate its burden of excess toxic lipids by oxidation, storage in lipid droplets, or secretion in lipoproteins. Akin to the liver and the intestine, the heart expresses both apolipoprotein B (ApoB) and microsomal triglyceride (TG) transfer protein (MTTP). This allows the packaging of intracellular cholesterol ester and TG in the endoplasmic reticulum into nascent lipoproteins. The nascent lipoprotein is then released into the bloodstream; cardiac lipotoxicity is reduced by this ‘reverse lipid transport’ process.
Streptozotocin-treated diabetic mice have a dramatic increase in heart TG content, which is prevented by overexpression of apoB100.134 This finding confirms that TG accumulation in the heart is important for the development of diabetic cardiomyopathy, and that lipoprotein generation by cardiomyocytes contributes to cardiac lipid metabolism. This is further supported by the observation that increased expression of MTTP and ApoB is observed under different disease conditions known to be related to cardiac lipotoxicity, including hypoxia135 and obesity.136 In humans, homozygosity for null mutations in the ApoB gene (homozygous hypobetalipoproteinemia) or in the MTTP gene (as in abetalipoproteinemia) dampens the secretion of ApoB-containing lipoproteins by the liver and intestine and results in the accumulation of cytosolic LDs in hepatocytes and enterocytes. Whether cardiac lipoprotein production is also affected in these patients is unknown; although there are no reports of heart LD accumulation in abetalipoproteinemia, there have been reports of cardiomyopathy and arrhythmias in these patients.132 This finding, however, might also relate to the defects in delivery of fat-soluble vitamins, esp. retinoids. On the same line, the promotion of lipoprotein production (achieved in MTTP overexpressing heart) protects against lipotoxic cardiomyopathy.137 Changes in MTTP activity (observed in carriers of different polymorphisms on the MTTP gene) affect cardiac remodelling and the risk of coronary heart disease independently of plasma lipoprotein levels.138 These findings suggest a need for a deeper characterization of human carriers of MTTP loss of function mutations.
9. Conclusion
Although not usually viewed as an important organ in lipid metabolism, evidence has accumulated that defective lipolysis in the heart modifies circulating TG levels.139,140 The heart is a major site of NEFA uptake, as lipids are the primary substrate for cardiac energy production. Metabolic alteration and imbalances in lipid oxidation can cause cardiac energy depletion and morphological alteration finally promoting the development of HF. Lipid uptake, storage, and metabolic regulation play also a central role during HF development. While many processes involved in lipid metabolism are altered prior to and during the development of HF, it is likely that abnormal accumulation of toxic lipids alters mitochondria function and creates a feed-forward process leading to reduced lipid oxidation not compensated by reduced lipid uptake. It has been suggested that during HF, the sympathetic system could drive a sustained adipose lipolysis with increased circulating NEFA levels that could exacerbate HF.141 In support of this hypothesis, the inhibition of adipose NEFA release was shown to reduce catecholamine-induced heart failure.142,143 While elevated plasma lipid levels are seen primarily as the key driver of atherosclerosis, these molecules do much more and serve as both the fuel and poison for the heart. Furthermore, from the evolutional point of view, the fact that the heart is one of the three organs in the body able to produce lipoproteins suggests that this mechanism is in place to limit lipid and cholesterol accumulation and to unload toxic lipids from cardiomyocytes.
Author’s contributions
All authors have made equal intellectual contributions to the writing of this manuscript. All authors read and approved the final manuscript.
This manuscript was handled by Reviews Deputy Editor Ali J. Marian.
Contributor Information
Lorenzo Da Dalt, Department of Pharmacological and Biomolecular Sciences, University of Milan, Via Balzaretti 9, Milan, Italy.
Ainara G Cabodevilla, Division of Endocrinology, Diabetes and Metabolism, Department of Medicine, New York University Grossman School of Medicine, 550 1st Ave., New York, NY, USA.
Ira J Goldberg, Division of Endocrinology, Diabetes and Metabolism, Department of Medicine, New York University Grossman School of Medicine, 550 1st Ave., New York, NY, USA.
Giuseppe Danilo Norata, Department of Pharmacological and Biomolecular Sciences, University of Milan, Via Balzaretti 9, Milan, Italy; Center for the Study of Atherosclerosis, E. Bassini Hospital, Via Massimo Gorki 50, Cinisello Balsamo, Italy.
Funding
The authors of this work are supported by: Telethon Foundation (GGP19146 to G.D.N.); Progetti di Rilevante Interesse Nazionale (PRIN 2017 K55HLC to G.D.N.); Ricerca Finalizzata, Ministry of Health (RF-2019-12370896 to G.D.N.); PNRR, Centro Nazionale Di Ricerca 3 (Sviluppo Di Terapia Genica E Farmaci Con Tecnologia a RNA to G.D.N.) and PNRR Ecosistemi per L’Innovazione, (Multilayered Urban Sustainability Action to G.D.N.), and HL45095, 160891, and 151326 from the National Heart Blood and Lung Institute (I.J.G.). A.G.C. is supported by the American Heart Association Postdoctoral Fellowship.
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Sisakian HS, Isayev E, Kurlianskaya A, Mullens W, Tokmakova M, Agathangelou P, Melenovsky V, Wiggers H, Hassanein M, Uuetoa T, Lommi J, Kostovska ES, Juilliere Y, Aladashvili A, Luchner A, Chrysohoou C, Nyolczas N, Thorgeirsson G, Weinstein JM, di Lenarda A, Aidargaliyeva N, Bajraktari G, Beishenkulov M, Kamzola G, Abdel-Massih T, Celutkiene J, Noppe S, Cassar A, Vataman E, AbirKhalil S, van Pol P, Mo R, Straburzynska-Migaj E, Fonseca C, Chioncel O, Shlyakhto E, Zavatta M, Otasevic P, Goncalvesova E, Lainscak M, Molina BD, Schaufelberger M, Suter T, Yılmaz MB, Voronkov L, Davies C. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2016;37:2129–2200.27206819 [Google Scholar]
- 2. Kong MG, Jang SY, Jang J, Cho H-J, Lee S, Lee SE, Kim KH, Yoo B-S, Kang S-M, Baek SH, Choi D-J, Jeon E-S, Kim J-J, Cho M-C, Chae SC, Oh B-H, Lim S, Park SK, Lee H-Y. Impact of diabetes mellitus on mortality in patients with acute heart failure: a prospective cohort study. Cardiovasc Diabetol 2020;19:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kumar AA, Kelly DP, Chirinos JA. Mitochondrial dysfunction in heart failure with preserved ejection fraction. Circulation 2019;139:1435–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kadkhodayan A, Coggan AR, Peterson LR. A “PET” area of interest: myocardial metabolism in human systolic heart failure. Heart Fail Rev 2013;18:567–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ballard FB, Danforth WH, Naegle S, Bing RJ. Myocardial metabolism of fatty acids. J Clin Invest 1960;39:717–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Danforth WH, Ballard FB, Kako K, Choudhury BR. Metabolism of the heart in failure. Circulation 1960;21:112–123. [DOI] [PubMed] [Google Scholar]
- 7. Fucho R, Casals Ń, Serra D, Herrero L. Ceramides and mitochondrial fatty acid oxidation in obesity. FASEB J 2017;31:1263–1272. [DOI] [PubMed] [Google Scholar]
- 8. Abourjaili G, Shtaynberg N, Wetz R, Costantino T, Abela GS. Current concepts in triglyceride metabolism, pathophysiology, and treatment. Metabolism 2010;59:1210–1220. [DOI] [PubMed] [Google Scholar]
- 9. Boutagy NE, Singh AK, Sessa WC. Targeting the vasculature in cardiometabolic disease. J Clin Invest 2022;132:e148556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abumrad NA, Cabodevilla AG, Samovski D, Pietka T, Basu D, Goldberg IJ. Endothelial cell receptors in tissue lipid uptake and metabolism. Circ Res 2021;128:433–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Allard MF, Schonekess BO, Henning SL, English DR, Lopaschuk GD. Contribution of oxidative metabolism and glycolysis to ATP production in hypertrophied hearts. Am J Physiol Heart Circ Physiol 1994;267:H742–H750. [DOI] [PubMed] [Google Scholar]
- 12. Rizzuto R, de Stefani D, Raffaello A, Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol 2012;13:566–578. [DOI] [PubMed] [Google Scholar]
- 13. Wong HS, Dighe PA, Mezera V, Monternier PA, Brand MD. Production of superoxide and hydrogen peroxide from specific mitochondrial sites under different bioenergetic conditions. J Biol Chem 2017;292:16804–16809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mills SE, Foster DW, McGarry JD. Interaction of malonyl-CoA and related compounds with mitochondria from different rat tissues. Relationship between ligand binding and inhibition of carnitine palmitoyltransferase I. Biochemical J 1983;214:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang W, Zhang L, Battiprolu PK, Fukushima A, Nguyen K, Milner K, Gupta A, Altamimi T, Byrne N, Mori J, Alrob OA, Wagg C, Fillmore N, Wang S, Liu DM, Fu A, Lu JY, Chaves M, Motani A, Ussher JR, Reagan JD, Dyck JRB, Lopaschuk GD. Malonyl CoA decarboxylase inhibition improves cardiac function post-myocardial infarction. JACC Basic Transl Sci 2019;4:385–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dávila-Román VG, Vedala G, Herrero P, de las Fuentes L, Rogers JG, Kelly DP, Gropler RJ. Altered myocardial fatty acid and glucose metabolism in idiopathic dilated cardiomyopathy. J Am Coll Cardiol 2002;40:271–277. [DOI] [PubMed] [Google Scholar]
- 17. Weiss RG, Gerstenblith G, Bottomley PA. ATP Flux through creatine kinase in the normal, stressed, and failing human heart. Proc Natl Acad Sci 2005;102:808–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Neglia D, de Caterina A, Marraccini P, Natali A, Ciardetti M, Vecoli C, Gastaldelli A, Ciociaro D, Pellegrini P, Testa R, Menichetti L, L’Abbate A, Stanley WC, Recchia FA. Impaired myocardial metabolic reserve and substrate selection flexibility during stress in patients with idiopathic dilated cardiomyopathy. Am J Physiol Heart Circ Physiol 2007;293:H3270–H3278. [DOI] [PubMed] [Google Scholar]
- 19. Rosca MG, Tandler B, Hoppel CL. Mitochondria in cardiac hypertrophy and heart failure. J Mol Cell Cardiol 2013;55:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen L, Gong Q, Stice JP, Knowlton AA. Mitochondrial OPA1, apoptosis, and heart failure. Cardiovasc Res 2009;84:91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yu H, Guo Y, Mi L, Wang X, Li L, Gao W. Mitofusin 2 inhibits angiotensin II-induced myocardial hypertrophy. J Cardiovasc Pharmacol Ther 2011;16:205–211. [DOI] [PubMed] [Google Scholar]
- 22. Xin T, Lv W, Liu D, Jing Y, Hu F. Opa1 reduces hypoxia-induced cardiomyocyte death by improving mitochondrial quality control. Front Cell Dev Biol 2020;8:853. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23. Schulze PC, Drosatos K, Goldberg IJ. Lipid use and misuse by the heart. Circ Res 2016;118:1736–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Diakos NA, Navankasattusas S, Abel ED, Rutter J, McCreath L, Ferrin P, McKellar SH, Miller DV, Park SY, Richardson RS, Deberardinis R, Cox JE, Kfoury AG, Selzman CH, Stehlik J, Fang JC, Li DY, Drakos SG. Evidence of glycolysis up-regulation and pyruvate mitochondrial oxidation mismatch during mechanical unloading of the failing human heart. JACC Basic Transl Sci 2016;1:432–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Abel ED, Kaulbach HC, Tian R, Hopkins JCA, Duffy J, Doetschman T, Minnemann T, Boers M-E, Hadro E, Oberste-Berghaus C, Quist W, Lowell BB, Ingwall JS, Kahn BB. Cardiac hypertrophy with preserved contractile function after selective deletion of GLUT4 from the heart. J Clin Invest 1999;104:1703–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rutter MK, Parise H, Benjamin EJ, Levy D, Larson MG, Meigs JB, Nesto RW, Wilson PWF, Vasan RS. Impact of glucose intolerance and insulin resistance on cardiac structure and function. Circulation 2003;107:448–454. [DOI] [PubMed] [Google Scholar]
- 27. Wisneski JA, Gertz EW, Neese RA, Gruenke LD, Cymerman Craig J. Dual carbon-labeled isotope experiments using D-[6-14C] glucose and L-[1,2,3-13C3] lactate: a new approach for investigating human myocardial metabolism during ischemia. J Am Coll Cardiol 1985;5:1138–1146. [DOI] [PubMed] [Google Scholar]
- 28. Lopaschuk GD, Wambolt RB, Barr RL. An imbalance between glycolysis and glucose oxidation is a possible explanation for the detrimental effects of high levels of fatty acids during aerobic reperfusion of ischemic hearts. J Pharmacol Exp Ther 1993;264:135–144. [PubMed] [Google Scholar]
- 29. Kato T, Niizuma S, Inuzuka Y, Kawashima T, Okuda J, Tamaki Y, Iwanaga Y, Narazaki M, Matsuda T, Soga T, Kita T, Kimura T, Shioi T. Analysis of metabolic remodeling in compensated left ventricular hypertrophy and heart failure. Circ Heart Fail 2010;3:420–430. [DOI] [PubMed] [Google Scholar]
- 30. Murashige D, Jang C, Neinast M, Edwards JJ, Cowan A, Hyman MC, Rabinowitz JD, Frankel DS, Arany Z. Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science 2020;370:364–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Son NH, Basu D, Samovski D, Pietka TA, Peche VS, Willecke F, Fang X, Yu SQ, Scerbo D, Chang HR, Sun F, Bagdasarov S, Drosatos K, Yeh ST, Mullick AE, Shoghi KI, Gumaste N, Kim KJ, Huggins LA, Lhakhang T, Abumrad NA, Goldberg IJ. Endothelial cell CD36 optimizes tissue fatty acid uptake. J Clin Invest 2018;128:4329–4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Glatz JFC, Luiken JJFP. Dynamic role of the transmembrane glycoprotein CD36 (SR-B2) in cellular fatty acid uptake and utilization. J Lipid Res 2018;59:1084–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Trent CM, Yu S, Hu Y, Skoller N, Huggins LA, Homma S, Goldberg IJ. Lipoprotein lipase activity is required for cardiac lipid droplet production. J Lipid Res 2014;55:645–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Carley AN, Bi J, Wang X, Banke NH, Dyck JRB, O’Donnell JM, Lewandowski ED. Multiphasic triacylglycerol dynamics in the intact heart during acute in vivo overexpression of CD36. J Lipid Res 2013;54:97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nagendran J, Pulinilkunnil T, Kienesberger PC, Sung MM, Fung D, Febbraio M, Dyck JRB. Cardiomyocyte-specific ablation of CD36 improves post-ischemic functional recovery. J Mol Cell Cardiol 2013;63:180–188. [DOI] [PubMed] [Google Scholar]
- 36. Finck BN, Kelly DP. Peroxisome proliferator-activated receptor α (PPARα) signaling in the gene regulatory control of energy metabolism in the normal and diseased heart. J Mol Cell Cardiol 2002;34:1249–1257. [DOI] [PubMed] [Google Scholar]
- 37. Son NH, Park TS, Yamashita H, Yokoyama M, Huggins LA, Okajima K, Homma S, Szabolcs MJ, Huang LS, Goldberg IJ. Cardiomyocyte expression of PPARγ leads to cardiac dysfunction in mice. J Clin Invest 2007;117:2791–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Umbarawan Y, Syamsunarno MRAA, Koitabashi N, Obinata H, Yamaguchi A, Hanaoka H, Hishiki T, Hayakawa N, Sano M, Sunaga H, Matsui H, Tsushima Y, Suematsu M, Kurabayashi M, Iso T. Myocardial fatty acid uptake through CD36 is indispensable for sufficient bioenergetic metabolism to prevent progression of pressure overload-induced heart failure. Sci Rep 2018;8:12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bharadwaj KG, Hiyama Y, Hu Y, Huggins LA, Ramakrishnan R, Abumrad NA, Shulman GI, Blaner WS, Goldberg IJ. Chylomicron- and VLDL-derived lipids enter the heart through different pathways: in vivo evidence for receptor- and non-receptor-mediated fatty acid uptake. J Biol Chem 2010;285:37976–37986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yan X, Charette G, Delvin EE. Submitochondrial localization of kidney 25-hydroxycholecalciferol 1α-hydroxylase in vitamin D repleted weanling Guinea pigs. Biochem Cell Biol 1987;65:673–676. [DOI] [PubMed] [Google Scholar]
- 41. Basu D, Goldberg IJ. Regulation of lipoprotein lipase-mediated lipolysis of triglycerides. Curr Opin Lipidol 2020;31:154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang D, Wan A, Chiu AP-L, Wang Y, Wang F, Neumaier K, Lal N, Bround MJ, Johnson JD, Vlodavsky I, Rodrigues B. Hyperglycemia-induced secretion of endothelial heparanase stimulates a vascular endothelial growth factor autocrine network in cardiomyocytes that promotes recruitment of lipoprotein lipase. Arterioscler Thromb Vasc Biol 2013;33:2830–2838. [DOI] [PubMed] [Google Scholar]
- 43. Lee CS, Zhai Y, Shang R, Wong T, Mattison AJ, Cen HH, Johnson JD, Vlodavsky I, Hussein B, Rodrigues B. Flow-induced secretion of endothelial heparanase regulates cardiac lipoprotein lipase and changes following diabetes. J Am Heart Assoc 2022;11:e027958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Augustus AS, Buchanan J, Park TS, Hirata K, Noh HL, Sun J, Homma S, D’Armiento J, Abel ED, Goldberg IJ. Loss of lipoprotein lipase-derived fatty acids leads to increased cardiac glucose metabolism and heart dysfunction. J Biol Chem 2006;281:8716–8723. [DOI] [PubMed] [Google Scholar]
- 45. Yamashita H, Bharadwaj KG, Ikeda S, Park TS, Goldberg IJ. Cardiac metabolic compensation to hypertension requires lipoprotein lipase. Am J Physiol Endocrinol Metab 2008;295:E705–E713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yu X, Burgess SC, Ge H, Wong KK, Nassem RH, Garry DJ, Sherry AD, Malloy CR, Berger JP, Li C. Inhibition of cardiac lipoprotein utilization by transgenic overexpression of angptl4 in the heart. Proc Natl Acad Sci U S A 2005;102:1767–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Khan RS, Lin Y, Hu Y, Son NH, Bharadwaj KG, Palacios C, Chokshi A, Ji R, Yu S, Homma S, Christian Schulze P, Tian R, Goldberg IJ. Rescue of heart lipoprotein lipase-knockout mice confirms a role for triglyceride in optimal heart metabolism and function. Am J Physiol Endocrinol Metab 2013;305:E1339–E1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shang R, Rodrigues B. Lipoprotein lipase and its delivery of fatty acids to the heart. Biomolecules 2021;11:1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wilson CR, Tran MK, Salazar KL, Young ME, Taegtmeyer H. Western diet, but not high fat diet, causes derangements of fatty acid metabolism and contractile dysfunction in the heart of Wistar rats. Biochem J 2007;406:457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Park S-Y, Cho Y-R, Kim H-J, Higashimori T, Danton C, Lee M-K, Dey A, Rothermel B, Kim Y-B, Kalinowski A, Russell KS, Kim JK. Unraveling the temporal pattern of diet-induced insulin resistance in individual organs and cardiac dysfunction in C57bl/6 mice. Diabetes 2005;54:3530–3540. [DOI] [PubMed] [Google Scholar]
- 51. Belke DD, Betuing S, Tuttle MJ, Graveleau C, Young ME, Pham M, Zhang D, Cooksey RC, McClain DA, Litwin SE, Taegtmeyer H, Severson D, Kahn CR, Abel ED. Insulin signaling coordinately regulates cardiac size, metabolism, and contractile protein isoform expression. J Clin Invest 2002;109:629–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pereira RO, Wende AR, Olsen C, Soto J, Rawlings T, Zhu Y, Riehle C, Abel ED. GLUT1 Deficiency in cardiomyocytes does not accelerate the transition from compensated hypertrophy to heart failure. J Mol Cell Cardiol 2014;72:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yan J, Young ME, Cui L, Lopaschuk GD, Liao R, Tian R. Increased glucose uptake and oxidation in mouse hearts prevent high fatty acid oxidation but cause cardiac dysfunction in diet-induced obesity. Circulation 2009;119:2818–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nolan CJ, Ruderman NB, Kahn SE, Pedersen O, Prentki M. Insulin resistance as a physiological defense against metabolic stress: implications for the management of subsets of type 2 diabetes. Diabetes 2015;64:673–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fillmore N, Hou V, Sun J, Springer D, Murphy E. Cardiac specific knock-down of peroxisome proliferator activated receptor α prevents fasting-induced cardiac lipid accumulation and reduces perilipin 2. PLoS One 2022;17:e0265007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wu R, Chang HC, Khechaduri A, Chawla K, Tran M, Chai X, Wagg C, Ghanefar M, Jiang X, Bayeva M, Gonzalez F, Lopaschuk G, Ardehali H. Cardiac-specific ablation of ARNT leads to lipotoxicity and cardiomyopathy. J Clin Invest 2014;124:4795–4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Drosatos K, Pollak NM, Pol CJ, Ntziachristos P, Willecke F, Valenti MC, Trent CM, Hu Y, Guo S, Aifantis I, Goldberg IJ. Cardiac myocyte KLF5 regulates Ppara expression and cardiac function. Circ Res 2016;118:241–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tien YT, Chang MH, Chu PY, Lin CS, Liu CH, Liao AT. Downregulation of the KLF4 transcription factor inhibits the proliferation and migration of canine mammary tumor cells. Vet J 2015;205:244–253. [DOI] [PubMed] [Google Scholar]
- 59. Prosdocimo DA, John JE, Zhang L, Efraim ES, Zhang R, Liao X, Jain MK. KLF15 and PPARα cooperate to regulate cardiomyocyte lipid gene expression and oxidation. PPAR Res 2015;2015:201625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Leslie ND, Saenz-Ayala S. Very long-chain acyl-coenzyme a dehydrogenase deficiency. In Adam MP, Everman DB, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Amemiya A (eds.), GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2023. https://www.ncbi.nlm.nih.gov/books/NBK6816/ [Google Scholar]
- 61. Kurtz DM, Rinaldo P, Rhead WJ, Tian L, Millington DS, Vockley J, Hamm DA, Brix AE, Lindsey JR, Pinkert CA, O’Brien WE, Wood PA. Targeted disruption of mouse long-chain acyl-CoA dehydrogenase gene reveals crucial roles for fatty acid oxidation. Proc Natl Acad Sci U S A 1998;95:15592–15597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Exil VJ, Roberts RL, Sims H, McLaughlin JE, Malkin RA, Gardner CD, Ni G, Rottman JN, Strauss AW. Very-long-chain acyl-coenzyme a dehydrogenase deficiency in mice. Circ Res 2003;93:448–455. [DOI] [PubMed] [Google Scholar]
- 63. de Jong KA, Lopaschuk GD. Complex energy metabolic changes in heart failure with preserved ejection fraction and heart failure with reduced ejection fraction. Can J Cardiol 2017;33:860–871. [DOI] [PubMed] [Google Scholar]
- 64. Lopaschuk GD, Ussher JR, Folmes CDL, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev 2010;90:207–258. [DOI] [PubMed] [Google Scholar]
- 65. Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1963;281:785–789. [DOI] [PubMed] [Google Scholar]
- 66. Kienesberger PC, Pulinilkunnil T, Nagendran J, Dyck JRB. Myocardial triacylglycerol metabolism. J Mol Cell Cardiol 2013;55:101–110. [DOI] [PubMed] [Google Scholar]
- 67. Suzuki J, Shen WJ, Nelson BD, Patel S, Veerkamp JH, Selwood SP, Murphy GM, Reaven E, Kraemer FB. Absence of cardiac lipid accumulation in transgenic mice with heart-specific HSL overexpression. Am J Physiol Endocrinol Metab 2001;281:E857–E866. [DOI] [PubMed] [Google Scholar]
- 68. Marfella R, di Filippo C, Portoghese M, Barbieri M, Ferraraccio F, Siniscalchi M, Cacciapuoti F, Rossi F, D’Amico M, Paolisso G. Myocardial lipid accumulation in patients with pressure-overloaded heart and metabolic syndrome. J Lipid Res 2009;50:2314–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, Heldmaier G, Maier R, Theussl C, Eder S, Kratky D, Wagner EF, Klingenspor M, Hoefler G, Zechner R. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science 2006;312:734–737. [DOI] [PubMed] [Google Scholar]
- 70. Haemmerle G, Moustafa T, Woelkart G, Büttner S, Schmidt A, van de Weijer T, Hesselink M, Jaeger D, Kienesberger PC, Zierler K, Schreiber R, Eichmann T, Kolb D, Kotzbeck P, Schweiger M, Kumari M, Eder S, Schoiswohl G, Wongsiriroj N, Pollak NM, Radner FPW, Preiss-Landl K, Kolbe T, Rülicke T, Pieske B, Trauner M, Lass A, Zimmermann R, Hoefler G, Cinti S, Kershaw EE, Schrauwen P, Madeo F, Mayer B, Zechner R. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-α and PGC-1. Nat Med 2011;17:1076–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Fillmore N, Mori J, Lopaschuk GD. Mitochondrial fatty acid oxidation alterations in heart failure, ischaemic heart disease and diabetic cardiomyopathy. Br J Pharmacol 2014;171:2080–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Pulinilkunnil T, Kienesberger PC, Nagendran J, Waller TJ, Young ME, Kershaw EE, Korbutt G, Haemmerle G, Zechner R, Dyck JRB. Myocardial adipose triglyceride lipase overexpression protects diabetic mice from the development of lipotoxic cardiomyopathy. Diabetes 2013;62:1464–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Oluwadare J, Cabodevilla AG, Son NH, Hu Y, Mullick AE, Verano M, Alemán JO, Ramasamy R, Goldberg IJ. Blocking lipid uptake pathways does not prevent toxicity in adipose triglyceride lipase (ATGL) deficiency. J Lipid Res 2022;63:100274. doi: 10.1016/j.jlr.2022.100274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Harris CA, Haas JT, Streeper RS, Stone SJ, Kumari M, Yang K, Han X, Brownell N, Gross RW, Zechner R, Farese RV. DGAT Enzymes are required for triacylglycerol synthesis and lipid droplets in adipocytes. J Lipid Res 2011;52:657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Liu L, Trent CM, Fang X, Son NH, Jiang HF, Blaner WS, Hu Y, Yin YX, Farese RV, Homma S, Turnbull AV, Eriksson JW, Hu SL, Ginsberg HN, Huang LS, Goldberg IJ. Cardiomyocyte-specific loss of diacylglycerol acyltransferase 1 (DGAT1) reproduces the abnormalities in lipids found in severe heart failure. J Biol Chem 2014;289:29881–29891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Roe ND, Handzlik MK, Li T, Tian R. The role of diacylglycerol acyltransferase (DGAT) 1 and 2 in cardiac metabolism and function. Sci Rep 2018;8:4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chokshi A, Drosatos K, Cheema FH, Ji R, Khawaja T, Yu S, Kato T, Khan R, Takayama H, Knöll R, Milting H, Chung CS, Jorde U, Naka Y, Mancini DM, Goldberg IJ, Schulze PC. Ventricular assist device implantation corrects myocardial lipotoxicity, reverses insulin resistance, and normalizes cardiac metabolism in patients with advanced heart failure. Circulation 2012;125:2844–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Liu Q, Siloto RMP, Lehner R, Stone SJ, Weselake RJ. Acyl-CoA:diacylglycerol acyltransferase: molecular biology, biochemistry and biotechnology. Prog Lipid Res 2012;51:350–377. [DOI] [PubMed] [Google Scholar]
- 79. Kolwicz SC, Liu L, Goldberg IJ, Tian R. Enhancing cardiac triacylglycerol metabolism improves recovery from ischemic stress. Diabetes 2015;64:2817–2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Guo Y, Walther TC, Rao M, Stuurman N, Goshima G, Terayama K, Wong JS, Vale RD, Walter P, Farese RV. Functional genomic screen reveals genes involved in lipid-droplet formation and utilization. Nature 2008;453:657–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zhang X, Xu W, Xu R, Wang Z, Zhang X, Wang P, Peng K, Li M, Li J, Tan Y, Wang X, Pei H. Plin5 bidirectionally regulates lipid metabolism in oxidative tissues. Oxid Med Cell Longev 2022;2022:4594956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pollak NM, Jaeger D, Kolleritsch S, Zimmermann R, Zechner R, Lass A, Haemmerle G. The interplay of protein kinase A and perilipin 5 regulates cardiac lipolysis. J Biol Chem 2015;290:1295–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kuramoto K, Okamura T, Yamaguchi T, Nakamura TY, Wakabayashi S, Morinaga H, Nomura M, Yanase T, Otsu K, Usuda N, Matsumura S, Inoue K, Fushiki T, Kojima Y, Hashimoto T, Sakai F, Hirose F, Osumi T. Perilipin 5, a lipid droplet-binding protein, protects heart from oxidative burden by sequestering fatty acid from excessive oxidation. J Biol Chem 2012;287:23852–23863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zheng P, Xie Z, Yuan Y, Sui W, Wang C, Gao X, Zhao Y, Zhang F, Gu Y, Hu P, Ye J, Feng X, Zhang L. Plin5 alleviates myocardial ischaemia/reperfusion injury by reducing oxidative stress through inhibiting the lipolysis of lipid droplets. Sci Rep 2017;7:42574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Pollak NM, Schweiger M, Jaeger D, Kolb D, Kumari M, Schreiber R, Kolleritsch S, Markolin P, Grabner GF, Heier C, Zierler KA, Rülicke T, Zimmermann R, Lass A, Zechner R, Haemmerle G. Cardiac-specific overexpression of perilipin 5 provokes severe cardiac steatosis via the formation of a lipolytic barrier. J Lipid Res 2013;54:1092–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kolleritsch S, Kien B, Schoiswohl G, Diwoky C, Schreiber R, Heier C, Maresch LK, Schweiger M, Eichmann TO, Stryeck S, Krenn P, Tomin T, Schittmayer M, Kolb D, Rülicke T, Hoefler G, Wolinski H, Madl T, Birner-Gruenberger R, Haemmerle G. Low cardiac lipolysis reduces mitochondrial fission and prevents lipotoxic heart dysfunction in perilipin 5 mutant mice. Cardiovasc Res 2020;116:339–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Goldberg IJ, Trent CM, Schulze PC. Lipid metabolism and toxicity in the heart. Cell Metab 2012;15:805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wang H, Sreenivasan U, Gong DW, O’Connell KA, Dabkowski ER, Hecker PA, Ionica N, Konig M, Mahurkar A, Sun Y, Stanley WC, Sztalryd C. Cardiomyocyte-specific perilipin 5 overexpression leads to myocardial steatosis and modest cardiac dysfunction. J Lipid Res 2013;54:953–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Rambold AS, Cohen S, Lippincott-Schwartz J. Fatty acid trafficking in starved cells: regulation by lipid droplet lipolysis, autophagy, and mitochondrial fusion dynamics. Dev Cell 2015;32:678–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Suzuki J, Shen WJ, Nelson BD, Selwood SP, Murphy GM, Kanefara H, Takahashi S, Oida K, Miyamori I, Kraemer FB. Cardiac gene expression profile and lipid accumulation in response to starvation. Am J Physiol Endocrinol Metab 2002;283:E94–E102. [DOI] [PubMed] [Google Scholar]
- 91. Ueno M, Suzuki J, Hirose M, Sato S, Imagawa M, Zenimaru Y, Takahashi S, Ikuyama S, Koizumi T, Konoshita T, Kraemer FB, Ishizuka T. Cardiac overexpression of perilipin 2 induces dynamic steatosis: prevention by hormone-sensitive lipase. Am J Physiol Endocrinol Metab 2017;313:E699–E709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Mardani I, Tomas Dalen K, Drevinge C, Miljanovic A, Ståhlman M, Klevstig M, Scharin Täng M, Fogelstrand P, Levin M, Ekstrand M, Nair S, Redfors B, Omerovic E, Andersson L, Kimmel AR, Borén J, Levin MC. Plin2-deficiency reduces lipophagy and results in increased lipid accumulation in the heart. Sci Rep 2019;9:6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Tsai TH, Chen E, Li L, Saha P, Lee HJ, Huang LS, Shelness GS, Chan L, Chang BHJ. The constitutive lipid droplet protein PLIN2 regulates autophagy in liver. Autophagy 2017;13:1130–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Griffin JD, Bejarano E, Wang XD, Greenberg AS. Integrated action of autophagy and adipose tissue triglyceride lipase ameliorates diet-induced hepatic steatosis in liver-specific plin2 knockout mice. Cells 2021;10:1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wei J, Nelson MD, Szczepaniak EW, Smith L, Mehta PK, Thomson LEJ, Berman DS, Li D, Bairey Merz CN, Szczepaniak LS. Myocardial steatosis as a possible mechanistic link between diastolic dysfunction and coronary microvascular dysfunction in women. Am J Physiol Heart Circ Physiol 2016;310:H14–H19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. McGavock JM, Lingvay I, Zib I, Tillery T, Salas N, Unger R, Levine BD, Raskin P, Victor RG, Szczepaniak LS. Cardiac steatosis in diabetes mellitus. Circulation 2007;116:1170–1175. [DOI] [PubMed] [Google Scholar]
- 97. Nyman K, Granér M, Pentikäinen MO, Lundbom J, Hakkarainen A, Sirén R, Nieminen MS, Taskinen M-R, Lundbom N, Lauerma K. Cardiac steatosis and left ventricular function in men with metabolic syndrome. J Cardiovasc Magn Reson 2013;15:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. da Dalt L, Castiglioni L, Baragetti A, Audano M, Svecla M, Bonacina F, Pedretti S, Uboldi P, Benzoni P, Giannetti F, Barbuti A, Pellegatta F, Indino S, Donetti E, Sironi L, Mitro N, Catapano AL, Norata GD. PCSK9 deficiency rewires heart metabolism and drives heart failure with preserved ejection fraction. Eur Heart J 2021;42:3078–3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Listenberger LL, Han X, Lewis SE, Cases S, Farese RV, Ory DS, Schaffer JE. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci U S A 2003;100:3077–3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Cases S, Stone SJ, Zhou P, Yen E, Tow B, Lardizabal KD, Voelker T, Farese RV. Cloning of DGAT2, a second mammalian diacylglycerol acyltransferase, and related family members. J Biol Chem 2001;276:38870–38876. [DOI] [PubMed] [Google Scholar]
- 101. Listenberger LL, Schaffer JE. Mechanisms of lipoapoptosis: implications for human heart disease. Trends Cardiovasc Med 2002;12:134–138. [DOI] [PubMed] [Google Scholar]
- 102. Adrian L, Lenski M, Tödter K, Heeren J, Böhm M, Laufs U. AMPK Prevents palmitic acid-induced apoptosis and lipid accumulation in cardiomyocytes. Lipids 2017;52:737–750. [DOI] [PubMed] [Google Scholar]
- 103. Choi RH, Tatum SM, Symons JD, Summers SA, Holland WL. Ceramides and other sphingolipids as drivers of cardiovascular disease. Nat Rev Cardiol 2021;18:701–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Stratford S, Hoehn KL, Liu F, Summers SA. Regulation of insulin action by ceramide: dual mechanisms linking ceramide accumulation to the inhibition of Akt/protein kinase B. J Biol Chem 2004;279:36608–36615. [DOI] [PubMed] [Google Scholar]
- 105. Simmonds SJ, Cuijpers I, Heymans S, Jones EAV. Cellular and molecular differences between HFpEF and HFrEF: a step ahead in an improved pathological understanding. Cells 2020;9:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, Bui HH, Davis KE, Bikman BT, Halberg N, Rutkowski JM, Wade MR, Tenorio VM, Kuo MS, Brozinick JT, Zhang BB, Birnbaum MJ, Summers SA, Scherer PE. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med 2011;17:55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Zhang L, Ussher JR, Oka T, Cadete VJJ, Wagg C, Lopaschuk GD. Cardiac diacylglycerol accumulation in high fat-fed mice is associated with impaired insulin-stimulated glucose oxidation. Cardiovasc Res 2011;89:148–156. [DOI] [PubMed] [Google Scholar]
- 108. Yokoyama M, Seo T, Park T, Yagyu H, Hu Y, Son NH, Augustus AS, Vikramadithyan RK, Ramakrishnan R, Pulawa LK, Eckel RH, Goldberg IJ. Effects of lipoprotein lipase and statins on cholesterol uptake into heart and skeletal muscle. J Lipid Res 2007;48:646–655. [DOI] [PubMed] [Google Scholar]
- 109. Fielding CJ, Renston JP, Fielding PE. Metabolism of cholesterol-enriched chylomicrons. Catabolism of triglyceride by lipoprotein lipase of perfused heart and adipose tissues. J Lipid Res 1978;19:705–711. [PubMed] [Google Scholar]
- 110. Perman JC, Boström P, Lindbom M, Lidberg U, StÅhlman M, Hägg D, Lindskog H, Täng MS, Omerovic E, Hultén LM, Jeppsson A, Petursson P, Herlitz J, Olivecrona G, Strickland DK, Ekroos K, Olofsson SO, Borén J. The VLDL receptor promotes lipotoxicity and increases mortality in mice following an acute myocardial infarction. J Clin Invest 2011;121:2625–2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Soccio RE, Breslow JL. Intracellular cholesterol transport. Arterioscler Thromb Vasc Biol 2004;24:1150–1160. [DOI] [PubMed] [Google Scholar]
- 112. Hanada K, Kumagai K, Yasuda S, Miura Y, Kawano M, Fukasawa M, Nishijima M. Molecular machinery for non-vesicular trafficking of ceramide. Nature 2003;426:803–809. [DOI] [PubMed] [Google Scholar]
- 113. Sambandam N, Lopaschuk GD, Brownsey RW, Allard MF. Energy metabolism in the hypertrophied heart. Heart Fail Rev 2002;7:161–173. [DOI] [PubMed] [Google Scholar]
- 114. Cheng M-L, Tang H-Y, Wu P-T, Yang C-H, Lo C-J, Lin J-F, Ho H-Y. 7-Ketocholesterol Induces lipid metabolic reprogramming and enhances cholesterol ester accumulation in cardiac cells. Cells 2021;10:3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Olkkonen VM, Lehto M. Oxysterols and oxysterol binding proteins: role in lipid metabolism and atherosclerosis. Ann Med 2004;36:562–572. [DOI] [PubMed] [Google Scholar]
- 116. Björkhem I, Diczfalusy U. Oxysterols: friends, foes, or just fellow passengers? Arterioscler Thromb Vasc Biol 2002;22:734–742. [DOI] [PubMed] [Google Scholar]
- 117. Sozen E, Yazgan B, Sahin A, Ince U, Ozer NK. High cholesterol diet-induced changes in oxysterol and scavenger receptor levels in heart tissue. Oxid Med Cell Longev 2018;2018:8520746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Seye CI, Knaapen MWM, Daret D, Desgranges C, Herman AG, Kockx MM, Bult H. 7-Ketocholesterol induces reversible cytochrome c release in smooth muscle cells in absence of mitochondrial swelling. Cardiovasc Res 2004;64:144–153. [DOI] [PubMed] [Google Scholar]
- 119. Adachi J, Kudo R, Ueno Y, Hunter R, Rajendram R, Want E, Preedy VR. Heart 7-hydroperoxycholesterol and oxysterols are elevated in chronically ethanol-fed rats. J Nutr 2001;131:2916–2920. [DOI] [PubMed] [Google Scholar]
- 120. Tang HY, Wang CH, Ho HY, Wu PT, Hung CL, Huang CY, Wu PR, Yeh YH, Cheng ML. Lipidomics reveals accumulation of the oxidized cholesterol in erythrocytes of heart failure patients. Redox Biol 2018;14:499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Chandrakala AN, Sukul D, Selvarajan K, Sai-Sudhakar C, Sun B, Parthasarathy S. Induction of brain natriuretic peptide and monocyte chemotactic protein-1 gene expression by oxidized low-density lipoprotein: relevance to ischemic heart failure. Am J Physiol Cell Physiol 2012;302:C165–C177. [DOI] [PubMed] [Google Scholar]
- 122. Guo J, Li HZ, Zhang WH, Wang LC, Wang LN, Zhang L, Li GW, Li HX, Yang BF, Wu L, Wang R, Xu CQ. Increased expression of calcium-sensing receptors induced by ox-LDL amplifies apoptosis of cardiomyocytes during simulated ischaemia-reperfusion. Clin Exp Pharmacol Physiol 2010;37:e128–e135. [DOI] [PubMed] [Google Scholar]
- 123. Schlüter KD, Wolf A, Weber M, Schreckenberg R, Schulz R. Oxidized low-density lipoprotein (oxLDL) affects load-free cell shortening of cardiomyocytes in a proprotein convertase subtilisin/kexin 9 (PCSK9)-dependent way. Basic Res Cardiol 2017;112:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Maxfield FR, Tabas I. Role of cholesterol and lipid organization in disease. Nature 2005;438:612–621. [DOI] [PubMed] [Google Scholar]
- 125. Fernández A, Colell A, Caballero F, Matías N, García-Ruiz C, Fernández-Checa JC. Mitochondrial S-adenosyl-I-methionine transport is insensitive to alcohol-mediated changes in membrane dynamics. Alcohol Clin Exp Res 2009;33:1169–1180. [DOI] [PubMed] [Google Scholar]
- 126. Morimoto SI, Sekiguchi M, Hiramitsu S, Uemura A, Nishikawa T, Hishida H. Contribution of cardiac muscle cell disorganization to the clinical features of hypertrophic cardiomyopathy. Heart Vessels 2000;15:149–158. [DOI] [PubMed] [Google Scholar]
- 127. Maron BJ, Sato N, Roberts WC, Edwards JE, Chandra RS. Quantitative analysis of cardiac muscle cell disorganization in the ventricular septum. Comparison of fetuses and infants with and without congenital heart disease and patients with hypertrophic cardiomyopathy. Circulation 1979;60:685–696. [DOI] [PubMed] [Google Scholar]
- 128. Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev 2014;94:909–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Neubauer S. The failing heart—an engine out of fuel. N Engl J Med 2007;356:1140–1151. [DOI] [PubMed] [Google Scholar]
- 130. Chaanine AH, Joyce LD, Stulak JM, Maltais S, Joyce DL, Dearani JA, Klaus K, Nair KS, Hajjar RJ, Redfield MM. Mitochondrial morphology, dynamics, and function in human pressure overload or ischemic heart disease with preserved or reduced ejection fraction. Circ Heart Fail 2019;12:e005131. [DOI] [PubMed] [Google Scholar]
- 131. Sakatani T, Shirayama T, Suzaki Y, Yamamoto T, Mani H, Kawasaki T, Sugihara H, Matsubara H. The association between cholesterol and mortality in heart failure: comparison between patients with and without coronary artery disease. Int Heart J 2005;46:619–629. [DOI] [PubMed] [Google Scholar]
- 132. Nielsen LB, Véniant M, Borén J, Raabe M, Wong JS, Tam C, Flynn L, Vanni-Reyes T, Gunn MD, Goldberg IJ, Hamilton RL, Young SG. Genes for apolipoprotein B and microsomal triglyceride transfer protein are expressed in the heart: evidence that the heart has the capacity to synthesize and secrete lipoproteins. Circulation 1998;98:13–16. [DOI] [PubMed] [Google Scholar]
- 133. Borén J, Véniant MM, Young SG. Apo B100-containing lipoproteins are secreted by the heart. J Clin Invest 1998;101:1197–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Nielsen LB, Bartels ED, Bollano E. Overexpression of apolipoprotein B in the heart impedes cardiac triglyceride accumulation and development of cardiac dysfunction in diabetic mice. J Biol Chem 2002;277:27014–27020. [DOI] [PubMed] [Google Scholar]
- 135. Nielsen LB, Perko M, Arendrup H, Andersen CB. Microsomal triglyceride transfer protein gene expression and triglyceride accumulation in hypoxic human hearts. Arterioscler Thromb Vasc Biol 2002;22:1489–1494. [DOI] [PubMed] [Google Scholar]
- 136. Bartels ED, Nielsen JM, Hellgren LI, Ploug T, Nielsen LB. Cardiac expression of microsomal triglyceride transfer protein is increased in obesity and serves to attenuate cardiac triglyceride accumulation. PLoS One 2009;4:e5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Yokoyama M, Yagyu H, Hu Y, Seo T, Hirata K, Homma S, Goldberg IJ. Apolipoprotein B production reduces lipotoxic cardiomyopathy. J Biol Chem 2004;279:4204–4211. [DOI] [PubMed] [Google Scholar]
- 138. Ledmyr H, McMahon AD, Ehrenborg E, Nielsen LB, Neville M, Lithell H, MacFarlane PW, Packard CJ, Karpe F. The microsomal triglyceride transfer protein gene-493T variant lowers cholesterol but increases the risk of coronary heart disease. Circulation 2004;109:2279–2284. [DOI] [PubMed] [Google Scholar]
- 139. Levak-Frank S, Hofmann W, Weinstock PH, Radner H, Sattler W, Breslow JL, Zechner R. Induced mutant mouse lines that express lipoprotein lipase in cardiac muscle, but not in skeletal muscle and adipose tissue, have normal plasma triglyceride and high-density lipoprotein-cholesterol levels. Proc Natl Acad Sci U S A 1999;96:3165–3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Augustus A, Yagyu H, Haemmerle G, Bensadoun A, Vikramadithyan RK, Park SY, Kim JK, Zechner R, Goldberg IJ. Cardiac-specific knock-out of lipoprotein lipase alters plasma lipoprotein triglyceride metabolism and cardiac gene expression. J Biol Chem 2004;279:25050–25057. [DOI] [PubMed] [Google Scholar]
- 141. Rame JE. Chronic heart failure: a reversible metabolic syndrome? Circulation 2012;125:2809–2811. [DOI] [PubMed] [Google Scholar]
- 142. Takahara S, Ferdaoussi M, Srnic N, Maayah ZH, Soni S, Migglautsch AK, Breinbauer R, Kershaw EE, Dyck JRB. Inhibition of ATGL in adipose tissue ameliorates isoproterenol-induced cardiac remodeling by reducing adipose tissue inflammation. Am J Physiol Heart Circ Physiol 2021;320:H432–H446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Thiele A, Luettges K, Ritter D, Beyhoff N, Smeir E, Grune J, Steinhoff JS, Schupp M, Klopfleisch R, Rothe M, Wilck N, Bartolomaeus H, Migglautsch AK, Breinbauer R, Kershaw EE, Grabner GF, Zechner R, Kintscher U, Foryst-Ludwig A. Pharmacological inhibition of adipose tissue adipose triglyceride lipase by Atglistatin prevents catecholamine-induced myocardial damage. Cardiovasc Res 2022;118:2488–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]