Abstract
Background:
Single cycle induction chemotherapy (IC) with platinum and 5-flurouracil (PF) and treatment based on clinical response predicts organ preservation in laryngeal cancer. Other agents offer intriguing alternatives with potentially increased ease of administration, reduced risk for severe toxicities, and increased platinum sensitivity.
Methods:
We report the results of a phase II bioselection trial in advanced resectable laryngeal cancer utilizing an IC regimen of two cycles of platinum plus docetaxel (TP) with a Bcl-2 inhibitor. The primary endpoint was organ preservation rate at 12 weeks post chemoradiation.
Results:
54 patients were enrolled. Response to IC was 72%. The organ preservation rate was 59% with a laryngectomy free survival of 46%. Induction related grade ≥ 3 toxicities were observed in 56% of patients with two grade 5 events.
Conclusions:
Two cycles of TP IC plus a Bcl-2 inhibitor did not improve laryngeal preservation compared to a single cycle of PF.
Keywords: laryngeal cancer, organ preservation, neoadjuvant chemotherapy, chemoradiation, induction chemotherapy
Introduction
Laryngeal cancer affects more than 12,000 patients per annum in the United States[1]. Traditionally, the mainstay of treatment for locally advanced squamous cell carcinoma (LASCC) of the larynx has been total laryngectomy (TL)[2]. Although curative in 60% patients, TL is associated with long term functional impairment and impaired quality of life for many patients[3]. Organ preservation utilizing definitive chemoradiation has become a standard approach when treating LASCC of the larynx. Studies have demonstrated that survival rates for chemoradiation are equivalent to surgery, allowing for laryngeal preservation in approximately two thirds of patients[4, 5]. The remainder experience persistent disease necessitating a salvage laryngectomy, which is often associated with higher rates of long-term complications and poorer survival outcomes[6, 7].
A single cycle of induction chemotherapy (IC) is an effective method for selecting patients with LASCC of the larynx who will have favorable outcomes with chemoradiation[8]. Early data from our institution demonstrated that clinical response (defined as > 50% reduction in tumor volume on visual exam) after a single cycle of IC was predictive of successful laryngeal preservation. We performed a phase II trial, evaluating treatment stratification based on clinical response to a single cycle of IC (‘bioselection’). Patients underwent pre-treatment tumor visual assessment by a surgeon, were given one cycle of chemotherapy utilizing platinum and 5-flurouracil (PF), and then reassessed three weeks later to determine the degree of clinical response. If there was a > 50% reduction in tumor volume, patients were treated with chemoradiation; non-responders underwent upfront laryngectomy. This approach resulted in similar rates of laryngeal preservation but an improved overall survival when compared to other organ preservation strategies. In particular, patients undergoing upfront laryngectomy had excellent survival compared to historically inferior survival rates associated with delayed laryngectomy for tumor relapse[9]. Similarly, retrospective studies have favored bioselection over upfront chemoradiation[10].
Previous studies in patients with LASCC have demonstrated that an increased number of cycles of IC may result in a higher objective response rate [4]. Furthermore, trials in the metastatic setting have shown that cisplatin plus taxanes have equivalent response rates to PF with a more favorable toxicity profile[11]. This would suggest that a taxane might be substituted for 5-fluorouracil for improved tolerance. Taken together, bioselection using two cycles of platinum plus docetaxel (TP) offers a rational method to further increase laryngeal preservation in patients with advanced laryngeal cancer while decreasing treatment related toxicities.
B-cell lymphoma 2 (Bcl-2) and Bcl-XL proteins are anti-apoptotic regulators which have been shown to been implicated in the progression of head and neck squamous cell carcinoma and overexpressed in over 70% of patients with laryngeal cancer[12, 13]. Patients with low or absent expression of Bcl-XL have excellent rates of organ preservation[13]. Furthermore, preclinical evidence supports that Bcl-2/-XL inhibition increases platinum sensitivity in head and neck squamous cell carcinoma[14]. AT-101 is an oral Bcl-2 homolog domain 3 mimetic which has been shown to provided targeted inhibition and synergy with chemotherapy [15, 16]. Hence, combined inhibition of Bcl-2/-XL via AT-101 and IC is a rational means that could elicit a greater anti-tumor response in patients with laryngeal cancer undergoing bioselection.
We conducted a phase II trial to investigate the safety and efficacy of two cycles of IC with TP as bioselection in patients with LASCC of the larynx. We hypothesized that 1) two cycles of IC plus a Bcl-2 inhibitor would increase responses, allowing for a higher rate of organ preservation; and 2) induction with TP would improve tolerability compared to single cycle of bioselection with PF induction.
Materials and Methods
Patient Eligibility
This was a randomized phase 2, open label trial approved by the institutional review board at the University of Michigan Rogel Cancer Center (NCT01633541). This trial was performed in accordance with the ethical standards of the committee on human experimentation of the institution and in accord with the Helsinki Declaration of 1975 as revised in 1983. All patient’s provided written informed consent. Patients age 18 or older with pathologically confirmed previously untreated stage III or IVA squamous cell carcinoma of the larynx or hypopharynx were eligible. Staging was assigned per AJCC 7th edition. Patients must have had tumors that were amenable to surgically resection via total impaired absorptive function.
Patients with distant metastases or prior head and neck radiation and/or chemotherapy were not eligible. Additional exclusionary criteria included a history of grade 2 or greater peripheral neuropathy, hypersensitivity to docetaxel, NYHA class 3 or 4 heart disease, unstable angina or history of myocardial infarction within 6 months of enrollment, and gastrointestinal abnormalities resulting in impaired absorptive function.
Treatment Plan
Baseline studies were performed including assessment of the primary tumor and regional disease via direct examination and endoscopy of the primary tumor, diagnostic CT of the neck with perfusion, and laboratory studies.
Induction Chemotherapy
Patients were randomized to one of two arms of IC: TP (Arm A) or TP plus AT-101 (Arm B) (Figure 1) or one cycle in a 2:1 ratio in order to maximize the number of patients receiving AT101 for at least one cycle. Randomization was stratified by clinical stage and blocked to ensure balance in arm assignments. All patients without complete response received TP + AT-101 for their second cycle. The purpose of the randomization was to enable a comparison between the two treatments (TP+AT101 vs TP alone) based on clinical and laboratory measures assessed after one cycle. Carboplatin could be substituted for cisplatin as deemed necessary by the patient’s treating medical oncologist. The decision regarding patient eligibility for cisplatin based IC was made prior to randomization. Changes in the treatment plan could be made based on physician discretion prior to cycle 2. Docetaxel was administered at 75 mg/m2 and cisplatin 100 mg/m2 (Carboplatin AUC 6). AT-101 was initiated at 40 mg orally twice daily and administered on days 1–3 (total of 6 doses). Clinical response assessment was conducted by endoscopic laryngoscopy 23 days after initiation of IC and graded as complete response, partial response (≥50% but <100% reduction in primary tumor size), or no response (<50% reduction in primary tumor size). Response in nodal disease was not incorporated when evaluating response to IC. Patients with a complete response on both clinical exam and radiographic imaging post cycle 1 were directed to start chemoradiation whereas all other patients with partial or no responses were then treated with a second cycle of IC. With the second cycle, all received TP in combination with AT-101, regardless of the initial randomization arm. Repeat endoscopy for clinical response assessment was performed 23 days after cycle 2. Patients with complete or partial response to IC (≥50% reduction in tumor size after two cycles of induction) proceeded to definitive chemoradiation, whereas non-responders (< 50% reduction in tumor size after two cycles of induction) were directed to primary laryngectomy.
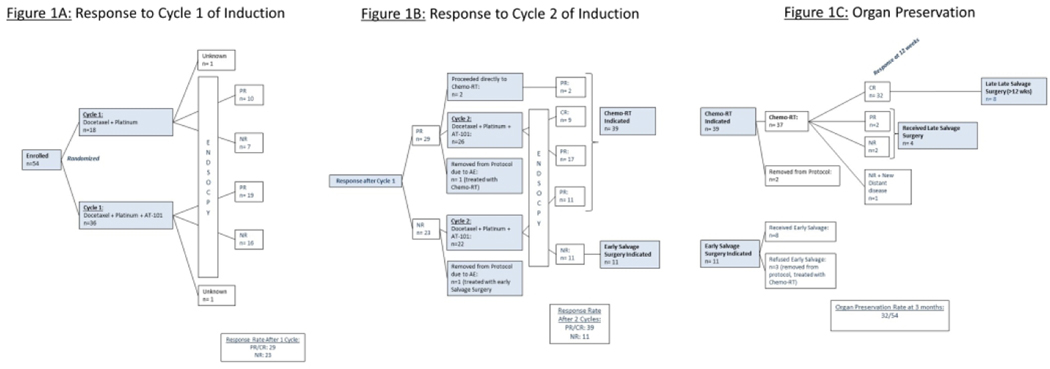
Figure 1:
Patient Enrollment These figures illustrate the trial schema, treatment, and patient response to induction chemotherapy by arm (1A), overall (1B), and long term organ preservation (1C)
1016×381mm (96 × 96 DPI)
Chemoradiation, Response Assessment, and Follow Up
Definitive radiotherapy began within 3 weeks after completion of IC. CT simulation was performed in a 5 point aquaplast mask with IV contrast. Gross Tumor volume (GTV) was defined on planning CT scans with the original staging CT and FDG-PET as well as MRI when available. All patients were treated with IMRT using 6MV photons on Varian linear accelerators. Dose was prescribed with simultaneous integrated boost (SIB) to 70Gy delivered to CTVhigh (GTV+ 5mm margin), 59.4Gy to CTVmid (nodal levels with positive LNs as well as whole larynx) and 56Gy to CTVlow (electively treated cervical LN levels) all in 35 fractions given 5 fractions per week. All patients were treated to cervical LN levels II-IV bilaterally, and level VI was added for evidence of subglottic extension. CTV to PTV margin was 3mm. Adjuvant radiation therapy doses were as follows: Patients were treated to 60–66Gy with SIB over 30–33 fractions. Post-operative bed and dissected neck were treated to 60Gy with boost to 66Gy for positive margins or to nodal levels of extracapsular extension. Non-dissected neck received 54–56Gy.
Cisplatin was delivered at 50 mg/m2 weekly concurrent with radiation therapy in patients receiving definitive chemoradiation for organ preservation. Patients were seen weekly for toxicity evaluation and management. Aggressive supportive care with anti-emetics and hydration was provided. Cisplatin-ineligible patients received weekly carboplatin (AUC 2.5). In patients who underwent an upfront laryngectomy, chemotherapy was added to adjuvant radiation for the presence of high-risk pathologic features (positive surgical margins and/or extra-nodal extension) as noted on surgical pathology.
Three months after completion of chemoradiation, patients underwent PET/CT imaging and laryngoscopy. Patients in whom persistent disease was suspected underwent a biopsy of the original tumor bed to assess response. Patients with a laryngeal biopsy positive for persistent disease underwent a late salvage laryngectomy. If neither imaging nor biopsy were concerning for persistent disease, patients were followed with active surveillance. Biopsies were performed for suspicion for recurrence by on physical exam or follow up imaging. If positive, patients were treated with salvage laryngectomy.
Quality of Life Assessment
Quality of life instruments (HN-QOL, V-RQOL) were administered prior to IC, then at 6, 12, and 24 months after completion of definite treatment (chemoradiation in responders or laryngectomy plus adjuvant radiation in non-responders).
Statistical Analysis
The primary clinical objective of the trial was to compare the larynx preservation rates in a treatment paradigm that uses up to 2 cycles of platinum and docetaxel (TP) induction chemotherapy to select patients for either concurrent chemoradiation or surgery. The optimal two-stage accrual design [Simon, Controlled Clinical Trials 10:1–10 1989] was adopted which provided 85% statistical power and 5% overall type I error rate for detecting early larynx preservation rates of 70% versus 50%. Secondary objectives included overall response rate to induction chemotherapy, progression-free survival, and treatment related toxicities.
Fifty-four patients were enrolled between 3/1/2012 and 7/2/2018. All subjects who received at least one cycle of IC (n=54) were included in the analysis describing laryngectomy-free, overall survival, overall response, and toxicity rates. Only subjects who underwent protocol defined treatment were included in the analysis of disease free survival. Quality of Life surveyed using validated instruments HNQOL and VRQOL. Treatment-related adverse events were graded according to the Common Terminology for Adverse Events version 3.0 (CTCAE v3).
Overall (OS) and Laryngectomy-Free (LFS) survival times were defined for all patients starting from date of enrollment. Disease Free Survival (DFS) was calculated among those treated per protocol indication (n=46) and was defined beginning from date of surgery for upfront surgery patients or date of CRT completion for CRT upfront patients. Events dates were defined as date of death (OS), laryngectomy or death (LFS); and disease progression or death (DFS) which ever came first. The Kaplan-Meier method was used to estimate OS, LFS, and DFS rates at 3 years with 95% confidence intervals of the point estimates using a log-log transformation. Analyses of Quality of Life measures were performed using Linear Mixed Effect models of the continuous QoL domains assuming a compound symmetric correlation structure. Separate models were fit that contained fixed effects for long term time points (screening, 6 mo, 12 mo, 24 mo), allowing a nonlinear longitudinal pattern to be estimated. Interactions with upfront treatment type were explored but failed to prove significant and therefore not reported in the final model results. All analyses were performed using SAS software, Version 9.4 (SAS Institute, Cary, NC) for Windows or R (version 4.0.0).
Results
Patient Characteristics
Fifty-four patients were enrolled and randomized between Arms A and B. All enrolled patients received at least one cycle of TP IC and were included in analysis for toxicities (Table 1). The mean age was 59.4 years old (range 19–82) with the primary site of disease was the supraglottic larynx (76%). 70% had Stage IVA disease and 48% of patients had T4 disease. Patient and tumor characteristics were balanced between the two arms (Table 2).
Table 1:
Patient and Tumor Characteristics
| Characteristic | Number of Patients= 54 |
|---|---|
|
| |
| Initial Randomization | |
| Arm A - TP alone | 18 |
| Arm B - TP + AT101 | 36 |
|
| |
| Age, years | 59.4 (19–82) |
|
| |
| Sex | |
| Male | 46 (85%) |
| Female | 8 (15%) |
|
| |
| Race | |
| White | 53 (98%) |
| Black or African American | 1 (2%) |
|
| |
| ECOG performance status | |
| 0 | 31 (57%) |
| 1 | 23 (43%) |
|
| |
| Cancer location | |
| Hypopharynx | 2 (4%) |
| Supraglottic | 38 (70%) |
| Glottic | 11 (20%) |
| Subglottic | 3 (6%) |
|
| |
| Stage | |
| III | 16 (30%) |
| IVA | 38 (70%) |
|
| |
| T Classification | |
| T2 | 2 (4%) |
| T3 | 26 (48%) |
| T4 | 26 (48%) |
|
| |
| N Classification | |
| N0 | 16 (30%) |
| N1 | 7 (13%) |
| N2a | 2 (4%) |
| N2b | 15 (28%) |
| N2c | 14 (26%) |
Table 2:
Induction Randomization and Response to Induction Chemotherapy
| Characteristic | Arm A - TP alone (n=18) | Arm B - TP + AT-101 (n=36) | p-value |
|---|---|---|---|
|
| |||
| Age, years | 61.4 (33–77) | 58.5 (19–82) | 0.34 |
|
| |||
| Sex | |||
| Male | 18 (100%) | 28 (78%) | 0.03 |
| Female | 0 | 8 (22%) | |
|
| |||
| Race | |||
| White | 18 (100%) | 35 (97%) | 0.48 |
| Black or African American | 0 | 1 (3%) | |
|
| |||
| ECOG performance status | |||
| 0 | 11 (61%) | 20 (56%) | 0.82 |
| 1 | 7 (41%) | 16 (44%) | |
|
| |||
| Cancer location | 0.16 | ||
| Supraglottic | 10 (53%) | 28 (78%) | |
| Glottic | 6 (32%) | 5 (14%) | |
| Subglottic | 0 | 3 (8%) | |
| Hypopharynx | 2 (10%) | 0 | |
|
| |||
| Stage | |||
| III | 6 (33%) | 10 (28%) | 0.67 |
| IVA | 12 (67%) | 26 (72%) | |
|
| |||
| ECOG performance status | |||
| 0 | 11 (61%) | 20 (56%) | 0.7 |
| 1 | 7 (39%) | 16 (44%) | |
|
| |||
| Response to First Cycle of IC | 0.76 | ||
| PR | 10 (56%) | 19 (53%) | |
| NR | 7 (39%) | 16 (44%) | |
|
| |||
| not assessed (death before) | 1 (6%) | 1 (3%) | |
Response to Induction Chemotherapy
Of the 54 patients, 2 patients were not evaluable for response as they died before the first response assessment (Figure 1A). Per physician discretion, carboplatin was used in lieu of cisplatin for 10 patients in Arm A (10/18, 55%) and 13 patients in Arm B (13/26, 36%). Twenty-nine responses were seen following the first cycle of IC, providing a response rate of 54% (29/54) with no difference between the arm treated with AT-101 plus TP versus TP alone (56% vs 50%, p=0.7). Two patients inadvertently proceeded directly to chemoradiation after a single cycle of IC. Two patients were removed from protocol after the first cycle of induction due to toxicities. One of the patients had a partial response and was treated with chemoradiation while the other did not have a response to therapy and proceeded to surgery.
The remaining 48 patients were treated with a second cycle of IC (Figure 1B). In Arm A, all patients who received cisplatin for the first cycle of IC proceeded to receive cisplatin for their second cycle of IC. In Arm B, one patient was switched from cisplatin to carboplatin for their second cycle of IC per treating physician discretion. The cumulative response rate after the second cycle of induction was 72% (39/54) with clinical complete response noted on direct tumor visualization in 9 patients (17%). Of the 22 non-responders to a single cycle who were treated with a second cycle of induction, 11 patients converted to responders.
Toxicity of Induction Chemotherapy
Forty three percent of patients experienced severe toxicities (defined as grade 3 or greater) during IC (Table 3). The rate or type of severe toxicities were not statistically different between patients randomized to arm A versus B (39% versus 44%). Two patients died after the first cycle of induction, one due to sudden cardiac arrest and the other due to intracranial hemorrhage, for which IC was thought to possibly have contributed to their demise. The most common severe toxicities included neutropenia (9%), diarrhea (7%), and nausea (7%). Four patients (7%, 4/54) did not receive the second cycle of induction due to toxicities.
Table 3:
Toxicities of Induction Chemotherapy
| CTCAE Grade | ||||
|---|---|---|---|---|
| 3 | 4 | 5 | Total | |
| Neutropenia | 2 | 3 | 0 | 5 (9%) |
| Diarrhea | 4 | 0 | 0 | 4 (7%) |
| Nausea | 4 | 0 | 0 | 4 (7%) |
| Vomiting | 3 | 0 | 0 | 3 (5%) |
| Dehydration | 2 | 0 | 0 | 2 (4%) |
| Hypokalemia | 2 | 0 | 0 | 2 (4%) |
| Alanine aminotransferase increased | 1 | 0 | 0 | 1 (2%) |
| Anemia | 1 | 0 | 0 | 1 (2%) |
| Anorexia | 1 | 0 | 0 | 1 (2%) |
| Decreased Glomerular Filtration Rate | 0 | 1 | 0 | 1 (2%) |
| Hand-and-foot syndrome | 1 | 0 | 0 | 1 (2%) |
| Hyponatremia | 1 | 0 | 0 | 1 (2%) |
| Pneumonia | 1 | 0 | 0 | 1 (2%) |
| Intracranial hemorrhage | 0 | 0 | 1 | 1 (2%) |
| Mucositis oral | 1 | 0 | 0 | 1 (2%) |
| Pruritus | 1 | 0 | 0 | 1 (2%) |
| Rash | 1 | 0 | 0 | 1 (2%) |
| Sepsis | 0 | 1 | 0 | 1 (2%) |
| Thrombosis/thrombus/embolism | 1 | 0 | 0 | 1 (2%) |
| Weight loss | 1 | 0 | 0 | 1 (2%) |
| Laryngospasm | 1 | 0 | 0 | 1 (2%) |
| Sudden Cardiac Arrest | 0 | 0 | 1 | 1 (2%) |
Includes all grade 3 or greater toxicities seen during induction chemotherapy and assessed by investigator to be possibly, probably, or definitely due to therapy
Toxicity of Chemoradiation
Thirty-seven patients were treated with definitive chemoradiotherapy per protocol. Per protocol, patients receiving definitive chemoradiation were planned to receive seven weekly doses of chemotherapy. Twenty patients (54%) initiated therapy with carboplatin based chemoradiation as they were deemed non-cisplatin eligible per the treating oncologist. An additional nine patients (24%) were started on concurrent cisplatin and radiotherapy, then switched mid treatment to carboplatin. The median number of doses administered was six (range: 3–7). Most toxicities were grade 1 or 2 however 28% of patients experienced severe toxicities during chemoradiation which included dysphagia (11%), pneumonia (8%), and mucositis (5%) (Table 4). Eleven patients (27%) had radiation delayed by more than five days.
Table 4:
Toxicities of Definitive Chemoradiation
| CTCAE Grade | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 or 5 | |
| Dysphagia | 13 | 5 | 4 | 0 |
| Pneumonia | 0 | 0 | 3 | 0 |
| Mucositis | 18 | 20 | 2 | 0 |
| Thrombocytopenia | 8 | 7 | 2 | 0 |
| Lymphopenia | 1 | 5 | 2 | 0 |
| Nausea | 14 | 9 | 1 | 0 |
| Weight loss | 9 | 2 | 1 | 0 |
| Neutropenia | 0 | 3 | 1 | 0 |
| Dehydration | 2 | 0 | 1 | 0 |
| Hyperkalemia | 1 | 1 | 1 | 0 |
| Allergic reaction | 0 | 0 | 1 | 0 |
| Hypertension | 0 | 0 | 1 | 0 |
| Seizure | 0 | 0 | 1 | 0 |
| Taste alteration | 12 | 11 | 0 | 0 |
| Xerostomia | 16 | 6 | 0 | 0 |
| Fatigue | 9 | 2 | 0 | 0 |
| Vomiting | 7 | 2 | 0 | 0 |
| Dermatitis radiation | 3 | 5 | 0 | 0 |
| Neuropathy | 4 | 0 | 0 | 0 |
| Anemia | 3 | 1 | 0 | 0 |
n=37 patients
Includes all toxicities seen during definitive chemoradiation regardless of investigator attribution
Organ Preservation
Early laryngectomy was indicated after two cycles of IC in 11 patients (Figure 1C). Three patients refused early laryngectomy, were removed from protocol, and treated with definitive chemoradiation. Of the 39 responders after two cycles of IC, 2 patients were removed from protocol one due to social barriers to ongoing trial participation and the other due to disease related decline in performance status. The remaining 37 patients were treated with concurrent chemoradiation. PET/CT response assessment at 12 weeks post chemoradiation demonstrated an 86% complete response rate. Four patients had residual disease for which late salvage surgery was indicated, and 1 patient had residual disease and new distant metastases. The organ preservation rate at 3 months post-chemoradiation was 59%.
Eight patients who were originally noted to have no evidence of disease on their post treatment PET/CT underwent a late salvage surgery for recurrent disease (defined as surgery > 12 weeks from completion of chemoradiation).
Survival
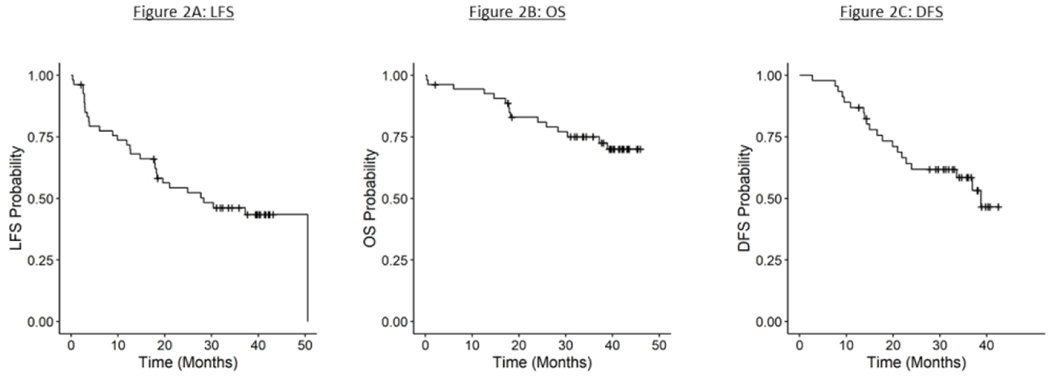
Twenty patients underwent a laryngectomy during the course of the trial. Eight patients underwent early laryngectomy having been identified to be non-responders after cycle 2 of IC. Four patients were treated with salvage surgery having been noted to have persistent disease on the 12 weeks post chemoradiation, and 8 patients underwent a late salvage surgery (>12 weeks from completion of definitive therapy) for recurrent disease. The estimated 3-year LFS was 46% (95% CI: 32, 59%) (Figure 2A). There was no significant difference between patients with T4 and non T4 disease (44% (95% CI: 25,62%) vs 48% (95% CI: 29,66)) (Table 5).
Figure 2:
Kaplan Meier Survival Analysis These figures illustrate the 3 year laryngectomy free survival (2A), 3 year overall survival (2B), and 3 year disease free survival (2C)
381×190mm (96 × 96 DPI)
Table 5:
Survival Estimates
| Population | No of Patients | KM Estimate | 95% CI |
|---|---|---|---|
|
| |||
| Overall Survival, 3 Year | |||
| Entire Group | 54 | 75% | (61,85) |
| Sub-Group | |||
| T4 | 26 | 72% | (50,86) |
| non T4 | 28 | 75% | (57,89) |
| Upfront Larynx indicated | 11 | 73% | (37,90) |
| Chemoradiaiton indicated | 39 | 78% | (61,88) |
| off protocol | 4 | ||
|
| |||
| Disease Free Survival, 3 Year | |||
| Entire Groupφ | 46 | 59% | (42,72) |
| Sub-Group | |||
| T4 | 22 | 48% | (25,67) |
| non T4 | 24 | 69% | (46,84) |
| Upfront Larynx indicated | 8 | 63% | (23,86) |
| Chemoradiaiton indicated | 37 | 56% | (38,71) |
|
| |||
| Laryngectomy Free Survival, 3 Year | |||
| Entire Group | 54 | 46% | (32, 59) |
| Sub-Group | |||
| T4 | 26 | 44% | (25,62) |
| non T4 | 28 | 48% | (29,66) |
| Chemoradiaiton indicated | 39 | 56% | (39,71) |
DFS defined for only those who received protocol defined treatment. Excludes: 2 early deaths, 2 off protocol during IC for AEs, 5 off protocol after IC completion for patient refusal of protocol defined treatment (3 early salvage refusals opted for CRT, 1 no CRT for performance status decline, and 1 no CRT at UM for follow-up to be treated closer to home).
The estimated 3-year OS was 75% (95% CI: 61,85%) (Figure 2B). The median follow-up duration among the study participants was 40 months (range: 0.3–46 months) and no patients remain on therapy. Follow-up on patients still alive ranges from 2 to 46 months (mean: 37.8 months). The estimated 3-year DFS among the 46 patients who received protocol defined treatment was 59% (95% CI: 42,72%)(Figure 2C). Ten patients died from their disease. Survival outcomes were compared between patients with T4 versus non T4 disease and there was found to be no significant difference in OS (72% (95% CI: 50, 86%) vs 75% (95% CI: 57,89%)) or DFS (48% (95% CI: 25,67%) vs 69% (95% CI: 46,84)). Similarly, there was no difference in OS between patients requiring planned early salvage surgery versus those where chemoradiation was indicated (73% (95% CI: 37,90%) vs 78% (95% CI: 61,88)).
Quality of Life
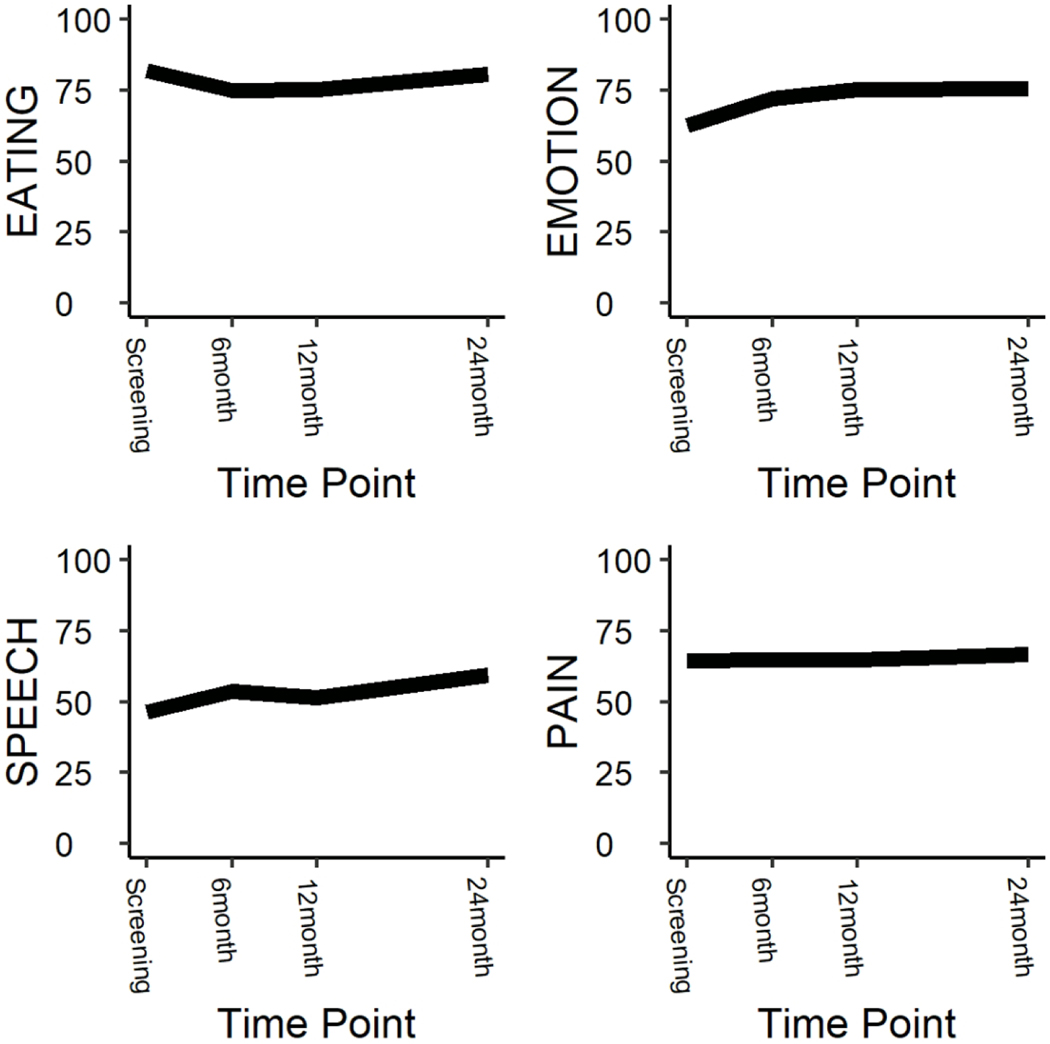
Quality of life was longitudinally assessed among all patients enrolled on the trial (Supp Table 1). Short term follow-up at 6 months post definitive treatment demonstrated no difference in any of the QOL domains compared to pre-treatment except a mild impairment in the eating domain (80.8 vs 74.8, p=0.03) and improvement in the emotion domain (62.2 vs 72.1, p < 0.001) on the HNQOL instrument (Figure 3). At 12 months, the impairment and eating resolved and on long term follow up at 24 months post completion of definitive treatment there was no difference in quality of life except for a significant improvement in the HNQOL emotion (62.2 vs 75.7, p < 0.001) and speech domains (47.9 vs 59.3, p=0.02) compared to pre-treatment.
Figure 3:
Long Term Quality of Life
These figures illustrate the results of longitudinal quality of life analysis as defined by the HN-QOL instrument.
101×101mm (300 × 300 DPI)
Due to the limited number of patients treated with early laryngectomy, we were limited in our ability to formally compare the quality of life of patients stratified to organ preservation versus surgery. However, as an exploratory analysis, interaction models were utilized. There was no interaction between upfront treatment and the trends in quality of life among any of the domains.
Discussion
In this randomized phase 2 study, we demonstrate that an alternate bioselection induction regimen with two cycles TP does not appear to improve the organ preservation rate or clinical response to chemotherapy compared to a single cycle of PF. No difference was seen in the response rate between patients treated with TP + AT-101 vs TP alone or toxicities. Our findings confirm the lack of clinical activity seen in other clinical trials throughout advanced malignancies including metastatic HNSCC combined with docetaxel[17].
Previous work suggests that clinical response to a single cycle of chemotherapy, defined as >50% reduction, is predictive of successful laryngeal organ preservation[9]. A bioselection paradigm stratifying patient treatment based on the degree of response offers impressive rates of larynx preservation and survival outcomes. The VA Larynx and RTOG 91–11 trials both evaluated IC followed by radiotherapy for locally advanced laryngeal cancer[4, 5]. Patients randomized to IC were given two cycles of PF induction and proceeded to a third cycle if they lacked disease progression. Those failing to respond after three cycles of therapy underwent laryngectomy. In RTOG 91–11, 24 patients had less than a partial response after cycle 2 and were given a third cycle. Seventeen of these patients experienced a complete response and were treated with larynx preservation with excellent long term outcomes, suggesting that additional cycles of chemotherapy may identify patients who will benefit from laryngeal organ preservation. In our trial, the administration of a second cycle of IC to nonresponders resulted in 48% patients developing a subsequent partial response. Disappointingly, 5 of the 11 patients eventually required a laryngectomy for recurrent disease. Thus, multiple cycles of chemotherapy does not enhance the identification of patients suitable for organ preservation through a bioselection paradigm.
Although bioselection offers the potential for improved outcomes in laryngeal cancer, administration of PF offers both logistical challenges and toxicity concerns. The E1395 study evaluated PF versus TP in unresectable recurrent or metastatic head and neck squamous cell carcinoma and showed no difference in response rates, supporting potential equivalence of the regimens[11]. Our study identified a lower response rate to one cycle of TP induction (54%) compared to PF (75%), although this difference resolved after a second cycle of TP (72%). Several factors could account for the difference in response rate between arms. Although equivalent activity was noted in previous studies, response assessment was performed by radiographic imaging after 3 cycles of treatment and fluorouracil was dosed lower than that given in our PF bioselection induction regimen (four-day infusion versus five-day infusion). Furthermore, there may be differential sensitivity to cytotoxic agents in locally advanced disease versus R/M disease. This finding merits further consideration for the design of further trials of IC in LASCC.
Our observed overall survival was similar to that of a previously reported retrospective study of laryngeal cancer outcomes with bioselection versus other modalities as part of routine clinical practice and correlates with our previous report of bioselection using single cycle PF. However, the organ preservation rate and 3 year LFS were inferior to that seen with single cycle PF induction (Organ Preservation Rate: 59% versus 70%, LFS: 46% versus 61%). There are several potential explanations of this finding. As previously noted, of the 11/22 patients who converted from non-responders to responders after a second cycle of induction, five of these eventually required a laryngectomy (2 for persistent disease post CRT, 3 for recurrence > 12 weeks after CRT). It is possible that a second cycle of IC does not add significant benefit. Compared to our previous laryngeal bioselection study, this study enrolled a higher proportion of patients with T4 tumors (48 vs 33%) or stage IVA disease (70 vs 54%) which have worse outcomes compared to non-T4 or non-stage IVA patients. As the previous bioselection study was utilized as our historical control, this difference could have contributed to the differential larynx preservation outcomes seen between the studies. However, subset analysis of our population demonstrated no difference in LFS or OS between patients with T4 versus non-T4, or stage IVA versus non-IVA. Hence, we do not believe the differences in study populations significantly contributed to the findings of these study.
Our study has several limitations. Response assessment in the bioselection treatment paradigm was determined by the treating otolaryngologist via direct visualization (direct laryngoscopy or office flexible endoscopy). Assessment of IC response by direct visualization has been shown to be highly predictive of successful laryngeal preservation, which is a specialty skill that requires a trained provider. All the providers participating in this study had extensive training in tumor response assessment via laryngoscopy. Variability in assessment, however, can exist among the surgeons providing assessments. Moreover, UMCC 9520 and the current study did not include exactly the same group of providers. Additionally, traditional radiographic response criteria, as used in this study, failed to capture treatment efficacy in its entirety[18]. Thus, improved non-invasive biomarkers are needed to help predict responses to induction therapy, ultimately leading to improvements in rates of organ preservation and overall survival.
In conclusion, two cycles of TP IC plus a Bcl-2 inhibitor did not improve the rate of laryngeal preservation compared to a single cycle of PF. Although patients treated with bioselection in this trial had excellent cure rates, their rates of laryngectomy free survival were lower than in previously published cohorts. If bioselection with IC is undertaken, a single cycle of PF should be utilized. Further research is needed to identify methods to optimize organ preservation while decreasing treatment related toxicities, including the possibilities of identifying predictive biomarkers, the incorporation of combined chemotherapy and immunotherapy, and the use of adaptive radiotherapy.
Supplementary Material
Funding Statement:
This work was supported by the University of Michigan Head and Neck Specialized Program of Research Excellence NIH/NCI P50CA097248 and NIH/NIDCD T32 DC005356, as well as University of Michigan Cancer Center Core Grant NIH/NCI P3O CA046592
References
- [1].Cosetti M, Yu GP, Schantz SP. Five-year survival rates and time trends of laryngeal cancer in the US population. Arch Otolaryngol Head Neck Surg. 2008;134:370–9. [DOI] [PubMed] [Google Scholar]
- [2].Forastiere AA, Ismaila N, Lewin JS, Nathan CA, Adelstein DJ, Eisbruch A, et al. Use of Larynx-Preservation Strategies in the Treatment of Laryngeal Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2018;36:1143–69. [DOI] [PubMed] [Google Scholar]
- [3].Singer S, Danker H, Guntinas-Lichius O, Oeken J, Pabst F, Schock J, et al. Quality of life before and after total laryngectomy: results of a multicenter prospective cohort study. Head Neck. 2014;36:359–68. [DOI] [PubMed] [Google Scholar]
- [4].Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091–8. [DOI] [PubMed] [Google Scholar]
- [5].Department of Veterans Affairs Laryngeal Cancer Study G, Wolf GT, Fisher SG, Hong WK, Hillman R, Spaulding M, et al. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. N Engl J Med. 1991;324:1685–90. [DOI] [PubMed] [Google Scholar]
- [6].Meulemans J, Debacker J, Demarsin H, Vanclooster C, Neyt P, Mennes T, et al. Oncologic Outcomes After Salvage Laryngectomy for Squamous Cell Carcinoma of the Larynx and Hypopharynx: A Multicenter Retrospective Cohort Study. Ann Surg Oncol. 2021;28:1751–61. [DOI] [PubMed] [Google Scholar]
- [7].Schuman AD, Birkeland AC, Farlow JL, Lyden T, Blakely A, Spector ME, et al. Predictors of Stricture and Swallowing Function Following Salvage Laryngectomy. Laryngoscope. 2021;131:1229–34. [DOI] [PubMed] [Google Scholar]
- [8].Eisbruch A, Thornton AF, Urba S, Esclamado RM, Carroll WR, Bradford CR, et al. Chemotherapy followed by accelerated fractionated radiation for larynx preservation in patients with advanced laryngeal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1996;14:2322–30. [DOI] [PubMed] [Google Scholar]
- [9].Urba S, Wolf G, Eisbruch A, Worden F, Lee J, Bradford C, et al. Single-cycle induction chemotherapy selects patients with advanced laryngeal cancer for combined chemoradiation: a new treatment paradigm. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:593–8. [DOI] [PubMed] [Google Scholar]
- [10].Wolf GT, Bellile E, Eisbruch A, Urba S, Bradford CR, Peterson L, et al. Survival Rates Using Individualized Bioselection Treatment Methods in Patients With Advanced Laryngeal Cancer. JAMA Otolaryngol Head Neck Surg. 2017;143:355–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gibson MK, Li Y, Murphy B, Hussain MH, DeConti RC, Ensley J, et al. Randomized phase III evaluation of cisplatin plus fluorouracil versus cisplatin plus paclitaxel in advanced head and neck cancer (E1395): an intergroup trial of the Eastern Cooperative Oncology Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:3562–7. [DOI] [PubMed] [Google Scholar]
- [12].Bauer JA, Kumar B, Cordell KG, Prince ME, Tran HH, Wolf GT, et al. Targeting apoptosis to overcome cisplatin resistance: a translational study in head and neck cancer. Int J Radiat Oncol Biol Phys. 2007;69:S106–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kumar B, Cordell KG, D’Silva N, Prince ME, Adams ME, Fisher SG, et al. Expression of p53 and Bcl-xL as predictive markers for larynx preservation in advanced laryngeal cancer. Arch Otolaryngol Head Neck Surg. 2008;134:363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bauer JA, Trask DK, Kumar B, Los G, Castro J, Lee JS, et al. Reversal of cisplatin resistance with a BH3 mimetic, (−)-gossypol, in head and neck cancer cells: role of wild-type p53 and Bcl-xL. Mol Cancer Ther. 2005;4:1096–104. [DOI] [PubMed] [Google Scholar]
- [15].Sonpavde G, Matveev V, Burke JM, Caton JR, Fleming MT, Hutson TE, et al. Randomized phase II trial of docetaxel plus prednisone in combination with placebo or AT-101, an oral small molecule Bcl-2 family antagonist, as first-line therapy for metastatic castration-resistant prostate cancer. Annals of oncology : official journal of the European Society for Medical Oncology. 2012;23:1803–8. [DOI] [PubMed] [Google Scholar]
- [16].Stein MN, Goodin S, Gounder M, Gibbon D, Moss R, Portal D, et al. A phase I study of AT-101, a BH3 mimetic, in combination with paclitaxel and carboplatin in solid tumors. Investigational new drugs. 2020;38:855–65. [DOI] [PubMed] [Google Scholar]
- [17].Swiecicki PL, Bellile E, Sacco AG, Pearson AT, Taylor JM, Jackson TL, et al. A phase II trial of the BCL-2 homolog domain 3 mimetic AT-101 in combination with docetaxel for recurrent, locally advanced, or metastatic head and neck cancer. Investigational new drugs. 2016;34:481–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Woolen S, Virkud A, Hadjiiski L, Cha K, Chan HP, Swiecicki P, et al. Prediction of Disease Free Survival Tomography. 2021;7:10–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.