Abstract
The attributes of the PCR allowed implementation of an assay for specific detection of Piscirickettsia salmonis from a few microliters of fish serum. This opens the way to less invasive modes of sampling for this microbial pathogen in salmonids.
Piscirickettsia salmonis is a novel intracellular pathogen that has been identified as the causative agent of an aggressive infectious disease affecting salmonid mariculture in Chile (7). Although this disease has also been reported in Canada (6), Norway (17), and Ireland (18), its pathogenesis in fish farms in Chile seems to be more severe than its pathogenesis elsewhere. Moreover, rickettsialike pathogens may be involved in epizootics affecting other cultured fish species (8). Similar agents have been found in diverse fish species, including tilapias (Tilapia spp.) (2), blue-eyed plecostomus (Panaque suttoni) (10), and dragonet (Callionymus lyra) (4). The results of in vitro studies in which nonsalmonid cell lines were infected by P. salmonis (1) seem to support the idea that the potential for infectivity of the agent is wider than previously thought. The salmonid immune system does not appear to exhibit a strong humoral response against the pathogen (11), which may partially explain the virulence of P. salmonis and suggests that the outlook for a classical prophylactic approach to prevent outbreaks of the disease is poor. Unfortunately, at present the biology of P. salmonis is almost completely unknown, as are its reservoir (if any) and mode of transmission. The techniques available for detection of this pathogen involve either sacrificing diseased specimens for analysis or postmortem characterization of tissues. Thus, at present it is not possible to survey for the presence and prevalence of P. salmonis in susceptible populations, which might help researchers understand P. salmonis behavior and design strategies for disease prevention.
Salmonid rearing in Chile has been seriously threatened by the appearance of the fastidious intracellular gram-negative organism P. salmonis. One of the conspicuous features of this agent is that it expresses itself 4 to 6 weeks after the fish have been transferred to seawater, thus spoiling the long and costly process of rearing that begins with fertilized eggs. There are currently no procedures that alert workers to the presence of the disease other than the symptoms in its terminal stages. Therefore, a nonlethal and minimally invasive alternative for early detection of P. salmonis would be useful for prevention and prophylactic strategies. We developed a nonlethal screening procedure to detect this pathogen in minute samples of fish serum (volume, <5 μl) by using the PCR. Mauel and colleagues (14) have described a PCR-based assay that they used to study internal tissues from infected fishes. We extended the PCR-based approach to detect the presence of amplifiable P. salmonis DNA in fish that do not display signs of disease. Also, we were interested in using PCR primers flanking a region of the ribosomal operon that is more variable than the region exploited earlier (14).
Specimens and DNA extraction.
Tissues collected in HN buffer (20 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 500 mM NaCl, 5 mM EDTA, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride), as well as sera, were obtained in 1996 and 1997 from farmed coho salmon (Oncorhynchus kisutch) and rainbow trout (Oncorhynchus mykiss) from southern Chile (Chiloé Island in Region Ten) and were stored at −15°C. Tissue from noninfected rainbow trout was received from a freshwater farm in central Chile (located in Region Five) with no history of P. salmonis infestation. Tilapia (Oreochromis niloticus) DNA was obtained from fresh muscle tissue. Initial optimization of the PCR with nucleic acids from fish and from P. salmonis EM-90 (originally isolated from Atlantic salmon [Salmo salar]) grown in chinook salmon (Oncorhynchus tshawytscha) embryo cell line CHSE-214 (12) was performed with DNA purified by a slight modification of a previously described protocol (7). For larger surveys, amplifiable DNA was prepared by the Chelex procedure (20–22) by using <0.5 mg of tissue or 5 to 20 μl of serum. Each biological sample was immersed in 100 μl of Instagene (6% [wt/vol] Chelex 100; catalog no. 732-6030; Bio-Rad) in a 1.5-ml microcentrifuge tube (G tube, catalog no. 4030; BIO PLAS Inc.) that is resistant to lid opening by internal vapor pressure; the mixture was vortexed briefly at the maximum speed and then incubated at 100°C for 15 min. Once cooled, the tube was vortexed briefly and centrifuged for 1 min at the maximum speed (16,000 × g) with a model 5415C Eppendorf centrifuge to collect the Chelex beads at the bottom of the tube. The supernatant (30 μl) was transferred to another tube for permanent storage at −15°C. All extractions included at least two negative controls that did not receive a biological sample and were taken through the extraction procedure exactly like the samples. DNAs from previously isolated or bacterial type species were obtained by the Chelex procedure by using single colonies grown on suitable solid agar media.
Primer design, amplification, and sequencing.
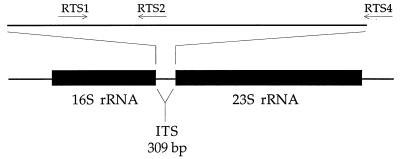
Primers for the PCR (Fig. 1) were designed based on alignments of sequences of the internal transcribed spacer (ITS) and the flanking 23S rRNA gene of the ribosomal operon. Sequences of P. salmonis LF-89 (= ATCC VR 1361) (type strain) (GenBank accession no. U36943), EM-90 (GenBank accession no. U36944), NOR-92 (GenBank accession no. U36946), ATL-4-91 (GenBank accession no. U36945), SLGO-94 (GenBank accession no. U62104), and C1-95 (GenBank accession no. U62103) and of Escherichia coli (GenBank accession no. AE000474) were aligned with the Sequencer 3.0 software (Gene Codes Corporation) and confirmed by visual examination. Primers RTS1 (5′-TGATTTTATTGTTTAGTGAGAATGA-3′; F-223), RTS2 (5′-AAATAACCCTAAATTAATCAAGGA-3′; R-266), and RTS4 (5′-ATGCACTTATTCACTTGATCATA-3′; R-459) (Fig. 1) were located at highly conserved sections of the P. salmonis ITS (the base at the 3′ end of each primer is indicated by F or R and the position in the LF-89 reference sequence; the direction of extension of each primer is indicated on the basis of whether extension produces the same strand as the LF-89 sequence [F, forward] or the other strand [R, reverse]). The expected lengths of amplification fragments obtained with the RTS1-RTS4 and RTS1-RTS2 primer pairs are 283 and 91 bp, respectively. The fragment flanked by primers RTS1 and RTS4 covers three-fourths of the P. salmonis ITS. Pairwise comparisons among the P. salmonis isolates listed above for this span of the ITS revealed levels of sequence difference ranging from 0 to 6.4% (including insertions and deletions), and this region therefore appears to be a promising region for monitoring genetic variation in P. salmonis. Amplification of nucleic acids of all bacterial species was possible with two universal eubacterial primers directed toward the 16S rRNA gene, with primers 358f and 517r of the E. coli 16S rRNA sequence as described by Murray et al. (15).
FIG. 1.
Map of the prokaryotic ribosomal operon and positions of primer pairs RTS1-RTS2 and RTS1-RTS4 for amplification by PCR of the P. salmonis ITS. The map is drawn to scale for the ITS section based on the P. salmonis LF-89 ITS sequence (the first 18 nucleotides at the 5′ end of primer RTS4 are located at the beginning of the 23S rRNA gene). The rRNA genes are drawn to a different scale, and the lengths are based on the lengths of these genes in the E. coli rRNA operon.
Each PCR was conducted in a total volume of 12.5 μl under a thin film of mineral oil by using GeneAmp 1× PCR buffer (Perkin-Elmer), which consists of 10 mM Tris-HCl (pH 8.3), 1.5 mM MgCl2, 50 mM KCl, and 0.001% (wt/vol) gelatin, plus each of the four deoxynucleoside triphosphates at a concentration of 0.2 mM, both oligonucleotide primers at a concentration of 0.5 μM, and 0.025 U of AmpliTaq DNA polymerase (Perkin-Elmer) per μl. Template DNA (5 to 25 ng) was delivered in a volume of 1 μl. A hot start (3) with a mixture containing both primers and the four deoxynucleoside triphosphates added after the other components of the reaction mixture reached 85°C was used to impede subsequent unwanted primer extension. An initial denaturation step (2 min at 94°C) preceded 36 to 39 cycles with the RTS1-RTS2 and RTS1-RTS4 primer pairs consisting of 94°C for 30 s, 50°C for 30 s, and 72°C for 30 s, with a final extension step at 72°C for 7 min. Products of the PCR were analyzed by electrophoresis in agarose gels (2.5% Seakem GTG; FMC) in 1× TBE buffer (19). The gels were stained with ethidium bromide (0.5 μg/ml), and the fluorescent bands visible with a UV transilluminator were photographed. Double-stranded PCR products were purified with carboxylated magnetic beads (5) prior to the cycle sequencing reaction, which was carried out with an ABI PRISM Ready Reaction dye terminator cycle sequencing kit by using one-half the volume recommended (AmpliTaq DNA Polymerase FS; protocol P/N 402078, revision A; Perkin-Elmer). Then for electrophoresis and sequence display we used an ABI PRISM model 377 DNA sequencer (Perkin-Elmer) with DNA Sequence Analysis software, version 2.1.2.
PCR detection of P. salmonis.
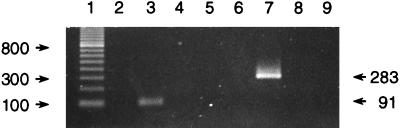
Amplification of DNA from P. salmonis EM-90 with the RTS1-RTS2 and RTS1-RTS4 primer pairs led to products of the expected lengths (Fig. 2, lanes 3 and 7, respectively), and no amplification was evident from equivalent amounts of templates from tilapia (Fig. 2, lanes 5 and 9) and a healthy trout (Fig. 2, lanes 4 and 8). Verification of the authenticity of the amplicon obtained with primers RTS1 and RTS4 came from the following two lines of evidence that were consistent with the previously reported sequence for P. salmonis EM-90: digestion of the product with restriction enzyme HinfI led to three fragments of the predicted lengths, and the sequence of the amplicon matched the EM-90 sequence at positions 225 to 428 (data not shown).
FIG. 2.
Specificity of the PCR for the P. salmonis ITS with primer pairs RTS1-RTS2 (lanes 2 through 5) and RTS1-RTS4 (lanes 6 through 9), as determined with DNA (5 ng) from P. salmonis EM-90 (lanes 3 and 7), rainbow trout (lanes 4 and 8), and tilapia (lanes 5 and 9). Amplicon sizes are indicated on the right. Lanes 2 and 6 contained extraction controls, while lane 1 contained a 100-bp ladder; the 800-bp fragment is highlighted, and sizes (in base pairs) are indicated on the left.
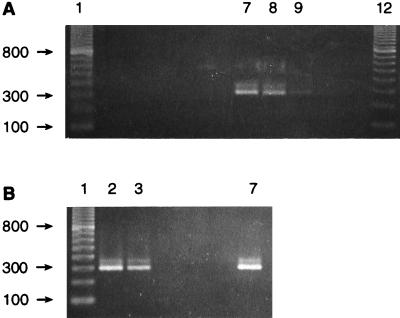
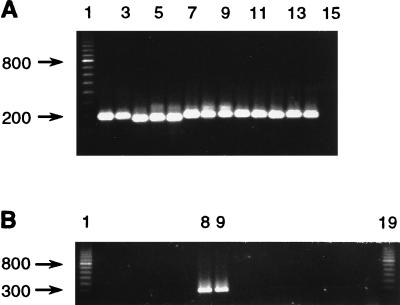
The capabilities of the PCR assay were examined more thoroughly by applying it to field-collected specimens. Animals displaying visible evidence of disease were PCR positive when a tissue sample (from trout R8) (Fig. 3A, lane 8) and serum samples (Fig. 3A, lane 9 and Fig. 3B, lane 7) were tested; however, there was disparity in the signal strengths of the two serum samples. The presence of P. salmonis was detected in tissue from an asymptomatic trout (individual C3) (Fig. 3B, lane 2) collected from a pen containing fish displaying clinical symptoms of disease (the same pen from which trout R8 was obtained), and, most rewardingly, serum from the same individual was PCR positive (Fig. 3B, lane 3). The sequences of the amplification products from fish R8 and C3 were identical to each other and to the LF-89 sequence at positions 224 to 458 except for an additional T in an otherwise successive sequence of four Ts in the previously reported sequence (positions 448 to 451). The results obtained with a dilution series of DNA obtained from fluids of a cell culture exhibiting full cytopathic effects following infection with EM-90 (16) suggest that the PCR assay used is capable of revealing the presence of 10 to 100 P. salmonis cells (data not shown). The culture fluid was treated with an excess of DNase I prior to extraction of the nucleic acid from the microorganism in order to eliminate remnants of the host genome that might contribute to the final DNA fraction.
FIG. 3.
PCR amplification with the RTS1-RTS4 primer pair and DNA. (A) Amplification of DNA from tilapia (5 ng) (lane 5), cell line CHSE-214 (25 ng) (lane 6), P. salmonis EM-90 propagated in CHSE-214 (5 ng) (lane 7), tissue from trout R8 with visible signs of piscirickettsiosis (lane 8), serum from trout R8 (lane 9), tissue from trout Rb1 with no signs of disease (probably not infected) (lane 10), and serum from trout Rb1 (lane 11). Lanes 1 and 12 contained a 100-bp ladder, and lanes 2 to 4 contained negative extraction controls. (B) Amplification of DNA from tissue from trout C3 with no signs of piscirickettsiosis (taken from a netpen containing individuals with the disease) (lane 2), serum from trout C3 (lane 3), tissue from coho salmon CM13 with no signs of disease (lane 4), serum from coho salmon CM13 (lane 5), serum from coho salmon CM6 with no signs of disease (lane 6), and serum from coho salmon CE1 with visible piscirickettsiosis (lane 7). Amplification reactions with coho salmon and trout tissues and sera were performed with 25 ng of total genomic DNA. Lane 1 contained a 100-bp ladder. Sizes (in base pairs) are indicated on the left. Lane numbers are given only for lanes in which the reactions produced the expected amplification product.
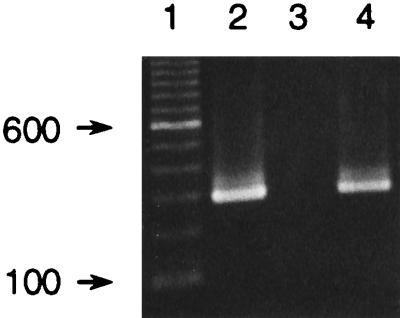
We were interested in developing a simpler extraction procedure, particularly in light of the earlier success in this regard with tissues (14, 16). Treatment of 20 μl of serum by the Chelex method (21, 22) yielded amplifiable template from infected individuals (Fig. 4, lanes 2 and 4). Further optimization revealed that 5 μl of serum was adequate for detection of the pathogen in infected animals and in asymptomatic animals (Table 1), and there was no increase in detection obtained by including protease K (22) during treatment with Chelex at 60°C (data not shown).
FIG. 4.
Amplification with the RTS1-RTS4 primer pair and DNA. Lane 2, DNA obtained by the Chelex method from serum from infected coho salmon CC3; lane 3, extraction control; lane 4, 10 times less DNA from infected coho salmon CC3 than was added to the PCR mixture loaded in lane 2. Lane 1 contained a 100-bp ladder, and the 600-bp fragment is highlighted. Sizes (in base pairs) are indicated on the left.
TABLE 1.
Screening of adult coho serum and tissue samples for P. salmonis by using PCR and DNA extracted by the Chelex method
| Fisha | Conditionb | PCR resultsc
|
|
|---|---|---|---|
| Tissue | Serum | ||
| VH15-1 | Sick | + | + |
| VH15-2 | Sick | + | + |
| VH15-3 | Sick | + | + |
| VH15-4 | Sick | + | + |
| VH15-5 | Sick | + | + |
| VH15-6 | Sick | + | + |
| VH15-7 | Healthy | + | + |
| VH15-8 | Healthy | +/− | − |
All fish were taken from the same pen during one collecting event.
Illness was evident from severe skin lesions, lethargy, and a propensity for hanging at the net sides (16).
PCR was conducted with primers RTS1 and RTS4. +, strong positive reaction; +/−, weak positive reaction; −, no synthesis of PCR product.
Primer specificity studies.
There is no basis to expect amplification of a fragment of the expected size from microorganisms other than P. salmonis at the annealing temperature employed in the PCR, as analysis of the available sequences from microbes belonging to the gamma subdivision of the proteobacteria (where P. salmonis is currently placed [7]) revealed no discernible sequence similarity with, for example, the site where primer RTS1 is located. Experimental confirmation of this was obtained by using representative gram-negative and gram-positive terrestrial microorganisms (E. coli [a member of the gamma subdivision of the proteobacteria] and Corynebacterium striatum) and aquatic microorganisms. Several of the aquatic microorganisms used were originally isolated from salmonids (Aeromonas salmonicida, Carnobacterium piscicola, Renibacterium salmoninarum, Vibrio anguillarum, and Yersinia ruckeri) and have been implicated in fish diseases. While amplification of all DNA templates with universal eubacterial primers 358f and 517r resulted in a fragment of the expected length (193 bp for E. coli; insertions and deletions are known for this section of the 16S rRNA gene) (Fig. 5A), only P. salmonis DNA was amplified with the RTS1-RTS4 primer pair (Fig. 5B). Discrimination of P. salmonis by the RTS1-RTS2 primer pair was also observed (results not shown).
FIG. 5.
Amplification of DNA obtained by the Chelex method. (A) Amplification with primers 358f and 517r. Lane 2, Aeromonas salmonicida ATCC 33658; lane 3, Aeromonas sp.; lane 4, Carnobacterium piscicola ATCC 35586; lane 5, Corynebacterium striatum; lane 6, Cytophaga sp. strain DSM 3660 (Deutsche Sammlung von Mikroorganismen und Zellkulturen); lane 7, E. coli; lane 8, P. salmonis LF-89; lane 9, P. salmonis EM-90; lane 10, Pseudoalteromonas antarctica CECT 4664 (Colección Española de Cultivos Tipos); lane 11, Pseudoalteromonas atlantica ATCC 19262; lane 12, Renibacterium salmoninarum ATCC 33209; lane 13, Vibrio anguillarum ATCC 43305; lane 14, Yersinia ruckeri ATCC 29473; lane 15, extraction control. Lane 1 contained a 100-bp ladder. (B) Amplification with primers RTS1 and RTS4. In lanes 1 to 15, the order of DNAs and the initial DNA concentration in each PCR are the same as in panel A. Lanes 16 through 18 contained additional negative controls, and lane 19 contained a 100-bp ladder.
PCR in aquaculture.
Efficient screening procedures for pathogens are valuable for ensuring good sanitation in aquaculture. Assays of serum samples with PCR without terminally compromising the fish open the way for more encompassing assessments of infections by P. salmonis in aquaculture settings.
The PCR assay described here is directed toward a region of the rRNA operon that is more variable than the region exploited by Mauel et al. (14), which promises finer discrimination in the description of new isolates of P. salmonis. In the present study the power of the PCR and the simplicity of direct fluorescent sequencing allowed us to document quickly the existence of what appeared to be a new strain of P. salmonis in rainbow trout in Chile. This recent isolate differs from LF-89 only by one T in a string of four Ts in the LF-89 sequence (positions 448 to 451; GenBank accession no. U36943). However, we also obtained the sequence of LF-89 by amplification and direct sequencing and found that it contains a sequence of five Ts at these positions, suggesting that recent isolates are identical to the type strain isolated in 1989 (7). The difference between our sequence and the previously reported sequence (GenBank accession no. U36943), although unexplained, does not deter us from speculating that strain LF-89 probably has been prevalent in the many cycles of infection that have occurred in the ensuing time in the salmon farms of Region Ten in southern Chile. Otherwise, the five Ts which we observed are shared with two previously described P. salmonis strains (C1-95 and SLGO-94). However, the isolate from trout described here differs at two positions (positions 297 and 427) and three positions (positions 297, 412, and 427) from C1-95 and SLGO-94, respectively.
One apparent failure of the assay emerged: a PCR-negative serum sample was obtained from an apparently healthy coho salmon, salmon VH15-8 (obtained from a pen containing diseased individuals), that was weakly positive when its tissue was examined (Table 1). This finding suggests that at very early stages of the disease during an evidently asymptomatic period, there may be a paucity of the pathogen in the blood, and the initial focus of infection may be the internal organs. A similar scenario is suggested by subsequent disease stages because of the distribution of signal between tissue and serum in trout R8 (Fig. 3A, lanes 8 and 9), although such a picture is in contrast to the similar signal strengths obtained with serum and tissue samples from an asymptomatic individual (Fig. 3B, lanes 2 and 3). Estimates of initial target sequences (in this case P. salmonis genomes) on the basis of the plateau of the PCR are qualitative at best (13). Therefore, sound conclusions concerning the relative burdens of the pathogen in different organs of fish will require a quantitative PCR approach (9).
We envision changing the current gel-based assay into a colorimetric format with microtiter plates for implementation of the assay at aquafarm sites as part of efforts to control the spread of the disease during artificial cultivation of salmonids. In this regard, the RTS1-RTS2 primer pair offers a very short amplicon (Fig. 2, lane 3), and increases in amplification efficiency are expected relative to generation of the threefold-longer fragment obtained with the RTS1-RTS4 primer pair. The colorimetric format coupled with miniaturization of the Chelex extraction procedure should encourage ambitious sampling with the following three objectives in mind: ascertaining the reliability of the PCR assay under field conditions; defining the progression of disease within an animal; and, in order to determine the path of transmission of this novel pathogen, pinpointing the presence of the P. salmonis genome both in the environment and in other organisms that coexist with salmonids in greater or lesser intimacy.
Acknowledgments
S.M. and V.H. were supported by Fomento al Desarrollo Científico y Tecnológico (FONDEF)/Comisión Nacional de Investigación Científica y Tecnológica (CONICYT) project 1038 and by the Dirección de Investigación y Postgrado, Universidad Católica de Valparaíso. S.H. and C.O. were supported by Minority Biomedical Research Support grant GM52588 and Minority International Research Training grant 1T37TW00078-01 from the National Institutes of Health to C.O. C.O. was also supported by National Science Foundation grant OCE-9315639 awarded to James T. Hollibaugh, San Francisco State University.
We are grateful to Enrique Madrid, Marine Harvest, Puerto Montt, Chile, for P. salmonis EM-90 from cell culture; Ana María Skármeta, Universidad Católica de Valparaíso, for salmonid samples; Gloria León, Universidad Austral de Chile, and Michael Mauel, University of Rhode Island, for DNAs from microorganisms other than P. salmonis; Randy Zebell and Sunny Pak, San Francisco State University, for assistance in sequencing and preparing figures; Ellen Prager, San Francisco State University, for superb editorial work; and two anonymous reviewers for helpful suggestions.
REFERENCES
- 1.Almendras F E, Jones S R M, Fuentealba C, Wright G M. In-vitro infection of a cell line from Ictalurus nebulosus with Piscirickettsia salmonis. Can J Vet Res. 1997;61:66–68. [PMC free article] [PubMed] [Google Scholar]
- 2.Chern R S, Chao C B. Outbreaks of a disease caused by a rickettsia-like organism in cultured tilapias in Taiwan. Fish Pathol. 1994;29:61–71. [Google Scholar]
- 3.D’Aquila R T, Bechtel L J, Viteler J A, Eron J J, Gorczyca P, Kaplin J C. Maximizing sensitivity and specificity of PCR by preamplification heating. Nucleic Acids Res. 1991;19:3749. doi: 10.1093/nar/19.13.3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis A J. A rickettsia-like organism from dragonets, Callionymus lyra L. (Teleostei:Callionymidae) in Wales. Bull Eur Assoc Fish Pathol. 1986;6:103–104. [Google Scholar]
- 5.DeAngelis M M, Wang D G, Hawkins T L. Solid-phase reversible immobilization for the isolation of PCR products. Nucleic Acids Res. 1995;23:4742–4743. doi: 10.1093/nar/23.22.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evelyn T P T. Salmonid rickettsial septicemia. In: Kent M L, editor. Diseases of seawater netpen-reared salmonid fishes in the Pacific Northwest. Canadian Special Publication of Fisheries and Aquatic Sciences no. 116. Nanaimo, Canada: Department of Fisheries and Oceans; 1992. pp. 18–19. [Google Scholar]
- 7.Fryer J L, Lannan C N, Giovannoni S J, Wood N D. Piscirickettsia salmonis gen. nov., sp. nov., the causative agent of an epizootic disease in salmonid fishes. Int J Syst Bacteriol. 1992;42:120–126. doi: 10.1099/00207713-42-1-120. [DOI] [PubMed] [Google Scholar]
- 8.Fryer J L, Mauel M J. The rickettsia: an emerging group of pathogens in fish. Emerg Infect Dis. 1997;3:137–144. doi: 10.3201/eid0302.970206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heid C A, Stevens J, Livak K J, Williams P M. Real time quantitative PCR. Genome Res. 1996;6:986–994. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- 10.Khoo L, Dennis P M, Lewbart G A. Rickettsia-like organisms in the blue-eyed plecostomus, Panaque suttoni (Eigenmann and Eigenmann) J Fish Dis. 1995;18:157–174. [Google Scholar]
- 11.Kuzyk M A, Thorton J C, Kay W W. Antigenic characterization of the salmonid pathogen Piscirickettsia salmonis. Infect Immun. 1996;64:5205–5210. doi: 10.1128/iai.64.12.5205-5210.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lannan C N, Winton J R, Fryer J L. Fish cell lines: establishment and characterization of nine cell lines from salmonids. In Vitro. 1984;20:671–676. doi: 10.1007/BF02618871. [DOI] [PubMed] [Google Scholar]
- 13.Livak K J. Quantitation of DNA/RNA using real-time PCR detection. Foster City, Calif: Perkin Elmer Applied Biosystems; 1996. [Google Scholar]
- 14.Mauel M J, Giovannoni S J, Fryer J L. Development of polymerase chain reaction assays for detection, identification, and differentiation of Piscirickettsia salmonis. Dis Aquat Org. 1996;26:189–195. [Google Scholar]
- 15.Murray A E, Hollibaugh J T, Orrego C. Phylogenetic compositions of bacterioplankton from two California estuaries compared by denaturing gradient gel electrophoresis of 16S rDNA fragments. Appl Environ Microbiol. 1996;62:2676–2680. doi: 10.1128/aem.62.7.2676-2680.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Office International des Epizooties. Diagnostic manual for aquatic animal diseases. Paris, France: Office International des Epizooties; 1995. pp. 1–5. [Google Scholar]
- 17.Olsen A B, Evensen O, Speilberg L, Melby H P, Hastein T. ‘Ny’ laksesykdom forärsaket av rickettsie. Nor Fiskeoppdrett. 1993;12:40–41. [Google Scholar]
- 18.Rodger H D, Drinan E M. Observation of a rickettsia-like organism in Atlantic salmon Salmo salar L. in Ireland. J Fish Dis. 1993;16:361–369. [Google Scholar]
- 19.Sambrook J, Fritsch E F, Maniatis J. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 20.Singer-Sam, J., R. L. Tanguay, and A. D. Riggs. 1989. Use of Chelex to improve the PCR signal from a small number of cells. Amplifications: A Forum for PCR Users, issue 3 (September):11.
- 21.Tan A-M, Orrego C. DNA stabilization and amplification from museum collections of extracts originally intended for allozyme analysis. Mol Ecol. 1992;1:195–197. doi: 10.1111/j.1365-294x.1992.tb00176.x. [DOI] [PubMed] [Google Scholar]
- 22.Walsh P S, Metzger D A, Higuchi R. Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. BioTechniques. 1991;10:506–513. [PubMed] [Google Scholar]