Abstract
Background
Invasive infections caused by Streptococcus pyogenes (invasive group A streptococcus [iGAS]) and Streptococcus pneumoniae (invasive pneumococcal disease [IPD]) decreased substantially at the beginning of the COVID-19 pandemic. Our study sought to evaluate the extent of this decrease and the trends of these infections since reversion of societal adjustments incident to the pandemic. We also wanted to compare the frequency of these infections with invasive community-onset Staphylococcus aureus (I-CO-SA) infections and common respiratory viral infections in this period.
Methods
Cases of iGAS, IPD, and I-CO-SA infections were identified prospectively and retrospectively at 2 large US children's hospitals by positive cultures from July 2018 through December 2022. Admission data were used to estimate frequency. For comparison, rates of respiratory syncytial virus (RSV), influenza, and SARS-CoV-2 were estimated by the number of positive viral test results at each institution.
Results
I-CO-SA infections showed little variation in the study period. Rates of iGAS infection and IPD decreased by 46% and 44%, respectively, from 2019 to 2020, coinciding with a substantial decrease in RSV and influenza. In 2022, RSV and influenza infection rates increased to prepandemic winter season rates, coinciding with a return to prepandemic rates of IPD (225% increase from 2021 to 2022) and a surge above prepandemic rates of iGAS infections (543% increase from 2021 to 2022).
Conclusions
The COVID-19 pandemic had an unexpected influence on IPD and iGAS infections that was temporally related to changes in rates of viral infections.
Invasive infections of Streptococcus pyogenes (invasive group A streptococcus [iGAS]) and Streptococcus pneumoniae (invasive pneumococcal disease [IPD]) decreased markedly at the start of the pandemic but have returned to prepandemic levels or higher.
WHAT'S KNOWN ON THIS SUBJECT
iGAS infections and IPD decreased significantly around March 2020, as did rates of common viral infections, especially respiratory syncytial virus and influenza. iGAS infections increased in 2 US states in late 2022.
WHAT THIS STUDY ADDS
Our study confirms a significant decrease in iGAS infections and IPD in 2020 and shows a return to prepandemic levels for IPD, while iGAS rates exceeded prepandemic levels in late 2022. Invasive community-onset Staphylococcus aureus infection rates remained stable.
The SARS-CoV-2 pandemic has affected the seasonality of other respiratory viruses [1]. This has been most apparent in the changes in incidence of influenza and respiratory syncytial virus (RSV). Certain invasive bacterial infections have been identified as being strongly associated with respiratory viral infections, such as invasive pneumococcal disease (IPD) and invasive group A streptococcus (iGAS) [2]. In a previous single-center study, we reported the influence of the SARS-CoV-2 pandemic on the incidence of invasive infections caused by the 3 most common gram-positive organisms in pediatric infections: Staphylococcus aureus, Streptococcus pneumoniae, and Streptococcus pyogenes [3]. In 2020 rates of iGAS and IPD decreased significantly as compared with 2017 to 2019, while rates of invasive community-onset S aureus (I-CO-SA) remained unchanged. This is thought to be related to the implementation of nonpharmaceutical interventions (NPIs), such as masking, social distancing, and enhanced hand hygiene, at the start of the COVID-19 pandemic. In late 2022, several European public health authorities reported increased iGAS infections in children [4, 5]. In the United States, Barnes et al [6] reported a significant decrease in pediatric iGAS infections during the COVID-19 pandemic, followed by an increase in rates above prepandemic rates in late 2022. Similarly, an increased incidence of IPD among children in England occurred during July through December 2021 after COVID-19 restrictions were relaxed [7]. This period is of particular interest due to the decrease in NPIs worldwide and the unpredictable seasonality of respiratory viruses during this time. We sought to update our previous work to provide more expanded and current data on these important infections. By including data from an additional institution, we sought to make our findings more generalizable.
METHODS
Cases were identified at Texas Children's Hospital, Houston, and St Louis Children's Hospital, Missouri. At Texas Children's Hospital, cases were identified through prospective active surveillance across all its campuses. All isolates of group A streptococcus (GAS), S pneumoniae, and S aureus identified by the clinical microbiology laboratory were captured by this surveillance [3]. Cases from St Louis Children's Hospital were identified retrospectively with generated reports within the electronic medical record, which were confirmed against previously collected prospective surveillance [8]. We examined culture-positive community-onset invasive infections from 1 July 2018 to 31 December 2022. Cases identified only by polymerase chain reaction (PCR) were excluded due to variation in available PCR testing between institutions. Invasive infections were regarded as those in which the organisms of interest were isolated from a normally sterile site [9]. Health care–associated cases were excluded (ie, those that had a clear underlying medical predisposition, such as medical hardware infection or significant immune compromise). The annual number of hospital admissions at each institution was obtained through institutional administrative data. The number of non-neonatal pediatric admissions (patients <18 years old admitted from the community) was used as the denominator in calculations of frequency.
The primary comparison of interest was the total number of cases of iGAS, IPD, and I-CO-SA from July 2018 through December 2022 as well as the rate ratio of infection from year to year from 2019 to 2022. To adjust for changes in health care access during the early period of the pandemic, rates of cases per 10 000 hospital admissions per year were calculated and used as a surrogate for annual incidence. Rate ratio from year to year was calculated with corresponding 95% CI and P value. Data analysis was performed with Stata version 15 (StataCorp LLC). Since data from both institutions for the full year of 2018 were not available, this year was excluded from our analysis of annual rates but included in our monthly data. Cases of RSV, influenza, and SARS-CoV-2 detection were also collected from each institution's prospective active viral testing to include the number of positive test results per month for each virus. The institutional review boards of both sites approved this study.
RESULTS
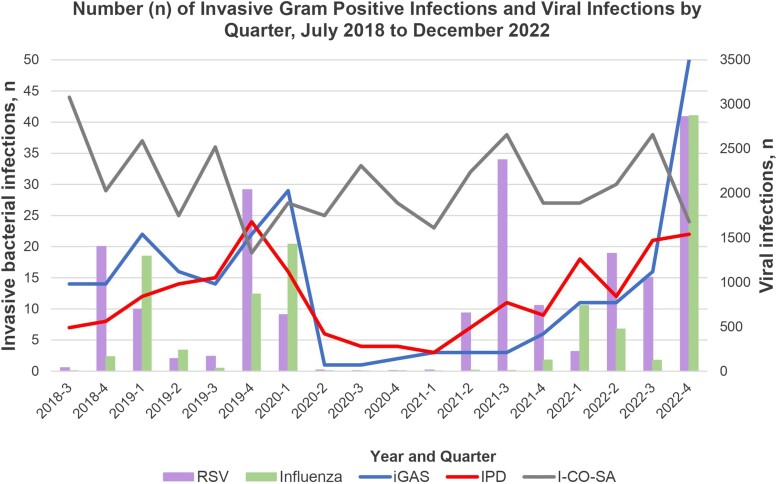
Demographics for patients with bacterial infections are shown in Table 1. The quarterly number of iGAS, IPD, and I-CO-SA cases and the number of positive influenza and RSV test results are presented in Figure 1 with monthly data presented in Supplementary Figure 1A–F. iGAS and IPD cases, as well as RSV and influenza infections, decreased dramatically in 2020 as compared with 2018 to 2019. In 2021, RSV had a summer surge that was associated with a gradual increase in IPD, but iGAS remained low. In 2022, RSV and influenza had a typical seasonal spike that was associated with increased rates of IPD and especially iGAS. In contrast, I-CO-SA cases did not change over these same periods.
Table 1.
Demographic Data by Organism and Year by Number of Patients
| Year, No. (%) | ||||
|---|---|---|---|---|
| 2019 | 2020 | 2021 | 2022 | |
| Group A Streptococcus | ||||
| Gender | ||||
| Male | 40 (54) | 19 (58) | 4 (27) | 48 (55) |
| Female | 34 (46) | 14 (42) | 11 (73) | 40 (45) |
| Ethnicity | ||||
| Hispanic | 35 (47) | 12 (36) | 4 (27) | 32 (36) |
| Non-Hispanic Black | 16 (22) | 7 (21) | 3 (20) | 13 (15) |
| Non-Hispanic White | 18 (24) | 11 (33) | 7 (47) | 32 (36) |
| Other | 3 (4) | 2 (6) | 1 (7) | 8 (9) |
| Declined/unknown | 2 (3) | 1 (3) | 0 (0) | 3 (3) |
| Age, y | ||||
| <1 | 6 (8) | 3 (9) | 1 (7) | 6 (7) |
| 1–5 | 24 (32) | 13 (39) | 3 (20) | 44 (50) |
| 6–12 | 37 (50) | 13 (39) | 5 (33) | 26 (30) |
| 13–17 | 7 (9) | 4 (12) | 6 (40) | 12 (14) |
| Streptococcus pneumoniae | ||||
| Gender | ||||
| Male | 38 (58) | 19 (63) | 15 (50) | 41 (56) |
| Female | 27 (42) | 11 (37) | 15 (50) | 32 (44) |
| Ethnicity | ||||
| Hispanic | 19 (29) | 11 (37) | 5 (17) | 29 (40) |
| Non-Hispanic Black | 14 (22) | 6 (20) | 10 (33) | 16 (22) |
| Non-Hispanic White | 22 (34) | 10 (33) | 13 (43) | 20 (27) |
| Other | 6 (9) | 1 (3) | 2 (7) | 6 (8) |
| Declined/unknown | 4 (6) | 2 (7) | 0 (0) | 2 (3) |
| Age, y | ||||
| <1 | 17 (26) | 7 (23) | 1 (3) | 9 (12) |
| 1–5 | 34 (52) | 15 (50) | 23 (77) | 45 (62) |
| 6–12 | 12 (18) | 4 (13) | 4 (13) | 16 (22) |
| 13–17 | 2 (3) | 4 (13) | 2 (7) | 3 (4) |
| Staphylococcus aureus | ||||
| Gender | ||||
| Male | 83 (71) | 59 (53) | 73 (61) | 68 (57) |
| Female | 34 (29) | 53 (47) | 47 (39) | 51 (43) |
| Ethnicity | ||||
| Hispanic | 29 (25) | 29 (26) | 30 (25) | 30 (25) |
| Non-Hispanic Black | 26 (22) | 18 (16) | 30 (25) | 25 (21) |
| Non-Hispanic White | 50 (43) | 58 (52) | 51 (43) | 50 (42) |
| Other | 4 (3) | 4 (4) | 4 (3) | 8 (7) |
| Declined/unknown | 8 (7) | 3 (3) | 5 (4) | 6 (5) |
| Age, y | ||||
| <1 | 16 (14) | 10 (9) | 21 (18) | 16 (13) |
| 1–5 | 31 (26) | 26 (23) | 31 (26) | 39 (33) |
| 6–12 | 51 (44) | 49 (44) | 47 (39) | 41 (34) |
| 13–17 | 19 (16) | 27 (24) | 21 (18) | 23 (19) |
Figure 1.
Number of iGAS (blue line), IPD (red line), and I-CO-SA (gray line) and positive tests for RSV (purple bar) and influenza (green bar) at 2 children's hospitals per quarter, July 2018–December 2022. I-CO-SA, invasive community-onset Staphylococcus aureus; iGAS, invasive group A streptococcus; IPD, invasive pneumococcal disease.
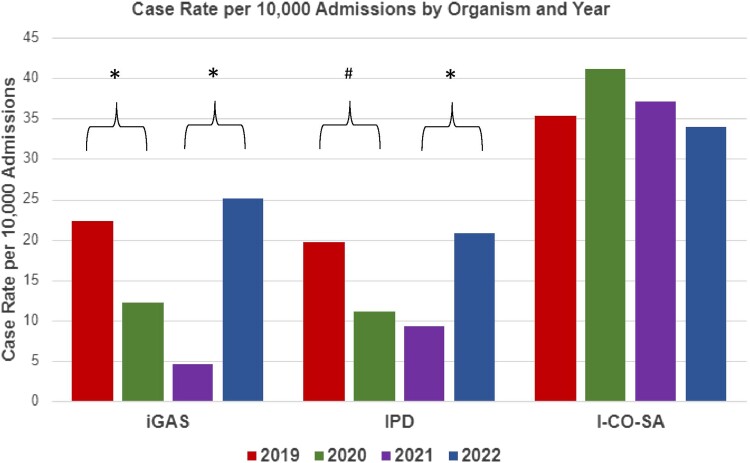
A comparison of case rates per 10 000 admissions per year for each organism is presented in Figure 2. Annual cases per 10 000 admissions of iGAS decreased from 22.4 in 2019 to 12.1 in 2020 and 4.6 in 2021 and then increased to 25.1 in 2022 (P < .001), with the largest increase at the end of 2022. A similar trend was noted in IPD, with annual cases per 10 000 admissions decreasing from 19.6 in 2019 to 11.0 in 2020 (P = .04) and 9.3 in 2021 (P > .05) and increasing to 20.8 in 2022 (P < .001). In contrast, the number of I-CO-SA cases per 10 000 admissions did not change significantly over the years 2019 to 2022 (P = .93). The rate of cases per 10 000 admissions did not differ significantly between the institutions for any of the organisms evaluated (P > .1 for all 3 organisms).
Figure 2.
The number of cases per 10 000 admissions for iGAS, IPD, and I-CO-SA from 2019 to 2022 at 2 children's hospitals. Combined annual hospital admissions were 33 102 in 2019, 27 200 in 2020, 32 337 in 2021, and 35 023 in 2022. *P < .001. #P = .01. I-CO-SA, invasive community-onset Staphylococcus aureus; iGAS, invasive group A streptococcus; IPD, invasive pneumococcal disease.
As compared with 2019, the rate ratios of iGAS and IPD were significantly decreased in 2020 and especially 2021, with a rebound to prepandemic levels by the end of 2022 (Table 2). This trend was not seen in I-CO-SA infections, which remained consistent throughout this period. Rates of methicillin-resistant S aureus were stable during the study period, accounting for 22% to 27% of the annual total I-CO-SA infections.
Table 2.
Rate Ratios of Invasive Infection Between Years by Organism
| Rate Ratio (95% CI) | ||||
|---|---|---|---|---|
| 2020 vs 2019 | 2021 vs 2020 | 2022 vs 2021 | 2022 vs 2019 | |
| Group A Streptococcus | 0.54 (.34–.83)a | 0.38 (.19–.72)a | 5.43 (3.12–10.11)a | 1.12 (.81–1.55) |
| Streptococcus pneumoniae | 0.56 (.35–.88)b | 0.84 (.48–1.4) | 2.25 (1.45–3.56)a | 1.06 (.75–1.51) |
| Staphylococcus aureus | 1.16 (.89–1.52) | 0.90 (.69–1.18) | 0.91 (.71–1.19) | 0.96 (.74–1.25) |
a P < .001.
b P = 0.04.
The number of each invasive bacterial infection across age groups did not vary over time. iGAS and I-CO-SA infections were most common in ages 1 to 12 years, with similar rates in the 1- to 5-year and 6- to 12-year groups. IPD occurred most frequently in the 1- to 5-year age group (Supplementary Figure 2A–C).
The proportion of patients with a given infectious etiology (eg, bloodstream infection, pneumonia, osteomyelitis) within each invasive bacterial infection group also showed no clear trend over time in the study period. The most common manifestation of iGAS infection (n = 238) was deep abscesses (n = 101, 42.4%), followed by bacteremia without a focus (n = 39, 16.4%). IPD (n = 213) was most likely to manifest as bacteremia without a focus (n = 79, 37.1%), complicated pneumonia (n = 58, 27.2%), or meningitis/brain abscess (n = 36, 16.9%). I-CO-SA infections (n = 541) were predominantly bone/joint infections (n = 299, 55.2%), with bacteremia (n = 103, 19.0%) being the next most common (Supplementary Figure 3A–C).
DISCUSSION
Our study shows that iGAS and IPD had a striking decrease in incidence in children starting in March 2020, followed by resurgence in the latter part of 2021 and especially in 2022 with a large spike in the fourth quarter. The most probable explanation for the initial decrease is the widespread implementation of NPIs. A decrease of viral infections including RSV and influenza occurred around March 2020 as well, with RSV demonstrating an atypical surge in the summer of 2021 and influenza returning early in 2022. IPD incidence started to increase also in the summer of 2021 after RSV cases increased, while iGAS did not start to increase again until early 2022 and most impressively at the end of 2022, when levels of RSV and influenza were very high as compared with before the pandemic. Our data do not show a temporal association between surges of SARS-CoV-2 and any of the studied bacterial infections.
Danino et al [10] evaluated rates of pneumococcal infections vs rates of intranasal carriage of pneumococcus from 2020 to 2021. They reported that while there was a significant decrease in pneumococcal infections, rates of intranasal carriage among children in Israel did not decrease significantly in the study period. The decreases in IPD were strongly associated with the virtual elimination of RSV, influenza, and human metapneumovirus. Rybak et al [11] reported similar findings in France between January 2007 and March 2021. It is unknown whether iGAS shares this same interesting pattern during this time. IPD rates found in our study were consistent with a report from the United Kingdom by Bertran et al [7], comparing 2017–2019 with the second half of 2021, as well as a report from Quebec, Canada, by Ouldali et al [12], which focused on the increase in IPD through January 2022. Our data expand on this, showing now a return of IPD to prepandemic levels.
Rates of iGAS identified in our study are consistent with recent studies from the Netherlands and France comparing iGAS rates from 2021 and 2022 with periods prior to 2020, which show a similar trend of increases in empyema and Streptococcal toxic shock syndrome/necrotizing fasciitis diagnoses in iGAS infections [13, 14]. The US Centers for Disease Control and Prevention also recently reported data from its Emerging Infections Program in Colorado and Minnesota, showing a sizable increase in iGAS in the October–December 2022 quarter (34 cases) as compared with the average in the same period from 2016 to 2019 (11 cases) and 2020 to 2021 (4 cases) [6]. Our data support this finding for iGAS, with the number of cases in the fourth quarter of 2022 being over twice as high as the same quarter in 2018 or 2019 (n = 52 in 2022, n = 22 in 2019, and n = 14 in 2018). These trends in iGAS infection seem to correlate with GAS pharyngitis rates in this period, as 2 sites in the United States reported decreases in GAS pharyngitis in 2020 [15, 16]; a report from the United Kingdom evaluating invasive and noninvasive GAS detections also demonstrated a similar trend for both [17]. However, a report from Spain showed similar trends in GAS pharyngitis and iGAS but without a surge above prepandemic levels [18]; thus, additional studies are needed to evaluate if there is a differential effect in this period between invasive and noninvasive GAS infections.
The incidence of I-CO-SA infections in children in our regions has not been influenced by the NPIs implemented for the COVID-19 pandemic or the changes in RSV or influenza patterns. Our results are consistent with rates of invasive S aureus reported in Kunming, China, from 2019 to 2022, as well as those previously reported by one of our institutions showing no significant variability in I-CO-SA rates before, during, and now after institution of NPIs during the COVID-19 pandemic [3, 19]. Of interest, overall S aureus nasal and/or axillary colonization remained relatively constant among 168 children enrolled in a Houston study during November 2019 to February 2020 (pre–COVID-19) and as reassessed every 3 months for a 12-month period through March 2021 (ongoing COVID-19) [20]. Prior to the COVID-19 pandemic, Choe et al [21] reported a coseasonality between RSV, coronaviruses (non–SARS-CoV), and influenza viruses and all-cause bacteremia (including S aureus) in children at Hasbro Children's Hospital, Providence, Rhode Island. They also showed that coinfection of viruses in patients with bacteremia was more than twice as likely to occur with influenza viruses or human metapneumoviruses than with RSV, although S aureus was not specifically analyzed.
It is uncertain why invasive S aureus infections did not exhibit the same temporal fluctuations seen with iGAS and IPD at least to some degree, as they are all gram-positive nasal colonizers with invasive potential. We suspect that this is likely related to ≥1 of the unique characteristics of S aureus vs S pneumoniae and GAS, such as skin colonization, different virulence factors, and different predominant sites of infection. Specifically, while all 3 organisms show associations with viral infections, for S aureus this association is primarily with pneumonia, which was a very small proportion of the total number of S aureus infections regardless of year in our study.
This study is limited by multiple factors. While the use of 2 large pediatric centers that demonstrated consistent trends adds validity to the study, the findings may not be broadly generalizable. Using total admissions as the denominator in calculations of frequency does not reflect incidence in the population but does help to control for the decreased seeking of medical care seen during periods of extensive social distancing in the early period of the pandemic [22]. Additionally, the infections in question are typically severe, so it is unlikely that a significant number of cases were missed due to hesitance to present to care; if anything, this would result in overestimating incidence during years with lower hospital admission rates. Culture data are more specific than insurance or billing data for identifying cases, but they may miss some cases that are not viable for culture; furthermore, we did not include cases diagnosed solely by PCR, which may result in some sampling bias, but this cannot be fully eliminated. Additional consideration could be given to more aspects of these infections (demographics, serotypes, vaccination rates, prescribing practices, etc), but these aspects were beyond the scope of this study. Finally, our data demonstrate temporal associations, and causality cannot be inferred.
CONCLUSION
This study aimed to evaluate unexpected changes in trends of invasive gram-positive infections seen following the start of the COVID-19 pandemic in 2020. All 3 organisms are common colonizers of the upper respiratory tract with the capacity for invasive disease. Despite their similarities, our data show a significant difference in the change in rates of iGAS and IPD as compared with I-CO-SA in the first 2 years of the pandemic. It also suggests a surge toward the end of 2022 of iGAS and IPD not seen in I-CO-SA. The exact implications and clinical impact of this differential trend are unknown and will require more consideration and investigation.
Supplementary Material
Contributor Information
Eric E Engstrom, Department of Pediatrics, Division of Infectious Diseases, Baylor College of Medicine and Texas Children's Hospital, Houston, Texas, USA.
Alexander S Plattner, Department of Pediatrics, Division of Infectious Diseases, Washington University in St Louis and St Louis Children's Hospital, St Louis, Missouri, USA.
J Chase McNeil, Department of Pediatrics, Division of Infectious Diseases, Baylor College of Medicine and Texas Children's Hospital, Houston, Texas, USA.
Kristina G Hulten, Department of Pediatrics, Division of Infectious Diseases, Baylor College of Medicine and Texas Children's Hospital, Houston, Texas, USA.
Patrick J Reich, Department of Pediatrics, Division of Infectious Diseases, Washington University in St Louis and St Louis Children's Hospital, St Louis, Missouri, USA.
Mary G Boyle, Department of Pediatrics, Division of Infectious Diseases, Washington University in St Louis and St Louis Children's Hospital, St Louis, Missouri, USA.
James J Dunn, Department of Pediatrics, Division of Infectious Diseases, Baylor College of Medicine and Texas Children's Hospital, Houston, Texas, USA.
Stephanie A Fritz, Department of Pediatrics, Division of Infectious Diseases, Washington University in St Louis and St Louis Children's Hospital, St Louis, Missouri, USA.
Sheldon L Kaplan, Department of Pediatrics, Division of Infectious Diseases, Baylor College of Medicine and Texas Children's Hospital, Houston, Texas, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. E. E. E. conceptualized and designed the study, coordinated data collection, carried out the initial analyses, drafted the initial manuscript, and critically reviewed and revised the manuscript. S. L. K. conceptualized and designed the study and critically reviewed and revised the manuscript. J. C. M. collected data, carried out subsequent analyses of the data, and critically reviewed and revised the manuscript. K. G. H., J. J. D., P. J. R., A. S. P., S. A. F., and M. G. B. collected data and critically reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Patient consent statement. Patient consent was waived as the deidentified data posed no more than minimal risk to the subjects of the study. The institutional review boards of both sites approved this study.
References
- 1.National Respiratory and Enteric Virus Surveillance System, Centers for Disease Control and Prevention. RSV national trends. 2020. Available at: https://www.cdc.gov/surveillance/nrevss/rsv/natl-trend.html. Accessed January 9, 2023.
- 2. Klein EY, Monteforte B, Gupta A, et al. The frequency of influenza and bacterial coinfection: a systematic review and meta-analysis. Influenza Other Respir Viruses 2016; 10:394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McNeil JC, Flores AR, Kaplan SL, Hulten KG. The indirect impact of the SARS-CoV-2 pandemic on invasive group A Streptococcus, Streptococcus pneumoniae and Staphylococcus aureus infections in Houston area children. Pediatr Infect Dis J 2021; 40:e313–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization . Disease outbreak news: increased incidence of scarlet fever and invasive group A Streptococcus infection—multi-country. 15 December 2022. Available at: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON429
- 5.European Centre for Disease Prevention and Control. Increase in invasive group A streptococcal infections among children in Europe, including fatalities. Published 12 December 2022. Available at: https://www.ecdc.europa.eu/en/news-events/increase-invasive-group-streptococcal-infections-among-children-europe-including. Accessed 12 January 2023.
- 6. Barnes M, Youngkin E, Zipprich J, et al. Increase in invasive group A strep infections—Colorado and Minnesota, October-December 2022. MMWR 2023; 72:265–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bertran M, Amin-Chowdhury Z, Sheppard CL, et al. Increased incidence of invasive pneumococcal disease among children after COVID-19 pandemic, England. Emerg Infect Dis 2022; 28:1669–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McNeil JC, Sommer LM, Boyle M, et al. Cefazolin inoculum effect and methicillin-susceptible Staphylococcus aureus osteoarticular infections in children. Antimicrob Agents Chemother 2020; 64:e00703-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miller KM, Lamagni T, Cherian T, et al. Standardization of epidemiological surveillance of invasive group A streptococcal infections. Open Forum Infect Dis 2022; 9(suppl 1):S31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Danino D, Ben-Shimol S, van der Beek BA, et al. Decline in pneumococcal disease in young children during the coronavirus disease 2019 (COVID-19) pandemic in Israel associated with suppression of seasonal respiratory viruses, despite persistent pneumococcal carriage: a prospective cohort study. Clin Infect Dis 2022; 75:e1154–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rybak A, Levy C, Angoulvant F, et al. Association of nonpharmaceutical interventions during the COVID-19 pandemic with invasive pneumococcal disease, pneumococcal carriage, and respiratory viral infections among children in France. JAMA Netw Open 2022; 5:e2218959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ouldali N, Deceuninck G, Lefebvre B, et al. Increase of invasive pneumococcal disease in children temporally associated with RSV outbreak in Quebec: a time-series analysis. Lancet Reg Health Am 2023; 19:100448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Kempen EB, Bruijning-Verhagen PCJ, Borensztajn D, et al. Increase in invasive group A streptococcal infections in children in the Netherlands: a survey among 7 hospitals in 2022. Pediatr Infect Dis J 2023; 42:e122–4. [DOI] [PubMed] [Google Scholar]
- 14. Lassoued Y, Assad Z, Ouldali N, et al. Unexpected increase in invasive group A streptococcal infections in children after respiratory viruses outbreak in France: a 15-year time-series analysis. Open Forum Infect Dis 2023; 10:ofad188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McBride JA, Eickhoff J, Wald ER. Impact of COVID-19 quarantine and school cancelation on other common infectious diseases. Pediatr Infect Dis J 2020; 39:e449–52. [DOI] [PubMed] [Google Scholar]
- 16. Boyanton BL, Snowden JN, Frenner RA, Rosenbaum ER, Young HL, Kennedy JL. SARS-CoV-2 infection mitigation strategies concomitantly reduce group A Streptococcus pharyngitis. Clin Pediatr (Phila) 2023; 62:683–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alcolea-Medina A, Snell LB, Alder C, et al. The ongoing Streptococcus pyogenes (group A Streptococcus) outbreak in London, United Kingdom, in December 2022: a molecular epidemiology study. Clin Microbiol Infect 2023; 29:887–90. [DOI] [PubMed] [Google Scholar]
- 18. de Ceano-Vivas M, Molina Gutiérrez MÁ, Mellado-Sola I, et al. Streptococcus pyogenes infections in Spanish children before and after the COVID pandemic: coming back to the previous incidence. Enferm Infecc Microbiol Clin (Engl Ed). Published online 30 June 2023. doi: 10.1016/j.eimce.2023.04.021 [DOI] [PubMed] [Google Scholar]
- 19. Ma M, Tao L, Li X, et al. Changes in molecular characteristics and antimicrobial resistance of invasive Staphylococcus aureus infection strains isolated from children in Kunming, China during the COVID-19 epidemic. Front Microbiol 2022; 13:944078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McNeil JC, Joseph M, Sommer LM, Flores AR. Staphylococcus aureus colonization in healthy children during the first year of the severe acute respiratory syndrome coronavirus 2 pandemic. J Pediatr 2022; 249:101–5.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Choe YJ, Park S, Michelow IC. Co-seasonality and co-detection of respiratory viruses and bacteraemia in children: a retrospective analysis. Clin Microbiol Infect 2020; 26:1690.e5–.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moynihan R, Sanders S, Michaleff ZA, et al. Impact of COVID-19 pandemic on utilisation of healthcare services: a systematic review. BMJ Open 2021; 11:e045343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.