Abstract
Purpose:
This study aims to evaluate the efficacy of preemptive embolization (PE) of the lumbar arteries (LAs) and inferior mesenteric artery (IMA) (PELI) for preventing abdominal aortic aneurysm (AAA) enlargement associated with type 2 endoleak (T2EL).
Material and Methods:
Patients who underwent endovascular aneurysm repair (EVAR) between January 2015 and December 2020 were classified into the control (without PE), IMA (PE of a patent IMA with a diameter ≥2.5 mm), and PELI (PE of patent LAs with a diameter ≥2 mm and IMA) groups. The rate of freedom from AAA enlargement following EVAR (enlargement ≥5 mm from pre-EVAR) was compared using the log-rank test. The prevalence of T2EL at 6 months and 1 year after EVAR was compared using Fisher's exact test.
Results:
The cumulative rates of freedom from AAA enlargement at 54 months after EVAR (maximum observational period in the PELI group) were as follows: control group, 77.5%; IMA group, 62.5%; and PELI group, 100%. The mean CT follow-up periods of the control, IMA, and PELI groups were 46.4 ± 22.3, 31.1 ± 20.6, and 22.9 ± 15.5 months, respectively. None of the 31 patients in the PELI group experienced AAA enlargement after EVAR, whereas 2 out of the 16 patients in the IMA group and 20 out of the 98 patients in the control group had AAA enlargement. No significant differences were observed in the rate of freedom from AAA enlargement (PELI group vs. IMA group, P = 0.11; PELI group vs. control group, P = 0.11). The prevalence of T2EL was significantly lower in the PELI group than in the control group at 6 months (13.6% in PELI group vs. 42.1% in control group, P = 0.02) and 1 year (14.3% in PELI group vs. 40.0% in control group, P = 0.04).
Conclusions:
PELI was significantly associated with a low prevalence of T2EL and may prevent T2EL-associated AAA enlargement.
Keywords: endoleak; endovascular procedures; aortic aneurysm, abdominal
Introduction
Endovascular aneurysm repair (EVAR) has become the standard treatment for abdominal aortic aneurysm (AAA). Endoleak is a common complication after EVAR, with type 2 endoleak (T2EL) comprising the majority of the endoleak (28.1%-32.7%) [1, 2]. Persistent T2EL is associated with late adverse events, including AAA enlargement and rupture and AAA-related mortality after EVAR [3]. The Society for Vascular Surgery suggests that secondary intervention for T2EL should be considered on the basis of the size and expansion (>5 mm) of the aneurysm [4]. Nonetheless, the T2EL rate remains at 71%-87.5% after the secondary intervention [1, 5], and there is little evidence to support the efficacy of the secondary intervention [6]. Therefore, preemptive embolization (PE) of the aortic branches has been suggested. The efficacy of inferior mesenteric artery (IMA) embolization has been described in a meta-analysis and prospective randomized controlled trial [7, 8], and the prevalence of both T2EL and aneurysm sac growth was significantly reduced by IMA embolization. Lumbar arteries (LAs) are another common source of T2EL [9, 10]. PE of both LAs and IMA (PELI) might be more effective than IMA embolization alone to prevent T2EL-related complications. A previous study reported the efficacy of PELI in reducing both the prevalence of T2EL and aneurysm diameter at a particular time [11]. However, few previous studies have compared the two embolization methods. Moreover, to the best of our knowledge, time-to-event analyses about the occurrence of AAA enlargement, which are considered suitable for assessing the effectiveness of PELI, have not been conducted. Therefore, this study aimed to evaluate the efficacy of PELI in preventing AAA enlargement associated with T2EL using time-to-event analyses.
Material and Methods
This retrospective cohort study was approved by our institutional review board (Approval number: 1459) and was conducted at a single center. The requirement for written informed consent was waived.
Patients' eligibility criteria
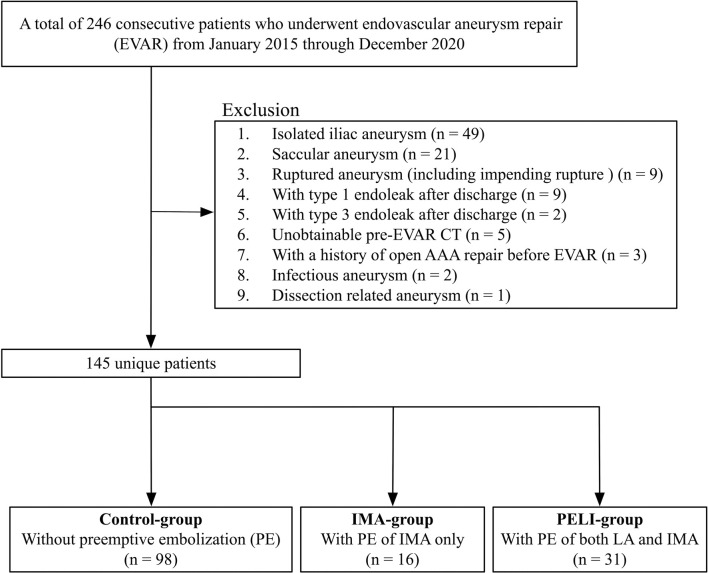
Fig. 1 shows a flowchart of the patients' selection process and classification. A total of 246 consecutive patients who underwent EVAR at our institution between January 2015 and December 2020 were included in this study. The exclusion criteria were as follows: (1) isolated iliac aneurysm, (2) saccular aneurysm, (3) ruptured AAA (including impending rupture), (4) type 1 endoleak and (5) type 3 endoleaks detected in any follow-up period, (6) unobtainable pre-EVAR computed tomography (CT), (7) prior history of open AAA repair before EVAR, (8) infectious or inflammatory AAA, and (9) dissection-related AAA. The included patients were classified into three groups based on the types of PE as follows: (1) control (without PE), (2) IMA (PE of a patent IMA with a diameter ≥2.5 mm), and (3) PELI (PE of patent LAs with a diameter ≥2 mm and a patent IMA with a diameter ≥2.5 mm) groups.
Figure 1.
Patients’ flowchart.
AAA, abdominal aortic aneurysm; CT, computed tomography; EVAR, endovascular aneurysm repair; IMA, inferior mesenteric artery; LA, lumbar artery; PE, preemptive embolization; PELI, preemptive embolization of both LAs and IMA
CT protocols
All CT angiography scans were performed using 64- or 320-detector row CT scanners (LightSpeed PRO 16, GE Healthcare or Aquilion One, Canon Medical Systems) at a 2-mm slice thickness. The CT protocols involved a triple-phase technique that included the unenhanced, arterial, and delayed phases. Contrast materials containing 600 mg iodine/kg total body weight were injected at a flow rate of 3 mL/s. Arterial phase images were obtained using a bolus-tracking program with an attenuation threshold of 250 Hounsfield units in the aorta. Delayed phase images were obtained 90 s after the arterial phase.
Interventional procedures
PE of the side branches during EVAR was started in August 2016 at our institution. Initially, we embolized only a patent IMA with a diameter ≥2.5 mm, referring to previous studies showing that a patent IMA with a diameter of 2-3 mm is a risk factor of T2EL [9, 12-15]. Since March 2017, we have expanded the indications and embolized patent LAs with a diameter ≥2 mm [9, 10]. Accessory renal arteries from the aortic neck or aneurysm sac were embolized since August 2016. The median sacral artery was embolized on the basis of the same criteria as those of LA embolization. PE was performed by board-certified interventional radiologists before the deployment of stent grafts. A 4- or 5-Fr shepherd-hook angiographic catheter (Medikit, Tokyo, Japan; or Cook, Bloomington, IN, USA) was placed in the ostium of the IMA/LA. The following catheters were also used when necessary: 4.5-Fr guiding sheath (Parent Plus 45; Medikit), 5-Fr J-shaped sheath (Medikit), or a 4- or 5-Fr cobra angiographic catheter (Medikit). A microcatheter (Estream 2 marks; Toray, Tokyo, Japan) was inserted through a 0.016-inch microguidewire (Meister; Asahi Intecc, Nagoya, Japan), and embolization was performed using coils (Tornado; Cook or Interlock-Fibered IDC Occlusion System; Boston Scientific, Marlborough, MA, USA) until blood stagnation from microcatheter injection. After PE, standard aortic stent grafts were implanted using the conventional method, at the operator's discretion. All procedures were supervised by an interventional radiologist and performed with surgical cutdown of the femoral arteries under general anesthesia.
Follow-up and endpoints
Follow-up CT angiography was commonly scheduled before discharge, at 6 months and 1 year after EVAR, and every 6 months thereafter. Unenhanced CT with duplex ultrasonography examinations was alternatively used for patients with an estimated glomerular filtration rate <30 mL/min/1.73 m2. The primary endpoint was the rate of freedom from AAA enlargement following EVAR, which was defined as a growth of ≥5 mm in the maximal diameter of the AAA from pre-EVAR. The secondary endpoint was the prevalence of T2EL at 6 months and 1 year after EVAR.
Data collection
Two interventional radiologists retrospectively reviewed all EVAR-related images and developed the endpoint data in consensus. The AAA diameter was measured on axial CT images and was assessed from pre-EVAR to the latest CT. T2EL was diagnosed on CT angiography or duplex ultrasonography when persistent aneurysm sac filling through the side branches was confirmed, without signs of any other type of endoleak. Moreover, we collected the following data as supplemental information: radiation exposure dose, volume of the contrast material, procedure time, and the frequency of contrast-induced acute kidney injury (a 25% relative increase or a 0.5-mg/dL absolute increase in serum creatinine within 72 h of contrast exposure) [16].
Statistical analysis
Time-to-event analysis of the occurrence of AAA enlargement was performed using the Kaplan-Meier method and compared between each group using the log-rank test. Fisher's exact test was used to compare the prevalence of T2EL and contrast-induced acute kidney injury. One-way analysis of variance was used for other comparisons. The significance level was set at alpha = 0.05. Python programming language (version 3.8.5; https://www.python.org/), rpy2 package (version 3.4.5; https://rpy2.github.io/), and lifelines package (version 0.26.0; https://lifelines.readthedocs.io/en/latest/) were used for statistical analysis.
Results
Two hundred and forty-six patients met the inclusion criteria, and 101 of them were excluded. Finally, 98, 16, and 31 patients were classified into the control, IMA, and PELI groups, respectively (Fig. 1). Patient characteristics are shown in Table 1. Table 2 lists the details of the PE of IMA and LA. The embolization success rate of IMA was 87.5% (14/16) and 94.4% (17/18) in the IMA and PELI groups, respectively. The embolization success rate of LA was 95.3% (82/86) in the PELI group. The number of patients who underwent accessary renal artery embolization was 1 (6.3%) and 4 (12.9%) in the IMA and PELI groups, respectively. Three patients (9.7%) underwent median sacral artery embolization in the PELI group. The mean number of embolized arteries in the PELI group was 3.4 ± 2.1. The mean number of patent LAs after EVAR was as follows: control group, 4.7 ± 1.5; IMA group, 4.6 ± 1.7 months; and PELI group, 2.3 ± 1.3 months (P < 0.01). There were no PE-related complications.
Table 1.
Patients’ Characteristics at the Time of EVAR.
| Control group
(n = 98) |
IMA group
(n = 16) |
PELI group
(n = 31) |
P-value | |
|---|---|---|---|---|
| Age (years) * | 77.2 ± 7.1 | 76.1 ± 8.0 | 78.0 ± 6.2 | 0.66 |
| Sex | 0.78 | |||
| Male | 83 (84.7%) | 14 (87.5%) | 25 (80.6%) | |
| Female | 15 (15.3%) | 2 (12.5%) | 6 (19.4%) | |
| AAA diameter (mm) * | 54.7 ± 10.7 | 53.1 ± 8.8 | 53.3 ± 9.8 | 0.71 |
| Stent graft | 0.36 | |||
| Excluder | 63 (64.3%) | 10 (62.5%) | 16 (51.6%) | |
| Endurant | 26 (26.5%) | 3 (18.8%) | 12 (38.7%) | |
| AFX | 5 (5.1%) | 2 (12.5%) | 3 (9.7%) | |
| Zenith | 4 (4.1%) | 1 (6.2%) | 0 (0.0%) | |
| The number of anatomical factors outside the instructions for use | 0.40 | |||
| 0 | 54 (55.1%) | 8 (50.0%) | 12 (38.7%) | |
| 1 | 31 (31.6%) | 6 (37.5%) | 16 (51.6%) | |
| 2 | 13 (13.3%) | 2 (12.5%) | 3 (9.7%) | |
| Anatomical factors outside the instructions for use | ||||
| Neck angle | 28 (28.6%) | 3 (18.8%) | 12 (38.7%) | 0.37 |
| Neck length | 2 (2.0%) | 0 (0.0%) | 1 (3.2%) | 0.69 |
| Neck diameter | 10 (10.2%) | 1 (6.2%) | 0 (0.0%) | 0.16 |
| Neck calcification | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1.00 |
| Neck thrombus | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1.00 |
| Reverse taper neck | 0 (0.0%) | 0 (0.0%) | 1 (3.2%) | 0.32 |
| Iliac artery diameter | 1 (1.0%) | 0 (0.0%) | 0 (0.0%) | 1.00 |
| Bilateral iliac artery occlusion | 16 (16.3%) | 6 (37.5%) | 8 (25.8%) | 0.10 |
| Chronic kidney disease** | 43 (43.9%) | 9 (56.2%) | 14 (45.2%) | 0.66 |
| Smoking history | 54 (65.9%) | 14 (87.5%) | 22 (71.0%) | 0.22 |
| Hypertension | 22 (22.4%) | 6 (37.5%) | 16 (51.6%) | 0.01 |
| Dyslipidemia | 10 (10.2%) | 1 (6.2%) | 3 (9.7%) | 1 |
| Diabetes mellitus | 12 (12.2%) | 2 (12.5%) | 3 (9.7%) | 1 |
| Cerebrovascular disease | 10 (10.2%) | 4 (25.0%) | 1 (3.2%) | 0.07 |
| Coronary arterial disease | 18 (18.4%) | 2 (12.5%) | 9 (29.0%) | 0.33 |
Data are shown as n (%) unless indicated otherwise. P values were calculated with Fisher’s exact test.
*Data are mean ± standard deviation. P values were calculated with the one-way analysis of variance.
**Chronic kidney disease is defined as an estimated glomerular filtration rate <60 mL/min/1.73 m2.
AAA, abdominal aortic aneurysm; Control group, patients without PE; EVAR, endovascular aneurysm repair; IMA, inferior mesenteric artery; IMA group, patients who had PE of the IMA only; PE, preemptive embolization; PELI, preemptive embolization of both lumbar arteries and IMA; PELI group, patients who had PE of both lumbar arteries and IMA
Table 2.
Details of the Preemptive Embolization of Side Branches.
| IMA group
(n = 16) |
PELI group
(n = 31) |
|||
|---|---|---|---|---|
| Side branch | IMA | LAs | IMA | LAs |
| Chronic occlusion rate* | 0.0% (0/16) | 21.9% (21/96) | 16.1% (5/31) | 15.6% (29/186) |
| Embolization success rate** | 87.5% (14/16) | (–) | 94.4% (17/18) | 95.3% (82/86) |
Data are shown as mean ± standard deviation unless indicated otherwise.
*In parentheses, the numerator and denominator represent the number of occluded vessels and the total number of side branches, respectively. The total number of LAs is six times the number of patients in the PELI group (bilateral second to fourth LAs).
**In parentheses, the numerator and denominator represent the numbers of embolized vessels and vessels in which embolization was attempted, respectively.
IMA, inferior mesenteric artery; LAs, lumbar arteries; PELI, preemptive embolization of both LAs and IMA
Rate of freedom from AAA enlargement following EVAR
Fig. 2 shows the Kaplan-Meier curves of the rate of freedom from AAA enlargement following EVAR. The mean CT follow-up periods were as follows: control group, 46.4 ± 22.3 months; IMA group, 31.1 ± 20.6 months; and PELI group, 22.9 ± 15.5 months. None of the 31 patients in the PELI group experienced AAA enlargement of ≥5 mm after EVAR, whereas 2 out of the 16 patients in the IMA group and 20 of the 98 patients in the control group had AAA enlargement. No significant difference was found in the rate of freedom from AAA enlargement between the groups. The cumulative rates of freedom from AAA enlargement at 54 months after EVAR (maximum observational period in the PELI group) were as follows: control group, 77.5% (95% confidence interval [CI], 65.6%-85.8%); IMA group, 62.5% (95% CI, 14.2%-89.3%); and PELI group, 100% (95% CI, 100%-100%).
Figure 2.
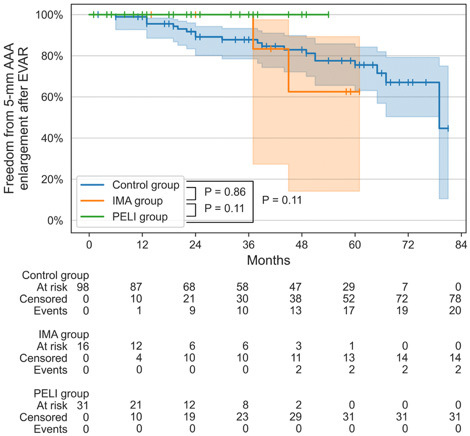
Kaplan–Meier analysis of freedom from 5-mm AAA enlargement after EVAR.
The plot shows the rate of freedom from 5-mm AAA enlargement after EVAR. The shaded areas indicate the 95% confidence interval, and the vertical lines represent censoring. The data at the bottom show the cumulative number of patients at risk, those who were censored, and those with stent occlusion in each group. P-values were calculated using the log-rank test.
AAA, abdominal aortic aneurysm; EVAR, endovascular aneurysm repair; IMA, inferior mesenteric artery; PE, preemptive embolization; PELI, preemptive embolization of both LAs and IMA
Prevalence of T2EL
The prevalence of T2EL at 6 months and 1 year after EVAR was as follows: control group, 42.1% (32/76) and 40.0% (30/75); IMA group, 33.3% (4/12) and 27.3% (3/11); and PELI group, 13.6% (3/22) and 14.3% (3/21), respectively. Duplex ultrasonography was alternatively used to evaluate T2EL in 9, 1, and 3 patients in the control, IMA, and PELI groups, respectively. The prevalence of T2EL was significantly lower in the PELI group than in the control group at 6 months (P = 0.02; odds ratio, 0.22; 95% CI, 0.04-0.84) and 1 year (P = 0.04; odds ratio, 0.26; 95% CI, 0.04-0.99) (Table 3).
Table 3.
Prevalence of T2EL at 6 Months and 1 Year after EVAR.
| PELI group vs. control group | PELI group vs. IMA group | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Control group
(n = 98) |
IMA group
(n = 16) |
PELI group
(n = 31) |
P-value | Odds ratio
(95% CI) |
P-value | Odds ratio
(95% CI) |
|||
| 6 months later | 42.1% (32/76) | 33.3% (4/12) | 13.6% (3/22) | .02 | 0.22 (0.04–0.84) | .21 | 0.33 (0.04–2.43) | ||
| 1 year later | 40.0% (30/75) | 27.3% (3/11) | 14.3% (3/21) | .04 | 0.25 (0.04–0.98) | .39 | 0.46 (0.05–4.18) | ||
Data show the prevalence of T2EL. In parentheses, the numerator and denominator represent the numbers of examinations with T2EL and times that computed tomography angiography or duplex ultrasonography was performed, respectively.
CI, confidence interval; EVAR, endovascular aneurysm repair; IMA, inferior mesenteric artery; PELI, preemptive embolization of both lumbar arteries and IMA; T2EL, type 2 endoleak
Supplemental information in the interventional procedures
Fig. 3 shows the box plots of the radiation exposure dose, volume of the contrast material, and procedure time. All median values were highest in the PELI group, followed by the IMA and control groups (P < 0.01). The frequency of contrast-induced acute kidney injury was as follows: control group, 3.1% (3/98); IMA group, 6.3% (1/16); and PELI group, 6.5% (2/31). No significant difference was seen in the frequency of contrast-induced acute kidney injury (P = 0.52).
Figure 3.
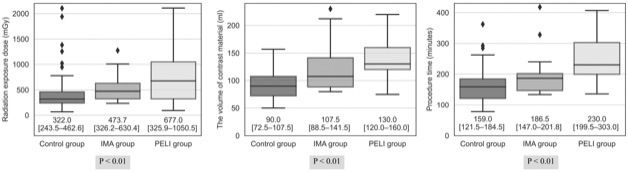
Box plots of the radiation exposure dose, volume of the contrast material, and procedure time in interventional procedures.
The boxes represent the inner quartiles, and the horizontal lines within the box indicate the median. The diamonds represent outliers (below the first quartile − 1.5 interquartile range, or above the third quartile + 1.5 interquartile range). P-values were calculated using one-way analysis of variance. The numbers under each box plot indicate the median and interquartile range (in square brackets).
IMA, inferior mesenteric artery; PELI, preemptive embolization of both lumbar arteries and IMA
Discussion
In this study, we investigated the efficacy of PELI in preventing T2EL-associated AAA enlargement. The time-to-event analysis showed no significant difference in the rate of freedom from AAA enlargement. None of the 31 patients who underwent PELI experienced AAA enlargement ≥5 mm after EVAR during 22.9 ± 15.5 months of CT follow-up. The prevalence of T2EL was the lowest in the patients who underwent PELI and was significantly lower than that in patients who did not undergo PE. There were no PE-related complications.
PE of the side branches has been shown to be a safe and effective method to reduce T2EL-related complications [17]. Embolization strategies varied, but they were divided into two types: PE of IMA alone or of both LAs and IMA. Several studies have indicated that IMA-only embolization is effective in reducing the prevalence of T2EL and AAA sizes [7, 8, 13]. However, another study reported that this significant difference disappeared after 6 months, and LA patency was a significant risk factor for persistent T2EL [18]. PELI might be more efficient than IMA-only embolization in preventing T2EL-related complications. A previous study reported the efficacy of PELI, with AAA sac shrinkage in 86.7% of the patients [11]. To the best of our knowledge, time-to-event analyses of AAA enlargement after PELI have not been reported. AAA enlargement is a representative late adverse event after EVAR and is an indicator for additional treatment considerations. Therefore, we aimed to evaluate the efficacy of PELI in preventing T2EL-associated AAA enlargement.
The time-to-event analysis did not show any significant difference between the groups. However, none of the 31 patients with PELI experienced an AAA enlargement of ≥5 mm after EVAR. These findings are similar to those of previous reports [19], which indicated that none of the 37 patients who underwent PE of the side branches with diameters >2.5 mm experienced AAA enlargement during 30.1 ± 5.3 months of follow-up. In contrast, 8 out of the 38 patients without PE experienced AAA enlargement ≥5 mm during 30.2 ± 5.7 months of follow-up. Briefly, none of the patients with PELI experienced AAA enlargement ≥5 mm in either our study or a previous study [19]. This fact may reflect the efficacy of PELI in preventing T2EL-associated AAA enlargement, although our study might lack the statistical power of time-to-event analysis. (We could not calculate the statistical power because no previous results of hazard ratios in PELI regarding aneurysm diameter were obtainable.) Additional large-scale studies are necessary to confirm the efficacy of PELI in preventing T2EL-associated AAA enlargement.
In this study, T2EL was most infrequent in the PELI group, followed by the IMA and control groups. A previous meta-analysis showed that patients with PELI had a significantly lower T2EL rate than those who did not undergo embolization (odds ratio, 0.21; 95% CI, 0.06-0.77; P = 0.02) [17]. Another meta-analysis revealed that patients with PELI had a lower prevalence of T2EL than those who only had embolization of the IMA (9.8% vs. 21.3%) [20]. As expected, the higher number of embolized side branches, the lower the T2EL rates. However, our study showed no significant difference in T2EL rates between the PELI and IMA groups, which might be due to the small number of patients, especially in the IMA group. The prevalence of T2EL in the PELI group (13.6%-14.3%) was similar to that in previous results [20]. Unembolized small side branches might be involved in the residual T2EL, but massive T2EL leading to aneurysmal sac growth may be controlled by PELI.
Our embolization success rate was high, especially in LA embolization, compared with that reported in a meta-analysis (LA, 95.3% vs. 69.1%; IMA, 91.1% vs. 82.3%, respectively) [18]. One of the reasons for failed embolization was the instability of the microcatheter. The proximal LA is frequently more tortuous than IMA, and without stable catheter cannulation, a microcatheter can easily fall into the aorta while advancing the microguidewire. To cannulate a catheter as stably as possible, we used not only multiple catheters with different widths but also a curved sheath (J- or cobra-shaped) when necessary to bring the catheter tip closer to the AAA wall. These techniques may explain the high success rate of LA embolization.
Evidence on the benefit of PELI for patients is lacking. Routine PELI could be feasible with a high technical success rate, but it was accompanied by increased radiation exposure dose, volume of the contrast material, and procedure time. We assumed that the radiation exposure dose remained within the acceptable range, even in the PELI group, and no significant difference was observed in the frequency of contrast-induced acute kidney injury. Nonetheless, the indications for PELI need to be optimized.
Our study had several limitations. First, because of the retrospective study design, the patients and procedures were heterogeneous and selection bias may be present. Second, the sample size was small. Subsequent studies with larger sample sizes are warranted to validate the efficacy of PELI and the incidence of contrast-induced acute kidney injury. Third, the follow-up period may not be long enough, especially in the PELI group. Fourth, the analyses were based on manually developed data, which may have added some variability. Finally, a subgroup analysis according to the type of stent graft was not conducted because of the small sample size.
In summary, the time-to-event analysis showed no significant difference in the rate of freedom from AAA enlargement following EVAR between the groups. However, none of the patients with PELI experienced AAA enlargement ≥5 mm during follow-up. PELI may have the potential to prevent T2EL-associated AAA enlargement.
Conflict of Interest
None
Funding
The authors received no financial support for preparing this article for publication.
Author Contribution
HN established the methods and wrote the draft SI and TT helped the study's design; ST was an advisor of the project. All the authors performed the procedures and approved the final version of the manuscript.
IRB
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The institutional review board of our hospital (Tenri Hospital) approved this study (Approval number: 1491).
Informed Consent
With approvals from the institutional review board, the informed consent requirement was waived because of the retrospective nature of the study.
Acknowledgement
We thank Editage (www.editage.com) for English language editing.
References
- 1.Fujimura N, Obara H, Matsubara K, et al. Characteristics and risk factors for type 2 endoleak in an East Asian population from a Japanese multicenter database. Circ J. 2016; 80: 118-123. doi: 10.1253/circj.CJ-15-0850 [DOI] [PubMed] [Google Scholar]
- 2.Morisaki K, Yamaoka T, Iwasa K, Ohmine T, Guntani A. Preoperative risk factors for aneurysm sac expansion caused by type 2 endoleak after endovascular aneurysm repair. Vascular. 2017; 25: 533-541. doi: 10.1177/1708538117702787 [DOI] [PubMed] [Google Scholar]
- 3.Seike Y, Matsuda H, Shimizu H, et al. Nationwide analysis of persistent type II Endoleak and late outcomes of endovascular abdominal aortic aneurysm repair in Japan: A propensity-matched analysis. Circulation. 2022; 145: 1056-1066. doi: 10.1161/CIRCULATIONAHA.121.056581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaikof EL, Dalman RL, Eskandari MK, et al. The society for vascular surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018; 67: 2-77.e2. doi: 10.1016/j.jvs.2017.10.044 [DOI] [PubMed] [Google Scholar]
- 5.Horinouchi H, Okada T, Yamaguchi M, et al. Mid-term outcomes and predictors of transarterial embolization for type II endoleak after endovascular abdominal aortic aneurysm repair. Cardiovasc Intervent Radiol. 2020; 43: 696-705. doi: 10.1007/s00270-020-02436-2 [DOI] [PubMed] [Google Scholar]
- 6.Ultee KHJ, Büttner S, Huurman R, et al. Editor's choice-systematic review and meta-analysis of the outcome of treatment for type II endoleak following endovascular aneurysm repair. Eur J Vasc Endovasc Surg. Elsevier BV; 2018; 56: 794-807. [DOI] [PubMed] [Google Scholar]
- 7.Biancari F, Mäkelä J, Juvonen T, Venermo M. Is inferior mesenteric artery embolization indicated prior to endovascular repair of abdominal aortic aneurysm? Eur J Vasc Endovasc Surg. 2015; 50: 671-674. doi: 10.1016/j.ejvs.2015.06.116 [DOI] [PubMed] [Google Scholar]
- 8.Samura M, Morikage N, Otsuka R, et al. Endovascular aneurysm repair with inferior mesenteric artery embolization for preventing type II endoleak: A prospective randomized controlled trial. Ann Surg. 2020; 271: 238-244. doi: 10.1097/SLA.0000000000003299 [DOI] [PubMed] [Google Scholar]
- 9.Aoki A, Maruta K, Hosaka N, Omoto T, Masuda T, Gokan T. Evaluation and coil embolization of the aortic side branches for prevention of type II endoleak after endovascular repair of abdominal aortic aneurysm. Ann Vasc Dis. The Editorial Committee of Annals of Vascular Diseases; 2017; 10: 351-358. doi: 10.3400/avd.oa.17-00088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samura M, Morikage N, Mizoguchi T, et al. Identification of anatomical risk factors for type II endoleak to guide selective inferior mesenteric artery embolization. Ann Vasc Surg. 2018; 48: 166-173. doi: 10.1016/j.avsg.2017.10.016 [DOI] [PubMed] [Google Scholar]
- 11.Branzan D, Geisler A, Steiner S, et al. Type II endoleak and aortic aneurysm sac shrinkage after preemptive embolization of aneurysm sac side branches. J Vasc Surg. 2021; 73: 1973-1979.e1. doi: 10.1016/j.jvs.2020.11.032 [DOI] [PubMed] [Google Scholar]
- 12.Fukuda T, Matsuda H, Sanda Y, et al. CT findings of risk factors for persistent type II endoleak from inferior mesenteric artery to determine indicators of preoperative IMA embolization. Ann Vasc Dis. 2014; 7: 274-279. doi: 10.3400/avd.oa.14-00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Otsu M, Ishizaka T, Watanabe M, et al. Analysis of anatomical risk factors for persistent type II endoleaks following endovascular abdominal aortic aneurysm repair using CT angiography. Surg Today. 2016; 46: 48-55. doi: 10.1007/s00595-015-1115-5 [DOI] [PubMed] [Google Scholar]
- 14.Liu Z, Chen Y, Qin Y, et al. A nomogram risk assessment model to predict the possibility of type II endoleak-related re-intervention after endovascular aneurysm repair (EVAR). Sci Rep. 2023; 13: 14. doi: 10.1038/s41598-022-27356-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huh U, Lee CW, Chung SW, et al. Risk factors of secondary intervention for type II endoleaks in endovascular aneurysm repair: An 8-year single institution study. Asian J Surg. 2019; 42: 106-111. doi: 10.1016/j.asjsur.2017.10.003 [DOI] [PubMed] [Google Scholar]
- 16.Rundback JH, Nahl D, Yoo V. Contrast-induced nephropathy. J Vasc Surg. 2011; 54: 575-579. doi: 10.1016/j.jvs.2011.04.047 [DOI] [PubMed] [Google Scholar]
- 17.Li Q, Hou P. Sac Embolization and side branch embolization for preventing type II endoleaks after endovascular aneurysm repair: A meta-analysis. J Endovasc Ther. 2020; 27: 109-116. doi: 10.1177/1526602819878411 [DOI] [PubMed] [Google Scholar]
- 18.Chew DK, Dong S, Schroeder AC, Hsu HW, Franko J. The role of the inferior mesenteric artery in predicting secondary intervention for type II endoleak following endovascular aneurysm repair. J Vasc Surg. 2019; 70: 1463-1468. doi: 10.1016/j.jvs.2019.01.090 [DOI] [PubMed] [Google Scholar]
- 19.Burbelko M, Kalinowski M, Heverhagen JT, et al. Prevention of type II endoleak using the AMPLATZER vascular plug Vndovascular aneurysm repair. Eur J Vasc Endovasc Surg. 2014; 47: 28-36. doi: 10.1016/j.ejvs.2013.10.003 [DOI] [PubMed] [Google Scholar]
- 20.Yu HYH, Lindström D, Wanhainen A, Tegler G, Hassan B, Mani K. Systematic review and meta-analysis of prophylactic aortic side branch embolization to prevent type II endoleaks. J Vasc Surg. 2020; 72: 1783-1792.e1. doi: 10.1016/j.jvs.2020.05.020 [DOI] [PubMed] [Google Scholar]