Abstract
Osteoblastic bone reaction, the occurrence of new osteoblastic lesions, is a paradoxical phenomenon during the treatment of cancers and can be defined as disease progression or bone metastases. Osteoblastic bone reactions usually occur in patients who receive treatments such as chemotherapy or hormonal or targeted therapy; however, it is difficult to differentiate them from disease progression or an increase in osteoblastic activity in response to therapy. Although osteoblastic bone reaction in lung cancer has been described in a few reports, it has never been reported in patients with KRASG12V-mutant lung adenocarcinoma treated with immunotherapy and antiangiogenesis. Here, we describe a case of a 77-year-old male with KRASG12V-mutant lung adenocarcinoma whose osteoblastic bone response was found during treatment with sintilimab and bevacizumab. We showed the course of the disease as well as systematic imaging manifestations of lung cancer with osteoblastic bone reaction and discussed their mechanisms.
Keywords: Osteoblastic bone reaction, KRASG12V mutation, Lung adenocarcinoma
Introduction
Bone metastases, especially spinal metastasis, are common in lung cancer, whose metastatic lesions are usually osteolytic with poor prognosis [1]. Therefore, the lesions are usually osteolytic and hardly ever osteoblastic in radiological imaging or magnetic resonance imaging (MRI) scans [2]. Furthermore, osteoblastic metastases have mostly been reported in small-cell or adenocarcinoma of the lung [3, 4]. Unlike some tumors have spontaneous calcification, an osteoblastic reaction/response is a phenomenon in which new osteoblastic lesions appear that are not visible at baseline and are detected radiographically after treatment, such as targeted therapy and chemotherapy [5, 6]. This osteoblastic bone reaction has been described in reports of lung cancer; however, most of them were found in previously undetectable and undocumented bone metastases [7]. Compared with previous findings of osteoblastic bone reaction, we also found an osteoblastic bone reaction in a patient with lung adenocarcinoma treated with sintilimab and bevacizumab, which indicates responses to the therapy rather than disease progression. Therefore, we showed a positive therapeutic outcome of KRASG12V-mutant lung adenocarcinoma treated with sintilimab and bevacizumab, and we would like to share this with investigators in this field.
Case Report
Investigations
We report a 77-year-old smoking Asian male who presented with stage IVB (T2aN2M1c) adenocarcinoma of the right upper lobe with multiple bone, liver, and pulmonary metastases. In June 2022, he was admitted to our orthopedic department due to progressive back pain. Computed tomography (CT) confirmed multiple osteolytic bone metastases in the lumbar vertebrae and liver and pulmonary metastases (Fig. 1a-c). Then, the patient was further transferred to the oncology department for further management.
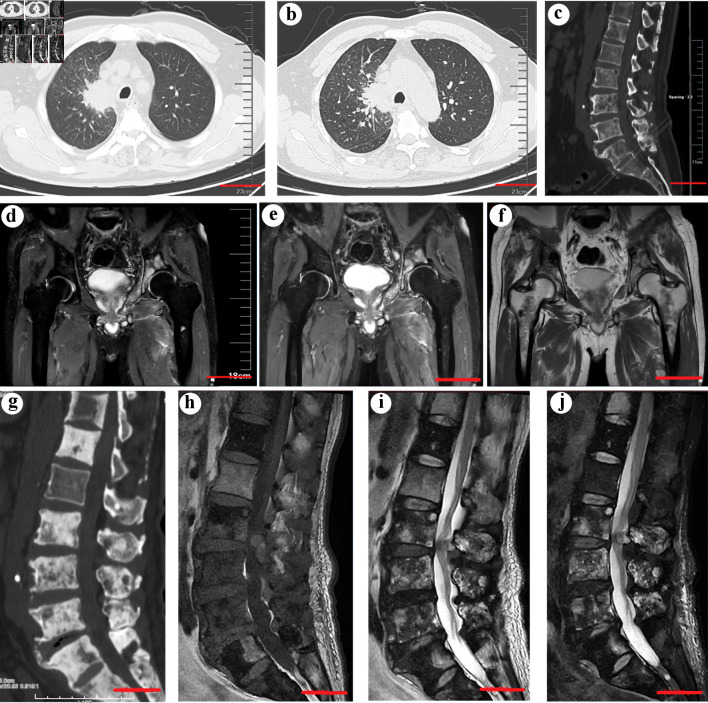
Figure 1.
(a) Computed tomography (CT) scan showing a neoplasm in the right upper lobe (scale bar = 5 cm). (b) An enhanced CT scan confirmed the existence of a neoplasm (scale bar = 5 cm). (c) CT scans of the spine before treatment with sintilimab, bevacizumab, and chemotherapy showed multiple osteolytic lesions in the thoracic and lumbar vertebrae (scale bar = 5 cm). (d, e, f) An MRI study showed new pelvic and bilateral femoral metastases (scale bar = 5 cm). (g) CT scans of the spine showed marked osteoblastic activity and osteosclerosis in the vertebra with metastases after the treatment (scale bar = 5 cm). (h, i, j) The corresponding MRI scans of the spine after treatment. (h) T1W sagittal spin-echo sequence of the spine showed hypointense lesions in multiple thoracic and lumbar vertebrae. The T2W sagittal spin-echo sequence (i) and T2W sagittal spin-echo sequence with fat suppression (j) showed both hyperintense and hypointense lesions in multiple thoracic and lumbar vertebrae, which indicates osteoblastic bone lesions (scale bar = 5 cm).
Diagnosis and treatment
Mutational analysis of the tumor revealed an activating KRASG12V mutation, and he subsequently completed eight cycles of sintilimab, and bevacizumab combined with paraplatin and pemetrexed for chemotherapy. In March 2023, he complained of pain in the bilateral hip, and a follow-up MRI scan showed new metastases in the pelvis and bilateral femur (Fig. 1d-f). He then received radiotherapy at the tumor site, which achieved pain relief. He continued immunotherapy, antiangiogenesis therapy, and chemotherapy. A CT scan 12 weeks later showed marked diffuse osteoblastic activity in the metastases in the lumbar vertebra and osteosclerosis surrounding the metastasis in the vertebrae with metastases (Fig. 1g). A corresponding MRI after the treatment confirmed the existence of abnormal osteoblastic bone lesions (Fig. 1h-j). Considering his improvement in bone pain, the increased osteosclerosis may be regarded as an osteoblastic response to immunotherapy [4, 5]. Therefore, the treatment was continued.
Follow-up and outcomes
Currently, he is 1 year following the initial treatment and remains stable with the disease.
Discussion
Bone metastasis, especially spinal metastasis, frequently develops from lung adenocarcinomas [1]. Kirsten rat sarcoma (KRAS) mutations occur in approximately 11% of the Asian population with lung cancer and account for 35% of lung adenocarcinomas [8]. Patients with KRAS mutations frequently have poor prognosis and low survival rates, whereas efficient therapies for KRAS-mutant non-small cell lung cancer (NSCLC) still fail to reach a consensus widely [9]. Among the various subtypes of mutations, KRASG12V mutations are predominant in smokers [8]. NSCLC with the KRASG12V mutation has distinct metastatic mechanisms compared with other subtypes, which can drive metastasis in a Wnt-dependent manner [8]. Moreover, a recent study revealed that the KRASG12V mutation in NSCLC can upregulate programmed death ligand 1 (PD-L1) expression and promote immune escape by regulating the transforming growth factor-β/epithelial-mesenchymal transition (EMT) signaling pathway, which can serve as a potential therapeutic target for KRAS mutations in NSCLC [10].
As an immune checkpoint inhibitor, sintilimab is a programmed cell death protein 1 (PD-1) inhibitor that is approved for patients with NSCLC, and studies have proven that squamous cell NSCLC exhibits a superior response to sintilimab compared with adenocarcinoma [11]. However, the mechanism by which sintilimab induces an osteoblastic response in our patients with spinal metastases from lung adenocarcinoma is unclear and needs further investigation. Bone metastasis, especially spinal metastasis, is frequently related to lung cancer, which is usually osteolytic due to enhanced osteoclast differentiation [1, 12, 13]. Activated osteoclasts have been shown to be associated with activation of the PD-1 pathway, whose blockade can inhibit osteoclast formation and cancer-related pain in bones [14, 15]. Considering the activation of the Wnt signaling pathway in NSCLC with the KRASG12V mutation, we hypothesized that PD-1 inhibition can inhibit osteoclastogenesis and facilitate Wnt-dependent osteogenesis in cancer bone metastases during bone remodeling [8, 16, 17]. Therefore, osteoblastosis can be regarded as an indirect sign of the response to efficient PD-1 inhibition within bone metastases of KRASG12V-mutant lung adenocarcinoma.
Increasing evidence has demonstrated that inflammation, especially chronic inflammation, highly correlates with cancer, which can promote oncogenesis by upregulating immune checkpoints to induce a state of immunosuppression in the tumor microenvironment [18, 19]. Thus, immunotherapy, such as immune checkpoint inhibitors, has emerged as an effective tool for disease control in patients with advanced NSCLC; however, antiangiogenic therapy also plays a vital role in fighting cancers [20, 21]. In our case, we showed promising clinical outcomes by synergistically administering immunotherapy and antiangiogenesis combined with chemotherapy. Although the safety of the regimen is acceptable in patients, further studies are still needed to discover more promising and effective chemotherapy-free options for the treatment of patients with advanced NSCLC, especially KRASG12V-mutant lung adenocarcinoma, to improve therapeutic efficacy and tolerability. Therefore, it is important for clinicians to be aware of this phenomenon of osteoblastic reaction/response, which may predict a good response to treatment, especially immunotherapy with PD-1 inhibitors, for patients with KRASG12V-mutant lung adenocarcinoma.
In conclusion, this case report documents a rare osteoblastic bone reaction developing during treatment with sintilimab and bevacizumab in a patient with KRASG12V-mutant lung adenocarcinoma, which represents a good response to treatment rather than disease progression. However, our case has several limitations. First, we only reported a phenomenon and preliminarily investigated its potential mechanisms; thus, a mechanistic study in depth is needed. Second, our study had a limited number of patients; thus, a large-scale study is needed. These limitations are the bases and directions for further studies. Therefore, to compensate for these shortcomings, further studies should be conducted to verify our findings.
Acknowledgments
None to declare.
Funding Statement
This work was supported by the Natural Science Foundation of Shandong Province (grant no. ZR2023QH517; ZR2022QH252; ZR2020QH077; ZR2020MH087); Shandong Qianfoshan Hospital Cultivation Fund (QYPY2019NSFC1011); and Academic Promotion Program of Shandong First Medical University (2019LJ001).
Conflict of Interest
The authors declared no conflict of interest.
Informed Consent
This study was approved by our institution, and written informed consent was obtained from the patient.
Author Contributions
XJM, CJC and XZ participated in the drafting, writing, and revising of the manuscript. JWZ, WHX, YBQ, JKL, and QWM participated in the data selection and analysis. CJC, LZ, and YY contributed to the study concept and acquired and analyzed the data. All authors contributed to the drafting of the manuscript and figure preparation.
Data Availability
The data sets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
- 1.Chen CJ, Yan TB, Liu YD, Wang Y, Zhao X, Qi YB. et al. Multiple myeloma rather than metastatic lung cancer: an unexpected cause of spinal cord compression. World Journal of Oncology. 2023;14(5):438–442. doi: 10.14740/wjon1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen C, Ma Q, Qi Y, Wu Y, Li J, Ren Y, Osteolytic schwannoma in an older patient with lumbar degenerative disk disease: a case report. HSS Journal®. 15563316231200862.
- 3.Napoli LD, Hansen HH, Muggia FM, Twigg HL. The incidence of osseous involvement in lung cancer, with special reference to the development of osteoblastic changes. Radiology. 1973;108(1):17–21. doi: 10.1148/108.1.17. [DOI] [PubMed] [Google Scholar]
- 4.Lind JS, Postmus PE, Smit EF. Osteoblastic bone lesions developing during treatment with erlotinib indicate major response in patients with non-small cell lung cancer: a brief report. J Thorac Oncol. 2010;5(4):554–557. doi: 10.1097/JTO.0b013e3181d3e47e. [DOI] [PubMed] [Google Scholar]
- 5.Comito F, Ambrosini V, Sperandi F, Melotti B, Ardizzoni A. Osteoblastic bone response mimicking bone progression during treatment with pembrolizumab in advanced cutaneous melanoma. Anticancer Drugs. 2018;29(10):1026–1029. doi: 10.1097/CAD.0000000000000689. [DOI] [PubMed] [Google Scholar]
- 6.Chen C, Ren Y, Qi Y, Zhang G, Hu H, Zhang K, Wu Y. et al. Congenital lumbosacral malformations with low-grade neuroepithelial tumor causing progressive postpoliomyelitis paralytic limb. Ann Neurol. 2023;94(1):160–162. doi: 10.1002/ana.26667. [DOI] [PubMed] [Google Scholar]
- 7.Fink C, Hasan B, Deleu S, Pallis AG, Baas P, O'Brien M. High prevalence of osteoblastic bone reaction in computed tomography scans of an European Organisation for Research and Treatment of Cancer prospective randomised phase II trial in extensive stage small cell lung cancer. Eur J Cancer. 2012;48(17):3157–3160. doi: 10.1016/j.ejca.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 8.Hung PS, Huang MH, Kuo YY, Yang JC. The inhibition of wnt restrain KRAS(G12V)-driven metastasis in non-small-cell lung cancer. Cancers (Basel) 2020;12(4):837. doi: 10.3390/cancers12040837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ricciuti B, Leonardi GC, Metro G, Grignani F, Paglialunga L, Bellezza G, Baglivo S. et al. Targeting the KRAS variant for treatment of non-small cell lung cancer: potential therapeutic applications. Expert Rev Respir Med. 2016;10(1):53–68. doi: 10.1586/17476348.2016.1115349. [DOI] [PubMed] [Google Scholar]
- 10.Pan LN, Ma YF, Li Z, Hu JA, Xu ZH. KRAS G12V mutation upregulates PD-L1 expression via TGF-beta/EMT signaling pathway in human non-small-cell lung cancer. Cell Biol Int. 2021;45(4):795–803. doi: 10.1002/cbin.11524. [DOI] [PubMed] [Google Scholar]
- 11.Gao S, Li N, Gao S, Xue Q, Ying J, Wang S, Tao X. et al. Neoadjuvant PD-1 inhibitor (Sintilimab) in NSCLC. J Thorac Oncol. 2020;15(5):816–826. doi: 10.1016/j.jtho.2020.01.017. [DOI] [PubMed] [Google Scholar]
- 12.da Silva GT, Bergmann A, Santos Thuler LC. Prognostic factors in patients with metastatic spinal cord compression secondary to lung cancer: a systematic review of the literature. Eur Spine J. 2015;24(10):2107–2113. doi: 10.1007/s00586-015-4157-x. [DOI] [PubMed] [Google Scholar]
- 13.Wang M, Zhao M, Guo Q, Lou J, Wang L. Non-small cell lung cancer cell-derived exosomal miR-17-5p promotes osteoclast differentiation by targeting PTEN. Exp Cell Res. 2021;408(1):112834. doi: 10.1016/j.yexcr.2021.112834. [DOI] [PubMed] [Google Scholar]
- 14.Tai YT, Cho SF, Anderson KC. Osteoclast immunosuppressive effects in multiple myeloma: role of programmed cell death ligand 1. Front Immunol. 2018;9:1822. doi: 10.3389/fimmu.2018.01822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang K, Gu Y, Liao Y, Bang S, Donnelly CR, Chen O, Tao X. et al. PD-1 blockade inhibits osteoclast formation and murine bone cancer pain. J Clin Invest. 2020;130(7):3603–3620. doi: 10.1172/JCI133334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen C, Fu L, Luo Y, Zeng W, Qi X, Wei Y, Chen L. et al. Engineered exosome-functionalized extracellular matrix-mimicking hydrogel for promoting bone repair in glucocorticoid-induced osteonecrosis of the femoral head. ACS Appl Mater Interfaces. 2023;15(24):28891–28906. doi: 10.1021/acsami.3c01539. [DOI] [PubMed] [Google Scholar]
- 17.Wang Q, Miao Y, Qian Z, Chen L, Lu T, Xu Y, Jiang X. et al. MicroRNA-15a-5p plays a role in osteogenic MC3T3-E1 cells differentiation by targeting PDCD4 (programmed cell death 4) via Wnt/beta-catenin dependent signaling pathway. Bioengineered. 2021;12(1):8173–8185. doi: 10.1080/21655979.2021.1977766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sara A, Ruff SM, Noonan AM, Pawlik TM. Real-world use of immunotherapy for hepatocellular carcinoma. Pragmat Obs Res. 2023;14:63–74. doi: 10.2147/POR.S397972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen C, Si M, Gao X, Wang W, Wang S, Pan X. Primary cutaneous diffuse large B-cell lymphoma after total knee arthroplasty: a case study and a systematic review of its cutaneous manifestations and treatment options. Postepy Dermatol Alergol. 2022;39(3):545–552. doi: 10.5114/ada.2021.108444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chu T, Zhong R, Zhong H, Zhang B, Zhang W, Shi C, Qian J. et al. Phase 1b study of sintilimab plus anlotinib as first-line therapy in patients with advanced NSCLC. J Thorac Oncol. 2021;16(4):643–652. doi: 10.1016/j.jtho.2020.11.026. [DOI] [PubMed] [Google Scholar]
- 21.Chen C, Wang S, Chen J, Liu X, Zhang M, Wang X, Xu W. et al. Escin suppresses HMGB1-induced overexpression of aquaporin-1 and increased permeability in endothelial cells. FEBS Open Bio. 2019;9(5):891–900. doi: 10.1002/2211-5463.12622. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.