Abstract
Background:
Uncontrolled blood pressure (BP) is a leading preventable cause of death that remains common in the US population despite the availability of effective medications. New technology and program innovation has high potential to improve BP, but may be expensive and burdensome for patients, clinicians, health systems and payers, and may not produce desired results or reduce existing disparities in BP control.
Methods and Results:
The National Patient-Centered Outcomes Research Network (PCORnet) Blood Pressure Control Laboratory is a platform designed to enable national surveillance, and facilitate quality improvement and comparative effectiveness research. The platform uses PCORnet for engagement of health systems and collection of electronic health record data, and the Eureka Research Platform for eConsent and collection of patient-reported outcomes and mHealth data from wearable devices and smartphones. Three demonstration projects are underway: BP Track will conduct national surveillance of BP control and related clinical processes by measuring theory-derived pragmatic BP control metrics using electronic health record data, with a focus on tracking disparities over time; BP MAP will conduct a cluster-randomized trial comparing effectiveness of two versions of a BP control quality improvement program; BP Home will conduct an individual patient-level randomized trial comparing effectiveness of Smartphone-linked versus standard home BP monitoring. Thus far, BP Track has collected electronic health record data from over 826,000 eligible patients with hypertension who completed approximately 3.1 million ambulatory visits. Preliminary results demonstrate substantial room for improvement in BP control (<140/90 mmHg), which was 58% overall, and in the clinical processes relevant for BP control. For example, only 12% of hypertensive patients with a high BP measurement during an ambulatory visit received an order for a new antihypertensive medication.
Conclusions:
The PCORnet BP Control Lab is designed to be a reusable platform for efficient surveillance and comparative effectiveness research; results from demonstration projects are forthcoming.
Uncontrolled blood pressure (BP) is a leading preventable cause of death1, causing over 450,000 deaths per year in the US2, 3. While effective and affordable medications are available to control BP, multiple rounds of medication adjustment and intensification are typically required, and BP control is often not achieved4, 5. With the 2017 American College of Cardiology (ACC) / American Heart Association (AHA) Hypertension Guideline defining lower BP thresholds for diagnosis, treatment and control6, the prevalence of hypertension now approaches 50% of all US adults, and millions more Americans already treated for hypertension are now considered to have uncontrolled BP6, 7. Achieving optimal BP control at the population-level could save thousands of lives per year8.
It is unclear, however, how best to improve BP control rates in the US. Some organizations have reconfigured care delivery and achieved improvements in control9–13, but many of these approaches are resource intensive and may not be feasible or sustainable in all settings, particularly in resource-poor settings such as safety net clinics14–16. Home BP monitoring can be effective, but generally requires “additional support” to produce significant and lasting reductions in BP17–19. It remains unclear what types of additional support will be both effective and sustainable across varying healthcare delivery settings. Emerging technologies including smartphone apps and wearables20–23 could facilitate BP measurement, tracking, interpretation, patient-clinician communication, medication decision-making and adherence, and thereby improve the various healthcare processes required for BP24, 25 control, but few studies of technology effectiveness measuring BP control outcomes have been conducted22.
Randomized controlled trials (RCTs) are required to accurately assess and compare effectiveness of different strategies for improving BP control. RCTs, however, are difficult, time-consuming and expensive to conduct26–30. The National Heart, Lung and Blood Institute alone spent over $90 million in 2018 ($1.6 billion since 1985) on RCTs relevant to BP (National Institutes of Health (NIH) RePORTER query31 and examples29, 30). New methods that improve RCT efficiency could help accelerate evidence generation and translation of innovative healthcare delivery solutions into major population health benefits.
Large simple real-world pragmatic trials that leverage existing resources may help streamline evidence generation and reduce burden on both investigators and patients28, 32–35. These methods may be particularly useful for measuring effectiveness of BP control interventions. Unlike many phenotypes, BP is measured routinely during healthcare delivery and recorded systematically in electronic health records (EHRs). Controlling High Blood Pressure, which can be constructed using data in the EHR, is a National Quality Forum endorsed performance measure (NQF 001836) recognized by the Centers for Medicare and Medicaid Services for use in clinical incentive programs37–39. While this metric has limitations, it has direct relevance to public health; demonstrating even a small average improvement in BP control from a scalable intervention would likely translate to significant health benefits when implemented broadly40.
We aim to establish a platform for conducting large, simple, patient-centered real-world RCTs designed to demonstrate and compare effectiveness of BP control interventions, and a national surveillance system for monitoring both population-wide and local improvements in BP control. Our platform leverages EHR systems now ubiquitous in the US, the National Patient-Centered Outcomes Research Network (PCORnet) funded by the Patient-Centered Outcomes Research Institute (PCORI)41, 42, and an NIH-funded digital system designed to enable direct-to-participant research including collection of patient-reported outcomes and mHealth data from wearable devices and smartphones43, 44. In this design and methods paper, we describe the structural features of the platform – which we call the PCORnet Blood Pressure Control Laboratory (BP Control Lab) – and the three projects now underway using the platform that will demonstrate its utility. We end with a description of the data assets of the network of PCORnet organizations currently participating and preliminary results from our surveillance project.
STRUCTURAL FEATURES
The BP Control Lab brings together a set of established resources that can be leveraged to support efficient research and surveillance. Below we describe these modular resources, and how they are configured to support large simple real-world pragmatic RCTs, local quality improvement efforts, and national surveillance. We also describe the collaborative networks that support the BP Control Lab.
The BP Control Lab uses PCORnet specifically for access to EHR data, and more broadly for access to a networked clinical research infrastructure. PCORnet was jointly envisioned by PCORI and NIH45, launched in 2013 by PCORI41, 45, 46, and transitioned to the People-Centered Research Foundation in 2018 for management, administration and business development47. PCORnet’s Clinical Data Research Networks support curation of EHR data in a common data model48 that allows querying of EHR data across organizations using standardized queries. Patient-level data are retained locally at each organization/network, and are queried via a distributed research analysis system administered by the PCORnet Coordinating Center (Figure 1). Along with systolic and diastolic BP measurements made in the context of healthcare encounters, the PCORnet Common Data Model includes information about patient demographics, encounters, diagnoses, medications, select laboratory measurements, and other domains potentially useful in evaluating effectiveness of BP control interventions48, and has demonstrated utility for hypertension surveillance49.
Figure 1. Electronic Health Record Data Flow and Distributed Querying in PCORnet.
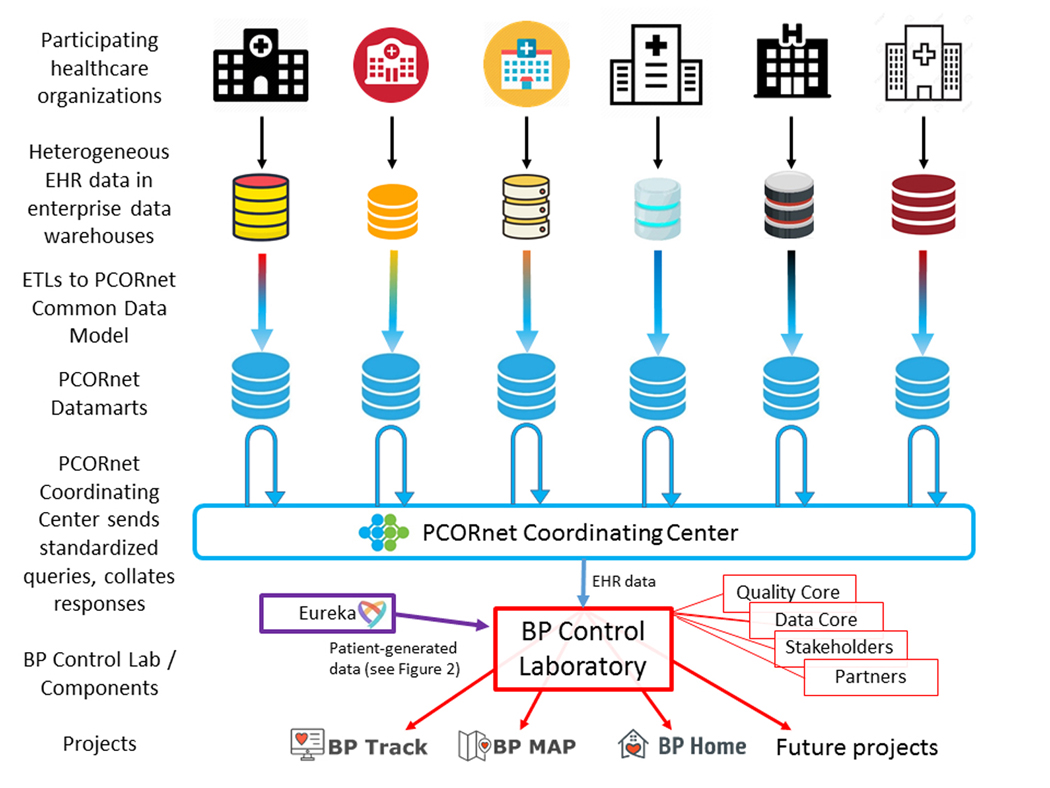
Electronic health record (EHR) data are generated by participating healthcare organizations and stored in local, heterogeneously-structured comprehensive enterprise data warehouses (e.g., Clarity™ for Epic™ EHR systems). To support PCORnet, each organization executes regular extract, transform and load (ETL) operations that transform those EHR data into a homogeneous and simplified/intuitive set of relational data tables – the PCORnet Common Data Model48 – that are maintained on locally-controlled servers – PCORnet Datamarts – at each participating healthcare organization (blue database icons). The data are not stored centrally by PCORnet or by the Blood Pressure (BP) Control Laboratory. To access these data, The BP Control Laboratory Data Core, with input from the Quality Core, Partners, and Stakeholders, develops Common Data Model queries (written in SAS) that are distributed by the PCORnet Coordinating Center to each participating datamart. Datamarts then run the query locally and return results to the PCORnet Coordinating Center, which collates results and delivers them to the BP Control Lab. The BP Control Lab then links the EHR data with patient-generated data from Eureka as needed (e.g. for BP Home, see Figure 2), and delivers it to the project teams supported by the Laboratory. Ongoing projects include BP Track, BP MAP and BP Home (see Figure 3).
To complement data collected during healthcare encounters, the BP Control Lab uses the Eureka Research Platform for direct patient engagement and collection of patient-generated health data (Figure 2). Eureka (originally named the Health ePeople Resource for Mobilized Research) was funded by the NIH in 201543, 50 to accelerate use of mHealth data in research and evaluation of mHealth technology for improving health. Its multitenant cloud-based platform currently supports development of web- and mobile app-based patient portals that directly engage patients in eConsent, eligibility assessment, online surveys, and data collection from wearable devices and smartphones for prospective research studies including RCTs44. The platform supports secure tracking and data linkage of Eureka enrollees recruited across different systems (e.g., patients recruited from a healthcare delivery organization), retrieval of BP measurements from home BP monitoring devices via electronic data transfer, and a study management portal with customizable reports.
Figure 2. Patient-Generated Health Data Collection via the Eureka Research Platform.
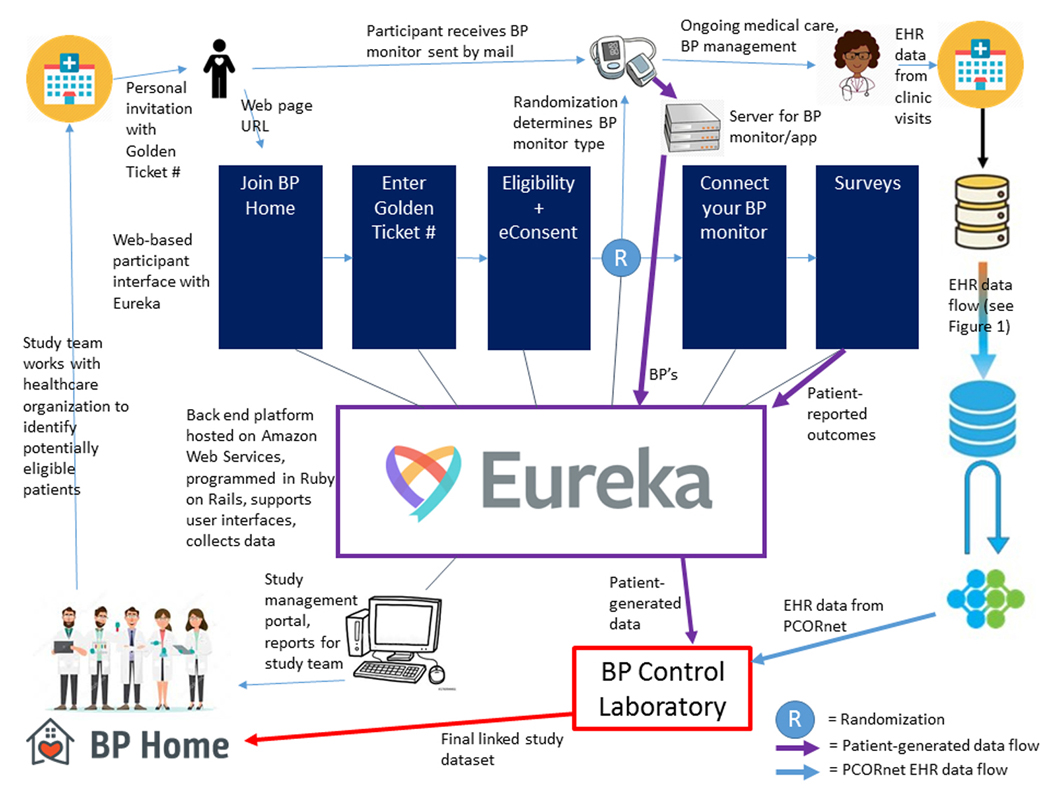
For BP Home and future projects like it, patient-generated health data including patient-reported outcomes and blood pressure (BP) measurements from home BP monitors are collected via the Eureka Research Platform. Study teams (lower left corner) work with site personnel at healthcare organizations to identify potentially eligible patients and send them personal invitations to join the study. Interested patients go to a Eureka-hosted web portal (optimized for viewing on either a desktop or smartphone) that takes them through study information, eligibility and eConsent procedures. They also enter a “Golden Ticket #” provided with the personal invitation that enables future identity linkage. Eligible and consenting participants are randomized to one of the two study arms, and are mailed a home blood pressure (BP) monitor (Smartphone-linked or standard depending on randomization arm, see Figure 3), which they use for ongoing medical care and BP management with their clinician. Participants in the Smartphone-linked arm then authorize connecting their home BP monitor to Eureka, allowing Eureka to obtain their BP measurements from the device company server. Participants in both arms fill out surveys that allow Eureka to gather patient-reported outcomes and other information. The study team can access reports and participant information through a study management portal hosted by Eureka. The BP Control Laboratory uses the Golden Ticket # to link patient-generated data from Eureka with electronic health record (EHR) data from PCORnet (see Figure 1), and provides the final linked study dataset to the study team for analysis.
The BP Control Lab represents a collaborative effort and partnership between PCORnet entities, the American Medical Association (AMA), and the AHA. The project was conceived by investigators and patients participating in the PCORnet Cardiovascular Health Collaborative Research Group51, 52, including representatives from 4 Clinical Data Research Networks (OneFlorida53, REACHnet54, ADVANCE55 and STAR56), a Patient-Powered Research Network focused on cardiovascular health (the Heart Research Alliance57, formerly named the Health eHeart Alliance58), an active Patient Advisory Board, and the PCORnet Coordinating Center41. To build the BP Control Lab, a collaborative partnership including these PCORnet entities, the AMA, and the AHA applied for and received funding through PCORI’s “Partnerships to Conduct Clinical Research within PCORnet” Funding Announcement59. The three projects funded by our award, described below, demonstrate BP Control Lab functionality and utility.
DEMONSTRATION PROJECTS
Overview
Our PCORI award supports 3 projects, each designed to answer scientific research questions and demonstrate different aspects of BP Control Lab functionality. These 3 projects, named “BP Track”, “BP MAP” and “BP Home”, are described below, and summarized in Figure 360, 61.
Figure 3. Design Features of Three Projects Currently Supported by the Blood Pressure Control Laboratory.
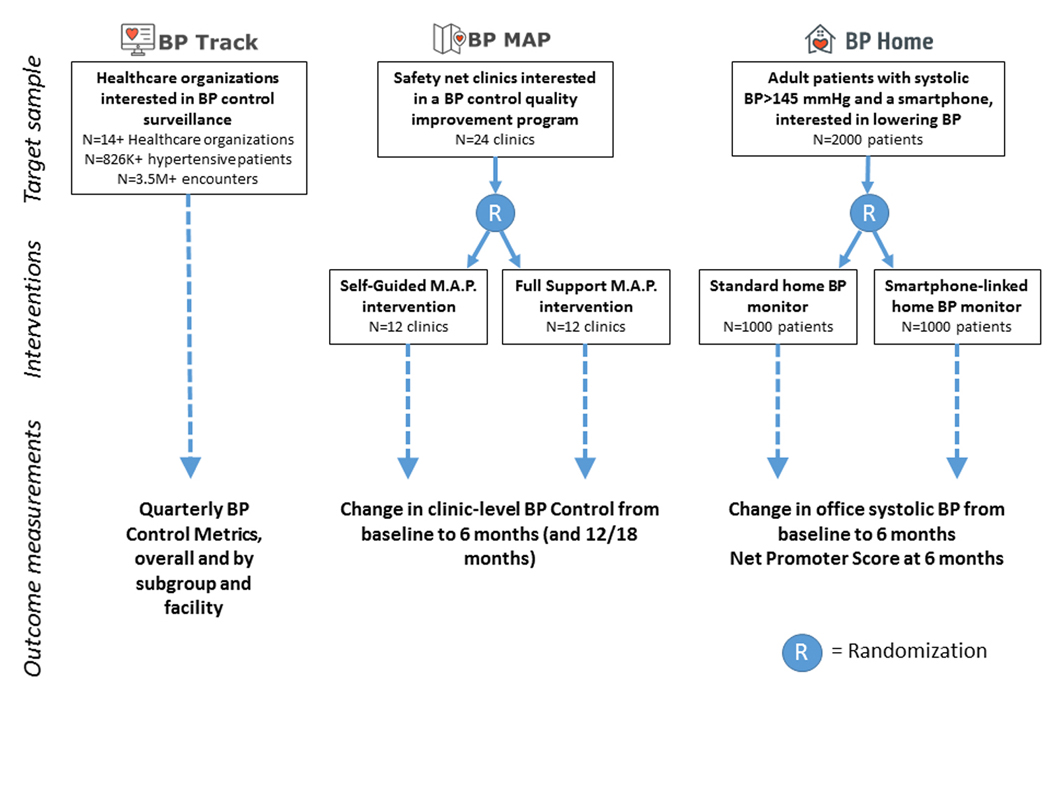
The Blood Pressure (BP) Control Laboratory currently supports BP Track (a national surveillance project), BP MAP (a cluster randomized controlled trial [RCT]), and BP Home (an individual-level RCT). In the figure, we describe the target sample (target numbers and characteristics of the sample units), interventions (for the two RCTs), and the primary outcome measurement(s) for each project. BP Control Metrics, including overall BP Control and 9 other BP-related quality metrics, are described in Table 1. M.A.P. – Measure Accurately, Act Rapidly, Partner with Patients (a quality improvement program for BP control managed by the American Medical Association)24, 60, 61
BP Track: A National Surveillance System
BP Track aims to establish a national BP control surveillance system that generates statistics on BP control and BP-related quality metrics for participating healthcare organizations. Organizations must contribute data to a PCORnet datamart that agrees to respond to quarterly queries written in SAS (Statistical Analysis Systems, Cary, North Carolina) against the PCORnet Common Data Model, and in return receives access to metric performance reports. The queries will produce a set of quality metrics relevant to improving BP control (BP Control Metrics), including Controlling High Blood Pressure (NQF 001836, 62, 63) and Improvement in Blood Pressure (CMS65v764) and additional process measures relevant to clinical management and treatment practices for BP control (Table 1). Metric design and development is guided by two frameworks – the AMA’s M.A.P. framework (Measure Accurately, Act Rapidly, and Partner with Patients)24, 60, 61 and the Blood Pressure Control Model25, 65 – that specify relevant clinical processes. Additional metrics perceived to be useful to stakeholders can be added over time.
Table 1.
Blood Pressure Control Metrics to be Tracked by the PCORnet Blood Pressure Control Laboratory
| # | Metric | Description | Implementation plan |
|---|---|---|---|
| 1 | Blood Pressure Control, % of patients | This overall measure of BP control implements NQF 0018, which defines BP Control as the percent of eligible hypertensive patients for whom the BP measurements at their most recent ambulatory care visit were at goal, defined as systolic BP (SBP) < 140 mmHg and diastolic BP (DBP) < 90 mmHg. | Wave 1 |
| 2 | Blood Pressure Control to 2017 Guideline Goal, % of patients | This alternative overall measure of BP control is identical to Metric 1, except that attainment of BP Control is defined by SBP < 130 mmHg and DBP < 80 mmHg, as per the goal stated in the 2017 ACC/AHA Hypertension Guideline. Note that while the treatment threshold varies in the Guideline, depending on cardiovascular risk, the goal applies to all patients. | Wave 2 |
| 3 | Improvement in Blood Pressure, % of patients | This overall measure of BP improvement implements CMS065v4, which defines BP improvement as either a reduction of 10 mmHg in SBP or achievement of SBP that is “adequately controlled” (SBP < 140 mmHg) in months 10–12 of the measurement period, among hypertensive patients not previously controlled. | Wave 1 |
| 4 | Confirmatory Repeated Blood Pressure Measurement, % of visits | This process measure is designed to capture the practice of repeating a BP measurement in the same visit when the first measurement done in clinic is high (SBP≥140 mmHg or DBP≥90 mmHg). | Wave 1 |
| 5 | Terminal Digit = Zero, % of measurements | Inappropriate rounding of BP measurements (usually to zero) leads to measurement error and worse treatment decisions. This metric is designed to measure the extent of this behavior, which would lead to a terminal digit of zero greater than 10% of the time (if an automated BP monitor is used) or greater than 20% (if a manual BP monitor is used with recommended rounding to even digits). Unlike most of our metrics, lower is better, down to an ideal value of 10–20%, which would be expected if no rounding were occurring. | Wave 2 |
| 6 | Medication Intensification, % of visits | This process measure captures the proportion of visits where BP is uncontrolled where a BP medication is ordered that is of a different class of medication than had previously been used. Note that this explicitly does not give credit for ordering a simple refill or medication dose increase, or use of a different medication in the same class. | Wave 1 |
| 7 | Repeat Visit in 4 Weeks After Uncontrolled HTN, % of visits | This process measure captures the proportion of persons who had uncontrolled HTN who made a subsequent visit within the following 4 weeks. | Wave 2 |
| 8 | Average SBP Reduction After Medication Intensification, mmHg | This continuous metric describes the change in SBP observed between a visit with a medication intensification to the subsequent visit occurring at least 10 days later. We will collect both the average and the standard deviation for this metric. | Wave 1 |
| 9 | Use of a CCB or Thiazide or Thiazide-Like Diuretic among African-American Patients on At Least One Medication, % of patients | Use of calcium channel blockers (CCB) OR a thiazide or thiazide-like diuretic medication classes is recommended to treat black or African American patients as first line monotherapy due to increased efficacy. This metric, which is limited to African-American patients with a diagnosis of hypertension taking at least one medication class, describes the prevalence of those receiving the recommended drug class. | Wave 2 |
| 10 | Use of Fixed Dose Combination Product among Patients Taking 2 or More Classes of Medications, % of patients | Use of fixed dose combination medications helps with adherence, promotes rational combinations of medications, and increases likelihood of achieving BP control. This metric, which is limited to patients taking more than one BP medication class, describes the prevalence of fixed dose combination pill use. | Wave 2 |
BP Control Metric results will be produced overall for each participating PCORnet datamart, and for any number of individual clinical units within the datamart (e.g., a particular general internal medicine clinic). Each PCORnet datamart will specify clinical units with at least one identified clinician stakeholder, to whom clinical unit-specific metrics will be provided. Each metric will be produced for the overall relevant patient population (in the datamart, or the clinical unit) and for subgroups of those patients defined by categories of age (18–44, 45–64, and 65+ years), sex (male, female, and other) and race/ethnicity (Non-Hispanic Asian, Black, White and Other, and Hispanic any race). Reports and interactive data visualization will allow stakeholders to view their results and compare to blinded results from other participating organizations.
BP Track will support quality improvement efforts by providing systematic measurements of the specified quality metrics over time. Using these quality metrics, quality improvement programs can target particular processes in need of improvement, implement interventions, and use BP Track to assess change in metrics over time in relevant clinical units and patient subgroups compared to control units without the same exposure. This approach will be used for BP MAP (see below). BP Track will also support participation in the Target:BP™ Program66, a national initiative formed by the AHA and the AMA that aims to help healthcare organizations prioritize and improve BP control and recognize organizations for achieving BP control rates of 70 percent or higher.
Although organizations participating in BP Track will not represent a random sample of either the US population or US healthcare organizations, the relatively broad participation in the program (currently 14 PCORnet datamarts with healthcare organizations in 15 different states) and scalability (additional organizations can participate if they support the PCORnet Common Data Model and participate in the distributed research network) makes it a potentially useful platform for national surveillance. BP Track represents the first use of PCORnet for national surveillance, and a testbed for PCORnet surveillance methodology.
BP MAP: A Cluster Randomized Quality Improvement Trial
BP MAP (Improving Blood Pressure Control in Diverse Populations by Measuring Accurately, Acting Rapidly, and Partnering with Patients) is a cluster randomized RCT that will compare effectiveness of a “Full Support” versus a “Self-Guided” version of a clinic-level hypertension quality improvement intervention. The quality improvement intervention is based on the AMA’s M.A.P. framework24 and six-month M.A.P. BP Improvement Program, which includes quality improvement materials and a protocolized program with support from dedicated practice change facilitators. Interventions based on the M.A.P. framework have shown evidence of effectiveness, with improved BP control and process metrics in pre-post analyses60, 61 with sustainability at 12 months60. It is unclear, however, how much the support from the dedicated practice change facilitators (who are trained centrally by the AMA in a train-the-trainer model) is required for the program to be successful.
BP MAP will randomize 24 clinics from two PCORnet research networks (REACHnet and OCHIN) to one of two versions of the AMA’s M.A.P. BP Improvement Program: Full Support versus Self-Guided. The Full Support arm will include an on-site practice assessment, an in-person launch meeting, training and personalized support for dedicated practice change facilitators from experienced AMA staff, and access to an online Digital Guide containing resources and training materials. The Self-Guided arm will have access to the Digital Guide and an informational webinar at launch, but will not receive in person site visits or practice change facilitator support. Both arms will run BP Track queries (Table 1) on a monthly basis and use the results to guide and target their quality improvement efforts. The primary outcome will be change in BP control (NQF 0018) from baseline to 6 months, as assessed using BP Track queries of the PCORnet Common Data Model (Table 1, Metric 1). This outcome will be compared between randomized arms; and each arm will also be compared against Usual Care (non-randomized), which will include other PCORnet datamarts participating concurrently in BP Track (excluding datamarts with clinical units participating in BP MAP). Additional features of BP MAP are published on clinicaltrials.gov67. The protocol, including a description of the Digital Guide and resources required to implement the Full Support versus the Self-Guided versions of the intervention at scale, will be published separately.
BP Home: A Randomized Controlled Trial of Home Monitoring Technology
BP Home (The PCORnet Blood Pressure Home Monitoring Study) will compare effectiveness of Smartphone-linked versus standard home BP monitors for helping patients with uncontrolled hypertension achieve a reduction in systolic BP. Home BP monitoring by itself has been shown to have only a small overall impact on BP control17, 19; home monitoring combined with “additional support” seems to provide more robust gains in BP control17–19. While additional support that requires reconfiguration of care and extra resources may not be achievable in low-resource settings, emerging technology that is more user-friendly in diverse patient populations (including elderly and low-income patients who tend to be late adopters of technology) may help bridge this gap68–70. Developing and testing new devices, smartphone apps, and other support systems that effectively engage patients in home BP monitoring and help patients and clinicians achieve subsequent BP control remains an active area of research and development in the public and private sectors; while consumer-focused technology reviews are available71, these typically do not rely on evidence produced by robust comparative effectiveness methods. It is critically important to demonstrate and compare effectiveness of emerging technologies designed to enhance BP control.
BP Home will test whether having a Smartphone-linked home BP monitor (that connects via Bluetooth to a smartphone and works in tandem with a commercially available smartphone app) constitutes “additional support” that improves BP control more effectively than a standard device without Bluetooth connectivity. Our pragmatic design features online enrollment, eConsent and survey delivery via the Eureka Research Platform (Figure 2), simple eligibility criteria (systolic BP >145, stated desire to lower systolic BP by 10 mmHg, and owning a smartphone), scalable interventions (two consumer devices) provided with minimal study-specific support, and an imperfect but pragmatic outcome (reduction in systolic BP measured at most recent clinic visit, at 6 months after enrollment). Four PCORnet networks will help recruit the planned 2000 BP Home participants required for the study, and will query their PCORnet datamarts for office-based BP measurements from EHR data, which will be linked with patient-reported outcomes and home BP measurements collected by Eureka (Figure 2). The Patient Advisory Board helped design the protocol and the web portal hosted on the Eureka platform, reviews all patient-facing material, and advises the study team on all matters impacting participant experience. Additional features of BP Home are published on clinicaltrials.gov72.
Description of BP Control Lab data assets and preliminary results from BP Track, Wave 1
Fourteen “Wave 1” datamarts fully executed contracts with the PCORnet Coordinating Center in time to participate in the first quarterly BP Track queries; additional datamarts are expected to participate in subsequent Waves, which are currently funded to continue through mid-2021. Table 2 describes the totals and ranges in the number of observations (eligible patients and ambulatory visits) contributed by each Wave 1 datamart. A total of 826,392 adult patients with hypertension met eligibility criteria across datamarts, many with comorbid diagnoses relevant to hypertension control and cardiovascular disease. Those patients completed over 3 million qualifying ambulatory visits where BP was measured during the year-long observation periods selected by each datamart. One datamart reported results from four specific clinical units of interest within their datamarts; more will participate in this aspect of BP Track in Wave 2.
Table 2.
Total patients and encounters available for analysis in Wave 1 of BP Track
| Patient/Encounter Type | Total numbers of patients and observations from participating datamarts | |
|---|---|---|
| Median (range) of totals within each datamart N=14 Datamarts |
Total across all datamarts | |
| Patients | ||
| - All adult patients with hypertension meeting eligibility criteria* for BP Control metrics | 35,719 (1,042 – 178,132) | 826,392 |
| - …with diabetes diagnosis† | 7,826 (206 – 63,327) | 240,753 |
| - …with coronary heart disease diagnosis† | 5,261 (43 – 29,116) | 112,456 |
| - …with heart failure diagnosis† | 2,187 (24 – 12,993) | 47,677 |
| - …with depression diagnosis† | 5,484 (260 – 31,717) | 116,626 |
| - …with COPD diagnosis† | 2,067 (51 – 12,008) | 50,788 |
| Encounters | ||
| - All ambulatory encounters made by eligible patients* | 130,956 (3,704 – 757,235) | 3,570,311 |
| - …with a BP measurement available | 119,078 (3,655 – 714,894) | 3,103,423 |
During a defined 1-year measurement period, the following criteria are met: at least one ambulatory visit occurs, patient is age 18–85 at the end of the period; a diagnosis of hypertension during the first six months of the period or at any time prior; no hospice services provided to the patient; no diagnosis of end-stage renal disease, dialysis, or renal transplant during or prior; no diagnosis of pregnancy; not residing in a long-term care facility36.
Defined by a diagnosis assigned during the 1-year measurement period.
BP Track – PCORnet Blood Pressure Control Registry; BP – Blood pressure; COPD – Chronic obstructive pulmonary disease
BP Control Metrics from datamarts reporting in Wave 1 showed substantial room for improvement in BP control and relevant clinical processes (Table 3). Overall results (weighted for numbers of observations) demonstrated that 58% of adult patients with hypertension were controlled to <140/90 mmHg, confirmatory re-measurement of BP after a high ambulatory measurement was uncommon (16%), and only 12% of patients with a high BP at an ambulatory visit were subsequently prescribed a new class of BP medication. When this occurred, however, the average reduction in systolic BP was relatively large (14.6 mmHg). We observed substantial variation in these metrics between datamarts, and between clinical units within datamarts, that indicates marked room for improvement. BP Track data will be available for analysis and publication, subject to a forthcoming BP Track Publication Policy; requests may be submitted to the corresponding author.
Table 3.
Aggregate Blood Pressure Control Metrics in BP Track, Wave 1
| BP Control Metric | Result | ||||
|---|---|---|---|---|---|
| # | Name | N1 | Weighted result* | Datamart Range† (Min-Max) N=14 datamarts n=826,392 patients |
Clinical Unit Range‡ (Min-Max) N=4 clinical units‡ n=144,432 patients |
| 1 | Blood Pressure Control, % of patients | 826,392 | 58% | 40% - 65% | 31% - 60% |
| 3 | Improvement in Blood Pressure, % of patients | 213,240 | 28% | 16% - 37% | 13% - 28% |
| 4 | Confirmatory Repeated Blood Pressure Measurement, % of visits | 254,820 | 16% | 0% - 92% | 14% - 51% |
| 6 | Medication Intensification, % of visits | 244,526 | 12% | 0.5% - 16% | 8% - 11% |
| 8 | Average SBP Reduction After Medication Intensification, mmHg | 14,928 | 14.6 mmHg | 11.1 – 16.5 | 11.2 – 16.1 |
Overall results are calculated as weighted averages of datamart-specific results, weighted by the total number of observations (patients or visits) meeting eligibility criteria for metric calculation (N). Note: For confidentiality, all counts reported to the BP Control Lab (both numerators and denominators in proportion metrics) are masked when cell sizes are between 1–10. Results are reported as missing when the denominator (N) is <11. For metrics 1, 3, 4 and 6, which are proportions, we imputed a numerator of 5 if the denominator was 100 or greater and the numerator was between 1 and 10. N’s represent total eligible observations contributing to non-missing (including imputed) results.
Datamart Range represents the minimum value and the maximum value across the 14 reporting PCORnet datamarts. Two values were imputed for Metric 4*.
Clinical Unit Range represents the minimum value and the maximum value across the 4 individual clinical units within PCORnet datamarts specifically tracked in BP Track by specifying FACILITYID in the ENCOUNTER table of the PCORnet Common Data Model.
SBP – Systolic blood pressure; BP - Blood pressure
SUMMARY
The BP Control Lab represents a new national infrastructure for BP control surveillance, evaluation of healthcare quality improvement efforts, and pragmatic RCTs. Results from the three demonstration projects will be reported in coming years. Along with the main scientific outcomes, each project will include assessments of engagement from target stakeholders including patients and trial participants, clinicians, local policymakers (e.g., medical system leadership), and researchers interested in BP control. We also plan to analyze efficiency and sustainability of the BP Control Lab, and potential cost savings to research projects using the core infrastructure (PCORnet and Eureka). Our goal is to provide results useful to our stakeholders, and to reuse the BP Control Lab infrastructure that now exists for future efficient research that helps improve BP control, reduce disparities, guide evidence-based use of technology, and improve cardiovascular outcomes for the US population. Investigators interested in using the BP Control Lab are encouraged to contact the study authors.
ACKNOWLEDGMENTS
The PCORnet Blood Pressure Control Laboratory Team would like to acknowledge the engagement, input, moral support and other contributions of our patient advisory board (including author CM and also Kathi Sigona, Greg Merritt, Debbie Holmes, Patrice Williams, and Patty Poston) and our clinical nurse stakeholder (Judith Sansone).
SOURCES OF FUNDING
The PCORnet Blood Pressure Control Lab is funded by a partnership including the Patient-Centered Outcomes Research Institute (PCORI Contract PaCR-2017C2-8153), the American Medical Association (funding and in-kind support), and the American Heart Association (in-kind support). The American Medical Association and the American Heart Association are represented on the Steering Committee. The findings and conclusions are those of the authors and do not necessarily represent the views of PCORI, the American Medical Association, or the American Heart Association.
Two of us (MR and GW) are employees of the American Medical Association, and one (CMS) is an employee of the American Heart Association The funding sources described above partially support salaries (MJP, VF, TC, KMS, MS, JT, AMC, ECO, MF, CM, RMCD) or consulting income (CM) that allows us to complete the demonstration projects; and all authors hope to reuse the infrastructure developed here for other funding applications.
Footnotes
DISCLOSURES
The authors have no other conflicts to report.
REFERENCES
- 1.Global Burden of Disease Risk Factors. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1659–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, Murray CJ and Ezzati M. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009;6:e1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Institute for Health Metrics and Evaluation. GBD Compare. https://vizhub.healthdata.org/gbd-compare/. Accessed July 5, 2019.
- 4.Nwankwo T, Yoon SS, Burt V and Gu Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011–2012. NCHS Data Brief. 2013:1–8. [PubMed] [Google Scholar]
- 5.Wald DS, Law M, Morris JK, Bestwick JP and Wald NJ. Combination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trials. Am J Med. 2009;122:290–300. [DOI] [PubMed] [Google Scholar]
- 6.Whelton PK, Carey RM, Aranow WS, Casey DE, Collins KJ, Himmelfarb CD, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SCS, C. C., Stafford RS, Taler SJ, Thomas RJ, Williams KA, Williamson JD and Wright JT. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13–e115. [DOI] [PubMed] [Google Scholar]
- 7.Muntner P, Carey RM, Gidding S, Jones DW, Taler SJ, Wright JT, Jr. and Whelton PK. Potential US Population Impact of the 2017 ACC/AHA High Blood Pressure Guideline. Circulation. 2018;137:109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moise N, Huang C, Rodgers A, Kohli-Lynch CN, Tzong KY, Coxson PG, Bibbins-Domingo K, Goldman L and Moran AE. Comparative Cost-Effectiveness of Conservative or Intensive Blood Pressure Treatment Guidelines in Adults Aged 35–74 Years: The Cardiovascular Disease Policy Model. Hypertension. 2016;68:88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark CE, Smith LF, Taylor RS and Campbell JL. Nurse led interventions to improve control of blood pressure in people with hypertension: systematic review and meta-analysis. BMJ. 2010;341:c3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glynn LG, Murphy AW, Smith SM, Schroeder K and Fahey T. Interventions used to improve control of blood pressure in patients with hypertension. The Cochrane database of systematic reviews. 2010:CD005182. [DOI] [PubMed]
- 11.Jaffe MG, Lee GA, Young JD, Sidney S and Go AS. Improved blood pressure control associated with a large-scale hypertension program. JAMA. 2013;310:699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morgado MP, Morgado SR, Mendes LC, Pereira LJ and Castelo-Branco M. Pharmacist interventions to enhance blood pressure control and adherence to antihypertensive therapy: Review and meta-analysis. Am J Health Syst Pharm. 2011;68:241–53. [DOI] [PubMed] [Google Scholar]
- 13.Victor RG, Lynch K, Li N, Blyler C, Muhammad E, Handler J, Brettler J, Rashid M, Hsu B, Foxx-Drew D, Moy N, Reid AE and Elashoff RM. A Cluster-Randomized Trial of Blood-Pressure Reduction in Black Barbershops. N Engl J Med. 2018;378:1291–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landon BE, Hicks LS, O’Malley AJ, Lieu TA, Keegan T, McNeil BJ and Guadagnoli E. Improving the management of chronic disease at community health centers. N Engl J Med. 2007;356:921–34. [DOI] [PubMed] [Google Scholar]
- 15.Mueller M, Purnell TS, Mensah GA and Cooper LA. Reducing racial and ethnic disparities in hypertension prevention and control: what will it take to translate research into practice and policy? American journal of hypertension. 2015;28:699–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogedegbe G, Tobin JN, Fernandez S, Cassells A, Diaz-Gloster M, Khalida C, Pickering T and Schwartz JE. Counseling African Americans to Control Hypertension: cluster-randomized clinical trial main effects. Circulation. 2014;129:2044–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duan Y, Xie Z, Dong F, Wu Z, Lin Z, Sun N and Xu J. Effectiveness of home blood pressure telemonitoring: a systematic review and meta-analysis of randomised controlled studies. J Hum Hypertens. 2017;31:427–437. [DOI] [PubMed] [Google Scholar]
- 18.Margolis KL, Asche SE, Bergdall AR, Dehmer SP, Groen SE, Kadrmas HM, Kerby TJ, Klotzle KJ, Maciosek MV, Michels RD, O’Connor PJ, Pritchard RA, Sekenski JL, Sperl-Hillen JM and Trower NK. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control: a cluster randomized clinical trial. JAMA. 2013;310:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uhlig K, Patel K, Ip S, Kitsios GD and Balk EM. Self-measured blood pressure monitoring in the management of hypertension: a systematic review and meta-analysis. Annals of Internal Medicine. 2013;159:185–94. [DOI] [PubMed] [Google Scholar]
- 20.OMRON. HeartGuide: Take your blood pressure anytime, anywhere. https://omronhealthcare.com/products/heartguide-wearable-blood-pressure-monitor-bp8000m/. Accessed Jan 24, 2019.
- 21.Dey J, Gaurav A and Tiwari VN. InstaBP: Cuff-less Blood Pressure Monitoring on Smartphone using Single PPG Sensor. Conf Proc IEEE Eng Med Biol Soc. 2018;2018:5002–5005. [DOI] [PubMed] [Google Scholar]
- 22.Parati G, Torlasco C, Omboni S and Pellegrini D. Smartphone Applications for Hypertension Management: a Potential Game-Changer That Needs More Control. Curr Hypertens Rep. 2017;19:48. [DOI] [PubMed] [Google Scholar]
- 23.Kumar N, Khunger M, Gupta A and Garg N. A content analysis of smartphone-based applications for hypertension management. J Am Soc Hypertens. 2015;9:130–6. [DOI] [PubMed] [Google Scholar]
- 24.Boonyasai RT, Rakotz MK, Lubomski LH, Daniel DM, Marsteller JA, Taylor KS, Cooper LA, Hasan O and Wynia MK. Measure accurately, Act rapidly, and Partner with patients: An intuitive and practical three-part framework to guide efforts to improve hypertension control. J Clin Hypertens (Greenwich). 2017;19:684–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fontil V, Bibbins-Domingo K, Kazi DS, Sidney S, Coxson PG, Khanna R, Victor RG and Pletcher MJ. Simulating Strategies for Improving Control of Hypertension Among Patients with Usual Source of Care in the United States: The Blood Pressure Control Model. J Gen Intern Med. 2015;30:1147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Speich B, von Niederhausern B, Schur N, Hemkens LG, Furst T, Bhatnagar N, Alturki R, Agarwal A, Kasenda B, Pauli-Magnus C, Schwenkglenks M, Briel M and Group MARTA. Systematic review on costs and resource use of randomized clinical trials shows a lack of transparent and comprehensive data. J Clin Epidemiol. 2018;96:1–11. [DOI] [PubMed] [Google Scholar]
- 27.DiMasi JA, Grabowski HG and Hansen RW. Innovation in the pharmaceutical industry: New estimates of R&D costs. J Health Econ. 2016;47:20–33. [DOI] [PubMed] [Google Scholar]
- 28.Lauer MS and Bonds D. Eliminating the “expensive” adjective for clinical trials. Am Heart J. 2014;167:419–20. [DOI] [PubMed] [Google Scholar]
- 29.NIH. Project Information: 5R01HL127215–02. 2017. https://projectreporter.nih.gov/project_info_description.cfm?aid=9271229&icde=36919625&ddparam=&ddvalue=&ddsub=&cr=1&csb=default&cs=ASC&pball=. Accessed Nov 14, 2017
- 30.NIH. Project Information: 5UH3HL130688–03. 2017. https://projectreporter.nih.gov/project_info_details.cfm?aid=9344671&icde=36919637. Accessed Nov 14, 2017.
- 31.NIH. NIH Research Portfolio Online Reporting Tools (RePORT). http://projectreporter.nih.gov/. Accessed Feb 23, 2019.
- 32.Agency for Healthcare Research and Quality. Using Pragmatic Clinical Trials: to Test the Effectiveness of Patient-Centered Medical Home Models in Real-World Settings. 2013. Available at https://pcmh.ahrq.gov/sites/default/files/attachments/UsingPragmatic_032513comp.pdf. Accessed Feb 14, 2020.
- 33.Califf RM, Robb MA, Bindman AB, Briggs JP, Collins FS, Conway PH, Coster TS, Cunningham FE, De Lew N, DeSalvo KB, Dymek C, Dzau VJ, Fleurence RL, Frank RG, Gaziano JM, Kaufmann P, Lauer M, Marks PW, McGinnis JM, Richards C, Selby JV, Shulkin DJ, Shuren J, Slavitt AM, Smith SR, Washington BV, White PJ, Woodcock J, Woodson J and Sherman RE. Transforming Evidence Generation to Support Health and Health Care Decisions. N Engl J Med. 2016;375:2395–2400. [DOI] [PubMed] [Google Scholar]
- 34.Lauer MS and D’Agostino RB Sr. The randomized registry trial--the next disruptive technology in clinical research? N Engl J Med. 2013;369:1579–81. [DOI] [PubMed] [Google Scholar]
- 35.Sherman RE, Anderson SA, Dal Pan GJ, Gray GW, Gross T, Hunter NL, LaVange L, Marinac-Dabic D, Marks PW, Robb MA, Shuren J, Temple R, Woodcock J, Yue LQ and Califf RM. Real-World Evidence - What Is It and What Can It Tell Us? N Engl J Med. 2016;375:2293–2297. [DOI] [PubMed] [Google Scholar]
- 36.National Quality Forum. 0018: Controlling High Blood Pressure. 2017; http://www.qualityforum.org/News_And_Resources/Press_Releases/2012/NQF_Endorses_Cardiovascular_Measures.aspx. Accessed October 29, 2017.
- 37.California Department of Health Care Services. California Medi-Cal 2020 Demontration. Available at https://www.dhcs.ca.gov/provgovpart/Documents/CAMedi-Cal2020STCsAmended04052018.pdf. Accessed 2/12/2020.
- 38.Centers for Medicare and Medicaid Services. Accountable Care Organization (ACO) 2018 Quality Measures, Narrative Specifications Document, January 20, 2018. In: Program CMSS, ed2018. [Google Scholar]
- 39.Merit-Based Incentive Payment System (MIPS) Overview. https://qpp.cms.gov/mips/overview. Accessed May 17, 2019.
- 40.Hardy ST, Loehr LR, Butler KR, Chakladar S, Chang PP, Folsom AR, Heiss G, MacLehose RF, Matsushita K and Avery CL. Reducing the Blood Pressure-Related Burden of Cardiovascular Disease: Impact of Achievable Improvements in Blood Pressure Prevention and Control. J Am Heart Assoc. 2015;4:e002276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fleurence RL, Curtis LH, Califf RM, Platt R, Selby JV and Brown JS. Launching PCORnet, a national patient-centered clinical research network. J Am Med Inform Assoc. 2014;21:578–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Selby JV, Krumholz HM, Kuntz RE and Collins FS. Network news: powering clinical research. Sci Transl Med. 2013;5:182fs13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Department of Health and Human Services. Mobilizing Research: A Research Resource to Enhance mHealth Research (U2C). https://grants.nih.gov/grants/guide/rfa-files/RFA-od-15-129.html. Accessed Oct 29, 2017.
- 44.Eureka Mobile Research: Now, everyone can contribute to research that makes the world healthier. http://info.eurekaplatform.org/. Accessed Oct 29, 2017.
- 45.Collins FS, Hudson KL, Briggs JP and Lauer MS. PCORnet: turning a dream into reality. J Am Med Inform Assoc. 2014;21:576–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.PCORI. PCORnet: The National Patient-Centered Clinical Research Network. http://www.pcori.org/content/pcornet-national-patient-centered-clinical-research-network. Accessed Oct 1, 2014.
- 47.People-Centered Research Foundation. https://pcrfoundation.org/. Accessed Feb 14, 2020.
- 48.PCORI. PCORnet Common Data Model (CDM). https://pcornet.org/pcornet-common-data-model/. Accessed Feb 14, 2020.
- 49.Smith SM, McAuliffe K, Hall JM, McDonough CW, Gurka MJ, Robinson TO, Sacco RL, Pepine C, Shenkman E and Cooper-DeHoff RM. Hypertension in Florida: Data From the OneFlorida Clinical Data Research Network. Prev Chronic Dis. 2018;15:E27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.University of California, San Francisco. UCSF Awarded $9.75 Million to Create Platform Accelerating Mobile Health Research. 2015; https://www.ucsf.edu/news/2015/10/131911/ucsf-awarded-975-million-create-platform-accelerating-mobile-health-research. Accessed October 29, 2017.
- 51.Pletcher MJ, Forrest CB and Carton TW. PCORnet’s Collaborative Research Groups. Patient Relat Outcome Meas. 2018;9:91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.PCORI. Collaborative Research Groups. http://www.pcornet.org/collaborative-research-groups/. Accessed Oct 4, 2017.
- 53.University of Florida Clinical and Translational Science Institute. OneFlorida Clinical Research Consortium. https://www.ctsi.ufl.edu/ctsa-consortium-projects/oneflorida/. Accessed Aug 12, 2019.
- 54.REACHnet. https://reachnet.org/. Accessed Aug 12, 2019.
- 55.ADVANCE Collaborative. http://advancecollaborative.org/. Accessed Aug 12, 2019.
- 56.STAR: Stakeholders, Technology, and Research CRN. https://starcrn.org/. Accessed Aug 12, 2019.
- 57.The Heart Research Alliance. https://www.heartresearchalliance.org/. Accessed Aug 10, 2019.
- 58.PCORI. The Health eHeart Alliance: Project Summary. Available at https://www.pcori.org/research-results/2013/health-eheart-alliance. Accessed Aug 17, 2019.
- 59.PCORI. Using PCORnet to Compare Blood Pressure Control Strategies. https://www.pcori.org/research-results/2018/using-pcornet-compare-blood-pressure-control. Accessed Feb 14, 2020.
- 60.Egan BM, Sutherland SE, Rakotz M, Yang J, Hanlin RB, Davis RA and Wozniak G. Improving Hypertension Control in Primary Care With the Measure Accurately, Act Rapidly, and Partner With Patients Protocol. Hypertension. 2018;72:1320–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hanlin RB, Asif IM, Wozniak G, Sutherland SE, Shah B, Yang J, Davis RA, Bryan ST, Rakotz M and Egan BM. Measure Accurately, Act Rapidly, and Partner With Patients (MAP) improves hypertension control in medically underserved patients: Care Coordination Institute and American Medical Association Hypertension Control Project Pilot Study results. J Clin Hypertens (Greenwich). 2018;20:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.American Medical Association. Quality ID #236 (NQF 0018): Controlling High Blood Pressure – National Quality Strategy Domain: Effective Clinical Care. https://www.acr.org/-/media/ACR/NOINDEX/Measures/2018_Measure_236_Registry.pdf. Accessed Feb 14, 2020.
- 63.US Department of Health and Human Services Health Resources and Services Administration. Hypertension Control. https://www.hrsa.gov/sites/default/files/quality/toolbox/508pdfs/hypertensioncontrol.pdf. Accessed Feb 14, 2020.
- 64.eCQI. Hypertension: Improvement in Blood Pressure. 2018; https://ecqi.healthit.gov/ecqm/ep/2018/cms065v7. Accessed Aub 17, 2019.
- 65.Bellows BK, Ruiz-Negron N, Bibbins-Domingo K, King JB, Pletcher MJ, Moran AE and Fontil V. Clinic-Based Strategies to Reach United States Million Hearts 2022 Blood Pressure Control Goals. Circ Cardiovasc Qual Outcomes. 2019;12:e005624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.American Heart Association, American Medical Association. TARGET:BP. https://targetbp.org/. Accessed Aug 15, 2017.
- 67.ClinicalTrials.gov. Improving Blood Pressure Control in Diverse Populations by Measuring Accurately, Acting Rapidly, and Partnering With Patients (BP MAP). https://clinicaltrials.gov/ct2/show/NCT03818659. Accessed Aug 17, 2019.
- 68.Thiboutot J, Sciamanna CN, Falkner B, Kephart DK, Stuckey HL, Adelman AM, Curry WJ and Lehman EB. Effects of a web-based patient activation intervention to overcome clinical inertia on blood pressure control: cluster randomized controlled trial. J Med Internet Res. 2013;15:e158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aboumatar HJ, Carson KA, Beach MC, Roter DL and Cooper LA. The impact of health literacy on desire for participation in healthcare, medical visit communication, and patient reported outcomes among patients with hypertension. J Gen Intern Med. 2013;28:1469–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fontil V, McDermott K, Tieu L, Rios C, Gibson E, Sweet CC, Payne M and Lyles CR. Adaptation and Feasibility Study of a Digital Health Program to Prevent Diabetes among Low-Income Patients: Results from a Partnership between a Digital Health Company and an Academic Research Team. J Diabetes Res. 2016;2016:8472391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.TheSweetHome.com. The Best Blood Pressure Monitors for Home Use. 2017; http://thesweethome.com/reviews/best-blood-pressure-monitors-for-home-use/#how-we-picked-and-tested. Accessed October 29, 2017.
- 72.ClinicalTrials.gov. The PCORnet Blood Pressure Home Monitoring Study (BP HOME). https://clinicaltrials.gov/ct2/show/NCT03796689. Accessed Feb 14, 2020.


