Abstract
Bacterial vaginosis (BV) is a dysbiotic condition of the vaginal microbiome associated with higher risk of infection by Neisseria gonorrhoeae—the cause of gonorrhea. Here we test if one known facet of BV—the presence of bacterial cytolysins—leads to mobilization of intracellular contents that enhance gonococcal virulence. We cloned and expressed recombinant vaginolysin (VLY), a cytolysin produced by the BV-associated bacterium Gardnerella, verifying that it liberates contents of cervical epithelial (HeLa) cells, while vector control preparations did not. We tested if VLY mediates a well-known gonococcal virulence mechanism—the molecular mimicry of host glycans. To evade host immunity, N. gonorrhoeae caps its lipooligosaccharide (LOS) with α2-3-linked sialic acid. For this, gonococci must scavenge a metabolite made inside host cells. Flow cytometry-based lectin-binding assays showed that gonococci exposed to vaginolysin-liberated contents of HeLa cells displayed greater sialic acid capping of their LOS. This higher level of bacterial sialylation was accompanied by increased binding of the complement regulatory protein factor H, and greater resistance to complement attack. Together these results suggest that cytolytic activities present during BV may enhance the ability of N. gonorrhoeae to capture intracellular metabolites and evade host immunity via glycan molecular mimicry.
Keywords: bacterial vaginosis, complement, cytolysin, factor H, Gardnerella, gonorrhea, sialic acid, vaginolysin
Gonococci can capture intracellular metabolites and use them to mimic host glycans. Here we show that cytolysins present during bacterial vaginosis can enhance this gonococcal virulence mechanism by freeing cervical epithelial intracellular contents.
In most humans the vaginal microbiome is dominated by Lactobacillus species [1]. High levels of Lactobacillus are associated with optimal sexual and reproductive health outcomes in a wide variety of settings globally [2]. In contrast, nearly one-third of reproductive age women in the United States are affected by bacterial vaginosis (BV), a dysbiotic condition of vaginal microbiota [3]. In BV there is a lower relative abundance of Lactobacillus, especially L. crispatus. and outgrowth of diverse anaerobic bacteria [1, 4]. This shift in microbial composition is often accompanied by higher vaginal pH (>4.5), a fishy odor, and changes in vaginal mucus characteristics and epithelial phenotypes [4, 5]. BV has been associated with numerous adverse health outcomes, including increased risk of infection by the bacterium Neisseria gonorrhoeae [6].
Neisseria gonorrhoeae is the causative agent of the uniquely human gonorrhea, a prolific sexually transmitted infection with upwards of 80 million new cases per year worldwide [7]. Some individuals with gonococcal infection of the cervicovaginal tract can experience severe consequences, including pelvic inflammatory disease [8], ectopic pregnancy [9], and, in rare cases, bacteremia [10]. There is currently no commercially available vaccine for N. gonorrhoeae infection, and resistant strains have been found against almost every available antibiotic [7]. There have been numerous clinical studies indicating that BV is a risk factor for coincident and new gonococcal infection, even after adjusting for relevant confounders [6, 11–13]. However, the mechanisms by which BV predisposes individuals to N. gonorrhoeae infection have not been well defined.
Some of the abundant bacteria in BV produce cytolytic proteins. In particular, vaginolysin (VLY) is a cytolysin produced by members of the genus Gardnerella, which are often identified as the most abundant members of the BV consortium [1, 14]. In other biological contexts, cytolytic proteins can aid bacterial colonization or virulence, for example by lysing host red blood cells (RBCs) and promoting iron acquisition by liberating intracellular heme [15]. We hypothesized that cytolytic proteins in BV may enhance gonococcal colonization of the female reproductive tract by disrupting genital epithelial cells and freeing host intracellular contents that aid in acquisition of virulence traits. To test this hypothesis mechanistically, we specifically investigated if Gardnerella VLY enhances resistance of N. gonorrhoeae to attack by the host complement system using a well-described mechanism of immune evasion in which the bacterium scavenges carbohydrate building blocks from host cells in a ploy to decorate its own surface with host-like glycans.
N. gonorrhoeae engages in a feat of molecular mimicry by directly transferring sialic acid residues to its surface lipooligosaccharide (LOS) [16]. N-acetylneuraminic acid or Neu5Ac is the major sialic acid found in humans. It is present on all cell surfaces and particularly abundant at mucosal surfaces, including the genital tract. Factor H is found in mammalian body fluids and inhibits the host complement system by recognizing sialic acids and preventing the complement system from targeting self-cells as foreign [17]. N. gonorrhoeae mimics the appearance of host glycans by scavenging a metabolically activated form of sialic acid, cytidine-5′-monophospho-N-acetyl neuraminic acid (CMP-Neu5Ac) to cap its LOS with Neu5Ac [16]. N. gonorrhoeae lacks the ability to synthesize CMP-Neu5Ac, and therefore must scavenge this molecule from host cells [18].
In this study we use an in vitro model to test if VLY treatment of cervical epithelial cells potentiates N. gonorrhoeae survival by enabling resistance to the complement system via glycan molecular mimicry. If VLY treatment of host cells is able to enhance CMP-Neu5Ac capture and LOS sialylation, we expect to see (1) capping of surface galactose residues, (2) higher α2-3 surface sialylation that is dependent on its surface sialyltransferase (Lst), (3) more binding to Factor H, and (4) greater resistance to complement attack.
METHODS
Bacterial Strains and Cell Lines
N. gonorrhoeae F62 and F62 Δlst were grown on 15% chocolate agar plates (GC agar base [Remel] plus 5% laked sheep's blood) and incubated for 17–19 hours at 37°C, 5% CO2. Gardnerella American Type Culture Collection (ATCC) 14019 was grown either on New York City (NYC) III agar or liquid medium supplemented with 10% heat-inactivated horse serum. ClearColi BL21(DE3) cells (Biosearch Technologies) were cultured per manufacturer instructions.
Protein Cloning and Expression
Truncated VLY gene, vly(tr), without the signal sequence was constructed by amplification of the vly gene from Gardnerella vaginalis ATCC14019 beginning at position A45, similar to a previously described method, but using a 6-His tag [19]. This construct was ligated into pET28a vector and transformed into ClearColi BL21(DE3) cells.
For purification of VLY, cells carrying vly(tr)-pET28a or the vector only (empty) plasmid were grown in Terrific Broth plus kanamycin (50 µg/mL) shaking at 37°C to an OD600 of 0.4. Protein expression was induced with 1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) for 16 hours at 18°C before being pelleted and resuspended in Tris-HCL buffer (50 mM Tris HCl plus 300 mM NaCl, 5 mM imidazole; pH 8.0), containing protease inhibitors (Roche EDTA free, 0.2 mg/mL lysozyme, and 160 µM phenylmethylsulfonyl fluoride). Cells were lysed in this buffer via sonication on ice 26 times, 15 second each, at 10% amplitude. Clarified lysates were incubated with TALON Metal Affinity Resin cobalt beads (Takara), and beads were then washed with Tris-HCL (pH 8.0) buffers with increasing concentrations of imidazole. The bound proteins were eluted using Tris-HCL buffer (pH 8.0) with 300 mM imidazole. Preparations were evaluated for purity using Coomassie sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and protein concentrations were estimated using BCA Protein Assay Kit (Pierce). Prior to use in assays, protein preparations were buffer exchanged into 1x Dulbecco's phosphate-buffered saline (DPBS) using PD Midi-Trap G-25 columns (Cytiva).
Hemolysis
To wash and isolate intact red blood cells, 5–10 mL of washed single-donor human RBCs in Alsever's solution (Innovative Research, Inc) were added to a 50-mL conical tube, followed by 30–40 mL of cold DPBS (no calcium, no magnesium). The tube was gently inverted several times to mix RBCs with DPBS, before pelleting the RBCs at 375g, 4°C, for 10 minutes. DPBS wash of the pellet was repeated until the supernatant was clear rather than pink, indicating the absence of lysed RBCs and free hemoglobin. After the final wash, RBCs were resuspended in cold DPBS to a calculated OD640 of 10, and 62.5 µL of this suspension was added to each well of a 96-well V-bottom plate. Dilutions of recombinant VLY (2.5–1000 ng/mL) or the vector-only control were made in DPBS and 62.5 µL of each dilution was added (in triplicate) to wells containing RBCs, to create a final protein concentration range of 1.25–500 ng/mL. The plate was incubated aerobically at 37°C, shaking at approximately 300 rpm for 40 minutes, followed by centrifugation at 375g, 4°C, for 15 minutes. Supernatants were transferred to a clear, half-area polystyrene plate and heme release was determined by reading absorbance at 545 nm.
Cytolysis (LDH Release)
HeLa cells (purchased from ATCC) were seeded in a 96-well sterile, tissue culture treated plate and grown to approximately 85%–99% confluency in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS). Prior to the assay, medium was removed and cells were washed twice with DPBS (no calcium, no magnesium). Dilutions of recombinant VLY (0.25–6 µg/mL) or parallel dilutions of the vector-only control were made in DMEM without FBS or phenol red, and 150 µL of each dilution was added (in triplicate) to wells containing cells. The plate was incubated for 1.5 hours, shaking gently at approximately 175 rpm in 37°C, 5% CO2. High-lysis control wells had no VLY added, but received 7.5 µL of Lysis Solution from the Roche Cytotoxicity Detection Kit Plus 15 minutes before the end of incubation. Supernatants were removed and centrifuged at 200g for 5 minutes to pellet any lifted cells. Lactate dehydrogenase (LDH) analysis was conducted on supernatants using a Roche Cytotoxicity Detection Kit Plus, per manufacturer instructions. Percent LDH release was determined by comparing sample values against high-lysis control.
VLY Treatment of HeLa Cells to Generate Supernatants With Intracellular Contents
HeLa cells were grown to 85%–99% confluence in a 100-mm sterile tissue culture treated Petri dish. Prior to treatment, cells were washed twice with DPBS (no calcium, no magnesium). A 1 µg/mL solution of recombinant VLY (or equivalently diluted empty-vector preparation) was prepared in DMEM (no FBS, no phenol red), and 4 mL of this solution was added to washed cells. Plates were incubated at 37°C, 5% CO2, shaking at approximately 175 rpm, for 1.5 hours. Following incubation, supernatants were collected into conical tubes and centrifuged at 200g for 5 minutes to remove any lifted cells. Supernatants were then passed through a 0.22-µm cutoff filter to remove any cell debris before being used in gonococcal sialylation experiments.
Gonococcal Sialylation
Sterile-filtered HeLa cell supernatants (or exogenous CMP-Neu5Ac dilutions in DMEM) were combined 3:1 with 4× GC media, and 315 µL was added to wells of a 48-well tissue culture treated plate (Corning). An overnight plate of N. gonorrhoeae was scraped to make an OD600 0.5 suspension in assay medium (DMEM plus 1× GC media) and 35 µL was added to each well for a starting OD600 of 0.05. The plate was then incubated for 3 hours at 37°C, 5% CO2, shaking at approximately 300 rpm. After incubation, bacteria were collected and transferred to a 96-deepwell (2 mL) plate (Fisher Scientific) and washed 3 times with wash buffer (1 mL of DPBS [no calcium, no magnesium] plus 0.2% bovine serum albumin [BSA]). To minimize any contaminating signal from sialylated glycoproteins in the wash buffer, immunoglobulin G (IgG)-free BSA (Sigma) was used. After the final wash, bacterial samples were resuspended in wash buffer and each sample was split into 2–3 groups for use in different downstream assays.
Lectin Binding
To create lectin preconjugation solution, biotinylated lectins (Maackia amurensis lectin I [MAL-I] and Erythrina cristagalli lectin [ECA]) were diluted 1:500 into staining buffer (DPBS [with calcium and magnesium] plus 0.1% BSA) containing Cy5-streptavidin (1:1000). Lectins and Cy5-SA were incubated at room temperature for approximately 1 hour, with end-over-end mixing. Following incubation with HeLa cell contents, washed bacteria were transferred to a 96-well V-bottom plate, spun down, and resuspended in 50 µL preconjugated lectin solution. Bacteria were incubated, shaking at 37°C for approximately 50 minutes, then fixed with 1% formalin. Lectin binding was assessed via flow cytometry using the BD FACSCanto II High Throughput Sampler option.
Factor H Binding
Following incubation with HeLa cell contents, bacteria were transferred to a 96-well V-bottom plate, spun down, and resuspended in wash buffer containing either 0.1 mg/mL Fc3/FH* (Fc region of human IgG3 fused to domains 18–20 of human Factor H), or 0.1 mg/mL Fc3 alone [20]. The plate was incubated, shaking at 37°C for 1–1.5 hours before cells were spun down and resuspended in a 1:000 dilution of fluorescein isothiocyanate (FITC)-conjugated anti-human IgG in wash buffer. Secondary incubation was done shaking at room temperature for 30–40 minutes. Bacteria were then fixed in 1% formalin and analyzed by flow cytometry as described for lectin binding.
Serum Resistance
Following incubation with HeLa cell contents, 10 µL of washed bacterial samples (approximately 6 × 105 colony-forming units [CFU]) were transferred to a 96-well plate and exposed to 4% pooled normal human serum (Complement Technologies) in wash buffer. From each well, 10 µL was immediately removed and used to perform serial dilution and colony enumeration (t = 0). The 96-well plate was incubated at 37°C, 5% CO2, shaking at approximately 300 rpm for 40 minutes. At the end of the incubation, 10 µL was again removed from each well and used for CFU enumeration (t = 40). Percent survival was determined by dividing the CFU at t = 40 by the CFU at t = 0, and multiplying by 100.
RESULTS
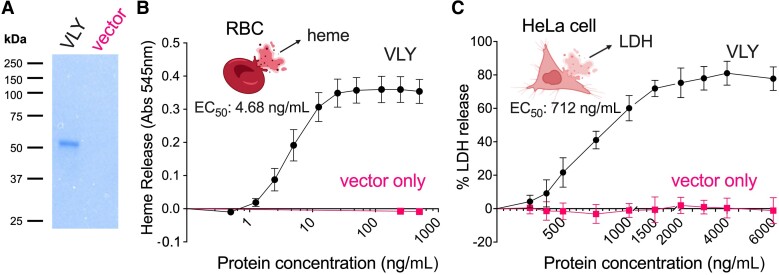
To test our working hypothesis, that Gardnerella VLY potentiates a well-known mechanism of gonococcal molecular mimicry, we first exposed human RBCs (hRBCs) and cervical epithelial cells to purified recombinant VLY expressed in Escherichia coli and measured the release of intracellular contents. As described in the “Methods” section, we began by cloning and optimizing expression of VLY (sequence shown in Supplementary Figure 1). To avoid biological effects that might be elicited by endotoxin, a modified E. coli strain containing a detoxified form of lipopolysaccharide was used [21]. As an additional control, the empty plasmid vector was transformed into the same E. coli expression strain and identical parallel purification procedures were performed. Preparations from cells containing the VLY construct revealed a single band at the expected molecular weight (about 53 kD) in SDS-PAGE, while the vector-only control preparation yielded no bands (Figure 1A). As expected, incubating recombinant VLY with hRBCs led to heme release (Figure 1B). Maximum heme release from hRBCs was achieved between 50 and 100 ng/mL VLY, with a 50% maximum effective concentration (EC50) of 4.68 ng/mL (95% confidence interval [CI], 4.18–5.26 ng/mL, hillslope 1.95).
Figure 1.
Exposure of human cells to Gardnerella VLY leads to a release of intracellular contents from RBCs and cervical epithelial cells. A, Coomassie blue SDS-PAGE gel showing recombinant purified VLY and a parallel control preparation using cells containing the empty expression vector. B, Cytolytic activity of VLY against human RBCs determined by heme release (detected as absorbance at 545 nm). The EC50 was calculated using a 4-parameter nonlinear regression model. The data are a combination of 3 independent experiments, with 3 biological replicates per experiment. C, Cytolytic activity of VLY against HeLa cells determined by LDH release. The EC50 was calculated using a 4-parameter nonlinear regression model. Data are a combination of 2 independent experiments, with 3 to 4 biological replicates per experiment. B and C, error bars represent standard deviation. Abbreviations: Abs, absorbance; EC50, 50% maximum effective concentration; LDH, lactate dehydrogenase; RBC, red blood cell; SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis; VLY, vaginolysin.
VLY was also active against HeLa cells, a human cervical epithelial cell line, as measured by the release of a cytosolic enzyme, LDH, often used as a marker of cell membrane damage. Compared to hRBCs, HeLa cells required 20- to 30-fold more VLY (1500–2000 ng/mL VLY) to release intracellular contents. Likewise, the EC50 of VLY against HeLa cells (712 ng/mL) was about 150-fold higher compared to hRBCs (95% CI, 670–757 ng/mL, hillslope 3.02).
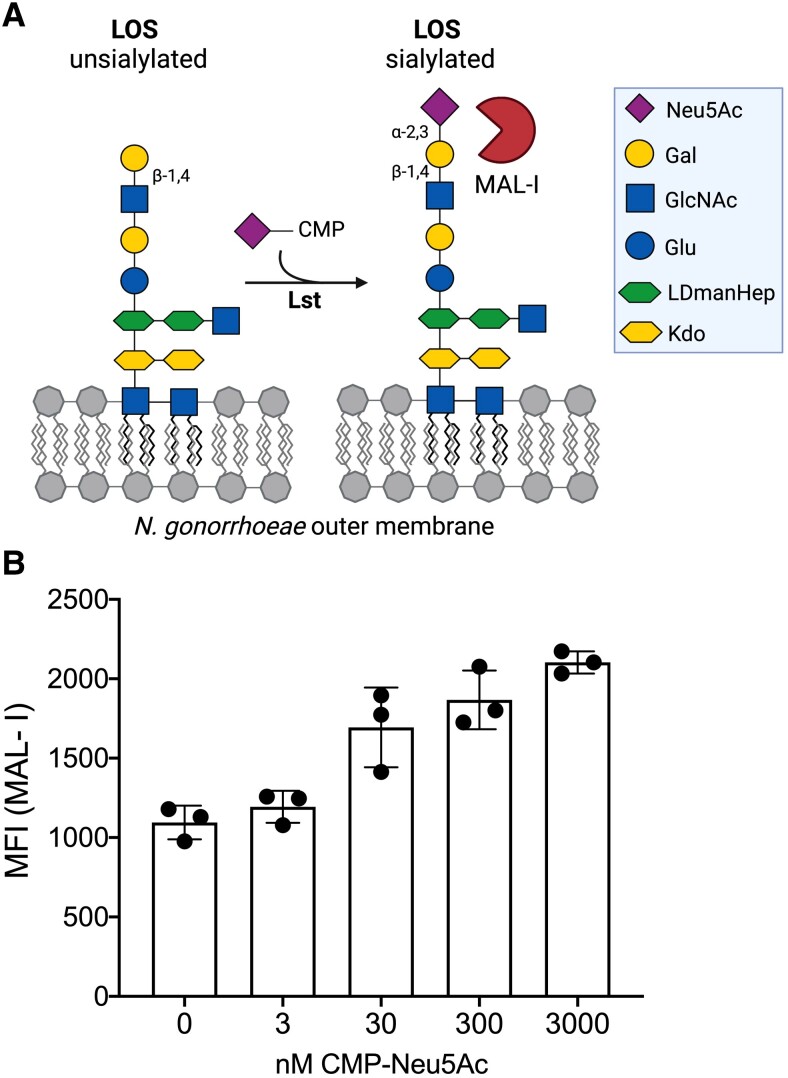
To study structural elements of the gonococcal LOS relevant to our hypothesis, we developed a lectin-binding assay in flow cytometry to monitor terminal α2-3-linked sialic acids (Figure 2A). Prior studies have shown that at baseline, gonococcal lacto-N-neotetraose (LNnT) LOS terminates with galactose (Gal) in a β1-4 linkage to N-acetylglucosamine (GlcNAc). When CMP-Neu5Ac is provided or becomes available in the host, the gonococcal LOS-sialyltransferase (Lst) adds Neu5Ac to the terminal Gal in an α2-3 linkage (Figure 2A). MAL-I recognizes the Neu5Acα2-3Galβ1-4GlcNAc moiety that terminates sialylated LOS (Figure 2A). This flow cytometry-based assay shows that MAL-I binds to N. gonorrhoeae (strain F62) provided with exogenous CMP-Neu5Ac in a dose-dependent manner (Figure 2B). Additional experiments demonstrated that this increase in MAL-I binding requires Lst (Supplementary Figure 2).
Figure 2.
MAL-I binds to sialylated gonococcal LOS. A, Schematic of Neu5Ac addition to the terminal lactosamine of gonococcal LOS. MAL-I binding site is indicated. B, MAL-I binding to Neisseria gonorrhoeae F62 wild type following bacterial incubation with increasing concentrations of CMP-Neu5Ac, presented as MFI. Data are from a single experiment, with 3 biological replicates per CMP-Neu5Ac concentration. Error bars represent standard deviation. Abbreviations: CMP-Neu5Ac, cytidine-5′-monophospho-N-acetyl neuraminic acid; Gal, galactose; GlcNAc, N-acetylglucosamine; LDmanHep, L-glycero-D-manno-heptose; Kdo, 2-keto-3-deoxy-D-mannooctanoic acid; LOS, lipooligosaccharide; Lst, LOS-sialyltransferase; MAL-I, Maackia amurensis lectin I; MFI, median fluorescence intensity.
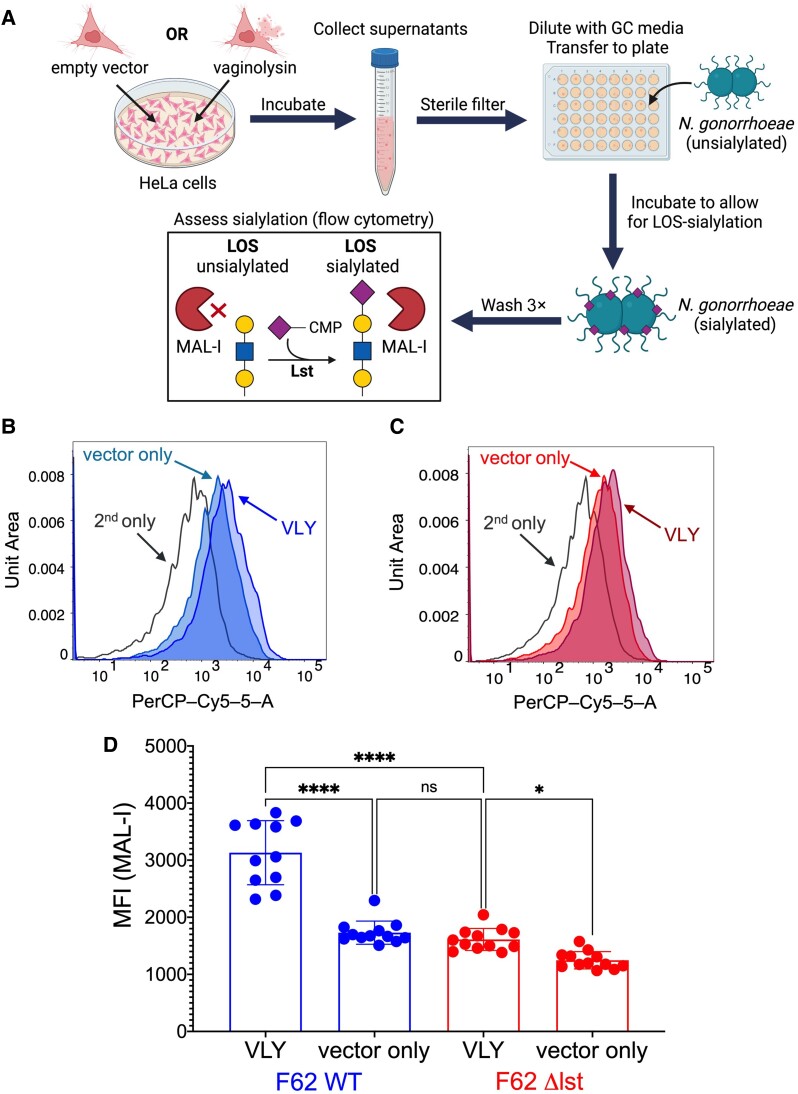
We hypothesized that exposure of epithelial cells to VLY would create conditions in which the gonococcus can more readily place sialic acids onto its LOS. To test this, N. gonorrhoeae was incubated with supernatants of cervical epithelial cells treated with VLY. The general method for this schematic is represented in Figure 3A. Briefly, HeLa cells were treated with 1 µg/mL VLY, or with an equivalent dilution of the control preparation from cells harboring the empty vector. After 90 minutes of incubation, HeLa supernatants were collected, centrifuged, and filtered to remove cell debris >0.22 µm. N. gonorrhoeae strain F62 was then incubated with supernatants from cells treated with VLY or the vector control preparation and MAL-I binding was evaluated. Consistent with our hypothesis, gonococcus exhibited greater binding to MAL-I after exposure to VLY-extracted cellular contents compared to vector controls (Figure 3B and 3D). The VLY-mediated increase in MAL-I binding was almost entirely abrogated by deleting the gene encoding Lst (Figure 3C and 3D). We also observed a small but significant source of Lst-independent MAL-I binding, which is discussed in the “Conclusions” section.
Figure 3.
Treatment of cervical epithelial cells with Gardnerella VLY potentiates Lst-dependent Neisseria gonorrhoeae sialylation. N. gonorrhoeae F62 WT and Δlst were incubated with the supernatants of HeLa cells that had been exposed to either 1 μg/mL VLY or a parallel dilution of the vector control preparation. A, Schematic of the method for Figure 3 experiments, and those shown in Figure 4C and 4D. B–D, MAL-I binding to N. gonorrhoeae F62 WT and Δlst after incubation with HeLa cell supernatants. B and C, Representative histograms of MAL-I binding to (B) F62 WT or (C) Δlst. D, MAL-I binding presented as MFI. Data are a combination of 2 independent experiments, with 3–4 biological replicates per experiment. Error bars represent standard deviation. Statistical significance was evaluated using ordinary 1-way ANOVA. *P < .05, ****P < .0001. Abbreviations: CMP, cytidine-5′-monophosphate; LOS, lipooligosaccharide; Lst, LOS-sialyltransferase; MAL-I, Maackia amurensis lectin I; MFI, median fluorescence intensity; PerCP-Cy5-5-A, flow cytometer channel detecting red light emissions; ns, not significant; VLY, vaginolysin; WT, wild type.
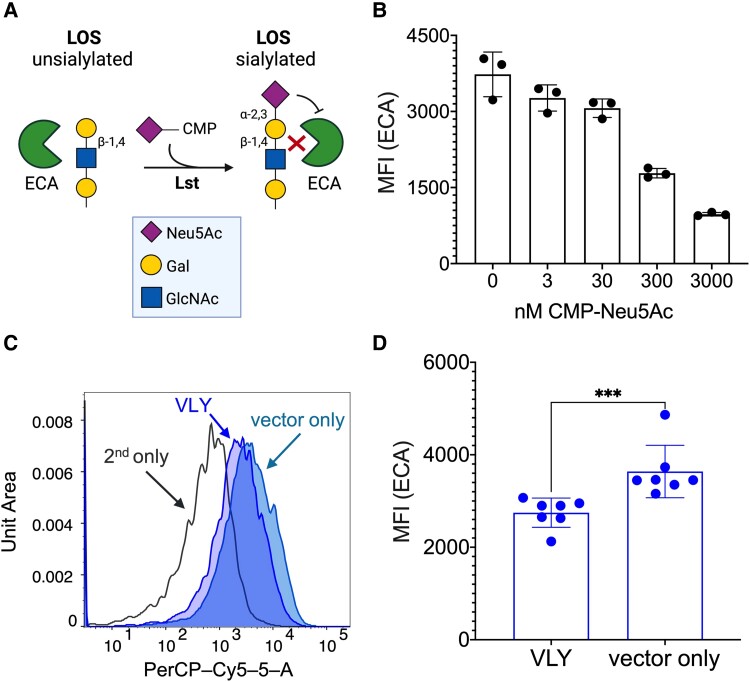
In other experiments, ECA was used to probe surface glycan structures of N. gonorrhoeae. ECA recognizes the nonsialylated LOS structure containing terminal Galβ1-4GlcNAc (Figure 4A). As expected, ECA binding to N. gonorrhoeae F62 was highest in the absence of CMP-Neu5Ac, while incubation with increasing concentrations of CMP-Neu5Ac resulted in a dose-dependent reduction in ECA binding as the galactose residues became capped with sialic acids (Figure 4B). Likewise, binding of ECA to gonococci was lower after exposure to VLY-extracted cellular contents compared to vector controls (Figure 4C and 4D). In both instances, the difference in ECA binding was dependent on Lst activity (Supplementary Figure 3). Together, these data suggest that the exposure of cervical epithelial cells to Gardnerella VLY enhances gonococcal LOS sialylation, indicated by higher MAL-I binding and lower ECA binding.
Figure 4.
Neisseria gonorrhoeae caps LOS galactose when exposed to VLY-liberated HeLa contents. ECA recognizes the unsialylated terminal epitope of gonococcal LOS, and therefore binding of ECA acts as a negative readout of gonococcal sialylation. A, Diagram of N. gonorrhoeae unsialylated LOS, with ECA-binding epitope indicated. B, ECA binding to N. gonorrhoeae F62 WT following bacterial incubation with increasing concentrations of CMP-Neu5Ac, presented as MFI. Data are from a single experiment, with 3 biological replicates per concentration. Error bars represent standard deviation. C and D, ECA binding after N. gonorrhoeae F62 WT was incubated with the supernatants of HeLa cells that had been exposed to either 1 μg/mL VLY or a parallel dilution of the pET28a vector-only control. C, Representative histogram of ECA binding to F62 WT. D, ECA binding presented as MFI. Data are a combination of 2 independent experiments, with 3–4 biological replicates per experiment. Error bars represent standard deviation. Significance determined by Mann-Whitney U-test. ***P < .001. Abbreviations: CMP-Neu5Ac, cytidine-5′-monophospho-N-acetyl neuraminic acid; ECA, Erythrina cristagalli lectin; Gal, galactose; LOS, lipooligosaccharide; Lst, LOS-sialyltransferase; MFI, median fluorescence intensity; PerCP-Cy5-5-A, flow cytometer channel detecting red light emissions; VLY, vaginolysin; WT, wild type.
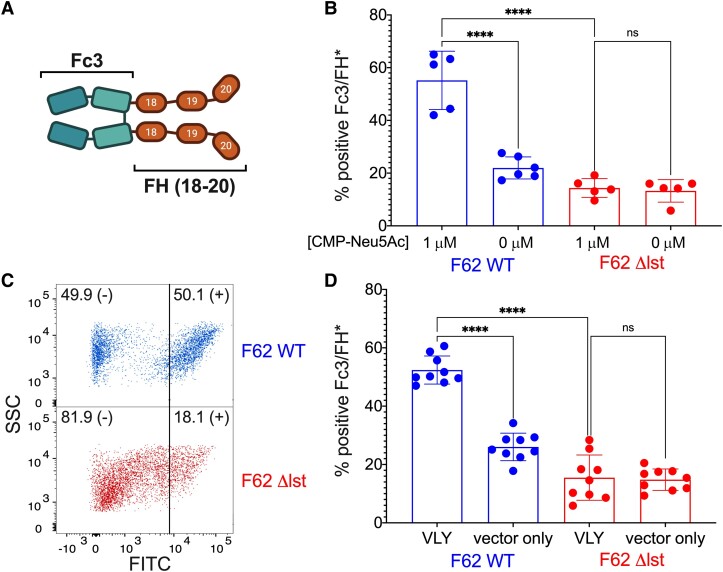
One of the major mechanisms by which LOS sialylation potentiates gonococcal virulence is via recruitment of host Factor H (FH). The addition of α2-3-linked Neu5Ac to LNnT LOS increases binding of FH to the gonococcal surface [22], where it inhibits activation of the alternative complement pathway and protects the gonococcus from killing by the complement cascade [17]. We hypothesized that the enhanced gonococcal sialylation observed in Figure 3 and Figure 4 upon exposure to VLY-liberated HeLa cell contents would also lead to increased recruitment of FH. To test this hypothesis, we used a chimeric FH protein (Fc3/FH*or S2534) [20] comprised of the Fc region of human IgG3 fused to domains 18–20 of human Factor H, which are known to interact with the sialylated gonococcal surface (Figure 5A) [23]. Because S2534 is being developed as an antigonococcal immunotherapeutic, a point mutation (D to G at position 1119 in domain 19) was introduced to abrogate binding to host cells without affecting binding to sialylated gonococci. Prior studies have shown that Fc3/FH* binds to N. gonorrhoeae in a sialylation-dependent manner [20], which we confirmed in our assay system (Figure 5B).
Figure 5.
Exposure of Neisseria gonorrhoeae to VLY-liberated HeLa contents increases sialylation-dependent recruitment of human FH. A, Schematic of chimeric FH protein S2534 (Fc3/FH*). S2534 consists of human IgG3 Fc receptor (Fc3) fused to the 3 C-terminal domains 18–20 of human FH. A point mutation in FH domain 19 (D to G at position 1119), which was introduced to abrogate binding to host cells, does not interfere with binding of the C-terminus of FH to sialylated N. gonorrhoeae. B, Binding of Fc3/FH* to N. gonorrhoeae F62 WT and Δlst following incubation with either 0 μM or 1 μM CMP-Neu5Ac. Data are presented as the percentage of gated events positive for FITC. C and D, Fc3/FH* binding after N. gonorrhoeae F62 WT and Δlst were incubated with the supernatants of HeLa cells that had been exposed to either 1 μg/mL VLY or a parallel dilution of the pET28a vector-only control. C, Representative dot-plot of Fc3/FH* binding to N. gonorrhoeae WT and Δlst after exposure to VLY-extracted HeLa contents. D, Fc3/FH* binding presented as the percentage of gated events positive for FITC. B and D, Data are a combination of 2 independent experiments, with 2–3 (B) or 4–5 (D) biological replicates per experiment. Error bars represent standard deviation. Significance determined by 1-way ANOVA. ****P < .0001. Abbreviations: CMP-Neu5Ac, cytidine-5′-monophospho-N-acetyl neuraminic acid; FH, Factor H; FITC, fluorescein isothiocyanate; lst, lipooligosaccharide-sialyltransferase; ns, not significant; SSC, side scatter; VLY, vaginolysin; WT, wild type.
Using the same method depicted in Figure 2A, we assessed recruitment of chimeric Fc3/FH* to N. gonorrhoeae F62 following bacterial exposure to VLY-liberated HeLa contents. As predicted, Fc3/FH* binding was greatly increased after exposure to the VLY-extracted HeLa contents compared to the vector control, and this effect was Lst dependent (Figure 5C and 5D).
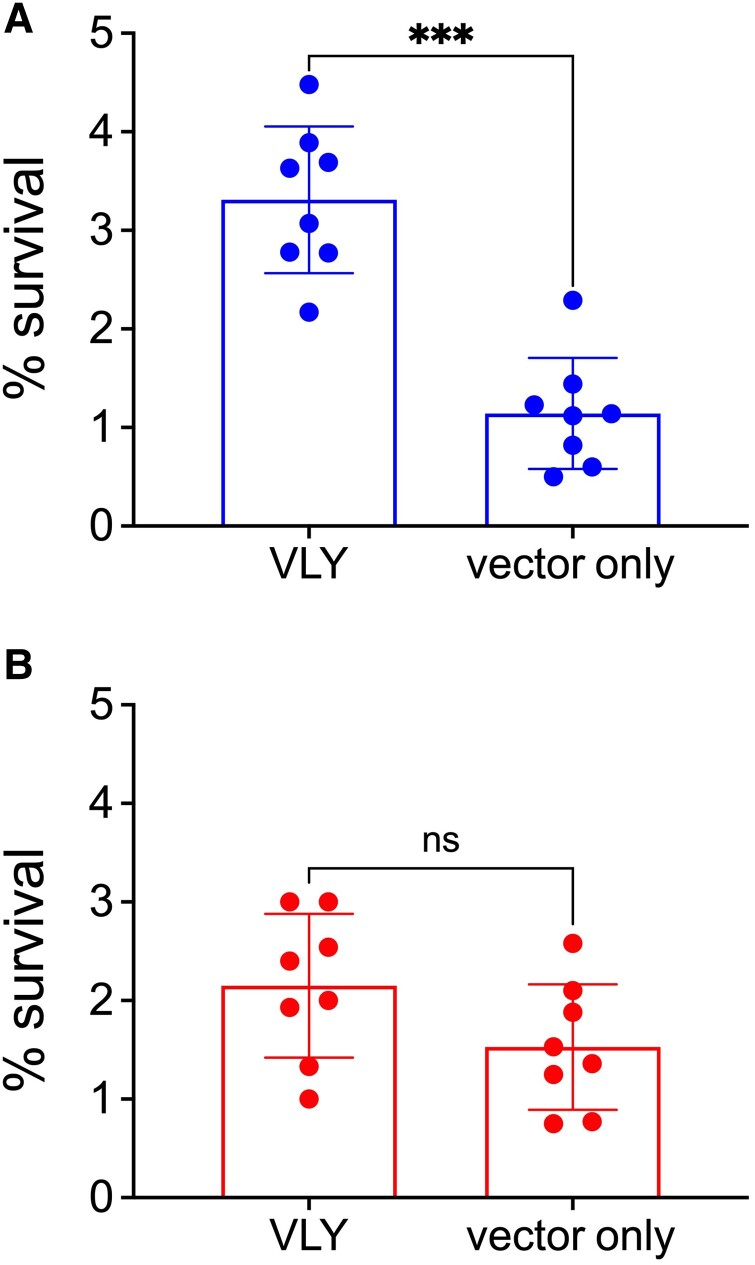
Finally, we assessed how exposure to VLY-liberated HeLa contents impacts gonococcal immune evasion. It is well established that LOS sialylation and FH recruitment increase gonococcal resistance to killing by human serum [24]. Therefore, we hypothesized that exposure to VLY-extracted HeLa contents would potentiate N. gonorrhoeae resistance in a serum-killing assay. N. gonorrhoeae F62 was incubated in the presence of 4% normal human serum and colony enumeration was used to assess survival. As expected, there was a significant increase in gonococcal survival after exposure to the VLY-liberated HeLa cell contents compared to vector-only controls (Figure 6A). This difference was not observed when N. gonorrhoeae F62 Δlst was tested (Figure 6B), suggesting that the survival depended on LOS sialylation.
Figure 6.
Exposure of Neisseria gonorrhoeae to VLY-liberated HeLa contents increases sialyaltion-dependent survival in human serum. N. gonorrhoeae F62 WT and Δlst were incubated with the supernatants of HeLa cells that had been exposed to either 1 μg/mL VLY or a parallel dilution of the vector-control preparation. A and B, Survival of N. gonorrhoeae F62 WT (A) and Δlst (B) after 40-minute incubation in 4% normal human serum. Percent survival determined by colony enumeration at 0 and 40 minutes. Error bars represent standard deviation. Significance determined by Mann-Whitney U-test. ***P < .001. Abbreviations: lst, lipooligosaccharide-sialyltransferase; ns, not significant; VLY, vaginolysin; WT, wild type.
CONCLUSIONS
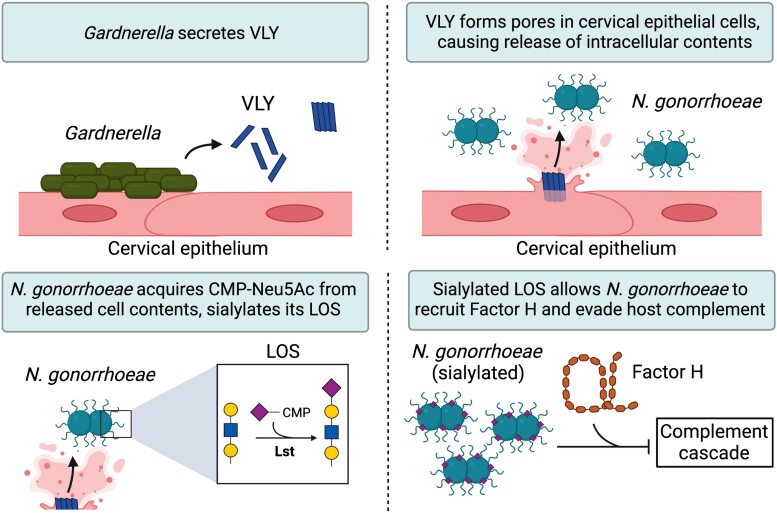
In this study, we asked if Gardnerella, an abundant bacterial genus in BV, could enhance the virulence of N. gonorrhoeae by providing access to intracellular contents. Exposure of epithelial cells to the Gardnerella cytolysin, VLY, enhanced gonococcal acquisition of LOS sialic acid, engagement in factor H binding, and evasion of complement killing. A limitation of our study is that we were unable to directly observe and measure CMP-Neu5Ac release from the HeLa cells upon treatment with VLY. However, CMP-Neu5Ac is the activated nucleotide sugar required for the gonococcal sialyltransferase, Lst. Thus, the data strongly support a model in which VLY-induced membrane damage result in enhanced access of gonococcal Lst to CMP-Neu5Ac otherwise contained inside host cells (Figure 7).
Figure 7.
Proposed model of Gardnerella VLY potentiating Neisseria gonorrhoeae virulence. Gardnerella secretes VLY, which forms pores in cervical epithelial cells. Pore formation leads to a release of intracellular contents, including CMP-Neu5Ac. N. gonorrhoeae scavenges released CMP-Neu5Ac to sialylate its LOS, which enhances its ability to evade host immunity. Abbreviations: CMP-Neu5Ac, cytidine-5′-monophospho-N-acetyl neuraminic acid; LOS, lipooligosaccharide; Lst, LOS-sialyltransferase; VLY, vaginolysin.
MAL-I recognizes the α2-3-sialylated N-acetylactosamine of LOS (Figure 2A) and showed higher binding to the bacteria exposed to supernatants of VLY-liberated cervical epithelial cells compared to controls. Interestingly, MAL-I binding to F62 Δlst was still modestly higher on cells exposed to VLY-liberated supernatants compared to the vector controls (Figure 3C and 3D). This could indicate the presence of molecules on the gonococcal surface with α2-3-linked sialic acids independent of Lst activity. Alternative explanations include (1) the presence of mammalian glycans that were not fully removed by washing in the presence of VLY, (2) VLY treatment releasing mammalian glycosyltransferases capable of sialylating gonococcal LOS [25], or (3) the bacteria produce a sialic acid-independent glycan epitope that binds to MAL-I (eg, the lectin also binds 3-O-sulfated galactose [26]). Despite the uncertainty about the remaining MAL-I binding, the majority of the MAL-I binding was both VLY and Lst specific.
Molecular mimicry of host glycans is a well-characterized gonococcal virulence factor. LOS sialylation counters multiple host defense mechanisms including the classical and alternative pathways of complement, cationic antimicrobial peptides, and additionally dampens the inflammatory response by engaging Siglec receptors [22, 27–29]. In women, N. gonorrhoeae is believed to acquire CMP-Neu5Ac by invading epithelial cells. Our findings suggest that cell damage driven by cytolysins present in BV may augment the ability of N. gonorrhoeae to scavenge this resource from the host, even during extracellular occupation of the niche. Future work should investigate if this finding is replicated using more clinically relevant, non-cell line tissues, such as cervical biopsy samples.
A recent study showed that sialidases found in cervicovaginal secretions of women with BV can remove LOS sialic acids [30]. While desialylation of gonococcal LOS could render gonococci more susceptible to host defenses, it may confer other advantages. In fact, experimental evidence suggests that sialic acid removal may be required for asialoglycoprotein receptor binding and infection in men [31]. While Gardnerella sialidases can desialylate gonococci, our data show that their VLY may enhance the availability of CMP-Neu5Ac to promote LOS sialylation. Thus, the balance between sialidase and VLY activity may ultimately determine the net amount of LOS sialylation. Additionally, while almost all complete genomes of Gardnerella contain the gene for VLY, only a subset of strains encode sialidase genes and have sialidase activity in culture [32–34]. Gardnerella is also not the only BV-associated microbe with cytolytic activity. Lactobacillus iners, for example, is another frequent member of the BV consortium that encodes its own cytolysin [35]. Future work should incorporate new experimental systems to investigate the interplay between sialidases and cytolytic activities from Gardnerella and other BV-associated bacteria in the gonococcal life cycle.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Sydney R Morrill, Department of Molecular Microbiology, Washington University School of Medicine, St Louis, Missouri, USA; Department of Obstetrics, Gynecology, and Reproductive Sciences, University of California San Diego, La Jolla, California, USA; Glycobiology Research and Training Center, University of California San Diego, La Jolla, California, USA.
Sudeshna Saha, Department of Obstetrics, Gynecology, and Reproductive Sciences, University of California San Diego, La Jolla, California, USA; Glycobiology Research and Training Center, University of California San Diego, La Jolla, California, USA.
Ajit P Varki, Glycobiology Research and Training Center, University of California San Diego, La Jolla, California, USA; Department of Cellular and Molecular Medicine, University of California San Diego, La Jolla, California, USA; Department of Pathology, University of California San Diego, La Jolla, California, USA; Center for Academic Research and Training in Anthropogeny, University of California San Diego, La Jolla, California, USA.
Warren G Lewis, Department of Obstetrics, Gynecology, and Reproductive Sciences, University of California San Diego, La Jolla, California, USA; Glycobiology Research and Training Center, University of California San Diego, La Jolla, California, USA.
Sanjay Ram, Division of Infectious Diseases and Immunology, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
Amanda L Lewis, Department of Obstetrics, Gynecology, and Reproductive Sciences, University of California San Diego, La Jolla, California, USA; Glycobiology Research and Training Center, University of California San Diego, La Jolla, California, USA.
Notes
Author contributions. S. M. helped conceive and develop the project, performed experiments, data analysis, and wrote the first draft of the manuscript. S. S. performed experiments and data analysis, guided experiments, and participated in editing the manuscript. A. V. helped develop the project and participated in editing the manuscript. W. G. helped conceive and develop the project, guided experiments and data analysis, and participated in editing the manuscript. S. R. helped develop the project and provided key reagents and funding for the project, and participated in editing the manuscript. A. L. helped conceive the project, guided experiments and data analysis, provided funding for the project, and participated in editing the manuscript.
Acknowledgments. We thank Dr Keith L. Wycoff and Y Tran (Plant Biotechnology, Inc) for providing Fc3/FH* (S2534). All schematics were generated using BioRender.com.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (grant numbers R01AI160247 to S. R. and R01Al114635 to A. L.); and startup funds from the University of California San Diego.
References
- 1. Ravel J, Gajer P, Abdo Z, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A 2011; 108:4680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen X, Lu Y, Chen T, Li R. The female vaginal microbiome in health and bacterial vaginosis. Front Cell Infect Microbiol 2021; 11:631972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Koumans EH, Sternberg M, Bruce C, et al. The prevalence of bacterial vaginosis in the United States, 2001–2004; associations with symptoms, sexual behaviors, and reproductive health. Sex Transm Dis 2007; 34:864–9. [DOI] [PubMed] [Google Scholar]
- 4. Srinivasan S, Hoffman NG, Morgan MT, et al. Bacterial communities in women with bacterial vaginosis: high resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS One 2012; 7:e37818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med 1983; 74:14–22. [DOI] [PubMed] [Google Scholar]
- 6. Bautista CT, Wurapa E, Sateren WB, Morris S, Hollingsworth B, Sanchez JL. Bacterial vaginosis: a synthesis of the literature on etiology, prevalence, risk factors, and relationship with chlamydia and gonorrhea infections. Mil Med Res 2016; 3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organization . Multi-drug resistant gonorrhoea. https://www.who.int/news-room/fact-sheets/detail/multi-drug-resistant-gonorrhoea. Accessed 30 March 2023.
- 8. Reekie J, Donovan B, Guy R, et al. Risk of pelvic inflammatory disease in relation to chlamydia and gonorrhea testing, repeat testing, and positivity: a population-based cohort study. Clin Infect Dis 2018; 66:437–43. [DOI] [PubMed] [Google Scholar]
- 9. Reekie J, Donovan B, Guy R, et al. Risk of ectopic pregnancy and tubal infertility following gonorrhea and chlamydia infections. Clin Infect Dis 2019; 69:1621–3. [DOI] [PubMed] [Google Scholar]
- 10. Lee MH, Byun J, Jung M, et al. Disseminated gonococcal infection presenting as bacteremia and liver abscesses in a healthy adult. Infect Chemother 2015; 47:60–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gallo MF, Macaluso M, Warner L, et al. Bacterial vaginosis, gonorrhea, and chlamydial infection among women attending a sexually transmitted disease clinic: a longitudinal analysis of possible causal links. Ann Epidemiol 2012; 22:213–20. [DOI] [PubMed] [Google Scholar]
- 12. Brotman RM, Klebanoff MA, Nansel TR, et al. Bacterial vaginosis assessed by gram stain and diminished colonization resistance to incident gonococcal, chlamydial, and trichomonal genital infection. J Infect Dis 2010; 202:1907–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Martin HL, Richardson BA, Nyange PM, et al. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis 1999; 180:1863–8. [DOI] [PubMed] [Google Scholar]
- 14. Morrill S, Gilbert NM, Lewis AL. Gardnerella vaginalis as a cause of bacterial vaginosis: appraisal of the evidence from in vivo models. Front Cell Infect Microbiol 2020; 10:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Caza M, Kronstad JW. Shared and distinct mechanisms of iron acquisition by bacterial and fungal pathogens of humans. Front Cell Infect Microbiol 2013; 3:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ram S, Shaughnessy J, de Oliveira RB, Lewis LA, Gulati S, Rice PA. Gonococcal lipooligosaccharide sialylation: virulence factor and target for novel immunotherapeutics. Pathog Dis 2017; 75:ftx049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kopp A, Hebecker M, Svobodová E, Józsi M. Factor H: a complement regulator in health and disease, and a mediator of cellular interactions. Biomolecules 2012; 2:46–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mandrell RE, Apicella MA. Lipo-oligosaccharides (LOS) of mucosal pathogens: molecular mimicry and host-modification of LOS. Immunobiology 1993; 187:382–402. [DOI] [PubMed] [Google Scholar]
- 19. Randis TM, Zaklama J, Larocca TJ, et al. Vaginolysin drives epithelial ultrastructural responses to Gardnerella vaginalis. Infection and Immunity 2013; 81:4544–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shaughnessy J, Chabeda A, Tran Y, et al. An optimized factor H-Fc fusion protein against multidrug-resistant Neisseria gonorrhoeae. Front Immunol 2022; 13:975676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mamat U, Woodard RW, Wilke K, et al. Endotoxin-free protein production—clearColi™ technology. Nature Methods 2013; 10:916. [Google Scholar]
- 22. Gulati S, Cox A, Lewis LA, et al. Enhanced factor H binding to sialylated gonococci is restricted to the sialylated lacto-N-neotetraose lipooligosaccharide species: implications for serum resistance and evidence for a bifunctional lipooligosaccharide sialyltransferase in gonococci. Infect Immun 2005; 73:7390–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shaughnessy J, Ram S, Bhattacharjee A, et al. Molecular characterization of the interaction between sialylated Neisseria gonorrhoeae and factor H. J Biol Chem 2011; 286:22235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ram S, Sharma AK, Simpson SD, et al. A novel sialic acid binding site on factor H mediates serum resistance of sialylated Neisseria gonorrhoeae. J Exp Med 1998; 187:743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mandrell RE, Lesse AJ, Sugai JV, et al. In vitro and in vivo modification of Neisseria gonorrhoeae lipooligosaccharide epitope structure by sialylation. J Exp Med 1990; 171:1649–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Byrd-Leotis L, Jia N, Matsumoto Y, et al. Sialylated and sulfated N-glycans in MDCK and engineered MDCK cells for influenza virus studies. Sci Rep 2022; 12:12757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gulati S, Schoenhofen IC, Whitfield DM, et al. Utilizing CMP-sialic acid analogs to unravel Neisseria gonorrhoeae lipooligosaccharide-mediated complement resistance and design novel therapeutics. PLoS Pathogens 2015; 11:e1005290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gulati S, Schoenhofen IC, Lindhout-Djukic T, et al. Efficacy of antigonococcal CMP-nonulosonate therapeutics require cathelicidins. J Infect Dis 2020; 222:1641–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Landig CS, Hazel A, Kellman BP, et al. Evolution of the exclusively human pathogen Neisseria gonorrhoeae: human-specific engagement of immunoregulatory siglecs. Evol Appl 2019; 12:337–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ketterer MR, Rice PA, Gulati S, et al. Desialylation of Neisseria gonorrhoeae lipooligosaccharide by cervicovaginal microbiome sialidases: the potential for enhancing infectivity in men. J Infect Dis 2016; 214:1621–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Edwards JL, Apicella MA. The molecular mechanisms used by Neisseria gonorrhoeae to initiate infection differ between men and women. Clin Microbiol Rev 2004; 17:965–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Garcia EM, Serrano MG, Edupuganti L, Edwards DJ, Buck GA, Jefferson KK. Sequence comparison of vaginolysin from different Gardnerella species. Pathogens 2021; 10:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lewis WG, Robinson LS, Gilbert NM, Perry JC, Lewis AL. Degradation, foraging, and depletion of mucus sialoglycans by the vagina-adapted actinobacterium Gardnerella vaginalis. J Biol Chem 2013; 288:12067–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schellenberg JJ, Paramel Jayaprakash T, Withana Gamage N, Patterson MH, Vaneechoutte M, Hill JE. Gardnerella vaginalis subgroups defined by cpn60 sequencing and sialidase activity in isolates from Canada, Belgium and Kenya. PLoS One 2016; 11:e0146510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rampersaud R, Planet PJ, Randis TM, et al. Inerolysin, a cholesterol-dependent cytolysin produced by Lactobacillus iners. J Bacteriol 2011; 193:1034–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.