Abstract
Background
Human immunodeficiency virus (HIV) infection remains incurable due to the persistence of a viral reservoir despite antiretroviral therapy (ART). Cannabis (CB) use is prevalent amongst people with HIV (PWH), but the impact of CB on the latent HIV reservoir has not been investigated.
Methods
Peripheral blood cells from a cohort of PWH who use CB and a matched cohort of PWH who do not use CB on ART were evaluated for expression of maturation/activation markers, HIV-specific T-cell responses, and intact proviral DNA.
Results
CB use was associated with increased abundance of naive T cells, reduced effector T cells, and reduced expression of activation markers. CB use was also associated with reduced levels of exhausted and senescent T cells compared to nonusing controls. HIV-specific T-cell responses were unaffected by CB use. CB use was not associated with intact or total HIV DNA frequency in CD4 T cells.
Conclusions
This analysis is consistent with the hypothesis that CB use reduces activation, exhaustion, and senescence in the T cells of PWH, and does not impair HIV-specific CD8 T-cell responses. Longitudinal and interventional studies with evaluation of CB exposure are needed to fully evaluate the impact of CB use on the HIV reservoir.
Keywords: HIV, antiretroviral, cannabis, function, immune, inflammation, persistence, reservoir, suppressed
In a cross-sectional study of people with HIV on antiretroviral therapy, cannabis use was associated with reduction of T-cell activation, exhaustion, and senescence without compromise of HIV-specific immune responses. No association with HIV reservoir size or composition was observed.
While antiretroviral therapy (ART) suppresses human immunodeficiency virus (HIV) replication, continuous therapy is required due to a latently infected reservoir that fuels viral rebound upon ART cessation [1]. ART mitigates CD4 T-cell depletion associated with untreated infection and leads to reconstitution of pathogen-specific immune response, and decreases in immune activation [2–4]. However, sequelae of immune activation and dysfunction, such as cancers and cardiopulmonary disease, contribute to morbidity and mortality in treated individuals [5, 6]. The mechanisms of this persistent pathologic inflammation during ART suppression are unclear but may involve residual proviral expression, impaired gut mucosal barrier function, and long-lasting immune system alterations induced during untreated infection [7, 8].
Cannabis (CB) use is prevalent among people with HIV (PWH), with close to 80% of PWH reporting any lifetime CB use, and up to 50% reporting daily to weekly use [9]. Despite widespread use and increasing legality, the impact of CB use on immune function in PWH during suppressive ART is incompletely understood [10–21]. Delta-9-tetrahydrocannabinol (THC), the major psychoactive cannabinoid constituent, exerts its effects via 2 G-protein coupled receptors CB1 and CB2. CB1 is largely present in neurons. CB2, by contrast, is abundantly expressed in immune cells, including CD4 T cells [22–24]. At the molecular level, activation of cannabinoid and potentially other receptors leads to complex pleotropic cell-type and context-dependent cellular signaling [22–24]. Canonically, cannabinoids are characterized as anti-inflammatory through several mechanisms, including suppression of inflammatory cytokines, decreased T-cell proliferation, and modulation of T helper responses [22–24].
In the context of HIV infection in humans, there are mixed results regarding the immune impacts of CB use, reviewed in detail elsewhere [10, 25]. Across multiple studies, CB use did not impact CD4 and CD8 counts or increase the risk for HIV disease progression in untreated disease [10, 25, 26]. Some but not all human studies report a variety of anti-inflammatory effects of CB use, including decreases in levels of activated T cells and inflammatory cytokines [10–21]. Notably, hazardous CB use or CB use paired with concomitant use of other substances may decrease the anti-inflammatory effect of CB use [13, 15, 19].
The virologic effects of CB use on HIV infection are also poorly understood. In vitro studies demonstrate that synthetic cannabinoids can inhibit HIV replication, and some but not all animal and human studies suggest reduced viral loads in PWH who use CB [10, 24, 27, 28]. During treated HIV infection, CB use has been associated with more rapid decay of total HIV DNA during the first 2 years of ART [20]. CB use also influences T-cell activation and proliferation [10, 25]. Given that these factors are implicated in HIV reservoir dynamics, further evaluation of the immune-virologic effects of CB use in the context of persistent HIV infection is needed to inform strategies to deplete the HIV reservoir and mitigate persistent inflammation [29].
In this cross-sectional study, high-dimensional immunophenotyping, measurement of HIV-specific T-cell responses, and HIV reservoir quantification using the intact proviral DNA assay (IPDA) were performed on peripheral blood samples from PWH on suppressive ART with or without CB use to assess impact of CB use on inflammation, immune function, and the HIV reservoir.
METHODS
Study Approval and Samples
This study was reviewed and approved by the institutional review boards from both Duke University and University of North Carolina (00107328). All participants provided written, informed consent. Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood and viably cryopreserved.
Peptide Stimulation and Flow Cytometry
Thawed PBMCs were rested for 6 hours prior to stimulation with or without HIV Gag, Pol, and Env PepMixes (JPT Laboratories) at 0.2 µg/mL final concentration of each with dimethyl sulfoxide solvent, and in the presence of brefeldin A, monensin, and CD107a-PE (H4A3 clone; BL) prior to cytokine staining. A separate portion of unstimulated PBMCs were reserved for a high-dimensional immunophenotyping panel; detailed staining methods and antibody information is available in the Supplementary Methods. Sample acquisition was performed on a Cytek Aurora spectral flow cytometer. Flow cytometry analysis was performed in FlowJo version 10.8.
Intact Proviral DNA Assay
A portion of the viably thawed PBMCs described above underwent total CD4 T-cell negative selection and IPDA as previously described [30, 31]. Detailed assay performance parameters are reported in the Supplementary Methods.
Data Analysis
All statistical comparisons were subject to a false discovery rate (FDR) correction with q = 10%, except for the exploratory subgroup analysis for the IPDA data (Supplementary Materials). Comparisons were considered statistically significant if (1) P was < .05 and (2) the FDR correction q value was ≤10%.
Traditional statistical comparisons between the CB use and nonuse groups for flow cytometry and IPDA analysis were carried out using Mann-Whitney U tests. Evaluation of the relationship between years of self-reported CB use and IPDA and flow cytometry parameters were conducted using Spearman correlation.
Linear regression was employed to estimate the effect of self-reported years of CB use on the immunophenotypes observed while also accounting for the potential confounders of age, sex, race, years of HIV infection, years of treatment, CD4 T-cell nadir value, and recent CD4 T-cell count. Features were standardized prior to model fitting [32].
Comparison of functional T-cell responses was performed using a nonparametric multicomponent distribution statistical test [33]. As previously described, T-cell response data were subject to thresholding (75th percentile) and normalization to non–peptide-stimulated cells for each donor [33]. This facilitates visualization and ensures the comparison reflects true positive responses.
Multiple data science approaches were employed to further evaluate relationships between CB use and 142 immune/clinical/demographic parameters and are described in detail in the Supplementary Materials. Briefly, we leveraged these data science tools to provide the following main analyses: (1) receiver operating characteristic (ROC) curves for individual immune cell types, which finds the most important single immune cell type associated with CB use versus nonuse; (2) sparse decision trees, which find combinations of immune cell types that best distinguish PWH who use CB versus those who do not [34]; and (3) dimension reduction, an unsupervised technique for visualization of high-dimensional data on a 2-dimensional plot to visualize assess if there are global difference in PWH who use CB versus PWH who do not use CB [35].
RESULTS
Cohort Characteristics
Seventy-five PWH participated in this cross-sectional study; detailed study inclusion and exclusion criteria are published elsewhere [36]. Briefly, all participants had tested positive for HIV for more than 2 years (median, 12 years; range, 2–34 years) and were ART suppressed (<50 RNA copies/mL) for at least 12 months (median, 10 years; range, 2–28 years). Overall, the 2 groups had similar characteristics in terms of age, sex, race, years of infection and therapy, and CD4 nadir (Table 1). Individuals with recent or previous regular use of illicit substances other than CB, as well as individuals with alcohol use disorder, were excluded.
Table 1.
Participant Demographic and Clinical Characteristics
| Characteristic | Cannabis Use (n = 33) |
No Cannabis Use (n = 42) |
|---|---|---|
| Age, y | 42 (33, 48.5) | 45.5 (37, 53.25) |
| Sex, % male (No.) | 87.88 (29) | 73.81 (31) |
| Race/ethnicity, % (No.) | ||
| African American | 66.67 (22) | 71.43 (30) |
| Caucasian | 30.30 (10) | 28.57 (12) |
| Hispanic | 0 (0) | 0 (0) |
| Mixed | 3.03 (1) | 0 (0) |
| Years HIV positive | 12 (7, 21) | 12 (8, 21.5) |
| Years ART treatment | 10 (6, 17.5) | 10 (7, 19) |
| CD4 count at study entry, cells/mm3 | 815 (626, 1141) | 779.5 (658, 932.3) |
| Nadir CD4 count, cells/mm3 | 285 (133.5, 431.8) | 245.5 (102.5, 360.8) |
| Current antiretroviral class, % (No.) | ||
| Combined | 57.58 (19) | 47.62 (20) |
| NNRTI | 33.33 (11) | 23.81 (10) |
| PI | 9.09 (3) | 11.9 (5) |
| II | 0 (0) | 16.67 (7) |
Unless otherwise specified, quantitative data are median (Q1, Q3). There are missing values in the data: years HIV positive, 1 missing; years ART treatment, 8 missing; and nadir CD4 count, 3 missing.
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; II, integrase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Criteria for the CB-use group (n = 33) were greater than 12 days of self-reported CB use within the last 90 days and a positive urine test, while PWH who did not use CB (n = 42) were defined as having no reported CB or illicit substance use in the last 12 months and a negative urine test. Additionally, estimates of self-reported years of lifetime CB use for all participants were also obtained to estimate cumulative exposure, because reliance on analysis of CB metabolites verifies only recent use and is limited by marked variation related to host metabolism, route of administration, and heterogeneity of CB [37, 38]. Median lifetime self-reported years of CB use for the cohort was 19 (Q1, 11; Q3, 24) years.
CB Use Is Associated With Alterations to T-Cell Maturation, Activation, and Exhaustion
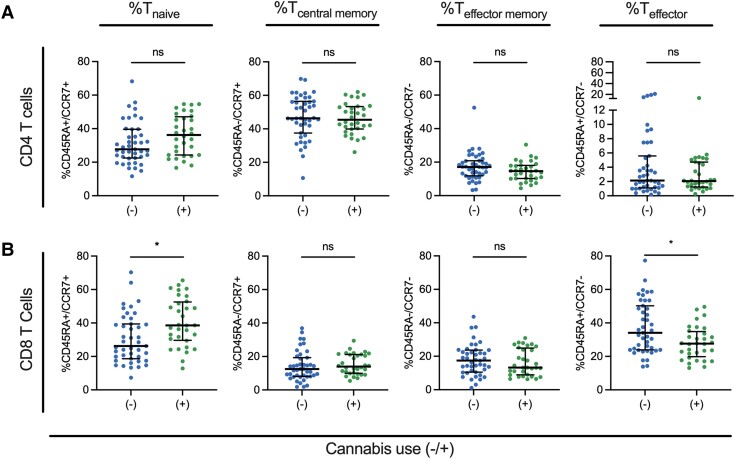
High-dimensional flow cytometry immunophenotyping was performed for each participant (Supplementary Figure 1A and Supplementary Table 1). Use of CB was not associated with statistically significant (FDR-corrected Mann-Whitney U test) changes in abundance of CD4 T cells (median difference = 5.2%), CD8 T cells (median difference = 3%), NK cells (median difference = 0.3%), or NKT cells (median difference = 0.4%) (Supplementary Figure 1B–E). No statistically significant alterations to CD4 maturation subsets were observed (Figure 1A). Evaluation of T-cell maturation subsets demonstrated that CB use was associated with statistically significantly increased CD8 T naive cells (median difference = 12.4%) and decreased CD8 T effector cells (median difference = 6.5%) (Figure 1B).
Figure 1.
Cannabis (CB) use is associated with increased CD8 naive T cells and decreased CD8 T effector cells. A and B, Proportional abundance of T cells in naive (Tn), central memory (Tcm), effector memory (Tem), and effector (Teff) subsets for CD4 T cells (A) and CD8 T cells (B). Cell subsets assigned by expression of the surface markers CD45RA and CCR7: Tn, CD45RA+/CCR7+; Tcm, CD45RA−/CCR7+; Tem, CD45RA−/CCR7−; and Teff, CD45RA+/CCR7−. Each dot represents an individual participant (CB use n = 33; no CB use n = 42). Comparisons were considered statistically significant (designated with a single asterisk) if the (1) P value from the Mann-Whitney U test was <.05 and (2) the FDR q value was <10%. Lines represent the median and error bars the interquartile range.
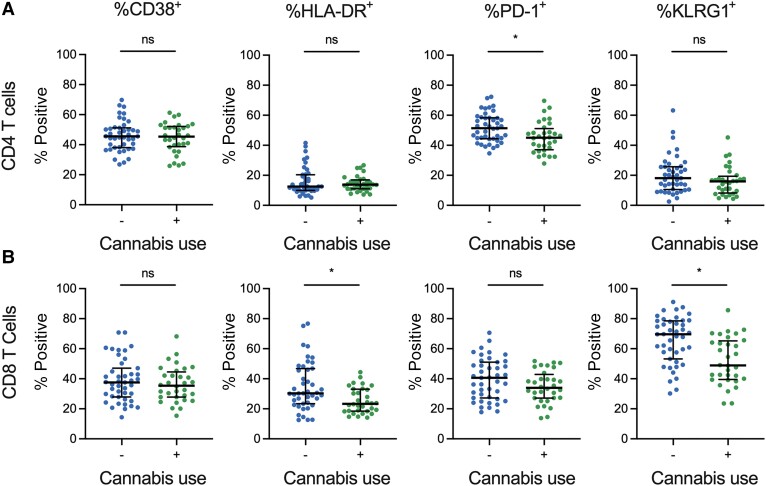
Next, we conducted an analysis of classical activation and exhaustion markers (CD38, HLA-DR, and PD-1) and an immune senescence marker (killer cell lectin-like receptor subfamily G member 1 [KLRG1]) on total CD4 and CD8 cells (Figure 2) [39–41]. CB use was associated with statistically significant (FDR-corrected Mann-Whitney U test) reduced frequencies of CD4+PD1+ T cells (median difference = 6.4%) (Figure 2A). Similarly, in CD8 T cells, significantly decreased HLA-DR+ (median difference = 7.0%) and KLRG1+ levels were observed for PWH who use CB (median difference = 20.7% (Figure 2B). Additional analysis of activation/exhaustion/senescence markers in CD4 and CD8 T-cell subsets demonstrated that CB use was associated with statistically significantly (FDR-corrected Mann-Whitney U test) reduced frequencies of CD38+ cells in the CD4 T naive (median difference = 4.7%) and T central memory (median difference = 4.3%) compartments (Supplementary Figures 2 and 3).
Figure 2.
Cannabis (CB) use is associated with decreased activation and exhaustion markers on CD4 and CD8 T cells. A and B, Expression levels (percent positive) for 3 different immune activation markers (HLA-DR, CD38, and PD-1) are shown for total CD4 T cells (A) and CD8 T cells (B). Each dot represents an individual participant (CB use n = 33; no CB use n = 42). Comparisons were considered statistically significant (designated with a single asterisk) if (1) the P value from the Mann-Whitney U test was <.05 and (2) the FDR q value was <10%. Lines represent the median and error bars the interquartile range.
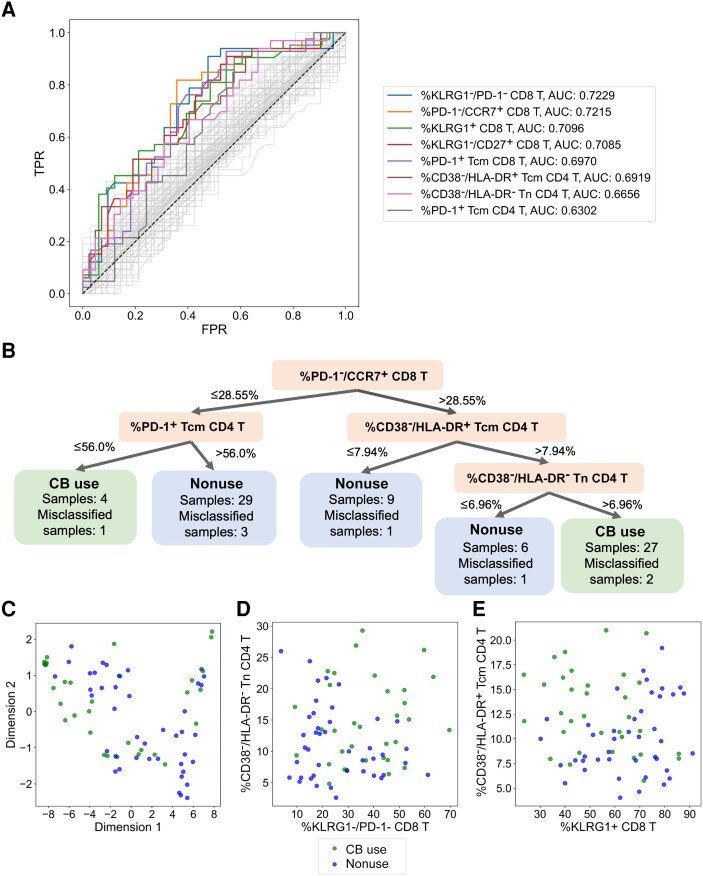
Figure 3.
Exploratory data science approaches identify complex immunophenotype classifications that can predict CB use status. A, ROC curves and corresponding AUC values for 133 features derived from demographics, IPDA, and flow cytometry analysis of the cohort. Axes represent TPR and the FPR for each feature-derived model for classifying study participants into the CB use versus nonuse groups. Dashed line corresponds to the ROC curve of an uninformative model. B, Visualization of the dataset using the decision tree algorithm GOSDT. This tree correctly describes 89.3% of the dataset. C, Visualization of the dataset based on application of PaCMAP dimension reduction. D and E, Scatter plot of 2 sets of feature combination. We constructed pairs of features with high AUC values combined with features from GOSDT tree and chose the 2 pairs with lowest Spearman correlation: the frequency of CD38−/HLA-DR− cells within the CD4 Tn population versus the frequency of KLRG1−/PD-1− cells within the total CD8 T-cell gate (D), and the frequency of CD38−/HLA-DR+ cells within the CD4 Tcm population versus the frequency of KLRG1+ cells within the CD8 T-cell population (E). Abbreviations: AUC, area under the curve; CB, cannabis; FPR, false positive rate; GOSDT, generalized and scalable optimal sparse decision trees; IPDA, intact proviral DNA assay; ROC, receiver operating characteristic; Tcm, central memory T cell; Tn, naive T cell; TPR, true positive rate.
To assess for a potential dose-response effect of CB use on inflammation, years of lifetime self-reported CB use (Supplementary Figure 4A) was correlated with immune markers identified to be statistically significantly different between CB-use and nonuse groups in Figure 1 and Figure 2 using FDR-corrected Spearman correlations. Years of CB use weakly positively correlated with CD8 naive T-cell frequencies (r = 0.20), albeit without statistical significance. A statistically significant negative correlation was observed for CD8 effector T-cell frequencies (r = −0.27) (Supplementary Figure 4B and 4C). Years of CB use was statistically significantly negatively correlated with CD4+PD-1+ T cells (r = −0.29), CD8+HLA-DR+ T cells (r = −0.27), and CD8+KLRG1+ T cells (r = −0.29) (Supplementary Figure 4D–F).
Linear Regression With Adjustment for Confounders Show Self-reported Years of CB Is Directly Related to Altered Immunophenotypes
Linear regression was employed to estimate the effect of self-reported years of CB use on the immunophenotypes observed while also accounting for the potential confounders of age, sex, race, years of HIV infection, years of treatment, CD4 T-cell nadir value, and recent CD4 T-cell count. For the 8 statistically significant cell populations identified in Figure 1 and Figure 2, and Supplementary Figures 2 and 3, the best fit linear models included those for percent CD8 T KLRG1+, percent CD8 T naive, and percent CD8 T central memory PD-1+ populations: more than 22% of the variance in these populations was explained by the demographics, clinical information, and CB use (Supplementary Figure 5 and Supplementary Table 2). To determine the relevance of self-reported years of CB use to these models, we also computed adjusted R2 scores without self-reported years of CB use in the models. We observed a reduction in R2 scores for all 8 linear models (median R2 = 0.15 with years of CB use, median R2 = 0.07 without years of CB use; Supplementary Table 2). This finding lends support to the notion that self-reported lifetime years of CB use partially explains the variance in several immunophenotypes related to inflammation, even after accounting for several potential confounders.
Data Science Approaches Identify Immunophenotypes That Distinguish PWH Who Use CB From Those Who Do Not
We leveraged several data science approaches to identify features associated with CB use that may not be apparent in traditional analyses (Supplementary Methods). First, the ability of each of 142 single-immunophenotype frequencies from the flow cytometry analysis or clinical/demographic data (Supplementary Table 1 and Supplementary Methods) to classify individuals correctly into the CB-use versus nonuse groups was evaluated using ROC curves. The top 2 predictive features (greatest area under the ROC curve [AUC]) were (1) percent KLRG1−/PD-1− CD8 T cells and (2) percent PD-1−/CCR7+ CD8 T cells (Figure 3A and Supplementary Table 3).
Next, generalized and scalable optimal sparse decision trees (GOSDT) [34] analysis was performed using the 46 features with AUC > 0.6 identified in the ROC analysis. This approach generated a classification tree that correctly identifies the CB-use status of 67 of 75 participants using only 4 specific immunophenotypes (Figure 3B).
As an additional approach to identify unique features associated with CB use, CB use agnostic high-dimensional reduction techniques were performed using the 46 features with an AUC > 0.6 from the ROC analysis. Superimposition of CB use status after clustering revealed some separation of PWH who use CB and those who do not (Figure 3C). Remarkably, visualization of participants using combinations of 2 minimally correlated immunophenotype features identified in the ROC analysis and GOSDT algorithm (Figure 3D and 3E) resulted in similar cluster separation as the high dimension analysis (Figure 3C). Overall, the data science approach corroborates the traditional analysis in Figure 1, Figure 2, Figure 3, and Figure 4, and identifies combinations of a limited number of specific immune parameters that highlight the anti-inflammatory signature associated with CB use.
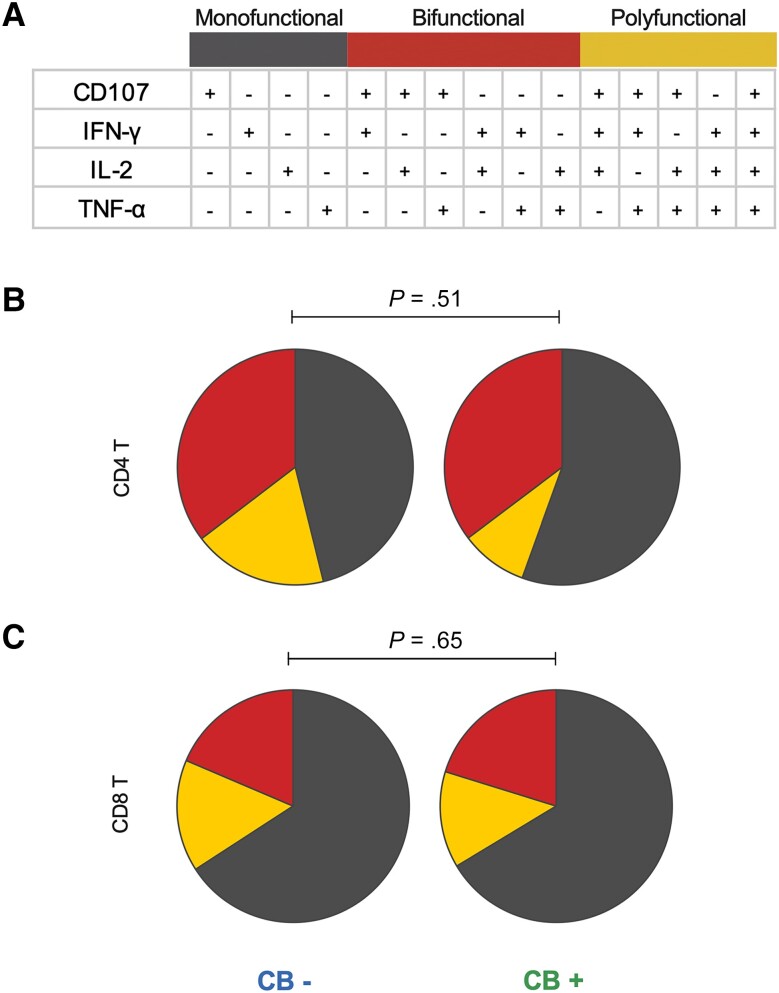
Figure 4.
Cannabis use does not impair HIV-specific T-cell responses. PBMCs from each study participant were stimulated with HIV peptides and analyzed for expression of CD107, IFN-γ, IL-2, and TNF-α by flow cytometry. A, Cells were classified as monofunctional, bifunctional, or polyfunctional based on expression patterns of these 4 proteins. B and C, The frequency of monofunctional, bifunctional, and polyfunctional CD4 T cells (B) or CD8 T cells (C) was compared between PWH who use CB and PWH who do not, using a previously described nonparametric statistical test for groups with multicomponent measurements [33]. Abbreviations: CB, cannabis; HIV, human immunodeficiency virus; IFN-γ, interferon-γ; IL-2, interleukin 2; PBMC, peripheral blood mononuclear cell; PWH, people with HIV; TNF-α, tumor necrosis factor-α.
CB Use Is Not Associated With a Reduction in HIV-Specific T-Cell Responses
Given the associations of CB use with suppression of inflammatory cytokines, decreased T-cell activation, and modulation of T helper responses [22–24], an important question is whether functional T-cell responses to HIV are impaired in PWH on ART who use CB. To address this, HIV-specific CD4 and CD8 T-cell functional responses were quantified using PBMCs from each study participant as described in the “Methods” section. CD4 and CD8 T-cell responses were assessed using collapsed analysis groups: monofunctional (1/4 markers positive), bifunctional (2/4 markers positive), or polyfunctional (3/4 or 4/4 markers positive) (Figure 4A). There was a similar distribution of mono-, bi-, or polyfunctional CD4 (Figure 4B) and CD8 (Figure 4C) T-cell responses to the overall functional T-cell response across PWH who use CB versus those who do not. A previously described statistical test [33] for groups with multicomponent measurements demonstrated no statistically significant association of CB use with altered CD4 or CD8 functional T-cell responses.
Evaluation of the Relationship Between CB Use and HIV Reservoir
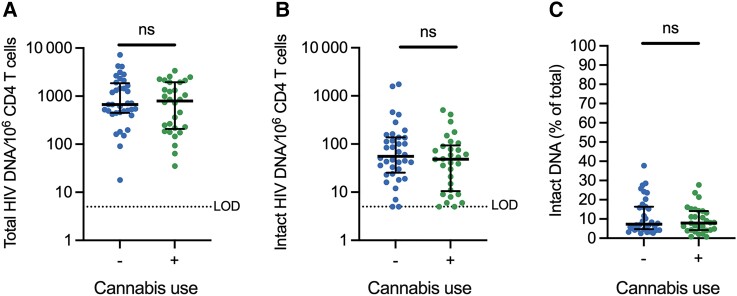
As CB use may influence HIV reservoir dynamics [20], we evaluated whether CB use impacts the latent HIV reservoir in durably ART-suppressed PWH. Total CD4 T cells from each participant were evaluated with the IPDA, which quantifies intact proviruses per million CD4 T cells; 5′-defective, 3′-defective, and total (sum of intact, 5′-defective, and 3′-defective) proviral frequencies are also quantified in the same sample [30]. Total HIV DNA frequency was not statistically significantly different between PWH who use CB and those who do not (Figure 5A). Frequency of intact proviruses were also not statistically different when assessed as absolute frequencies/106 CD4 T cells or as a percentage of total HIV DNA frequency (Figure 5B and 5C). No statistically significant correlation between years of CB use and intact or total HIV DNA was observed (Supplementary Figure 2G and 2H). A hypothesis-generating exploratory subgroup analysis was performed to further examine if CB use was associated with reservoir frequency (Supplementary Methods and Supplementary Figure 6). This analysis identified that in individuals with NK cell expression of NKG2A > 28%, CB use may be associated with a lower level of intact HIV DNA.
Figure 5.
Cannabis use is not associated with differences in intact or total HIV DNA reservoir frequency in CD4 T cells. IPDA was carried out on purified CD4 T cells from subjects without IPDA amplification failures (66/75, n = 30 CB use and n = 36 nonuse) to quantify (A) total viral DNA per million CD4 T cells (total HIV DNA/106 CD4 T cells), (B) intact proviruses per million CD4 T cells (intact HIV DNA/106 CD4 T cells), and (C) percent intact proviruses of total proviruses detected by IPDA for each study participant (intact DNA % of total). Each dot represents an individual participant. Comparisons were considered statistically significant (designated with a single asterisk) if (1) the P value from the Mann-Whitney U test was <.05 and (2) the FDR q value was <10%. Lines represent the median and error bars the interquartile range. Abbreviations: HIV, human immunodeficiency virus; IPDA, intact proviral DNA assay; LOD, limit of detection; ns, not significant.
DISCUSSION
In this observational cross-sectional study, we found that CB use was associated with alterations in CD8 T-cell subset abundance, as well as reduced expression of a variety of T-cell immune activation, exhaustion, and senescence markers. Notably, the magnitude of the aforementioned parameters correlated with self-reported years of lifetime regular CB use, suggesting a potential dose responsive impact of CB use on immune activation. Data science approaches were confirmatory of the anti-inflammatory effect of CB use, and identified a limited number of complex immunophenotypes that could predict CB use. Importantly, HIV-specific functional T-cell responses were unaffected by CB use. Finally, CB use was not associated with altered frequency of intact or total HIV DNA.
Canonically, cannabinoids are thought to exhibit anti-inflammatory effects [22, 23]. However, despite the high prevalence of CB use in PWH, only a handful of studies have examined the impact of CB use on the immune system in PWH on durable suppressive ART [10]. Some human studies have demonstrated that CB use is associated with anti-inflammatory effects, including lower levels of inflammatory monocytes, plasma inflammatory cytokines, and T-cell activation markers [10–15, 21]. However, this broad anti-inflammatory phenotype has not been reproduced in all studies [16–20].
This study supports the notion that CB use is associated with anti-inflammatory effects on T cells. A notable strength of the study is the focus on individuals who use CB but do not have other substance use disorders, as other substance use has been suggested to confound the anti-inflammatory effects of CB [13, 15, 19]. Further, several anti-inflammatory phenotypes associated with CB correlated with the cumulative lifetime years of self-reported CB use. Additionally, using data science approaches, a limited combination of specific immunophenotype parameters was identified that predicted CB use in PWH. Common among these parameters were markers of T-cell inflammation/exhaustion, including CD38, HLA-DR, PD-1, and KLRG1.
There are some limitations of this study and remaining questions regarding the impact of CB use on inflammation in PWH on ART. Future appropriately powered studies will be required to understand the interactions of CB use with other substances (such as tobacco, alcohol, illicit drugs, and/or polysubstance use), biological sex, and the immune system [13, 15, 19, 27–29]. Additional studies might also further define the mechanisms underlying the anti-inflammatory effect of CB use in the context of PWH on ART [22–24]. The specific CB constituents and exposures that mediate these anti-inflammatory effects also remain to be determined. CB contains several cannabinoids, including THC, cannabidiol (CBD), and minor cannabinoids. Self-reported lifetime years of CB use correlated with several metrics of immune activation/exhaustion, suggesting a dose-responsive effect; however, the correlation was weak and self-reported exposures are subject to response bias. Therefore, controlled animal model experiments or clinical trials will be necessary to establish dosing and the specific CB constituents or derivatives that could provide the most immune benefit to PWH [21]. Thorough assessment of the benefits and risks of CB use on nonimmune tissues and physiological and psychological processes will also be required [9].
Given the anti-inflammatory signature associated with CB use, an important question is whether functional T-cell responses to HIV are impaired in PWH on ART who use CB [11, 22–24]. Short-term CB exposure in PWH on ART did not alter mitogenic and non-HIV functional immune responses; however, no study has evaluated the impact of CB use on HIV-specific functional T-cell responses during ART [42]. Importantly, in this study CB use was not associated with altered functional CD4 and CD8 T-cell responses to HIV peptide pools ex vivo in PWH on suppressive ART. However, it remains possible that CB use could impact adaptive responses in a manner that is not captured using ex vivo assays or exhibit immunosuppressive effects during only certain stages of infection such as prior to ART initiation [43]. Nonetheless, these results provide some reassurance that CB use does not adversely impact HIV-specific T-cell functionality.
In addition to addressing immune activation and T-cell functionality, our study represents the first assessment of the impact of CB use on the intact and total HIV DNA reservoir in participants on durable suppressive ART, when reservoir frequencies are relatively more stable and less subject to the dynamics of initial reservoir decline and CD4 reconstitution following ART initiation [20, 44, 45]. Interestingly, a clear association between CB use and the frequency or composition (intact vs defective proviruses) of the HIV reservoir in CD4 T cells was not observed in this cross-sectional study. An exploratory subgroup analysis suggested that CB use may be associated with lower HIV reservoir frequency in PWH who use CB that have higher NK cell NKG2A expression, but this requires additional follow-up and validation. The absence of a clear relationship between CB use and HIV reservoir size across the entire cohort may be a true negative finding but could also reflect limitations of the cross-sectional design. For instance, the impact of CB use on the HIV reservoir may be dose-dependent; however, no correlation was observed between lifetime years of self-reported CB use and intact or total HIV DNA frequencies. That said, longitudinal cohort studies or clinical trials are required to fully elucidate the impact of CB on long-term HIV reservoir frequency and dynamics, as CB use may impact the HIV reservoir through pre-ART (eg, impacts on viral replication) and post-ART (eg, influences on reservoir decay/expansion) mechanisms [20, 29, 44].
In summary, CB use was associated with a specific anti-inflammatory immunophenotypic signature and preserved HIV-specific T-cell responses during long-term ART. While no robust association of CB use and the HIV reservoir was observed, this observational cross-sectional study provides the scientific basis for several hypotheses that could be explored in longitudinal cohort or interventional studies to fully dissect the interactions between CB use, inflammation, and the HIV reservoir. This might ultimately be leveraged clinically to mitigate persistent inflammation and associated sequelae in PWH, as well as inform strategies for an HIV cure.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Shane D Falcinelli, Department of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA; Department of Microbiology and Immunology, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA; HIV Cure Center, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA.
Alicia D Cooper-Volkheimer, Department of Medicine, Duke University, Durham, North Carolina, USA.
Lesia Semenova, Department of Computer Science, Duke University, Durham, North Carolina, USA.
Ethan Wu, Department of Computer Science, Duke University, Durham, North Carolina, USA.
Alexander Richardson, Department of Computer Science, Duke University, Durham, North Carolina, USA.
Manickam Ashokkumar, Department of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA; HIV Cure Center, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA.
David M Margolis, Department of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA; Department of Microbiology and Immunology, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA; HIV Cure Center, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA.
Nancie M Archin, Department of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA; HIV Cure Center, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA.
Cynthia D Rudin, Department of Computer Science, Duke University, Durham, North Carolina, USA.
David Murdoch, Department of Medicine, Duke University, Durham, North Carolina, USA.
Edward P Browne, Department of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA; Department of Microbiology and Immunology, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA; HIV Cure Center, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA.
Notes
Author contributions. E. P. B. and D. M. Murdoch designed the study. S. D. F., A. D. C. V., and A. M. conducted experiments. E. P. B., D. M. Murdoch, A. R., E. W., L. S., C. D. R., and S. D. F. analyzed data. E. P. B., S. D. F., N. A., L. S., C. D. R., D. M. Murdoch, and D. M. Margolis wrote the manuscript.
Acknowledgment. The authors thank the participants who made this study possible.
Disclaimer. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health.
Financial support. This work was supported by the National Institutes of Health, National Institute of Allergy and Infectious Diseases (grant numbers R01 AI143381 to E. P. B., UM1 AI164567 to D. M. Margolis, and F30 AI145588 to S. D. F.); and National Institute on Drug Abuse (grant numbers R61 DA047023 to E. P. B. and R01 DA054994 to C. D. R.).
References
- 1. Eisinger RW, Fauci AS. Ending the HIV/AIDS pandemic. Emerg Infect Dis 2018; 24:413–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Powderly WG, Landay A, Lederman MM. Recovery of the immune system with antiretroviral therapy: the end of opportunism? JAMA 1998; 280:72–7. [DOI] [PubMed] [Google Scholar]
- 3. Guihot A, Bourgarit A, Carcelain G, Autran B. Immune reconstitution after a decade of combined antiretroviral therapies for human immunodeficiency virus. Trends Immunol 2011; 32:131–7. [DOI] [PubMed] [Google Scholar]
- 4. Gandhi RT, McMahon DK, Bosch RJ, et al. Levels of HIV-1 persistence on antiretroviral therapy are not associated with markers of inflammation or activation. PLoS Pathog 2017; 13:e1006285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nasi M, De Biasi S, Gibellini L, et al. Ageing and inflammation in patients with HIV infection. Clin Exp Immunol 2017; 187:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marcus JL, Leyden WA, Alexeeff SE, et al. Comparison of overall and comorbidity-free life expectancy between insured adults with and without HIV infection, 2000–2016. JAMA Netw Open 2020; 3:e207954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Paiardini M, Muller-Trutwin M. HIV-associated chronic immune activation. Immunol Rev 2013; 254:78–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Klatt NR, Chomont N, Douek DC, Deeks SG. Immune activation and HIV persistence: implications for curative approaches to HIV infection. Immunol Rev 2013; 254:326–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Montgomery L, Bagot K, Brown JL, Haeny AM. The association between marijuana use and HIV continuum of care outcomes: a systematic review. Curr HIV/AIDS Rep 2019; 16:17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Costiniuk CT, Jenabian MA. Cannabinoids and inflammation: implications for people living with HIV. AIDS 2019; 33:2273–88. [DOI] [PubMed] [Google Scholar]
- 11. Manuzak JA, Gott TM, Kirkwood JS, et al. Heavy cannabis use associated with reduction in activated and inflammatory immune cell frequencies in antiretroviral therapy-treated human immunodeficiency virus-infected individuals. Clin Infect Dis 2018; 66:1872–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rizzo MD, Crawford RB, Henriquez JE, et al. HIV-infected cannabis users have lower circulating CD16+ monocytes and IFN-gamma-inducible protein 10 levels compared with nonusing HIV patients. AIDS 2018; 32:419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yin L, Dinasarapu AR, Borkar SA, et al. Anti-inflammatory effects of recreational marijuana in virally suppressed youth with HIV-1 are reversed by use of tobacco products in combination with marijuana. Retrovirology 2022; 19:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ellis RJ, Peterson SN, Li Y, et al. Recent cannabis use in HIV is associated with reduced inflammatory markers in CSF and blood. Neurol Neuroimmunol Neuroinflamm 2020; 7:e809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Castro FOF, Silva JM, Dorneles GP, et al. Distinct inflammatory profiles in HIV-infected individuals under antiretroviral therapy using cannabis, cocaine or cannabis plus cocaine. AIDS 2019; 33:1831–42. [DOI] [PubMed] [Google Scholar]
- 16. Murray CH, Javanbakht M, Cho GD, Gorbach PM, Fulcher JA, Cooper ZD. Changes in immune-related biomarkers and endocannabinoids as a function of frequency of cannabis use in people living with and without HIV [published online ahead of print 20 April 2023]. Cannabis Cannabinoid Res doi: 10.1089/can.2022.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ghosn J, Leruez-Ville M, Blanche J, et al. HIV-1 DNA levels in peripheral blood mononuclear cells and cannabis use are associated with intermittent HIV shedding in semen of men who have sex with men on successful antiretroviral regimens. Clin Infect Dis 2014; 58:1763–70. [DOI] [PubMed] [Google Scholar]
- 18. Krsak M, Wada NI, Plankey MW, et al. Self-reported cannabis use and markers of inflammation in men who have sex with men with and without HIV. Cannabis Cannabinoid Res 2021; 6:165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vidot DC, Manuzak JA, Klatt NR, et al. Brief report: hazardous cannabis use and monocyte activation among methamphetamine users with treated HIV infection. J Acquir Immune Defic Syndr 2019; 81:361–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chaillon A, Nakazawa M, Anderson C, et al. Effect of cannabis use on human immunodeficiency virus DNA during suppressive antiretroviral therapy. Clin Infect Dis 2020; 70:140–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bouassa RS M, Comeau E, Alexandrova Y, et al. Effects of oral cannabinoids on systemic inflammation and viral reservoir markers in people with HIV on antiretroviral therapy: results of the CTN PT028 pilot clinical trial. Cells 2023; 12:1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Turcotte C, Blanchet MR, Laviolette M, Flamand N. The CB(2) receptor and its role as a regulator of inflammation. Cell Mol Life Sci 2016; 73:4449–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nichols JM, Kaplan BLF. Immune responses regulated by cannabidiol. Cannabis Cannabinoid Res 2020; 5:12–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eisenstein TK, Meissler JJ. Effects of cannabinoids on T-cell function and resistance to infection. J Neuroimmune Pharmacol 2015; 10:204–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ellis RJ, Wilson N, Peterson S. Cannabis and inflammation in HIV: a review of human and animal studies. Viruses 2021; 13:1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kaslow RA, Blackwelder WC, Ostrow DG, et al. No evidence for a role of alcohol or other psychoactive drugs in accelerating immunodeficiency in HIV-1-positive individuals. A report from the Multicenter AIDS Cohort Study. JAMA 1989; 261:3424–9. [PubMed] [Google Scholar]
- 27. Slawek DE, Arnsten J, Sohler N, et al. Daily and near-daily cannabis use is associated with HIV viral load suppression in people living with HIV who use cocaine. AIDS Care 2021; 33:1350–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Milloy MJ, Marshall B, Kerr T, et al. High-intensity cannabis use associated with lower plasma human immunodeficiency virus-1 RNA viral load among recently infected people who use injection drugs. Drug Alcohol Rev 2015; 34:135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cohn LB, Chomont N, Deeks SG. The biology of the HIV-1 latent reservoir and implications for cure strategies. Cell Host Microbe 2020; 27:519–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bruner KM, Wang Z, Simonetti FR, et al. A quantitative approach for measuring the reservoir of latent HIV-1 proviruses. Nature 2019; 566:120–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Falcinelli SD, Kilpatrick KW, Read J, et al. Longitudinal dynamics of intact HIV proviral DNA and outgrowth virus frequencies in a cohort of individuals receiving antiretroviral therapy. J Infect Dis 2021; 224:92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gelman A. Data analysis using regression and multilevel/hierarchical models. Cambridge: Cambridge University Press, 2006. [Google Scholar]
- 33. Roederer M, Nozzi JL, Nason MC. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A 2011; 79:167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lin J, Zhong C, Hu D, Rudin C, Seltzer M. Generalized and scalable optimal sparse decision trees. Proc Machine Learning Res 2020; 119. doi: 10.48550/arXiv.2006.08690. [DOI] [Google Scholar]
- 35. Wang Y, Huang H, Rudin C, Shaposhnik Y. Understanding how dimension reduction tools work: an empirical approach to deciphering t-SNE, UMAP, TriMap, and PaCMAP for data visualization. J Mach Learn Res 2021; 22:1–73. [Google Scholar]
- 36. Murdoch DM, Barfield R, Chan C, et al. Neuroimaging and immunological features of neurocognitive function related to substance use in people with HIV. J Neurovirol 2023; 29:78–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hjorthoj CR, Hjorthoj AR, Nordentoft M. Validity of timeline follow-back for self-reported use of cannabis and other illicit substances–systematic review and meta-analysis. Addict Behav 2012; 37:225–33. [DOI] [PubMed] [Google Scholar]
- 38. Karschner EL, Swortwood-Gates MJ, Huestis MA. Identifying and quantifying cannabinoids in biological matrices in the medical and legal cannabis era. Clin Chem 2020; 66:888–914. [DOI] [PubMed] [Google Scholar]
- 39. Rodriguez IJ, Lalinde Ruiz N, Llano Leon M, et al. Immunosenescence study of T cells: a systematic review. Front Immunol 2020; 11:604591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang S, Zhang Q, Hui H, Agrawal K, Karris MAY, Rana TM. An atlas of immune cell exhaustion in HIV-infected individuals revealed by single-cell transcriptomics. Emerg Microbes Infect 2020; 9:2333–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fenwick C, Joo V, Jacquier P, et al. T-cell exhaustion in HIV infection. Immunol Rev 2019; 292:149–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bredt BM, Higuera-Alhino D, Shade SB, Hebert SJ, McCune JM, Abrams DI. Short-term effects of cannabinoids on immune phenotype and function in HIV-1-infected patients. J Clin Pharmacol 2002; 42:82S–9S. [DOI] [PubMed] [Google Scholar]
- 43. Chen W, Kaplan BL, Pike ST, et al. Magnitude of stimulation dictates the cannabinoid-mediated differential T cell response to HIVgp120. J Leukoc Biol 2012; 92:1093–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Siliciano JD, Siliciano RF. In vivo dynamics of the latent reservoir for HIV-1: new insights and implications for cure. Annu Rev Pathol 2022; 17:271–94. [DOI] [PubMed] [Google Scholar]
- 45. White JA, Simonetti FR, Beg S, et al. Complex decay dynamics of HIV virions, intact and defective proviruses, and 2LTR circles following initiation of antiretroviral therapy. Proc Natl Acad Sci U S A 2022; 119:e2120326119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.