Abstract
Background
The aim of this study was to determine whether neurometabolite abnormalities indicating neuroinflammation and neuronal injury are detectable in individuals post–coronavirus disease 2019 (COVID-19) with persistent neuropsychiatric symptoms.
Methods
All participants were studied with proton magnetic resonance spectroscopy at 3 T to assess neurometabolite concentrations (point-resolved spectroscopy, relaxation time/echo time = 3000/30 ms) in frontal white matter (FWM) and anterior cingulate cortex–gray matter (ACC-GM). Participants also completed the National Institutes of Health Toolbox cognition and motor batteries and selected modules from the Patient-Reported Outcomes Measurement Information System.
Results
Fifty-four participants were evaluated: 29 post–COVID-19 (mean ± SD age, 42.4 ± 12.3 years; approximately 8 months from COVID-19 diagnosis; 19 women) and 25 controls (age, 44.1 ± 12.3 years; 14 women). When compared with controls, the post–COVID-19 group had lower total N-acetyl compounds (tNAA; ACC-GM: −5.0%, P = .015; FWM: –4.4%, P = .13), FWM glutamate + glutamine (–9.5%, P = .001), and ACC-GM myo-inositol (−6.2%, P = .024). Additionally, only hospitalized patients post–COVID-19 showed age-related increases in myo-inositol, choline compounds, and total creatine (interaction P = .029 to <.001). Across all participants, lower FWM tNAA and higher ACC-GM myo-inositol predicted poorer performance on several cognitive measures (P = .001–.009), while lower ACC-GM tNAA predicted lower endurance on the 2-minute walk (P = .005).
Conclusions
In participants post–COVID-19 with persistent neuropsychiatric symptoms, the lower-than-normal tNAA and glutamate + glutamine indicate neuronal injury, while the lower-than-normal myo-inositol reflects glial dysfunction, possibly related to mitochondrial dysfunction and oxidative stress in Post-COVID participants with persistent neuropsychiatric symptoms.
Keywords: COVID-19, myo-inositol, N-acetylaspartate, neuropsychiatric symptoms, proton magnetic resonance spectroscopy
Brain metabolites were compared between participants with long COVID and healthy controls using proton magnetic resonance spectroscopy. We found neuronal injury and glial dysfunction, possibly due to mitochondrial dysfunction and oxidative stress in participants ∼8 months after acute COVID-infection.
The clinical syndrome coronavirus disease 2019 (COVID-19) ranges from asymptomatic infections to severe life-threatening pneumonia and multiorgan involvement [1]. Furthermore, depending on disease severity and vaccination status, 10% to 70% of patients with COVID-19 may develop post–COVID-19 conditions (PCCs), or “long COVID,” with highly variable symptoms involving multiple organs or systems, which may persist beyond 2 years [2]. Neuropsychiatric symptoms are common in PCCs, including hypogeusia, hyposmia or anosmia, dizziness, headaches, and fatigue, as well as complaints of both cognitive problems (“brain fog”) and psychiatric symptoms (anxiety, depression) [2]. Despite the high prevalence of PCCs, the neurologic sequelae [3] and the extent of recovery remain unclear.
Acutely, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is associated with a proinflammatory cytokine response, including a “cytokine storm” of interleukins (IL-2, IL-6, and IL-7), acute phase reactants (C-reactive protein and ferritin), chemoattractants, and effector molecules [4]. However, the pathogenic factors mediating PCCs remain unclear [3]. Autopsy studies of patients and nonhuman primates with COVID-19 show prominent neuroinflammation and activated microglia [5–8]. A mouse model evaluated acute and long COVID-19 seven weeks after infection and found persistent microglial activation, white matter (WM) myelin loss, and elevated cerebral spinal fluid (CSF) cytokines and chemokines [9]. Additionally, patients with acute COVID-19 showed elevated plasma glial fibrillary acidic protein (GFAp; glial activation marker) and neurofilament light chain protein (NfL; neuronal damage marker) [10–12]. In recovered patients with COVID-19, plasma GFAp or NfL levels remained elevated at 4 months [12, 13] but normalized by 7 months [11].
Since glial and neuronal metabolites can be assessed noninvasively with proton magnetic resonance spectroscopy (1H-MRS), we used 1H-MRS to evaluate possible neuronal and glial metabolite abnormalities in patients with post–acute COVID-19 who had persistent neuropsychiatric symptoms. Neuronal injury or loss is associated with reduced neuronal metabolites: total N-acetyl-compounds (tNAA; N-acetylaspartate + N-acetylaspartyl-glutamate) and glutamate + glutamine (Glu + Gln). Neuroinflammation is typically shown by elevated glial marker myo-inositol (mI) and often concomitantly elevated choline compounds (Cho) and total creatine (tCr) [14], due to their higher concentrations in glia than neurons [15]. Multiple 1H-MRS studies not only showed age-related increases in glial metabolites [16] but delineated neuronal injury and neuroinflammation in various viral infections, including HIV, John Cunningham virus, hepatitis C virus, Epstein-Barr virus, and cytomegalovirus [14]. These metabolite abnormalities in patients with HIV [17] or hepatitis C virus [18] often predicted disease severity and correlated with clinical variables.
Based on findings of neuroinflammation and neuronal injury in clinical and postmortem studies of patients with COVID-19 [5, 6, 9–13] and in many patients with neuropsychiatric disorders [19], we hypothesized that, when compared with healthy controls, participants post–COVID-19 would show (1) evidence of neuronal injury with lower neuronal markers (tNAA and Glu + Gln) and (2) persistent neuroinflammation with elevated glial metabolites (mI, Cho, and tCr). Furthermore, since COVID-19 is typically more severe in older and hospitalized patients, we secondarily analyzed how age and prior hospitalization might affect brain metabolite concentrations. Finally, we explored whether neuronal and glial metabolite levels would predict neuropsychiatric symptom severity.
METHODS
Participants
Participants were recruited via local community advertisements and referrals. An overall 169 interested participants were screened by telephone: 60 were ineligible, 26 were not interested, 7 were lost to follow-up, and 15 were not matched controls. Hence, 61 (36%) were screened in person and provided written informed consent (Supplementary Figure 1). Each had a structured physical and neuropsychiatric evaluation, urine toxicology to screen for substance use disorders, and electrocardiogram and blood tests to evaluate overall health and exclude confounding medical conditions. After this screening, 2 more were ineligible, 2 were no longer interested, and 3 could not complete the 1H-MRS. The final sample included 54 participants who fulfilled all study criteria and completed the behavioral assessments and magnetic resonance imaging (MRI) with 1H-MRS: 29 patients post–COVID-19 and 25 healthy controls matched by age, sex, and education. Participants were studied between February 2021 and February 2022. The study was approved by the Institutional Review Board of the University of Maryland School of Medicine.
Inclusion criteria were men or women aged 18 to 75 years who were willing and able to provide informed consent. Participants post–COVID-19 had at least 1 neuropsychiatric symptom that emerged after COVID-19 and documented COVID-19 diagnosis ≥6 weeks earlier. Healthy controls had no prior COVID-19 symptoms or diagnosis, a negative polymerase chain reaction test result for SARS-CoV-2 within 1 week, or a negative rapid antigen test result on the day of evaluation. Exclusion criteria were as follows: any significant confounding neurologic or psychiatric disorder (eg, stroke, encephalitis from any cause except COVID-19, neurodegenerative disorder, schizophrenia, uncontrolled major depression or anxiety disorder requiring medication prior to COVID-19, traumatic brain injury with loss of consciousness for >1 hour requiring hospitalization), severe substance use disorders (per the DSM-5) except for tobacco or cannabis use, or any contraindication for MRI and 1H-MRS.
All 54 participants were assessed by using quantitative neurobehavioral measures from the National Institutes of Health (NIH) Toolbox–Cognition Battery and Motor Battery [20] and selected surveys from the Patient-Reported Outcomes Measurement Information System (PROMIS) [21]. All participants post–COVID-19 completed a standardized symptom severity questionnaire (Table 1).
Table 1.
Participant Characteristics and Post–COVID-19 Symptoms
| Post–COVID-19 (n = 29) | Controls (n = 25) | P Value | |
|---|---|---|---|
| Age, y | 42.4 ± 12.3 | 44.1 ± 12.3 | .61a |
| Sex | |||
| Men | 10 (34.5) | 11 (44) | .47b |
| Women | 19 (65.5) | 14 (56) | |
| Race/ethnicity: Asian, Biracial, Black, Hispanic, White | 0, 1, 8, 1, 19 | 2, 0, 10, 4, 9 | .08b |
| Education: graduate, undergraduate, some college, high school | 8, 8, 8, 5 | 12, 7, 3, 3 | .34b |
| Body mass index | 30.7 ± 8.0 | 27.3 ± 6.7 | .10a |
| Index of Social Positionc | 30.4 ± 14.1 | 29.0 ± 14.6 | .73a |
| Substance use history: lifetime, past month | |||
| Tobacco use | 10, 1 | 10, 1 | >.99,b >.99d |
| Marijuana usee | 14, 3 | 5, 1 | .04,b .36d |
| Alcohol use | 23, 23 | 21, 21 | >.99,b >.99b |
| Comorbid medical illnesses pre–COVID-19 or vaccination status at study | |||
| Hypertension | 6 | 1 | .11d |
| Diabetes | 4 | 0 | .12d |
| Overweight/obese | 9/13 | 6/8 | .91b |
| Chronic obstructive pulmonary disease | 4 | 0 | .12d |
| SARS-CoV-2 vaccination: yes, no, unknown | 16, 13, 0 | 20, 2, 3 | .05b |
| Post–COVID-19 participant history and symptoms | |||
| Days since diagnosis | 242 ± 156 (42–484) | ||
| Hospitalized, nonhospitalized | 9, 20 | ||
| COVID-19 treatments received | |||
| Nasal cannula O2, hi-flow, or BiPAP | 5, 3 | ||
| Ventilation, ECMO | 2, 1 | ||
| Steroid,f remdesivir, monoclonal antibodyg | 15, 5, 3 | ||
| Other treatments (apixaban, antibioticsh) | 1, 5 | ||
| Post–COVID-19 symptoms, %: total (mild, moderate, severe) | |||
| Neurologic | |||
| Concentration problems | 92.9 (14.3, 53.6, 25.0) | ||
| Memory problems | 78.6 (17.9, 39.3, 21.4) | ||
| Confusion | 64.3 (42.9, 14.3, 7.1) | ||
| Headaches | 57.1 (7.1, 35.7, 14.3) | ||
| Dizziness | 57.1 (28.6, 14.3, 14.3) | ||
| Gait disturbance | 50.0 (28.6, 17.9, 3.6) | ||
| Visual disturbances | 50.0 (21.4, 21.4, 7.1) | ||
| Paresthesia | 42.9 (17.9, 10.7, 14.3) | ||
| Coordination problems | 39.3 (14.3, 25.0, 0.0) | ||
| Hyposmia | 28.6 (14.3, 10.7, 3.6) | ||
| Dysgeusia | 28.6 (14.3, 7.1, 7.1) | ||
| Postural instability | 14.3 (3.6, 7.1, 3.6) | ||
| Other neurologic | 14.3 (10.7, 3.6, 0.0) | ||
| Psychological/other | |||
| Fatigue | 85.7 (10.7, 28.6, 46.4) | ||
| Depression or anxiety | 67.9 (21.4, 32.1, 14.3) | ||
| Sleep disturbances | 64.3 (17.9, 21.4, 25.0) | ||
| Myalgia | 60.7 (21.4, 32.1, 7.1) | ||
| Lightheadedness | 46.4 (17.9, 17.9, 10.7) | ||
| Urinary problems | 25.0 (17.9, 3.6, 3.6) |
Data are presented as mean ± SD (range) or No. (%) unless noted otherwise.
Abbreviations: BiPAP, bilevel positive airway pressure; ECMO, extracorporeal membrane oxygenation; hi-flow, high-flow oxygen.
a T test.
bChi-square test.
cIndex of Social Position calculated from the Hollingshead Four Factor Index of Socioeconomic Status.
dFisher exact test.
eIncludes 2 post–COVID-19 using cannabidiol.
fDexamethasone, prednisone, methylprednisolone, or hydrocortisone.
gBamlanivimab or etesevimab.
hAzithromycin or ceftriaxone.
Neuroimaging and Brain 1H-MRS Data Acquisition
All 54 participants completed MRI and 1H-MRS on a 3-T Prisma MRI scanner (Siemens) with a 64-channel phased array head-neck coil. Structural MRI included a T1-weighted volume-navigated 3-dimensional MP-RAGE sequence (magnetization-prepared rapid gradient echo; relaxation/echo/inversion time = 2500/2.88/1060 ms, flip angle = 8°, slab thickness = 176 mm, isotropic resolution = 1 mm, in-plane acceleration = 2) and a T2-weighted fluid-attenuated inversion recovery sequence. All structural scans were reviewed by a board-certified neuroradiologist (independent of the study) and a board-certified neurologist (L. C.). T1-weighted images were used for MRS voxel placement and post hoc segmentation of gray matter (GM), WM, and CSF within each voxel, as covariates for partial volume effects on brain metabolite concentrations.
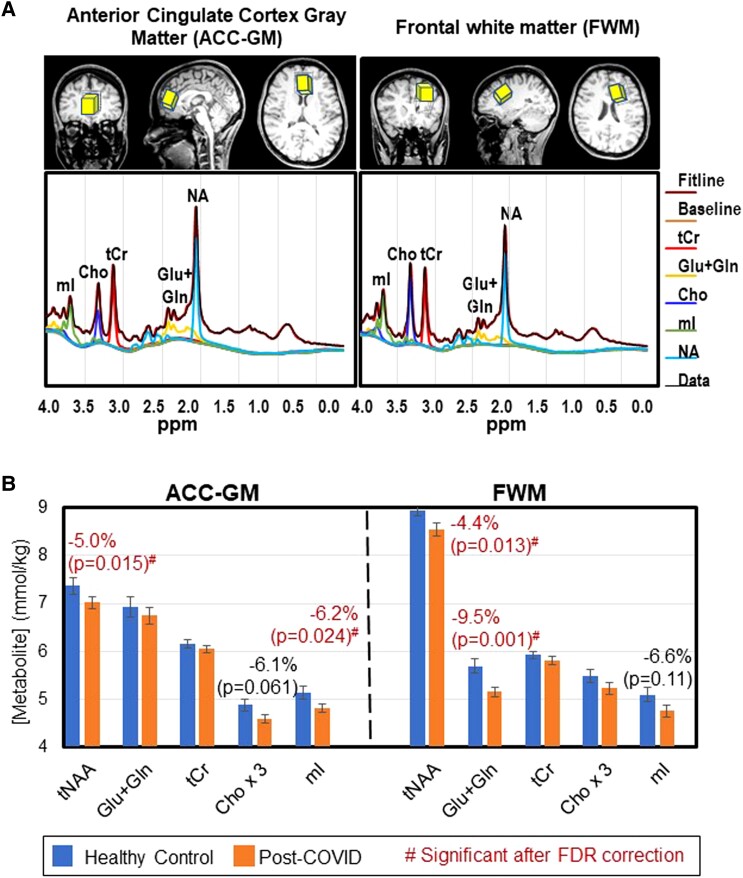
Localized 1H-MRS data were acquired in the anterior cingulate cortex (ACC) with predominantly GM (ACC-GM; 30 × 25 × 25 mm3) and frontal WM (FWM; 20 × 30 × 25 mm3; Figure 1A). The ACC-GM was chosen because this is a major node for the attention network, which is required for all cognitive tasks, while the FWM was selected since this brain region often shows neuroinflammation in other viral neuroinfections (eg, HIV, John Cunningham virus, and hepatitis C virus) [14]. Metabolite concentrations in each voxel were measured with point-resolved spectroscopy [22] (relaxation/echo time = 3000/30 ms, 128 averages) plus 7 water acquisitions at variable echo time for quantitation [23]. A fast-shimming routine was performed with a mapping projection technique. 1H-MRS acquisitions in 2 controls (1 FWM and 1 ACC-GM) with poor shim initially were repeated within the same scanning session.
Figure 1.
Voxel locations, typical magnetic resonance spectra, and brain metabolite concentrations (mean ± SE) in participants post–COVID-19 and healthy controls. A, Axial and sagittal magnetic resonance imaging scans show the 2 voxel locations in the ACC-GM and FWM regions. Typical spectra for each brain region are shown below the scans. B, Metabolite concentrations of each group: healthy control (blue and left bar of each pair) and post–COVID-19 (orange and right bar of each pair). The corresponding P values are from group comparisons for each metabolite concentration (covaried for age, sex, and percentage gray matter in each voxel as needed). ACC-GM, anterior cingulate cortex–gray matter; Cho × 3, choline compounds *3 (choline + phosphocholine + glyceryl phosphorylcholine); FDR, false discovery rate; FWM, frontal white matter; Glu + Gln, glutamate + glutamine; mI, myo-inositol; NA, N-acetyl compounds; tCr, total creatine (creatine + phosphocreatine); tNAA, N-acetyl compounds. #Red indicates significant group differences after FDR correction.
MRS Data Processing
Spectral data were processed through the Linear Combination Model program (LC Model 6.3) to determine the peak areas for tNAA, tCr, Cho, mI, and Glu + Gln. The amplitude from the brain water acquisition (interpolated to echo time = 0) was used as a reference to calculate metabolite concentrations corrected for the percentage CSF in each voxel. Quality assurance was based on visual inspection to exclude spectra with visible artifacts and automated rejection of spectra with a tCr line width >0.07 ppm or Cramer-Rao lower bound >15% (see Figure 1A for typical spectra). Overall, the tCr line widths were excellent and similar between groups (Supplementary Table 1). Additionally, the MP-RAGE scans were segmented in Gannet [24] to calculate percentage GM and WM in each voxel. To avoid potential processing bias, each participant’s status was blinded, and the LC Model fitting was automated.
Sample Size and Statistical Analyses
With 20 subjects per group and assuming α = .05 and a standard deviation of 50% per group, we would have 89% power to detect a 1.5-fold change in outcome variables between groups. Similar sample sizes yielded significant group effects in prior 1H-MRS studies [14, 25–27].
Statistical analyses were performed in R (version 4.1.2). Metabolite concentrations were analyzed through stepwise regression based on the Akaike information criterion. We included post–COVID-19 status, age, and their interaction as primary independent variables and sex and percentage GM in each voxel as covariates. If the COVID-19 status × age interaction or covariates in the initial model did not meet P < .1, a reduced model without nonsignificant terms was applied. We performed additional analysis of covariance in the post–COVID-19 group to assess hospitalization status on metabolite concentrations, with sex and percentage GM as covariates. We also assessed whether the days since COVID-19 diagnosis predicted metabolite concentrations. Statistical significance for these main analyses was defined per the Benjamini-Hochberg procedure based on a false discovery rate (FDR) of 0.05. Furthermore, we explored whether metabolite levels predicted neurobehavioral T-scores (NIH Toolbox–Cognition Battery and Motor Battery, PROMIS), fully adjusted for age, sex, race/ethnicity, and education, using analysis of covariance (covaried for percentage GM).
RESULTS
Participant Characteristics
By design, the post–COVID-19 and control groups had similar age, sex, self-reported race/ethnicity proportion, education, and Index of Social Position (Table 1) [25]. They also had comparable prevalence of prior comorbid medical illnesses and past-month tobacco, marijuana, or alcohol use. The post–COVID-19 group was evaluated at a mean ± SD 242 ± 156 days (median, 183; range, 42–484) after acute illness. Nine patients post–COVID-19 were hospitalized and required supplemental oxygen, extracorporeal membrane oxygenation, and/or ventilation. Fifteen received steroids, 5 had remdesivir, and 3 had monoclonal antibodies. More controls than patients were vaccinated against SARS-CoV-2.
The commonest post–COVID-19 neuropsychiatric complaints were problems with concentration (92.9%) and memory (78.6%), significant fatigue (85.7%), and depression or anxiety (67.9%). Of the 4 participants post–COVID-19 reporting severe anxiety or depression, 2 required prescription medications to manage symptoms (1 used duloxetine; 1 used levomilnacipran and bupropion).
NIH Toolbox and PROMIS
Despite the high prevalence of concentration and memory complaints, participants post–COVID-19 had similar performance as controls for all domains assessed by the NIH Toolbox–Cognition Battery (Table 2). However, on the NIH Toolbox–Motor Battery, the post–COVID-19 group had poorer endurance on the 2-Minute Endurance Walk Test (−28.3%, P = .001) and were slower on the 4-Meter Walk Gait Speed Test (−21.4%, P = .01) and 9-Hole Pegboard Dexterity Test (dominant hand, −13.0%, P = .01). Furthermore, on the PROMIS surveys, patients post–COVID-19 endorsed more symptoms, including depression, fatigue, anxiety, and pain, which led to poorer global mental and physical health scores. We previously reported similar NIH Toolbox and PROMIS findings, including the NIH Toolbox–Emotional Battery, in a larger sample [26]; the current subset is presented for correlation with the 1H-MRS data.
Table 2.
T-Scores From the NIH Toolbox Batteries and Selected Modules From PROMIS
| Post–COVID-19 (n = 29) | Controls (n = 25) | P Value | |
|---|---|---|---|
| NIH Toolbox: Cognition Battery | |||
| Flanker Inhibitory Control and Attention Test | 44.7 ± 10.7 | 47.7 ± 11.5 | .33 |
| Picture Sequence Memory Test | 51.3 ± 10.5 | 53.6 ± 9.2 | .40 |
| List Sorting Working Memory Test | 51.9 ± 8.6 | 49.1 ± 9.8 | .26 |
| Picture Vocabulary Test | 51.5 ± 9.6 | 52.0 ± 13.4 | .86 |
| Dimensional Change Card Sort Test | 52.0 ± 12.4 | 50.5 ± 13.5 | .67 |
| Pattern Comparison Processing Speed Test | 57.6 ± 12.8 | 55.2 ± 16.4 | .55 |
| Oral Symbol Digit Test | 84.3 ± 18.5 | 84.0 ± 17.0 | .96 |
| Auditory Verbal Learning Test (Rey) | 25.7 ± 5.5 | 24.8 ± 7.7 | .62 |
| Oral Reading Recognition Test | 52.6 ± 8.2 | 54.4 ± 7.2 | .38 |
| Fluid Cognition | 52.1 ± 10.8 | 51.5 ± 12.9 | .86 |
| Crystallized Cognition | 52.6 ± 9.1 | 53.4 ± 10.1 | .74 |
| Total Cognition | 52.9 ± 10.1 | 52.9 ± 9.4 | .99 |
| NIH Toolbox: Motor Battery | |||
| 4-Meter Walk Gait Speed Test | 1.1 ± 0.3 | 1.4 ± 0.3 | .01 |
| 2-Minute Walk Endurance Test | 31.1 ± 12.9 | 43.4 ± 11.0 | .001 |
| Grip Strength | |||
| Dominant | 49.1 ± 9.2 | 51.2 ± 11.0 | .45 |
| Nondominant | 48.0 ± 9.9 | 48.7 ± 11.7 | .81 |
| 9-Hole Pegboard Dexterity Test | |||
| Dominant | 45.3 ± 10.2 | 52.1 ± 9.1 | .01 |
| Nondominant | 44.8 ± 9.4 | 47.9 ± 8.1 | .21 |
| Standing Balance Test | 47.0 ± 8.4 | 42.0 ± 13.5 | .12 |
| PROMIS | |||
| Depression | 55.1 ± 9.4 | 45.6 ± 7.0 | .0001 |
| Anxiety | 58.1 ± 10.1 | 47.0 ± 8.6 | 8.04 × 10−5 |
| Fatigue | 57.6 ± 9.2 | 39.7 ± 8.0 | 6.66 x10−10 |
| Pain | |||
| Interference | 56.7 ± 9.3 | 41.6 ± 6.0 | 5.81 × 10−9 |
| Intensity | 46.2 ± 8.9 | 34.1 ± 5.8 | 3.41 × 10−7 |
| Quality | 47.4 ± 8.4 | 32.7 ± 5.8 | 1.17 × 10−9 |
| Behavior | 55.8 ± 8.1 | 41.1 ± 8.3 | 2.58 ×10−8 |
| Global Health | |||
| Mental | 40.6 ± 10.0 | 53.3 ± 8.6 | 8.24 ×10−6 |
| Physical | 36.5 ± 7.5 | 57.8 ± 8.3 | 1.34 × 10−13 |
Data are presented as mean ± SD. Bolded P-values indicate significance (P < .05).
Abbreviations: NIH, National Institutes of Health; PROMIS, Patient-Reported Outcomes Measurement Information System.
Brain Metabolite Concentrations
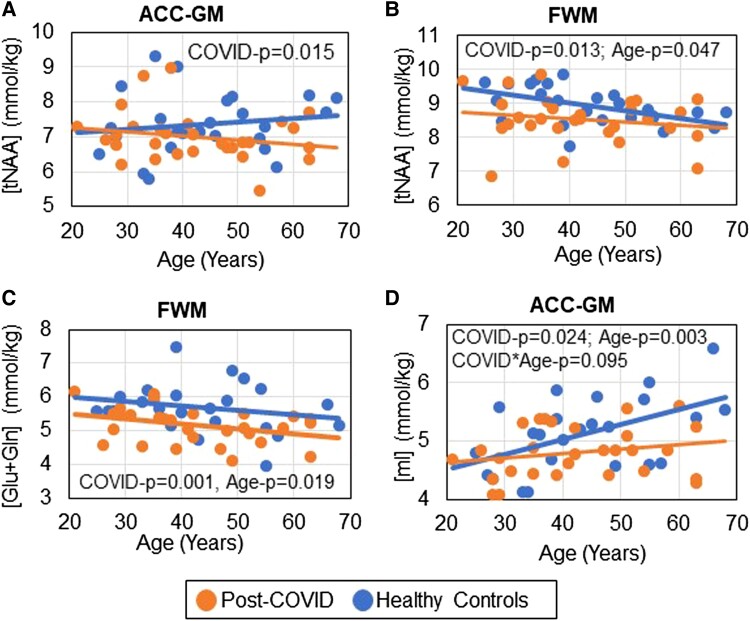
The post–COVID-19 group had lower tNAA than controls in ACC-GM (−5.0%, P = .015) and FWM (−4.4%, P = .013), with both significant after FDR correction (Figure 1B, left; Figure 2A and 2B, Supplementary Table 1). Likewise, FWM Glu + Gln was lower in participants post–COVID-19 (−9.5%, P = .001; Figure 1B, right; Figure 2C). tNAA and Glu + Gln in the FWM both declined with age independent of COVID-19 status (Figure 2B and 2C).
Figure 2.
Lower-than-normal brain metabolite concentrations for each participant across the age span. A–D, Participants post–COVID-19 show lower brain metabolite levels than healthy controls. B, C, The neuronal metabolite concentrations of tNAA and Glu + Gln in FWM declined with age. D, The glial metabolite ACC-GM mI increased with age for all participants. However, the age-related increase in ACC-GM mI is attenuated in those with a post–COVID-19 condition. ACC-GM, anterior cingulate cortex–gray matter; FWM, frontal white matter; Glu + Gln, glutamate + glutamine; mI, myo-inositol; tNAA, N-acetyl compounds.
Additionally, as compared with controls, the post–COVID-19 group showed trends for lower mI in the ACC-GM (−6.2%, P = .024) and FWM (−6.6%, P = .11; Figures 1B and 2D). The ACC-GM mI demonstrated the expected age-related increases in controls (r = 0.52, P = .01) but not subjects post–COVID-19 (r = 0.21, P = .28, interaction P = .095; Figure 2D). However, none of the metabolite levels correlated with days since COVID-19 diagnosis (not shown).
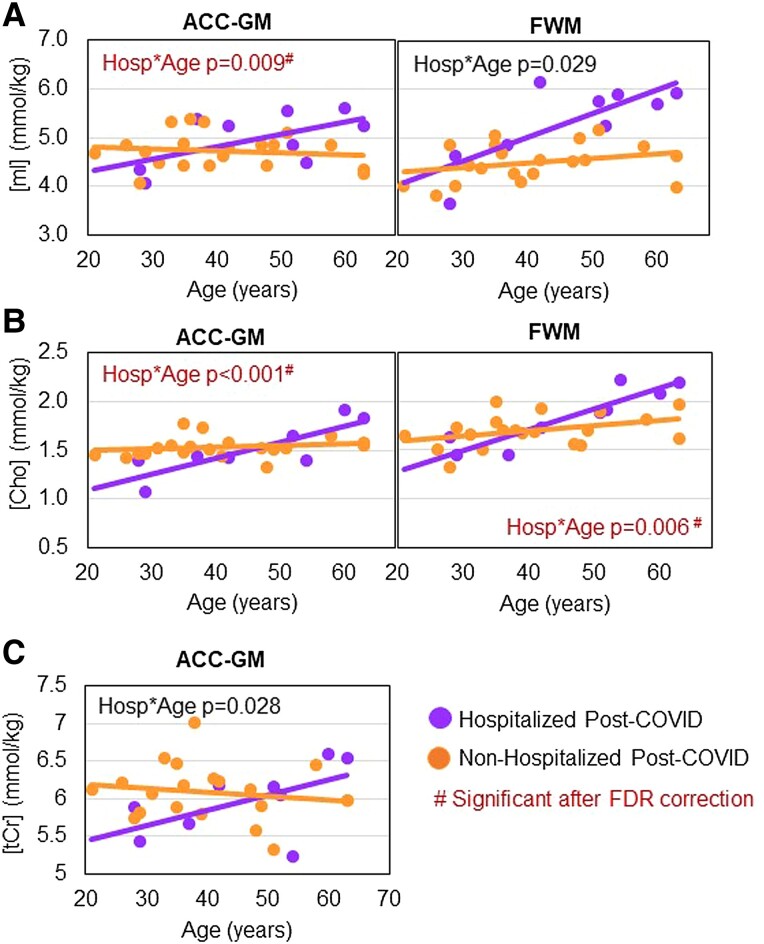
Among participants post–COVID-19, interactions between hospitalization status and age were observed for Cho and mI in both brain regions and for ACC-GM tCr (interaction P = .029 to <.001). Specifically, these glial metabolite levels increased with age only in hospitalized participants (Figure 3A–C). The interactions for ACC-GM Cho, ACC-GM mI, and FWM Cho remained significant after FDR correction.
Figure 3.
Age-related glial metabolite levels in hospitalized and nonhospitalized patients post–COVID-19. As compared with nonhospitalized participants (orange), hospitalized participants (purple) had greater age-related increases in (A) ACC-GM and FWM mI, (B) ACC-GM and FWM Cho, and (C) ACC-GM tCr. #Group × age interaction P values remained significant after FDR correction for ACC-GM Cho (P = .002), FWM Cho (P = .030), and ACC-GM mI (P = .031). ACC-GM, anterior cingulate cortex–gray matter; Cho, soluble choline compounds (choline + phosphocholine + glyceryl phosphorylcholine); FDR, false discovery rate; FWM, frontal white matter; mI, myo-inositol; tCr, total creatine (creatine + phosphocreatine).
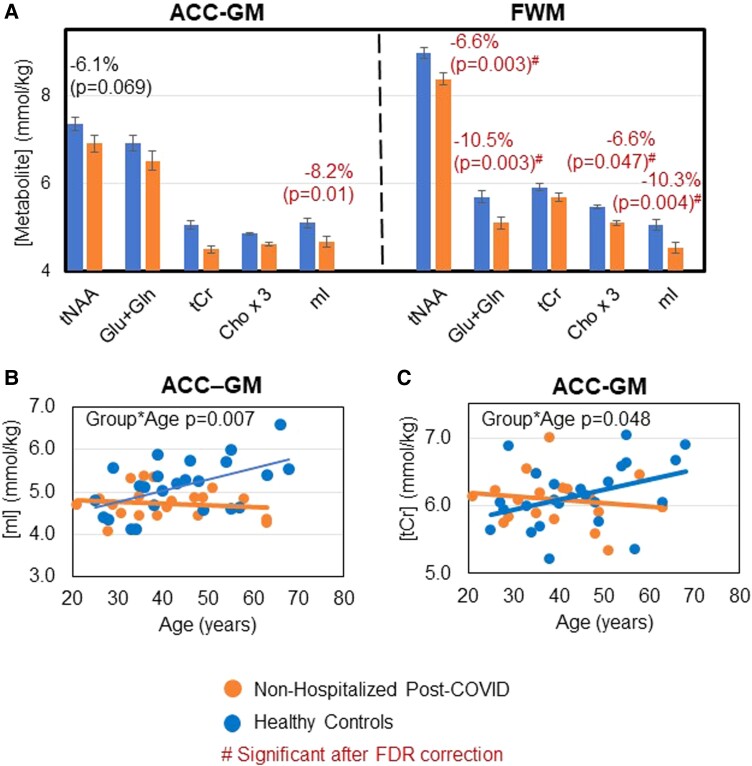
Furthermore, nonhospitalized patients post–COVID-19 (∼70% of post–COVID-19 group) had even lower levels of brain metabolites than controls (−6.1% to -10.5%), especially in FWM (Figure 4A). While controls showed normal age-related increases in ACC-GM glial metabolites mI and tCr, nonhospitalized participants post–COVID-19 lacked age-related increases in mI (interaction P = .007) and tCr (interaction P = .048; Figures 4B and 4C).
Figure 4.
Lower-than-normal levels of, and lack of normal age-related increases in, glial metabolites in nonhospitalized patients post–COVID-19. A, As compared with healthy controls (blue and left bar of each pair), nonhospitalized participants post–COVID-19 (orange and right bar of each pair) showed even lower levels of brain metabolites than those observed across all patients post–COVID-19. Mean values are adjusted per the covariates as needed; error bars indicate SE. B and C, Nonhospitalized patients post–COVID-19 showed a lack of normal age-related increases in ACC-GM mI and ACC-GM tCr. #Indicates significant group differences after FDR correction. ACC-GM, anterior cingulate cortex–gray matter; Cho × 3, choline compounds *3 (choline + phosphocholine + glyceryl phosphorylcholine); FDR, false discovery rate; FWM, frontal white matter; Glu + Gln, glutamate + glutamine; mI, myo-inositol; tCr, total creatine (creatine + phosphocreatine); tNAA, N-acetyl compounds.
Brain Metabolite Levels Predicted Cognitive and Motor Performance and Pain Measures
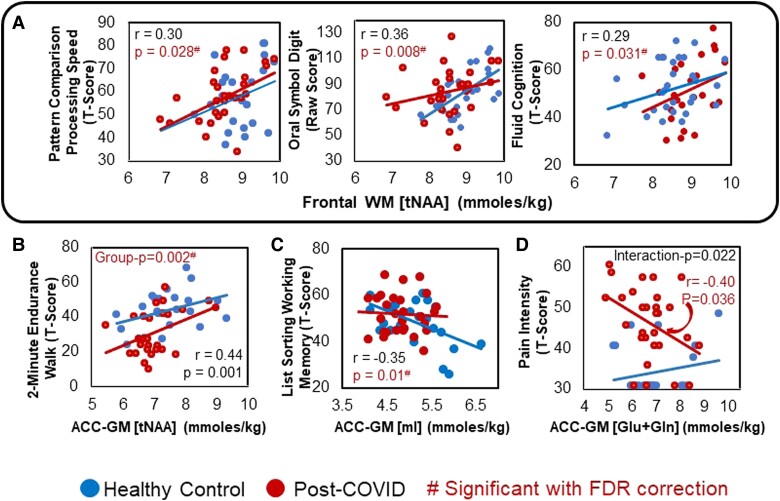
On our exploratory analyses, regardless of COVID-19 status, lower FWM tNAA predicted poorer cognitive performance on the Pattern Comparison Processing Speed Test (r = 0.30, P = .028), Oral Symbol Digit Test (r = 0.36, P = .008), and overall Fluid Cognition (r = 0.29; P = .031; Figure 5A). Furthermore, lower ACC-GM tNAA predicted poorer endurance on the 2-Minute Walk Endurance Test (r = 0.44, P = .001; Figure 5B). Conversely, higher ACC-GM mI predicted lower performance on the List Sorting Working Memory Test (r = −0.35, P = .01; Figure 5C). Finally, lower ACC-GM Glu + Gln predicted greater pain intensity only in participants with COVID-19 (r = −0.40, P = .036, interaction P = .022; Figure 5D).
Figure 5.
Brain metabolite levels predicted cognitive, motor, and pain scores. A, Across all participants, lower FWM tNAA predicted slower performance on Pattern Comparison Processing Speed, less accuracy on the Oral Symbol Digit Test, and reduced overall T-scores on Fluid Cognition. B, Lower ACC-GM tNAA predicted poorer performance (lower endurance) on the 2-Minute Endurance Walk Test in both groups. C, Higher ACC-GM mI predicted poorer recall on the List Sorting Working Memory Test in both groups. D, Lower ACC-GM Glu + Gln predicted greater pain intensity only in patients post–COVID-19. #Interaction P values that remained significant after FDR correction. ACC-GM, anterior cingulate cortex–gray matter; FWM, frontal white matter; Glu + Gln, glutamate + glutamine; mI, myo-inositol; tNAA, N-acetyl compounds.
DISCUSSION
This first 1H-MRS study in participants post–COVID-19 with persistent neuropsychiatric symptoms found lower neuronal (tNAA, Glu + Gln) and glial (mI) metabolite concentrations as compared with controls. The lower neuronal markers indicate neuronal injury or loss, whereas the lower mI suggests glial dystrophy or dysfunction, rather than neuroinflammation, in the frontal brain regions of these patients post–COVID-19. The lower-than-normal neurometabolite levels were even more pronounced in nonhospitalized patients post–COVID-19. Furthermore, the altered neurometabolite concentrations remained detectable approximately 8 months after acute illness.
Persistently Lower Neuronal Markers Post–COVID-19
The lower-than-normal tNAA in both brain regions and Glu + Gln in the FWM are consistent with our hypothesis and provide evidence of neuronal injury in these participants post–COVID-19. tNAA and Glu + Gln are reduced in many neurologic disorders, such as Alzheimer disease [27], advanced stages of HIV-associated neurocognitive disorders, and multiple sclerosis [14]. Since N-acetylaspartate, the major component of the tNAA peak, is synthesized in mitochondria [28], lower tNAA in our patients with COVID-19 might have resulted from mitochondrial dysfunction. An edited MRS study in our post–COVID-19 group revealed reduced frontal lobe glutathione, indicating decreased antioxidant capacity and oxidative stress, another indicator for mitochondrial dysfunction [29].
The markedly reduced FWM Glu + Gln in our post–COVID-19 group provides further evidence for neuronal injury, since most neurons (∼80%) are glutamatergic. Also, the Glu + Gln peak observed on MRS is dominated by Glu, which has a 2- to 3-fold higher concentration than Gln [30]. Similarly, reduced Glu + Gln levels were found in the early stages of other brain disorders, including Alzheimer disease [14] and HIV-associated neurocognitive disorders [31]. Since N-acetylaspartyl-glutamate serves as a reservoir for glutamate [32], the lower tNAA might have contributed to the lower Glu + Gln in our participants post–COVID-19. Our MRS evidence for neuronal injury is consistent with findings from postmortem studies of patients with COVID-19 that consistently demonstrated neuronal damage, likely because of systemic inflammation with synergistic contributions from hypoxia and ischemia due to acute respiratory distress syndrome [6, 33]. Furthermore, NfL, a neuronal injury marker, was elevated in patients with acute COVID-19—both younger (12–25 years) [10] and older (31–73 years) individuals [11, 12, 33, 34].
While our participants post–COVID-19 with or without prior hospitalization had similarly reduced tNAA, other studies suggest that neuronal injury may be related to COVID-19 severity. For instance, during acute COVID-19, CSF NfL levels were elevated only in patients with significant CNS disorders (ie, encephalitis, encephalopathy, acute disseminated encephalomyelitis, or stroke), whereas plasma NfL levels were elevated in patients with central or peripheral nervous system disorders (ie, Guillain-Barre syndrome) and in hospitalized patients [34]. Another study found persistently elevated plasma NfL levels only in patients with COVID-19 who developed acute respiratory distress syndrome and required oxygen therapy, again suggesting that brain injury is more related to COVID-19 severity (and hypoxia) than viral neuroinvasiveness [33].
Altered Glial Metabolites in Participants Post–COVID-19
Contrary to our hypothesis, the post–COVID-19 group had lower-than-normal glial marker mI and lacked the normal age-dependent increases of ACC-GM mI. These findings suggest that our participants post–COVID-19 approximately 8 months after acute illness, especially the nonhospitalized participants, lacked significant glial activation. Glial markers mI and, to a lesser extent, Cho and tCr are typically elevated in neurodegenerative disorders associated with neuroinflammation, including Alzheimer disease, neuroinfectious disorders (eg, HIV, hepatitis C virus, John Cunningham virus), and multiple sclerosis [14]. Therefore, the lower-than-normal mI in patients post–COVID-19 is an uncommon finding but was reported in elderly patients with schizophrenia [35]. The reduced glial metabolites suggest glial dysfunction or glial dystrophy, which was observed on neuropathology of Alzheimer disease, Lewy body disease, vascular dementia [36], and schizophrenia [35, 37]. Whether PCC is associated with glial dystrophy or dysfunction warrants further investigation.
One mechanism that can cause glial dysfunction is iron dyshomeostasis–mediated oxidative damage [36, 38]. Consistent with this hypothesis, our participants with COVID-19 with lower-than-normal FWM mI had lower-than-normal levels of the antioxidant glutathione in this brain region, suggesting oxidative damage [29]. Oxidative stress can also lead to dysregulation of the osmotic control in astrocytes, which is accompanied by markedly reduced mI, taurine, and hypotaurine, as demonstrated by 1H-MRS and carbon (13C) MRS in glial and neuronal cultures [39]. Furthermore, brain diffusion tensor imaging demonstrated higher-than-normal fractional anisotropy in participants post–COVID-19 [40, 41], again suggesting greater magnetic susceptibility, likely from higher iron content in ferritin, an acute-phase reactant of COVID-19 [4].
Last, the reduced mI in patients post–COVID-19 may be a compensatory response to glial swelling (ie, cytotoxic edema). Since mI is a major intracellular osmolyte, reducing mI would lower the intracellular water content, thereby partially reducing swelling. Residual swelling could lead to the reduced diffusivities observed on brain diffusion tensor imaging of patients post–COVID-19 [40, 41].
Altered Glial Metabolite Levels Varied by COVID-19 Severity
The small subset of our hospitalized patients post–COVID-19 had higher FWM mI than the nonhospitalized patients and greater age-related increases in mI in both brain regions; hence, the hospitalized group attenuated the lower-than-normal mI across the entire post–COVID-19 group. The relatively higher mI in the hospitalized patients suggests that neuroinflammation with glial activation remained prominent, consistent with the neuropathology reporting increased microglial activation in patients with severe COVID-19 [5, 7, 8]. However, the nonhospitalized patients with post–COVID-19 had even lower FWM mI, without age-related increases in mI in both brain regions, as well as more pronounced reductions of neurometabolites that are abundant in glia (ie, tCr and Cho). Therefore, the nonhospitalized patients post–COVID-19 likely had glial dystrophy, which might be an important contributory factor to the persistent neuropsychiatric symptoms.
Metabolite Abnormalities Did Not Vary With Duration Since COVID-19 Diagnosis
The abnormal brain metabolites did not normalize in those further from COVID-19 diagnosis, suggesting that neuronal injury or loss and glial abnormalities are either irreversible or still present. These findings are somewhat discordant with those from plasma or CSF studies. For instance, during acute infection, plasma GFAp levels increased early and preceded increases in NfL, and both elevated levels correlated with COVID-19 disease severity [11, 12, 42]. However, GFAp and NfL levels normalized 4 to 7 months after acute infection despite persistent neurologic symptoms in some patients with post–COVID-19 [11, 12]. Four months after COVID-19 diagnosis, elevated NfL was present only in those with moderate to severe COVID-19 [12], while higher GFAp was observed in patients who had long COVID-19 with persistent neurologic symptoms [13]. Together, these findings suggest that ongoing neuronal injury and inflammation may persist in those with moderate to severe COVID-19 and, based on our 1H-MRS data, patients with post–COVID-19 who were hospitalized. However, neuronal injury with glial dysfunction appeared to persist even in patients with long COVID-19 who had a milder clinical course without hospitalization. Based on prior NfL and GFAp findings, acute or subacute neuronal injury processes may stop after approximately 6 months, without further leakage of NfL and GFAp into the CSF or plasma, whereas the neuronal damage and glial dysfunction remain detectable around 8 months after COVID-19 diagnosis with highly sensitive In Vivo 1H-MRS. Future longitudinal studies are needed to determine whether these altered neurometabolite levels may normalize.
Brain Metabolite Levels Predicted Cognition, Motor Function, and Pain
Our exploratory analyses showed that regardless of COVID-19 status, lower FWM tNAA predicted poorer performance on several cognitive measures, indicating that lower neuronal marker levels might negatively affect cognition. In addition, the poorer physical endurance (2-Minute Endurance Walk Test) in those with lower ACC-GM tNAA is consistent with a similar association found in patients with multiple sclerosis [43]. Furthermore, despite the overall lower-than-normal mI in our participants post–COVID-19, those with higher ACC-GM mI had poorer results on the List Sorting Working Memory Test. These findings are consistent with prior 1H-MRS studies in healthy controls [44] and persons with HIV [17, 45] or mild cognitive impairment [46]. Last, in the post–COVID-19 group, lower ACC-GM Glu + Gln predicted greater pain intensity, as reported in other pain syndromes [14], including spinal cord injury [47], low back pain [48], and major depression [49]. However, given the many exploratory analyses performed, these findings can indicate only trends for such relationships.
Limitations
This study has several limitations. First, the cross-sectional design does not provide a causal inference that our findings are due to COVID-19. Second, we cannot predict whether some of the brain metabolite alterations in these participants post–COVID-19 might normalize over time. Third, to determine whether these findings are unique to long COVID-19, a second control group of patients post–COVID-19 without persistent neuropsychiatric symptoms could clarify our findings. Finally, given the range of neuropsychiatric symptoms, having multiple symptoms might lead to interactive or synergistic effects on neurometabolite abnormalities; however, our sample size precluded us from performing further subanalyses.
This study has several strengths. First, the main findings remained significant after FDR correction for multiple comparisons. Second, we enrolled only participants who had long COVID-19 with neuropsychiatric symptoms. Third, we performed a standardized battery of computerized cognitive, motor, and emotional, measures that yielded T-scores adjusted for age, sex, race/ethnicity, and education. Fourth, rather than measuring metabolite ratios (eg, relative to tCr), we used a customized MRS protocol to measure concentrations, corrected for the proportion of CSF and GM-WM in each voxel for variations in the water T2 value.
CONCLUSIONS
The findings from this study suggest that SARS-CoV-2 infection may cause neuronal injury or loss, as well as glial dysfunction or dystrophy, in frontal brain regions, which may contribute to the lingering neuropsychiatric symptoms in patients with PCCs. These alterations are still evident approximately 8 months after the acute infection. However, participants with severe COVID-19 that required hospitalization, especially older individuals, may have residual neuroinflammation.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Thomas Ernst, Department of Diagnostic Radiology and Nuclear Medicine, School of Medicine, University of Maryland; Department of Neurology, School of Medicine, Johns Hopkins University.
Meghann C Ryan, Department of Diagnostic Radiology and Nuclear Medicine, School of Medicine, University of Maryland; Program in Neuroscience, School of Medicine, University of Maryland.
Hua-Jun Liang, Department of Diagnostic Radiology and Nuclear Medicine, School of Medicine, University of Maryland.
Justin P Wang, Department of Diagnostic Radiology and Nuclear Medicine, School of Medicine, University of Maryland.
Eric Cunningham, Department of Diagnostic Radiology and Nuclear Medicine, School of Medicine, University of Maryland.
Muhammad G Saleh, Department of Diagnostic Radiology and Nuclear Medicine, School of Medicine, University of Maryland.
Shyamasundaran Kottilil, Institute of Human Virology, Division of Infectious Disease, Department of Medicine, School of Medicine, University of Maryland.
Linda Chang, Department of Diagnostic Radiology and Nuclear Medicine, School of Medicine, University of Maryland; Department of Neurology, School of Medicine, Johns Hopkins University; Department of Neurology, School of Medicine, University of Maryland, Baltimore.
Notes
Acknowledgments. We thank our research participants for their participation. We also thank Dr Andrea Levine for referring some of the participants post–COVID-19 to the study, Dr Eleanor Wilson for data collection, and Dr Edward Herskovits for reviewing the structural MRI scans.
Financial support. This work was supported by a grant from the National Institute of Neurological Disorders and Stroke (R21-NS121615).
References
- 1. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol 2023; 21:133–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Spudich S, Nath A. Nervous system consequences of COVID-19. Science 2022; 375:267–9. [DOI] [PubMed] [Google Scholar]
- 4. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020; 395:1033–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rutkai I, Mayer MG, Hellmers LM, et al. Neuropathology and virus in brain of SARS-CoV-2 infected non-human primates. Nat Commun 2022; 13:1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thakur KT, Miller EH, Glendinning MD, et al. COVID-19 neuropathology at Columbia University Irving Medical Center/New York Presbyterian Hospital. Brain 2021; 144:2696–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Colombo D, Falasca L, Marchioni L, et al. Neuropathology and inflammatory cell characterization in 10 autoptic COVID-19 brains. Cells 2021; 10:2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee MH, Perl DP, Nair G, et al. Microvascular injury in the brains of patients with COVID-19. N Engl J Med 2021; 384:481–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fernández-Castañeda A, Lu P, Geraghty AC, et al. Mild respiratory COVID can cause multi-lineage neural cell and myelin dysregulation. Cell 2022; 185:2452–68.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Havdal LB, Berven LL, Selvakumar J, et al. Neurological involvement in COVID-19 among non-hospitalized adolescents and young adults. Front Neurol 2022; 13:915712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kanberg N, Simrén J, Edén A, et al. Neurochemical signs of astrocytic and neuronal injury in acute COVID-19 normalizes during long-term follow-up. EBioMedicine 2021; 70:103512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Needham EJ, Ren AL, Digby RJ, et al. Brain injury in COVID-19 is associated with dysregulated innate and adaptive immune responses. Brain 2022; 145:4097–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peluso MJ, Sans HM, Forman CA, et al. Plasma markers of neurologic injury and inflammation in people with self-reported neurologic postacute sequelae of SARS-CoV-2 infection. Neurol Neuroimmunol Neuroinflamm 2022; 9:e200003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chang L, Munsaka SM, Kraft-Terry S, Ernst T. Magnetic resonance spectroscopy to assess neuroinflammation and neuropathic pain. J Neuroimmune Pharmacol 2013; 8:576–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brand A, Richter-Landsberg C, Leibfritz D. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev Neurosci 1993; 15:289–98. [DOI] [PubMed] [Google Scholar]
- 16. Chang L, Ernst T, Poland RE, Jenden DJ. In vivo proton magnetic resonance spectroscopy of the normal aging human brain. Life Sci 1996; 58:2049–56. [DOI] [PubMed] [Google Scholar]
- 17. Chang L, Jiang C, Cunningham E, et al. Effects of APOE epsilon4, age, and HIV on glial metabolites and cognitive deficits. Neurology 2014; 82:2213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thames AD, Castellon SA, Singer EJ, et al. Neuroimaging abnormalities, neurocognitive function, and fatigue in patients with hepatitis C. Neurol Neuroimmunol Neuroinflamm 2015; 2:e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dunn GA, Loftis JM, Sullivan EL. Neuroinflammation in psychiatric disorders: an introductory primer. Pharmacol Biochem Behav 2020; 196:172981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gershon RC, Wagster MV, Hendrie HC, Fox NA, Cook KF, Nowinski CJ. NIH Toolbox for assessment of neurological and behavioral function. Neurology 2013; 80:S2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol 2010; 63:1179–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bottomley PA. Spatial localization in NMR spectroscopy in vivo. Ann NY Acad Sci 1987; 508:333–48. [DOI] [PubMed] [Google Scholar]
- 23. Ernst T, Kreis R, Ross BD. Absolute quantitation of water and metabolites in the human brain; part I: compartments and water. J Magn Reson 1993; 102:1–8. [Google Scholar]
- 24. Edden RA, Puts NA, Harris AD, Barker PB, Evans CJ. Gannet: a batch-processing tool for the quantitative analysis of gamma-aminobutyric acid–edited MR spectroscopy spectra. J Magn Reson Imaging 2014; 40:1445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hollingshead A. Four-Factor Index of Social Status. New Haven: Yale University, 1975. [Google Scholar]
- 26. Ryan M, Liang H, Wilson E, et al. Quantifying the neuropsychiatric symptoms in post-acute sequelae of COVID-19 (PASC) using the NIH Toolbox and PROMIS. NeuroImmune Pharm Ther 2022; 2:95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ernst T, Chang L, Melchor R, Mehringer CM. Frontotemporal dementia and early Alzheimer disease: differentiation with frontal lobe H-1 MR spectroscopy. Radiology 1997; 203:829–36. [DOI] [PubMed] [Google Scholar]
- 28. Ariyannur PS, Madhavarao CN, Namboodiri AM. N-acetylaspartate synthesis in the brain: mitochondria vs microsomes. Brain Res 2008; 1227:34–41. [DOI] [PubMed] [Google Scholar]
- 29. Saleh MG, Chang L, Liang H, et al. Ongoing oxidative stress in individuals with post-acute sequelae of COVID-19. NeuroImmune Pharm Ther 2022; 2:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Goryawala MZ, Sheriff S, Maudsley AA. Regional distributions of brain glutamate and glutamine in normal subjects. NMR Biomed 2016; 29:1108–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ernst T, Jiang CS, Nakama H, Buchthal S, Chang L. Lower brain glutamate is associated with cognitive deficits in HIV patients: a new mechanism for HIV-associated neurocognitive disorder. J Magn Reson Imaging 2010; 32:1045–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Clark JF, Doepke A, Filosa JA, et al. N-acetylaspartate as a reservoir for glutamate. Med Hypotheses 2006; 67:506–12. [DOI] [PubMed] [Google Scholar]
- 33. Zingaropoli MA, Iannetta M, Piermatteo L, et al. Neuro-axonal damage and alteration of blood-brain barrier integrity in COVID-19 patients. Cells 2022; 11:2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Paterson RW, Benjamin LA, Mehta PR, et al. Serum and cerebrospinal fluid biomarker profiles in acute SARS-CoV-2–associated neurological syndromes. Brain Commun 2021; 3:fcab099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chang L, Friedman J, Ernst T, Zhong K, Tsopelas ND, Davis K. Brain metabolite abnormalities in the white matter of elderly schizophrenic subjects: implication for glial dysfunction. Biol Psychiatry 2007; 62:1396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shahidehpour RK, Higdon RE, Crawford NG, et al. Dystrophic microglia are associated with neurodegenerative disease and not healthy aging in the human brain. Neurobiol Aging 2021; 99:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Uranova NA, Vikhreva OV, Rakhmanova VI. Abnormal microglial reactivity in gray matter of the prefrontal cortex in schizophrenia. Asian J Psychiatr 2021; 63:102752. [DOI] [PubMed] [Google Scholar]
- 38. Streit WJ, Sammons NW, Kuhns AJ, Sparks DL. Dystrophic microglia in the aging human brain. Glia 2004; 45:208–12. [DOI] [PubMed] [Google Scholar]
- 39. Brand A, Leibfritz D, Richter-Landsberg C. Oxidative stress-induced metabolic alterations in rat brain astrocytes studied by multinuclear NMR spectroscopy. J Neurosci Res 1999; 58:576–85. [PubMed] [Google Scholar]
- 40. Liang H, Ernst T, Oishi K, et al. Abnormal brain diffusivity in participants with persistent neuropsychiatric symptoms after COVID-19. NeuroImmune Pharm Ther 2022; 2:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lu Y, Li X, Geng D, et al. Cerebral micro-structural changes in COVID-19 patients—an MRI-based 3-month follow-up study. EClinicalMedicine 2020; 25:100484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kanberg N, Ashton NJ, Andersson LM, et al. Neurochemical evidence of astrocytic and neuronal injury commonly found in COVID-19. Neurology 2020; 95:e1754–9. [DOI] [PubMed] [Google Scholar]
- 43. Mueller C, Baird JF, Motl RW. Whole-brain metabolic abnormalities are associated with mobility in older adults with multiple sclerosis. Neurorehabil Neural Repair 2022; 36:286–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lind A, Boraxbekk CJ, Petersen ET, Paulson OB, Siebner HR, Marsman A. Regional myo-inositol, creatine, and choline levels are higher at older age and scale negatively with visuospatial working memory: a cross-sectional proton MR spectroscopy study at 7 Tesla on normal cognitive ageing. J Neurosci 2020; 40:8149–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chang L, Ernst T, Witt MD, Ames N, Gaiefsky M, Miller E. Relationships among brain metabolites, cognitive function, and viral loads in antiretroviral-naïve HIV patients. Neuroimage 2002; 17:1638–48. [DOI] [PubMed] [Google Scholar]
- 46. Mitolo M, Stanzani-Maserati M, Capellari S, et al. Predicting conversion from mild cognitive impairment to Alzheimer's disease using brain (1)H-MRS and volumetric changes: a two-year retrospective follow-up study. Neuroimage Clin 2019; 23:101843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Widerström-Noga E, Pattany PM, Cruz-Almeida Y, et al. Metabolite concentrations in the anterior cingulate cortex predict high neuropathic pain impact after spinal cord injury. Pain 2013; 154:204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gussew A, Rzanny R, Güllmar D, Scholle HC, Reichenbach JR. 1H-MR spectroscopic detection of metabolic changes in pain processing brain regions in the presence of non-specific chronic low back pain. Neuroimage 2011; 54:1315–23. [DOI] [PubMed] [Google Scholar]
- 49. Yüksel C, Öngür D. Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. Biol Psychiatry 2010; 68:785–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.