Abstract
Background
No overall estimate of respiratory syncytial virus (RSV)-associated hospitalizations in children aged under 5 years has been published for the European Union (EU). We aimed to estimate the RSV hospitalization burden in children aged under 5 years in EU countries and Norway, by age group.
Methods
We collated national RSV-associated hospitalization estimates calculated using linear regression models via the RESCEU project for Denmark, England, Finland, Norway, the Netherlands, and Scotland, 2006–2018. Additional estimates were obtained from a systematic review. Using multiple imputation and nearest neighbor matching methods, we estimated overall RSV-associated hospitalizations and rates in the EU.
Results
Additional estimates for 2 countries (France and Spain) were found in the literature. In the EU, an average of 245 244 (95% confidence interval [CI], 224 688–265 799) yearly hospital admissions with a respiratory infection per year were associated with RSV in children aged under 5 years, with most cases occurring among children aged under 1 year (75%). Infants aged under 2 months represented the most affected group (71.6 per 1000 children; 95% CI, 66.6–76.6).
Conclusions
Our findings will help support decisions regarding prevention efforts and represent an important benchmark to understand changes in the RSV burden following the introduction of RSV immunization programs in Europe.
Keywords: Europe, burden of disease, hospitalization, modelling, respiratory hospitalization, respiratory syncytial virus
The study estimated that an average of 245 244 children aged under 5 years are hospitalized annually due to RSV in the EU, with the highest hospitalization rates in children aged less than 2 months (71.6 per 1000 children).
It is globally estimated that respiratory syncytial virus (RSV) is associated with about 22% of all acute lower respiratory infections [1], and this results in approximately 84 500–125 200 deaths per year in young children [2]. Several studies have been conducted to understand the burden of RSV-associated infections, hospitalizations, and deaths in children in Europe. For example, Reeves et al explored routinely collected hospital data on RSV in children aged <5 years in 7 European countries and compared these to RSV-associated admission rates [3], while Demont et al provided information on the clinical and economic burden of RSV-associated hospitalization in children aged <5 years in France between 2010 and 2018 [4]. Despite these efforts, no estimates for RSV-associated hospitalizations are available for children in the European Union (EU) as a whole.
RSV-associated hospitalization estimates are important for public health purposes, as they can help allocate resources, and provide important insights and inputs for prevention measures and strategies. The establishment of a robust age-specific burden of disease estimates, which have often been limited due to a lack of routine testing for RSV [5], has also been underlined by the World Health Organization (WHO) [6].
In this article, we present overall estimates of RSV-associated respiratory hospitalizations (absolute numbers and rates) by age group in children aged less than 5 years in the EU that were obtained by calculating country-specific estimates (EU-28: includes the United Kingdom as it was part of EU when the data were collected). The national estimates were also used to calculate the proportion of RSV-associated hospitalizations among all-cause hospitalizations and respiratory hospitalizations in this age group, for each country. Our results will support efforts to communicate the RSV disease burden and provide important data for decisions regarding future prevention and control measures linked to various immunization programs (such as vaccines and/or monoclonal antibodies).
METHODS
Data Sources
We searched for published and unpublished national estimates of RSV-associated hospitalizations (defined as any admission that contained at least 1 respiratory infection-specific International Classification of Diseases-Tenth Revision [ICD-10] code at any point during admission) in children aged under 5 years in EU countries that were calculated using regression models as input data for the statistical analysis. The EU was chosen as the preferred region as it is a highly integrated political and economic union of 28 member states, making it a more homogeneous and consistent entity than Europe as a whole.
RSV-Associated Hospitalization Estimates From the RESCEU Project
The data sources for Denmark, England, Finland, Norway, the Netherlands, and Scotland have been described in papers that were previously published by the Respiratory Syncytial Virus Consortium in Europe (RESCEU) [3, 7]. A retrospective study of overall respiratory hospital admissions (ie, respiratory tract infections with or without an associated pathogen), RSV-related respiratory admissions, and other pathogen-respiratory admissions in children <5 years of age using routinely collected hospital admissions databases was conducted by Reeves and colleagues in these 6 countries of the EU [3]. These data were then used by Johannesen and colleagues to calculate age-specific estimates of RSV-associated hospitalizations in children during 2006–2018 in these countries using a linear regression approach, with estimates available for the following age groups: 0–2, 3–5, 6–11, 12–35, and 36–59 months [7].
Literature Review Estimates to Identify Estimates in Other Countries
To increase the geographical representativeness of this work, we searched the scientific literature for additional data points in EU countries by adopting the same search strategy as a previously published systematic review that aimed to estimate the global incidence, hospital admission rate, and mortality due to RSV in young children based on national estimates [2]. The systematic review by Li et al [2] was broader in scope and inclusion criteria (ie, they also included studies reporting incidence and in-hospital and out-of-hospital mortality), therefore, we considered that the included records needed to be further screened and assessed for eligibility in our review.
The new search was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [8]. The same search string as the systematic review conducted by Li and colleagues was used [2] (see Supplementary Material); MEDLINE and EMBASE databases were searched from 1 January 2019 to 30 November 2021 for original articles. Papers published before 2019 that had been included by Li and colleagues were added to the reference list of relevant papers and further assessed for eligibility. No language restrictions were applied as long as an English abstract was available. To be included, a study had to (1) report national estimates of RSV-associated hospitalizations in children in EU countries calculated by using linear regression models (this criterion was chosen in order to have a homogeneous pool of estimates for the EU [9]); (2) report details (ie, ICD codes) on the diagnosis (ie, bronchiolitis, lower respiratory infections, etc.); and (3) analyze the same age bands used by Johannesen and colleagues [7].
After removing duplicates, titles and abstracts were independently screened by 2 researchers (M. D. R. and R. O. Y.), who then retrieved and independently read in full copy the articles that were not excluded. The papers that had been included in the previous systematic review were further assessed for eligibility, and the reference list of all eligible papers was checked by means of backward citation chaining for further relevant references. Data extraction was organized using an internally piloted spreadsheet [2]. The estimates reported by the included articles were then extracted and used as input data for the statistical modelling, along with other information (data sources, details on the primary diagnosis, years in which the study was conducted, etc.). The quality assessment of the included studies was conducted by using a tool designed by Li et al [2] (see Supplementary Material). Based on the assessment of different questions (on study testing, subjects, case definition, sampling strategy, etc.) an overall score was calculated.
Statistical Analysis
A 2-stage modelling approach was used to estimate the RSV-associated hospitalizations and rates in the EU in children under 5 years old. This method was adapted from prior work focused on influenza-associated mortality during the 2009 pandemic [9] and for seasonal influenza [10]. Because the period covered by the eligible studies was 2006–2018, the United Kingdom was included in the EU estimates.
In stage 1, we identified annual age-specific estimates of RSV-associated hospitalizations from respiratory causes that were calculated using linear regression models. Data from the 6 RESCEU countries, plus those that were found in the literature and matched the inclusion criteria (see “Results” section), were used as input data for stage 2.
In stage 2, we used the country estimates to extrapolate the hospitalization burden and generate plausible values for all EU countries using 2 different modelling approaches, each involving 2 steps: (1) a data creation step using the matching approach or the multiple imputation approach, and (2) a data analysis step where a hierarchical linear random effects model is used to project the burden in all EU countries. For both approaches, the data creation step relied on 10 country-specific indicators representing health conditions at a demographic, geographic, and population level (see Supplementary Material) [9]. As 2 different sets of 10 indicators were used, the statistical modelling produced 4 sets of results (see Supplementary Material), each related to the combination of 1 set of indicators and 1 modelling approach. An average of the 4 different models was calculated and used to calculate the absolute annual number and rates of RSV-associated hospitalizations (with uncertainty intervals) by country for the following age groups: 0–2, 3–5, 6–11, 12–35, and 36–59 months, which are consistent with the previously published age groups used by Johannesen and colleagues [7]. We also assessed the hospitalizations rate for the EU in children aged 0–59 months and calculated the ratio of hospitalizations that occurred in each age group, considering our estimated total number of RSV-associated hospitalizations as denominator.
Finally, to compare RSV-associated hospitalizations to total respiratory and all-cause hospitalizations, the estimated absolute number of RSV-associated hospitalizations was used as the numerator to calculate the proportion of RSV-associated hospitalizations among respiratory and all-cause hospitalizations (data related to 2015) occurring in children aged under 5 years. The population denominator (roughly 25 900 000 children under 59 months of age in the EU in 2015) and other demographic indicators used for the analysis were obtained from Eurostat [11]. Statistical analyses were conducted using Stata version 16 (Stata Corp).
RESULTS
Results of the Literature Review and Stage 1
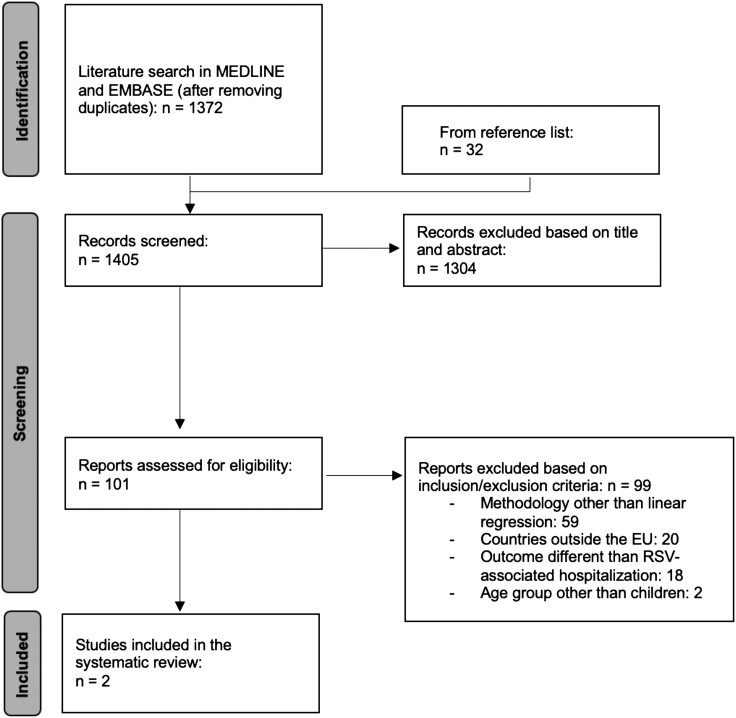
The literature search in MEDLINE and EMBASE produced 1372 unique entries, and an additional 33 articles were found by backward citation chaining or because they had been included by Li and colleagues in the previous systematic review [2] (Figure 1). Of these, 1304 were excluded based on title and abstract and 101 were read in full text: 99 were excluded for not matching the inclusion criteria; the main reason for exclusion was not presenting national RSV-associated hospitalization estimates calculated by using regression methods. Two studies, in particular, were excluded as they focused on different age groups [12, 13] but they provided useful estimates as they covered a country (England) that was included in stage 1 (estimates for England were already available) and were therefore comparable to the data reported by Johannesen et al [7]. Finally, 2 studies [14, 15] reporting RSV-associated hospitalization estimates for Spain from 1997 to 2011 and for France during 2010–2018 were included in the review and their estimates were used as input data for the statistical modelling (stage 2). Both studies reported RSV-associated hospitalization rates per 100 000 children in age groups that were slightly different to those used by Johannesen et al; we therefore only used data related to the age groups that were consistent with the estimates produced by Johannesen et al [7] (12–35 months and 36–59 months for Spain, and 0–2 months, 3–5 months, and 6–11 months for France). Moreover, the estimates for France were not annual but based on the epidemic periods only (October to March), so we recalculated the estimates for the whole year by arbitrarily assuming little RSV activity during April-September (10% of the activity observed from October to March [16]). In total, data from 8 countries were used as stage 1 inputs: Denmark, England, Finland, Norway, Netherlands, Scotland, France (age groups 0–2 months, 3–5 months, 6–11 months), and Spain (age groups 12–35 months, 36–59 months) (Table 1).
Figure 1.
Systematic review flow diagram.
Table 1.
Description of the Data Sources That Provided Stage 1 Estimates
| Author, year | Country | Period of Observation | Age Groups | Age Groups Whose Estimates Were Used as Inputs | Outcome Coding |
|---|---|---|---|---|---|
| Johannesen, 2022 [7] | Denmark | 2010–2017 | 0–2 mo, 3–5 mo, 6–11 mo, 12–35 mo, 36–59 mo | 0–2 mo, 3–5 mo, 6–11 mo, 12–35 mo, 36–59 mo | ICD-10; J00, J02–06 acute URTI; J09–18 pneumonia and influenza; J20–21, J40 bronchiolitis and bronchitis; J22 unspecified LRTI |
| Johannesen, 2022 [7] | England | 2007–2017 | 0–2 mo, 3–5 mo, 6–11 mo, 12–35 mo, 36–59 mo | 0–2 mo, 3–5 mo, 6–11 mo, 12–35 mo, 36–59 mo | ICD-10; J00, J02–06 acute URTI; J09–18 pneumonia and influenza; J20–21, J40 bronchiolitis and bronchitis; J22 unspecified LRTI |
| Johannesen, 2022 [7] | Finland | 2006–2016 | 0–2 mo, 3–5 mo, 6–11 mo, 12–35 mo, 36–59 mo | 0–2 mo, 3–5 mo, 6–11 mo, 12–35 mo, 36–59 mo | ICD-10; J00, J02–06 acute URTI; J09–18 pneumonia and influenza; J20–21, J40 bronchiolitis and bronchitis; J22 unspecified LRTI |
| Johannesen, 2022 [7] | Netherlands | 2013–2017 | 0–2 mo, 3–5 mo, 6–11 mo, 12–35 mo, 36–59 mo | 0–2 mo, 3–5 mo, 6–11 mo, 12–35 mo, 36–59 mo | ICD-10; J00, J02–06 acute URTI; J09–18 pneumonia and influenza; J20–21, J40 bronchiolitis and bronchitis; J22 unspecified LRTI |
| Johannesen, 2022 [7] | Norway | 2008–2017 | 0–2 mo, 3–5 mo, 6–11 mo, 12–35 mo, 36–59 mo | 0–2 mo, 3–5 mo, 6–11 mo, 12–35 mo, 36–59 mo | ICD-10; J00, J02–06 acute URTI; J09–18 pneumonia and influenza; J20–21, J40 bronchiolitis and bronchitis; J22 unspecified LRTI |
| Johannesen, 2022 [7] | Scotland | 2010–2016 | 0–2 mo, 3–5 mo, 6–11 mo, 12–35 mo, 36–59 mo | 0–2 mo, 3–5 mo, 6–11 mo, 12–35 mo, 36–59 mo | ICD-10; J00, J02–06 acute URTI; J09–18 pneumonia and influenza; J20–21, J40 bronchiolitis and bronchitis; J22 unspecified LRTI |
| Demont, 2020 [15] | France | 2010–2018 | 0–2 mo, 3–5 mo, 6–11 mo, 12–23 mo, 24–59 mo | 0–2 mo, 3–5 mo, 6–11 mo | ICD-10; J121, J205, J210, J219 |
| Gil-Prieto, 2015 [14] | Spain | 1997–2011 | 0 y, 1 y, 2 y, 3 y, 4 y, < 5 y, < 2 y | 2 y, 3 y, 4 y | ICD-9-CM; 466, acute bronchitis and bronchiolitis; 480.1, pneumonia due to RSV; 079.6, RSV infection |
Abbreviations: ICD-9-CM, International Classification of Diseases Nineth Revision-Clinical Modification; LRTI, lower respiratory tract infection; RSV, respiratory syncytial virus; URTI, upper respiratory tract infection.
Stage 2 Estimates
We included stage 1 estimates from 8 countries in our analysis, roughly representing 40% of the population of the EU and Norway [17]. The results produced by the 4 models were consistent across the age groups, the highest rates being calculated with the multiple imputation approach and the lowest rates being calculated with the nearest neighbor matching approach (5% variation between the highest and lowest estimates of RSV hospitalization rates in children aged 0–2 months) (see Supplementary Material). Here, we present the results for the average of the 4 models (Table 2 and Table 3).
Table 2.
Average RSV-Associated Hospitalization Rates per 1000 Population per Age Group per Year
| Country | 0–2 mo (95% CI)a | 3–5 mo (95% CI)a | 6–11 mo (95% CI)a | 12–35 mo (95% CI)b | 36–59 mo (95% CI)b |
|---|---|---|---|---|---|
| EU-28c | 71.6 (66.6–76.6) | 38.9 (36–41.9) | 17.6 (16.1–19.1) | 5 (4.4–5.5) | 1 (0.9–1.1) |
| Austria | 65 (55.2–74.8) | 33.1 (27.7–38.5) | 13.5 (10.7–16.4) | 4.5 (3.5–5.5) | 0.9 (0.7–1.1) |
| Belgium | 68.6 (58.8–78.4) | 36.3 (30.9–41.7) | 15.9 (13.1–18.7) | 4.8 (3.8–5.9) | 1.2 (0.9–1.4) |
| Bulgaria | 81.8 (72–91.6) | 48.9 (43.5–54.3) | 21.2 (18.4–24) | 5.5 (4.5–6.6) | 1 (0.8–1.2) |
| Croatia | 79.4 (69.7–89.2) | 42.6 (37.2–48) | 19 (16.2–21.8) | 4.8 (3.8–5.8) | 0.9 (0.7–1.1) |
| Cyprus | 78.4 (68.6–88.2) | 41.3 (35.9–46.7) | 16.7 (13.9–19.5) | 5.5 (4.5–6.5) | 0.9 (0.7–1.1) |
| Czech Republic | 73.9 (64.1–83.7) | 41 (35.6–46.4) | 18.4 (15.6–21.2) | 5.7 (4.6–6.7) | 1.1 (0.9–1.3) |
| Denmark | 59.2 (49.3–69) | 40.7 (35.3–46.2) | 20.4 (17.5–23.2) | 7.5 (6.4–8.5) | 1.6 (1.4–1.8) |
| Estonia | 69.8 (60–79.7) | 37.3 (31.8–42.7) | 17.2 (14.4–20.1) | 5.1 (4–6.1) | 1 (0.8–1.2) |
| Finland | 77.8 (67.9–87.7) | 43.2 (37.7–48.6) | 16.6 (13.8–19.4) | 5.2 (4.2–6.3) | 0.8 (0.6–1) |
| France | 98.3 (88.5–108.1) | 48.8 (43.4–54.2) | 26 (23.2–28.8) | 5 (4–6.1) | 1.1 (0.9–1.3) |
| Germany | 72.5 (62.7–82.2) | 38.6 (33.2–44) | 17.5 (14.6–20.3) | 5.2 (4.2–6.3) | 1 (0.8–1.2) |
| Greece | 82.6 (72.8–92.4) | 44.3 (38.9–49.7) | 19.1 (16.3–21.9) | 4.5 (3.4–5.5) | 0.9 (0.7–1.1) |
| Hungary | 75.3 (65.5–85.1) | 44.7 (39.3–50.1) | 19.8 (16.9–22.6) | 5.4 (4.4–6.4) | 1.1 (0.9–1.3) |
| Ireland | 70.1 (60.3–79.9) | 47 (41.6–52.4) | 22.6 (19.8–25.4) | 7.1 (6–8.1) | 1.3 (1–1.5) |
| Italy | 80.9 (71.1–90.7) | 41.7 (36.3–47.1) | 18.1 (15.3–20.9) | 4.3 (3.2–5.3) | 0.9 (0.7–1.1) |
| Latvia | 75 (65.2–84.8) | 41.3 (35.9–46.7) | 18.4 (15.6–21.2) | 4.6 (3.6–5.7) | 1.1 (0.8–1.3) |
| Lithuania | 73.6 (63.8–83.4) | 38.5 (33.1–43.9) | 17 (14.2–19.8) | 4 (3–5) | 0.9 (0.7–1.1) |
| Luxembourg | 63.5 (53.7–73.3) | 32.6 (27.2–38) | 13.1 (10.3–15.9) | 5 (4–6.1) | 0.9 (0.7–1.1) |
| Malta | 64.8 (54.8–74.7) | 35.2 (29.7–40.7) | 17.9 (15–20.7) | 5.4 (4.4–6.5) | 1.3 (1.1–1.5) |
| Netherlands | 47.4 (37.6–57.3) | 19.9 (14.5–25.4) | 8.5 (5.7–11.3) | 1.9 (0.8–2.9) | 1.1 (0.9–1.3) |
| Norway | 54.6 (44.5–64.7) | 34.8 (29.3–40.3) | 15.4 (12.5–18.2) | 6.6 (5.6–7.7) | 0.5 (0.3–0.7) |
| Poland | 71.4 (61.6–81.2) | 36.5 (31.1–41.9) | 16.2 (13.4–19.1) | 4.6 (3.5–5.6) | 0.9 (0.7–1.1) |
| Portugal | 70.2 (60.4–80) | 31.7 (26.3–37.1) | 15.2 (12.4–18) | 4.5 (3.4–5.5) | 1.2 (0.9–1.4) |
| Romania | 68.8 (59–78.6) | 37.7 (32.3–43.1) | 19.4 (16.6–22.2) | 4.1 (3.1–5.2) | 1 (0.8–1.2) |
| Slovakia | 74.6 (64.8–84.3) | 42 (36.6–47.4) | 18.8 (16–21.6) | 4.7 (3.7–5.8) | 0.8 (0.6–1) |
| Slovenia | 75.4 (65.6–85.2) | 42.6 (37.2–48) | 17.6 (14.8–20.4) | 5.2 (4.2–6.2) | 1.1 (0.9–1.3) |
| Spain | 69.4 (59.6–79.2) | 32.6 (27.2–38) | 16.8 (14–19.6) | 3 (2–4.1) | 0.8 (0.6–1) |
| Sweden | 63 (53.1–72.8) | 35.5 (30.1–41) | 16.9 (14.1–19.7) | 5.3 (4.3–6.3) | 1.2 (1–1.4) |
| United Kingdom | 63.4 (47.8–79.1) | 38.9 (29.7–48.1) | 18.9 (14.2–23.6) | 6.1 (4.4–7.9) | 1.3 (1–1.6) |
Abbreviations: CI, confidence interval; EU, European Union; RSV, respiratory syncytial virus.
aRSV-associated hospitalization rates in these 3 age groups for the 29 countries are estimated by also including data from France reported by Demont and colleagues [15].
bRSV-associated hospitalization rates in these 2 age groups for the 29 countries are estimated by also including data from Spain reported by Gil-Prieto and colleagues [14].
cIncludes the United Kingdom and excludes Norway.
Table 3.
Average RSV-Associated Hospitalizations per Age Group per Year
| Country | 0–2 mo (95% CI)a | 3–5 mo (95% CI)a | 6–11 mo (95% CI)a | 12–35 mo (95% CI)b | 36–59 mo (95% CI)b |
|---|---|---|---|---|---|
| EU-28c | 90 200 (83 923–96 476) | 49 052 (45 328–52 776) | 44 369 (40 529–48 208) | 50 852 (45 249–56 456) | 10 771 (9659–11 883) |
| Austria | 1308 (1111–1505) | 667 (558–775) | 545 (432–658) | 732 (563–902) | 147 (112–182) |
| Belgium | 2141 (1836–2446) | 1133 (965–1302) | 992 (816–1167) | 1235 (973–1497) | 306 (250–362) |
| Bulgaria | 1374 (1210–1539) | 822 (732–913) | 714 (620–808) | 733 (596–869) | 141 (112–171) |
| Croatia | 783 (687–880) | 420 (366–473) | 375 (320–431) | 391 (306–477) | 77 (59–95) |
| Cyprus | 181 (159–204) | 95 (83–108) | 77 (64–90) | 106 (86–126) | 18 (13–22) |
| Czech Republic | 2031 (1762–2300) | 1128 (979–1276) | 1012 (858–1167) | 1237 (1012–1462) | 258 (209–308) |
| Denmark | 846 (704–986) | 582 (504–660) | 582 (500–662) | 864 (744–985) | 199 (172–225) |
| Estonia | 238 (205–271) | 127 (108–146) | 118 (98–137) | 141 (112–170) | 32 (25–38) |
| Finland | 1122 (980–1264) | 622 (544–701) | 479 (398–561) | 625 (501–750) | 97 (71–124) |
| France | 18 145 (16 336–19 952) | 9018 (8021–10 015) | 9587 (8548–10 626) | 7573 (5998–9148) | 1704 (1368–2040) |
| Germany | 12 977 (11 223–14 731) | 6906 (5939–7874) | 6252 (5244–7260) | 7250 (5802–8699) | 1334 (1039–1629) |
| Greece | 1895 (1670–2119) | 1015 (891–1139) | 875 (746–1003) | 862 (661–1063) | 193 (147–239) |
| Hungary | 1748 (1521–1975) | 1038 (913–1163) | 917 (787–1048) | 976 (788–1164) | 199 (161–237) |
| Ireland | 1139 (980–1298) | 764 (676–851) | 735 (643–826) | 950 (810–1091) | 176 (146–206) |
| Italy | 10 111 (8888–11 334) | 5213 (4538–5888) | 4534 (3832–5236) | 4475 (3387–5563) | 1021 (787–1256) |
| Latvia | 407 (353–459) | 224 (195–253) | 200 (169–230) | 190 (148–232) | 40 (32–48) |
| Lithuania | 559 (485–633) | 292 (251–333) | 258 (215–300) | 241 (179–304) | 54 (42–67) |
| Luxembourg | 96 (82–111) | 49 (41–58) | 40 (31–49) | 64 (51–77) | 12 (9–15) |
| Malta | 69 (59–80) | 38 (32–44) | 38 (32–44) | 47 (38–57) | 11 (9–13) |
| Netherlands | 2071 (1641–2502) | 870 (633–1108) | 741 (494–988) | 651 (292–1011) | 398 (319–475) |
| Norway | 811 (661–961) | 517 (435–599) | 456 (370–542) | 812 (682–941) | 61 (34–89) |
| Poland | 6542 (5646–7439) | 3346 (2852–3842) | 2979 (2464–3494) | 3459 (2669–4249) | 730 (557–903) |
| Portugal | 1444 (1243–1645) | 651 (540–762) | 627 (511–742) | 769 (590–949) | 226 (185–268) |
| Romania | 3300 (2830–3769) | 1807 (1548–2066) | 1860 (1590–2129) | 1540 (1150–1929) | 389 (305–472) |
| Slovakia | 1035 (900–1170) | 583 (509–658) | 523 (445–600) | 531 (414–648) | 98 (72–123) |
| Slovenia | 399 (347–451) | 225 (197–254) | 186 (156–216) | 225 (180–270) | 48 (38–57) |
| Spain | 7399 (6356–8442) | 3473 (2897–4048) | 3574 (2975–4172) | 2670 (1762–3578) | 788 (585–992) |
| Sweden | 1824 (1538–2110) | 1030 (872–1187) | 980 (816–1144) | 1229 (987–1471) | 288 (237–339) |
| United Kingdom | 12 333 (9291–15 375) | 7565 (5778–9351) | 7352 (5515–9188) | 9890 (7128–12 652) | 2156 (1614–2698) |
Abbreviations: CI, confidence interval; EU, European Union; RSV, respiratory syncytial virus.
aRSV-associated hospitalization in these 3 age groups are estimated by also including data from France reported by Demont and colleagues [15].
bRSV-associated hospitalization in these 2 age groups are estimated by also including data from Spain reported by Gil-Prieto and colleagues [14].
cIncludes the United Kingdom and excludes Norway.
We estimated that an average of 245 244 (95% confidence interval [CI], 224 688–265 799) hospital admissions with respiratory infection were associated with RSV in the 28 EU countries per year in children under the age of 5 years, with most cases occurring among children aged less than 1 year (74.9%) and those aged 1–2 years (20.7%) (Table 4). Infants aged less than 2 months represented the most affected group (71.6 per 1000 population; 95% CI, 66.6–76.6), with the rates declining as the children got older: 38.9 per 1000 in children aged 3–5 months, 17.6 (6–11 months), 5.0 (12–35 months), and 1.0 (36–59 months). Overall, we estimated that an average of 10 children per 1000 living in the EU are hospitalized due to RSV annually (average rates in children aged 0–59 months, 10.06 per 1000 population; 95% CI, 9.90–10.21).
Table 4.
Ratio of RSV-Associated Hospitalization Occurring in Children Aged Less Than 1 Year, From 1 to 2 Years, and From 3 to 4 Years
| Country | 0–2 moa | 3–5 moa | 6–11 moa | 0–11 moa,b | 12–35 moc | 36–59 moc |
|---|---|---|---|---|---|---|
| EU-28d | 36.8 | 20.0 | 18.1 | 74.9 | 20.7 | 4.4 |
| Austria | 38.5 | 19.6 | 16.0 | 74.1 | 21.6 | 4.3 |
| Belgium | 36.9 | 19.5 | 17.1 | 73.4 | 21.3 | 5.3 |
| Bulgaria | 36.3 | 21.7 | 18.9 | 76.9 | 19.4 | 3.7 |
| Croatia | 38.3 | 20.5 | 18.3 | 77.1 | 19.1 | 3.8 |
| Cyprus | 37.9 | 19.9 | 16.1 | 74.0 | 22.2 | 3.8 |
| Czech Republic | 35.8 | 19.9 | 17.9 | 73.6 | 21.8 | 4.6 |
| Denmark | 27.5 | 18.9 | 18.9 | 65.4 | 28.1 | 6.5 |
| Estonia | 36.3 | 19.4 | 18.0 | 73.6 | 21.5 | 4.9 |
| Finland | 38.1 | 21.1 | 16.3 | 75.5 | 21.2 | 3.3 |
| France | 39.4 | 19.6 | 20.8 | 79.8 | 16.5 | 3.7 |
| Germany | 37.4 | 19.9 | 18.0 | 75.3 | 20.9 | 3.8 |
| Greece | 39.2 | 21.0 | 18.1 | 78.2 | 17.8 | 4.0 |
| Hungary | 35.8 | 21.3 | 18.8 | 75.9 | 20.0 | 4.1 |
| Ireland | 30.3 | 20.3 | 19.5 | 70.1 | 25.2 | 4.7 |
| Italy | 39.9 | 20.6 | 17.9 | 78.3 | 17.7 | 4.0 |
| Latvia | 38.4 | 21.1 | 18.9 | 78.3 | 17.9 | 3.8 |
| Lithuania | 39.8 | 20.8 | 18.4 | 79.0 | 17.2 | 3.8 |
| Luxembourg | 36.8 | 18.8 | 15.3 | 70.9 | 24.5 | 4.6 |
| Malta | 34.0 | 18.7 | 18.7 | 71.4 | 23.2 | 5.4 |
| Netherlands | 43.8 | 18.4 | 15.7 | 77.8 | 13.8 | 8.4 |
| Norway | 30.5 | 19.5 | 17.2 | 67.1 | 30.6 | 2.3 |
| Poland | 38.4 | 19.6 | 17.5 | 75.4 | 20.3 | 4.3 |
| Portugal | 38.8 | 17.5 | 16.9 | 73.2 | 20.7 | 6.1 |
| Romania | 37.1 | 20.3 | 20.9 | 78.3 | 17.3 | 4.4 |
| Slovakia | 37.4 | 21.0 | 18.9 | 77.3 | 19.2 | 3.5 |
| Slovenia | 36.8 | 20.8 | 17.2 | 74.8 | 20.8 | 4.4 |
| Spain | 41.3 | 19.4 | 20.0 | 80.7 | 14.9 | 4.4 |
| Sweden | 34.1 | 19.2 | 18.3 | 71.6 | 23.0 | 5.4 |
| United Kingdom | 31.4 | 19.3 | 18.7 | 69.3 | 25.2 | 5.5 |
Data are percent; 100% is represented by all RSV-associated hospitalization occurring in children aged under 5 years.
aRSV-associated hospitalizations in this age group are estimated by also including data from France reported by Demont and colleagues [15].
bCalculated as a total of the previous 3 age groups (0–2 months, 3–5 months, 6–11 months).
cRSV-associated hospitalizations in these 2 age groups are estimated by also including data from Spain reported by Gil-Prieto and colleagues [14].
dIncludes the United Kingdom and excludes Norway.
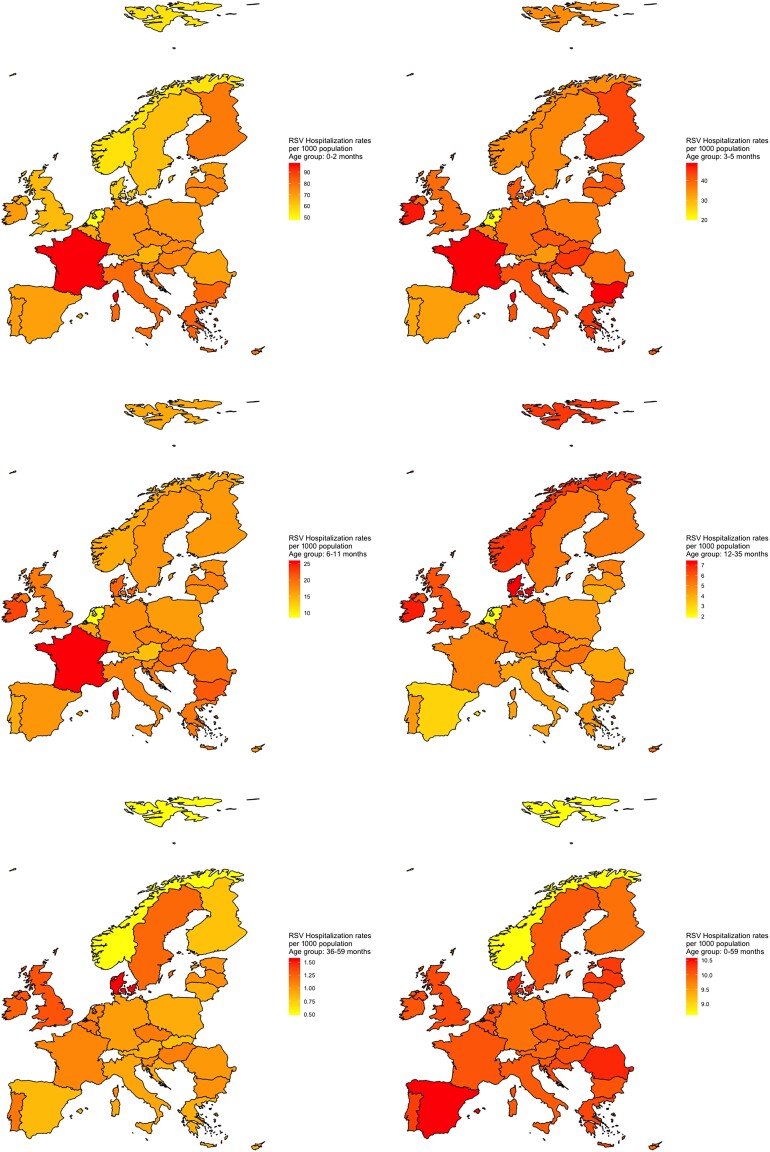
We also estimated country-specific RSV-associated respiratory hospitalizations for each of the 29 countries and rates per 1000 children (Figure 2, Table 2, and Table 3). The countries that had the highest absolute number of estimated hospitalizations were France (46 027 hospitalizations per year in children aged under 5 years), the UK (39 296 hospitalizations), and Germany (34 719 hospitalizations).
Figure 2.
RSV-associated hospitalisation rates per 1000 population in 28 EU countries and Norway.
The hospitalization rates varied widely across the EU: in the first age group (0–2 months) they ranged from 47.4 (95% CI, 37.6–57.3) per 1000 population in the Netherlands to 98.3 (95% CI, 88.5–108.1) in France. The lowest rates for the age group 36–59 months were estimated for Norway (0.5, 95% CI, .3–.7). In the age group 0–5 years (0–59 months), the rates ranged from 8.61 (95% CI, 8.31–8.92) in Norway to 10.58 (95% CI, 10.30–18.86) in Spain (Figure 2).
Most RSV-associated hospitalizations occurred in children aged less than 1 year (74.9% averaged, ranging from 65.4% in Denmark to 80.7% in Spain; Table 4). The youngest group (0–2 months) was the most affected, with percentages ranging from 27.5% in Denmark to 43.8% in the Netherlands. RSV-associated hospitalizations were less likely in children aged from 3 to 4 years (36–59 months), with the percentage ranging from 3.3% in Finland to 8.4% in the Netherlands.
Comparison With Total Pediatric Hospitalizations and Respiratory Pediatric Hospitalizations
We compared the country estimates to total national pediatric hospitalizations and respiratory pediatric hospitalizations in the EU and Norway and found that RSV-associated hospitalizations represented from 1.8% (95% CI, 1.5%–2.1%; Lithuania) to 9.9 (95% CI, 8.4%–11.5%; Finland) of total hospitalizations in children younger than 5 years (Table 5) [11]. This percentage was higher for pediatric respiratory hospitalizations, ranging from 6.8% in Lithuania to 51.6% in Sweden, and these percentages are likely to be much higher during the winter, especially during the weeks when RSV circulates.
Table 5.
Hospitalizations in Children Aged Under 5 Years (all Causes and Respiratory Causes) in EU-28 Countries and Norway, and Percent of all Hospitalizations and all Respiratory Hospitalizations That Were due to RSV
| Country | Hospitalizations in Children Under 5 y, all Causesa | Hospitalizations in Children Under 5 y, Respiratory Causesa | RSV-Associated Hospitalizations in Children Under 5 yb | % of all Hospitalizations in Children Under 5 y due to RSV | % of all Respiratory Hospitalizations in Children Under 5 y due to RSV |
|---|---|---|---|---|---|
| Austria | NA | 16 305 | 3399 (2776–4022) | … | 20.8 (17.0–24.7) |
| Belgium | NA | NA | 5807 (4840–6774) | … | … |
| Bulgaria | NA | NA | 3784 (3270–4300) | … | … |
| Croatia | 62 972 | 8060 | 2046 (1738–2356) | 3.2 (2.8–3.7) | 25.4 (21.6–29.2) |
| Cyprus | 6839 | 1568 | 477 (405–550) | 7.0 (5.9–8.0) | 30.4 (25.8–35.1) |
| Czech Republic | 196 900 | 26 813 | 5666 (4820–6513) | 2.9 (2.4–3.3) | 21.1 (18.0–24.3) |
| Denmark | 47 695 | 7672 | 3073 (2624–3518) | 6.4 (5.5–7.4) | 40.1 (34.2–45.9) |
| Estonia | NA | NA | 656 (548–762) | … | … |
| Finland | 29 637 | 6854 | 2945 (2494–3400) | 9.9 (8.4–11.5) | 43.0 (36.4–49.6) |
| France | 1 229 788 | 127 538 | 46 027 (40 271–51 781) | 3.7 (3.3–4.2) | 36.1 (31.6–40.6) |
| Germany | 1 253 873 | 162 515 | 34 719 (29 247–40 193) | 2.8 (2.3–3.2) | 21.4 (18.0–24.7) |
| Greece | NA | NA | 4840 (4115–5563) | … | … |
| Hungary | 175 698 | 29 555 | 4878 (4170–5587) | 2.8 (2.4–3.2) | 16.5 (14.1–18.9) |
| Ireland | 53 504 | 11 637 | 3764 (3255–4272) | 7.0 (6.1–8.0) | 32.3 (28.0–36.7) |
| Italy | 731 993 | 62 922 | 25 354 (21 432–29 277) | 3.5 (2.9–4.0) | 40.3 (34.1–46.5) |
| Latvia | NA | 9608 | 1061 (897–1222) | … | 11.0 (9.3–12.7) |
| Lithuania | 78 166 | 20 663 | 1404 (1172–1637) | 1.8 (1.5–2.1) | 6.8 (5.7–7.9) |
| Luxembourg | NA | 777 | 261 (214–310) | … | 33.6 (27.5–39.9) |
| Malta | 7030 | 642 | 203 (170–238) | 2.9 (2.4–3.4) | 31.6 (26.5–37.1) |
| Netherlands | NA | 18 201 | 4731 (3379–6084) | … | 26.0 (18.6–33.4) |
| Norway | 84 850 | 7251 | 2657 (2182–3132) | 3.1 (2.6–3.7) | 36.6 (30.1–43.2) |
| Poland | 664 693 | 120 409 | 17 056 (14 188–19 927) | 2.6 (2.1–3.0) | 14.2 (11.8–16.5) |
| Portugal | 98 167 | 8069 | 3717 (3069–4366) | 3.8 (3.1–4.4) | 46.1 (38.0–54.1) |
| Romania | 424 339 | 123 377 | 8896 (7423–10 365) | 2.1 (1.7–2.4) | 7.2 (6.0–8.4) |
| Slovakia | 113 651 | 19 071 | 2770 (2340–3199) | 2.4 (2.1–2.8) | 14.5 (12.3–16.8) |
| Slovenia | 46 511 | 7346 | 1083 (918–1248) | 2.3 (2.0–2.7) | 14.7 (12.5–17.0) |
| Spain | NA | 58 598 | 17 904 (14 575–21 232) | … | 30.6 (24.9–36.2) |
| Sweden | NA | 10 362 | 5351 (4450–6251) | … | 51.6 (42.9–60.3) |
| United Kingdom | 979 392 | 116 819 | 39 296 (29 326–49 264) | 4.0 (3.0–5.0) | 33.6 (25.1–42.2) |
Abbreviation: NA, not available.
aData are related to 2015 [11].
bEstimates calculated in the present article.
DISCUSSION
Understanding the burden of disease caused by RSV, and specifically the incidence of hospitalizations and deaths, will help assess the impact of RSV prevention programs (new monoclonal antibodies and vaccines [18–21]). Our study estimated that an average of roughly 250 000 respiratory hospitalizations in children aged 5 years and younger were associated with RSV each year in the 28 EU countries included in the analysis, with 3 out of 4 hospitalizations (ranging from 65.4% in Denmark to 80.7% Spain) occurring on average in children aged 0–11 months and 96% in those aged less than 0–23 months (ranging from 93.9% in Portugal to 97.7% in Norway) (Table 4).
We applied 4 extrapolation methods to obtain these estimates and saw small differences across the outcomes (eg, less than 5% difference when comparing estimates in the age group 0–2 months): this was reassuring as it suggests that our results are not driven by the choice of a specific model. Consistent with previous studies, our results show an increase in RSV hospital admissions with a decrease in patient age, with infants under 1 year having the highest burden of RSV hospitalizations (especially those aged 0–2 months) [22]. Glatman-Freedman et al (Israel), Saravanos et al (Australia), and Arriola et al (United States) have also found the highest age-specific hospitalization rates in children aged 0–2 months, with reductions in the other age groups [23–25]. This confirms how RSV immunization programs targeting the first 6 months of life could be highly effective in reducing most of the RSV hospitalization burden [2].
Our estimates (and specifically the hospitalization rates) varied strongly across the different EU countries; this finding is not entirely surprising as these results reflect the stage 1 data inputs that were entered into the stage 2 modelling procedure, where the Netherlands had the lowest [7] and France the highest rates [15]. Differences in the outcome coding and in the study design (the French study was conducted during the winter season, and we therefore needed to recalculate the estimates for a whole year) may explain the higher rates reported in France. From a methodological perspective, these results highlight the importance of having stage 1 estimates that are calculated in a harmonized manner as the stage 2 extrapolations are sensitive to the stage 1 inputs [26]. We also used 2 sets of 10 indicators to produce the extrapolations (see Supplementary Material): these sets were chosen based on the availability of data in all included countries and are not always specific to RSV. From a statistical perspective, this is not likely to influence the estimates (the indicators only aim to capture variability across countries), but it would be more elegant to develop indicator sets that are better aligned with RSV, as was done for influenza [9]. We did not further explore these differences, as doing so would require more advanced analytical methods and would be beyond the scope of this study. However, calculating and reporting country-specific hospitalizations and rates was not only important to estimate the hospitalization burden of RSV in the EU, but it will also serve as a potential reference for future studies, and this should further improve country-specific estimates.
In fact, whilst it is important to properly understand the real burden of disease associated with RSV in the EU and to estimate the potential impact of prevention efforts, it is not easy to compare our country-specific results with findings from the literature, due to the use of other methods to calculate these rates and the paucity of published studies. The recent, large perspective study by Wildenbeest and colleagues [27], conducted in 5 European countries, reported lower but comparable hospitalization rates (1.8% RSV-associated hospitalization in the first year of life in healthy term-born infants, 3.3% in children <3 months). The lower rates reported by Wildenbeest and colleagues might be related to the exclusion of preterm infants or those at highest risk for severe illness [28], which were included in the studies used in our analysis [7, 14, 15]. Lower rates of hospitalizations reported by another national study conducted in Spain [29] may reflect not only the different outcome coding (they only included children with bronchiolitis [ICD-9 code 466.11] instead of all respiratory hospitalizations) but also the observed general increase in RSV-associated admissions over the years that may be due to changes in health care policies (an increase in hospital bed availability or a change in the admission threshold), as reported by Green et al [30]. Our study also shows how hospitalizations due to RSV in children aged under 5 years represent one of the leading causes of EU infant hospitalizations (Table 5): based on our estimates, up to 1 in 10 hospitalized children under 5 years of age may be associated to RSV, and this number is larger (approximately 4 per 10 children in Italy, Portugal, Denmark, and Finland, and 1 per 2 children in Sweden) if we only consider respiratory hospitalizations (Table 5). Accurate and reliable patient-based data on hospitalizations for multiple pathogens in children aged under 5 years and the related cause(s) of the hospitalization will be fundamental in assessing whether RSV is actually the leading cause of infant hospitalizations in Europe, as recently demonstrated for the United States [31].
Our study has a number of limitations. First, our extrapolations would benefit from more countries with RSV-associated estimates to populate the statistical models (eg, additional country estimates in southern and eastern Europe); moreover, it has to be acknowledged that the EU countries for which we had national estimates are not entirely representative of the whole of Europe (eg, the WHO European region), and this is one of the reasons why we decided to focus on an EU-wide estimate. A second limitation is that the estimates used for stage 1 are regression-based and this holds inherent uncertainties related to country-specific collection methods of laboratory data and ICD codes for hospital admissions (ICD-10 for all countries included except the study conducted in Spain, in which ICD-9-CM was used) [14]. Without uniform reporting systems and consistent coding practices, it is hard to generalize results to other countries. Despite this, whilst differences in coding can be profound when looking at a single code, they are reduced when the modelling builds on a wider range of codes (eg, all respiratory codes, as done by Johannesen and colleagues [7]), as clinical practices and coding guidelines are less affected. Another limitation is that our estimates are based on country-specific hospitalization rates that were calculated for different time periods (see Table 1), thus are possibly influenced by differences in RSV circulation (eg, types) over the years. Finally, our extrapolations are based on a period in which coronavirus disease 2019 (COVID-19) was not present: it would be preferable to have more recent estimates to understand the impact of the COVID-19 pandemic on RSV circulation [32] and its burden in terms of infections, hospitalizations, and deaths.
Despite these limitations, our study is, to our knowledge, the first attempt to estimate the RSV hospitalization burden in children under the age of 5 years across the EU, and in EU countries for which no estimates have been produced so far. These estimates should help optimize public health responses (eg, the allocation of more resources to pediatric hospitals during the winter season) and support planning for future immunization programs [33]. Additionally, they could help gain a better understanding of the impact of RSV-associated hospitalizations on the increased risk of premature adult deaths from respiratory disease [34]. Finally, they represent a benchmark to understand changes in the RSV burden after the COVID-19 pandemic and in the future following the introduction of RSV immunization programs in Europe.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Marco Del Riccio, Netherlands Institute for Health Services Research, Utrecht, The Netherlands; Department of Health Sciences, University of Florence, Florence, Italy.
Peter Spreeuwenberg, Netherlands Institute for Health Services Research, Utrecht, The Netherlands.
Richard Osei-Yeboah, Centre for Global Health, Usher Institute, University of Edinburgh, Edinburgh, United Kingdom.
Caroline K Johannesen, Statens Serum Institut, Copenhagen, Denmark.
Liliana Vazquez Fernandez, Department of Methods Development and Analytics, Norwegian Institute of Public Health, Oslo, Norway.
Anne C Teirlinck, Centre for Infectious Disease Control, National Institute for Public Health and the Environment, Bilthoven, The Netherlands.
Xin Wang, School of Public Health, Nanjing Medical University, Nanjing, China.
Terho Heikkinen, Department of Pediatrics, University of Turku and Turku University Hospital, Turku, Finland.
Mathieu Bangert, Sanofi Vaccines, Lyon, France.
Saverio Caini, Netherlands Institute for Health Services Research, Utrecht, The Netherlands.
Harry Campbell, Centre for Global Health, Usher Institute, University of Edinburgh, Edinburgh, United Kingdom.
John Paget, Netherlands Institute for Health Services Research, Utrecht, The Netherlands.
the RESCEU Investigators:
Harish NAIR, Harry CAMPBELL, Philippe Beutels, Louis Bont, Andrew Pollard, Peter Openshaw, Federico Martinon-Torres, Terho Heikkinen, Adam Meijer, Thea K Fischer, Maarten van den Berge, Carlo Giaquinto, Michael Abram, Kena Swanson, Bishoy Rizkalla, Charlotte Vernhes, Scott Gallichan, Jeroen Aerssens, Veena Kumar, and Eva Molero
Notes
Acknowledgments . The RESCEU investigators are: Harish NAIR (University of Edinburgh), Harry CAMPBELL (University of Edinburgh), Philippe Beutels (Universiteit Antwerpen), Louis Bont (University Medical Center Utrecht), Andrew Pollard (University of Oxford), Peter Openshaw (Imperial College London), Federico Martinon-Torres (Servicio Galego de Saude), Terho Heikkinen (University of Turku and Turku University Hospital), Adam Meijer (National Institute for Public Health and the Environment), Thea K. Fischer (Statens Serum Institut), Maarten van den Berge (University of Groningen), Carlo Giaquinto (PENTA Foundation), Michael Abram (AstraZeneca), Kena Swanson (Pfizer), Bishoy Rizkalla (GlaxoSmithKline), Charlotte Vernhes (Sanofi Pasteur), Scott Gallichan (Sanofi Pasteur), Jeroen Aerssens (Janssen), Veena Kumar (Novavax), and Eva Molero (Team-It Research).
Disclaimer. Data from the Norwegian Patient Registry have been used in this publication. The interpretation and reporting of these data are the sole responsibility of the authors, and no endorsement by the Norwegian Patient Registry is intended nor should be inferred. This work reflects only the author's views and opinions. The EC is not responsible for any use that may be made of the information it contains.
Financial support. This work was supported by RESCEU. RESCEU has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No 116019. This Joint Undertaking receives support from the European Union Horizon 2020 Research and Innovation Programme and European Federation of Pharmaceutical Industries and Associations. Funding to pay the Open Access publication charges for this article was provided by the Netherlands Institute for Health Services Research (Nivel).
References
- 1. Shi T, McAllister DA, O’Brien KL, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 2017; 390:946–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li Y, Wang X, Blau DM, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet 2022; 399:2047–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reeves RM, van Wijhe M, Tong S, et al. Respiratory syncytial virus-associated hospital admissions in children younger than 5 years in 7 European countries using routinely collected datasets. J Infect Dis 2020; 222:S599–605. [DOI] [PubMed] [Google Scholar]
- 4. Demont C, Petrica N, Bardoulat I, et al. Economic and disease burden of RSV-associated hospitalisations in young children in France, from 2010 through 2018. BMC Infect Dis 2021; 21:730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee N, Walsh EE, Sander I, et al. Delayed diagnosis of respiratory syncytial virus infections in hospitalized adults: individual patient data, record review analysis and physician survey in the United States. J Infect Dis 2019; 220:969–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Modjarrad K, Giersing B, Kaslow DC, et al. WHO consultation on respiratory syncytial virus vaccine development report from a world health organization meeting held on 23–24 March 2015. Vaccine 2016; 34:190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Johannesen CK, van Wijhe M, Tong S, et al. Age-specific estimates of respiratory syncytial virus-associated hospitalizations in 6 European countries: a time series analysis. J Infect Dis 2022; 226:S29–37. [DOI] [PubMed] [Google Scholar]
- 8. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Simonsen L, Spreeuwenberg P, Lustig R, et al. Global mortality estimates for the 2009 influenza pandemic from the GLaMOR project: a modeling study. PLoS Med 2013; 10:e1001558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Paget J, Spreeuwenberg P, Charu V, et al. Global mortality associated with seasonal influenza epidemics: new burden estimates and predictors from the GLaMOR project. J Glob Health 2019; 9:20421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eurostat . Hospital discharges and length of stay statistics. https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Hospital_discharges_and_length_of_stay_statistics&oldid=561104#Hospital_discharges_by_sex_and_age. Accessed 10 July 2022.
- 12. Reeves RM, Hardelid P, Gilbert R, et al. Estimating the burden of respiratory syncytial virus (RSV) on respiratory hospital admissions in children less than five years of age in England, 2007–2012. Influenza Other Respir Viruses 2017; 11:122–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cromer D, van Hoek AJ, Newall AT, et al. Burden of paediatric respiratory syncytial virus disease and potential effect of different immunisation strategies: a modelling and cost-effectiveness analysis for England. Lancet Public Health 2017; 2:e367–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gil-Prieto R, Gonzalez-Escalada A, Marín-García P, et al. Respiratory syncytial virus bronchiolitis in children up to 5 years of age in Spain: epidemiology and comorbidities: an observational study. Medicine 2015; 94:e831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Demont C, Bizouard G, Watier L, et al. Excess hospitalisations associated with RSV among children under 5 years old in France from 2010 to 2018. ESCAIDE, Barcelona, 24–27 November 2020. https://www.escaide.eu/en/general-information/abstract-books. Accessed 30 June 2022. [Google Scholar]
- 16. Li Y, Wang X, Broberg EK, et al. Seasonality of respiratory syncytial virus and its association with meteorological factors in 13 European countries, week 40 2010 to week 39 2019. Euro Surveill 2022; 27:2100619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eurostat . Demography, population stock, and balance. https://ec.europa.eu/eurostat/web/population-demography/demography-population-stock-balance/database. Accessed 10 July 2022 .
- 18. Hammitt LL, Dagan R, Yuan Y, et al. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N Engl J Med 2022; 386:837–46. [DOI] [PubMed] [Google Scholar]
- 19. Karron RA. Preventing respiratory syncytial virus (RSV) disease in children. Science 2021; 372:686–7. [DOI] [PubMed] [Google Scholar]
- 20. Mazur NI, Terstappen J, Baral R, et al. Respiratory syncytial virus prevention within reach: the vaccine and monoclonal antibody landscape. Lancet Infect Dis 2023; 23:e2–e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Teirlinck AC, Broberg EK, Stuwitz Berg A, et al. Recommendations for respiratory syncytial virus surveillance at the national level. Eur Respir J 2021; 58:2003766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bont L, Checchia PA, Fauroux B, et al. Defining the epidemiology and burden of severe respiratory syncytial virus infection among infants and children in western countries. Infect Dis Ther 2016; 5:271–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Glatman-Freedman A, Kaufman Z, Applbaum Y, et al. Respiratory syncytial virus hospitalisation burden: a nation-wide population-based analysis, 2000–2017. J Infect 2020; 81:297–303. [DOI] [PubMed] [Google Scholar]
- 24. Saravanos GL, Sheel M, Homaira N, et al. Respiratory syncytial virus-associated hospitalisations in Australia, 2006–2015. Med J Aust 2019; 210:447–53. [DOI] [PubMed] [Google Scholar]
- 25. Arriola CS, Kim L, Langley G, et al. Estimated burden of community-onset respiratory syncytial virus-associated hospitalizations among children aged <2 years in the United States, 2014–15. J Pediatric Infect Dis Soc 2020; 9:587–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Paget J, Danielle Iuliano A, Taylor RJ, et al. Estimates of mortality associated with seasonal influenza for the European union from the GLaMOR project. Vaccine 2022; 40:1361–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wildenbeest JG, Billard M-N, Zuurbier RP, et al. The burden of respiratory syncytial virus in healthy term-born infants in Europe: a prospective birth cohort study. Lancet Respir Med 2022; 11:341–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. American Lung Association . Learn about respiratory syncytial virus (RSV), 2021. https://www.lung.org/lung-health-diseases/lung-disease-lookup/rsv/learn-about-rsv. Accessed 20 December 2022.
- 29. Sanchez-Luna M, Elola FJ, Fernandez-Perez C, et al. Trends in respiratory syncytial virus bronchiolitis hospitalisations in children less than 1 year: 2004–2012. Curr Med Res Opin 2016; 32:693–8. [DOI] [PubMed] [Google Scholar]
- 30. Green CA, Yeates D, Goldacre A, et al. Admission to hospital for bronchiolitis in England: trends over five decades, geographical variation and association with perinatal characteristics and subsequent asthma. Arch Dis Child 2016; 101:140–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Suh M, Movva N, Jiang X, et al. Respiratory syncytial virus is the leading cause of United States infant hospitalizations, 2009–2019: a study of the national (nationwide) inpatient sample. J Infect Dis 2022; 226:S154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van Summeren J, Meijer A, Aspelund G, et al. Low levels of respiratory syncytial virus activity in Europe during the 2020/21 season: what can we expect in the coming summer and autumn/winter? Euro Surveill 2021; 26:2100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sande CJ. Implementation strategies for passive respiratory syncytial virus immunisation. Lancet Infect Dis 2021; 21:1200–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Allinson JP, Chaturvedi N, Wong A, et al. Early childhood lower respiratory tract infection and premature adult death from respiratory disease in Great Britain: a national birth cohort study. Lancet 2023; 401:1183–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.