Abstract
The majority of patients with lung squamous cell carcinoma are diagnosed at an advanced stage, which poses a challenge to the efficacy of chemotherapy. Therefore, the search for an early biomarker needs to be addressed. CD36 is a scavenger receptor expressed in various cell types. It has been reported that it is closely related to the occurrence and development of many kinds of tumours. However, its role in lung squamous cell carcinoma has not been reported. Our research aims to reveal the role of CD36 in lung squamous cell carcinoma by integrating single-cell RNA sequencing (scRNA-seq) and bulk RNA sequencing data. We used bioinformatics methods to explore the potential carcinogenicity of CD36 by analysing the data from the cancer genome map (TCGA), gene expression comprehensive map (GEO), human protein map (HPA) comparative toxicology genomics database (CTD) and other resources. Our study dissected the relationship between CD36 and prognosis and gene correlation, functional analysis, mutation of different tumours, infiltration of immune cells and exploring the interaction between CD36 and chemicals. The results showed that the expression of CD36 was heterogeneous. Compared with normal patients, the expression was low in lung squamous cell carcinoma. In addition, CD36 showed early diagnostic value in four kinds of tumours (LUSC, BLCA, BRCA and KIRC) and was positively or negatively correlated with the prognosis of different tumours. The relationship between CD36 and the tumour immune microenvironment was revealed by immunoinfiltration analysis, and many drugs that might target CD36 were identified by the comparative toxicological genomics database (CTD). In summary, through pancancer analysis, we found and verified for the first time that CD36 may play a role in the detection of lung squamous cell carcinoma. In addition, it has high specificity and sensitivity in detecting cancer. Therefore, CD36 can be used as an auxiliary index for early tumour diagnosis and a prognostic marker for lung squamous cell carcinoma.
Keywords: CD36, LUSC, Immune, Prognosis, Metabolism
1. Introduction
Nearly 1.8 million people are diagnosed with lung cancer every year [1,2]. Lung cancer has become the leading cause of death, and the number of people who die each year exceeds the sum of deaths caused by colorectal cancer, breast cancer, prostate cancer and pancreatic cancer [3]. Lung squamous cell carcinoma (LUSC) is a subtype of non-small cell carcinoma, accounting for approximately 40 % of all lung cancers according to age or tobacco exposure. Compared with lung adenocarcinoma (LUAD), LUSC is associated with poor clinical prognosis and a lack of available targeted drugs [4,5]. To sustain growth, proliferation, and viability, cancer cells necessitate the generation of ATP via metabolic pathways. The Warburg effect, occurring even in oxygen-rich environments, compels cancer cells to heavily depend on glycolysis for ATP production. Extensive aerobic glycolysis becomes imperative to fulfil the energy demands and supports biosynthesis in rapidly multiplying cancer cells [[6], [7], [8]]. This phenomenon occurs not only in tumour cells but also in other cells, such as immune cells. When immune cells are activated and differentiated in response to relevant factors from the tumour microenvironment, their metabolism is reprogrammed. This may be caused by metabolic changes in cancer cells [9]. In recent years, immune metabolism has attracted increasing attention, which highlights that energy metabolism reprogramming affects the function of immune cells [10]. However, it has been suggested that changes in the lipid metabolic pathway are related to the improvement of haematopoietic activity and immune function [11]. The main function of the scavenger receptor CD36 is to take up long-chain fatty acids that are used by cells for lipid synthesis or oxidative phosphorylation, resulting in a large amount of ATP. In addition, CD36 is a regulator of some biological activities, such as immune recognition, inflammation, molecular adhesion, inflammatory reaction, apoptosis and phagocytosis, angiogenesis, and energy metabolism tumour metastasis [[12], [13], [14]]. Studies have reported that CD36 leads to the death of tumour microvascular endothelial cells by binding to TSP-1 or inhibiting vascular endothelial growth factor receptor 2 [[15], [16], [17]]. Several studies have shown that CD36 plays a pivotal role in various cancers [14,[18], [19], [20]]. However, we still know nothing about the function of CD36 in lung squamous cell carcinoma.
In this study, we evaluated the expression of CD36 in various malignant tumours from two cohorts, the Cancer Genome Atlas (TCGA) and the Gene Expression Omnibus (GEO). Furthermore, we analysed the relationship between the expression of CD36 and the prognosis of tumour patients. This comprehensive study aims to clarify the potential function and regulatory mechanism of CD36 in the pathogenesis or clinical prognosis of lung squamous cell carcinoma. This study will apply evidence to confirm CD36 as a diagnostic biomarker and therapeutic target for lung squamous cell carcinoma treatment.
2. Materials and methods
2.1. Data collection and analysis
The LUSC scRNA-seq datasets were downloaded from the GEO Database (https://www. ncbi. nlm. nih.gov/geo.), including GSE127465. The GSE127465 dataset includes data from 6 patients with lung squamous cell carcinoma. Bulk RNA-seq datasets were downloaded from The University of California Santa Cruz (UCSC) (https://xenabrowser.net/) and GEO Database, including TCGA-PANCAN, GSE73403 and GSE18842. Sample information of the dataset is shown in Table S1, and the research workflow is shown in Fig. S1.
2.2. Differential expression and mutation analysis of CD36 in cancer
We sourced RNA-seq and clinical data from the UCSC database, followed by log2(counts+1) normalization of the data. Subsequently, we examined the divergence in CD36 gene expression between tumour samples and normal samples. Taking into consideration the notable disparity in expression between tumour and normal samples, we opted to focus on an existing dataset of lung squamous cell carcinoma. Next, we further verified the expression of CD36 in normal and lung squamous cell carcinoma patients through a GEO external cohort. Immunohistochemical images of healthy lung tissues and lung squamous cell carcinoma tissues were downloaded from the HPA database (http://www.proteinatlas.org/). To further evaluate the difference in CD36 expression between normal and lung squamous cell carcinoma patients in the TCGA database. Finally, we conducted an investigation into the single nucleotide variations occurring within the CD36 gene across 19 distinct types of cancers.
2.3. Survival prognosis analysis
Based on the median expression level of CD36, the cancer patients were categorized into a high-expression group and a low-expression group. We employed the Kaplan‒Meier method to analyse the survival outcomes of patients with cancers within the TCGA cohort. Subsequently, we proceeded to validate the correlation between CD36 expression and the survival outcomes of tumour patients using an external cohort sourced from the Gene Expression Omnibus (GEO) database. Then, we used Cox regression to analyse the relationship between the expression of CD36 in the TCGA cohort and the prognosis of tumour patients. In addition, the relationship between CD36 expression and tumour staging was discussed. Finally, we employed both receiver operating characteristic (ROC) curves and a nomogram to examine the link between CD36 expression and the survival rate among patients afflicted with lung squamous cell carcinoma.
2.4. Correlation analysis
Genes associated with CD36 were calculated using Spearman correlation. The top 100 genes that CD36 was coexpressed with were discovered and confirmed in the TCGA cohort. R software's “clusterProfiler” package (ver. 3.9.2) was used to conduct an analysis of Gene Ontology (GO) enrichment and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis.
2.5. Differential analysis and functional enrichment analysis
Based on the median CD36 expression levels in pancancer, patients were segregated into a high-expression group and a low-expression group. Using the “limma” package (ver. 3.54.2) for difference analysis, DEGs were discovered. The following standards were used to determine which DEGs were significant: I. p value < 0.01; II. log2-fold change (FC) absolute value > 2. Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) enrichment analyses of DEGs were carried out using the R packages “clusterProfiler”, “enrichplot”, and ggplot2 (ver. 3.4.2) to investigate the biological roles and signalling pathways. The “pheatmap” package (ver. 1.0.12) was used to create heatmaps of DEGs.
2.6. Immune infiltration analysis
To explore the relationship between CD36 expression and immune infiltration in cancer, we used the “ssGSEA” algorithm in R to calculate the degree of immune infiltration of 28 kinds of immune cells in the TCGA cohort and observed the relationship between CD36 expression and immune infiltration [21].
2.7. Comprehensive analysis of single-cell data clustering and annotation
The scRNA-seq dataset was analysed with the “Seurat” software package (ver. 4.3.0.1). We used the “IntegrateData” function to integrate and correct six purified lung squamous cell carcinoma samples in batches. The unqualified cells are subsequently eliminated from the dataset using the quality control standards that are stated below. 500< nFeature_RNA <5000; 200< nCount_RNA <35,000; and percentage.mt < 10 %. Finally, 6232 cells were used for further study. This was then used for cell type annotation using the singleR (ver. 1.8.1) and CellMarker 2.0 databases.
2.8. Annotating cell types in bulk RNA-seq datasets
The CIBERSORT algorithm calculates a nonnegative gene expression matrix from the expression of marker genes for various cell types to estimate the relative proportions of various cell subsets [22]. Here, we compare the differences between different cell types and between normal tissues and tumour tissues with all marker genes of ten cell clusters as inputs.
2.9. Expression of CD36 in different cell groups and determination of the differentiation status of macrophage subsets
Utilizing singleR and CellMarker, the cells were categorized into distinct cell types. Subsequently, an investigation was conducted into the expression of the CD36 gene within these various cellular clusters. Monocle (ver. 2.26.0) was used to construct the pseudotime trajectory of macrophages. The algorithm uses machine learning technology to arrange cells into trajectories with branching points according to a set of specific genes as inputs. The results show that different branches are cell populations with different differentiation states. Here, we studied the expression changes of the CD36 gene in the pseudotime sequence of macrophages.
2.10. Targeted drug screening
We used Autodock (Linux, ver. 4.2) for molecular docking and studied the small molecular compounds interacting with the CD36 gene. First, we downloaded the catalogue of small molecules interacting with the CD36 gene in the CTD database and then downloaded the structure of small molecules in the PubChem database (https://pubchem.ncbi.nlm.nih.gov/). Next, we downloaded the biomacromolecule structure of CD36 gene translation from the UniProt database (https://www.uniprot.org). Finally, according to the standard docking process, biological macromolecules and small molecular compounds are automatically docked. Small molecular compounds with stable binding to biological macromolecules have low binding energy. In addition, the results were visualized by PyMol (ver. 2.6, open source).
2.11. Statistical analysis
R: The R Project for Statistical Computing (r-project.org) software version 4.2.1 was used to conduct all statistical analyses. The Spearman correlation test was used to perform a correlation analysis. Nonparametric tests were employed for conducting statistical comparisons between groups, while the log-rank test was utilized to assess the differences in survival probabilities among the samples. p < 0.05 was considered a significant difference.
3. Results
3.1. Expression and mutation of CD36 in different types of cancer
We downloaded the RNA-seq data of TCGA-PANCAN from the UCSC database. Fig. 1A shows the significant difference in CD36 transcription levels between cancer patients and adjacent normal patients in 17 kinds of cancers. CD36 expression was significantly lower in BLCA, BRCA, CHOL, COAD, ESCA, HNSC, KIRP, LUAD, LUSC, PRAD, READ, STAD, THCA, THYM and UCEC than in normal patients. Conversely, there was a significant increase in the expression of CD36 observed in patients with GBM and KIRC.
Fig. 1.
Expression and mutation of CD36 in different types of cancer. (A) CD36 expression in 25 types of cancer. (B) CD36 expression in tumour and normal patients in lung squamous cell carcinoma from GSE18842 (C) Expression of CD36 protein in normal and lung squamous cell carcinoma. (D) CD36 mutation in 19 types of cancer. *p < 0.05, **p < 0.01, ***p < 0.001. ****p < 0.0001.
Furthermore, the expression of CD36 in lung squamous cell carcinoma was further verified by a GEO external cohort and immunohistochemical analysis. The results showed that the expression of CD36 in patients with lung squamous cell carcinoma was lower than that in normal patients (Fig. 1B–C).
The occurrence and development of tumours is closely related to genome mutations [23]. Finally, we analysed the types and sites of CD36 gene mutations in 19 types of cancers in the TCGA database (Fig. 1D). The results showed that the frequency of CD36 mutations was different among different cancer types, and missense mutations were the main type of mutation.
3.2. Prognostic value of CD36 expression
We analysed the expression of CD36 in different cancers, and the results showed that the expression of CD36 in different cancers was heterogeneous. Next, we studied the relationship between CD36 expression and cancer prognosis and used TCGA and GEO datasets to evaluate OS. According to the median expression level of CD36, cancer patients were divided into a high-expression group and a low-expression group. Subsequently, we analysed the survival results of tumour patients in the TCGA cohort by the Kaplan‒Meier method. Our results showed that among patients with BLCA, BRCA, and LUSC, patients with low CD36 expression levels lived longer. Among KIRC patients, patients with high CD36 expression levels had a longer survival time (Fig. 2A). Then, we further verified the relationship between the expression of CD36 and the survival of patients with lung squamous cell carcinoma using an external cohort from the Gene Expression Synthesis (GEO) database (Fig. 2B). Next, we used Cox regression to analyse the relationship between the expression of CD36 in the TCGA cohort and the prognosis of tumour patients (Fig. 2C and Fig. S2). The time-varying ROC curve was used to evaluate the relationship between CD36 expression and prognosis in patients with lung squamous cell carcinoma. The AUCs of the GEO dataset at 1 year, 3 years, 5 years and 7 years were all greater than 0.7, suggesting that CD36 expression has a strong predictive effect on the survival of LUSC patients (Fig. 2D). In addition, we established a nomogram using prognostic factors (Fig. 2E). Finally, we studied the relationship between CD36 expression and clinical features (Fig. S3).
Fig. 2.
Prognostic value of CD36 expression. (A) Bladder urothelial carcinoma (BLCA); breast invasive carcinoma (BRCA); kidney renal clear cell carcinoma (KIRC) and lung squamous cell carcinoma (LUSC) by Kaplan-Meier analysis. (B) Kaplan-Meier analysis of lung squamous cell carcinoma in GSE73403. (C) Univariate Cox regression analysis of overall survival in pancancer. (D) ROC curves of CD36 expression on 1-, 3-, 5- and 7-year survival of LUSC patients. (E) Nomogram of prognostic factors.
3.3. Correlation and enrichment analysis
We studied the relationship between the expression of CD36 and the prognosis of cancer. The results showed that the expression of CD36 was positively or negatively correlated with the prognosis of different cancers. Next, we calculated the top 100 coexpressed genes of CD36 by Spearman correlation (Fig. 3A). Based on these coexpressed genes, KEGG and GO functional enrichment analyses were performed (Fig. 3B). These genes were primarily enriched in Epstein-Barr virus infection, the IL-17 signalling pathway, the cell cycle, and human T-cell leukaemia virus type 1 illness, according to KEGG enrichment analysis. According to GO enrichment analysis, these genes are mainly enriched in the positive regulation of phagocytosis, haemostasis and defence response and the combination of GTP and carbohydrate. These results emphasize the central role of CD36 in immunity.
Fig. 3.
Correlation and enrichment analysis. (A) Spearman correlation between CD36 expression. (B) Enrichment analysis of KEGG- and GO-related genes.
3.4. Biological functions associated with CD36
We separated the patients into high-expression and low-expression groups based on the median CD36 expression since CD36 expression is related to the prognosis of cancer. Genes with differential expression, including those that were up- and downregulated, were found (Fig. 4A). The heatmap of the top 200 DEGs is shown in Fig. 4B. GO enrichment analysis showed that these genes were enriched in the development of embryonic organs, monocyte differentiation, gland development and the response to exogenous substances (Fig. 4C). KEGG enrichment analysis showed that these genes were enriched in PPARα and the cholesterol metabolism pathway (Fig. 4D). The results show that CD36 plays an important role in organ development, immune cell differentiation and the energy metabolism pathway.
Fig. 4.
Biological functions associated with CD36. (A) The DEGs between the high- and low-CD36 expression groups. (B) Heatmap of the top 200 DEGs. (C) The dotplot of gene GO analysis of DEGs. (D) The dotplot of gene KEGG analysis of DEGs.
3.5. The association between CD36 expression and immunity
We found that CD36 plays an important role in immune regulation through functional enrichment analysis. Next, we studied the relationship between CD36 and immunity. According to the median expression of CD36, the patients were divided into a high-expression group and a low-expression group, and the relationship between CD36 expression and immunity and matrix score was discussed. The results showed that the immune and matrix scores of the CD36 high-expression group were significantly higher than those of the CD36 low-expression group (Fig. 5A). Next, we evaluated the difference in immune cell content between the high and low CD36 expression groups by ssGSEA. The results showed that the contents of seven kinds of immune cells were different between the high and low CD36 expression groups (Fig. 5B). Then, we also observed the correlation between CD36 and 28 kinds of immune cells, and the results showed that CD36 was related to many kinds of immune cells (Fig. 5C). Finally, we evaluated the relationship between the high and low expression of CD36 and immune score in 25 kinds of cancers. Our research shows that there are significant differences in immune scores between the high and low CD36 expression groups in various cancers (Fig. 5D). These results provided more evidence for the association between CD36 and immune infiltration.
Fig. 5.
The association between CD36 expression and immunity. (A) The association of CD36 expression with immune score. (B) The association of CD36 expression with the abundance of immune cells. (C). Spearman correlation between CD36 and immune cells. (D) The association of CD36 expression with the tumour microenvironment. *p < 0.05, **p < 0.01, ***p < 0.001. ****p < 0.0001.
3.6. Identification of cell types and analysis of immunoinfiltration in LUSC
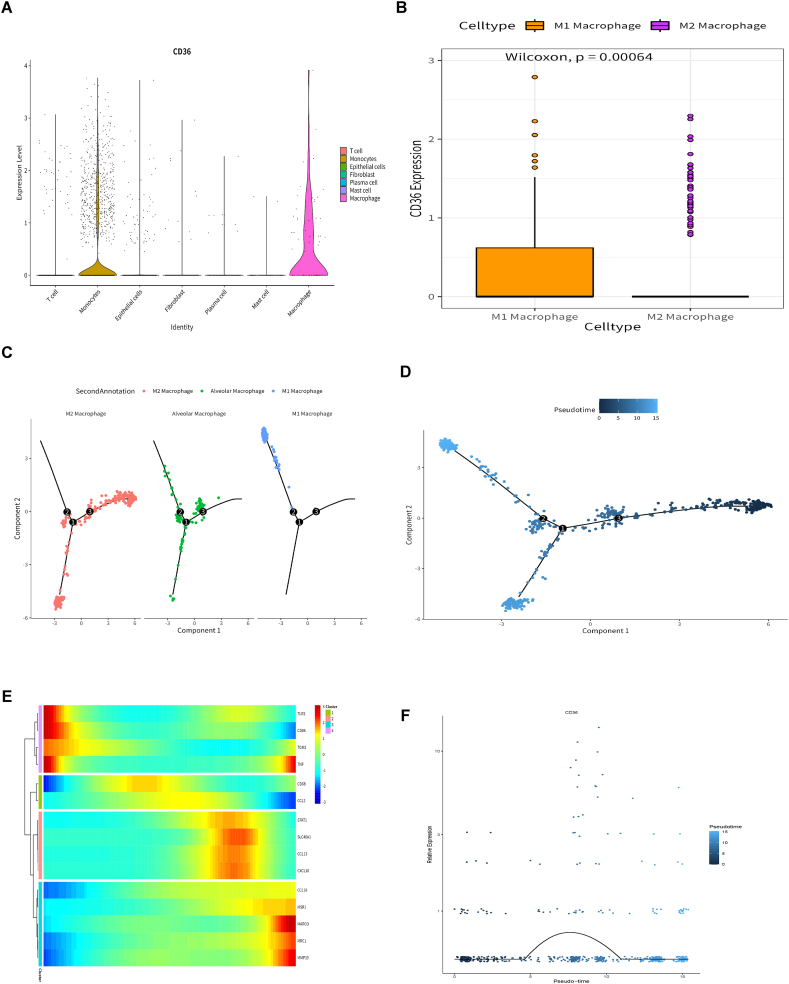
We confirmed the role and prognostic value of CD36 in lung squamous cell carcinoma by bulk RNA-seq analysis. Next, we further verified the role of CD36 in lung squamous cell carcinoma by single-cell data. By using the standard single-cell procedure, all cells were divided into 13 cell clusters, and after cell annotation, they were divided into 7 cell types: macrophages, monocytes, fibroblasts, plasma cells, epithelial cells, T cells and mast cells (Fig. 6A). Next, a more exact subcluster analysis of monocytes, T cells, and macrophages was performed. The monocyte cells were divided into 11 cell clusters, and after cell annotation, they were divided into 3 cell types: monocytes, macrophages, and neutrophils (Fig. 6B). The macrophages were divided into 8 cell clusters, and after cell annotation, they were divided into 6 cell types: M1 macrophages, M2 macrophages, alveolar macrophages, plasma cells, NKT cells, and epithelial cells (Fig. 6C). The T cells were divided into 9 cell clusters, and after cell annotation, they were divided into 4 cell types: T cells, B cells, NK cells and dendritic cells (Fig. 6D). Then, we annotated ten cell clusters in the TCGA cohort and found significant cell differences between normal and tumour patients. Notably, the number of macrophages in lung squamous cell carcinoma patients was significantly higher than that in regular patients (Fig. 6E). Fig. S4 shows the information of different cell groups and marker genes of different cell types.
Fig. 6.
Single-cell analysis: (A) Seven cell types were identified by marker gene annotation. (B) Three cell types were identified by marker gene annotation. (C) Six cell types were identified by marker gene annotation. (D) Four cell types were identified by marker gene annotation. (E) The abundance of 10 cell types in bulk RNA-seq: Ten cell types were annotated to the TCGA queue by CIBERSORT. (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001).
3.7. Differential expression of CD36 and differentiation of macrophages
After cell grouping and annotation, we studied the expression of CD36 in seven cell groups. The results showed that CD36 was expressed in macrophages and monocytes (Fig. 7A). CIBERSORT annotated the TCGA cohort of lung squamous cell carcinoma, and there was a significant difference in the number of macrophages between normal and lung squamous cell carcinoma patients. Then, we studied the expression of CD36 in M1 and M2 macrophages. The results showed that the expression level of CD36 in M2 macrophages was significantly higher than that in M1 macrophages (Fig. 7B). Then, using Monocle, a pseudotemporal trajectory analysis was performed on the macrophage subpopulation. The results showed that macrophages can exist in three different differentiation states, and the differentiation process is from M2 macrophages to alveolar macrophages, M1 macrophages and M2 macrophages (Fig. 7C and D). The heatmap displays the expression changes of macrophage marker genes in the whole time series (Fig. 7E). Finally, we studied the expression changes of CD36 in the whole pseudotime series, and the expression changes of CD36 in the whole pseudotime series are shown in Fig. 7F. These results indicate that CD36 is expressed in macrophages and participates in regulating the differentiation of macrophages.
Fig. 7.
Differential expression of CD36 in different cell types and pseudotime analysis of macrophages: (A) Differential expression of CD36 in 7 cell types. (B) Differential expression of CD36 in M1 and M2 macrophages. (C–D) According to the pseudotime of macrophages, the cell population was divided into three different differentiation states. (E) Expression of macrophage marker genes throughout the pseudosequence. (F) The expression of CD36 in the entire pseudotime sequence.
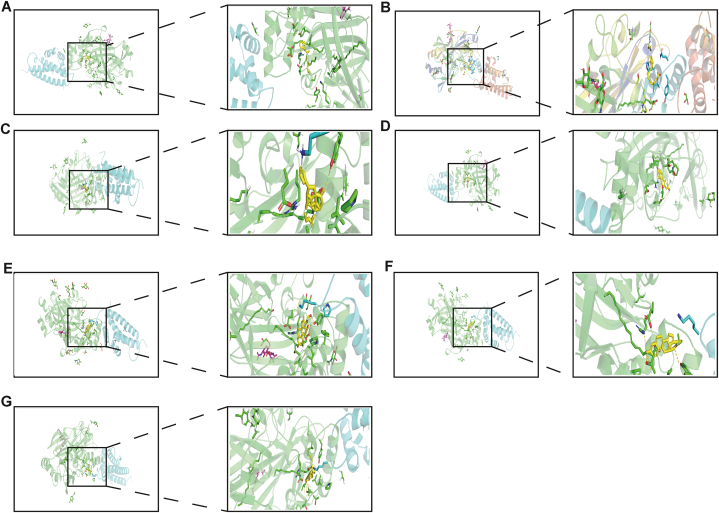
3.8. Potential drug therapy
Our study shows that the high expression of CD36 is related to the poor prognosis of patients with lung squamous cell carcinoma. Therefore, we screened small molecular compounds that can reduce the expression of CD36 from the CTD database and used AutoDock for molecular docking. Seven small molecular compounds can dock with CD36 and reduce the expression of CD36. The docking results of small molecular compounds with CD36 are shown in Fig. 8A–G. They had simulated binding energies of −11.17 (kcal/mol), −7.77 (kcal/mol), −7.76 (kcal/mol), −6.97 (kcal/mol), −6.9 (kcal/mol), −6.79 (kcal/mol), and −5.8 (kcal/mol). In summary, we screened seven kinds of small molecular compounds that are beneficial for improving the poor prognosis caused by the CD36 gene, providing a new research idea for targeted therapy of lung squamous cell carcinoma.
Fig. 8.
Potential drug therapy (A) Alitretinoin (B) Retinol acetate (C) Diosbulbin B (D) Oestradiol (E) Dexamethasone (F) Acetylene oestrogen (G) Cholesterol.
4. Discussion
Previous studies have shown that CD36 plays a role in many cancers [14,[18], [19], [20]]. Based on the expected function of CD36 in human cancer, we carried out pancancer analysis, characterized the expression characteristics of CD36 in 25 cancer types, and further discussed its prognostic value, genetic variation, functional analysis and correlation with the cancer immune microenvironment to better understand the potential characteristics of CD36 in human cancer.
Our research shows that compared with normal tissues, the CD36 gene is abnormally expressed in 17 cancer types (25 assessed in total). In BLCA, BRCA, CHOL, COAD, ESCA, HNSC, KIRP, LUAD, LUSC, PRAD, READ, STAD, THCA, THYM and UCEC, the expression level of CD36 increased significantly. However, in GBM and KIRC, the expression of CD36 decreased significantly (Fig. 1A). We further verified the expression of CD36 in lung squamous cell carcinoma using the GEO external cohort and HPA database (Fig. 1B and C). These results show that the expression of CD36 in different cancers is heterogeneous, which is consistent with previous studies [[24], [25], [26]]. CD36 may have carcinogenic/anticancer effects and may be used as a diagnostic biomarker for these cancers.
Cancer is the result of one or more genetic changes. Gene changes may determine the prognosis of cancer [27]. These genetic changes include gene mutation, structural variation, amplification, deep deletion and multiple changes. The mutation mode of CD36 in many cancers is mainly missense mutation (Fig. 1D), so modifying the CD36 gene may be a promising method to treat cancer. In addition, combined with Kaplan-Meier survival analysis results, these results show the prognostic value of CD36 in four cancer types. Among them, highly expressed CD36 predicted that the OS of patients with BLCA, BRCA and LUSC was unfavourable, while that of patients with KIRC was favourable. These results indicate that CD36 has important prognostic value in the survival of patients with BLCA, BRCA, LUSC and KIRC (Fig. 2, Fig. S2 and Fig. S3). Based on these observations, we can recognize the role of CD36 in the carcinogenesis and cancer development of LUSC and the correlation between CD36 and patient survival.
KEGG and GO enrichment analyses revealed the molecular mechanism of CD36. The results showed that CD36 and its related genes were mainly enriched in immune and metabolic signalling pathways (Fig. 3, Fig. 4). This is consistent with previous research results [[28], [29], [30], [31], [32]]. Our enrichment analysis shows that CD36 may play a carcinogenic or anticancer role in tumours by regulating immune and energy metabolism signals.
The tumour immune microenvironment (TME) is an important part of tumour biology [33]. The tumour immune microenvironment is a complex structure composed of immune cells, endothelial cells, fibroblasts and other substances that plays an important role in tumour progression [34]. The interaction between cancer cells and TME components is beneficial to the immune escape of tumours, which eventually leads to the activation, proliferation and invasion of cancer cells, which is related to the recurrence of tumours and the survival of patients [35,36]. Our research shows that the expression of CD36 is significantly related to the infiltration levels of monocytes, CD56bright natural killer cells, effector memory CD4 T cells, memory B cells, type 17 helper cells, eosinophils and neutrophils (Fig. 5). The role of CD36 in immune cells was confirmed, which increased the possibility of using CD36 for cancer immunotherapy.
The role and prognostic value of CD36 in lung squamous cell carcinoma were confirmed by bulk RNA-seq. Next, we further verified the role of CD36 in lung squamous cell carcinoma by integrating scRNA-seq data. Previous studies have shown that CD36 can control tumour growth by reprogramming the metabolism of glucose and fatty acids [37]. Most myeloid cells infiltrating solid tumours are tumour-associated macrophages (TAMs), also known as macrophages in the microenvironment of solid tumours. TAMs are related to the poor prognosis of cancer patients. TAMs are functionally equivalent to the M2-like macrophage phenotype but not completely equivalent. They promote microenvironment immunosuppression and tumour growth [38]. The most common forms of macrophages are M1 and M2. As a rapid energy source, M1 macrophages are more likely to use glycolysis to produce a large amount of lactic acid. On the other hand, M2 macrophages usually proliferate. An important energy source is the oxidative phosphorylation of fatty acids to produce a large amount of ATP [39]. Studies have shown that TAMs produced from human melanoma and colorectal cancer absorb a large amount of lipids through CD36, a key driver of cell energy metabolism, and maintain accelerated fatty acid oxidation. The phosphorylation of STAT6 promotes the production of MRC1, TGM2, ARG1 and other genes, and increased fatty acid oxidation helps TAMs maintain their M2-like macrophage phenotype and promote tumour proliferation [40]. Our research shows that CD36 is expressed in macrophages and participates in regulating the differentiation of macrophages (Fig. 7). This confirmed the role of CD36 in immune metabolism and increased the possibility of using CD36 for cancer immunotherapy.
Our research indicates a significant correlation between high CD36 expression and poor prognosis in patients with lung squamous cell carcinoma. Therefore, we screened several small molecular compounds from the CTD database that can downregulate CD36 expression, including alitretinoin, retinol acetate, diosbulbin B, oestradiol, dexamethasone, acetylene oestrogen, and cholesterol. Through molecular docking, it was shown that these small molecular compounds can be stably combined with CD36 (Fig. 8). Aliretinoic acid (9-cis retinoic acid) is a unique all-agonist retinoid that can bind to all six known retinoid receptors (RAR-α, -β, -γ and RXR-α, -β, -γ). Studies have shown that alitretinoin can be used to treat chronic hand dermatitis and other diseases [41]. Oestradiol is an important oestrogen, also known as 17β-oestradiol. It is one of the key oestrogens naturally produced in humans and other mammals. More than 95 % of oestradiol in blood binds to sex hormone binding globulin (SHBG) and alumina, which is often used to treat diseases related to oestrogen reduction [42]. Dexamethasone is a synthetic steroid that has been used in clinical practice for many years because of its anti-inflammatory, anti-allergic and immunosuppressive properties. In addition, dexamethasone has been used for a long time to prevent and treat nausea and vomiting caused by chemotherapy [43]. Cholesterol is an integral part of the eukaryotic cell membrane and a key molecule in controlling membrane fluidity, tissue and other physical and chemical parameters. By stabilizing the membrane structure to resist injury, it also plays a regulatory role in antibiotic resistance and the cellular immune response to viruses [44]. A drug targeting CD36 was found by molecular docking. However, how to make the drug target cancer is a challenge. Recent research shows that a series of innovative materials can be used to deliver drugs to appropriate tumour cells and reduce the side effects of drugs on cells. For example, drugs are delivered to corresponding target cells or organs through nanomaterials [45], aptamer systems [46], and colloidal delivery systems [47] to reduce the toxic side effects of drugs.
In this study, we first revealed in detail the correlation between CD36 expression and the clinical prognosis and immune cell infiltration of lung squamous cell carcinoma, which may help to better understand the role of CD36 in the occurrence of lung squamous cell carcinoma and provide a new perspective for immunotherapy of lung squamous cell carcinoma. Finally, we screened drugs targeting the CD36 gene, which provided new insights for the targeted treatment of lung squamous cell carcinoma. However, there are still some limitations in this study, and we have not explored potential confounding factors that could influence the expression of CD36 and cancer prognosis. These factors include lifestyle variables such as smoking and diet, as well as comorbid conditions such as diabetes and hypertension. Therefore, the influence of these confounding factors needs to be further discussed.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Data availability statement
The datasets generated and analysed during the current study are available in the UCSC repository and GEO database, including TCGA-PANCAN, GSE18842, GSE127465 and GSE73403.
Funding
This research was supported by the Yunnan High-level Personnel Training Support Program (YNWR-QNBJ-2020-243), Kunming University of Science and Technology Medical joint project (grant number KUST-PE2022005Y), and Special Fund Project for Central Government Leading Local Science and Technology Development (202207AB110015).
CRediT authorship contribution statement
Hui Wang: Investigation, Writing – original draft, Methodology. Jianyu Pang: Methodology, Visualization. Shuojie Zhang: Visualization. Qian Yu: Methodology. Yongzhi Chen: Visualization. Lulin Wang: Methodology. Miaomiao Sheng: Funding acquisition, Writing – review & editing. Juhua Dan: Methodology, Writing – review & editing. Wenru Tang: Formal analysis, Funding acquisition, Methodology, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Thanks to the patients who provided clinical data for this medical study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e22201.
Contributor Information
Juhua Dan, Email: danjuhua177@163.com.
Wenru Tang, Email: 20080059@kust.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics. CA: a cancer journal for clinicians. 2015;65(2):87–108. doi: 10.3322/caac.21262. 2012. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M., et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Li Y., Gu J., Xu F., Zhu Q., Ge D., Lu C. Transcriptomic and functional network features of lung squamous cell carcinoma through integrative analysis of GEO and TCGA data. Sci. Rep. 2018;8(1) doi: 10.1038/s41598-018-34160-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirsch F.R., Scagliotti G.V., Mulshine J.L., Kwon R., Curran W.J., Wu Y.L., et al. Lung cancer: current therapies and new targeted treatments. Lancet (London, England) 2017;389(10066):299–311. doi: 10.1016/S0140-6736(16)30958-8. [DOI] [PubMed] [Google Scholar]
- 5.Kulasingam V., Diamandis E.P. Strategies for discovering novel cancer biomarkers through utilization of emerging technologies. Nat. Clin. Pract. Oncol. 2008;5(10):588–599. doi: 10.1038/ncponc1187. [DOI] [PubMed] [Google Scholar]
- 6.Pearce E.L., Pearce E.J. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38(4):633–643. doi: 10.1016/j.immuni.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun L., Suo C., Li S.T., Zhang H., Gao P. Metabolic reprogramming for cancer cells and their microenvironment: beyond the Warburg Effect. Biochimica et biophysica acta Reviews on cancer. 2018;1870(1):51–66. doi: 10.1016/j.bbcan.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Teng X., Li W., Cornaby C., Morel L. Immune cell metabolism in autoimmunity. Clin. Exp. Immunol. 2019;197(2):181–192. doi: 10.1111/cei.13277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacIver N.J., Michalek R.D., Rathmell J.C. Metabolic regulation of T lymphocytes. Annu. Rev. Immunol. 2013;31:259–283. doi: 10.1146/annurev-immunol-032712-095956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ganeshan K., Chawla A. Metabolic regulation of immune responses. Annu. Rev. Immunol. 2014;32:609–634. doi: 10.1146/annurev-immunol-032713-120236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soehnlein O., Swirski F.K. Hypercholesterolemia links hematopoiesis with atherosclerosis. Trends in endocrinology and metabolism: TEM (Trends Endocrinol. Metab.) 2013;24(3):129–136. doi: 10.1016/j.tem.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J., Li Y. CD36 tango in cancer: signaling pathways and functions. Theranostics. 2019;9(17):4893–4908. doi: 10.7150/thno.36037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang M., Wu N., Xu B., Chu Y., Li X., Su S., et al. Fatty acid-induced CD36 expression via O-GlcNAcylation drives gastric cancer metastasis. Theranostics. 2019;9(18):5359–5373. doi: 10.7150/thno.34024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ladanyi A., Mukherjee A., Kenny H.A., Johnson A., Mitra A.K., Sundaresan S., et al. Adipocyte-induced CD36 expression drives ovarian cancer progression and metastasis. Oncogene. 2018;37(17):2285–2301. doi: 10.1038/s41388-017-0093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshida T., Yokobori T., Saito H., Kuriyama K., Kumakura Y., Honjo H., et al. CD36 expression is associated with cancer aggressiveness and energy source in esophageal squamous cell carcinoma. Ann. Surg Oncol. 2021;28(2):1217–1227. doi: 10.1245/s10434-020-08711-3. [DOI] [PubMed] [Google Scholar]
- 16.Cheng Q., Jabbari K., Winkelmaier G., Andersen C., Yaswen P., Khoshdeli M., et al. Overexpression of CD36 in mammary fibroblasts suppresses colony growth in breast cancer cell lines. Biochemical and biophysical research communications. 2020;526(1):41–47. doi: 10.1016/j.bbrc.2020.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang R., Tao B., Fan Q., Wang S., Chen L., Zhang J., et al. Fatty-acid receptor CD36 functions as a hydrogen sulfide-targeted receptor with its Cys333-Cys272 disulfide bond serving as a specific molecular switch to accelerate gastric cancer metastasis. EBioMedicine. 2019;45:108–123. doi: 10.1016/j.ebiom.2019.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan J., Fan Z., Wang Z., Dai Q., Xiang Z., Yuan F., et al. CD36 mediates palmitate acid-induced metastasis of gastric cancer via AKT/GSK-3β/β-catenin pathway. Journal of experimental & clinical cancer research : CR. 2019;38(1):52. doi: 10.1186/s13046-019-1049-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng W.W., Wilkins O., Bang S., Ung M., Li J., An J., et al. CD36-Mediated metabolic rewiring of breast cancer cells promotes resistance to HER2-targeted therapies. Cell Rep. 2019;29(11) doi: 10.1016/j.celrep.2019.11.008. 3405-20.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun Q., Zhang W., Wang L., Guo F., Song D., Zhang Q., et al. Hypermethylated CD36 gene affected the progression of lung cancer. Gene. 2018;678:395–406. doi: 10.1016/j.gene.2018.06.101. [DOI] [PubMed] [Google Scholar]
- 21.Chen Z., Yu M., Yan J., Guo L., Zhang B., Liu S., et al. PNOC expressed by B cells in cholangiocarcinoma was survival related and LAIR2 could Be a T cell exhaustion biomarker in tumor microenvironment: characterization of immune microenvironment combining single-cell and bulk sequencing technology. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.647209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu J., Chen Y., Zhang X., Guo J., Xu K., Li L. A novel prognostic model based on single-cell RNA sequencing data for hepatocellular carcinoma. Cancer Cell Int. 2022;22(1):38. doi: 10.1186/s12935-022-02469-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mei J., Liu Y., Xu R., Hao L., Qin A., Chu C., et al. Characterization of the expression and prognostic value of 14-3-3 isoforms in breast cancer. Aging. 2020;12(19):19597–19617. doi: 10.18632/aging.103919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen X., Su C., Ren S., Zhou C., Jiang T. Pan-cancer analysis of KEAP1 mutations as biomarkers for immunotherapy outcomes. Ann. Transl. Med. 2020;8(4):141. doi: 10.21037/atm.2019.11.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang T., Chen X., Su C., Ren S., Zhou C. Pan-cancer analysis of ARID1A alterations as biomarkers for immunotherapy outcomes. J. Cancer. 2020;11(4):776–780. doi: 10.7150/jca.41296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J., Wen T., Li Z., Che X., Gong L., Jiao Z., et al. CD36 upregulates DEK transcription and promotes cell migration and invasion via GSK-3β/β-catenin-mediated epithelial-to-mesenchymal transition in gastric cancer. Aging. 2020;13(2):1883–1897. doi: 10.18632/aging.103985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leclerc J., Flament C., Lovecchio T., Delattre L., Ait Yahya E., Baert-Desurmont S., et al. Diversity of genetic events associated with MLH1 promoter methylation in Lynch syndrome families with heritable constitutional epimutation. Genet. Med. : official journal of the American College of Medical Genetics. 2018;20(12):1589–1599. doi: 10.1038/gim.2018.47. [DOI] [PubMed] [Google Scholar]
- 28.Vander Heiden M.G., DeBerardinis R.J. Understanding the intersections between metabolism and cancer biology. Cell. 2017;168(4):657–669. doi: 10.1016/j.cell.2016.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Q., Luo Q., Halim A., Song G. Targeting lipid metabolism of cancer cells: a promising therapeutic strategy for cancer. Cancer letters. 2017;401:39–45. doi: 10.1016/j.canlet.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Snaebjornsson M.T., Janaki-Raman S., Schulze A. Greasing the wheels of the cancer machine: the role of lipid metabolism in cancer. Cell Metabol. 2020;31(1):62–76. doi: 10.1016/j.cmet.2019.11.010. [DOI] [PubMed] [Google Scholar]
- 31.Silverstein R.L., Febbraio M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci. Signal. 2009;2(72):re3. doi: 10.1126/scisignal.272re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tandon N.N., Lipsky R.H., Burgess W.H., Jamieson G.A. Isolation and characterization of platelet glycoprotein IV (CD36) J. Biol. Chem. 1989;264(13):7570–7575. [PubMed] [Google Scholar]
- 33.Liu C., Zhou X., Zeng H., Wu D., Liu L. HILPDA is a prognostic biomarker and correlates with macrophage infiltration in pan-cancer. Frontiers in oncology. 2021;11 doi: 10.3389/fonc.2021.597860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 35.Ohtani H. Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in human colorectal cancer. Cancer Immun. 2007;7:4. [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshihara K., Shahmoradgoli M., Martínez E., Vegesna R., Kim H., Torres-Garcia W., et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat. Commun. 2013;4:2612. doi: 10.1038/ncomms3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuijpers M.J., de Witt S., Nergiz-Unal R., van Kruchten R., Korporaal S.J., Verhamme P., et al. Supporting roles of platelet thrombospondin-1 and CD36 in thrombus formation on collagen. Arterioscler. Thromb. Vasc. Biol. 2014;34(6):1187–1192. doi: 10.1161/ATVBAHA.113.302917. [DOI] [PubMed] [Google Scholar]
- 38.Rabold K., Netea M.G., Adema G.J., Netea-Maier R.T. Cellular metabolism of tumor-associated macrophages - functional impact and consequences. FEBS Lett. 2017;591(19):3022–3041. doi: 10.1002/1873-3468.12771. [DOI] [PubMed] [Google Scholar]
- 39.Vitale I., Manic G., Coussens L.M., Kroemer G., Galluzzi L. Macrophages and metabolism in the tumor microenvironment. Cell Metabol. 2019;30(1):36–50. doi: 10.1016/j.cmet.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 40.Su P., Wang Q., Bi E., Ma X., Liu L., Yang M., et al. Enhanced lipid accumulation and metabolism are required for the differentiation and activation of tumor-associated macrophages. Cancer Res. 2020;80(7):1438–1450. doi: 10.1158/0008-5472.CAN-19-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng C., Michaels J., Scheinfeld N. Alitretinoin: a comprehensive review. Expet Opin. Invest. Drugs. 2008;17(3):437–443. doi: 10.1517/13543784.17.3.437. [DOI] [PubMed] [Google Scholar]
- 42.Pang J., Yu Q., Chen Y., Yuan H., Sheng M., Tang W. Integrating Single-cell RNA-seq to construct a Neutrophil prognostic model for predicting immune responses in non-small cell lung cancer. J. Transl. Med. 2022;20(1):531. doi: 10.1186/s12967-022-03723-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sinner B. [Perioperative dexamethasone] Anaesthesist. 2019;68(10):676–682. doi: 10.1007/s00101-019-00672-x. [DOI] [PubMed] [Google Scholar]
- 44.Chakraborty S., Doktorova M., Molugu T.R., Heberle F.A., Scott H.L., Dzikovski B., et al. How cholesterol stiffens unsaturated lipid membranes. Proceedings of the National Academy of Sciences of the United States of America. 2020;117(36):21896–21905. doi: 10.1073/pnas.2004807117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng X., Wei J., Ge Q., Xing D., Zhou X., Qian Y., et al. The optimized drug delivery systems of treating cancer bone metastatic osteolysis with nanomaterials. Drug Deliv. 2021;28(1):37–53. doi: 10.1080/10717544.2020.1856225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang X., Zhou Q., Li X., Gan X., Liu P., Feng X., et al. Insights into aptamer-drug delivery systems against prostate cancer. Molecules. 2022;27(11) doi: 10.3390/molecules27113446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mohammadi K., Sani M.A., Azizi-Lalabadi M., McClements D.J. Recent progress in the application of plant-based colloidal drug delivery systems in the pharmaceutical sciences. Adv. Colloid Interface Sci. 2022;307 doi: 10.1016/j.cis.2022.102734. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analysed during the current study are available in the UCSC repository and GEO database, including TCGA-PANCAN, GSE18842, GSE127465 and GSE73403.