Abstract
Flavonoids are a highly abundant class of secondary metabolites present in plants. Isoflavonoids, in particular, are primarily synthesized in leguminous plants within the subfamily Papilionoideae. Numerous reports have established the favorable role of isoflavonoids in preventing a range of human diseases. Among the isoflavonoid components, glyceollins are synthesized specifically in soybean plants and have displayed promising effects in mitigating the occurrence and progression of breast and ovarian cancers as well as other diseases. Consequently, glyceollins have become a sought-after natural component for promoting women's health. In recent years, extensive research has focused on investigating the molecular mechanism underlying the preventative properties of glyceollins against various diseases. Substantial progress has also been made toward elucidating the biosynthetic pathway of glyceollins and exploring potential regulatory factors. Herein, we provide a review of the research conducted on glyceollins since their discovery five decades ago (1972–2023). We summarize their pharmacological effects, biosynthetic pathways, and advancements in chemical synthesis to enhance our understanding of the molecular mechanisms of their function and the genes involved in their biosynthetic pathway. Such knowledge may facilitate improved glyceollin synthesis and the creation of health products based on glyceollins.
Keywords: Glyceollins, Isoflavonoids, Soybean, Breast cancer, Angiogenesis, Biosynthesis
1. Introduction
Isoflavonoids, a unique class of plant secondary metabolites, possess a wide range of biological functions in both plant defense against pathogens [1] and improvement of human health [2]. Plant species that contain flavonoids are present in almost all families of the plant kingdom [3], but isoflavonoids are predominantly synthesized in species in the subfamily Papilionoideae of Fabaceae, distinguishing them from other subclasses of flavonoids [4]. Isoflavonoids can be divided into several groups based on their structural skeletons, including isoflavone, isoflavanone, isoflavanonol, isoflavan, isoflav-3-ene, isoflavan-4-ol, rotenoid or pterocarpan (Fig. 1), with isoflavones being the most abundant [5]. However, the types and quantities of isoflavonoids can vary greatly among different plant species.
Fig. 1.
The structures of isoflavonoids and glyceollins.
Soybean, which is a member of the subfamily Papilionoideae, is one of the most important crop plants in the world for protein and oil production, and is known for its high content of isoflavonoids [6]. Daidzin and genistin are the primary isoflavonoids found in soybean seeds [7], making it a prominent source of these compounds in people's diets, particularly in East Asian countries [8]. Even within the Fabaceae family, soybean contains a relatively high amount of isoflavonoids and is easily accessible for natural isoflavonoid production.
Apart from daidzein and genistein, glyceollins, a class of pterocarpan derivatives from daidzein, are synthesized only when germinated soybean seeds are stimulated by pathogens [9]. The first glyceollin chemical structure was discovered in soybean in 1972, but was initially misidentified as hydroxyphaseollin [10]. The other two main isomers were identified four years later [11], and were eventually named glyceollins in 1978 [12]. At least six natural glyceollins have now been identified, and they are believed to inhibit the growth of fungi in plants, serving as soybean phytoalexins [13]. Additionally, their effective antiestrogenic roles in animals have attracted special attention in medicine [14]. Consequently, a plethora of preclinical research has been conducted on the medicinal roles of glyceollins, and their recent advances in human health have been summarized in several reviews [[15], [16], [17], [18], [19]].
As a primary phytoalexin in soybeans, glyceollins are not produced under normal conditions. They are only synthesized when soybean tissues are infected by fungi or exposed to other elicitors [20,21]. Despite the limited production of glyceollins in soybeans, their potential as estrogen receptor (ER)-independent inhibitors for several cancers has generated significant interest. Some chemists have attempted to develop chemical synthesis methods for glyceollin production, achieving yields at the multigram level [22]. However, complex synthetic pathways, environmental pollution, and high manufacturing cost associated with chemical synthesis remain unresolved issues, leading researchers to explore alternative methods for glyceollin production. Recent developments in the field of biosynthesis have provided new possibilities for large-scale production of glyceollins [23,24].
This review provides an overview of the current knowledge on the pharmacological effects of glyceollins and related mechanisms, with a focus on their target genes and pathways, including ER and some other newly identified proteins. The de novo biosynthetic pathway of glyceollins in soybeans and related transcription regulation factors are also summarized and reviewed. Additionally, all reported elicitors that stimulate glyceollin synthesis are listed, and the advances in chemical synthesis and possible glyceollin production by microorganisms are discussed.
2. Glyceollins play various medicinal roles through multiple signaling pathways
2.1. ER signaling pathway
Isoflavonoids are compounds that have been found to have estrogen-mimicking properties since the 1950s [25,26]. However, it was not until the identification of two estrogen receptors, ERα and ERβ, that more detailed research on their mechanisms of action was carried out [27,28]. Many studies have evaluated the binding affinity of various isoflavonoids to these two receptors [[29], [30], [31], [32]]. While many isoflavonoids were found to have ER binding activity, their affinity was much lower than that of the natural estrogen estradiol.
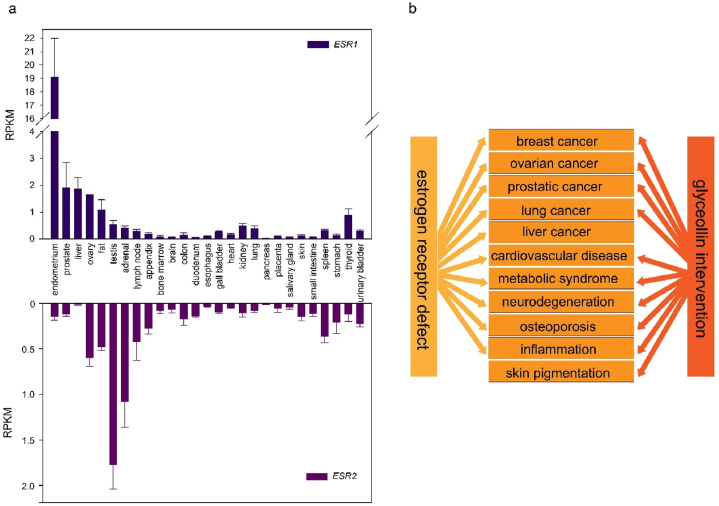
Both ERα and ERβ in humans are expressed in multiple tissues, but with different patterns (the expression data from genes with IDs of 2099 and 2100 were in the NCBI database). For example, the gene ESR1 (encoding ERα) is expressed at much higher levels in the endometrium than in all other tissues, and is also expressed at relatively high levels in the liver, ovary, prostate, and fat. In contrast, the gene ESR2 (encoding ERβ) is expressed highest in the testis and has relatively high expression in the adrenal, ovary, fat, and lymph node tissues (Fig. 2a) [33]. Additionally, ERα and ERβ respond differently to various estrogens or ER inhibitors [34]. These differences make the ER pathway complex and influenced by multiple factors.
Fig. 2.
The relationship between estrogen receptors and glyceollins. (a) The expression levels of both estrogen receptor alpha (ESR1) and estrogen receptor beta (ESR2) in different human tissues. (b) The overlapped diseases caused by estrogen receptor defect and prevented by glyceollins.
Defects in ER signaling can lead to many diseases, including breast cancer [35], ovarian cancer [36], prostatic cancer [37], lung cancer [38], liver cancer [39], cardiovascular disease [40], metabolic syndrome [41], neurodegeneration [42], inflammation [43], and osteoporosis [44]. Detailed mechanisms of ER signaling can be found in various reviews [45,46].
Glyceollins, a group of pterocarpan-type isoflavonoids, have been shown to exhibit binding affinity to ERs [[47], [48], [49]]. Compared to other isoflavonoids, such as daidzein and genistein, their competitive binding affinity for ERs was significantly lower. However, glyceollin I demonstrated superior affinity to ERα when compared to ERβ [47,49]. Additionally, glyceollin I exhibited a much higher absorption rate than daidzein or genistein [50].
These ER-binding isoflavonoids are typically classified into two groups: agonists or antagonists. ER bound to glyceollin I inhibits the gene expression dependent on two known estrogen response element (ERE) sequences, indicating that glyceollin I is a specific ER antagonist [49]. Among the three main glyceollins, glyceollin I demonstrated higher ER-binding activity than glyceollin II or III and was considered the primary active molecule in the glyceollin mixture [51]. Glyceollins have demonstrated remarkable antiestrogenic properties, while their precursor daidzein exhibits the opposite effect [47]. The antiestrogenic effects of glyceollins may be attributed to their prenylated structure. Some evidence suggests that prenylated isoflavonoids more frequently function as antagonists, while non-prenylated isoflavonoids prefer to act as agonists [52]. The known health benefits of glyceollins include the prevention and intervention of breast cancer, ovarian cancer, prostate cancer [[53], [54], [55]], cardiovascular disease [56], regulation of glucose metabolism [57], treatment of osteoporosis [58], and improvement of skin pigmentation [59], among others. Notably, many diseases prevented by glyceollins overlap with those caused by ER defects (Fig. 2b). Therefore, it is believed that glyceollins exert important medicinal functions through the ER signaling pathway. Further investigations into the detailed mechanisms of these functions have revealed that the ER is a key target of glyceollins.
Breast cancer is a prevalent malignancy affecting women globally and poses a significant threat to their health. It is categorized into three subtypes: estrogen/progesterone receptor-positive, HER2-positive, or triple-negative [60]. The majority, approximately 70 %, of breast cancer patients fall under the first subtype, and endocrine therapy serves as the standard treatment approach [61]. Furthermore, approximately 50 % of ovarian cancers can also be managed with endocrine therapy [62]. Endocrine therapy comprises two oral medications: the ER inhibitor [63] or estrogen-synthesis inhibitor [64]. However, prolonged use of these inhibitors may lead to drug resistance [65]. Hence, the discovery of potent and safer ER inhibitors is crucial for the prevention and treatment of breast cancer. Despite having ER-binding activity, most isoflavonoids, including daidzein and genistein, may act as negative regulators for ER-positive breast cancer [66]. Consequently, mixed soybean isoflavonoids should be cautiously considered for breast cancer patients. Current evidence suggests that ERα plays a significant role in breast cancer proliferation compared to ERβ [67,68]. Glyceollins, as rare products with higher affinity to ERα than ERβ, show specific antiestrogenic activity and are among the most promising natural products for intervention in breast or ovarian cancer. Currently, most research on the function of glyceollins revolves around breast or ovarian cancer and is dependent on the ER pathway.
The MCF-7 cell line is a widely utilized ER-positive breast cancer cell line whose proliferation is dependent on ER. Administration of exogenous estradiol significantly increases the proliferation of these cells, but glyceollin supplementation has been shown to effectively reduce the proliferation induced by estradiol [47]. When these cells were transplanted into ovariectomized (ovx) mice, exogenous estradiol was found to increase the growth of tumor tissue, while glyceollin-feeding significantly inhibited tumor growth. The changes in the expression of the progesterone receptor (PgR) protein, a key target of ER, confirmed that glyceollins inhibit the growth of breast cancer cells via the ER pathway [48,51,54]. Glyceollins have also been shown to have antagonistic effects on the increase in uterus weight and volume induced by estradiol in mice. Similarly, in postmenopausal female monkeys, glyceollins inhibited the effect of estradiol by decreasing the expression of the PgR gene, which in turn increased the proliferation of mammary duct cells [69]. Furthermore, glyceollins have been shown to inhibit the growth of explanted ovarian BG-1 cells induced by estradiol [51,54]. In addition to breast and ovarian cancer cell proliferation, glyceollins also inhibit the proliferation of human prostate cancer cells LNCaP through the ER pathway [55]. A cluster of noncoding RNAs was activated and promoted the expression of ESR1 in LTED breast cancer cells, and glyceollins could inhibit the expression of these noncoding RNAs [70]. Glyceollin I has been shown to significantly inhibit the expression of BCL2 but increase the expression of NGFR in MCF-7 cells [48]. Moreover, explanted MCF-7 cells in mice also expressed lower levels of BAG1, TFF1, BCL2, CCND1, and RAC2 after glyceollin treatment, revealing its ability to promote cell apoptosis [51]. However, whether the expression of these genesis ER-dependent or ER-independent has yet to be determined. In addition to the inhibition of ER-dependent breast and ovarian cancer cell proliferation, glyceollins also promote ER-dependent osteogenesis [58]. A large-scale molecular docking analysis predicted that glyceollin III could bind to sex hormone-binding globulin, which is essential for estrogen transport in blood vessels [71]. This result suggests that glyceollin I is not the only effective product in the glyceollin mixture.
2.2. ER-independent signaling pathways in breast cancer
Letrozole, the most commonly used estrogen synthesis inhibitor, has been found to reduce ER-dependent cancer cell proliferation. In contrast, letrozole-resistant breast cancer cells, specifically LTLT-Ca cells, express higher levels of ZEB1 and EGFR, but lower levels of ESR1 and TFF1 than letrozole-sensitive AC-1 cells. This suggests that the resistant cell line has obtained the ability to proliferate independently of ER signaling. However, glyceollin treatment was still effective in decreasing the proliferation of LTLT-Ca cells. Glyceollin not only decreased the expression of ZEB1 and EGFR, but also further decreased the expression of ESR1 and TFF1, indicating that the regulatory effect of glyceollins is both dependent and independent of ER signaling. Moreover, glyceollins were able to reverse the epithelial to mesenchymal transition (EMT) process, possibly through a significant increase in the E-cadherin level and a decrease in the N-cadherin level. These results were further confirmed in LTLT-Ca explanted tumor tissue in mice [72]. Molecular docking analysis predicts that glyceollins can bind to TGFBR1 and TGFBR2, which are two receptors of TGFβ, a key protein in EMT [73].
In the letrozole-resistant breast cancer cell line T47DaromLR, the levels of HER2, EGFR, and MAPK were higher than those in the letrozole-sensitive cell line T47Darom. However, glyceollin treatment was found to inhibit the proliferation of resistant cells without changing the levels of HER2 and MAPK. Glyceollins inhibited the cell entry into G2 phase and accelerated apoptosis [74]. Molecular docking analysis further suggests that glyceollins can bind to the aryl hydrocarbon receptor (AhR). Subsequent experiments in triple-negative breast cancer MDA-MB-231 cells confirmed that glyceollins activate the gene expression of AhR target genes, such as CYP1A1 and CYP1B1, while decreasing the cell migration ability. Meanwhile, the expression of N-cadherin (CDH2) and CCL2 decreased, but the expression of PDLIM4 increased after glyceollin treatment [75].
2.3. HIF-1α and VEGF signaling pathways
Glyceollins have been found to inhibit the proliferation of endothelial progenitor cells (EPCs), which are capable of differentiating into mature endothelial cells for neovascularization. Additionally, glyceollins inhibit the expression of SDF-1α, which is known to play a crucial role in the migration of endothelial progenitor cells. In mixed cultures of EPCs and human umbilical vein endothelial cells, glyceollin treatment resulted in a decrease in the number of tube structures similar to vessels. Furthermore, glyceollin treatment resulted in a decrease in the transcription levels of SDF-1, CXCR4, ANG-1, TIE-2, and eNOS, as well as a decrease in the phosphorylation levels of eNOS, Akt, and ERK induced by SDF-1α or VEGF. In experiments with mice, glyceollin administration led to a decrease in the proliferation of lung cancer LLC cells that had been transplanted, a reduction in vessel density, and a decrease in the number of EPCs when compared to controls [76,77]. In vitro and in vivo experiments have both confirmed the efficacy of glyceollins in inhibiting angiogenesis. The proliferation and migration of human umbilical vein endothelial cells can be induced by VEGF or bFGF, but glyceollins have been found to reduce the VEGF-induced phosphorylation of VEGFR2 and bFGF-induced phosphorylation of bFGFR1, as well as the phosphorylation of ERK, JNK, p38 MAPK, Akt and FAK [77]. Hypoxia-induced gastric cancer MKNI cells have shown increased expression of VEGF, CA9 and ALDOC, but glyceollins have been found to inhibit this increase. Additionally, glyceollins have been found to decrease the protein level of HIF-1α, without changing its transcription level. The inhibition of HIF-1α by glyceollins has been found to be independent of the p53 and IGF-1 pathways but rather occurs through a decrease in HIF-1α synthesis via the PI3K-Akt-mTOR pathway. Furthermore, glyceollins have been found to decrease the phosphorylation levels of mTOR, p70S6K and Akt, while a PI3K inhibitor can reverse the glyceollin-induced inhibition of HIF-1α protein synthesis. In addition, the results from molecular docking analysis indicated that glyceollins had a high affinity for HSP90, which is known to interact with HIF-1α to prevent its stabilization. Experimental findings confirmed that glyceollins blocked the interaction between HSP90 and HIF-1α, leading to the inhibition of the growth of lung cancer LLC cells implanted in mice. Furthermore, glyceollins reduced the phosphorylation level of mTOR and Akt, resulting in a lower vessel density in tumor tissue [78]. Similarly, the phosphorylation levels of p70S6K, pS6, eIF4, and eEF2K in MCF-7 or T47D breast cancer cells were also reduced after glyceollin treatment [79]. Moreover, in arterial smooth muscle cells, the induction of proliferation by PDGF-BB was attenuated with glyceollin treatment, which led to lower levels of CDK2 and cyclin E. The treatment also increased the levels of p27 and p53 and decreased the phosphorylation levels of PDGFR-B, PLCg1, Akt, and ERK, which were induced by PDGF-BB. Additionally, glyceollin treatment inhibited the increased level of ROS induced by PDGF-BB and decreased the synthesis of actin filaments, which facilitated cell migration. Overall, glyceollins exhibited an antagonistic effect in this regard [80].
2.4. AMPK and insulin signaling pathways
An in vitro study revealed the inhibitory effect of glyceollin I on the activity of ACAT-1 and ACAT-2, which are both cholesterol synthetases in humans [81]. This implies that glyceollin may play a potential role in lipid metabolism. Treatment with glyceollin increased the glucose uptake ability of adipocytes differentiated from 3T3-L1 fibroblasts in an insulin-dependent manner [82,83]. PPAR-γ can enhance cells' insulin sensitivity to increase insulin-dependent glucose uptake and triacylglycerol storage. However, glyceollin treatment did not increase the activity of PPAR-γ or the level of triacylglycerol, suggesting that glyceollin's effect is independent of PPAR-γ. The synthesis of insulin in insulinoma Min6 cells was stimulated by glyceollins only under high glucose conditions, and this induction was not affected by palmitate, an insulin synthesis inhibitor. Additionally, glyceollins inhibited the apoptosis induced by palmitate, possibly by decreasing the expression of endoplasmic reticulum stress response genes such as XBP-1, ATF-4, ATF-6, and CHOP. The level of GLP-1 was increased in the enteroendocrine cell line NCI–H716 under high glucose conditions, and glyceollin treatment further increased its level [83].
Glyceollins increased insulin content and decreased free fatty acid levels in the serum of a type II diabetic mouse model, along with an increase in the number of pancreatic β cells. In mouse liver, glyceollin feeding increased triglyceride and glycogen content, as well as the phosphorylation levels of Akt, AMPK, and ACC, but decreased PEPCK expression [57]. The fasting blood glucose level of ZDSD/Pco rats significantly decreased and adipocytes expressed higher levels of GLUT1 and GLUT4 after glyceollin feeding [82]. Glyceollin feeding reduced blood glucose levels and insulin content in C57BL/KsJ-db/db mice and increased high-density lipoprotein cholesterol content. In skeletal muscle cells, the phosphorylation level of JNK decreased, but AMPK, ACC, and Akt phosphorylation increased after glyceollin treatment. In rat L6 myotube cells, glucose uptake and fatty acid oxidation increased after glyceollin treatment, in a AMPK phosphorylation-dependent manner. Glyceollins restored endoplasmic reticulum stress-induced AMPK, ACC, and IRS-1 phosphorylation inhibition and improved glucose uptake ability. The knockout of CaMKK or addition of PI3K or CaMKK inhibitor could dismiss glyceollin's role in blood glucose regulation [84].
A high-fat diet caused an increase in low-density lipoprotein (LDL), very low-density lipoprotein (VLDL), and total cholesterol in the serum, as well as cholesterol accumulation in the liver of Golden Syrian hamsters. However, glyceollin feeding reversed the process and inhibited the expression of LPL [85]. Molecular docking analysis predicted that glyceollin II could bind to LXRα and LXRβ. Genes related to the LXR pathway, including ABCG1 and APOA1, also showed increased expression. Furthermore, glyceollins increased the expression of genes involved in cholesterol homeostasis, including c-FOS, IGF1, ABCA1, ABCA2, RUNX2, and OPN [58].
2.5. Toll-like receptor and HMGB1 signaling pathways
After lipopolysaccharide (LPS) induction, the level of HMGB1 in human umbilical vein endothelial cells significantly increased, along with TLR2 and TLR4. HMGB1 reduced the phosphorylation level of MAPK p38, while increasing the content of ICAM-1, VCAM-1 and E-selectin, thus promoting leukocyte migration to the site of inflammation. Moreover, HMGB1 elevated the levels of TNFα and IL-6 cytokines, and NF-κB and ERK proteins. Similar results were observed in a CLP-induced sepsis mouse model. However, glyceollin treatment in human umbilical vein endothelial cells or CLP-induced mice led to a lower level of HMGB1, restored the changes caused by increased HMGB1, and ultimately improved the survival rate of mice [86].
In mice feda high-fat diet, the expression levels of TLR3 and TLR4 in the spleen were increased, which further elevated the NF-κB level and ultimately led to increased levels of IL-17 and TNFα, along with a reduction in naive T cells. However, glyceollins were found to counteract the effects induced by a high-fat diet. Molecular docking analysis predicted that glyceollins could bind directly to TLR3 and TLR4, thereby preventing the inflammatory response through the Toll-like receptor signaling pathway [87].
2.6. Nrf2 and NF-κB signaling pathways
Glyceollin treatment in mouse hepatoma cells Hepa1c1c7 or BPRc1 increased the ratio of Nrf2 translocation into the nucleus, resulting in the activation of gene transcription dependent on the promoter ARE element. Additionally, the protein level and activity of NQO1, HO-1, and GR increased as well. Molecular docking analysis predicted that glyceollin I could bind to Keap1, which interacted with Nrf2, blocking its translocation to the cell nucleus. Furthermore, glyceollins increased the phosphorylation level of Akt, but a PI3K inhibitor abolished glyceollin-induced Nrf2 translocation and downstream gene expression [[88], [89], [90]]. After glyceollin treatment, these cells' extracellular glutathione levels increased. Glyceollins also increased the levels of QR and γGCS but decreased HIF-1α levels [88]. Glyceollins increased the ROS level and promoted cell apoptosis. Using NAC, an antioxidant, to eliminate ROS could decrease glyceollin-induced apoptosis. Glyceollin increased the levels of caspase-3 and cytochrome C while decreasing BCL2 levels. Additionally, glyceollin decreased cyclin D, cyclin E, CDK2, and CDK4 levels, increased CIP1 and KIP1 levels, and resulted in G1 arrest [89]. In addition, 7,12-dimethylbenz(a)anthracene (DMBA) increased the tumor occurrence and death rate of C57BL/6J female mice. It increased the expression of MUC1, and increased the levels of the phase I detoxifying enzymes CYP1A1 and CYP1B1, but inhibited the phase II detoxifying pathway by blocking Nrf2 translocation and increasing Keap1 expression. Glyceollin feeding efficiently reduced DMBA-induced changes and recovered multiple parameters involved in oxidative stress and hepatic injury in serum [91]. Glutamine induced excitotoxicity in primary cortical neuron cells in C57BL/6 mice and inhibited the proliferation HT22 of hippocampal neuronal cells. Glyceollins could dismiss the damaging role of glutamine by interacting with the Nrf2 protein. ERK or JNK inhibitors could eliminate the function of glyceollins, but p38 or PI3K inhibitors could not. Glyceollins can decrease the level of ROS through the ERK or JNK pathway. Glyceollins significantly increased the levels of Nrf2 and HO-1 through the ERK- and JNK-dependent pathways. Scopolamine reduced the learning and memory ability of mice, and glyceollins could restore these abilities by interacting with Nrf2 [92]. Glyceollin feeding increased the tumor size and volume of colorectal cancer HCT116 cells explanted in mice. The function of glyceollins in tumor growth was p53 dependent, and the proliferation of p53-deleted HCT116 cells was not influenced by glyceollins. Glyceollins increased the levels of Nrf2, HO-1, and NQO1 in p53-wild type cells but did not change their expression in p53-deleted cells [93]. Glyceollins have been demonstrated to exhibit inhibitory effects on LPS-induced IL-6 and COX-2 elevation in mouse macrophage RAW264.7. Additionally, LPS induction was shown to increase the production of NO, but this effect was reversed by glyceollin treatment. In addition, glyceollins were found to increase HO-1 expression. LPS exposure resulted in an increase in the content and phosphorylation level of the NF-κB p65 subunit, but this response was attenuated by glyceollin treatment [94,95]. Glyceollins were also found to repress LPS-induced cytokines IL-1β, IL-18, and TNFα, and to inhibit the phosphorylation level of IKK and downstream IKB, while promoting the production of anti-inflammatory cytokines such as IL-10 [94]. In a mouse model of ulcerative colitis induced by dextran sulfate sodium (DSS), epithelial damage occurred, accompanied by an increase in the serum levels of TNFα and IL6, as well as NO. Furthermore, the NF-κB p65 subunit and iNOS also increased. However, glyceollins were able to attenuate the DSS-induced inflammatory response and lipid peroxidation [96].
2.7. Melanogenesis pathway
The addition of α-MSH protein was found to promote the production of melanin in B16 melanoma cells. However, the use of glyceollin treatment could restore melanin levels to those seen in α-MSH nontreated conditions. Both in vitro and in vivo studies have shown that glyceollins can inhibit the activity of tyrosinase. At high concentrations, glyceollins can decrease the levels of TRP1 and MITF, as well as cAMP independent of forskolin stimulation [59]. In addition, glyceollins can also reduce the accumulation of melanin in zebrafish embryos by reducing tyrosinase activity. Glyceollin treatment has been found to reduce the expression of SCF and SOX10, decrease downstream c-kit and p44/42 ERK phosphorylation, and to downregulate the cAMP level [97].
2.8. RhoA pathway
Sodium fluoride (NaF) has been found to induce vasoconstriction in rats. However, glyceollin treatment has been shown to reduce NaF-induced vasoconstriction. This effect is attributed to the inhibitory action of glyceollins on the phosphorylation level of MLC20 protein, which is a known biomarker of vasoconstriction. Furthermore, glyceollins have been found to suppress the expression of RhoA and downstream MYPT1 phosphorylation [56].
2.9. Multiple omics analysis
It is widely acknowledged that the effects of glyceollins in cells are diverse and intricate, and transcriptome and proteome results have led to a comprehensive understanding of cell signaling changes after glyceollin treatment. In female nude mice, glyceollins have been shown to inhibit the proliferation of triple-negative breast cancer cells, MDA-MB-231 or MDA-MB-468, independent of the ER pathway. Microarray analysis revealed that glyceollin treatment caused a decrease in the expression of many cancer-promoting microRNAs, whereas the expression of several cancer-inhibiting microRNAs increased. Additionally, the proteomic results demonstrated that glyceollin led to an increase in NME1 and a decrease in vimentin, both of which resulted in a lower risk of cancer cell migration [98]. The effect of glyceollins on the mammary gland was evaluated in postmenopausal cynomolgus monkeys, and showed a significant decrease in the levels of LDL, VLDL, and total cholesterol in serum after glyceollin feeding. Transcriptome analysis showed significant changes in the expression of many genes involved in fatty acid metabolism, particularly in the triglyceride synthetic pathway. The genes involved in the PPAR pathway, including ADIPOQ, DGAT2, GPD1, GYS1, LEP, LPIN1, PLIN, and PPARγ, were significantly upregulated [99]. Transcriptome analysis of ovx CFW mouse brain tissue with glyceollin treatment revealed more than 200 differentially expressed genes involving the cranial nerve. Similar results were obtained from transcriptome analysis of the other samples [100,101]. Glyceollins were shown to inhibit the proliferation of both ER-positive and ER-negative breast cancer cells, and transcriptome analysis indicated that glyceollin treatment decreased the expression of genes involved in the cell cycle. Both ER-dependent and ER-independent pathways were enriched, with the FOXM1-related pathway involving most ER-dependent genes and the HIF-1α-related pathway involving most ER-independent genes [53].
For an easier and better understanding of the signaling pathways involved in glyceollin function and their potential crosstalk described above, we presented a complete interaction network of all related proteins in this section by database STRING [102] (Fig. 3 and Supplementary file 1), and listed all the molecular pathways and pharmacological activities of glyceollins in Table 1.
Fig. 3.
The interaction network of proteins regulated by glyceollins.
Table 1.
The diseases prevented by glyceollins and related signaling pathways.
| Diseases | Signaling pathways |
|---|---|
| Breast cancer | Estrogen-receptor dependent and independent pathways |
| Ovarian cancer | Estrogen-receptor pathway |
| Prostatic cancer | Estrogen-receptor pathway |
| Lung cancer | HIF-1α and VEGF signaling pathways |
| Cardiovascular disease | RhoA pathway |
| Metabolic syndrome | AMPK and insulin signaling pathways |
| Neurodegeneration | Nrf2 and NF-κB signaling pathways |
| Osteoporosis | Estrogen-receptor pathway |
| Inflammation | Toll-like receptor and HMGB1 signaling pathways |
| Skin pigmentation | Melanogenesis pathway |
3. De novo biosynthetic pathways of glyceollins in soybean
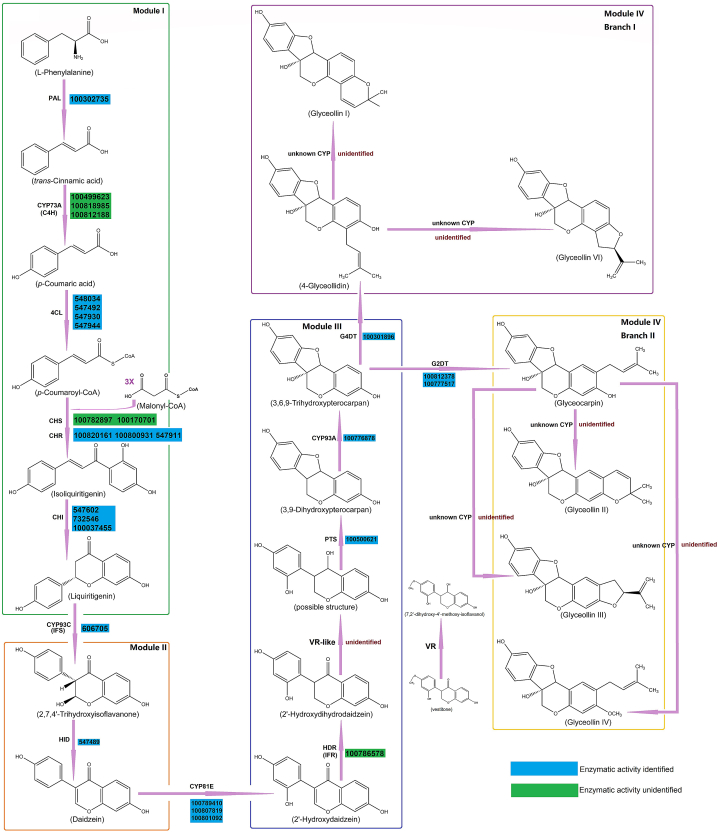
To provide a more detailed overview of the glyceollin synthetic pathway in soybean, we divided the complex pathways into four modules (Fig. 4). The first module involves the synthetic pathway of liquiritigenin, which is a shared route from flavonoids to isoflavonoids [103]. As a type of isoflavonoid, glyceollin synthesis shares this module with some other isoflavonoids. This module comprises six enzyme-dependent synthesis steps, which are presented in Fig. 4 and Table 2. The pathway starts with phenylalanine, and the first enzyme involved is phenylalanine ammonia lyase. In soybean, there are eight candidate genes that encode this enzyme, but only one of the encoded proteins has confirmed enzymatic activity [104]. This enzyme catalyzes the generation of trans-cinnamate (trans-cinnamic acid). The second enzyme is a trans-cinnamate 4-monooxygenase, which catalyzes the conversion of trans-cinnamate to p-coumaric acid. This enzyme belongs to the cytochrome P450 gene family and 73A subfamily, and the reaction is NADPH-dependent [105]. In soybean, there are three candidate genes that encode this enzyme, but to date, none of the proteins encoded by these genes have confirmed enzymatic activity. The third enzyme is a 4-coumarate--CoA ligase, which catalyzes the conversion of p-coumaric acid to p-coumaroyl-CoA. The reaction is ATP-dependent and requires CoA supplementation. There are possibly up to twenty-two candidate genes in soybean that encode this enzyme, and the enzymatic activity and substrate specificity of four gene-encoded proteins were identified by recombinant expression in Escherichia coli (E. coli) [106]. The fourth enzyme is a chalcone synthase, and in soybean, there are thirteen candidate genes that encode this protein, with two genes likely participating in isoflavonoid synthesis [107]. However, none of the encoded proteins have confirmed enzymatic activity. This reaction in this step requires the participation of three malonyl-CoAs. The fifth enzyme is a chalcone reductase, and in soybean, there are eight candidate genes that encode this enzyme, and the enzymatic activity of proteins encoded by three of the eight genes has been confirmed by recombinant expression in E. coli [108]. This reaction is also NADPH dependent. As the product from the fourth step is quickly transformed and difficult to identify, some references combine the fourth and fifth steps into a single step. The sixth enzyme is a chalcone isomerase, which catalyzes the production of liquiritigenin. In soybean, there are four candidate genes that encode this enzyme, and the enzymatic activity of three gene-encoded proteins was confirmed by recombinant expression in yeast [109]. This module is common in most plants, and the genes responsible for these steps in Arabidopsis thaliana were also identified [[110], [111], [112], [113]]. The pathway information was reviewed in previous reviews [[114], [115], [116]], and is not further discussed in this paper.
Fig. 4.
The biosynthetic pathway of glyceollins in soybean.
Table 2.
The candidate genes of soybean involved in glyceollin synthesis.
| Gene name | Candidate gene ID |
|---|---|
| PAL | 100302735; 100,811,101; 100,788,438; 100,787,902; 100,818,777; 100,803,857; 100,801,470; 100,787,872 |
| C4H | 100,499,623; 100,818,985; 100,812,188 |
| 4CL | 548,034; 547,492; 547,930; 547,944; 100,819,192; 100,810,418; 100,811,418; 100,814,237; 100,779,668; 100,780,741; 100,790,396; 100,811,344; 100,783,104; 100,796,728; 100,775,413; 100,804,808; 100,808,944; 100,797,843; 100,777,435; 100,784,234; 100,804,044; 100,801,143 |
| CHS | 100,782,897; 100,170,701; 100,775,264; 114,827,817; 732,575; 106,799,718; 100,791,524; 100,779,649; 100,785,554; 100,809,433; 100,790,997; 100,788,048; 114,827,817 |
| CHR | 100,820,161; 100,800,931; 547,911; 100,194,415; 100,788,544; 100,790,074; 100,787,305; 100,786,750 |
| CHI | 547,602; 100,037,455; 732,546; 732,545 |
| IFS | 606,705; 100,037,450 |
| HID | 547,489; 100,785,409; 100,814,777; 100,813,532; 100,795,872 |
| CYP81E | 100,789,410; 100,807,819; 100,801,092; 100,797,371; 100,812,102; 100,798,269; 100,790,507; 100,784,650; 100,811,727; 100,784,120; 100,804,509 |
| IFR | 100,786,578; 100,775,382; 100,780,465 |
| VR-like | 100,194,416; 100,793,687; 547,660; 100,794,209; 102,659,875; 547,570; 100,796,029; 100,786,878; 548,087; 732,626; 100,778,253; |
| PTS |
100,500,621; 100,817,155; 100,817,686; 100,500,576; 100,790,549; 100,816,095; 100,816,618; 100,500,048; 100,789,492; 100,306,267; 100,775,617; 100,798,988; 100,808,529; 100,781,542 |
| CYP93A |
100,776,878; 100,808,223; 100,797,067; 100,815,706; 100,779,342; 100,805,931; 100,807,524; 100,811,919; |
| G4DT |
100,301,896; 100,786,739; 100,787,246; 100,787,771; 100,777,408; 100,809,827; 100,786,181; 100,778,084; |
| G2DT | 100,812,378; 100,777,517 |
The genes in bold represented that the enzymatic activity of their encoded proteins was identified.
The second module pertains to the pathway from liquiritigenin to daidzein, which serves as the unique substrate for glyceollin production. This pathway involves two enzymatic reactions. The initial enzyme is a 2-hydroxyisoflavanone synthase, also known as IFS or 2-HIS, which belongs to the 93C subfamily of the cytochrome P450 gene family. In soybean, there are two candidate genes responsible for encoding this protein, with the enzymatic activity of one gene-encoded protein already confirmed through recombinant insect cell expression [117]. This reaction is NADPH dependent. The subsequent enzyme is a 2-hydroxyisoflavanone dehydratase, also referred to as HID or HIDH. In soybean, there are five candidate genes responsible for encoding this protein, with the enzymatic activity of one gene-encoded protein already confirmed [118]. Daidzein is one of the primary isoflavonoids found in soybean, and is typically stored in seeds in glycoside forms [119]. Additionally, daidzein can be converted into other isoflavonoids such as vestitone. Therefore, all methods that increase the daidzein content or reduce the proportion of daidzein in the bypass pathway can theoretically enhance the final glyceollin yield. Recently, daidzein was successfully synthesized in budding yeast through the transformation and reconstruction of some Arabidopsis and soybean isoflavonoid pathway genes [120], providing an excellent foundation for further glyceollin biosynthesis.
The third and fourth modules are specific to the production of glyceollins and are also the least understood. The third module comprises a pathway for the synthesis of 3,6,9-trihydroxypterocarpan, which is a common process for all types of glyceollins. This pathway is composed of five steps. The first step involves catalyzing daidzein to 2′-hydroxydaidzein by an isoflavone 2′-hydroxylase, which belongs to the cytochrome P450 family and 81E subfamily. This reaction is NADPH dependent. Soybean has eleven candidate genes that encode this enzyme, and recombinant E. coli expression confirmed the enzymatic activities of three encoded proteins. All three proteins showed high catalytic activity for converting daidzein to 2′-hydroxydaidzein. Additionally, they also displayed weaker activity toward genistein and formononetin [121]. The homologous gene for this enzyme in licorice (GeCYP81E1, NCBI accession: AB001379.1) was also cloned and identified, and the enzymatic activity was confirmed by recombinant yeast microsomes. The recombinant enzyme could convert not only daidzein but also genistein and formononetin to their corresponding 2′-hydroxy forms, but the transformation rates varied [122]. The homologous gene in alfalfa (MtCYP81E7, NCBI accession: 25493449) encoded a protein with weak catalytic activity to convert daidzein to 2′-hydroxydaidzein, but it most frequently utilized formononetin as the substrate [123]. This suggests that the protein isoflavone 2′-hydroxylase in different species have substrate preferences, and the proteins in soybean tend to use daidzein for glyceollin production. The second step involves transforming 2′-hydroxydaidzein to 2′-hydroxy-2,3-dihydrodaidzein by a reductase called IFR or HDR, and this reaction also requires NADPH. The enzyme in soybean has been purified and identified, and it can catalyze 2′-hydroxydaidzein in vitro with high activity. It also utilizes the substrates 2′-hydroxyformononetin or 2′-hydroxygenistein, but with relatively lower activity [124]. There are three possible genes that could encode this protein, and the function of one gene was elucidated by overexpressing it in soybean. In the seeds of transgenic soybeans, the content of glyceollins increased, and that of daidzein decreased, while genistein and glycitein contents were not changed. The expression of this gene was induced by some stress factors and several associated hormones. However, the enzymatic function of soybean has yet to be determined [125]. A candidate gene found in the QTL of soybean isoflavone content suggests its vital role in isoflavone synthesis [126]. Reduced expression of IFR in Phaseolus vulgaris resulted in decreased shoot and root growth, as well as an impact on nodulation [127]. The crystal structure of its homologous protein in alfalfa was determined, providing a foundation to comprehend its catalytic mechanism [128]. The first knowledge gap in the pathway is in the third step, which likely catalyzes 2′-hydroxy-2,3-dihydrodaidzein from the isoflavanone structure to the isoflavanol structure, by an unidentified reductase enzyme. There is currently no report about this gene or its functional description. Nevertheless, a similar pathway involved in the transformation of vestitone to medicarpin may offer clues to discover the candidate genes of the third step. The catalytic process from vestitone to 7,2′-dihydroxy-4′-methoxy-isoflavanol is similar to the third step, both converting 2′-hydroxyisoflavanone to 2′-hydroxyisoflavanol (Fig. 4). Vestitone's catalysis is dependent on an enzyme called VR, and it is probable that 2′-hydroxy-2,3-dihydrodaidzein transformation depends on an enzyme with a highly homologous protein sequence. The vestitone reductase gene in alfalfa was cloned, and its function was confirmed by recombinant expression in E. coli [129]. The protein's crystal structure was also determined [130]. The putative vestitone reductase gene in soybean was cloned, but its functions were not confirmed [131]. Through a homogenous gene search, eleven candidate genes were found, but the enzymatic activity of the gene-encoded proteins has yet to be determined. In the fourth step, the product from the third step is catalyzed by a pterocarpan synthase, producing 3,9-dihydroxypterocarpan. The protein in soybean was purified, and its function was confirmed [132]. In that study, it was found that 3,9-dihydroxypterocarpan can be directly produced by pterocarpan synthase from 2′-hydroxy-2,3-dihydrodaidzein. Meanwhile, the enzyme showed equal efficiency for vestitone catalysis. It was likely that the study used crude enzyme extracts. However, later enzymatic activity tests of pterocarpan synthase of licorice, soybean, or Lotus japonicus by expression in E. coli exhibited preference for various substrates [133]. It is plausible that multiple gene paralogs were present and exhibited a preference for converting various substrate types. Moreover, the expression of this gene is significantly upregulated in response to elicitors. In soybean, fourteen candidate genes were anticipated to encode pterocarpan synthase, but the enzymatic activity for only one protein was confirmed [133]. The fifth step involves an enzymatic reaction from 3,9-dihydroxypterocarpan to glycinol, catalyzed by a cytochrome P450 monooxygenase. The protein belongs to the CYP450 gene family and 93A subfamily, and its enzymatic activity also requires NADPH. The activity of this enzyme was first identified in 1984, but at that time, the protein was not purified, and its sequence was not determined [134]. In soybean, nine candidate genes were predicted to encode this enzyme, and the enzymatic activity of the CYP93A1 gene was identified in soybean by recombinant expression in budding yeast, and demonstrated to catalyze the substrate 3,9-dihydroxypterocarpan to glycinol [135]. Similarly, this expression of this gene is also regulated by some elicitors or stress-associated hormones.
The last module comprises two branched parts that are responsible for the production of different types of glyceollins. For branch I, glyceollin I is synthesized, while for branch II, glyceollins II and III are synthesized. There are six known types of glyceollins, but glyceollins I, II, and III account for the majority of glyceollins [136]. In the first step of both branches, a dimethylallyl is introduced to the C-2 or C-4 position of glycinol by the paralogous dimethylallyl transferases (G4DT or G2DT). For branch I, in the first step, a dimethylallyl is added to the C-4 position of glycinol, forming the product of 4-glyceollidin. Eight candidate genes were identified in soybean to encode this protein, and the function of one gene-encoded protein was identified using recombinant yeast microsomes [137]. For branch II, the first step involves the catalysis of glycinol into glyceocarpin by the addition of a dimethylallyl to the C-2 position of glycinol. The gene encoding this protein was cloned, and its function was identified by a yeast expression system [138]. The paralogous proteins responsible for two different types of catalysis are from the genes generated by a whole-genome duplication event. Furthermore, a new G2DT gene was identified by an in vitro enzyme assay [139]. The second step for both branches includes the process of cyclization of dimethylallyl. This is the second knowledge gap in the glyceollin synthetic pathway. The current literature suggests that the enzyme responsible for this step is a cytochrome P450 monooxygenase. The enzyme's function is confirmed by microsomes from soybean cell cultures, which can use both 4-glyceollidin and glyceocarpin as substrates to synthesize all three types of glyceollins [140]. As none of the genes encoding this enzyme protein have yet been cloned and identified, it is difficult to determine whether the enzyme is encoded by a single gene or by several paralogous genes. The expression of the protein also requires induction by pathogen elicitors, similar to some other enzymes in this pathway. The eight cytochrome P450 genes corresponding to Y92437 (CYP73A11), Y10489 (CYP71A9), Y10490 (CYP71D9), Y10491 (CYP82A2), Y10492 (CYP93A3), Y10493 (CYP71D8), Y10982 (CYP82A3), and Y10983 (CYP82A4) were identified to be induced by elicitors [141]. The publication of numerous soybean transcriptomes induced by pathogens has provided additional insight that could help us complete the knowledge gap in the final step of the pathway [142,143]. Our laboratory has undertaken gene mining of the glyceollin synthetic pathway through transcriptome analysis and has discovered that the gene expression of CYP71D8s and CYP82As is consistently upregulated in soybean plants challenged by pathogen infections (unpublished data). Consequently, we posited that these cytochrome P450 genes most likely encode the ultimate protein enzyme of the aforementioned pathway.
4. Elicitors and transcription factors that regulate glyceollin biosynthesis
Ever since the discovery of glyceollins, scientists have been relentlessly searching for a means to enhance their concentration in soybean. On the one hand, numerous exogenous elicitors have been identified to effectively increase glyceollins content, including various kinds of fungi and bacteria, diverse polysaccharides or oligosaccharides, several kinds of hormones, acidic growth medium, herbicides, metallic elements, light regulation, and wound treatment. These elicitors are outlined in Table 3. Notably, groundbreaking work was conducted in 1978. The accumulation of glyceollins was first identified to be induced by Phytophthora megasperma var. sojae [144]. It was discovered that the mycelial walls of this fungus could cause the accumulation of glyceollins without causing any damage to soybeans. Certain components produced by P. megasperma were shown to act as elicitors to activate the accumulation of glyceollins [145]. The existence of glyceollin elicitors was further validated by the activation of glyceollin accumulation through extracts from nonpathogenic Saccharomyces cerevisiae [146]. The accumulation of glyceollins was observed to be induced by a variety of biotic and abiotic components. Chemical components, including HgCl2, CdCl2, AgNO3, CuSO4, K2Cr2O7, Triton X-100, and Nonidet P-40, as well as the biotic components of the P. megasperma cell wall, extracellular metabolites, and cytoplasmic supernatant, could effectively induce the accumulation of glyceollins. Moreover, glyceollin accumulation mainly depends on an increase in synthesis induced by biotic components or a decrease in degradation induced by abiotic components. After induction by these factors, the content of glyceollins could reach approximately 0.4–2.0 mg [147]. Glucan from P. megasperma or S. cerevisiae has been shown to be an elicitor in activating glyceollin synthesis [148], while the cell wall and cytoplasmic extracts of soybean itself are not capable of activating glyceollin synthesis [147]. This suggests that fungus-specific cell wall polysaccharides are active components that stimulate glyceollin synthesis. Additionally, bacteria such as Pseudomonas glycinea can also activate glyceollin synthesis, and specific elicitor components have been detected from the cell envelope of this bacterium [149]. Inoculation with Meloidogyne incognita stimulated the synthesis of glyceollin in the root system [150]. Additionally, exposure to UV light at 254 nm with an intensity of 8 × 102 Jm−2 was found to activate glyceollin synthesis [151]. The presence of ascospores and mycelium of Sclerotinia sclerotiorum was also observed to induce the synthesis of glyceollins [152]. Infection with Rhizobium japonicum (61-A-24 strain) resulted in a significant increase in glyceollin synthesis in the root system [153]. Furthermore, a single mechanical injury was also found to trigger glyceollin synthesis [154]. The secretion of oxalic acid by Rhizoctonia sclerotica was identified as another activator of glyceollin synthesis [155]. In comparison to glucan, laminarin and polytran have demonstrated a better ability to activate glyceollin synthesis in soybeans [156]. It was discovered that the application of gibberellic acid alone to soybeans is sufficient to induce glyceollin synthesis [157]. Infection with Rhizoctonia solani was also observed to induce glyceollin accumulation in soybeans, whereas the endophytic fungus Glomus mosseae could not [158]. Furthermore, oligogalacturonic acid has also been found to induce the synthesis of glyceollins [159]. The synthesis of glyceollins was also observed to be induced by treatment with hydrogen peroxide [160]. Treatment of soybean cotyledons by four different Aspergillus species was also found to stimulate glyceollin synthesis [161]. The herbicide lactofen was also discovered to be a potent inducer of glyceollin synthesis [162]. Among eight different fungal strains evaluated for their ability to activate glyceollin synthesis, it was observed that the NRRL 3653 strain of Trichoderma reesei was able to cause glyceollin accumulation in soybean cotyledons to reach 3.763 mg/g [163]. Evaluation of the synthesis ability of 60 different soybean varieties under various conditions revealed that treatment with Aspergillus sojae resulted in the highest yield of glyceollin, exceeding 8 mg/g [164]. Under acidic conditions (pH 3.0), glyceollin synthesis was effectively promoted [165]. Soybean pretreated with reactive oxygen species and then infected with Rhizopus were found to obtain higher yields of glyceollin compared to Rhizopus treatment alone [166].
Table 3.
The list of elicitors that can stimulate the synthesis of glyceollins.
| Elicitor types |
Reference No. |
|---|---|
| Fungi | |
| Phytophthora megasperma var. sojae | 143 |
| Saccharomyces cerevisiae | 145 |
| Sclerotinia sclerotiorum | 151 |
| Rhizoctonia sclerotica | 154 |
| Rhizoctonia Solani | 157 |
| Aspergillus | 160 |
| Trichoderma reesei (NRRL 3653 strains) | 162 |
| Rhizopus | 165 |
| Bacteria | |
| Pseudomonas glycinea | 148 |
| Rhizobium japonicum (61-A-24 strain) | 152 |
| Animals | |
| Meloidogyne incognita | 149 |
| Polysaccharides or oligosaccharides | |
| Glucan from P. megasperma or S. cerevisiae | 147 |
| Laminarin | 155 |
| Polytran | 155 |
| Oligogalacturonic acid | 158 |
| Phytohormones | |
| Gibberellic acid | 156 |
| pH value | |
| pH 3.0 | 164 |
| Herbicides | |
| Lactofen | 161 |
| Metallic elements | |
| HgCl2 | 146 |
| CdCl2 | 146 |
| AgNO3 | 146 |
| CuSO4 | 146 |
| K2Cr2O7 | 146 |
| Light regulation | |
| 254 nm ultraviolet light | 150 |
| Chemicals | |
| Triton X-100 | 146 |
| Nonidet P-40 | 146 |
| Hydrogen peroxide | 159 |
In addition to enhancing the synthesis level of glyceollins through exogenous treatment, genetic factors governing glyceollin synthesis have been identified. Evaluation of 60 soybean cultivars suggests that the content of glyceollins is not correlated with that of daidzein [164]. Engineering the expression of soybean transcription factors is a more modern approach for improving glyceollin concentrations in soybean. Thus, it is important to identify transcription factors that specifically regulate the pathway of glyceollin synthesis. The GmNAC42-1 gene was the first identified transcription factor that promote glyceollin synthesis in soybean [167]. Then, the transcription factors GmMYB29A1 and GmMYB29A2 have been identified as the key transcriptional regulators for the glyceollin synthetic pathway genes. Overexpression of GmMYB29A2 promoted the expression of GmMYB29A1 and GmNAC42-1, and increased the glyceollins’ contents [168]. However, either individual or co-overexpression of GmMYB29A2 and GmNAC42-1 was insufficient to activate the glyceollin accumulation when the elicitors were absent [[167], [168], [169]]. A third transcription factor GmHSF6-1 was also identified to promote glyceollin accumulation, and it still could not activate glyceollin synthesis alone in the absence of elicitors [169]. Metabolite-based genome-wide association methods have also led to the identification of some promising candidate transcription factors implicated in glyceollin synthesis, whereas their functions were not identified [170]. It suggested that a complex transcription factor network for biosynthesis regulation of glyceollin and other phytoalexins may exist, and its recent advance was reviewed by Ahmed and Kovinich [171].
5. Chemical synthesis of glyceollins
In 2008, the chemical synthesis of glyceollin I was first achieved using a 15-step methodology, with a yield of approximately 3.4 % [172]. Subsequently, in 2011, a successful 14-step route for glyceollin I synthesis was established, with a yield of 1.5 % [173]. In the same year, an 11-step synthetic pathway for glyceollin I was introduced, yielding approximately 12 % [22]. Later, the synthesis steps were further optimized through shortening [174]. In 2015, a new 12-step synthesis method was employed to synthesize glyceollin II with a yield of approximately 7 % [175]. In a recent report, an eight-step method for the synthesis of glyceollin I and II from the substrate (2,4-dimethoxyphenyl) acetic acid was established, with transformation rates reaching 15 % and 4.9 %, respectively [176]. Following acid and heat treatment, glyceollins can be transformed into their dehydroxylated forms, which are more stable and exhibit specific antagonistic effects on ERα. These compounds may be more effective drugs for the prevention of breast cancer [177].
6. Future development of glyceollins
After more than half a century of relentless pursuit, scientists have made significant strides in unraveling the effects of glyceollins, particularly their impact on diseases such as breast cancer, which heavily rely on the estrogen receptor pathway. Nonetheless, the full extent of the pharmacological effects exerted by glyceollins remains elusive. Current investigations suggest the existence of several independent action pathways for glyceollins, although only the estrogen receptor pathway has been understood relatively well. The inhibitory effects of glyceollins on certain tumor types, independent of the estrogen receptor pathway, are remarkably compelling, thus accentuating their medicinal value. Notably, glyceollins have demonstrated promising preventive effects against the triple-negative subtype of breast cancer, notorious for its propensity for recurrence and therapeutic resistance. However, the underlying mechanisms through which glyceollins operate independently of the estrogen receptor pathway are yet to be fully elucidated. In leveraging molecular docking technology, several novel putative target proteins that may interact with glyceollins have been identified, yet these potential bindings have not been experimentally validated. By substantiating these prospective targets of glyceollins, our understanding of their mode of action will be significantly enhanced. Concurrently, this endeavor will facilitate the determination of whether the multifaceted effects of glyceollins stem from a singular signaling pathway or divergent pathways in various diseases. Moreover, research on unexplored pathways governing the actions of glyceollins, such as the melanogenesis pathway, remains limited, necessitating an evaluation of whether glyceollins exhibit advantages over other compounds in these contexts. Should superior effects be observed, further investigations should be pursued.
Considering the favorable outcomes associated with glyceollins in multiple diseases, it becomes increasingly imperative to study their production to ensure an ample supply. Currently, the complete biosynthesis pathway of glyceollins remains largely uncharted, thereby impeding the genetic regulation of glyceollin production. Consequently, the identification of unknown genes within the glyceollin synthesis pathway is urgently needed. The wealth of available transcriptome data presents a valuable resource for identifying these candidate genes, and it is anticipated that the biosynthesis pathway will be completely understood in the coming years. Building upon this foundation, two viable approaches for enhancing current glyceollin production have emerged through genetic manipulation methods and synthetic biology techniques. The first approach involves modifying the expression of transcription factor genes that regulate the production of glyceollins in soybean, transitioning from elicitor-induced expression to constitutive expression. Substituting the promoters of these genes with constitutive counterparts is a viable option, yet the expression of a least one unidentified negative transcription regulator must be removed. Furthermore, exploring homologous genes in wild soybeans to determine their potential for higher enzymatic activity could boost synthesis efficiency. Consequently, elevated glyceollins and a more feasible production method can be achieved while circumventing the need for additional elicitor-induced processes. The second approach entails heterologous expression of genes involved in the glyceollin biosynthesis pathway within engineered microbial strains, thereby enabling the direct microbial fermentation of glyceollins. Both approaches may promise convenient, efficient, and cost-effective glyceollin production. Notably, the heterologous biosynthesis pathway for daidzein, the precursor of glyceollin, has already been achieved in Saccharomyces cerevisiae, significantly simplifying the challenge of microbial heterologous synthesis of glyceollins. Adequate supply of glyceollins is necessary for future clinical trials and development of specific semi-synthetic glyceollin derivatives. Thus, a stable technological process for large-scale glyceollin production must be established.
Since the glyceollins are soybean-derived molecules, they are promising with safety. However, as a kind of phytoalexins, glyceollins may be toxic for human at certain concentrations, and their safety should be determined. Especially, the safety of long-term glyceollin exposure is critical for glyceollins’ application in health products and medicines. The fermented soybean products are abundant in glyceollins, with a long history as food. Before large-scale glyceollin production is achieved, glyceollin-included soybean products could be used for clinical trials on preventing breast cancer and some other related diseases.
It is anticipated that future research on glyceollins will continue to focus on unraveling their mechanisms of anticancer activity and metabolic regulation, with particular emphasis on exploring the biosynthesis of glyceollins as a burgeoning area of interest, supplanting chemical synthesis as a preferred avenue of exploration.
7. Conclusions
Glyceollins, a class of phytoalexins present in soybeans, have been demonstrated to possess remarkable efficacy in combating a range of ailments, particularly breast cancer and its recurrence. The manifold therapeutic benefits of glyceollins have stimulated the development of a plethora of health and medicinal products incorporating these phytochemicals. Notwithstanding the comprehensive understanding of the biosynthetic pathway of glyceollins, the specific genetic determinants responsible for their complete biosynthesis remain elusive. Consequently, it is of paramount importance to unravel these aspects and fill the knowledge gap in the synthetic pathway. With the emergence of innovative synthetic biology techniques, it is conceivable that an optimized cellular factory for the biosynthesis of glyceollins can be established, predicated on the elucidation of the entire biosynthetic pathway. Such a paradigm shift in production would confer enhanced reliability, efficiency, and sustainability to glyceollin synthesis, culminating in a significant upsurge in their therapeutic applications.
Data availability statement
Data availability is not applicable to this article as no new data were created or analyzed in this study.
CRediT authorship contribution statement
Zhiyong Yue: Writing – original draft. Shanhong He: Investigation. Jinpei Wang: Writing – review & editing. Qi Jiang: Writing – review & editing. Hanping Wang: Writing – review & editing. Jia Wu: Writing – review & editing. Chenxi Li: Investigation. Zixian Wang: Investigation. Xuan He: Data curation, Visualization. Nannan Jia: Data curation, Visualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This paper was supported by Key Research and Development Program of Shaanxi Province (2022NY-016, China), Science and Research Special Project of Education Department of Shaanxi Provincial Project (22JK0527, China), and Shaanxi University Student Innovation and Entrepreneurship Training Program (S202112713053, China).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e21874.
Appendix A. Supplementary data
The following is/are the supplementary data to this article.
References
- 1.Dakora F.D., Phillips D.A. Diverse functions of isoflavonoids in legumes transcend anti-microbial definitions of phytoalexins. Physiol Mol Plant P. 1996;49:1–20. [Google Scholar]
- 2.Miadoková E. Isoflavonoids - an overview of their biological activities and potential health benefits. Interdiscip Toxicol. 2009;2(4):211–218. doi: 10.2478/v10102-009-0021-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samanta A., Das G., Das S.K. Roles of flavonoids in plants. Carbon. 2011;100(6):12–35. [Google Scholar]
- 4.Al-Maharik N. Isolation of naturally occurring novel isoflavonoids: an update. Nat. Prod. Rep. 2019;36(8):1156–1195. doi: 10.1039/c8np00069g. [DOI] [PubMed] [Google Scholar]
- 5.Foudah A.I., Abdel-Kader M.S. In: Flavonoids - from Biosynthesis to Human Health. Justino J., editor. UK IntechOpen Ltd; London: 2017. Isoflavonoids; pp. 61–95. [Google Scholar]
- 6.Anderson E.J., Ali M.L., Beavis W.D., Chen P., Clemente T.E., Diers B.W. Springer; Cham: 2019. Soybean [Glycine Max (L.) Merr.] Breeding: History, Improvement, Production and Future Opportunities. Advances in Plant Breeding Strategies: Legumes; pp. 431–516. [Google Scholar]
- 7.Medic J., Atkinson C., Hurburgh C.R., Jr. Current knowledge in soybean composition. J Am Oil Chen Soc. 2014;91:363–384. [Google Scholar]
- 8.Kimira M., Arai Y., Shimoi K., Watanabe S. Japanese intake of flavonoids and isoflavonoids from foods. J. Epidemiol. 1998;8(3):168–175. doi: 10.2188/jea.8.168. [DOI] [PubMed] [Google Scholar]
- 9.Lee M.R., Kim J.Y., Chun J., Park S., Kim H.J., Kim J.S., et al. Induction of glyceollins by fungal infection in varieties of Korean soybean. J. Microbiol. Biotechnol. 2010;20(8):1226–1229. doi: 10.4014/jmb.1005.03047. [DOI] [PubMed] [Google Scholar]
- 10.Sims J.J., Keen N.T., Honwad V.K. Hydroxyphaseollin, an induced antifungal compound from soybeans. Phytochemistry. 1972;11(2):827–828. [Google Scholar]
- 11.Lyne R.L., Mulheirn L.J., Leworthy D.P. New pterocarpinoid phytoalexins of soybean. J. Chem. Soc. 1976;13:497–498. [Google Scholar]
- 12.Grisebach H., Ebel J. Phytoalexins, chemical defense substances of higher plants? Angew Chem. Int. Ed. Engl. 1978;17(9):635–647. [Google Scholar]
- 13.Smith D.A., Banks S.W. Biosynthesis, elicitation and biological activity of isoflavonoid phytoalexins. Phytochemistry. 1986;25(5):979–995. [Google Scholar]
- 14.Tilghman S.L., Boué S.M., Burow M.E. Glyceollins, a novel class of antiestrogenic phytoalexins. Mol. Cell. Pharmacol. 2010;2(4):155–160. [Google Scholar]
- 15.Ng T.B., Ye X.J., Wong J.H., Fang E.F., Chan Y.S., Pan W., et al. Glyceollin, a soybean phytoalexin with medicinal properties. Appl. Microbiol. Biotechnol. 2011;90(1):59–68. doi: 10.1007/s00253-011-3169-7. [DOI] [PubMed] [Google Scholar]
- 16.Kim H.J., Lim J.S., Kim W.K., Kim J.S. Soyabean glyceollins: biological effects and relevance to human health. Proc. Nutr. Soc. 2012;71(1):166–174. doi: 10.1017/S0029665111003272. [DOI] [PubMed] [Google Scholar]
- 17.Nwachukwu I.D., Luciano F.B., Udenigwe C.C. The inducible soybean glyceollin phytoalexins with multifunctional health-promoting properties. Food Res. Int. 2013;54(1):1208–1216. [Google Scholar]
- 18.Bamji S.F., Corbitt C. Glyceollins: soybean phytoalexins that exhibit a wide range of health-promoting effects. J. Funct.Foods. 2017;34:98–105. [Google Scholar]
- 19.Pham T.H., Lecomte S., Efstathiou T., Ferriére F., Pakdel F. An update on the effects of glyceollins on human health: possible anticancer effects and underlying mechanisms. Nutrients. 2019;11(1):79. doi: 10.3390/nu11010079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lygin A.V., Hill C.B., Zernova O.V., Crull L., Widholm J.M., Hartman G.L., et al. Response of soybean pathogens to glyceollin. Phytopathology. 2010;100(9):897–903. doi: 10.1094/PHYTO-100-9-0897. [DOI] [PubMed] [Google Scholar]
- 21.Peng Q., Zhang M., Gao L., Eromosele O., Qiao Y., Shi B. Effects of alginate oligosaccharides with different molecular weights and guluronic to mannuronic acid ratios on glyceollin induction and accumulation in soybeans. J. Food Sci. Technol. 2018;55(5):1850–1858. doi: 10.1007/s13197-018-3101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luniwal A., Khupse R., Reese M., Liu J., El-Dakdouki M., Malik N., et al. Multigram synthesis of glyceollin I. Org.process Res.dev. 2011;15(5):1149–1162. [Google Scholar]
- 23.Yu O., Mcgonigle B. Metabolic engineering of isoflavone biosynthesis. Adv. Agron. 2005;86(5):147–190. [Google Scholar]
- 24.Zhang J., Yu O. Metabolic engineering of isoflavone biosynthesis in seeds. Modif Seed Compos Promot Health Nutr. 2009;51:151–176. [Google Scholar]
- 25.Cheng E., Yoder L., Story C.D., Burroughs W. Estrogenic activity of some isoflavone derivatives. Science. 1954;120(3119):575–576. doi: 10.1126/science.120.3119.575. [DOI] [PubMed] [Google Scholar]
- 26.Bradbury R.B., White D.E. Estrogens and related substances in plants. Vitam Horm. 1954;12(2):207–233. doi: 10.1016/s0083-6729(08)61013-4. [DOI] [PubMed] [Google Scholar]
- 27.Greene L., Gilna P., Waterfield M., Baker A., Hort Y., Shine J. Sequence and expression of human estrogen receptor complementary DNA. Science. 1986;231(4742):1150–1154. doi: 10.1126/science.3753802. [DOI] [PubMed] [Google Scholar]
- 28.Kuiper G.G., Enmark E., Pelto-Huikko M., Nilsson S., Gustafsson J.A. Cloning of a novel receptor expressed in rat prostate and ovary. Proc. Natl. Acad. Sci. U.S.A. 1996;93(12):5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halabalaki M., Alexi X., Aligiannis N., Lambrinidis G., Pratsinis H., Florentin I., et al. Estrogenic activity of isoflavonoids from Onobrychis ebenoides. Planta Med. 2006;72(6):488–493. doi: 10.1055/s-2005-916261. [DOI] [PubMed] [Google Scholar]
- 30.Pfitscher A., Reiter E., Jungbauer A. Receptor binding and transactivation activities of red clover isoflavones and their metabolites. J. Steroid Biochem. Mol. Biol. 2008;112(1–3):87–94. doi: 10.1016/j.jsbmb.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 31.Djiogue S., Halabalaki M., Alexi X., Njamen D., Fomum Z.T., Alexis M.N., et al. Isoflavonoids from Erythrina poeppigiana: evaluation of their binding affinity for the estrogen receptor. J Nat Prod. 2009;72(9):1603–1607. doi: 10.1021/np900271m. [DOI] [PubMed] [Google Scholar]
- 32.De-Eknamkul W., Umehara K., Monthakantirat O., Toth R., Frecer V., Knapic L., et al. QSAR study of natural estrogen-like isoflavonoids and diphenolics from Thai medicinal plants. J. Mol. Graph. Model. 2011;29(6):784–794. doi: 10.1016/j.jmgm.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 33.Fagerberg L., Hallström B.M., Oksvold P., Kampf C., Djureinovic D., Odeberg J., et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteomics. 2014;13(2):397–406. doi: 10.1074/mcp.M113.035600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barkhem T., Carlsson B.O., Nilsson Y., Enmark E., Gustafsson J., Nilsson S. Differential response of estrogen receptor alpha and estrogen receptor beta to partial estrogen agonists/antagonists. Mol. Pharmacol. 1998;54(1):105–112. doi: 10.1124/mol.54.1.105. [DOI] [PubMed] [Google Scholar]
- 35.Sommer S., Fuqua S.A. Estrogen receptor and breast cancer. Semin. Cancer Biol. 2001;11(5):339–352. doi: 10.1006/scbi.2001.0389. [DOI] [PubMed] [Google Scholar]
- 36.Chan K.K., Wei N., Liu S.S., Xiao-Yun L., Cheung A.N., Ngan H.Y. Estrogen receptor subtypes in ovarian cancer: a clinical correlation. Obstet. Gynecol. 2008;111(1):144–151. doi: 10.1097/01.AOG.0000296715.07705.e9. [DOI] [PubMed] [Google Scholar]
- 37.Bonkhoff H. Estrogen receptor signaling in prostate cancer: implications for carcinogenesis and tumor progression. Prostate. 2018;78(1):2–10. doi: 10.1002/pros.23446. [DOI] [PubMed] [Google Scholar]
- 38.Hsu L.H., Chu N.M., Kao S.H. Estrogen, estrogen receptor and lung cancer. International J Mol Sci. 2017;18(8):1713. doi: 10.3390/ijms18081713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao Y., Li Z. Interplay of estrogen receptors and FOXA factors in the liver cancer. Mol. Cell. Endocrinol. 2015;418:334–339. doi: 10.1016/j.mce.2015.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aryan L., Younessi D., Zargari M., Banerjee S., Agopian J., Rahman S., et al. The role of estrogen receptors in cardiovascular disease. Int. J. Mol. Sci. 2020;21(12):4314. doi: 10.3390/ijms21124314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hevener A.L., Clegg D.J., Mauvais-Jarvis F. Impaired estrogen receptor action in the pathogenesis of the metabolic syndrome. Mol. Cell. Endocrinol. 2015;418:306–321. doi: 10.1016/j.mce.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chakrabarti M., Haque A., Banik N.L., Nagarkatti P., Nagarkatti M., Ray S.K. Estrogen receptor agonists for attenuation of neuroinflammation and neurodegeneration. Brain Res. Bull. 2014;109:22–31. doi: 10.1016/j.brainresbull.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pelekanou V., Kampa M., Kiagiadaki F., Deli A., Theodoropoulos P., Agrogiannis G., et al. Estrogen anti-inflammatory activity on human monocytes is mediated through cross-talk between estrogen receptor ERα36 and GPR30/GPER1. J. Leukoc. Biol. 2016;99(2):333–347. doi: 10.1189/jlb.3A0914-430RR. [DOI] [PubMed] [Google Scholar]
- 44.Komm B.S., Chines A.A. An update on selective estrogen receptor modulators for the prevention and treatment of osteoporosis. Maturitas. 2012;71(3):221–226. doi: 10.1016/j.maturitas.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 45.Yaşar P., Ayaz G., User S.D., Güpür G., Muyan M. Molecular mechanism of estrogen-estrogen receptor signaling. Reprod. Med. Biol. 2017;16(1):4–20. doi: 10.1002/rmb2.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fuentes N., Silveyra P. Estrogen receptor signaling mechanisms. Adv Protein Chem Struct Biol. 2019;116:135–170. doi: 10.1016/bs.apcsb.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burow M.E., Boue S.M., Collins-Burow B.M., Melnik L.I., Duong B.N., Carter-Wientjes C.H., et al. Phytochemical glyceollins, isolated from soy, mediate antihormonal effects through estrogen receptor alpha and beta. J. Clin. Endocrinol. Metab. 2001;86(4):1750–1758. doi: 10.1210/jcem.86.4.7430. [DOI] [PubMed] [Google Scholar]
- 48.Payton-Stewart F., Khupse R.S., Boué S.M., Elliott S., Zimmermann M.C., Skripnikova E.V. Glyceollin I enantiomers distinctly regulate ER-mediated gene expression. Steroids. 2010;75(12):870–878. doi: 10.1016/j.steroids.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 49.Nikov G.N., Hopkins N.E., Boue S., Alworth W.L. Interactions of dietary estrogens with human estrogen receptors and the effect on estrogen receptor-estrogen response element complex formation. Environ. Health Perspect. 2000;108(9):867–872. doi: 10.1289/ehp.00108867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Y., Takao K., Abe C., Sasaki K., Ochiai K., Matsui T. Intestinal absorption of prenylated isoflavones, glyceollins, in sprague-dawley rats. J. Agric. Food Chem. 2020;68(31):8205–8211. doi: 10.1021/acs.jafc.0c02475. [DOI] [PubMed] [Google Scholar]
- 51.Zimmermann M.C., Tilghman S.L., Boué S.M., Salvo V.A., Elliott S., Williams K.Y., et al. Glyceollin I, a novel antiestrogenic phytoalexin isolated from activated soy. J Pharmacol Exp Ther. 2010;332(1):35–45. doi: 10.1124/jpet.109.160382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simons R., Gruppen H., Bovee T.F., Verbruggen M.A., Vincken J.P. Prenylated isoflavonoids from plants as selective estrogen receptor modulators (phytoSERMs) Food Funct. 2012;3(8):810–827. doi: 10.1039/c2fo10290k. [DOI] [PubMed] [Google Scholar]
- 53.Lecomte S., Chalmel F., Ferriere F., Percevault F., Plu N., Saligaut C., et al. Glyceollins trigger anti-proliferative effects through estradiol-dependent and independent pathways in breast cancer cells. Cell Commun. Signal. 2017;15(1):1–18. doi: 10.1186/s12964-017-0182-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Salvo V.A., Boué S.M., Fonseca J.P., Elliott S., Corbitt C., Collins-Burow B.M., et al. Antiestrogenic glyceollins suppress human breast and ovarian carcinoma tumorigenesis. Clin. Cancer Res. 2006;12(23):7159–7164. doi: 10.1158/1078-0432.CCR-06-1426. [DOI] [PubMed] [Google Scholar]
- 55.Payton-Stewart F., Schoene N.W., Kim Y.S., Burow M.E., Cleveland T.E., Boue S.M., et al. Molecular effects of soy phytoalexin glyceollins in human prostate cancer cells LNCaP. Mol. Carcinog. 2009;48(9):862–871. doi: 10.1002/mc.20532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Song M.J., Baek I., Jeon S.B., Seo M., Kim Y.H., Cui S., et al. Effects of glyceollin I on vascular contraction in rat aorta. N-S Arch Pharmacoli. 2010;381(6):517–528. doi: 10.1007/s00210-010-0513-x. [DOI] [PubMed] [Google Scholar]
- 57.Park S., Kim D.S., Kim J.H., Kim J.S., Kim H.J. Glyceollin-containing fermented soybeans improve glucose homeostasis in diabetic mice. Nutrition. 2012;28(2):204–211. doi: 10.1016/j.nut.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 58.Bateman M.E., Strong A.L., Hunter R.S., Bratton M.R., Komati R., Sridhar J., et al. Osteoinductive effects of glyceollins on adult mesenchymal stromal/stem cells from adipose tissue and bone marrow. Phytomedicine. 2017;27:39–51. doi: 10.1016/j.phymed.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 59.Lee Y.S., Kim H.K., Lee K.J., Jeon H.W., Cui S., Lee Y.M., et al. Inhibitory effect of glyceollin isolated from soybean against melanogenesis in B16 melanoma cells. BMB Rep. 2010;43(7):461–467. doi: 10.5483/bmbrep.2010.43.7.461. [DOI] [PubMed] [Google Scholar]
- 60.Waks A.G., Winer E.P. Breast cancer treatment: a review. JAMA. 2019;321(3):288–300. doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- 61.Visvanathan K., Fabian C.J., Bantug E., Brewster A.M., Davidson N.E., DeCensi A., et al. Use of endocrine therapy for breast cancer risk reduction: ASCO clinical practice guideline update. J. Clin. Oncol. 2019;37(33):3152–3165. doi: 10.1200/JCO.19.01472. [DOI] [PubMed] [Google Scholar]
- 62.Langdon S.P., Gourley C., Gabra H., Stanley B. Endocrine therapy in epithelial ovarian cancer. Expert Rev. Anticancer Ther. 2017;17(2):109–117. doi: 10.1080/14737140.2017.1272414. [DOI] [PubMed] [Google Scholar]
- 63.Begam A.J., Jubie S., Nanjan M.J. Estrogen receptor agonists/antagonists in breast cancer therapy: a critical review. Bioorg. Chem. 2017;71:257–274. doi: 10.1016/j.bioorg.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 64.Chumsri S., Howes T., Bao T., Sabnis G., Brodie A. Aromatase, aromatase inhibitors, and breast cancer. J. Steroid Biochem. Mol. Biol. 2011;125(1–2):13–22. doi: 10.1016/j.jsbmb.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mills J.N., Rutkovsky A.C., Giordano A. Mechanisms of resistance in estrogen receptor positive breast cancer: overcoming resistance to tamoxifen/aromatase inhibitors. Curr. Opin. Pharmacol. 2018;41:59–65. doi: 10.1016/j.coph.2018.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen S.I., Tseng H.T., Hsieh C.C. Evaluating the impact of soy compounds on breast cancer using the data mining approach. Food Funct. 2020;11(5):4561–4570. doi: 10.1039/c9fo00976k. [DOI] [PubMed] [Google Scholar]
- 67.Ali S., Coombes R.C. Estrogen receptor alpha in human breast cancer: occurrence and significance. J. Mammary Gland Biol. Neoplasia. 2000;5(3):271–281. doi: 10.1023/a:1009594727358. [DOI] [PubMed] [Google Scholar]
- 68.Haldosén L.A., Zhao C., Dahlman-Wright K. Estrogen receptor beta in breast cancer. Mol. Cell. Endocrinol. 2014;382(1):665–672. doi: 10.1016/j.mce.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 69.Wood C.E., Clarkson T.B., Appt S.E., Franke A.A., Boue S.M., Burow M.E., et al. Effects of soybean glyceollins and estradiol in postmenopausal female monkeys. Nutr. Cancer. 2006;56(1):74–81. doi: 10.1207/s15327914nc5601_10. [DOI] [PubMed] [Google Scholar]
- 70.Yamamoto T., Sakamoto C., Tachiwana H., Kumabe M., Matsui T., Yamashita T., et al. Endocrine therapy-resistant breast cancer model cells are inhibited by soybean glyceollin I through Eleanor non-coding RNA. Sci. Rep. 2018;8(1) doi: 10.1038/s41598-018-33227-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Montes-Grajales D., Martínez-Romero E., Olivero-Verbel J. Phytoestrogens and mycoestrogens interacting with breast cancer proteins. Steroids. 2018;134:9–15. doi: 10.1016/j.steroids.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 72.Carriere P.P., Llopis S.D., Naiki A.C., Nguyen G., Phan T., Nguyen M.M., et al. Glyceollin I reverses epithelial to mesenchymal transition in letrozole resistant breast cancer through ZEB1. Int J Environ Res Public Health. 2015;13(1):10. doi: 10.3390/ijerph13010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hermanto F.E., Rifa’i M., Widodo . vol. 2155. AIP Publishing LLC; 2019. (Potential Role of Glyceollin as Anti-metastatic Agent through Transforming Growth Factor-β Receptors Inhibition Signaling Pathways: A Computational Study). [Google Scholar]
- 74.Walker R.R., Patel J.R., Gupta A., Davidson A.M., Williams C.C., Payton-Stewart F., et al. Glyceollins trigger anti-proliferative effects in hormone-dependent aromatase-inhibitor-resistant breast cancer cells through the induction of apoptosis. Int. J. Mol. Sci. 2022;23(5):2887. doi: 10.3390/ijms23052887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pham T.H., Lecomte S., Le Guevel R., Lardenois A., Evrard B., Chalmel F., et al. Characterization of glyceollins as novel aryl hydrocarbon receptor ligands and their role in cell migration. Int. J. Mol. Sci. 2020;21(4):1368. doi: 10.3390/ijms21041368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Choi J.H., Nguyen M.P., Jung S.Y., Kwon S.M., Jee J.G., Bae J.S., et al. Inhibitory effect of glyceollins on vasculogenesis through suppression of endothelial progenitor cell function. Mol. Nutr. Food Res. 2013;57(10):1762–1771. doi: 10.1002/mnfr.201200826. [DOI] [PubMed] [Google Scholar]
- 77.Lee S.H., Lee J., Jung M.H., Lee Y.M. Glyceollins, a novel class of soy phytoalexins, inhibit angiogenesis by blocking the VEGF and bFGF signaling pathways. Mol. Nutr. Food Res. 2013;57(2):225–234. doi: 10.1002/mnfr.201200489. [DOI] [PubMed] [Google Scholar]
- 78.Lee S.H., Jee J.G., Bae J.S., Liu K.H., Lee Y.M. A group of novel HIF-1α inhibitors, glyceollins, blocks HIF-1α synthesis and decreases its stability via inhibition of the PI3K/AKT/mTOR pathway and Hsp90 binding. J. Cell. Physiol. 2015;230(4):853–862. doi: 10.1002/jcp.24813. [DOI] [PubMed] [Google Scholar]
- 79.Bratton M.R., Martin E.C., Elliott S., Rhodes L.V., Collins-Burow B.M., McLachlan J.A., et al. A novel regulator of mTOR/p70S6 in estrogen receptor positive breast cancer. J. Steroid Biochem. Mol. Biol. 2015;150:17–23. doi: 10.1016/j.jsbmb.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim H.J., Cha B.Y., Choi B., Lim J.S., Woo J.T., Kim J.S. Glyceollins inhibit platelet-derived growth factor-mediated human arterial smooth muscle cell proliferation and migration. Br. J. Nutr. 2012;107(1):24–35. doi: 10.1017/S0007114511002571. [DOI] [PubMed] [Google Scholar]
- 81.Lee J.H., Seo W.D., Jeong S.H., Jeong T.S., Lee W.S., Park K.H. Human acyl-CoA: cholesterol acyltransferase inhibitory effect of flavonoids from roots of Glycine max (L.) merr. J Appl Biol Chem. 2006;49(2):57–61. [Google Scholar]
- 82.Boué S.M., Isakova I.A., Burow M.E., Cao H., Bhatnagar D., Sarver J.G., et al. Glyceollins, soy isoflavone phytoalexins, improve oral glucose disposal by stimulating glucose uptake. J. Agric. Food Chem. 2012;60(25):6376–6382. doi: 10.1021/jf301057d. [DOI] [PubMed] [Google Scholar]
- 83.Park S., Ahn I.S., Kim J.H., Lee M.R., Kim J.S., Kim H.J. Glyceollins, one of the phytoalexins derived from soybeans under fungal stress, enhance insulin sensitivity and exert insulinotropic actions. J. Agric. Food Chem. 2010;58(3):1551–1557. doi: 10.1021/jf903432b. [DOI] [PubMed] [Google Scholar]
- 84.Yoon E.K., Jeong Y.T., Li X., Song C., Park D.C., Kim Y.H., et al. Glyceollin improves endoplasmic reticulum stress-induced insulin resistance through CaMKK-AMPK pathway in L6 myotubes. J. Nutr. Biochem. 2013;24(6):1053–1061. doi: 10.1016/j.jnutbio.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 85.Huang H., Xie Z., Boue S.M., Bhatnagar D., Yokoyama W., Yu L.L., et al. Cholesterol-lowering activity of soy-derived glyceollins in the golden Syrian hamster model. J. Agric. Food Chem. 2013;61(24):5772–5782. doi: 10.1021/jf400557p. [DOI] [PubMed] [Google Scholar]
- 86.Lee W., Ku S.K., Lee Y.M., Bae J.S. Anti-septic effects of glyceollins in HMGB1-induced inflammatory responses in vitro and in vivo. Food Chem. Toxicol. 2014;63:1–8. doi: 10.1016/j.fct.2013.10.034. [DOI] [PubMed] [Google Scholar]
- 87.Atho'illah M.F., Safitri Y.D., Nur'aini F.D., Widyarti S., Tsuboi H., Rifa'i M. Elicited soybean extract attenuates proinflammatory cytokines expression by modulating TLR3/TLR4 activation in high-fat, high-fructose diet mice. J. Ayurveda Integr. Med. 2021;12(1):43–51. doi: 10.1016/j.jaim.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jung C.L., Kim H.J., Park J.H., Kong A.N., Lee C.H., Kim J.S. Synergistic activation of the Nrf2-signaling pathway by glyceollins under oxidative stress induced by glutathione depletion. J. Agric. Food Chem. 2013;61(17):4072–4078. doi: 10.1021/jf303948c. [DOI] [PubMed] [Google Scholar]
- 89.Kim H.J., Jung C.L., Jeong Y.S., Kim J.S. Soybean-derived glyceollins induce apoptosis through ROS generation. Food Funct. 2014;5(4):688–695. doi: 10.1039/c3fo60379b. [DOI] [PubMed] [Google Scholar]
- 90.Kim H.J., di Luccio E., Kong A.N., Kim J.S. Nrf2-mediated induction of phase 2 detoxifying enzymes by glyceollins derived from soybean exposed to Aspergillus sojae. Biotechnol. J. 2011;6(5):525–536. doi: 10.1002/biot.201100010. [DOI] [PubMed] [Google Scholar]
- 91.Kim B.R., Seo J.Y., Sung M.K., Park J.H., Suh H.J., Liu K.H., et al. Suppression of 7,12-dimethylbenz(a)anthracene-induced mammary tumorigenesis by glyceollins. Mol. Nutr. Food Res. 2015;59(5):907–917. doi: 10.1002/mnfr.201400726. [DOI] [PubMed] [Google Scholar]
- 92.Seo J.Y., Kim B.R., Oh J., Kim J.S. Soybean-derived phytoalexins improve cognitive function through activation of Nrf2/HO-1 signaling pathway. Int. J. Mol. Sci. 2018;19(1):268. doi: 10.3390/ijms19010268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jeong G., Oh J., Kim J.S. Glyceollins modulate tumor development and growth in a mouse xenograft model of human colon cancer in a p53-dependent manner. J. Med. Food. 2019;22(5):521–528. doi: 10.1089/jmf.2018.4290. [DOI] [PubMed] [Google Scholar]
- 94.Yoon E.K., Kim H.K., Cui S., Kim Y.H., Lee S.H. Soybean glyceollins mitigate inducible nitric oxide synthase and cyclooxygenase-2 expression levels via suppression of the NF-κB signaling pathway in RAW 264.7 cells. Int. J. Mol. Med. 2012;29(4):711–717. doi: 10.3892/ijmm.2012.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim H.J., Sung M.K., Kim J.S. Anti-inflammatory effects of glyceollins derived from soybean by elicitation with Aspergillus sojae. Inflamm. Res. 2011;60(10):909–917. doi: 10.1007/s00011-011-0351-4. [DOI] [PubMed] [Google Scholar]
- 96.Seo H., Oh J., Hahn D., Kwon C.S., Lee J.S., Kim J.S. Protective effect of glyceollins in a mouse model of dextran sulfate sodium-induced colitis. J. Med. Food. 2017;20(11):1055–1062. doi: 10.1089/jmf.2017.3960. [DOI] [PubMed] [Google Scholar]
- 97.Shin S.H., Lee Y.M. Glyceollins, a novel class of soybean phytoalexins, inhibit SCF-induced melanogenesis through attenuation of SCF/c-kit downstream signaling pathways. Exp. Mol. Med. 2013;45(4):e17. doi: 10.1038/emm.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rhodes L.V., Tilghman S.L., Boue S.M., Wang S., Khalili H., Muir S.E., et al. Glyceollins as novel targeted therapeutic for the treatment of triple-negative breast cancer. Oncol. Lett. 2012;3(1):163–171. doi: 10.3892/ol.2011.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wood C.E., Boue S.M., Collins-Burow B.M., Rhodes L.V., Register T.C., Cline J.M., et al. Glyceollin-elicited soy protein consumption induces distinct transcriptional effects as compared to standard soy protein. J. Agric. Food Chem. 2012;60(1):81–86. doi: 10.1021/jf2034863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bamji S.F., Page R.B., Patel D., Sanders A., Alvarez A.R., Gambrell C., et al. Soy glyceollins regulate transcript abundance in the female mouse brain. Funct. Integr. Genomics. 2015;15(5):549–561. doi: 10.1007/s10142-015-0442-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bamji S.F., Rouchka E., Zhang Y., Li X., Kalbfleisch T., Corbitt C. Next generation sequencing analysis of soy glyceollins and 17-β estradiol: effects on transcript abundance in the female mouse brain. Mol. Cell. Endocrinol. 2018;471:15–21. doi: 10.1016/j.mce.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 102.Szklarczyk D., Kirsch R., Koutrouli M., Nastou K., Mehryary F., Hachilif R., Annika G.L., Fang T., Doncheva N.T., Pyysalo S., Bork P., Jensen L.J., von Mering C. The STRING database in 2023: protein–protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023;51(D1):D638–D646. doi: 10.1093/nar/gkac1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yonekura-Sakakibara K., Higashi Y., Nakabayashi R. The origin and evolution of plant flavonoid metabolism. Front. Plant Sci. 2019;10:943. doi: 10.3389/fpls.2019.00943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang C., Wang X., Zhang F., Dong L., Wu J., Cheng Q., et al. Phenylalanine ammonia-lyase2.1 contributes to the soybean response towards Phytophthora sojae infection. Sci. Rep. 2017;7(1):7242. doi: 10.1038/s41598-017-07832-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang B., Lewis K.M., Abril Davydov DR., Vermerris W., Sattler S.E., et al. Structure and function of the cytochrome P450 monooxygenase cinnamate 4-hydroxylase from sorghum bicolor. Plant Physiol. 2020;183(3):957–973. doi: 10.1104/pp.20.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lindermayr C., Möllers B., Fliegmann J., Uhlmann A., Lottspeich F., Meimberg H., et al. Divergent members of a soybean (Glycine max L.) 4-coumarate: coenzyme A ligase gene family. Eur. J. Biochem. 2002;269(4):1304–1315. doi: 10.1046/j.1432-1033.2002.02775.x. [DOI] [PubMed] [Google Scholar]
- 107.Dhaubhadel S., Gijzen M., Moy P., Farhangkhoee M. Transcriptome analysis reveals a critical role of CHS7 and CHS8 genes for isoflavonoid synthesis in soybean seeds. Plant Physiol. 2007;143(1):326–338. doi: 10.1104/pp.106.086306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mameda R., Waki T., Kawai Y., Takahashi S., Nakayama T. Involvement of chalcone reductase in the soybean isoflavone metabolon: identification of GmCHR5, which interacts with 2-hydroxyisoflavanone synthase. Plant J. 2018;96(1):56–74. doi: 10.1111/tpj.14014. [DOI] [PubMed] [Google Scholar]
- 109.Ralston L., Subramanian S., Matsuno M., Yu O. Partial reconstruction of flavonoid and isoflavonoid biosynthesis in yeast using soybean type I and type II chalcone isomerases. Plant Physiol. 2005;137(4):1375–1388. doi: 10.1104/pp.104.054502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cochrane F.C., Davin L.B., Lewis N.G. The Arabidopsis phenylalanine ammonia lyase gene family: kinetic characterization of the four PAL isoforms. Phytochemistry. 2004;65(11):1557–1564. doi: 10.1016/j.phytochem.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 111.Ehlting J., Büttner D., Wang Q., Douglas C.J., Somssich I.E., Kombrink E. Three 4-coumarate: coenzyme A ligases in Arabidopsis thaliana represent two evolutionarily divergent classes in angiosperms. Plant J. 1999;19(1):9–20. doi: 10.1046/j.1365-313x.1999.00491.x. [DOI] [PubMed] [Google Scholar]
- 112.Mizutani M., Ohta D., Sato R. Isolation of a cDNA and a genomic clone encoding cinnamate 4-hydroxylase from Arabidopsis and its expression manner in planta. Plant Physiol. 1997;113(3):755–763. doi: 10.1104/pp.113.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Feinbaum R.L., Ausubel F.M. Transcriptional regulation of the Arabidopsis thaliana chalcone synthase gene. Mol. Cell Biol. 1988;8(5):1985–1992. doi: 10.1128/mcb.8.5.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Saito K., Yonekura-Sakakibara K., Nakabayashi R., Higashi Y., Yamazaki M., Tohge T., et al. The flavonoid biosynthetic pathway in Arabidopsis: structural and genetic diversity. Plant Physiol Biochem. 2013;72:21–34. doi: 10.1016/j.plaphy.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 115.Tohge T., de Souza L.P., Fernie A.R. Current understanding of the pathways of flavonoid biosynthesis in model and crop plants. J. Exp. Bot. 2017;68(15):4013–4028. doi: 10.1093/jxb/erx177. [DOI] [PubMed] [Google Scholar]
- 116.Liu W., Feng Y., Yu S., Fan Z., Li X., Li J., et al. The flavonoid biosynthesis network in plants. Int. J. Mol. Sci. 2021;22(23) doi: 10.3390/ijms222312824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Steele C.L., Gijzen M., Qutob D., Dixon R.A. Molecular characterization of the enzyme catalyzing the aryl migration reaction of isoflavonoid biosynthesis in soybean. Arch. Biochem. Biophys. 1999;367(1):146–150. doi: 10.1006/abbi.1999.1238. [DOI] [PubMed] [Google Scholar]
- 118.Akashi T., Aoki T., Ayabe S. Molecular and biochemical characterization of 2-hydroxyisoflavanone dehydratase. Involvement of carboxylesterase-like proteins in leguminous isoflavone biosynthesis. Plant Physiol. 2005;137(3):882–891. doi: 10.1104/pp.104.056747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang H., Murphy P.A. Isoflavone content in commercial soybean foods. J. Agric. Food Chem. 1994;42(8):1666–1673. [Google Scholar]
- 120.Liu Q., Liu Y., Li G., Savolainen O., Chen Y., Nielsen J. De novo biosynthesis of bioactive isoflavonoids by engineered yeast cell factories. Nat. Commun. 2021;12(1):6085. doi: 10.1038/s41467-021-26361-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Uchida K., Akashi T., Aoki T. Functional expression of cytochrome P450 in Escherichia coli: an approach to functional analysis of uncharacterized enzymes for flavonoid biosynthesis. Plant Biotech. 2015;32(3):205–213. [Google Scholar]
- 122.Akashi T., Aoki T., Ayabe S. CYP81E1, a cytochrome P450 cDNA of licorice (Glycyrrhiza echinata L.), encodes isoflavone 2'-hydroxylase. Biochem. Biophys. Res. Commun. 1998;251(1):67–70. doi: 10.1006/bbrc.1998.9414. [DOI] [PubMed] [Google Scholar]
- 123.Liu C.J., Huhman D., Sumner L.W., Dixon R.A. Regiospecific hydroxylation of isoflavones by cytochrome p450 81E enzymes from Medicago truncatula. Plant J. 2003;36(4):471–484. doi: 10.1046/j.1365-313x.2003.01893.x. [DOI] [PubMed] [Google Scholar]
- 124.Fischer D., Ebenau-Jehle C., Grisebach H. Phytoalexin synthesis in soybean: purification and characterization of NADPH:2'-hydroxydaidzein oxidoreductase from elicitor-challenged soybean cell cultures. Arch. Biochem. Biophys. 1990;276(2):390–395. doi: 10.1016/0003-9861(90)90737-j. [DOI] [PubMed] [Google Scholar]
- 125.Cheng Q., Li N., Dong L., Zhang D., Fan S., Jiang L., et al. Overexpression of soybean isoflavone reductase (GmIFR) enhances resistance to Phytophthora sojae in soybean. Front. Plant Sci. 2015;6:1024. doi: 10.3389/fpls.2015.01024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pei R., Zhang J., Ling T., Zhang S., Sun J. Identification of novel QTL associated with soybean isoflavone content. The Crop Journal. 2018;6(3):244–252. [Google Scholar]
- 127.Rípodas C., Via V.D., Aguilar O.M., Zanetti M.E., Blanco F.A. Knock-down of a member of the isoflavone reductase gene family impairs plant growth and nodulation in Phaseolus vulgaris. Plant Physiol Biochem. 2013;68:81–89. doi: 10.1016/j.plaphy.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 128.Wang X., He X., Lin J., Shao H., Chang Z., Dixon R.A. Crystal structure of isoflavone reductase from alfalfa (Medicago sativa L.) J. Mol. Biol. 2006;358(5):1341–1352. doi: 10.1016/j.jmb.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 129.Guo L., Paiva N.L. Molecular cloning and expression of alfalfa (Medicago sativa L.) vestitone reductase, the penultimate enzyme in medicarpin biosynthesis. Arch. Biochem. Biophys. 1995;320(2):353–360. doi: 10.1016/0003-9861(95)90019-5. [DOI] [PubMed] [Google Scholar]
- 130.Shao H., Dixon R.A., Wang X. Crystal structure of vestitone reductase from alfalfa (Medicago sativa L.) J. Mol. Biol. 2007;369(1):265–276. doi: 10.1016/j.jmb.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 131.Liu G.Y. Isolation, sequence identification and tissue expression profile of two novel soybean (glycine max) genes-vestitone reductase and chalcone reductase. Mol. Biol. Rep. 2009;36(7):1991–1994. doi: 10.1007/s11033-008-9409-y. [DOI] [PubMed] [Google Scholar]
- 132.Fischer D., Ebenau-Jehle C., Grisebach H. Purification and characterization of pterocarpan synthase from elicitor-challenged soybean cell cultures. Phytochemistry. 1990;29(9):2879–2882. doi: 10.1016/0003-9861(90)90737-j. [DOI] [PubMed] [Google Scholar]
- 133.Uchida K., Akashi T., Aoki T. The missing link in leguminous pterocarpan biosynthesis is a dirigent domain-containing protein with isoflavanol dehydratase activity. Plant Cell Physiol. 2017;58(2):398–408. doi: 10.1093/pcp/pcw213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hagmann M.L., Heller W., Grisebach H. Induction of phytoalexin synthesis in soybean. Stereospecific 3,9-dihydroxypterocarpan 6a-hydroxylase from elicitor-induced soybean cell cultures. Eur. J. Biochem. 1984;142(1):127–131. doi: 10.1111/j.1432-1033.1984.tb08259.x. [DOI] [PubMed] [Google Scholar]
- 135.Schopfer C.R., Kochs G., Lottspeich F., Ebel J. Molecular characterization and functional expression of dihydroxypterocarpan 6a-hydroxylase, an enzyme specific for pterocarpanoid phytoalexin biosynthesis in soybean (Glycine max L.) FEBS Lett. 1998;432(3):182–186. doi: 10.1016/s0014-5793(98)00866-7. [DOI] [PubMed] [Google Scholar]
- 136.Simons R., Vincken J.P., Roidos N., Bovee T.F., van Iersel M., Verbruggen M.A., et al. Increasing soy isoflavonoid content and diversity by simultaneous malting and challenging by a fungus to modulate estrogenicity. J. Agric. Food Chem. 2011;59(12):6748–6758. doi: 10.1021/jf2010707. [DOI] [PubMed] [Google Scholar]
- 137.Akashi T., Sasaki K., Aoki T., Ayabe S., Yazaki K. Molecular cloning and characterization of a cDNA for pterocarpan 4-dimethylallyltransferase catalyzing the key prenylation step in the biosynthesis of glyceollin, a soybean phytoalexin. Plant Physiol. 2009;149(2):683–693. doi: 10.1104/pp.108.123679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yoneyama K., Akashi T., Aoki T. Molecular characterization of soybean pterocarpan 2-dimethylallyltransferase in glyceollin biosynthesis: local gene and whole-genome duplications of prenyltransferase genes led to the structural diversity of soybean prenylated isoflavonoids. Plant Cell Physiol. 2016;57(12):2497–2509. doi: 10.1093/pcp/pcw178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sukumaran A., McDowell T., Chen L., Renaud J., Dhaubhadel S. Isoflavonoid-specific prenyltransferase gene family in soybean: GmPT01, a pterocarpan 2-dimethylallyltransferase involved in glyceollin biosynthesis. Plant J. 2018;96(5):966–981. doi: 10.1111/tpj.14083. [DOI] [PubMed] [Google Scholar]
- 140.Welle R., Grisebach H. Induction of phytoalexin synthesis in soybean: enzymatic cyclization of prenylated pterocarpans to glyceollin isomers. Arch. Biochem. Biophys. 1988;263(1):191–198. doi: 10.1016/0003-9861(88)90627-3. [DOI] [PubMed] [Google Scholar]
- 141.Schopfer C.R., Ebel J. Identification of elicitor-induced cytochrome P450s of soybean (Glycine max L.) using differential display of mRNA. Mol. Gen. Genet. 1998;258(4):315–322. doi: 10.1007/s004380050736. [DOI] [PubMed] [Google Scholar]
- 142.Guttikonda S.K., Trupti J., Bisht N.C., Chen H., An Y.Q., Pandey S., et al. Whole genome co-expression analysis of soybean cytochrome P450 genes identifies nodulation-specific P450 monooxygenases. BMC Plant Biol. 2010;10:243. doi: 10.1186/1471-2229-10-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang H., Waller L.C., Tripathy S., St, Martin S.K., Zhou L., Krampis K., Tucker D.M., et al. Analysis of genes underlying soybean quantitative trait loci conferring partial resistance to Phytophthora sojae. Plant Genome. 2010;3(1):23–40. [Google Scholar]
- 144.Yoshikawa M., Yamauchi K., Masago H. Glyceollin: its rle in restricting fungal growth in resistant soybean hypocotyls infected with Phytophthora megasperma var. sojae. Physiol Plant Path. 1978;12(1):73–82. [Google Scholar]
- 145.Zähringer U., Ebel J., Grisebach H. Induction of phytoalexin synthesis in soybean. Elicitor-induced increase in enzyme activities of flavonoid biosynthesis and incorporation of mevalonate into glyceollin. Arch. Biochem. Biophys. 1978;188(2):450–455. doi: 10.1016/s0003-9861(78)80029-0. [DOI] [PubMed] [Google Scholar]
- 146.Hahn M.G., Albersheim P. Host-pathogen interactions: XIV. Isolation and partial characterization of an elicitor from yeast extract. Plant Physiol. 1978;62(1):107–111. doi: 10.1104/pp.62.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yoshikawa M. Diverse modes of action of biotic and abiotic phytoalexin elicitors. Nature. 1978;275(5680):546–547. [Google Scholar]
- 148.Cline K., Wade M., Albersheim P. Host-pathogen interactions: XV. Fungal glucans which elicit phytoalexin accumulation in soybean also elicit the accumulation of phytoalexins in other plants. Plant Physiol. 1978;62(6):918–921. doi: 10.1104/pp.62.6.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bruegger B.B., Keen N.T. Specific elicitors of glyceollin accumulation in the Pseudomonas glycinea-soybean host-parasite system. Physiol Plant Path. 1979;15(1):43–51. [Google Scholar]
- 150.Kaplan D.T., Keen N.T., Thomason I.J. Association of glyceollin with the incompatible response of soybean roots to Meloidogyne incognita. Physiol Plant Path. 1980;16(3):309–318. [Google Scholar]
- 151.Reilly J.J., William L.K. Thymine dimer and glyceollin accumulation in U.V.-irradiated soybean suspension cultures. Environ. Exp. Bot. 1980;20(2):131–134. [Google Scholar]
- 152.Sutton D.C., Deverall B.J. Phytoalexin accumulation during infection of bean and soybean by ascospores and mycelium of Sclerotinia sclerotiorum. Plant Pathol. 2010;33(3):377–383. [Google Scholar]
- 153.Werner D., Mellor R.B., Hahn M.G., Grisebach H. Soybean root response to symbiotic infection glyceollin I accumulation in an ineffective type of soybean nodules with an early loss of the peribacteroid membrane. Z Naturforsch C. 1985;40c:179–181. [Google Scholar]
- 154.Bhattacharyya M.K., Ward E. Biosynthesis and metabolism of glyceollin I in soybean hypocotyls following wounding or inoculation with Phytophthora megasperma f. sp. glycinea. Physiol. Mol. Plant Pathol. 1987;31(3):387–405. [Google Scholar]
- 155.Favaron F., Alghisi P., Marciano P., Magro P. Polygalacturonase isoenzymes and oxalic acid produced by Sclerotinia sclerotiorum in soybean hypocotyls as elicitors of glyceollin. Physiol. Mol. Plant Pathol. 1988;33(3):385–395. [Google Scholar]
- 156.Bonhoff A., Grisebach H. Elicitor-induced accumulation of glyceollin and callose in soybean roots and localized resistance against Phytophthora megasperma f.sp. glycinea. Plant Sci. 1988;54(3):203–209. [Google Scholar]
- 157.Chakraborty U., Chakraborty B.N., Purkayastha R.P. Application of growth substances and mineral nutrition affecting disease development and glyceollin production of soybean. Folia Microbiol. 1989;34(6):490–497. [Google Scholar]
- 158.Wyss P., Boller T., Wiemken A. Glyceollin production in soybean during the process of infection by Glomus mosseae and Rhizoctonia solani. Agr Ecosyst Environ. 1990;29:451–456. [Google Scholar]
- 159.Komae K., Komae A., Misaki A. A 4,5-unsaturated low molecular oligogalacturonide as a potent phytoalexin-elicitor isolated from polygalacturonide of Ficus awkeotsang. Agr Biol Chem. 1990;54:1477–1484. [Google Scholar]
- 160.Montillet J.L., Norbert D. Hydroperoxides induce glyceollin accumulation in soybean. Plant Physiol Biochem. 1992;29(6):689–694. [Google Scholar]
- 161.Boué S.M., Carter C.H., Ehrlich K.C., Cleveland T.E. Induction of the soybean phytoalexins coumestrol and glyceollin by Aspergillus. J. Agric. Food Chem. 2000;48(6):2167–2172. doi: 10.1021/jf9912809. [DOI] [PubMed] [Google Scholar]
- 162.Landini S., Graham M.Y., Graham T.L. Lactofen induces isoflavone accumulation and glyceollin elicitation competency in soybean. Phytochemistry. 2003;62(6):865–874. doi: 10.1016/s0031-9422(02)00709-4. [DOI] [PubMed] [Google Scholar]
- 163.Isaac I.C., Johnson T.J., Berhow M., Baldwin E.L., Karki B., Woyengo T., et al. Evaluating the efficacy of fungal strains to stimulate glyceollin production in soybeans. Mycol. Prog. 2017;16(3):1–8. [Google Scholar]
- 164.Park I.S., Kim H.J., Jeong Y.S., Kim W.K., Kim J.S. Differential abilities of Korean soybean varieties to biosynthesize glyceollins by biotic and abiotic elicitors. Food Sci. Biotechnol. 2017;26(1):255–261. doi: 10.1007/s10068-017-0034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jahan M.A., Kovinich N. Acidity stress for the systemic elicitation of glyceollin phytoalexins in soybean plants. Plant Signal. Behav. 2019;14(7) doi: 10.1080/15592324.2019.1604018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kalli S., Araya-Cloutier C., Lin Y., de Bruijn W.J.C., Chapman J., Vincken J.P. Enhanced biosynthesis of the natural antimicrobial glyceollins in soybean seedlings by priming and elicitation. Food Chem. 2020;317 doi: 10.1016/j.foodchem.2020.126389. [DOI] [PubMed] [Google Scholar]
- 167.Jahan M.A., Harris B., Lowery M., Coburn K., Infante A.M., Percifield R.J., Ammer A.G., Kovinich N. The NAC family transcription factor GmNAC42–1 regulates biosynthesis of the anticancer and neuroprotective glyceollins in soybean. BMC Genom. 2019;20:149. doi: 10.1186/s12864-019-5524-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jahan M.A., Harris B., Lowery M., Infante A.M., Percifield R.J., Kovinich N. Glyceollin transcription factor GmMYB29A2 regulates soybean resistance to Phytophthora sojae. Plant Physiol. 2020;183(2):530–546. doi: 10.1104/pp.19.01293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lin J., Monsalvo I., Ly M., Jahan M.A., Wi D., Martirosyan I., et al. RNA-seq dissects incomplete activation of phytoalexin biosynthesis by the soybean transcription factors GmMYB29A2 and GmNAC42-1. Plants. 2023;12(3):545. doi: 10.3390/plants12030545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yasmin F., Zhang H., Leamy L., Wang B., Winnike J., Reid R.W., et al. Genetic basis and selection of glyceollin induction in wild soybean. bioRxiv. 2022;17 doi: 10.3389/fpls.2024.1240981. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ahmed S., Kovinich N. Regulation of phytoalexin biosynthesis for agriculture and human health. Phytochem Rev. 2021;20:483–505. [Google Scholar]
- 172.Khupse R.S., Erhardt P.W. Total syntheses of racemic, natural (-) and unnatural (+) glyceollin I. Org. Lett. 2008;10(21):5007–5010. doi: 10.1021/ol802112r. [DOI] [PubMed] [Google Scholar]
- 173.Khupse R.S., Sarver J.G., Trendel J.A., Bearss N.R., Reese M.D., Wiese T.E., et al. Biomimetic syntheses and antiproliferative activities of racemic, natural (-), and unnnatural (+) glyceollin I. J. Med. Chem. 2011;54(10):3506–3523. doi: 10.1021/jm101619e. [DOI] [PubMed] [Google Scholar]
- 174.Kohno Y., Koso M., Kuse M., Takikawa H. Formal synthesis of soybean phytoalexin glyceollin I. Tetrahedron Lett. 2014;55(10):1826–1828. [Google Scholar]
- 175.Malik N., Zhang Z., Erhardt P. Total synthesis of (±)-Glyceollin II and a dihydro derivative. J Nat Prod. 2015;78(12):2940–2947. doi: 10.1021/acs.jnatprod.5b00607. [DOI] [PubMed] [Google Scholar]
- 176.Ciesielski P., Metz P. Asymmetric one-pot transformation of isoflavones to pterocarpans and its application in phytoalexin synthesis. Nat. Commun. 2020;11(1):3091. doi: 10.1038/s41467-020-16933-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.van de Schans M.G., Vincken J.P., Bovee T.F., Cervantes A.D., Logtenberg M.J., Gruppen H. Structural changes of 6a-hydroxy-pterocarpans upon heating modulate their estrogenicity. J. Agric. Food Chem. 2014;62(43):10475–10484. doi: 10.1021/jf503127c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data availability is not applicable to this article as no new data were created or analyzed in this study.