Abstract
Adverse events (AEs) of antipsychotic drugs include neuroleptic malignant syndrome (NMS), which presents complex clinical symptoms, resulting in a fatal outcome. In this study, the association between antipsychotic drugs and NMS was comprehensively evaluated by cluster and association analyses using the Japanese Adverse Drug Event Report (JADER) database.
The analyses were performed using 20 typical antipsychotics (TAPs) alongside 9 atypical antipsychotics (AAPs). The Standardised MedDRA Queries (SMQ) database was used to analyze NMS (SMQ code: 20000044). Reporting odds ratios (RORs) were used for AE signal detection. The relationship between antipsychotic drugs and AEs for NMS was investigated by performing hierarchical cluster analysis using Ward's method.
Between April 2004 and September 2021, the total number of JADER reports was 705,294. RORs (95 % confidence interval) of NMS for haloperidol, chlorpromazine, risperidone, and aripiprazole were 12.1 (11.1–13.3), 6.3 (5.7–7.0), 6.2 (5.8–6.6), and 4.7 (4.4–5.1), respectively. Three clusters were formed, with characteristics as follows: Cluster 1 consisted of only TAPs, such as bromperidol and fluphenazine, whilst having a high reporting rate of hypotension, tachycardia, dyskinesia, and dystonia. Cluster 2 consisted of all AAPs alongside several TAPs, such as haloperidol and chlorpromazine, with higher reporting rates of disturbance of consciousness, extrapyramidal disorders (excluding dyskinesia and dystonia), and serotonin syndrome. Cluster 3 consisted of only perphenazine, whilst having a higher reporting rate of coma, leukocytosis, and Parkinsonism.
The results of this study may therefore aid in the management of NMS using antipsychotic drugs.
1. Introduction
Neuroleptic malignant syndrome (NMS) is primarily caused by the administration, reduction, or cessation of neuropsychiatric drugs and can be characterized by fever, disturbance of consciousness, extrapyramidal disorder, and autonomic nervous system imbalance, with an incidence of 0.07%–2.2 % [1]. Previously, NMS has had a high mortality rate; however, as medical professionals gradually became more aware of it with the progression of clinical research, the prognosis has improved remarkably. However, its etiology and pathophysiology have not yet been fully elucidated [1]. Although it requires prompt treatment, NMS is often diagnosed late in outpatient care. It has a special course, becomes severe, and requires attention in clinical settings. In addition to psychiatry, psychoneurotic drugs are used in psychosomatic medicine, neurology, internal medicine, surgery, and anesthesiology, alongside other clinical departments. Therefore, NMS is not only relevant to treating psychiatric problems but also affects health problems reported by other fields [[1], [2], [3], [4], [5]].
The clinical manifestations of NMS include acute fever and disturbance of consciousness, extrapyramidal disorder (muscle rigidity, tremors, dystonia, dyskinesia, etc.), autonomic nervous system imbalance (hyperhidrosis, tachycardia, abnormal blood pressure, etc.), myoclonus, and respiratory failure. In severe cases of NMS, skeletal muscle tissue dissolution occurs and progresses, whilst blood and urinary myoglobin levels increase, leading to acute renal failure [1]. Additionally, NMS can cause metabolic acidosis and disseminated intravascular coagulation [6], with muscle rigidity observed in most cases. Furthermore, some patients show no impairment of consciousness, whereas others present with delirium or coma [7]. As described above, the clinical symptoms are diverse; however, the characteristics of causative drugs remain unknown.
The Spontaneous Reporting System (SRS) for Adverse Events (AEs), published by regulatory authorities in the United States and Japan, has played a major role in the evaluation of drug safety. Based on AE signals obtained by data mining using the SRS database, it is possible to detect unknown AEs that have not been discovered in clinical trials as well as evaluate safety in specific populations and reflect actual clinical uses [8,9]. In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) has released the Japanese Adverse Drug Event Report (JADER) database, which contains more than 700,000 total AEs. We conducted a comprehensive drug safety evaluation of NMS using this database.
Cluster analysis (CA) [10,11] and association analysis (AA) [[12], [13], [14]] are useful statistical techniques in data mining. CA is used to find natural groupings within a dataset. Several uses of CA have been reported in the literature, such as characterizing psychiatric patients on the basis of clusters of symptoms [15], identifying the profile of optimal candidates for antipsychotic depot therapy [16], or identifying profiles of functioning and correlations of those profiles in a sample of patients with stable schizophrenia in a real-world setting [17]. This study aimed to comprehensively evaluate the incidence profile of NMS caused by antipsychotic drugs and find useful cluster information. A comprehensive evaluation of 29 types of antipsychotic drugs and 52 AEs was carried out by performing hierarchical CA.
AA is used to find frequent patterns and undetected clinical factor combinations between variables in huge databases, and it may be useful in decision-making by finding relationships between attributes in the database [[12], [13], [14]]. AA has been applied to evaluate the relationship between AEs and their risk factors [9,[18], [19], [20]]. Therefore, herein, we evaluated the relationship between NMS and antipsychotic drugs combinations by performing AA.
2. Methods
2.1. Data source
Data regarding AE reports were collected and fully anonymized by the PMDA to form the JADER database. The AE reports recorded in this database were downloaded from the PMDA website (www.pmda.go.jp). The JADER dataset was also downloaded from the PMDA website. The JADER data were divided into 4 files, “demo,” “drug,” “reac,” and “hist,” with each data table being linked by an identification number and integrated into a relational database. The content of the tables was as follows: The “demo” table contained basic patient information such as sex, age, and reporting year; the “drug” table comprised information on medication, such as the generic name of the drug administered, route of administration, and start and end dates of administration; the “reac” table contained AE, outcome, and date of onset, and the “hist” table included information on the patient's primary disease. We assessed this database for reports submitted between April 2004 and September 2021. The structure of this database complied with international safety reporting guidelines (International Council on Harmonization E2B).
The “drug” table described the presumed degree of drug involvement in the AEs as follows: “suspected drug,” “concomitant drug,” and “interacting drug.” In this retrospective pharmacovigilance study, data on the “suspected drugs” were extracted and analyzed. We subsequently integrated the relational database based on the four aforementioned tables using the FileMaker Pro 18 Advanced software (FileMaker, Inc. Santa Clara, CA, USA).
2.2. Definition of AEs
The AEs in the JADER database were coded using MedDRA ver. 23.1 (MedDRA/J, www.pmrj.jp/jmo/php/indexj.php). Additionally, the Standardised MedDRA Queries (SMQ) database was used for the analysis of SRSs. SMQ is a grouping of MedDRA terms for a defined condition or area of interest, usually at the Preferred Term (PT) level. We used the SMQ here for NMS (SMQ code: 20000044, containing 67 PTs). In this study, 52 PTs were analyzed, after excluding 15 that had reported no AEs (Table 1).
Table 1.
The number of cases for each preferred terms of standardised MedDRA Queries of neuroleptic malignant syndrome (SMQa code: 20000044).
| Category | Category for cluster analysis | Preferred terms code | Preferred terms | Case (n) | Case used for the cluster analysis (n) |
|---|---|---|---|---|---|
| Autonomic nervous system imbalance | – | 10003840 | Autonomic nervous system imbalance | 86 | 86 |
| i_Blood pressure abnormal | 10005728 | Blood pressure abnormal | 24 | 136 | |
| i_Blood pressure abnormal | 10023533 | Labile blood pressure | 17 | – | |
| i_Blood pressure abnormal | 10005746 | Blood pressure fluctuation | 95 | – | |
| – | 10019303 | Heart rate increased | 310 | 310 | |
| – | 10020642 | Hyperhidrosis | 473 | 473 | |
| i_Hypertension | 10005750 | Blood pressure increased | 2342 | 5402 | |
| i_Hypertension | 10020772 | Hypertension | 3060 | – | |
| i_Hypotension | 10005734 | Blood pressure decreased | 8003 | 9950 | |
| i_Hypotension | 10021097 | Hypotension | 1947 | – | |
| – | 10043071 | Tachycardia | 1508 | 1508 | |
| Cardiovascular insufficiency | – | 10065929 | Cardiovascular insufficiency | 23 | 23 |
| Disturbance of consciousness | – | 10001854 | Altered state of consciousness | 6138 | 6138 |
| – | 10010071 | Coma | 462 | 462 | |
| – | 10010305 | Confusional state | 441 | 441 | |
| – | 10050093 | Consciousness fluctuating | 10 | 10 | |
| – | 10012218 | Delirium | 3059 | 3059 | |
| – | 10012373 | Depressed level of consciousness | 2888 | 2888 | |
| – | 10013395 | Disorientation | 340 | 340 | |
| – | 10024855 | Loss of consciousness | 5375 | 5375 | |
| – | 10042264 | Stupor | 130 | 130 | |
| Extrapyramidal disorder | – | 10007776 | Catatonia | 62 | 62 |
| – | 10013916 | Dyskinesia | 956 | 956 | |
| – | 10013983 | Dystonia | 603 | 603 | |
| – | 10015832 | Extrapyramidal disorder | 469 | 469 | |
| – | 10060904 | Freezing phenomenon | 5 | 5 | |
| – | 10020651 | Hyperkinesia | 32 | 32 | |
| – | 10020852 | Hypertonia | 27 | 27 | |
| – | 10080149 | Malignant catatonia | 1 | 1 | |
| – | 10028330 | Muscle rigidity | 147 | 147 | |
| – | 10028622 | Myoclonus | 342 | 342 | |
| – | 10030071 | Oculogyric crisis | 140 | 140 | |
| – | 10030899 | Opisthotonus | 13 | 13 | |
| i_Parkinsonism | 10061536 | Parkinson's disease | 365 | 1157 | |
| i_Parkinsonism | 10034010 | Parkinsonism | 792 | – | |
| – | 10041045 | Slow response to stimuli | 3 | 3 | |
| – | 10044565 | Tremor | 1324 | 1324 | |
| – | 10045555 | Unresponsive to stimuli | 75 | 75 | |
| Hyperthermia malignant | – | 10020844 | Hyperthermia malignant | 298 | 298 |
| Leukocytosis | i_Leukocytosis | 10024378 | Leukocytosis | 74 | 1036 |
| i_Leukocytosis | 10047943 | White blood cell count increased | 962 | – | |
| Neuroleptic malignant syndrome | – | 10029282 | Neuroleptic malignant syndrome | 3461 | 3461 |
| Pyrexia | i_Pyrexia | 10005911 | Body temperature increased | 75 | 14812 |
| i_Pyrexia | 10020741 | Hyperpyrexia | 20 | – | |
| i_Pyrexia | 10037660 | Pyrexia | 14717 | – | |
| Rhabdomyolysis | – | 10005468 | Blood creatine phosphokinase abnormal | 11 | 11 |
| – | 10005470 | Blood creatine phosphokinase increased | 3136 | 3136 | |
| – | 10028625 | Myoglobin blood increased | 51 | 51 | |
| – | 10028631 | Myoglobin urine present | 10 | 10 | |
| – | 10028629 | Myoglobinuria | 32 | 32 | |
| – | 10039020 | Rhabdomyolysis | 6750 | 6750 | |
| Serotonin syndrome | – | 10040108 | Serotonin syndrome | 702 | 702 |
SMQ: Standardised MedDRA Queries.
2.3. Target drugs
In this study, the analyses were performed using 20 typical antipsychotics (TAPs) alongside 9 atypical antipsychotics (AAPs), according to classifications based on the Japanese Society of Neuropsychopharmacology (http://www.jsnp-org.jp/) and previous reports (Fig. 1) [4].
Fig. 1.
Flowchart of data analysis.
2.4. Signal detection
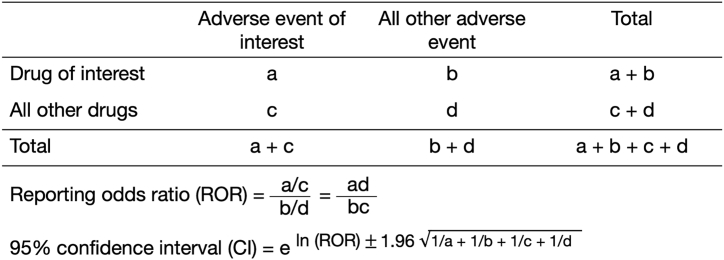
The reporting odds ratio (ROR), which is widely used in post-marketing drug safety evaluations, was used for signal detection [21]. The ROR was calculated from a 2 × 2 contingency table and was regarded as a signal when the lower limit of the 95 % confidence interval (CI) exceeded 1. Two or more cases were necessary to positively identify such signals (Fig. 2) [8].
Fig. 2.
Two-by-two contingency table for calculation of reporting odds ratio.
2.5. CA
CA was used to analyze the association between each PT of NMS and antipsychotic drugs. CA is an “unsupervised classification method” whereby classification criteria are not predetermined [10,11]. It assigns data to groups with similar properties. In this study, we used agglomerative hierarchical clustering to classify 29 drugs and subsequently analyzed the relationship between the RR of AEs for each drug. Clusters are typically generated from standardized data using Ward's method with Euclidean distance. Ward's method merges the clusters to minimize the sum of the squares within the cluster. The distance between clusters is defined as an increase in the sum of squares when two clusters are merged. Clusters of the antipsychotic drugs were formed, with the number of formations being determined based on the characteristics of each cluster. Considering that this analysis is generally not associated with a probabilistic evaluation, it is common for researchers to make appropriate decisions concerning the number of clusters with the greatest perceived significance.
Data analyses in this study were performed using JMP Pro 16.2 (SAS Institute Inc., Cary, NC, USA).
2.6. AA
AA focuses on finding frequent co-occurring associations among a collection of items. Given a set of transactions T (each transaction is a set of items), an association rule can be expressed as X [lhs: left-hand-side, the antecedent of the rule] → Y [rhs: right-hand-side, the consequent of the rule], where X and Y are mutually exclusive sets of items [[12], [13], [14]]. If the association rule of X → Y is true, then support, confidence, and lift values can be calculated to evaluate the correlation indicated by this rule. Support determines how often a rule, which in this case is the combination of X and Y, is observed in the database. Support was measured using the following formula:
where D is the total number of transactions in the database. Support is the probability that X and Y are simultaneously true.
The confidence value of the association rule demonstrates the rule's strength. Confidence was calculated by the following equation:
Lift represents the ratio of probability. Given the rule that X and Y occur together to the multiple of the two individual probabilities for X and Y, lift was calculated as follows:
Lift is a measure of the relationship between X and Y, indicating information regarding an increase in the probability of Y when it is provided with X. Higher lift values indicate stronger relationships. If X and Y are independent, lift equals 1. If X and Y are positively or negatively correlated, lift is > 1 or <1, respectively. AA was performed using the apriori function of the arules package of R version 4.3.1. In the first step, the apriori algorithm searches for item sets that have more than a given minimum support, whereas in the second step, rules are generated by selecting “confident” item sets from those found in the first step. The parameter maxlen (maximum length of item set/rule, a parameter in the arules package) is the maximum size of mined frequent item sets. To establish association rules efficiently, the thresholds for the optimized support, confidence, and maxlen are defined depending on factors such as the size of the data, the number of items, and the purpose of the research. Support and lift were visualized using the R-extension package arulesViz, which implements visualization techniques to explore association rules. The arguments of the plot in arulesViz were set as follows: method = “graph,” measure = “support,” shading = “lift.” The measures of support were used in visualization as the area of the circle. The measures of lift were used for the shading of the color of the circle.
3. Results
The JADER database contained 705,294 reports published between April 2004 and September 2021. The respective numbers of NMS (SMQ code: 20000044) reported for haloperidol, chlorpromazine, risperidone, and aripiprazole were 1011, 588, 1504, and 1079. The ROR (95 % CI) for each drug was 12.1 (11.1–13.3), 6.3 (5.7–7.0), 6.2 (5.8–6.6), and 4.7 (4.4–5.1), respectively. Additionally, the reporting ratio (RR) and ROR for each antipsychotic drug are summarized in Supplementary Tables S1–S52. The RRs of NMS for haloperidol, chlorpromazine, risperidone, and aripiprazole were 54.8 % (1011/1845), 38.7 % (588/1520), 38.1 % (1504/3950), and 32.0 % (1079/3369), respectively (Table 2).
Table 2.
Number of reports, reporting ratio, and reporting odds ratio of neuroleptic malignant syndrome (SMQa code: 20000044) associated with antipsychotics.
| Drugs | Total | Case (n) | Non-case (n) | Reporting ratio (%) | Reporting odds ratio (95 % conficdence interval) | |
|---|---|---|---|---|---|---|
| Typical antipsychotics | Bromperidol | 169 | 85 | 84 | 50.3 | 10.0 (7.4–13.5) |
| Chlorpromazine | 1520 | 588 | 932 | 38.7 | 6.3 (5.7–7.0) | |
| Clocapramine | 24 | 11 | 13 | 45.8 | 8.4 (3.7–18.7) | |
| Fluphenazine | 129 | 74 | 55 | 57.4 | 13.3 (9.4–18.9) | |
| Haloperidol | 1845 | 1011 | 834 | 54.8 | 12.1 (11.1–13.3) | |
| Levomepromazine | 1110 | 492 | 618 | 44.3 | 7.9 (7.0–8.9) | |
| Mosapramine | 25 | 12 | 13 | 48.0 | 9.1 (4.2–20.0) | |
| Nemonapride | 15 | 5 | 10 | 33.3 | 4.9 (1.7–14.5) | |
| Oxypertine | 18 | 6 | 12 | 33.3 | 4.9 (1.9–13.2) | |
| Perphenazine | 86 | 45 | 41 | 52.3 | 10.8 (7.1–16.6) | |
| Pimozide | 60 | 24 | 36 | 40.0 | 6.6 (3.9–11.0) | |
| Pipamperone | 19 | 6 | 13 | 31.6 | 4.6 (1.7–12.0) | |
| Prochlorperazine | 220 | 80 | 140 | 36.4 | 5.6 (4.3–7.4) | |
| Propericiazine | 124 | 59 | 65 | 47.6 | 9.0 (6.3–12.8) | |
| Reserpine | 18 | 4 | 14 | 22.2 | 2.8 (0.9–8.6) | |
| Spiperone | 2 | 1 | 1 | 50.0 | –b | |
| Sultopride | 82 | 49 | 33 | 59.8 | 14.7 (9.4–22.8) | |
| Tiapride | 472 | 192 | 280 | 40.7 | 6.8 (5.7–8.2) | |
| Timiperone | 39 | 18 | 21 | 46.2 | 8.5 (4.5–15.9) | |
| Zotepine | 456 | 213 | 243 | 46.7 | 8.7 (7.2–10.4) | |
| Atypical antipsychotics | Aripiprazole | 3369 | 1079 | 2290 | 32.0 | 4.7 (4.4–5.1) |
| Asenapine | 399 | 107 | 292 | 26.8 | 3.6 (2.9–4.5) | |
| Blonanserin | 914 | 398 | 516 | 43.5 | 7.7 (6.7–8.7) | |
| Clozapine | 2512 | 382 | 2130 | 15.2 | 1.8 (1.6–2.0) | |
| Olanzapine | 2623 | 812 | 1811 | 31.0 | 4.5 (4.1–4.9) | |
| Paliperidone | 1368 | 362 | 1006 | 26.5 | 3.6 (3.2–4.0) | |
| Perospirone | 534 | 246 | 288 | 46.1 | 8.5 (7.1–10.0) | |
| Quetiapine | 2972 | 852 | 2120 | 28.7 | 4.0 (3.7–4.3) | |
| Risperidone | 3950 | 1504 | 2446 | 38.1 | 6.2 (5.8–6.6) |
SMQ: Standardised MedDRA Queries.
n < 2.
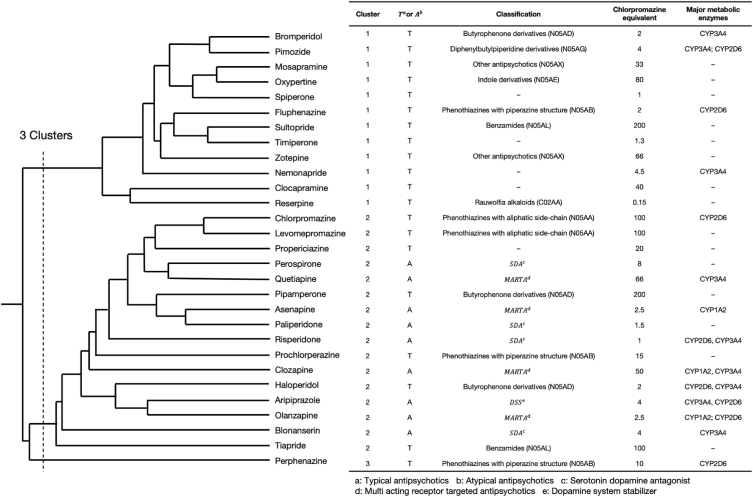
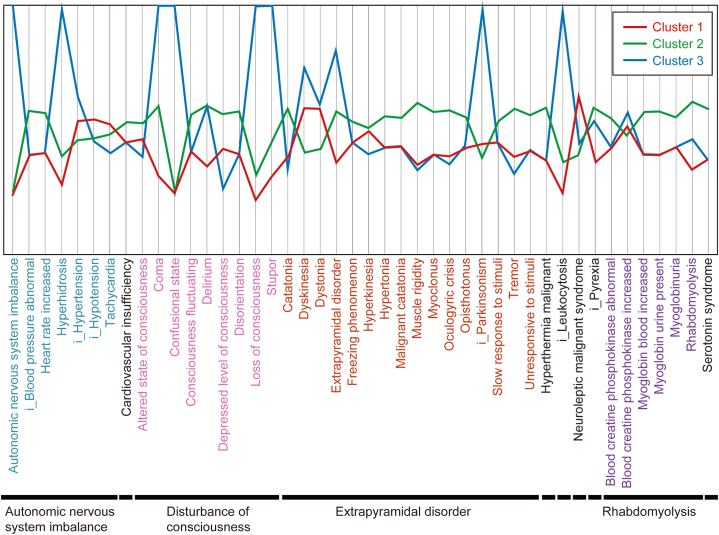
A total of three clusters were formed (Fig. 3), with the characteristics of each cluster being as follows: Cluster 1 consisted of only TAPs, such as bromperidol, fluphenazine, and sultopride, whilst having a high reporting rate of hypotension, tachycardia, dyskinesia, and dystonia; Cluster 2 consisted of all AAPs used in this study alongside several TAPs, such as haloperidol and chlorpromazine. The RRs of disturbance of consciousness, extrapyramidal disorder (excluding dyskinesia and dystonia), and serotonin syndrome were all high. Furthermore, Cluster 3 consisted of perphenazine only and had a high rate of coma, leukocytosis, and Parkinsonism (Fig. 4).
Fig. 3.
Dendrogram representing the clusters (Cluster 1: Consists only of typical antipsychotics, such as bromperidol, fluphenazine, and sultopride, whilst having a high reporting rate of hypotension, tachycardia, dyskinesia, and dystonia; Cluster 2: Consists of all atypical antipsychotics used in this study with several typical antipsychotics such as haloperidol and chlorpromazine. The reporting ratios for disturbance of consciousness, extrapyramidal symptoms (excluding dyskinesia and dystonia) and serotonin syndrome were high; Cluster 3: Consists of only perphenazine and has a high rate of reports of coma, leukocytosis, and Parkinsonism).
Fig. 4.
Cluster mean plots of cluster analysis. The axis ranges from two standard deviations above and below the mean. If a cluster mean falls beyond this range, the axis is extended to include it.
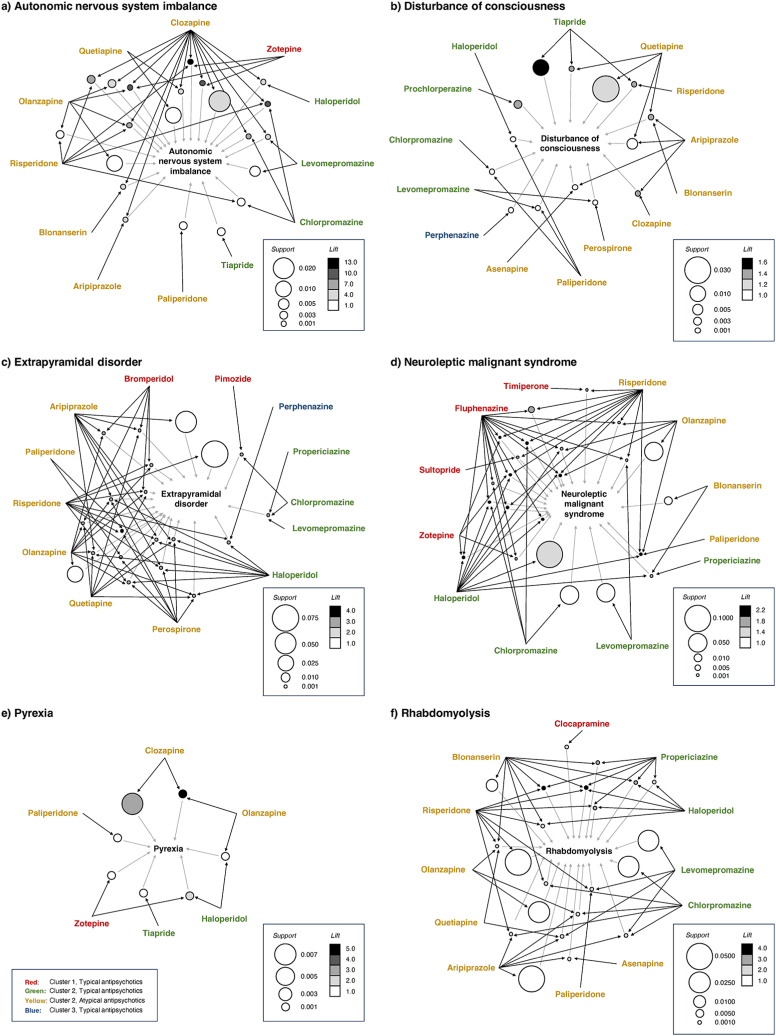
We evaluated the possible association between NMS and each antipsychotic. The apriori algorithm of AA identified a set of rules for autonomic nervous system imbalance, disturbance of consciousness, extrapyramidal disorder, NMS, pyrexia, and rhabdomyolysis (Fig. 5, Supplementary Tables S53–S58). The association rules {Clozapine, Risperidone, Zotepine} → {Autonomic nervous system imbalance} showed the high lift value (Fig. 5 (a), Table S53 (id [1])). The association rules {Chlorpromazine, Fluphenazine, Haloperidol} → {NMS}(Fig. 5 (d), Table S56 (id [1])), {Chlorpromazine, Fluphenazine, Risperidone} → {NMS}(Fig. 5 (d), Table S56 (id [2])), and {Fluphenazine, Haloperidol, Risperidone} → {NMS}(Fig. 5 (d), Table S56 (id [4])) exhibited high scores for lift. The association rules {Haloperidol} → {NMS} exhibited high scores for support (Fig. 5 (d), Table S56 (id [19])).
Fig. 5.
Visualization of correlation rules established by performing association rule analysis.
4. Discussion
Antipsychotics are classified as either TAPs or AAPs, with TAPs having a very high affinity for dopamine D2 receptors whilst being generally effective against positive symptoms, such as delusions and hallucinations. AAPs exhibit serotonin 5-HT2 receptor antagonistic activity in addition to dopamine D2 receptor antagonistic activity and are effective for negative symptoms such as emotional dullness and lack of spontaneity [22,23]. Although the etiology of NMS remains unknown, most NMS symptoms probably result from a rapid decrease in central dopaminergic activity because of the blockade of D2 receptors or the abrupt cessation of D2 receptor stimulation. In this regard, higher doses of neuropsychiatric drugs are correlated with a greater risk of developing NMS.
The reason for the higher risk of NMS when using TAPs is related to the high dopamine D2 receptor affinity of the drug and low binding dissociation from the receptor. AAPs are currently the drug of choice for treating the first episode of schizophrenia [24,25]. Considering that AAPs have fewer drawbacks than those of TAPs, they are more likely to exert an effect on negative symptoms and are less likely to cause extrapyramidal disorder [26]. However, no theory has been established for comparing TAP-related NMS risk with AAP-related NMS risk. A balanced dopamine D2 and 5-HT receptor blockade, particularly 5-HT2A receptor blockade [27], in AAPs has been proposed as a reason for lower NMS risk associated with AAPs than that with TAPs; however, it is not conclusive. Additionally, the possibility of publication bias has been proposed. All neuropsychiatric drugs have been reported to have the risk of NMS associated with their administration at standard doses and all routes of administration. Moreover, the present AA results showed that in extrapyramidal disorders, AAP-related rules were more common than TAP-related rules (Fig. 5 (c)), and in NMS, TAP-related rules tend to have higher lift values than those observed for AAP-related rules (Fig. 5 (d)).
Of the 29 drugs included in this study, the lower 95 % CI for the ROR of 27 NMS (SMQ code: 20000044) drugs, excluding reserpine and spiperone, exceeded 1. As a result, a relationship between antipsychotic drugs and NMS was proposed here. NMS was included among the serious AE items in the package inserts of all drugs used in this study, except for reserpine. Healthcare professionals should pay close attention to NMS in a patient who is administered not only TAPs but also AAPs, which are sought to have a relatively low risk of NMS compared with the risk associated with TAPs according to the previous reports [26,28].
CA defines similarities between multiple drugs and classifies them into homogeneous clusters, and it is an exploratory data analysis technique used to summarize the tendencies of sets such as unknown databases and to intuitively understand their content. Clustering is based on subjectivity or a point of view and can be divided into arbitrary clustering numbers by the researcher. The validity of clustering results can only be subsequently determined through external knowledge, such as the intended use of the division. If the user can interpret the division result, then it can be said that the correct number of clusters has been formed [10]. Therefore, the validity of clustering results must always be examined based on the purpose of their use. Here, we assigned a clustering number of three based on the similarity in expression profiles across the 52 AEs.
The characteristic information for each cluster shown in Fig. 3 demonstrates the trend in the occurrence of AEs common to drug groups belonging to the cluster information. This provides information on the reporting rates of AEs associated with NMS for each antipsychotic drug. Healthcare professionals can use Fig. 3, Fig. 4, Fig. 5 to obtain an overview of AEs that should be noted in advance for each drug used.
The extrapyramidal disorder is less likely to occur in patients receiving AAPs compared with those receiving TAPs [26,29]. However, several previous studies have indicated that AAPs can cause extrapyramidal disturbances to the same extent as TAPs [30]. However, multiple other studies have also suggested that AAPs and TAPs carry similar risks of extrapyramidal disturbance [31,32]. Cluster 2, which included all AAPs alongside several TAPs, was more likely to have AEs related to NMS (especially extrapyramidal symptoms, excluding dyskinesia and dystonia) compared with Cluster 1, which consisted of only TAPs. Cluster 2 included several TAPs in addition to AAPs; therefore, the results should be interpreted with caution.
Recently, several studies have investigated the association between NMS and CYP2D6 deletion. CYP2D6 activity has been shown to have genetic alterations that show differences in activity among ultra-rapid, extensive, intermediate, and poor metabolizers, with the percentage of intermediate mediators in Japan being 40%–50 % [33]. Several previous studies have also suggested that the delayed degradation of antipsychotic drugs metabolized by CYP2D6 may increase the risk of AEs [34]. Furthermore, one report found that those with CYP2D6*5 polymorphism (poor metabolizer type) were significantly more likely to develop NMS [35]. In addition, Kirchheiner et al. reported that it was better to adjust the dose of antipsychotic drugs by a factor of 0.33–1.5, based on the metabolic capacity of CYP2D6 [36]. Among the drugs used in this study, the TAPs pimozide, fluphenazine, chlorpromazine, perphenazine, and haloperidol, alongside AAP, such as aripiprazole, olanzapine, and risperidone, were all metabolized by CYP2D6 [37]. Because chlorpromazine (TAP) and asenapine (AAP) inhibit CYP2D6 [38,39], their concomitant use with other neuropsychiatric drugs metabolized by CYP2D6, such as risperidone and olanzapine, may increase blood concentration levels of these drugs and increase the risk of NMS. However, the present AA results showed no clear concomitant interactions. In addition to neuropsychiatric drugs, many other CYP2D6 inhibitors are known, including paroxetine (selective serotonin reuptake inhibitor), amiodarone (antiarrhythmic), cimetidine (H2-receptor antagonist), and quinidine (antiarrhythmic) [2,5,40]. We did not examine these concomitant medications in this study, and we believe that future studies should examine the mentioned CYP2D6 inhibitors.
An interesting approach could be investigating the relationship between NMS and the metabolic enzymes of antipsychotics. Whether the characteristics of the three clusters could be explained by metabolic enzymes or other genes is a subject for future investigation.
The limitations of analyses using SRS should be noted here. SRS uses insufficient information in terms of patient background, whilst under-reporting or over-reporting confounding factors, and no control population or reference group. To date, no widely accepted method has been established for adjusting the covariates in studies using SRS datasets. Multiple logistic regression methods have been reported to partially adjust for confounding factors [18]. Propensity scores may be used to reduce bias by equating groups based on possible confounders [41,42]. Alternately, some recent studies have provided some approaches to address these issues in a high-dimensional context [[43], [44], [45]]. Therefore, future studies should investigate problems such as adjusting covariates and reducing biases. Moreover, the present results must be carefully interpreted considering the limitations of this study. Furthermore, we need to consider external factors, such as the release of safety information from administrative authorities and market trends, and the possibility of overlooking delayed AEs. Considering the inherent problems of SRS, it should not be used for true risk assessment [8,21]. Drug therapy for patients with schizophrenia is based on the single-agent administration of AAPs. Depending on the efficacy and side effects of a drug, there are many cases of changes or additions to other drugs, as well as several cases of multidrug therapy [46,47]. Previous reports have shown that polypharmacy is a risk factor for NMS [48]. However, our analysis did not adequately consider polypharmacy. Malignant syndrome is considered to be less affected by the male-to-female ratio and age of onset [1]; therefore, we did not examine this by sex or age. In addition, we did not consider the potency or dosage of each antipsychotic drug. Therefore, an analysis that considers these factors will be conducted in the future.
Despite these analytical limitations, the findings obtained herein from JADER, a large-scale AE database that accumulates long-term cases collected from actual clinical settings, are considered useful. Therefore, it is important to continue medication for the treatment of schizophrenia. Owing to the long-term treatment, many patients stop taking the drug of their own accord. Additionally, AEs of antipsychotics can lead to their discontinuation. As a result of knowing the AE profile in advance, it is considered possible for medical professionals to predict characteristic NMSs that are likely to occur in a patient. The findings of this study can be further applied to CA and AA by incorporating datasets on individual susceptibility to NMS and psychological and social factors in order to develop a program to predict the risk factors of NMS in clinical practices. Overall, we believe that the results of this study will contribute to the treatment of schizophrenia.
5. Conclusion
Using the JADER database, we demonstrated the potential risks of NMS associated with antipsychotics based on RORs. Drugs were then classified based on their AE profiles by performing CA and AA. It is important to provide information to other healthcare professionals and patients, considering the characteristics of AEs reported in each cluster or the effects of concomitant antipsychotic drugs. Our study results may aid in the management of NMS using antipsychotic drugs.
Ethics approval
Ethical approval was not sought for this study because the study was a database-related observational study without directly involving any research subjects. All results were obtained from data openly available online from the PMDA website (www.pmda.go.jp). All data from the JADER database were fully anonymized by the relevant regulatory authority before we accessed them.
Funding statement
This research was partially supported by JSPS KAKENHI Grant Number, 21K06646 and 21K11100.
Data availability statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Mitsuhiro Nakamura reports financial support was provided by Japan Society for the Promotion of Science.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e21891.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.Ministry of Health, Labour and Welfare . 2008. Jutoku Fukusayo Shikkanbetsu Taiou Manual: Akusei Shoukougun (The Manual for Handling Disorders Due to Adverse Drug Reactions: Neuroleptic Malignant Syndrome)https://www.pmda.go.jp/files/000144356.pdf [Google Scholar]
- 2.Simon L.V., Hashmi M.F., Callahan A.L. StatPearls Publishing; 2023. Neuroleptic Malignant Syndrome.https://www.ncbi.nlm.nih.gov/books/NBK482282 [Google Scholar]
- 3.Pope G.H., Keck P.E., Mcelroy S.L. Frequency and presentation of neuroleptic malignant syndrome in a large psychiatric hospital. Am J Psychiatry. 1986;143:1227–1233. doi: 10.1176/ajp.143.10.1227. [DOI] [PubMed] [Google Scholar]
- 4.Anzai T., Takahashi K., Watanabe M. Adverse reaction reports of neuroleptic malignant syndrome induced by atypical antipsychotic agents in the Japanese Adverse Drug Event Report (JADER) database. Psychiatry Clin Neurosci. 2019;7:27–33. doi: 10.1111/pcn.12793. [DOI] [PubMed] [Google Scholar]
- 5.Tse L., Barr A.M., Scarapicchia V., Vila-Rodriguez F. Neuroleptic malignant syndrome: a review from a clinically oriented perspective. Curr. Neuropharmacol. 2015;13:395–406. doi: 10.2174/1570159x13999150424113345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamińska T., Szuster-Ciesielska A., Wysocka A., Marmurowska-Michałowska H., Dubas-Slemp H., Kandefer-Szerszeń M. Serum cytokine level and production of reactive oxygen species (ROS) by blood neutrophils from a schizophrenic patient with hypersensitivity to neuroleptics. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2003;9:CS71–75. [PubMed] [Google Scholar]
- 7.Fervienza A., López-Baamonde M., Jacas A., Muñoz G., Ibáñez C., Del Rio M.E. Neuroleptic malignant syndrome in a postoperative patient: a case report. Rev. Esp. Anestesiol. Reanim. 2022;69:364–367. doi: 10.1016/j.redare.2022.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Poluzzi E., Raschi E., Piccinni C., Ponti F.D. Data Mining Applications in Engineering and Medicine. Intec; 2012. Data mining techniques in pharmacovigilance: analysis of the publicly accessible FDA Adverse Event Reporting System (AERS) pp. 265–302. [DOI] [Google Scholar]
- 9.Hasegawa S., Ikesue H., Nakao S., Shimada K., Mukai R., Tanaka M., Matsumoto K., Inoue M., Satake R., Yoshida Y., Goto F., Hashida T., Nakamura M. Analysis of immune-related adverse events caused by immune checkpoint inhibitors using the Japanese Adverse Drug Event Report database. Pharmacoepidemiol. Drug Saf. 2020;29:1279–1294. doi: 10.1002/pds.5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Everitt B.S. fifth ed. John Wiley & Sons; New York: 2011. Cluster Analysis. [Google Scholar]
- 11.Kimes P.K., Liu Y., Neil Hayes D., Marron J.S. Statistical significance for hierarchical clustering. Biometrics. 2017;73:811–821. doi: 10.1111/biom.12647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yildirim P. Association patterns in open data to explore ciprofloxacin adverse events. Appl. Clin. Inf. 2015;6:728–747. doi: 10.4338/ACI-2015-06-RA-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harpaz R., Chase H.S., Friedman C. Mining multi-item drug adverse effect associations in spontaneous reporting systems. BMC Bioinf. 2010;11:S7. doi: 10.1186/1471-2105-11-S9-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu A.-, Li J., Leong T.- Automated knowledge extraction for decision model construction: a data mining approach. AMIA Annu Symp Proc. 2003;2003:758–762. https://www.ncbi.nlm.nih.gov/pubmed/14728275 Available: [PMC free article] [PubMed] [Google Scholar]
- 15.Blashfield R. first ed. Springer; New York: 1984. The Classification of Psychopathology: Neo-Kraepelinian and Quantitative Approaches, Softcover Reprint of the Original; p. 328. [Google Scholar]
- 16.Heres S., Hamann J., Mendel R., Wickelmaier F., Pajonk F.G., Leucht S., Kissling W. Identifying the profile of optimal candidates for antipsychotic depot therapy: a cluster analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2008;32:1987–1993. doi: 10.1016/j.pnpbp.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 17.Rocca P., Montemagni C., Mingrone C., Crivelli B., Sigaudo M., Bogetto F. A cluster-analytical approach toward real-world outcome in outpatients with stable schizophrenia. Eur. Psychiatr. 2016;32:48–54. doi: 10.1016/j.eurpsy.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka M., Hasegawa S., Nakao S., Shimada K., Mukai R., Matsumoto K., Nakamura M. Analysis of drug-induced hearing loss by using a spontaneous reporting system database. PLoS One. 2019;14 doi: 10.1371/journal.pone.0217951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hatahira H., Hasegawa S., Sasaoka S., Kato Y., Abe J., Motooka Y., Fukuda A., Naganuma M., Nakao S., Mukai R., Shimada K., Hirade K., Kato T., Nakamura M. Analysis of fall-related adverse events among older adults using the Japanese Adverse Drug Event Report (JADER) database. J Pharm Health Care Sci. 2018;4:32. doi: 10.1186/s40780-018-0129-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasegawa S., Matsui T., Hane Y., Abe J., Hatahira H., Motooka Y., Sasaoka S., Fukuda A., Naganuma M., Hirade K., Takahashi Y., Kinosada Y., Nakamura M. Thromboembolic adverse event study of combined estrogen-progestin preparations using Japanese Adverse Drug Event Report database. PLoS One. 2017;12 doi: 10.1371/journal.pone.0182045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Puijenbroek E.P., Bate A., Leufkens H.G., Lindquist M., Orre R., Egberts A.C. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol. Drug Saf. 2002;11:3–10. doi: 10.1002/pds.668. [DOI] [PubMed] [Google Scholar]
- 22.Meltzer H.Y., Gadaleta E. Contrasting typical and atypical antipsychotic drugs. Focus. 2021;19:3–13. doi: 10.1176/appi.focus.20200051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meltzer H.Y., Massey B.W. The role of serotonin receptors in the action of atypical antipsychotic drugs. Curr. Opin. Pharmacol. 2011;11:59–67. doi: 10.1016/j.coph.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Verdoux H., Tournier M., Bégaud B. Antipsychotic prescribing trends: a review of pharmaco-epidemiological studies. Acta Psychiatr. Scand. 2010;121:4–10. doi: 10.1111/j.1600-0447.2009.01425.x. [DOI] [PubMed] [Google Scholar]
- 25.Meltzer H.Y. Update on typical and atypical antipsychotic drugs. Annu. Rev. Med. 2013;64:393–406. doi: 10.1146/annurev-med-050911-161504. [DOI] [PubMed] [Google Scholar]
- 26.Tarsy D., Baldessarini R.J., Tarazi F.I. Effects of newer antipsychotics on extrapyramidal function. CNS Drugs. 2002;16:23–45. doi: 10.2165/00023210-200216010-00003. [DOI] [PubMed] [Google Scholar]
- 27.Meltzer H.Y., Li Z., Kaneda Y., Ichikawa J. Serotonin receptors: their key role in drugs to treat schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2003;27:1159–1172. doi: 10.1016/j.pnpbp.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 28.Bottlender R., Jäger M., Hofschuster E., Dobmeier P., Möller H.J. Neuroleptic malignant syndrome due to atypical neuroleptics: three episodes in one patient. Pharmacopsychiatry. 2002;35:119–121. doi: 10.1055/s-2002-31518. [DOI] [PubMed] [Google Scholar]
- 29.Caroff S.N., Mann S.C., Campbell E.C., Sullivan K.A. Movement disorders associated with atypical antipsychotic drugs. J. Clin. Psychiatry. 2002;63:12–19. [PubMed] [Google Scholar]
- 30.Hosomi K., Park P., Inose R., Fujimoto M., Takada M. Association between antipsychotic use and extrapyramidal syndrome: data mining of the FDA adverse event reporting system and the Japanese adverse drug event report database. Jpn J Drug Inform. 2015;17:125–132. [Google Scholar]
- 31.Peluso M.J., Lewis S.W., Barnes T.R., Jones P.B. Extrapyramidal motor side-effects of first- and second-generation antipsychotic drugs. Br. J. Psychiatry. 2012;200:387–392. doi: 10.1192/bjp.bp.111.101485. [DOI] [PubMed] [Google Scholar]
- 32.Carnahan R. No difference in extrapyramidal side effects between first-generation and second-generation antipsychotics. Evid. Base Ment. Health. 2012;15:91. doi: 10.1136/ebmental-2012-100850. [DOI] [PubMed] [Google Scholar]
- 33.Nishida Y., Fukuda T., Yamamoto I., Azuma J. CYP2D6 genotypes in a Japanese population: low frequencies of CYP2D6 gene duplication but high frequency of CYP2D6*10. Pharmacogenetics. 2000;10:567–570. doi: 10.1097/00008571-200008000-00010. [DOI] [PubMed] [Google Scholar]
- 34.Kato D., Kawanishi C., Kishida I., Furuno T., Matsumura T., Hasegawa H., Suzuki K., Hirayasu Y. CYP2D6 gene deletion allele in patients with neuroleptic malignant syndrome: preliminary report. Psychiatry Clin Neurosci. 2005;59:504–507. doi: 10.1111/j.1440-1819.2005.01405.x. [DOI] [PubMed] [Google Scholar]
- 35.Kato D., Kawanishi C., Kishida I., Furuno T., Suzuki K., Onishi H., Hirayasu Y. Effects of CYP2D6 polymorphisms on neuroleptic malignant syndrome. Eur. J. Clin. Pharmacol. 2007;63:991–996. doi: 10.1007/s00228-007-0355-8. [DOI] [PubMed] [Google Scholar]
- 36.Kirchheiner J., Nickchen K., Bauer M., Wong M.L., Licinio J., Roots I., Brockmöller J. Pharmacogenetics of antidepressants and antipsychotics: the contribution of allelic variations to the phenotype of drug response. Mol. Psychiatr. 2004;9:442–473. doi: 10.1038/sj.mp.4001494. [DOI] [PubMed] [Google Scholar]
- 37.Kanehisa laboratories. KEGG (the Kyoto encyclopedia of genes and genomes) https://www.kegg.jp/kegg/drug/jp08309.html
- 38.Highlights of prescribing information, SAPHRIS (asenapine) sublingual tablets. MERCK & CO., INC. 2007 www.accessdata.fda.gov/drugsatfda_docs/label/2011/022117s010lbl.pdf [Google Scholar]
- 39.Highlights of prescribing information. VERSACLOZ safely and effectively. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/203479s007s008lbl.pdf
- 40.Cicali E.J., Smith D.M., Duong B.Q., Kovar L.G., Cavallari L.H., Johnson J.A. A scoping review of the evidence behind cytochrome P450 2D6 isoenzyme inhibitor classifications. Clin. Pharmacol. Ther. 2020;108:116–125. doi: 10.1002/cpt.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakao S., Hasegawa S., Umetsu R., Shimada K., Mukai R., Tanaka M., Matsumoto K., Yoshida Y., Inoue M., Satake R., Nishibata Y., Liao J., Nakamura M. Pharmacovigilance study of anti-infective-related acute kidney injury using the Japanese Adverse Drug Event Report database. BMC Pharmacol Toxicol. 2021;22:47. doi: 10.1186/s40360-021-00513-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang X., Li L., Wang L., Feng W., Zhang P. Propensity score-adjusted three-component mixture model for drug-drug interaction data mining in FDA Adverse Event Reporting System. Stat. Med. 2020;39:996–1010. doi: 10.1002/sim.8457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schuemie M.J., Ryan P.B., Hripcsak G., Madigan D., Suchard M.A. arVix; New York: 2018. A Systematic Approach to Improving the Reliability and Scale of Evidence from Health Care Data.https://arxiv.org/pdf/1803.10791.pdf [Google Scholar]
- 44.Tian Y., Schuemie M.J., Suchard Marc A. Evaluating large-scale propensity score performance through real-world and synthetic data experiments. Int. J. Epidemiol. 2018;47:2005–2014. doi: 10.1093/ije/dyy120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hripcsak G., Ryan P.B., Duke J.D., Shah N.H., Park R.W., Huser V., Suchard M.A., Schuemie M.J., DeFalco F.J., Perotte A., Banda J.M., Reich C.G., Schilling L.M., Matheny M.E., Meeker D., Pratt N., Madigan D. Characterizing treatment pathways at scale using the OHDSI network. Proc Natl Acad Sci U S A. 2016;113:7329–7336. doi: 10.1073/pnas.1510502113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stuhec M. Antipsychotic treatment in elderly patients on polypharmacy with schizophrenia. Curr Opin Psychiatry. 2022;35:332–337. doi: 10.1097/YCO.0000000000000808. [DOI] [PubMed] [Google Scholar]
- 47.Kamei H. Polypharmacy management of antipsychotics in patients with Schizophrenia. Medicina (Kaunas) 2022;58:1584. doi: 10.3390/medicina58111584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Su Y.P., Chang C.K., Hayes R.D., Harrison S., Lee W., Broadbent M., Taylor D., Stewart R. Retrospective chart review on exposure to psychotropic medications associated with neuroleptic malignant syndrome. Acta Psychiatr. Scand. 2014;130:52–60. doi: 10.1111/acps.12222. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.