Abstract
Background/objective
Recent technological advances have allowed for the development of smart wearable devices (SmartWear) which can be used to monitor various aspects of patient healthcare. These devices provide clinicians with continuous biometric data collection for patients in both inpatient and outpatient settings. Although these devices have been widely used in fields such as cardiology and orthopedics, their use in the field of neurosurgery and neurology remains in its infancy.
Methods
A comprehensive literature search for the current and future applications of SmartWear devices in the above conditions was conducted, focusing on outpatient monitoring.
Findings
Through the integration of sensors which measure parameters such as physical activity, hemodynamic variables, and electrical conductivity - these devices have been applied to patient populations such as those at risk for stroke, suffering from epilepsy, with neurodegenerative disease, with spinal cord injury and/or recovering from neurosurgical procedures. Further, these devices are being tested in various clinical trials and there is a demonstrated interest in the development of new technologies.
Conclusion
This review provides an in-depth evaluation of the use of SmartWear in selected neurological diseases and neurosurgical applications. It is clear that these devices have demonstrated efficacy in a variety of neurological and neurosurgical applications, however challenges such as data privacy and management must be addressed.
Keywords: Body sensors, Wearable technology, Epilepsy, Stroke, Continuous monitoring, Neurosurgical outcomes, Neurodegenerative disease
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
1. Introduction
Neurological disorders are recognized as a global health challenge, accounting for the leading cause of disability and second leading cause of death globally.1 The economic burden of neurological disease in the US alone is estimated to be approximately $765 billion.2 The application of wearable body sensors known as SmartWear in this patient population may play an integral role in improving outcomes and reducing healthcare costs.3,4 By continuously and remotely monitoring patients, SmartWear has the potential to decrease inpatient length of stay, a major contributor to cost in both stroke and neurosurgical patients.5,6 Additionally, these devices can improve medication adherence, optimize treatment plans, decrease the number of in-person physician visits, and increase patient engagement.6, 7, 8 As the use of SmartWear becomes more widespread, their adoption in the treatment of neurological and neurosurgical patients is critical to improving patient care and reducing healthcare costs associated with these conditions.
Despite the significant progress made in this field over the last decade, there is still a lack of literature regarding the use of SmartWear in neurological and neurosurgical diseases. In this paper, we provide a comprehensive overview of the types of clinical data measured by SmartWear and its use in patients with specific neurological conditions or requiring neurosurgical intervention. We also present up-to-date information on innovative technologies and ongoing clinical trials that demonstrate potential future applications of SmartWear. Finally, we address barriers to the implementation of SmartWear which must be overcome to further accelerate their widespread use in healthcare.
1.1. Application to neurological disease prevention and recovery
1.1.1. Stroke
Globally, stroke is a leading cause of disability and mortality, and with an incidence of more than 795,000 per year in the United States alone, it imposes a significant burden on society.9, 10, 11 Timely intervention is crucial in the treatment of stroke and factors such as prehospital delay are a major barrier to receiving pharmacologic and surgical treatment.10, 11, 12 SmartWear has emerged as an easy-to-use and noninvasive tool to provide objective, quantifiable data for stroke surveillance and rehabilitation, with mounting evidence supporting its effectiveness.13,14 At the forefront are Zeit Medical's (Halo Alert System) wearable headband and the Neuralert wristband, which both use Electroencephalography (EEG) for stroke detection.15,16
These devices are being evaluated for use in high risk stroke patients, such as those with atrial fibrillation (AF).17,18 Kaisti et al conducted a study using a low-cost, wearable wristband designed for outpatient monitoring that showed favorable results in measuring heart rhythm and detecting irregularities using micro-electrical mechanical systems (MEMS).19 The device had almost identical pulse waveforms compared to the gold standard (invasive catheter recording) and effective classification accuracy between AF and sinus rhythm. More recently, the integration of machine learning algorithms and a wearable armband was able to continuously monitor for AF with high accuracy.20
While there is a paucity of evidence supporting screening asymptomatic carotid artery stenosis (CAS) for stroke prevention, targeted surveillance may be indicated in high-risk patients, such as those at risk for atheroma formation.21, 22, 23 A novel wearable carotid Doppler ultrasound neck patch (Foisonics Medical, Sudbury, Canada) showed favorable results in a study conducted with healthy volunteers.24 The patch was able to qualitatively and quantitatively follow blood flow metrics in the common carotid artery during both passive leg raise and quiet respiration. Its velocity measurement accuracy and ability to continuously record doppler spectrograms over many cardiac and respiratory cycles would prove useful in outpatient monitoring of high-risk CAS. The wearable Doppler patch can also minimize human measurement errors, monitor patients over extended periods, decreasing statistical limitations from inadequate beat sample size, all of which are limitations of handheld Doppler ultrasound.25,26 While the device is still in the proof-of-concept stage, it shows potential for use in outpatient stroke surveillance.
Evaluating motor impairments in stroke patients can be challenging due to variability among provider assessments and compensatory behavior from patients, however SmartWear is becoming increasingly popular in stroke rehabilitation to alleviate these issues.27, 28, 29, 30 Through, the use of Inertial measurement units (IMUs; measure kinetic variables) and Surface Electromyography (SEMG; measure muscle contraction), SmartWear has already been shown to identify and monitor hemi motor neglect, complex upper-limb movements, performance and posture, and whole-body tracking for patient evaluation and assessment of interventions like orthoses.14,31,32 Wei Chen et al reported 82 % and 90 % accuracy using a combined Apple watch (Apple, Cupertino, California, USA) IMU device to measure Activities of Daily Living (ADL) function in stroke patients based on ten and seven ADL tasks, respectively.33 Similarly, a randomized, parallel-controlled study of 120 motor impaired stroke patients investigated the use of remote rehabilitation with wearable IMUs attached to the affected arm, hand, thigh, and calf and demonstrated an increased motor ability with remote training with wearable IMU devices.34 Wearable SEMG devices can complement IMU data in motor assessment, and studies have shown that SEMG can enhance motor improvements, aid in diagnosis, and be used for outpatient stroke rehabilitation.14,35, 36, 37, 38, 39, 40, 41
The potential of SmartWear to make a significant impact on the management of stroke through surveillance, detection, and rehabilitation is clearly highlighted in the literature. Continued development of SmartWear, particularly for early detection, is critical to realizing the full potential of these devices in improving stroke outcomes.
1.1.2. Epilepsy
Epilepsy is one of the most common neurological conditions, affecting approximately 50 million people globally.42 Timely recognition and response to seizures can play a pivotal role in improving outcomes and reducing long-term sequelae for patients.42, 43, 44 Currently, there are two devices that have been approved by the Food & Drug Administration (FDA) for use in epilepsy patients, the Empatica Embrace (EE) biosensor and the SPEAC surface electromyography (SEMG) system developed by Brain Sentinel.45,46 The EE is a band worn on the wrist or ankle that can detect body temperature, blood volume pulse, and motion via an accelerometer and electrodermal sensor.45 The EE can also detect preictal states and notify caregivers and/or patients via call or text. Clinical trials of the EE band demonstrated successful detection of 53 of 54 total generalized tonic-clonic seizures (GTCS) across 141 patients, including 80 children.47
The SPEAC system is a SEMG patch applied to the bicep that continuously monitors motor activity to detect convulsions.48 Like the EE band, this is also a cloud-based data platform that sends alerts to caregivers' phones, however in addition to detection it is also capable of characterization. Clinical validation of SEMG resulted in detection in 95 % of patients with high specificity.49 A characterization study then demonstrated the SPEAC system to have a 72 % accuracy rate when differentiating between tonic-clonic, tonic, clonic, and complex motor seizures as compared with an 81 % accuracy rate by epileptologists reviewing the same data.50 Although SPEAC is not as accurate, further development may improve accuracy of this system and can be used to aid in diagnosis.
Although there are few FDA approved devices, there are a multitude of other SmartWear devices that have been reported in the literature. In a 2017 multicenter prospective study, an armband assessing accelerometry and ECG signals was able to detect clinically urgent seizures with a sensitivity of 71–87 %.51 Although these results are positive, this study also reported a high number of false alarms. Similar to this, Halford et al demonstrated that a SEMG monitoring patch worn over the biceps muscle could detect 100 % of generalized tonic-clonic seizures, with a false alarm rate of 1.44 per 24 h and a positive predictive value of 6.2 %.52 More recently, Naganur et al used a wrist-worn MEMS accelerometer to detect all seizures occurring in a cohort of 11. Further, this system classified epileptic and psychogenic non-epileptic seizures with sensitivity and specificity of 72.7 % and 100 %, respectively. Similarly to the previous study, the authors reported a rate of 2.4 false alarms per 24 h, thus posing a potential drawback to these devices.53 As multiple studies have shown the potential for wearable devices in the detection of epilepsy, focus has now started to shift to improving the accuracy of these devices through the use of machine learning. Nasseri et al showed that machine learning models applied to data collected by the EE band, were able to forecast seizure alerts on average 33 min before the onset.54 Similar results have been reported, with decreases in false alarms, increased predictive accuracy and sensitivity becoming a common trend55, 56, 57
1.1.3. Spinal cord injury
Annually, there are approximately 250,000 to 500,000 people who suffer from a spinal cord injury (SCI) worldwide.58 These patients have a high risk of medical complications due to motor deficits, disrupted regulation of bladder/bowel, cardiac, and/or respiratory functions, resulting in a decrease in functional improvement after discharge.59,60 Further, due to impaired activity and metabolic rate, patients with SCI are at increased risk for obesity which predisposes them to a multitude of health consequences.61 Therefore, the use of SmartWear to continuously monitor this population may improve outcomes and decrease preventable complications.
Loss of mobility makes regaining the ability to walk a key priority in the rehabilitation of patients with SCI; however, due to their injuries, these patients are also more likely to experience falls.62,63 This demonstrates the importance of remote monitoring using SmartWear technology. Lemay et al demonstrated through the use of a wearable IMU in SCI patients, that reliable and valid measurements of altered gait could be detected.64 Further, Noamani et al used accelerometers placed on the sacrum and tibia to identify changes in balance control after SCI.65 Although proof of concept, these studies are critical to the application of SmartWear for continuous monitoring of SCI patients to provide quantifiable data for optimizing rehabilitation and reducing falls.
Autonomic dysfunction is also common in SCI, with various manifestations and degrees of severity. Urinary dysfunction is one such symptom, causing both decreased quality of life and increased risk for severe infection.66,67 To address this issue, Fong et al proposed a wearable optical based sensor to continuously monitor bladder capacity and provide alerts to a mobile device.68 For direct modulation, Knight et al devised a novel wearable device, which when inserted into the anal canal, can use EMG to continuously monitor detrusor activity and stimulate nerves to modulate detrusor overactivity when detected.69 Although increased bladder capacity was achieved in a small cohort, side effects from stimulation (including autonomic dysreflexia) were observed. As bladder distension is a possible cause of autonomic dysreflexia, it is important to note that there is a lack of literature regarding the use of SmartWear in detecting this. Currently, Suresh et al.‘s use of a wrist-worn smart watch and machine learning model to accurately (94.10 %) detect early symptoms of autonomic dysreflexia remains one of the few studies demonstrating this application.70
Clinically, treatment of SCIs is complex and requires intensive monitoring to prevent further complications. Currently, the small body of literature regarding the use of SmartWear in SCI patients presents an important application of this technology, however there is a need for further studies.
1.1.4. Neurodegenerative disease and essential tremor
As the elderly population continues to rise globally, the incidence of neurodegenerative diseases, such as Parkinson's Disease, are likely to follow this trend.71 Parkinson's Disease (PD) is the fastest growing neurodegenerative disease in the world, with an estimated 90,000 patients diagnosed per year in the US alone.72 Tremor is one of the earliest manifestations of PD, however, it is also a nonspecific symptom of idiopathic essential tremor (ET). SmartWear can be used to aid current methods of detection which are unable to detect subtle fluctuations and amplitude of tremors, leading to delayed diagnosis or misdiagnosis.73 Lopez-Blanco et al demonstrated that data collected from a smartwatch and smartphone could be used to accurately detect tremors that were slower than the human eye can confidently perceive.74 Further, when combined with machine learning, a wearable bracelet was able to detect tremors with an accuracy of 91.7 %.75 Technologies such as these can be used to quantitatively identify tremors remotely in high-risk patients.
Sleep disturbances are also a significant symptom in early PD and can greatly affect the quality of life as the disease progresses.76 McGregor et al, demonstrated the use of accelerometers placed on each wrist to measure sleep abnormalities can effectively recognize PD patients from non–PD controls.77 Further, use of smartphones paired with machine learning demonstrated accurate differentiation between patients with PD and idiopathic REM sleep behavior disorder.78
Symptom improvement in parameters such as tremors, falls, and the freezing of gait are often used to determine efficacy of PD treatment, however these are often difficult to monitor in an outpatient setting.79, 80, 81 In a 2016 study of PD patients, accelerometers placed on the back were used to measure various parameters of gait in a free-living setting, finding it to be more sensitive in determining symptoms of PD compared to a clinical setting.82 Following this study, Burq et al in 2022 demonstrated that the use of a smartwatch containing multiple sensors (gyroscope, IMU, etc) could remotely monitor PD patients with motor tasks and provide information regarding pharmacodynamic responses to dopaminergic medications.83 This and similar studies, suggest that SmartWear actively monitors response to therapies and provides data through continuous monitoring for the development of biomarkers for disease progression.84
SmartWear is also being used to detect and monitor other neurodegenerative diseases such as Alzheimer's and dementia.85,86 With established baseline efficacy of certain quantitative measurements (ex. Tremor amplitude pattern for PD) for diagnosis and/or management of these diseases, the use of SmartWear will serve as a valuable tool in providing earlier detection, accurate staging, and optimal management, especially when combined with machine learning techniques.87,88
1.1.5. Recovery of neurosurgical patients post-operatively
Postoperative monitoring is a critical component of routine and high-risk neurosurgical care alike. While vital signs may provide important objective data in the immediate postoperative period, long-term outcomes of care are often described by subjective metrics such as Visual Analogue Score (VAS) and the Oswestry Disability Index (ODI). SmartWear enables providers to quantitatively understand parameters of patient recovery such as mobility, and posture, both of which are significant components to postoperative life.89, 90, 91
Reports of use in spinal stenosis, degenerative disc and spine disease, disc herniation, and spinal fusion procedures exist and these studies have found a correlation between the number of steps taken and improvement in patient outcomes. A 2019 study by Kim et al, was among the first to demonstrate the efficacy of using the popular Fitbit Charge (Google, San Francisco, California, USA) to measure the number of steps in postoperative laminectomy patients.92 The results of this study demonstrated a significant correlation between number of steps and improvement in both VAS and ODI scores. Similarly, the use of wearable accelerometers to measure step count, walking speed, step length and posture by Ghent et al, allowed for the establishment of a novel scoring tool which was positively correlated with change in ODI in lumbar surgery patients post-operatively.93 In regard to posture, Wang et al used a single tri-axis accelerometer amongst five patients with cervical spine injuries demonstrated 100 % accuracy in being able to classify different spinal postures.94 Moreover, smart garments and smart belts have shown promise in accurately determining improper posture and have even been shown to provide immediate feedback via vibratory pressure to users.91,95,96
Although much of the literature focuses on spinal surgery, these devices have been shown promise in other neurosurgical applications. In an endovascular application, a study analyzing postoperative carotid endarterectomy patients fitted with a belt containing an accelerometer demonstrated objective measurements in gait improvement post operatively.97 Additionally, evaluation of a smartwatch to monitor patients after transsphenoidal surgery found high adherence by patients and marked postoperative physiologic findings.98 Taken together, there is a clear role for SmartWear in the management of postoperative neurosurgical patients, however further evaluation of these devices in settings outside of spine surgery are warranted.
2. Discussion
Recently, digital health and biosensor companies have spent billions of dollars, driving clinical trials and development of innovative SmartWear technologies.99 The increasing application of SmartWear technology in the clinical setting is also reflected in the literature as well, as summarized in Table 1 and Fig. 1, with large increases over the past two decades in both neurosurgery specific applications and medical applications overall.100,101
Table 1.
Summary of SmartWear devices and applications.
| Clinical Applications | Location | Type of Sensor | Marker |
|---|---|---|---|
| Stroke | Wrist | MEMS19 | Arterial pressure waveform, arrhythmia detection, heart rate |
| Neck | Doppler Ultrasound24 | Carotid flow velocity | |
| Head | EEG13,15 | Brain Activity | |
| Wrist | Accelerometer16 | Asymmetric upper extremity weakness | |
| Wrist, Arm, Hip | Accelerometer, Gyroscope33 | Motor activity | |
| Arm, Hand, Thigh, Calf | Accelerometer, Gyroscope, Magnetometer34 | Motor activity | |
| Wrist or Ankle | Accelerometer, Electrodermal Sensor47 | Body temperature, blood volume pulse, motion | |
| Arm | Electromyography50 | Muscle activity | |
| Upper arm | Accelerometer and Electrocardiography51 | Heart rate, acceleration | |
| Epilepsy | Arm | Electromyography52 | Muscle activity |
| Wrist | Accelerometer53 | Motor activity | |
| Wrist | Accelerometer, photoplethysmography, electrodermal activity, temperature54 | Motor activity, blood volume pulse, electrodermal activity, heart rate, temperature | |
| Feet, Legs, and Sacrum | Accelerometer, Gyroscope64 | Gait Characteristics | |
| Sacrum, Tibia | Accelerometer, Gyroscope65 | Balance/Posture Control | |
| Spinal Cord Injury | Anal Canal | Electromyography69 | Muscle Activity |
| Wrist | Electrodermal Sensor70 | Galvanic skin response, heart rate, and skin temperature | |
| Wrist | Gyroscope74 | Tremor (angular motion) | |
| Wrist | Accelerometer, gyroscope75 | Tremor and bradykinesia detection (motor activity) | |
| Neurodegenerative Disease and Essential Tremor | Wrist | Accelerometer77 | Motor activity (used to calculate severity of bradykinesia) |
| Back | Accelerometer82 | Gait characteristics (motor activity) | |
| Wrist | IMU, gyroscope, photoplethysmography, skin conductance sensors83 | Acceleration and angular momentum during specified tasks | |
| Wrist | Accelerometer, altimeter, vibration motor92 | Ambulatory function, activity level | |
| Wrist | Accelerometers93 | Step count, gait velocity, mean step length | |
| Head | Accelerometer94 | Cervical curvature level | |
| Post-operative Recovery of Neurosurgical | Back | Accelerometer97 | Gait characteristics |
| Wrist | Photoplethysmography, accelerometer, gyroscope98 | Heart rate, heart rate variation, respiration, O2 saturation, calories, steps, distance | |
| Neck, claviclea | Temperature102 | Continuous monitoring of CSF flow | |
| Heada | Photoplethysmography103 | Oxygenation, heart rate, cerebral pulse pressure, vascular tone |
Currently only tested for inpatient use.
Fig. 1.
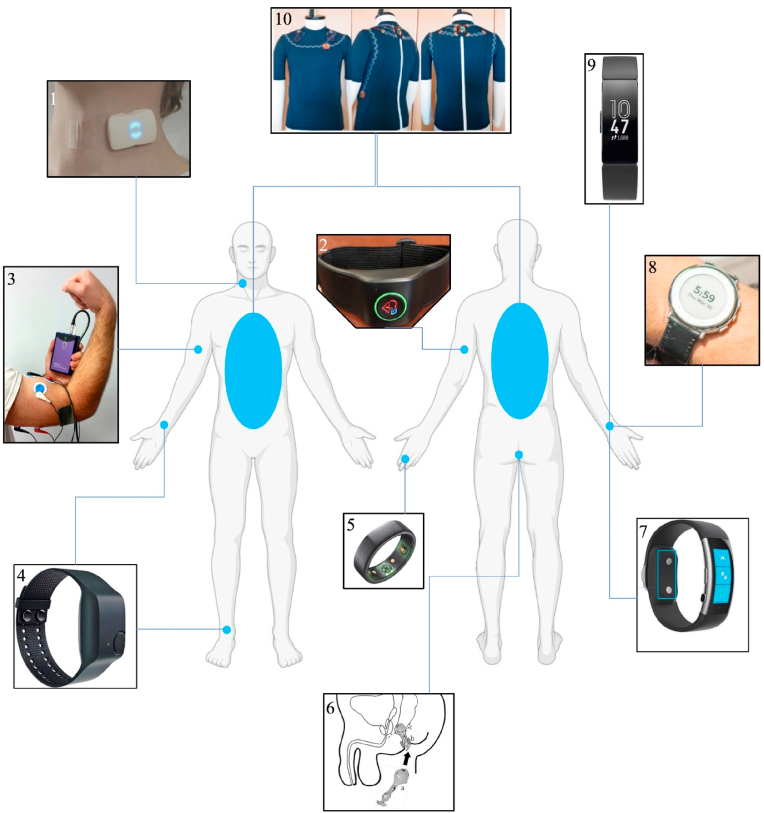
Visual summary of SmartWear devices for neurological and neurosurgical applications. Image 1 is a device used for continuous Doppler monitoring of the Carotid artery.24 Image 2 is a wearable armband for the detection of atrial fibrillation, a risk factor for stroke.20 Image 3 is Brain Sentinel's sEMG device worn on the biceps muscle for seizure monitoring/detection.49 Image 4 is the Empatica 4 which can be worn on the wrist or ankle for seizure monitoring/detection.104 Image 5 is the Oura ring which has been proposed for sleep monitoring in Alzheimer's disease.85,105 Image 6 is a neuromodulation device inserted into the anus for bladder control in patients with spinal cord injury.69 Image 7 is the Microsoft Band, which was used to detect autonomic dysreflexia in patients with SCI.70 Image 8 is smart wristwatch which can detect response to medication in PD patients.84 Image 9 is the FitBit Charge which was used to measure activity levels in patients post lumbar spinal fusion.92,105 Image 10 is a smart shirt which can detect postural changes.91 All image modifications have been cited and adaptations are protected by the Creative Commons license.
A critical factor for the widespread adoption of these devices in healthcare is validation through clinical trials. Currently, there are multiple studies investigating the efficacy of SmartWear in patients with various neurological/neurosurgical diseases. Examples of these include a smartwatch application used to monitor medication adherence in patients requiring pharmaceutical intervention for secondary stroke prevention (NCT04180475) and a neuromodulator device worn on the foot/ankle to provide bladder modulation in those with overactive bladder syndrome (NCT05381116). The use of SmartWear in Parkinson's Disease specifically has seen an increase in trials for various applications, ranging from the use of a smartwatch to evaluate response to pharmacologic therapy (NCT05351580) to improving gait using a wearable cueing device (NCT04459559). These and similar technologies, represent the application of SmartWear for monitoring and treating certain neurological conditions. Finally, there are also clinical trials underway to investigate the use of SmartWear in broader applications (such as the Chronolife TM smart shirt to monitor cancer patients), which may be applied to neurological/neurosurgical patients.
From a neurosurgical perspective, the use of SmartWear has largely been focused on monitoring postoperative patients.106, 107, 108 Many of these studies have specifically investigated gait function in patients who have recently undergone spinal surgery.106,102, 108, 109 For example, Inoue et al, used a Micro-Motion logger system to determine the postoperative activity of sixty patients that recently underwent lumbar spinal surgery.102 The study found significantly lower activity one month postoperatively, but this improved at 3 months and 12 months postoperatively. While objective postoperative data on patient activity does provide some benefit, some studies in the literature do not show a significant relationship between physical activity and improvement in subjective clinical outcomes.103, 110, 111 It is important to note that many tests of clinical outcomes are subjective pain rating scores, and perhaps do not provide an accurate representation of a patient's recovery. Furthermore, Schulte et al, suggests that objective activity level is only one aspect of recovery and other factors such as motivation or postoperative symptoms may play a role in clinical improvement as well.110 The results of these studies suggest the need to control for many factors (type of surgery, age, motivation of patient, etc.) in order to determine the true relationship between objective activity level subjective clinical outcomes.
Although there are many studies of SmartWear use for spinal pathology, studies have shown feasibility for use in patients treated neurosurgically for essential tremors and deep brain stimulation.112,113 For example, Chockalingam et al, used a smartphone application known as Lift Pulse to measure essential tremors post ventralis intermedius thalamic deep brain stimulation.113 The study found a significant correlation between Lift Pulse improvement and Marin Tremor Rating Scale for arm tremor. Wearable devices used to monitor cerebrospinal fluid flow and pediatric cerebral hemodynamics are also being investigated in inpatient settings, representing a possible avenue for monitoring these vital parameters remotely.114,115 These pilot studies demonstrate the potential future applications of SmartWear to measure complex physiologic parameters allowing for reduced postoperative complications and comprehensive monitoring.
Alongside clinical validation, development of novel sensors for continuous monitoring of endogenous biomarkers has also accelerated.116 Examples of these novel devices include wrist-worn devices which can monitor/detect cortisol levels and self-powered optical devices (similar to contact lenses) to monitor hemodynamic vital signs.117, 118, 119 Although promising technology, there are many barriers which must be addressed as well, such as integrating the large volume of data generated by SmartWear. Concerns persist over data integration into current healthcare practices and electronic health record (EHR) systems, as well as data overload of physicians.120, 121, 122, 123 Large EHR companies such as Epic are working to provide solutions to these issues, linking their system directly with popular SmartWear devices, however there are still many devices which are excluded from this and data visualization for clinicians remains an issue.122,124
Furthermore, due to the wearable technology industry's rapid expansion, there is a lack of industry-wide standards for the transmission and encryption of health data.121,122,124, 125, 126 The inadequacies of security measures may result in the exposure of private health data, potentially damaging the trust essential to the doctor–patient relationship.125,126 Moreover, since the private sector is responsible for a majority of SmartWear innovation, providers have raised concern over inadequate regulatory oversight to ensure that health data is being used solely for the benefit of patients and not corporate profits.122,125,127 These concerns have already been realized with pharmaceutical companies paying physicians to target vulnerable populations for financial gain and the purchase of healthcare data by advertising giants such as Amazon, Google, and Microsoft.128
Beyond lack of regulatory oversight, this technology may also create further barriers for marginalized and underserved populations. Currently, the majority of health monitoring devices are used by insured patients, living in suburban/urban areas, who own smart wireless technology.120 Patients of lower socioeconomic classes and rural areas may have less access to Wi-Fi and other technologies and therefore also have limited access to SmartWear devices, despite being the individuals who may benefit most from them.120,129,130 Such inequalities can exacerbate existing health disparities (e.g. race and class-based disparities) in regards to both access and outcomes.131,132 Finally, although incorporating machine learning is critical to the development and integration of SmartWear, it may further aggravate health disparities due to the greater representation of patients from higher socioeconomic/educational backgrounds in the datasets used for training these models.120,123,127,133 This presents a significant sampling and selection bias as the health profiles of lower socioeconomic status and rural patients may not be weighed as heavily when using machine learning and SmartWear to guide clinical decisions. Thus, as SmartWear represents the future of personalized data driven healthcare, there are a multitude of ethical, legal, and practical considerations which must be addressed to become fully integrated into patient care (Table 2).
Table 2.
Summary of merits and challenges/drawbacks of SmartWear devices and applications.
| Benefits of SmartWear | Challenges and Drawbacks of SmartWear |
|---|---|
| Clinical | |
|
|
| Technical | |
|
|
| Societal | |
|
|
3. Conclusion
The use of SmartWear is rapidly becoming adopted in the outpatient setting in order to monitor and treat neurological and neurosurgical patients. These devices can provide earlier detection, accurate diagnosis, precise treatment. The application of machine learning and artificial intelligence is critical to establishing predictive technology and improving sensitivity and specificity. Finally, although the future of SmartWear is promising, limitations such as integration and security must be addressed.
CRediT authorship contribution statement
Nithin Gupta: Writing – review & editing, Writing – original draft, Visualization, Investigation, Data curation. Varun Kasula: Writing – review & editing, Writing – original draft, Investigation, Data curation. Praveen Sanmugananthan: Writing – review & editing, Writing – original draft, Investigation, Data curation. Nicholas Panico: Writing – review & editing, Writing – original draft, Investigation, Data curation. Aimee H. Dubin: Writing – review & editing, Writing – original draft. David AW. Sykes: Writing – review & editing, Writing – original draft. Randy S. D'Amico: Writing – review & editing, Supervision, Conceptualization.
Declaration of competing interest
The authors have no relevant financial or non-financial interests to disclose.
Abbreviations
- ADL
Activities of Daily Living
- AF
Atrial fibrillation
- CAS
carotid artery stenosis
- EEG
Electroencephalography
- EHR
electronic health record
- EE
Empatica Embrace
- ET
essential tremor
- FDA
Food & Drug Administration
- GTCS
generalized tonic-clonic seizures
- IMU
Inertial measurement units
- MEMS
micro-electrical mechanical systems
- ODI
Oswestry Disability Index
- PD
Parkinson's Disease
- SCI
spinal cord injury
- SEMG
Surface Electromyography
- VAS
Visual Analogue Score
References
- 1.Feigin V.L., Vos T., Nichols E., et al. The global burden of neurological disorders: translating evidence into policy. The Lancet. Neurology. 2020;19(3):255–265. doi: 10.1016/S1474-4422(19)30411-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2017 US Neurological Disorders Collaborators. Feigin V.L., Vos T., Alahdab F., et al. Burden of neurological disorders across the US from 1990-2017: a global burden of disease study. JAMA Neurol. 2021;78(2):165–176. doi: 10.1001/jamaneurol.2020.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nherera L., Larson B., Cooley A., Reinhard P. An economic analysis of a wearable patient sensor for preventing hospital-acquired pressure injuries among the acutely ill patients. International journal of health economics and management. 2021;21(4):457–471. doi: 10.1007/s10754-021-09304-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Z., Cascioli V., McCarthy P.W. Healthcare monitoring using low-cost sensors to supplement and replace human sensation: does it have potential to increase independent living and prevent disease? Sensors. 2023;23(4):2139. doi: 10.3390/s23042139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan B., Haqqani U., Ullah S., Hamayun S., Bibi Z., Khanzada K. Duration of in-hospital stay for elective neurosurgical procedures in a tertiary care hospital. Cureus. 2021;13(6) doi: 10.7759/cureus.15745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smuck M., Odonkor C.A., Wilt J.K., Schmidt N., Swiernik M.A. The emerging clinical role of wearables: factors for successful implementation in healthcare. NPJ digital medicine. 2021;4(1):45. doi: 10.1038/s41746-021-00418-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greiwe J., Nyenhuis S.M. Wearable technology and how this can Be implemented into clinical practice. Curr Allergy Asthma Rep. 2020;20(8):36. doi: 10.1007/s11882-020-00927-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang H.S., Exworthy M. Wearing the future-wearables to empower users to take greater responsibility for their health and care: scoping review. JMIR mHealth and uHealth. 2022;10(7) doi: 10.2196/35684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y.H., Sawan M. Trends and challenges of wearable multimodal technologies for stroke risk prediction. Sensors. 2021;21(2):460. doi: 10.3390/s21020460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saini V., Guada L., Yavagal D.R. Global epidemiology of stroke and access to acute ischemic stroke interventions. Neurology. 2021;97(20 Suppl 2):S6–S16. doi: 10.1212/WNL.0000000000012781. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention . 2021, May 25. Stroke Facts. Centers for Disease Control and Prevention.https://www.cdc.gov/stroke/facts.htm [Google Scholar]
- 12.Soomann M., Vibo R., Kõrv J. Acute stroke: why do some patients arrive in time and others do not? Eur J Emerg Med: official journal of the European Society for Emergency Medicine. 2015;22(4):285–287. doi: 10.1097/MEJ.0000000000000206. [DOI] [PubMed] [Google Scholar]
- 13.Zeit Medical, Inc Zeit alert for stroke at home (ZASH) protocol. Clinicaltrials.gov. 2022, December 19. https://clinicaltrials.gov/ct2/show/NCT05669456
- 14.Maceira-Elvira P., Popa T., Schmid A.C., Hummel F.C. Wearable technology in stroke rehabilitation: towards improved diagnosis and treatment of upper-limb motor impairment. J NeuroEng Rehabil. 2019;16(1):142. doi: 10.1186/s12984-019-0612-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeit Medical. (n.d.) Www.zeitmedical.comhttps://www.zeitmedical.com/ Retrieved March 19, 2023, from.
- 16.Rapid stroke detection with Neuralert. (n.d.) 2023. Www.neuralerttechnologies.com Retrieved March 19.
- 17.Perez M.V., Mahaffey K.W., Hedlin H., et al. Large-Scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med. 2019;381(20):1909–1917. doi: 10.1056/NEJMoa1901183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lubitz S.A., Faranesh A.Z., Selvaggi C., et al. Detection of atrial fibrillation in a large population using wearable devices: the Fitbit heart study. Circulation. 2022;146(19):1415–1424. doi: 10.1161/CIRCULATIONAHA.122.060291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaisti M., Panula T., Leppänen J., et al. Clinical assessment of a non-invasive wearable MEMS pressure sensor array for monitoring of arterial pulse waveform, heart rate and detection of atrial fibrillation. NPJ digital medicine. 2019;2:39. doi: 10.1038/s41746-019-0117-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bashar S.K., Hossain M.B., Lázaro J., et al. Feasibility of atrial fibrillation detection from a novel wearable armband device. Cardiovasc Digit Health J. 2021 May 21;2(3):179–191. doi: 10.1016/j.cvdhj.2021.05.004. Erratum in: Cardiovasc Digit Health J. 2021 Sep 17;2(5):291. PMID: 35265907; PMCID: PMC8890073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.US Preventive Services Task Force. Krist A.H., Davidson K.W., Mangione C.M., et al. Screening for asymptomatic carotid artery stenosis: US preventive services task force recommendation statement. JAMA. 2021;325(5):476–481. doi: 10.1001/jama.2020.26988. [DOI] [PubMed] [Google Scholar]
- 22.Mortimer R., Nachiappan S., Howlett D.C. Carotid artery stenosis screening: where are we now? Br J Radiol. 2018;91(1090) doi: 10.1259/bjr.20170380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Redgrave J.N., Lovett J.K., Gallagher P.J., Rothwell P.M. Histological assessment of 526 symptomatic carotid plaques in relation to the nature and timing of ischemic symptoms: the Oxford plaque study. Circulation. 2006;113(19):2320–2328. doi: 10.1161/CIRCULATIONAHA.105.589044. [DOI] [PubMed] [Google Scholar]
- 24.Kenny J.S., Munding C.E., Eibl J.K., et al. A novel, hands-free ultrasound patch for continuous monitoring of quantitative Doppler in the carotid artery. Sci Rep. 2021;11(1):7780. doi: 10.1038/s41598-021-87116-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kenny J.S. Functional hemodynamic monitoring with a wireless ultrasound patch. J Cardiothorac Vasc Anesth. 2021;35(5):1509–1515. doi: 10.1053/j.jvca.2021.01.040. [DOI] [PubMed] [Google Scholar]
- 26.Kenny J.S., Barjaktarevic I., Eibl A.M., Parrotta M., Long B.F., Eibl J.K. A wearable carotid Doppler tracks changes in the descending aorta and stroke volume induced by end-inspiratory and end-expiratory occlusion: a pilot study. Health science reports. 2020;3(4):e190. doi: 10.1002/hsr2.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poole J.L., Whitney S.L. Assessments of motor function post stroke. Phys Occup Ther Geriatr. 2001;19:1–22. [Google Scholar]
- 28.Goldstein L.B., Bertels C., Davis J.N. Interrater reliability of the NIH stroke scale. Arch Neurol. 1989;46(6):660–662. doi: 10.1001/archneur.1989.00520420080026. [DOI] [PubMed] [Google Scholar]
- 29.Gladstone D.J., Danells C.J., Black S.E. The fugl-meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabilitation Neural Repair. 2002;16(3):232–240. doi: 10.1177/154596802401105171. [DOI] [PubMed] [Google Scholar]
- 30.Cirstea M.C., Levin M.F. Compensatory strategies for reaching in stroke. Brain : J Neurol. 2000;123(Pt 5):940–953. doi: 10.1093/brain/123.5.940. [DOI] [PubMed] [Google Scholar]
- 31.Gomez C., Oller J., Paradells J. Overview and evaluation of bluetooth low energy: an emerging low-power wireless technology. Sensors. 2012;12(9):11734–11753. doi: 10.3390/s120911734. [DOI] [Google Scholar]
- 32.Wittmann F., Held J.P., Lambercy O., et al. Self-directed arm therapy at home after stroke with a sensor-based virtual reality training system. J NeuroEng Rehabil. 2016;13(1):75. doi: 10.1186/s12984-016-0182-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen P.W., Baune N.A., Zwir I., Wang J., Swamidass V., Wong A.W.K. Measuring Activities of daily living in stroke patients with motion machine learning algorithms: a pilot study. Int J Environ Res Publ Health. 2021;18(4):1634. doi: 10.3390/ijerph18041634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo L., Wang J., Wu Q., et al. Clinical study of a wearable remote rehabilitation training system for patients with stroke: randomized controlled pilot trial. JMIR mHealth and uHealth. 2023;11 doi: 10.2196/40416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim J.H. The effects of training using EMG biofeedback on stroke patients upper extremity functions. J Phys Ther Sci. 2017;29(6):1085–1088. doi: 10.1589/jpts.29.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Repnik E., Puh U., Goljar N., Munih M., Mihelj M. Using inertial measurement units and electromyography to quantify movement during action research arm test execution. Sensors. 2018;18(9):2767. doi: 10.3390/s18092767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ablikim M., Achasov M.N., Adlarson P., et al. BESIII Collaboration Observation of a Resonant Structure in e^{+}e^{-}→K^{+}K^{-}π^{0}π^{0. Phys Rev Lett. 2020;124(11) doi: 10.1103/PhysRevLett.124.112001. [DOI] [PubMed] [Google Scholar]
- 38.Donoso Brown E.V., Dudgeon B.J., Gutman K., Moritz C.T., McCoy S.W. Understanding upper extremity home programs and the use of gaming technology for persons after stroke. Disability and health journal. 2015;8(4):507–513. doi: 10.1016/j.dhjo.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zardoshti-Kermani M., Wheeler B.C., Badie K., Hashemi R.M. EMG feature evaluation for movement control of upper extremity prostheses. IEEE Trans Rehabil Eng. 1995;3:324–333. [Google Scholar]
- 40.Nam C., Zhang B., Chow T., et al. Home-based self-help telerehabilitation of the upper limb assisted by an electromyography-driven wrist/hand exoneuromusculoskeleton after stroke. J NeuroEng Rehabil. 2021;18(1):137. doi: 10.1186/s12984-021-00930-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hung N.T., Paul V., Prakash P., et al. Wearable myoelectric interface enables high-dose, home-based training in severely impaired chronic stroke survivors. Annals of clinical and translational neurology. 2021;8(9):1895–1905. doi: 10.1002/acn3.51442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.WHO . World Health Organization; World Health Organization: WHO; 2022, February 9. Epilepsy.https://www.who.int/news-room/fact-sheets/detail/epilepsy [Google Scholar]
- 43.Silbergleit R., Durkalski V., Lowenstein D., et al. Intramuscular versus intravenous therapy for prehospital status epilepticus. N Engl J Med. 2012;366(7):591–600. doi: 10.1056/NEJMoa1107494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hill C.E., Parikh A.O., Ellis C., Myers J.S., Litt B. Timing is everything: where status epilepticus treatment fails. Ann Neurol. 2017;82(2):155–165. doi: 10.1002/ana.24986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rukasha T., I Woolley S., Kyriacou T., Collins T. Evaluation of wearable electronics for epilepsy: a systematic review. Electronics. 2020;9(6):968. doi: 10.3390/electronics9060968. [DOI] [Google Scholar]
- 46.SPEAC System. (n.d Epilepsy foundation. https://www.epilepsy.com/tools-resources/device-wiki/speac-system Retrieved March 19, 2023, from.
- 47.https://www.medtechdive.com/news/empatica-gets-fda-clearance-for-epilepsy-monitor-in-children/545834/#:∼:text=The%20study%20population%20included%2080
- 48.SPeAC System How the SPeAC system works. https://speacsystem.com/speac-system-seizure-monitor/how-the-speac-system-works/
- 49.Szabó C.Á., Morgan L.C., Karkar K.M., et al. Electromyography-based seizure detector: preliminary results comparing a generalized tonic–clonic seizure detection algorithm to video-EEG recordings. Epilepsia. 2015;56:1432–1437. doi: 10.1111/epi.13083. [DOI] [PubMed] [Google Scholar]
- 50.Baumgartner C., Whitmire L.E., Voyles S.R., Cardenas D.P. Using sEMG to identify seizure semiology of motor seizures. Seizure. 2021;86:52–59. doi: 10.1016/j.seizure.2020.11.013. [DOI] [PubMed] [Google Scholar]
- 51.van Andel J., Ungureanu C., Arends J., et al. Multimodal, automated detection of nocturnal motor seizures at home: is a reliable seizure detector feasible? Epilepsia open. 2017;2(4):424–431. doi: 10.1002/epi4.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Halford J.J., Sperling M.R., Nair D.R., et al. Detection of generalized tonic-clonic seizures using surface electromyographic monitoring. Epilepsia. 2017;58(11):1861–1869. doi: 10.1111/epi.13897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Naganur V.D., Kusmakar S., Chen Z., Palaniswami M.S., Kwan P., O'Brien T.J. The utility of an automated and ambulatory device for detecting and differentiating epileptic and psychogenic non-epileptic seizures. Epilepsia open. 2019;4(2):309–317. doi: 10.1002/epi4.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nasseri M., Pal Attia T., Joseph B., et al. Ambulatory seizure forecasting with a wrist-worn device using long-short term memory deep learning. Sci Rep. 2021;11(1) doi: 10.1038/s41598-021-01449-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Regalia G., Onorati F., Lai M., Caborni C., Picard R.W. Multimodal wrist-worn devices for seizure detection and advancing research: focus on the Empatica wristbands. Epilepsy Res. 2019;153:79–82. doi: 10.1016/j.eplepsyres.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 56.Meisel C., El Atrache R., Jackson M., Schubach S., Ufongene C., Loddenkemper T. Machine learning from wristband sensor data for wearable, noninvasive seizure forecasting. Epilepsia. 2020;61(12):2653–2666. doi: 10.1111/epi.16719. [DOI] [PubMed] [Google Scholar]
- 57.Zambrana-Vinaroz D., Vicente-Samper J.M., Manrique-Cordoba J., Sabater-Navarro J.M. Wearable epileptic seizure prediction system based on machine learning techniques using ECG, PPG and EEG signals. Sensors. 2022;22(23):9372. doi: 10.3390/s22239372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.World Health Organization: WHO . Who.int; World Health Organization: WHO; 2013, November 19. Spinal Cord Injury.https://www.who.int/news-room/fact-sheets/detail/spinal-cord-injury [Google Scholar]
- 59.van den Berg-Emons R.J., Bussmann J.B., Haisma J.A., et al. A prospective study on physical activity levels after spinal cord injury during inpatient rehabilitation and the year after discharge. Arch Phys Med Rehabil. 2008;89(11):2094–2101. doi: 10.1016/j.apmr.2008.04.024. [DOI] [PubMed] [Google Scholar]
- 60.Wirth B., van Hedel H., Kometer B., Dietz V., Curt A. Changes in activity after a complete spinal cord injury as measured by the spinal cord independence measure II (SCIM II) Neurorehabilitation Neural Repair. 2008;22(2):145–153. doi: 10.1177/1545968307306240. [DOI] [PubMed] [Google Scholar]
- 61.Shojaei M.H., Alavinia S.M., Craven B.C. Management of obesity after spinal cord injury: a systematic review. J Spinal Cord Med. 2017 Nov;40(6):783–794. doi: 10.1080/10790268.2017.1370207. Epub 2017 Sep 20. PMID: 28929907; PMCID: PMC5778942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Simpson L.A., Eng J.J., Hsieh J.T., Wolfe D.L. Spinal Cord Injury Rehabilitation Evidence Scire Research Team. The health and life priorities of individuals with spinal cord injury: a systematic review. J Neurotrauma. 2012 May 20;29(8):1548–1555. doi: 10.1089/neu.2011.2226. Epub 2012 Apr 18. PMID: 22320160; PMCID: PMC3501530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wannapakhe J., Arrayawichanon P., Saengsuwan J., Amatachaya S. Medical complications and falls in patients with spinal cord injury during the immediate phase after completing a rehabilitation program. J Spinal Cord Med. 2015 Jan;38(1):84–90. doi: 10.1179/2045772313Y.0000000173. Epub 2013 Nov 11. PMID: 24621026; PMCID: PMC4293538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lemay J.F., Noamani A., Unger J., Houston D.J., Rouhani H., Musselmann K.E. Using wearable sensors to characterize gait after spinal cord injury: evaluation of test-retest reliability and construct validity. Spinal Cord. 2021;59(6):675–683. doi: 10.1038/s41393-020-00559-4. [DOI] [PubMed] [Google Scholar]
- 65.Noamani A., Lemay J.F., Musselman K.E., et al. Postural control strategy after incomplete spinal cord injury: effect of sensory inputs on trunk–leg movement coordination. J NeuroEng Rehabil. 2020;17:141. doi: 10.1186/s12984-020-00775-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hansen R., Biering-Sørensen F., Kristensen J. Urinary incontinence in spinal cord injured individuals 10–45 years after injury. Spinal Cord. 2010;48:27–33. doi: 10.1038/sc.2009.46. [DOI] [PubMed] [Google Scholar]
- 67.Pavese C., Schneider M.P., Schubert M., et al. Prediction of bladder outcomes after traumatic spinal cord injury: a longitudinal cohort study. PLoS Med. 2016;13(6) doi: 10.1371/journal.pmed.1002041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fong D.D., Yu X., Mao J., et al. Restoring the sense of bladder fullness for spinal cord injury patients. Smart Health. 2018;9–10:12–22. doi: 10.1016/j.smhl.2018.07.014. [DOI] [Google Scholar]
- 69.Knight S.L., Edirisinghe N., Leaker B., Susser J., Craggs M.D. Conditional neuromodulation of neurogenic detrusor overactivity using transrectal stimulation in patients with spinal cord injury: a proof of principle study. Neurourol Urodyn. 2018;37(1):385–393. doi: 10.1002/nau.23310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Suresh S., Duerstock B.S. Automated detection of symptomatic autonomic dysreflexia through multimodal sensing. IEEE journal of translational engineering in health and medicine. 2020;8 doi: 10.1109/JTEHM.2019.2955947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hou Y., Dan X., Babbar M., et al. Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol. 2019;15(10):565–581. doi: 10.1038/s41582-019-0244-7. [DOI] [PubMed] [Google Scholar]
- 72.Parkinson's Foundation . Parkinson’s Foundation; 2019. Statistics.https://www.parkinson.org/Understanding-Parkinsons/Statistics [Google Scholar]
- 73.Cheng F., Duan Y., Jiang H., et al. Identifying and distinguishing of essential tremor and Parkinson's disease with grouped stability analysis based on searchlight-based MVPA. Biomed Eng Online. 2022;21(1):81. doi: 10.1186/s12938-022-01050-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.López-Blanco R., Velasco M.A., Méndez-Guerrero A., et al. Smartwatch for the analysis of rest tremor in patients with Parkinson's disease. J Neurol Sci. 2019;401:37–42. doi: 10.1016/j.jns.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 75.Channa A., Ifrim R.C., Popescu D., Popescu N. A-WEAR bracelet for detection of hand tremor and bradykinesia in Parkinson's patients. Sensors. 2021;21(3):981. doi: 10.3390/s21030981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schütz L., Sixel-Döring F., Hermann W. Management of sleep disturbances in Parkinson's disease. J Parkinsons Dis. 2022;12(7):2029–2058. doi: 10.3233/JPD-212749. PMID: 35938257; PMCID: PMC9661340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McGregor S., Churchward P., Soja K., et al. The use of accelerometry as a tool to measure disturbed nocturnal sleep in Parkinson's disease. NPJ Parkinson's disease. 2018;4:1. doi: 10.1038/s41531-017-0038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arora S., Baig F., Lo C., et al. Smartphone motor testing to distinguish idiopathic REM sleep behavior disorder, controls, and PD. Neurology. 2018;91(16):e1528–e1538. doi: 10.1212/WNL.0000000000006366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ramasubbu R., Lang S., Kiss Z.H.T. Dosing of electrical parameters in deep brain stimulation (dbs) for intractable depression: a review of clinical studies. Front Psychiatr. 2018;9:302. doi: 10.3389/fpsyt.2018.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gandhi K.R., Saadabadi A. StatPearls. StatPearls Publishing; 2022. Levodopa (L-Dopa) [PubMed] [Google Scholar]
- 81.Stocchi F., Vacca L., Radicati F.G. How to optimize the treatment of early stage Parkinson's disease. Transl Neurodegener. 2015;4:4. doi: 10.1186/2047-9158-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Del Din S., Godfrey A., Galna B., Lord S., Rochester L. Free-living gait characteristics in aging and Parkinson's disease: impact of environment and ambulatory bout length. J NeuroEng Rehabil. 2016;13(1):46. doi: 10.1186/s12984-016-0154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Burq M., Rainaldi E., Ho K.C., et al. Virtual exam for Parkinson's disease enables frequent and reliable remote measurements of motor function. NPJ digital medicine. 2022;5(1):65. doi: 10.1038/s41746-022-00607-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oyama G., Burq M., Hatano T., et al. Analytical and clinical validity of wearable, multi-sensor technology for assessment of motor function in patients with Parkinson's disease in Japan. Sci Rep. 2023 Mar 14;13(1):3600. doi: 10.1038/s41598-023-29382-6. PMID: 36918552; PMCID: PMC10015076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kourtis L.C., Regele O.B., Wright J.M., Jones G.B. Digital biomarkers for Alzheimer's disease: the mobile/wearable devices opportunity. NPJ digital medicine. 2019;2:9. doi: 10.1038/s41746-019-0084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mc Ardle R., Del Din S., Galna B., Thomas A., Rochester L. Differentiating dementia disease subtypes with gait analysis: feasibility of wearable sensors? Gait Posture. 2020;76:372–376. doi: 10.1016/j.gaitpost.2019.12.028. [DOI] [PubMed] [Google Scholar]
- 87.Nisticò R., Pirritano D., Salsone M., et al. Synchronous pattern distinguishes resting tremor associated with essential tremor from rest tremor of Parkinson's disease. Park Relat Disord. 2011;17(1):30–33. doi: 10.1016/j.parkreldis.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 88.Chandrabhatla A.S., Pomeraniec I.J., Ksendzovsky A. Co-evolution of machine learning and digital technologies to improve monitoring of Parkinson's disease motor symptoms. npj Digit. Med. 2022;5:32. doi: 10.1038/s41746-022-00568-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lightsey H.M., 4th, Yeung C.M., Samartzis D., Makhni M.C. The past, present, and future of remote patient monitoring in spine care: an overview. Eur Spine J. 2021 Aug;30(8):2102–2108. doi: 10.1007/s00586-021-06921-1. Epub 2021 Jul 9. PMID: 34241698. [DOI] [PubMed] [Google Scholar]
- 90.Lee T.J., Galetta M.S., Nicholson K.J., et al. Wearable technology in spine surgery. Clin Spine Surg. 2020 Jul;33(6):218–221. doi: 10.1097/BSD.0000000000000905. PMID: 31634172. [DOI] [PubMed] [Google Scholar]
- 91.Kang S.-W., Choi H., Park H.-I., et al. The development of an IMU integrated clothes for postural monitoring using conductive yarn and interconnecting technology. Sensors. 2017;17(11):2560. doi: 10.3390/s17112560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim D.H., Nam K.H., Choi B.K., Han I.H., Jeon T.J., Park S.Y. The usefulness of a wearable device in daily physical activity monitoring for the hospitalized patients undergoing lumbar surgery. Journal of Korean Neurosurgical Society. 2019;62(5):561–566. doi: 10.3340/jkns.2018.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ghent F., Mobbs R.J., Mobbs R.R., Sy L., Betteridge C., Choy W.J. Assessment and post-intervention recovery after surgery for lumbar disk herniation based on objective gait metrics from wearable devices using the gait posture Index. World neurosurgery. 2020;142:e111–e116. doi: 10.1016/j.wneu.2020.06.104. [DOI] [PubMed] [Google Scholar]
- 94.Wang Y., Zhou H., Yang Z., et al. vol. 2018. Annual International Conference of the IEEE Engineering in Medicine and Biology Society; 2018. An intelligent wearable device for human's cervical vertebra posture monitoring; pp. 3280–3283. (IEEE Engineering in Medicine and Biology Society. Annual International Conference). [DOI] [PubMed] [Google Scholar]
- 95.Lou E., Lam G.C., Hill D.L., Wong M.S. Development of a smart garment to reduce kyphosis during daily living. Med Biol Eng Comput. 2012;50(11):1147–1154. doi: 10.1007/s11517-011-0847-7. [DOI] [PubMed] [Google Scholar]
- 96.Tlili F., Haddad R., Bouallegue R., Shubair R. Internet of Things; 2021. Design and Architecture of Smart Belt for Real Time Posture Monitoring. [DOI] [Google Scholar]
- 97.Takahashi T., Fujiwara S., Igarashi S., et al. Comparison of subjective and objective assessments on improvement in gait function after carotid endarterectomy. Sensors. 2020;20(22):6590. doi: 10.3390/s20226590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cole T.S., Jahnke H., Godzik J., et al. Use of a wrist-mounted device for continuous outpatient physiologic monitoring after transsphenoidal surgery: a pilot study. Pituitary. 2019;22:156–162. doi: 10.1007/s11102-019-00946-y. [DOI] [PubMed] [Google Scholar]
- 99.Minen M.T., Stieglitz E.J. Wearables for neurologic conditions: considerations for our patients and research limitations. Neurology. Clin Pract. 2021;11(4):e537–e543. doi: 10.1212/CPJ.0000000000000971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kageyama I., Kurata K., Miyashita S., Lim Y., Sengoku S., Kodama K. A bibliometric analysis of wearable device research trends 2001-2022-A study on the reversal of number of publications and research trends in China and the USA. Int J Environ Res Publ Health. 2022;19(24) doi: 10.3390/ijerph192416427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mualem W., Durrani S., Lakomkin N., Van Gompel J., Quiñones-Hinojosa A., Bydon M. Utilizing data from wearable technologies in the era of telemedicine to assess patient function and outcomes in neurosurgery: systematic review and time-trend analysis of the literature. World neurosurgery. 2022;166:90–119. doi: 10.1016/j.wneu.2022.07.036. [DOI] [PubMed] [Google Scholar]
- 102.Inoue M., Orita S., Inage K., et al. Objective evaluation of postoperative changes in real-life activity levels in the postoperative course of lumbar spinal surgery using wearable trackers. BMC Muscoskel Disord. 2020;21(1):72. doi: 10.1186/s12891-020-3102-2. Published 2020 Feb 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stienen M.N., Rezaii P.G., Ho A.L., et al. Objective activity tracking in spine surgery: a prospective feasibility study with a low-cost consumer grade wearable accelerometer. Sci Rep. 2020;10(1):4939. doi: 10.1038/s41598-020-61893-4. Published 2020 Mar 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.van Lier H.G., Pieterse M.E., Garde A., et al. A standardized validity assessment protocol for physiological signals from wearable technology: methodological underpinnings and an application to the E4 biosensor. Behav Res Methods. 2020;52(2):607–629. doi: 10.3758/s13428-019-01263-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Deoni S.C.L., D'Sa V., Volpe A., et al. Remote and at-home data collection: considerations for the NIH HEALthy brain and cognitive development (HBCD) study. Dev Cogn Neurosci. 2022;54 doi: 10.1016/j.dcn.2022.101059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Aldemir K., Gürkan A. The effect of pedometer-supported walking and telemonitoring after disc hernia surgery on pain and disability levels and quality of life. Int J Nurs Pract. 2021;27(2) doi: 10.1111/ijn.12917. [DOI] [PubMed] [Google Scholar]
- 107.Barkley J.E., Vucetic H., Leone D., et al. Increased physical activity and reduced pain with spinal cord stimulation: a 12-month study. Int J Exerc Sci. 2020;13(3):1583–1594. doi: 10.70252/EVIW5224. . Published 2020 Dec 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Basil G.W., Sprau A.C., Eliahu K., Borowsky P.A., Wang M.Y., Yoon J.W. Using smartphone-based accelerometer data to objectively assess outcomes in spine surgery. Neurosurgery. 2021;88(4):763–772. doi: 10.1093/neuros/nyaa505. [DOI] [PubMed] [Google Scholar]
- 109.Gilmore S.J., Davidson M., Hahne A.J., McClelland J.A. The validity of using activity monitors to detect step count after lumbar fusion surgery. Disabil Rehabil. 2020;42(6):863–868. doi: 10.1080/09638288.2018.1509140. [DOI] [PubMed] [Google Scholar]
- 110.Schulte T.L., Schubert T., Winter C., et al. Step activity monitoring in lumbar stenosis patients undergoing decompressive surgery. Eur Spine J. 2010;19(11):1855–1864. doi: 10.1007/s00586-010-1324-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mobbs R.J., Phan K., Maharaj M., Rao P.J. Physical activity measured with accelerometer and self-rated disability in lumbar spine surgery: a prospective study. Global Spine J. 2016;6(5):459–464. doi: 10.1055/s-0035-1565259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cernera S., Alcantara J.D., Opri E., et al. Wearable sensor-driven responsive deep brain stimulation for essential tremor. Brain Stimul. 2021;14(6):1434–1443. doi: 10.1016/j.brs.2021.09.002. [DOI] [PubMed] [Google Scholar]
- 113.Chockalingam A., Boggs H., Prusik J., et al. Evaluation of quantitative measurement techniques for head tremor with thalamic deep brain stimulation. Neuromodulation. 2017;20(5):464–470. doi: 10.1111/ner.12566. [DOI] [PubMed] [Google Scholar]
- 114.Krishnan S.R., Arafa H.M., Kwon K., et al. Continuous, noninvasive wireless monitoring of flow of cerebrospinal fluid through shunts in patients with hydrocephalus. NPJ digital medicine. 2020;3:29. doi: 10.1038/s41746-020-0239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rwei A.Y., Lu W., Wu C., et al. A wireless, skin-interfaced biosensor for cerebral hemodynamic monitoring in pediatric care. Proc Natl Acad Sci USA. 2020;117(50):31674–31684. doi: 10.1073/pnas.2019786117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vijayan V., Connolly J.P., Condell J., McKelvey N., Gardiner P. Review of wearable devices and data collection considerations for connected health. Sensors. 2021;21(16):5589. doi: 10.3390/s21165589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kim H., Kim Y.S., Mahmood M., et al. Fully integrated, stretchable, wireless skin-conformal bioelectronics for continuous stress monitoring in daily life. Adv Sci. 2020;7(15) doi: 10.1002/advs.202000810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rice P., Upasham S., Jagannath B., Manuel R., Pali M., Prasad S. CortiWatch: watch-based cortisol tracker. Future science OA. 2019;5(9):FSO416. doi: 10.2144/fsoa-2019-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kaur B., Kumar S., Kaushik B.K. Novel wearable optical sensors for vital health monitoring systems—a review. Biosensors. 2023;13(2):181. doi: 10.3390/bios13020181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Smuck M., Odonkor C.A., Wilt J., Schmidt N., Swiernik M.A. The emerging clinical role of wearables: factors for successful implementation in healthcare. NPJ Digital Medicine. 2021;4 doi: 10.1038/s41746-021-00418-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Azodo I., Williams R., Sheikh A., Cresswell K. Opportunities and challenges surrounding the use of data from wearable sensor devices in health care: qualitative interview study. J Med Internet Res. 2020;22(10) doi: 10.2196/19542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jiang D., Shi G. Research on data security and privacy protection of wearable equipment in healthcare. J Healthc Eng. 2021 Feb 5;2021 doi: 10.1155/2021/6656204. PMID: 33628404; PMCID: PMC7884134. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 123.Bayoumy K., Gaber M., Elshafeey A., et al. Smart wearable devices in cardiovascular care: where we are and how to move forward. Nat Rev Cardiol. 2021 Aug;18(8):581–599. doi: 10.1038/s41569-021-00522-7. Epub 2021 Mar 4. PMID: 33664502; PMCID: PMC7931503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dinh-Le C., Chuang R., Chokshi S., Mann D. Wearable health technology and electronic health record integration: scoping review and future directions. JMIR Mhealth Uhealth. 2019 Sep 11;7(9) doi: 10.2196/12861. PMID: 31512582; PMCID: PMC6746089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Syagnik (Sy) Banerjee. Thomas Hemphill, Longstreet Phil. Wearable devices and healthcare: data sharing and privacy. Inf Soc. 2018;34(1):49–57. doi: 10.1080/01972243.2017.1391912. [DOI] [Google Scholar]
- 126.Li Z., Wang B., Li J., Hua Y., Zhang S. Local differential privacy protection for wearable device data. PLoS One. 2022;17(8) doi: 10.1371/journal.pone.0272766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Char Danton S., Abràmoff Michael D., Feudtner Chris. Identifying ethical considerations for machine learning healthcare applications. Am J Bioeth. 2020;20(11):7–17. doi: 10.1080/15265161.2020.1819469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chiruvella V., Guddati A.K. Ethical issues in patient data ownership. Interact J Med Res. 2021 May 21;10(2) doi: 10.2196/22269. PMID: 34018968; PMCID: PMC8178732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Drake Coleman, Zhang Yuehan, Chaiyachati Krisda H., et al. The limitations of poor broadband internet access for telemedicine use in rural America: an observational study. Ann Intern Med. 2019;171:382–384. doi: 10.7326/M19-0283. [Epub. [DOI] [PubMed] [Google Scholar]
- 130.Chen E., Miller G.E. Socioeconomic status and health: mediating and moderating factors. Annu Rev Clin Psychol. 2013;9(1):723–749. doi: 10.1146/annurev-clinpsy-050212-185634. [DOI] [PubMed] [Google Scholar]
- 131.Colvonen P.J., DeYoung P.N., Bosompra N.A., Owens R.L. Limiting racial disparities and bias for wearable devices in health science research. Sleep. 2020 Oct 13;43(10):zsaa159. doi: 10.1093/sleep/zsaa159. PMID: 32893865; PMCID: PMC8477341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Koerber D., Khan S., Shamsheri T., et al. The effect OF SKIN tone on accuracy of heart rate measurement in wearable devices: a systematic review. J Am Coll Cardiol. 2022 Mar;79(9_suppl ment) doi: 10.1016/S0735-1097(22)02981-3. 1990. [DOI] [Google Scholar]
- 133.Miller Portia, Votruba-Drzal Elizabeth, Levine Coley Rebekah. Poverty and academic achievement across the urban to rural landscape: associations with community resources and stressors. RSF: The Russell Sage Foundation Journal of the Social Sciences. 2019;5(2):106. doi: 10.7758/RSF.2019.5.2.06. [DOI] [PMC free article] [PubMed] [Google Scholar]