Abstract
The rpoN region of Pseudomonas stutzeri was cloned, and an rpoN null mutant was constructed. RpoN was not essential for denitrification in this bacterium but affected the expression levels and enzymatic activities of cytochrome cd1 nitrite reductase and nitric oxide reductase, whereas those of respiratory nitrate reductase and nitrous oxide reductase were comparable to wild-type levels. Since the transcription of the structural genes nirS and norCB, coding for nitrite reductase and the nitric oxide reductase complex, respectively, proceeded unabated, our data indicate a posttranslational process for the two key enzymes of denitrification depending on RpoN.
Denitrification is an alternative way of energy conservation for many facultative anaerobic bacteria. The process is thought to be organized in a tripartite modular way, in which the respiratory systems utilizing nitrate, nitrite and nitric oxide (NO), and nitrous oxide (N2O) have to be induced by environmental signals to form a functional unit for the complete denitrification pathway (for a review, see reference 24). To achieve this, the expression of the genes encoding the four reductases and accessory proteins have to be regulated in a concerted way, which may be controlled by a distinct sigma factor. Sigma factors have been found as coordinating elements for the transcription of specific sets of genes in response to environmental stimuli.
Sigma factor ς54 (encoded by the rpoN gene) was originally described as a factor involved in the expression of nitrogen-regulated genes; since then multiple and diverse physiological functions have been found to depend on this factor (12). The role of ς54 in denitrification is still insufficiently clarified. The factor is required in Ralstonia eutropha (formerly Alcaligenes eutrophus), a denitrifying hydrogen bacterium, for anaerobic growth on nitrate (18). However, it is not clear whether critical genes for denitrification depend on RpoN or whether the requirement is indirect in nature. In Pseudomonas aeruginosa RpoN controls diverse sets of genes, such as those for glutamine synthetase, urease, and flagellin, but an rpoN mutant grows anaerobically on nitrate (20). In this case it is not known whether the expression of genes for the entire denitrification pathway is independent of RpoN. A comparison of promoters of several denitrification genes of Pseudomonas stutzeri did not provide sequence-specific clues with respect to a dependence on RpoN (4). In Bradyrhizobium japonicum the expression of genes for nitrate respiration again is independent of rpoN (for a review, see reference 6), but as for P. aeruginosa the role of ς54 in the proper denitrification system remains to be investigated. An rpoN mutant of the diazotrophic denitrifier Azospirillum brasilense is defective in nitrate assimilation, yet a possible effect on denitrification has not been explored (17).
Here we describe the isolation of the rpoN gene region from P. stutzeri. To show which step of denitrification is regulated by RpoN we constructed an rpoN mutation by gene replacement and analyzed the mutant for the expression of the four terminal reductases of denitrification at the transcriptional and translational levels.
Isolation and cloning of rpoN.
The experimental strain was P. stutzeri MK21, a spontaneously streptomycin-resistant derivative of strain ATCC 14405. The presence of an rpoN gene was demonstrated by Southern hybridization of genomic DNA with a probe from rpoN of Pseudomonas putida. A genomic cosmid library of MK21 was screened with a 1.4-kb SacI-HindIII fragment derived from the rpoN-carrying plasmid pNTR1 (10). Cosmid DNA of 264 clones was isolated (5) and digested with SmaI. The DNA was separated on 0.75% agarose gels and blotted onto nitrocellulose membranes. Hybridization was performed at 65°C as described elsewhere (19). The rpoN gene of P. stutzeri was found on a 3.4-kb HindIII fragment on cosmid c167. It was cloned as two EcoRI-HindIII fragments of 1.6 and 1.8 kb into the vector pUC18. The 1.8-kb clone, pRpoN1.8, carried the complete rpoN gene. Occasionally a bacterium harbors two gene copies of rpoN (11). Since hybridization of genomic DNA with the homologous 1-kb XhoI probe (see below) at low stringency (45°C) gave a single signal, we assume that P. stutzeri possesses only one copy of rpoN.
Sequence analysis of rpoN and its flanking regions.
The nucleotide sequence of rpoN was determined by sequencing plasmid pRpoN1.8, and the rpoN-flanking regions were obtained by direct sequencing of cosmid c167. For this purpose the dideoxy chain termination method with universal and sequence-specific primers was used together with a Thermo-Sequenase kit (United States Biochemical Corp.) and [35S]dATP (Amersham). The sequence revealed four open reading frames (ORFs), whose derived products were similar to those derived from the rpoN region of other denitrifiers (Fig. 1). The rpoN sequence extends over 1,503 bp and encodes a protein of 502 amino acids with a Mr of 56,843. The derived amino acid sequence exhibits the three regions which have been determined to be typical of ς54 factors (for a review, see reference 15). The N-terminal region has the domain of 50 amino acids, rich in glutamine and leucine, followed by 110 residues with a prevalence of acidic amino acids. The carboxy-terminal region exhibits the helix-turn-helix structure (amino acid positions 387 to 412, P. stutzeri count) and the invariant sequence ARRTVAKYR (positions 479 to 487), known as the RpoN box (21).
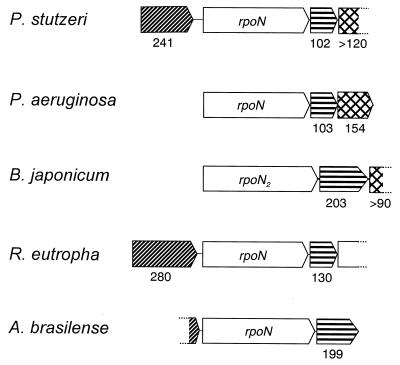
FIG. 1.
Organizational conservation of rpoN regions in denitrifying bacteria. ORFs are shown as arrow boxes to indicate the direction of transcription. Numbers of amino acids of the derived gene products are shown. Homologous components are indicated by identical patterns: ATP-binding proteins of ABC transporters (▨), modulators of ς54 function (▤), and PTS proteins, EIIA (▩). Information about the following organisms was taken from the following references: P. aeruginosa (9), B. japonicum (11), R. eutropha (22), A. brasilense (17). Incompletely sequenced ORFs are shown as open boxes.
ORF241, upstream of rpoN, is transcribed in the same direction and potentially encodes a 241-amino-acid protein with a Mr of 26,420. Downstream of rpoN, ORF102 may encode a 102-amino-acid polypeptide with a Mr of 11,752. The next ORF was sequenced only partially to cover 120 amino acids. The product of this ORF is a homolog of the protein EIIA of the phosphotransferase system. Mutations in the corresponding ORFs 95 and 154 downstream of rpoN of Klebsiella pneumoniae increase transcription from RpoN-dependent promoters, suggesting that the gene products may act as modulators of ς54 activity (16).
Primer extension analysis was used to locate the promoter of rpoN. Total RNA was prepared from MK21 cells grown in asparagine-citrate (AC) medium under oxygen-limited conditions and supplemented with 1 g of NaNO3 per liter (3). The RNA was extracted by a method described elsewhere (1), and primer extension was done by a standard protocol (2). We found two transcript initiation sites spaced by only two nucleotides. Upstream of those sites the sequences TATAAT and TAGGCA are thought to be −10 and −35 binding motifs, respectively. The −10 sequence is identical to the ς70 consensus sequence of Escherichia coli (8), whereas the −35 sequence varies somewhat from the TTGACA consensus sequence. The presence of these motifs suggests that rpoN of P. stutzeri is under the control of the principal sigma factor ς70, encoded by rpoD. Interestingly, the rpoN promoter of P. stutzeri is identical from positions +2 to −21 to the promoter sequence of rpoN of P. aeruginosa and extends further to an identical −35 motif (Fig. 2). Overall the derived P. stutzeri and P. aeruginosa RpoN proteins are 84.5% identical.
FIG. 2.
Nucleotide sequence of the rpoN promoter. The transcriptional start of rpoN was determined by primer extension analysis; the oligonucleotide used for primer extension is underlined. The 5′ ends of transcripts are marked by arrows and +1. Putative −35 and −10 motifs are boxed. RBS, ribosome binding site. The promoter regions identical to that of P. aeruginosa are printed in lowercase letters. The C-terminal and N-terminal amino acid sequences of ORF241 and RpoN, respectively, are given below the nucleotide sequence in one-letter code.
Construction of an rpoN mutation by gene replacement.
We constructed a null mutant to analyze the role of RpoN in denitrification of P. stutzeri. An internal 1-kb XhoI fragment which covers 66% of the rpoN gene was deleted from pRpoN1.8. The kanamycin resistance (Kmr) cassette from plasmid pUC4-Kiss (Pharmacia) with the aminoglycoside 3′-phosphotransferase gene of transposon Tn903 was inserted in the opposite orientation into rpoN, yielding plasmid pRpoN::Kmr. The replacement construct was linearized at the EcoRI site and electroporated into competent P. stutzeri cells. For electroporation the bacterium was grown in 3.2% Bacto Tryptone–2% yeast extract–0.5% NaCl, pH 7.5, for 16 h at 30°C. The cells were washed twice with 15% glycerol in 1 mM MOPS (morpholinepropanesulfonic acid), pH 7.5. Conditions for electroporation in a 0.2-cm cuvette were 2.5 kV, 25 μF, 200 Ω (Bio-Rad Gene Pulser). Disruption of the wild-type gene by homologous recombination in mutant MK516 was selected for on the basis of kanamycin resistance (final concentration, 200 μg ml−1) and ampicillin sensitivity (100 μg ml−1). Genomic DNA from MK516 was cleaved with HindIII. The digest was separated on a 0.8% agarose gel, transferred to a nylon membrane, and hybridized with the 1-kb XhoI fragment of rpoN and the 1.3-kb Kmr cassette to verify gene inactivation. Because of the effected deletion the XhoI probe gave no signal, whereas the Kmr probe yielded two signals of 1.5 and 2.6 kb, due to the internal HindIII site of the inserted cassette (data not shown).
Effect of the rpoN mutation on denitrification enzymes.
A striking phenotype of the rpoN mutant MK516 was its loss of growth on AC minimal medium. Since the structural gene for glutamine synthetase is under RpoN control in P. aeruginosa (20), we supplemented AC medium with glutamine but were unsuccessful in restoring growth with or without nitrate either under aerobic or anaerobic conditions. The mutant was also unable to use histidine or proline as the sole nitrogen source. The lack of growth of the rpoN mutant on minimal medium indicates that the absence of ς54 must affect amino acid metabolism and other routes of intermediary metabolism of P. stutzeri. MK516 grew in Luria-Bertani medium with nitrate, aerobically as well as anaerobically, but under the latter conditions grew more slowly than the wild type, demonstrating that rpoN exerts a general effect on denitrification. MK516 also lost its motility on 0.3% swarm agar, which indicates that RpoN may be required for flagellin synthesis, as had been demonstrated for P. aeruginosa (20).
To find out which step in the denitrification pathway was affected by rpoN, we measured the in vitro activities of the reductases for nitrate, nitrite, and NO of MK516 in comparison to those of MK21, representing wild-type traits (Table 1). Denitrification of MK516 was induced by a shift from aerobic to O2-limited growth conditions in the presence of nitrate. Luria-Bertani medium (500 ml in a 1-liter flask) was inoculated with an aerobic overnight culture to result in an optical density at 660 nm (OD660) of about 0.2. The culture was incubated for 5 h on a gyratory shaker at 240 rpm, 30°C, until the OD660 was ≈0.4. Denitrification was induced by adding NaNO3 (final concentration, 1 mg ml−1) and reducing the shaking speed to 120 rpm. Cells were harvested after 16 h by centrifugation and broken in a French press, and cell extract was obtained as the supernatant from centrifugation (10 min, 20,000 × g). MK516 showed reduced activities of cytochrome cd1 nitrite reductase and NO reductase, whereas the activity of nitrate reductase corresponded to that of the wild type (Table 1). Also, N2O reductase was not affected in MK516, measured as in vivo activity of whole cells by gas chromatography (data not shown). Next, we complemented the mutation in MK516 with the rpoN gene to verify that the lower activity found with nitrite and NO reductases was a direct effect of rpoN deletion and not a polar effect of genes located downstream of rpoN. The 1.8-kb EcoRI-HindIII fragment, containing the complete copy of rpoN, was cloned into vector pUCP24, a broad-host-range vector, that is able to replicate in the genus Pseudomonas (23). The construct was transferred to MK516 by electroporation. The complemented cell line MK516c exhibited increased enzyme activities, coming close to the wild-type level and sometimes even surpassing it, indicating that the diminished reductase activities of MK516 were a direct result of the rpoN mutation (Table 1).
TABLE 1.
In vitro activities of denitrification enzymes
Whether the effect on nitrite and NO reductases was caused by a decreased expression of the respective structural genes was investigated by comparing the amount of transcripts of nirS (nitrite reductase) and of norCB (NO reductase complex) of MK516 with that of the wild type (Fig. 3). Both structural gene sets are organized in independent transcriptional units (24). nirS is transcribed both from the nirSTB operon and as a monocistronic message and thus yields two signals on Northern blot analysis. Aerobically cultivated cells exhibited no transcripts. In cells grown under denitrifying conditions the nirS and norCB transcripts were readily detectable. The levels found in denitrification-induced MK516 were not decreased versus that in MK21. This rules out a direct dependence of nirS and norCB transcription on ς54. Finally, we determined the level of denitrification enzymes, since the lower activities of nitrite and NO reductases could be due to a posttranscriptional mechanism that depends on ς54 and affects the concentration of enzymes. Cells were shifted to denitrification, and a cell extract was prepared as described above. The protein pattern was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the reductases were detected with the respective polyclonal antisera by immunoblotting. The rpoN mutant showed substantially lower levels of both nitrite reductase and NO reductase, whereas those of nitrate reductase and N2O reductase corresponded to that of MK21 (Fig. 4). On complementation of MK516 by rpoN, wild-type levels of all four reductases were detected.
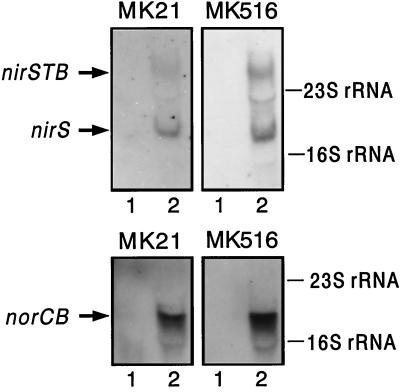
FIG. 3.
The rpoN null mutant is not affected in the transcription of nirS and norCB. Total RNA from MK516 and MK21 was prepared according to the method of Aiba et al. (1) from cells grown aerobically (lane 1 of each panel) or under denitrifying conditions (lane 2 of each panel). Induction of denitrification was as described in the text for the activity measurements. Samples (20 μg of RNA) were denatured by glyoxal-dimethyl sulfoxide treatment and separated on a 1.2% agarose gel (14). After transfer to a nylon membrane, the nirS and the norCB transcripts were detected by hybridization with digoxigenin-labeled probes (labeling kit from Boehringer Mannheim, following the instructions of the manufacturer). A 500-bp KpnI fragment of the nirS gene and a 2-kb PstI-BglII fragment of the norCB operon were used as probes. nirS exhibited mono- and polycistronic transcripts of 2 and 3.4 kb, respectively; the norCB transcript was 2 kb. Equal gel loading was verified by staining with acridine orange. The 16S and 23S rRNA species served as standards.
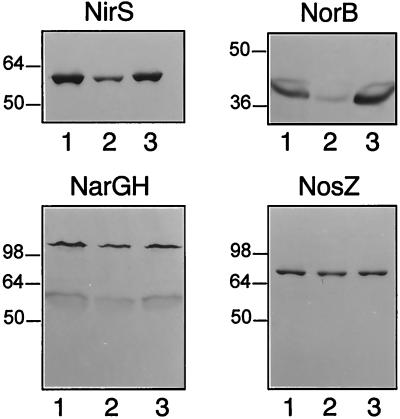
FIG. 4.
An rpoN mutation reduces the cellular level of nitrite reductase (NirS) and NO reductase (assayed as the NorB subunit). Cell extracts of strains MK21 (lanes 1), MK516 (lanes 2), and the complemented mutant MK516c (lanes 3), all induced for denitrification, were separated electrophoretically on a sodium dodecyl sulfate–12.5% polyacrylamide gel and blotted onto nitrocellulose, and the reductases were detected immunochemically. NarGH, nitrate reductase; NosZ, N2O reductase. Size markers (in kilodaltons) are indicated to the left of each panel.
In conclusion, ς54 is not involved in the expression of the denitrification system of P. stutzeri by acting as a transcription factor for one or several reductase genes, yet it affected the cellular concentrations of nitrite reductase and NO reductase. Since the transcription of both nirS and norCB proceeded unabated in the rpoN strain, the enzyme levels seem to be affected by a posttranslational mechanism involving one or several products of ς54-dependent gene expression, hence leading to diminished enzyme concentrations and concomitantly decreased denitrification rates. The effect is restricted to the two key enzymes of denitrification, nitrite reductase and NO reductase, and does not affect nitrate and nitrous oxide respiration. Our findings underline once more that the respiratory systems utilizing nitrite and NO are interlaced at several levels of regulation and further support the modular view of the denitrification process that we have detailed elsewhere (24).
Nucleotide sequence accession number.
The rpoN sequence reported here is available under the accession number AJ223088 in the EMBL/GenBank/DDBJ data banks.
Acknowledgments
We thank S. Harayama for a gift of rpoN from P. putida, H. Cuypers for his participation in the early stage of this project, and B. Schreckenberger for excellent technical assistance.
The work was supported by the Deutsche Forschungsgemeinschaft and Fonds der Chemischen Industrie.
REFERENCES
- 1.Aiba H, Adhya S, de Crombrugghe B. Evidence for two functional gal promoters in intact Escherichia coli. J Biol Chem. 1981;256:11905–11910. [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1995. [Google Scholar]
- 3.Coyle C L, Zumft W G, Kroneck P M H, Körner H, Jakob W. Nitrous oxide reductase from denitrifying Pseudomonas perfectomarina, purification and properties of a novel multicopper enzyme. Eur J Biochem. 1985;153:459–467. doi: 10.1111/j.1432-1033.1985.tb09324.x. [DOI] [PubMed] [Google Scholar]
- 4.Cuypers H, Berghöfer J, Zumft W G. Multiple nosZ promoters and anaerobic expression of nos genes necessary for Pseudomonas stutzerinitrous oxide reductase and assembly of its copper centers. Biochim Biophys Acta. 1995;1264:183–190. doi: 10.1016/0167-4781(95)00128-4. [DOI] [PubMed] [Google Scholar]
- 5.Feliciello I, Chinali G. A modified alkaline lysis method for the preparation of highly purified plasmid DNA from Escherichia coli. Anal Biochem. 1993;212:394–401. doi: 10.1006/abio.1993.1346. [DOI] [PubMed] [Google Scholar]
- 6.Fischer H-M. Genetic regulation of nitrogen fixation in rhizobia. Microbiol Rev. 1994;58:352–386. doi: 10.1128/mr.58.3.352-386.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frunzke K, Zumft W G. Rapid, single sample analysis of H2, O2, N2, NO, CO, N2O, and CO2by isothermal gas chromatography: applications to the study of bacterial denitrification. J Chromatogr. 1984;299:477–483. [Google Scholar]
- 8.Hawley D K, McClure W R. Compilation and analysis of Escherichia colipromoter DNA sequences. Nucleic Acids Res. 1983;11:2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin S, Ishimoto K, Lory S. Nucleotide sequence of the rpoN gene and characterization of two downstream open reading frames in Pseudomonas aeruginosa. J Bacteriol. 1994;176:1316–1322. doi: 10.1128/jb.176.5.1316-1322.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Köhler T, Harayama S, Ramos J-L, Timmis K N. Involvement of Pseudomonas putidaRpoN sigma factor in regulation of various metabolic functions. J Bacteriol. 1989;171:4326–4333. doi: 10.1128/jb.171.8.4326-4333.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kullik I, Fritsche S, Knobel H, Sanjuan J, Hennecke H, Fischer H-M. Bradyrhizobium japonicum has two differentially regulated, functional homologs of the ς54 gene (rpoN) J Bacteriol. 1991;173:1125–1138. doi: 10.1128/jb.173.3.1125-1138.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kustu S, Santero E, Keener J, Popham D, Weiss D. Expression of ς54 (ntrA)-dependent genes is probably united by a common mechanism. Microbiol Rev. 1989;53:367–376. doi: 10.1128/mr.53.3.367-376.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowe R H, Evans H J. Preparation and some properties of a soluble nitrate reductase from Rhizobium japonicum. Biochim Biophys Acta. 1964;85:377–389. doi: 10.1016/0926-6569(64)90301-3. [DOI] [PubMed] [Google Scholar]
- 14.McMaster G K, Carmichael G G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci USA. 1977;74:4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merrick M J. In a class of its own—the RNA polymerase sigma factor ς54 (ςN) Mol Microbiol. 1993;10:903–909. doi: 10.1111/j.1365-2958.1993.tb00961.x. [DOI] [PubMed] [Google Scholar]
- 16.Merrick M J, Coppard J R. Mutations in genes downstream of the rpoN gene (encoding ς54) of Klebsiella pneumoniae affect expression from ς54-dependent promoters. Mol Microbiol. 1989;3:1765–1775. doi: 10.1111/j.1365-2958.1989.tb00162.x. [DOI] [PubMed] [Google Scholar]
- 17.Milcamps A, Van Dommelen A, Stigter J, Vanderleyden J, Debruijn F J. The Azospirillum brasilense rpoNgene is involved in nitrogen fixation, nitrate assimilation, ammonium uptake, and flagellar biosynthesis. Can J Microbiol. 1996;42:467–478. doi: 10.1139/m96-064. [DOI] [PubMed] [Google Scholar]
- 18.Römermann D, Warrelmann J, Bender R A, Friedrich B. An rpoN-like gene of Alcaligenes eutrophus and Pseudomonas faciliscontrols expression of diverse metabolic pathways, including hydrogen oxidation. J Bacteriol. 1989;171:1093–1099. doi: 10.1128/jb.171.2.1093-1099.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 20.Totten P A, Lara J C, Lory S. The rpoN gene product of Pseudomonas aeruginosais required for expression of diverse genes, including the flagellin gene. J Bacteriol. 1990;172:389–396. doi: 10.1128/jb.172.1.389-396.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Slooten J C, Cervantes E, Broughton W J, Wong C H, Stanley J. Sequence and analysis of the rpoN sigma factor gene of Rhizobiumsp. strain NGR234, a primary coregulator of symbiosis. J Bacteriol. 1990;172:5563–5574. doi: 10.1128/jb.172.10.5563-5574.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warrelmann J, Eitinger M, Schwartz E, Römermann D, Friedrich B. Nucleotide sequence of the rpoN (hno) gene region of Alcaligenes eutrophus: evidence for a conserved gene cluster. Arch Microbiol. 1992;158:107–114. doi: 10.1007/BF00245213. [DOI] [PubMed] [Google Scholar]
- 23.West S E H, Schweizer H P, Dall C, Sample A K, Runyen-Janecky L J. Construction of improved Escherichia-Pseudomonas shuttle vectors derived from pUC18/19 and sequence of the region required for their replication in Pseudomonas aeruginosa. Gene. 1994;128:81–86. doi: 10.1016/0378-1119(94)90237-2. [DOI] [PubMed] [Google Scholar]
- 24.Zumft W G. Cell biology and molecular basis of denitrification. Microbiol Mol Biol Rev. 1997;61:533–616. doi: 10.1128/mmbr.61.4.533-616.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]