Abstract
Extremely cold habitats are a serious challenge for the existing there organisms. Inhabitants of these conditions are mostly microorganisms and lower mycetae. The mechanisms of microbial adaptation to extreme conditions are still unclear. Low temperatures cause significant physiological and biochemical changes in cells. Recently, there has been increasing interest in the relationship between low-temperature exposure and oxidative stress events, as well as the importance of antioxidant enzymes for survival in such conditions. The catalase is involved in the first line of the cells' antioxidant defense. Published information supports the concept of a key role for catalase in antioxidant defense against cold stress in a wide range of organisms isolated from the Antarctic. Data on representatives of microscopic fungi, however, are rarely found. There is scarce information on the characterization of catalase synthesized by adapted to cold stress organisms. Overall, this study aimed to observe the role of catalase in the survival strategy of filamentous fungi in extremely cold habitats and to identify the gene encoded catalase enzyme. Our results clearly showed that catalase is the main part of antioxidant enzyme defense in fungal cells against oxidative stress caused by low temperature exposure.
Keywords: Antarctic, Fungi, Catalase, Gene
Introduction
Enzymes are widely distributed in all living organisms and their important role is connected with lowering the energy of activation and accelerating numerous biological reactions.
Among them, a special place takes microbial enzymes, because of their faster, cost-effective, scalable, and amenable to genetic manipulation production [1]. Fungi are an important source of industrial enzymes. Recently, more than 50% of the total enzymes market belongs to fungal enzymes [2].
Fungal cells have the possibility of producing both intracellular and extracellular enzymes. Being natural decomposers, fungi produce extracellular enzymes needed for bioconversion of a variety of substrates and complexes [3]. Fungal enzymes have a significant role in fungal protection against hazardous compounds in the environment as well as in fungal cells' protection against stress caused by different stimuli. [4, 5]
Extremophiles are organisms that are able to live in extreme environments, i.e. environments with conditions approaching the limits of life. Life in these harmful conditions is characterized by a variety of stresses: additional ecological limiting factors, increased osmotic stress, high-pressure stress, UV irradiation, etc. [6]. Biological systems subjected to low temperatures are injured by an increase in cellular viscosity, a reduction in the fluidity of the cell membranes, reductions in molecular interactions, and many other oxidative stress events [7–9]. Filamentous fungi adapted to cold environments developed physiological and structural adaptations included in their survival strategies such as increasing the fluidity of the membrane, production of cold-active enzymes with high activity at low and moderate temperatures, and production of various compounds to protect themselves against intracellular freezing. Cold-adapted enzymes are one of the main elements of the Antarctic fungi survival strategy. They have some advantages and huge potential in biotechnological processes [10, 11].
Catalase is one of the main antioxidant enzymes that dismutate hydrogen peroxide (H2O2) into one molecule of oxygen and two molecules of water in a two-step reaction.
Tree families of catalases are known: as Mn-catalases, bifunctional catalase-peroxidases, and monofunctional catalases. Two distinct classes of catalases have been recognized within this family: enzymes with small units – between 50 and 65 kDa and enzymes with large units – 80 kDa. The first class included bacterial, plant, fungal, and animal catalases and the second class belonged to catalases in bacteria and filamentous fungi [12–14].
Catalase is an enzyme with serious potential for practical applications. In industrial processes, such as the production of textiles, the enzyme is used to break down hydrogen peroxide which is used as a bleaching agent. It is also used in the production of contact lens solutions, where it helps to break down hydrogen peroxide that is used to disinfect contact lenses [15].
A common application of the enzyme is in the food industry. Catalase is used in the production of cheese, where it helps to break down residual hydrogen peroxide that may be present in the milk or whey. This can help to prevent off-flavors and improve the quality of the final product [16]. Hydrogen peroxide is sometimes used as a wash or dip for meat products to reduce microbial contamination and it can be used as a preservative or disinfectant in the processing of fruits and vegetables. Catalase can be used to help break down any residual hydrogen peroxide before the product is packaged and sold.
Overall, catalase is an important enzyme in the food industry for ensuring product quality and safety, particularly in situations where hydrogen peroxide is used as a processing aid or preservative.
Catalase is an important enzyme implicated in mutagenesis and inflammation conditions as well as during the suppression of apoptosis [17] which are all known to be associated with oxidative stress conditions.
However, information on cold-adapted (CA) catalase from filamentous fungi is very scarce. In most cases, published data refer to CA enzyme synthesis by bacterial species. Fiedurek and Gromada (2000) published data on CA CAT from Antarctic fungi [18]. The second published information is from Krumova et al. (2021) for CA intracellular CAT produced by an Antarctic strain of P. griseofulvum [19, 20]. Our study aims to investigate the production of catalase by an Antarctic fungal strain, as well as to characterize the gene encoding an enzyme with catalase activity.
Materials and Methods
Strains, Media, and Growth Conditions
In the present experiments, the Antarctic fungal strain Aspergillus fumigatus I-9 was used. The strain belongs to the Mycological collection of the Stephan Angeloff Institute of Microbiology, Bulgarian Academy of Sciences. The cultivation was performed as follow: liquid media 4/4 as described in Angelova et al., (1996) was inoculated with 5 × 106 spores and was cultivated for 72, 96, and 120 h at four different temperatures 15, 20, 25, and 30 °C. [21].
Assay of Catalase and Superoxide Dismutase (SOD) Activity
Catalase activity was assayed by the method of Beers and Sizer, 1952 [22]. SOD activity was measured by the nitro-blue tetrazolium (NBT) reduction method of Beauchamp & Fridovich (1971) [23].
Isolation of DNA, PCR Conditions, and Sequencing Analysis
GeneMATRIX Plant & Fungi DNA Purification Kit (EURx Ltd.) were used for for DNA isolation from 48 h-old mycelium. PCR amplification was performed in a BioRad iCycler Thermal Cycler (Bio-Rad Laboratories, USA). The annealing temperature for each primer is presented in Table 1. The products were purified using the Gene JET PCR Purification Kit (Thermo Fisher Scientific Inc.). The primers were created with the Primer-BLAST program. The sequences were processed and analyzed with the following software: Chromas for sequence cleaning; ClustalW and BLASTX, and BLASTN to NCBI (National Center for Biotechnology Information, USA) to compare sequences. The studied genes were sequenced in parts, and the individual fragments were collected with the CAP3 Sequence Assembly Program. The putative protein structure of catalase was analyzed using the Conserved Domain Database (CDD) [24] and the Expasy program (Swiss Institute of Bioinformatics).
Table 1.
Specific primers for catalase genes created for this study
| Catalase-peroxidase (CAT2) | ||||
|---|---|---|---|---|
| CATFu | F | 5 ′-GATCCTCACCGGTAACGTCG-3 ′ | 60 °C | 800 bp |
| CATFu | R | 5 ′-GTGGGTGAGCTTGAACCAGG-3 ′ | ||
| 12 | F | 5 ′-TTAGGATTGGCCAGCAGCAA-3 ′ | 60 °C | 930 bp |
| 12 | R | 5 ′-GACATAGACTTGCCTCCCCG-3 ′ | ||
| 13 | F | 5 ′-TCCGTCATGGTGATTGGTCC-3 ′ | 60 °C | 900 bp |
| 13 | R | 5 ′-CAGGTAGTCTCTCGTCCCCA-3 ′ | ||
| 14 | F | 5 ′-CGAGCATCCGGACCAGTTC-3 ′ | 60 °C | 680 bp |
| 14 | R | 5 ′-CCAACCAGGACGGTCATCTC-3 ′ | ||
| 15 | F | 5 ′-CGAGATGACCGTCCTGGT-3 ′ | 60 °C | 520 bp |
| 15 | R | 5 ′-AATGAAAATGGGTACATCAGCATAA-3 ′ | ||
F/R forward or reverse primer
Nucleotide Sequence Accession Number
The obtained sequences were deposited in the public GenBank database with open access (https://www.ncbi.nlm.nih.gov/genbank/) under accession number: MW618007—catalase-peroxidase gene, complete cds; MT758189—ITS region Aspergillus fumigatus 1–9.
Results and Discussion
Growth and Enzyme Activity of A. fumigatus I-9
As a model strain in our observation, we used Antarctic fungal strain A. fumigatus I-9 (accession number MT758189) isolated from the Antarctic soil sample (Livingston island) at 4 °C. The strain was characterized as mesophilic. Experiments on its temperature characteristics showed that it develops well on 3 agar media (Beer Agar, Saburau Agar, and Chapek Dox Aagar) in a temperature range between 15 °C and 30 °C (Table 2).
Table 2.
Fungal growth on agar media at different temperatures
| Temperature | Diameter of fungal colony (cm) | ||
|---|---|---|---|
| 3 days | 5 days | 7 days | |
| 6 °C | – | – | – |
| 10 °C | – | – | – |
| 15 °C | 4.2 | 5.3 | 8.3 |
| 20 °C | 4.5 | 5.5 | 8.6 |
| 25 °C | 4.4 | 5.5 | 8.6 |
| 30 °C | 1.8 | 3 | 5.8 |
The influence of temperature on the catalase production of the model strain was studied. The changes in the activities of the second antioxidant enzyme during cultivation of the model strain at four different temperatures (15, 20, 25, and 30 °C) at the 72nd hour from the beginning of cultivation were monitored. Submerged cultivation of the tested strain at the investigated temperatures showed no changes in the growth (Fig. 1). Figure 1 shows that the studied strain developed well at the tested temperatures, which confirms its characteristic as a mesophilic strain.
Fig. 1.
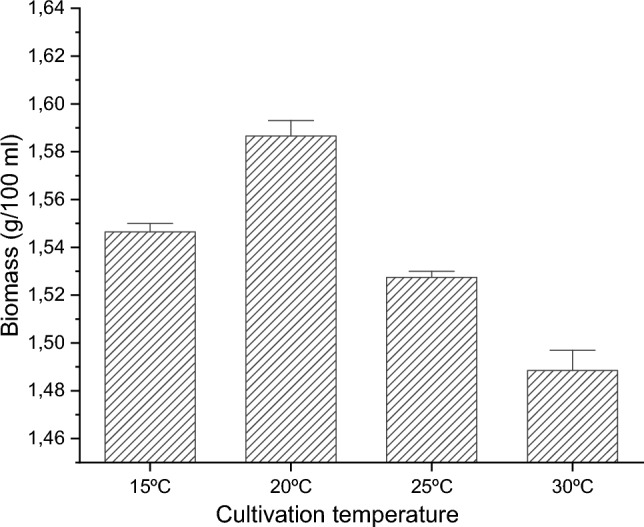
Biomass production of the model strain cultivated at different temperatures
The results of the conducted experiments showed the highest level of catalase and SOD enzyme activity when the strain was cultivated at 15 °C (Fig. 2). This is probably due to the activation of the enzymatic antioxidant defense in response to oxidative stress caused by low temperatures.
Fig. 2.
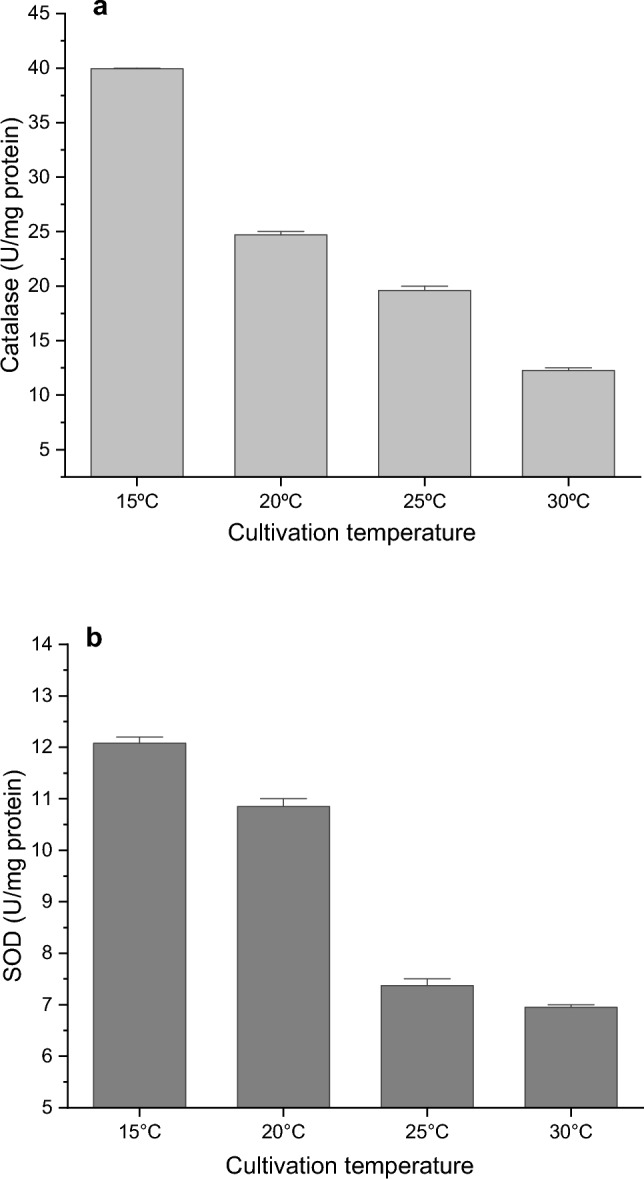
Catalase (a) and SOD (b) activity in dependence on growth temperature
Enzyme activity was also monitored at later stages of cultivation. We found that maximum extracellular activity was recorded at 72 h. The intracellular enzyme showed maximum activity at later hours of cultivation – at 96 h (Fig. 3). As is known, CAT is found in the cytoplasm or in peroxisomes, but there are also reports of an extracellularly synthesized enzyme. The extracellular enzyme that protects cells from exogenous oxidative damage is very rare. The most efficient producers of CAT in the extracellular space are microorganisms and especially strains of filamentous fungi [25–27]. Fiedurek et al. (2003) found that Penicillium cyclopium isolated from arctic tundra synthesized both enzymes and the ratio of intracellular catalase to extracellular catalase was 1:3 [28]. Similar results were reported by Kacem-Chaouche et al. (2005) [29].
Fig. 3.
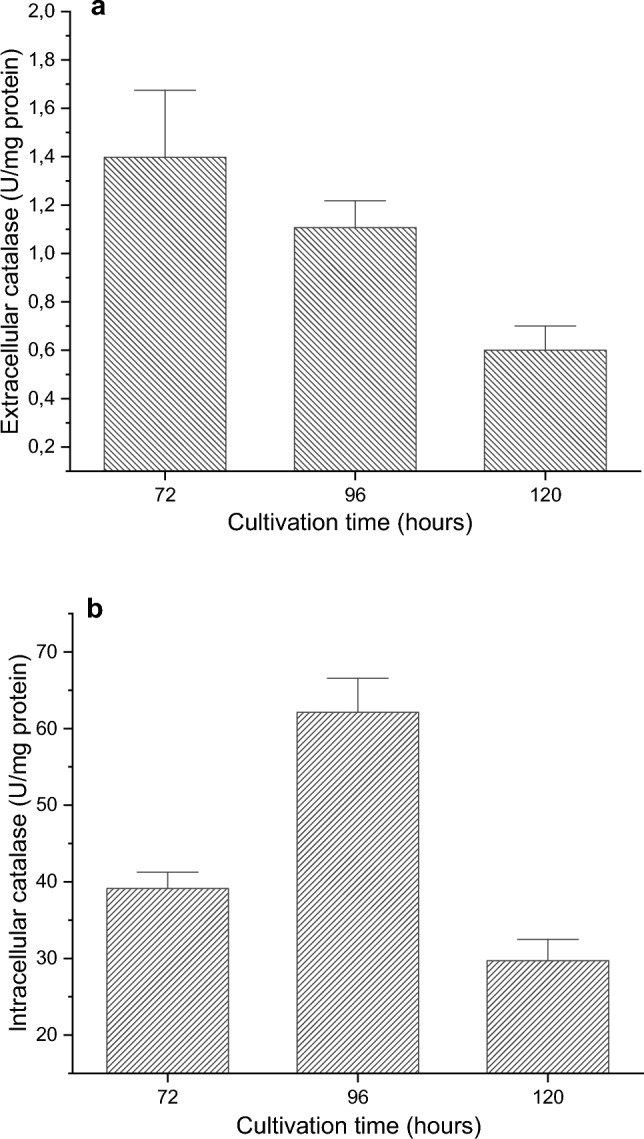
Time courses of CAT production (a) production of extracellular enzyme, (b) intracellular enzyme production
Nucleotide Sequence and Structural Analysis
The gene encoding the bifunctional catalase-peroxidase enzyme in A. fumigatus 1–9 consists of 2279 bp and encodes a protein of 759 amino acids. Based on a megablast search of NCBIs GenBank nucleotide database, the closest hits using our sequence (MW618007) of A fumigatus I-9 had the highest similarity to catalase-peroxidase CAT2 gene from A. fumigatus (GenBank AY125354; Identities = 3599/3610 (99.70%) and A. fumigatus CEA10 (GenBank CP097570; Identities = 3594/3610 (99.56%) [30]. The open reading frame (ORF) starts with the ATG codon.
The amino acid homology search showed the highest similarity (99.87%) with A. fumigatus Af293 catalase-peroxidase (GenBank XP_747039) with one substituted amino acid [31]. The comprehensive observation of the amino acid sequence of A. fumigatus I-9 catalase-peroxidases revealed an N-terminal catalytic domain. The active site is in the N-terminal domain and the active site includes heme-binding residues [24].
The analysis of the protein sequence of the catalase-peroxidase molecule by SWISS-MODEL [32] revealed that the enzyme is a dimer of two identical subunits. (Fig. 4). Each of the chains contains 759 amino acids and has a predicted molecular weight of 83 kDa (Table 2).
Fig. 4.
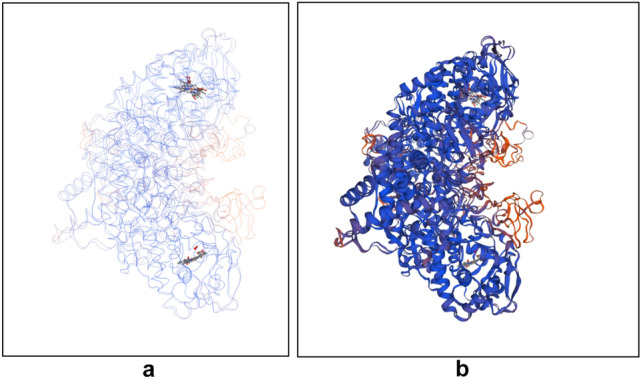
Three-dimensional model of catalase-peroxidase of Aspergillus fumigatus strain I-9 made by SWISS-MODEL Workspace [28]. (a) “Trace” model presentation of the chains revealing the formation of two active centers around the substrate; (b) “cartoon” model of the dimer
Discussion
Habitats with extremely low temperatures are a poorly explored niche for producers of biologically active substances. Antarctica is one such niche from which filamentous fungi have been isolated from different temperature classes [33]. They can survive a wide range of stressful conditions, such as drought, high salinity, solar radiation, and low temperatures, such as this continent provides. Simultaneously, they can be sources of new and valuable primary and secondary metabolites, including enzymes.
Cold-adapted microorganisms have developed different strategies to adapt to low temperatures [33]. Adaptation includes the synthesis of temperature-sensitive (cold-active, CA) enzymes [34–37], modulation of lipid composition to maintain cell membrane fluidity [38], the presence of RNA-chaperones that suppress the formation of unwanted RNA secondary structures [39], the synthesis of antifreeze proteins [40], etc. In addition, the increased tolerance to low-temperature stress is also a result of the higher level of the antioxidant defense system, because low temperatures accelerate the generation of ROS and cause oxidative stress [8, 41]. The first-line enzymes, SOD and CAT, play an essential role in the induced resistance to various stress factors, including temperature [42, 43]. Their combined action converts toxic oxy-radicals, and H2O2, into water and molecular oxygen. Simultaneously, with the dismutation of , the amount of the highly reactive hydroxyl radical decreases [44]. SOD is key to maintaining the balance between flux, H2O2 formation, and the Fenton reaction [45, 46]. Catalase is inactivated by [47], i.e. it cannot affect phenomena in a superoxide-generating system, mainly because of this inactivation [43].
Our results indicated an activated antioxidant defense in low-stress conditions. Lowering the growing temperature is accompanied by a typical antioxidant response—the expression of antioxidant enzymes is accelerated. An increasing activity of superoxide dismutase and catalase, which are the main enzymes for the direct removal of oxygen radicals, was observed. Similar data were reported by Smirnova et al. (2001) for E. coli, Zhang, et al. (2003) for S. cerevisiae, and Şahin & Gümüşlü (2004) for animal cells [48–50]. Activation of the antioxidant defense under low temperatures conditions has also been found in the plant Leucanthemum maximum [51].
Catalase protects aerobic organisms from H2O2 formed inside the cells, dismuting it before it diffuses into the environment. Even when not essential for a particular cell type, the enzyme plays an important role in increasing tolerance to various types of oxidants [52].
Catalases produced by fungi have some advantages such as rapid growth, absence of seasonal fluctuations, high yield, inexpensive media, etc. Most fungi have several monofunctional heme-containing catalases. They show a wide variety of enzymes in terms of their structure, location, and function and vary among genera. For example, the cat A gene of A. nidulans is specifically expressed in spores, and others are associated with metabolism. In A. niger, extracellular catalase protects cells from H2O2 produced by cell wall-associated glucose oxidase, and in Hansenula polymorpha, peroxisomal catalase is required for growth on methanol as a carbon source [53]. In Podospora anserina, catalases are strictly required for efficient utilization of more complex biomasses such as sawdust, allowing growth in the presence of lignin [54]. There is evidence for the presence of four catalase genes in Neurospora crassa, two catalase genes have been described in A. nidulans, and the catalase genes from yeast and filamentous fungi are placed in different gene families [12, 55, 56].
The present research contributes to the enrichment of knowledge about catalases produced by cold-adapted fungi and their encoding genes.
The main finding of our study is the identification and complete sequence of the catalase gene in the Antarctic fungus A. fumigatus I-9. The identified gene encodes the bifunctional catalase-peroxidase enzyme.
Catalase-peroxidases are bifunctional oxidoreductases. They accomplish both peroxidatic and catalytic activity. They have similarities with ascorbate peroxidases and cytochrome c peroxidases but they sole in their superfamily are capable of reduction and oxidation of hydrogen peroxide. Catalase-peroxidase genes have been found in eubacterial and archaebacterial genomes. detailed studies have only been performed with eubacterial and archaebacterial catalase-peroxidases [57–60]. Catalase-peroxidase genes can also be found in Ascomycota and Basidiomycota as well as in several protists [61, 62]. In contrast to prokaryotic, the corresponding eukaryotic proteins are less well described [61].
The molecular weight of this type of catalase is between 120 and 340 kDa. Catalase-peroxidases are synthesized by fungi and plants but are not found in animals. They have the same catalytic mechanism as monofunctional catalases [63]. Catalase-peroxidases evolved much later than monofunctional catalases [61, 64].
Proteins with catalase-peroxidase activity are described in P. nordicum [65], P. coprophilum, P. flavigenum, P. polonicum [66], P. digitatum [67]. According to CDD (NCBI) and Expasy, the enzyme is a dimer that possesses two identical subunits. Each subunit consists of two structurally homologous domains and its topology is similar to that of a class I peroxidase. The N-terminal domain houses the Active Site. the N-terminal domain contains the Chemobinding motif [59].
Conclusions
Antarctic fungal strain A. fumigatus I-9 includes antioxidant enzyme defense in its survival strategy against low-temperature stress. It identified catalase gene encoded protein with catalase-peroxidase activity. Probably, catalase-peroxidase enzyme is responsible for the cold stress tolerance of the fungus.
Research on catalases produced by Antarctic fungal strains is attractive for consideration in future potential applications in both medicine and biotechnology.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Singh RS, Singh T, Pandey A. Microbial enzymes—an overview. Adv Enzym Technol. 2019;72:1–40. doi: 10.1016/B978-0-444-64114-4.00001-72. [DOI] [Google Scholar]
- 2.Kango N, Jana UK, Choukade R. Fungal enzymes: sources and biotechnological applications. In: Satyanarayana T, Deshmukh S, Deshpande M, editors. Advancing frontiers in mycology and mycotechnology. Singapore: Springer; 2019. pp. 515–540. [Google Scholar]
- 3.Berbee ML, James TY, Strullu-Derrien C. Early diverging fungi: diversity and impact at the dawn of terrestrial life. Annu Rev Microbiol. 2017;71:41–60. doi: 10.1146/annurev-micro-030117-020324. [DOI] [PubMed] [Google Scholar]
- 4.El-Gendi H, Saleh AK, Badierah R, Redwan EM, El-Maradny YA, El-Fakharany EMA. Comprehensive insight into fungal enzymes: structure, classification, and their role in mankind’s challenges. J Fungi. 2022;8:23. doi: 10.3390/jof8010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan Y, Huang X, Zhou Y, Li J, Liu F, Li X, Zhou S. Cytosol peroxiredoxin and cell surface catalase differentially respond to H2O2 Stress in Aspergillus nidulans. Antioxidants. 2023;12(7):1333. doi: 10.3390/antiox12071333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins T, Margesin R. Psychrophilic lifestyles: mechanisms of adaptation and biotechnological tools. Appl microbiol biotechnol. 2019;103:2857–2871. doi: 10.1007/s00253-019-09659-5. [DOI] [PubMed] [Google Scholar]
- 7.Feller G. Psychrophilic enzymes: from folding to function and biotechnology. Sci Cairo. 2013;55:1–28. doi: 10.1155/2013/512840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gocheva YG, Tosi S, Krumova ET, et al. Temperature downshift induces antioxidant response in fungi isolated from Antarctica. Extremophiles. 2009;13:273–281. doi: 10.1007/s00792-008-0215-1. [DOI] [PubMed] [Google Scholar]
- 9.Margesin R, Feller G, Gerday C et al (2002) Cold-adapted microorganisms: adaptation strategies and biotechnological potential. In: Bitton, editor. The encyclopedia of environmental microbiology. Wiley, New York, pp 871–885. https://hdl.handle.net/2268/18203
- 10.Cavicchioli R, Charlton T, Ertan H, et al. Biotechnological uses of enzymes from psychrophiles. Microb Biotechnol. 2011;4:449–460. doi: 10.1111/j.1751-7915.2011.00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krishnan A, Alias AS, Wong CMVL, et al. Extracellular hydrolase enzyme production by soil fungi from King George Island, Antarctica. Polar Biol. 2011;34:1535–1542. doi: 10.1007/s00300-011-1012-3. [DOI] [Google Scholar]
- 12.Kawasaki L, Aguirre J. Multiple catalase genes are differentially regulated in Aspergillus nidulans. J Bacteriol. 2001;183:1434–1440. doi: 10.1128/jb.183.4.1434-1440.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nava-Ramírez T, Gutiérrez-Terrazas S, Hansberg W. The Molecular chaperone mechanism of the C-terminal domain of large-size subunit catalases. Antioxid Basel. 2023;12(4):839. doi: 10.3390/antiox12040839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karakus YY. Typical catalases: function and structure. London: IntechOpen; 2020. pp. 1–16. [Google Scholar]
- 15.Cook JN, Worsley JL (1996) Compositions and Method for Destroying Hydrogen Peroxide on Contact Lens. US Patent No. 5,521,091. https://patents.google.com/patent/US5521091A/en
- 16.Kaushal J, Mehandia S, Singh G, Raina A, Arya SK. Catalase enzyme: application in bioremediation and food industry. Biocatal Agric Biotechnol. 2018;16:192–199. doi: 10.1016/j.bcab.2018.07.035. [DOI] [Google Scholar]
- 17.Putnam CD, Arvai AS, Bourne Y, Tainer JA. Active and inhibited human catalase structures: ligand and NADPH binding and catalytic mechanism. J Mol Biol. 2000;296:295–309. doi: 10.1006/jmbi.1999.3458. [DOI] [PubMed] [Google Scholar]
- 18.Fiedurek J, Gromada A. Production of catalase and glucose oxidase by Aspergillus niger using unconventional oxygenation of culture. J Appl Microbiol. 2000;89:85–89. doi: 10.1046/j.1365-2672.2000.01085.x. [DOI] [PubMed] [Google Scholar]
- 19.Krumova E, Abrashev R, Dishliyska V, Stoyancheva G, Kostadinova N, Miteva-Staleva J, et al. Cold-active catalase from the psychrotolerant fungus Penicillium griseofulvum. J Basic Microbiol. 2021;61:782–794. doi: 10.1002/jobm.202100209. [DOI] [PubMed] [Google Scholar]
- 20.Stoyancheva G, Dishliyska V, Miteva-Staleva J, Kostadinova N, Abrashev R, Angelova M, Krumova E. Sequencing and gene expression analysis of catalase genes in Antarctic fungal strain Penicillium griseofulvum P29. Polar Biology. 2022;63:1–11. doi: 10.1007/s00300-021-03001-4. [DOI] [Google Scholar]
- 21.Angelova M, Genova L, Pashova S, Slokoska L, Dolashka P. Effect of cultural conditions on the synthesis of superoxide dismutase by Humicola lutea 110. J Ferment Bioeng. 1996;82:464–468. doi: 10.1016/S0922-338X(97)86984-X. [DOI] [Google Scholar]
- 22.Beers RF, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol chem. 1952;195:133–140. doi: 10.1016/S0021-9258(19)50881-X. [DOI] [PubMed] [Google Scholar]
- 23.Beauchamp C, Fridovich I. Superoxide dismutase: improved assay and an assay applicable to polyacrylamide gels. Anal Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- 24.Lu S, Wang J, Chitsaz F, Derbyshire MK, Geer RC, Gonzales NR, et al. CDD/SPARCLE: the conserved domain database in 2020. Nucleic Acids Res. 2020;48:D265–D268. doi: 10.1093/nar/gkz991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gromada A, Fiedurek J. Selective isolation of Aspergillus niger mutants with enhanced glucose oxidase production. J Appl Microbiol. 1997;82:648–652. doi: 10.1111/j.1365-2672.1997.tb03597.x. [DOI] [PubMed] [Google Scholar]
- 26.Fiedurek J, Gromada A. Screening and mutagenesis of molds for improvement of the simultaneous production of catalase and glucose oxidase. Enzyme Microb Technol. 1997;20:344–347. doi: 10.1016/S0141-0229(96)00148-2. [DOI] [Google Scholar]
- 27.Isobe K, Inoue N, Takamatsu Y, Kamada K, Wakao N. Production of catalase by fungi growing at low pH and high temperature. J Biosci Bioeng. 2006;101:73–76. doi: 10.1263/jbb.101.73. [DOI] [PubMed] [Google Scholar]
- 28.Fiedurek J, Gromada A, Słomka A, Korniłowicz-Kowalska T, Kurek E, Melke J. Catalase activity in arctic microfungi grown at different temperatures. Acta Biolog Hung. 2003;54:105–112. doi: 10.1556/abiol.54.2003.1.11. [DOI] [PubMed] [Google Scholar]
- 29.Kacem-Chaouche N, Maraihi Z, Destain J, Thonart P. Study of catalase production by an Aspergillus phoenicis mutant strain in date flour extract submerged cultures. Biotechnol Agron Soc Environ. 2005;9:173–178. [Google Scholar]
- 30.Paris S, Wysong D, Debeaupuis JP, Shibuya K, Philippe B, Diamond RD, Latgé JP. Catalases of Aspergillus fumigatus. Infect Immun. 2003;71:3551–3562. doi: 10.1128/iai.71.6.3551-3562.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nierman WC, Pain A, Anderson MJ, et al. Genomic sequence of the pathogenic and allergenic filamentous fungus Aspergillus fumigatus. Nature. 2005;438:1151–1156. doi: 10.1038/nature04332. [DOI] [PubMed] [Google Scholar]
- 32.Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G, Gumienny R, Heer FT, et al. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 2018;46:W296–W303. doi: 10.1093/nar/gky427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Onofri S, Selbmann L, de Hoog GS, Grube M, Barreca D, Ruisi S, Zucconi L. Evolution and adaptation of fungi at boundaries of life. Adv Space Res. 2007;40:1657–1664. doi: 10.1016/j.asr.2007.06.004. [DOI] [Google Scholar]
- 34.Feller G, Gerday C. Psychrophilic enzymes: hot topics in cold adaptation. Nat Rev Microbiol. 2003;1:200–208. doi: 10.1038/nrmicro773. [DOI] [PubMed] [Google Scholar]
- 35.Collins T, et al. Fundamentals of cold-adapted enzymes. In: Margesin R, Schinner F, Marx JC, Gerday C, et al., editors. Psychrophiles: from biodiversity to biotechnology. Berlin, Heidelberg: Springer; 2008. pp. 211–227. [Google Scholar]
- 36.Gatti-Lafranconi P, Natalello A, Rehm S, Doglia SM, Pleiss J, Lotti M. Evolution of stability in a cold-active enzyme elicits specificity relaxation and highlights substrate-related effects on temperature adaptation. J Mol Biol. 2010;395:155–166. doi: 10.1016/j.jmb.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, Zhang N, Ma J, Zhou Y, Wei Q, et al. Advances in cold-adapted enzymes derived from microorganisms. Front in Microbiol. 2023;14:1152847. doi: 10.3389/fmicb.2023.1152847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Russell NJ. Membrane components and cold sensing. In: Margesin R, Schinner F, Marx JC, Gerday C, editors. Psychrophiles: from biodiversity to biotechnology. Heidelberg: Springer; 2008. pp. 177–190. [Google Scholar]
- 39.Kwak KJ, Park SJ, Han JH, Kim MK, Oh SH, Han YS, Kang H. Structural determinants crucial to the RNA chaperone activity of glycine-rich RNA-binding proteins 4 and 7 in Arabidopsis thaliana during the cold adaptation process. J Exp Bot. 2011;62:4003–4011. doi: 10.1093/jxb/err101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.García-Arribas O, Mateo R, Tomczak MM, Davies PL, Mateu MG. Thermodynamic stability of a cold-adapted protein, type III antifreeze protein, and energetic contribution of salt bridges. Protein Sci. 2009;16:227–238. doi: 10.1110/ps.062448907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chattopadhyay MK. Low temperature and oxidative stress. Curr Sci. 2002;83:109–109. [Google Scholar]
- 42.Chattopadhyay MK, Raghu G, Sharma YVRK, Biju AR, Rajasekharan MV, Shivaji S. Increase in oxidative stress at low temperature in an Antarctic bacterium. Curr microbiol. 2011;62:544–546. doi: 10.1007/s00284-010-9742-y. [DOI] [PubMed] [Google Scholar]
- 43.Kostadinova N, Krumova E, Stefanova T, Dishliyska V, Angelova M. Transient cold shock induces oxidative stress events in Antarctic fungi. In: Lushchak V, editor. Oxidative stress/book 1. Houston: InTech; 2012. pp. 75–98. [Google Scholar]
- 44.Scandalios JG. Oxygen stress and superoxide dismutases. Plant Physiol. 1993;101:7–12. doi: 10.1104/pp.101.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fridovich I. Biological effects of the superoxide radical. Arch Biochem Biophys. 1986;247:1–11. doi: 10.1016/0003-9861(86)90526-6. [DOI] [PubMed] [Google Scholar]
- 46.Touati D. Regulation and protective role of the microbial superoxide dismutases. In: Touati D, editor. Molecular biology of free scavenging systems. Cold Spring Harbor Laboratory Press; 1992. pp. 231–264. [Google Scholar]
- 47.Kono Y, Fridovich I. Superoxide radical inhibits catalase. J Biol Chem. 1982;257:5751–5754. doi: 10.1016/S0021-9258(19)83842-5. [DOI] [PubMed] [Google Scholar]
- 48.Smirnova GV, Zakirova ON, Oktiabr'skiĭ ON. Role of the antioxidant system in response of Escherichia coli bacteria to cold stress [in Russian] Mikrobiologiia. 2001;70:55–60. [PubMed] [Google Scholar]
- 49.Zhang Y, Zhang J, Hoeflich KP, Ikura M, Qing G, Inouye M. MazF cleaves cellular mRNAs specifically at ACA to block protein synthesis in Escherichia coli. Mol Cell. 2003;12:913–923. doi: 10.1016/S1097-2765(03)00402-7. [DOI] [PubMed] [Google Scholar]
- 50.Şahin E, Gümüşlü S. Cold-stress-induced modulation of antioxidant defence: role of stressed conditions in tissue injury followed by protein oxidation and lipid peroxidation. Int J Biometeorol. 2004;48:165–171. doi: 10.1007/s00484-004-0205-7. [DOI] [PubMed] [Google Scholar]
- 51.Zhou XF, Cui J, DeStefano AL, Chazaro I, Baldwin CT, et al. Polymorphisms in the promoter region of catalase gene and essential hypertension. Dis Markers. 2005;21:3–7. doi: 10.1155/2005/487014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harris ED. Regulation of antioxidant enzymes. FASEB J. 1992;6:2675–2683. doi: 10.1096/fasebj.6.9.1612291. [DOI] [PubMed] [Google Scholar]
- 53.Witteveen CF, Veenhuis M, Visser J. Localization of glucose oxidase and catalase activities in Aspergillus niger. Appl Environ Microbiol. 1992;58:1190–1194. doi: 10.1128/aem.58.4.1190-1194.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bourdais A, Bidard F, Zickler D, Berteaux-Lecellier V, Silar P, Espagne E. Wood utilization is dependent on catalase activities in the filamentous fungus Podospora anserina. PLoS ONE. 2012;7:e29820. doi: 10.1371/journal.pone.0029820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He L, Duan Z, Yu M, Qi S, Wang Y, Lou H, He Q. HDA-2-containing complex is required for activation of catalase-3 expression in Neurospora crassa. mBio. 2022;13(4):e0135122. doi: 10.1128/mbio.01351-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yan Y, Huang X, Zhou Y, Li J, Liu F, Li X, Zhou S. Cytosol peroxiredoxin and cell surface catalase differentially respond to H2O2 Stress in Aspergillus nidulans. Antioxidants. 2023;12:1333. doi: 10.3390/antiox12071333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singh R, Wiseman B, Deemagarn T, Jha V, Switala J, Loewen PC. Comparative study of catalase-peroxidases (KatGs) Arch Biochem Biophys. 2008;471:207–214. doi: 10.1016/j.abb.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 58.Yamada Y, Fujiwara T, Sato T, Igarashi N, Tanaka N. The 2.0 A crystal structure of catalase-peroxidase from Haloarcula marismortui. Nat Struct Mol Biol. 2002;9:691–695. doi: 10.1038/nsb834. [DOI] [PubMed] [Google Scholar]
- 59.Bertrand T, Eady NAJ, Jones JN, Jesmin JM, Nagy JM, Jamart-Gregoire B, Raven EL, Brown KA. Crystal structure of mycobacterium tuberculosis catalase-peroxidase. J Biol Chem. 2004;279:38991–38999. doi: 10.1074/jbc.M402382200. [DOI] [PubMed] [Google Scholar]
- 60.Ten-I T, Kumasaka T, Higuchi W, Tanaka S, Yoshimatsu K, Fujiwara T, Sato T. Expression, purification, crystallization and preliminary X-ray analysis of the Met244Ala variant of catalase-peroxidase (KatG) from the haloarchaeon Haloarcula marismortui. Acta Cryst Sect F Struct Biol Cryst Commun. 2007;63:940–943. doi: 10.1107/S1744309107046489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zamocky M. Phylogenetic relationships in class I of the superfamily of bacterial, fungal, and plant peroxidases. Eur J Biochem. 2004;271:3297–3309. doi: 10.1111/j.1432-1033.2004.04262.x. [DOI] [PubMed] [Google Scholar]
- 62.Passardi F, Zamocky M, Favet J, Jakopitsch C, Penel C, Obinger C, Dunand C. Phylogenetic distribution of catalase-peroxidases: Are there patches of order in chaos? Gene. 2007;397:101–113. doi: 10.1016/j.gene.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 63.Carpena X, Wiseman B, Deemagarn T, Singh R, Switala J, Ivancich A, Fita I, Loewen PC. A molecular switch and electronic circuit modulate catalase activity in catalase-peroxidases. EMBO Rep. 2005;6:1156–1162. doi: 10.1038/sj.embor.7400550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chelikani P, Fita I, Loewen PC. Diversity of structures and properties among catalases. CMLS. 2004;61:192–208. doi: 10.1007/s00018-003-3206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wingfield BD, Barnes I, de Beer ZW, De Vos L, Duong TA, Kanzi AM, et al. Draft genome sequences of Ceratocystis eucalypticola, Chrysoporthe cubensis, C. deuterocubensis, Davidsoniella virescens, Fusarium temperatum, Graphilbum fragrans, Penicillium nordicum, and Thielaviopsis musarum. IMA Fungus. 2015;6:493–506. doi: 10.5598/imafungus.2015.06.02.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nielsen JC, Grijseels S, Prigent S, Ji B, Dainat J, Nielsen KF, Nielsen J. Global analysis of biosynthetic gene clusters reveals vast potential of secondary metabolite production in Penicillium species. Nat Microbiol. 2017;2:1–9. doi: 10.1038/nmicrobiol.2017.44. [DOI] [PubMed] [Google Scholar]
- 67.Wang W, Li T, Chen Q, Deng B, Deng L, Zeng K. Transcription factor CsWRKY65 participates in the establishment of disease resistance of citrus fruit to penicillium digitatum. J Agric Food Chem. 2021;69:5671–5682. doi: 10.1021/acs.jafc.1c01411c. [DOI] [PubMed] [Google Scholar]


