Abstract
Background
With hepatitis C (HCV) incidence rising due to injection drug use, people who inject drugs (PWID) are a priority population for direct-acting antivirals (DAA). However, significant barriers exist. At our institution, hospitalized PWID were screened for HCV but not effectively linked to care.
Aim
To improve retention in HCV care among hospitalized PWID.
Setting
Quaternary academic center in the Southeast US from August 2021 through August 2022.
Participants
Hospitalized PWID with HCV.
Program Description
E-consultation-prompted care coordination and HCV treatment with outpatient telehealth.
Program Evaluation
Care cascades were constructed to assess retention and HCV treatment, with the primary outcome defined as DAA completion or sustained virologic response after week 4. Of 28 patients, 11 started DAAs inpatient, 8 initiated outpatient, and 9 were lost to follow-up or transferred care. Overall, 82% were linked to care and 52% completed treatment. For inpatient initiators, 73% achieved the outcome. Of non-inpatient initiators, 71% were linked to care, 53% started treatment, and 36% achieved the outcome.
Discussion
Inpatient HCV treatment coordination, including DAA initiation, and telehealth follow-up, was feasible and highly effective for hospitalized PWID. Future steps should address barriers to inpatient DAA treatment and expand this model to other similar patient populations.
Supplementary Information:
The online version contains supplementary material available at 10.1007/s11606-023-08386-y.
KEY WORDS: hepatitis C, injection drug use, hospitalization, substance use
Introduction
New cases of hepatitis C virus (HCV) have risen in the USA every year since 2013, driven almost entirely by injection drug use (IDU).1 The American Association for the Study of Liver Diseases (AASLD) and Infectious Diseases Society of America (IDSA) recommend treating HCV in individuals with active substance use in virtually all instances.2 Treating people who inject drugs (PWID) can reduce transmission of HCV and provide community-level prevention for new HCV infections.3
HCV treatment has advanced substantially due to the development of highly effective direct-acting antivirals (DAAs), well-tolerated oral therapies with cure rates greater than 95%.4 More recent-pan-genotypic DAAs, given daily for 8 or 12 weeks with minimal side effects or need for lab monitoring, have allowed for further treatment simplification for antiviral-naïve patients. By limiting pre-treatment work-up and eliminating on-treatment monitoring, simplified treatment pathways reduce barriers to care.2, 5
Despite DAA advances and recommendations for widespread screening of HCV,6 linkage to care and initiation of DAAs remains limited.7 Recent data from the Centers for Disease Control and Prevention reported that only 35% of privately insured patients — and about 25% of patients insured by Medicaid or Medicare — initiated DAAs within 1 year of diagnosis. DAA initiation was lowest among persons younger than 40, despite having the cohort’s highest rates of HCV infection.8
PWID receive HCV treatment even more infrequently with several studies reporting PWID DAA initiation rates less than 15%.9–11 Historically, PWID experience unique barriers to HCV care, such as a lack of health insurance, stigma, difficulty obtaining labs, and transportation.12 When PWID have access to treatment, however, they are able to achieve sustained virologic response (SVR), or HCV cure, at similar rates as the general population in clinical trial and real-world settings.13, 14
Novel models of HCV care delivery are necessary to effectively scale HCV care to PWID, which will be paramount to achieving World Health Organization targets of reducing new HCV infections by 90% and treating 80% of eligible persons between 2016 and 2030.15 Unfortunately, as of 2021, the USA is not on target to meet 2030 HCV elimination goals.16 In March 2023, the Biden Administration proposed a 5-year plan to implement a national hepatitis C elimination program.17
Hospitalizations for IDU-related invasive infections, such as endocarditis and osteomyelitis, similar to HCV incidence, are also rising18, 19 and may present prime opportunity to engage PWID in HCV care. These infections often require long lengths of stay and are seen as “reachable moments” with data supporting inpatient initiation of medications for opioid use disorder (MOUD),20 for example. Research suggests this population may be interested in HCV education, care coordination, and inpatient initiation.21 Yet, there is no literature on using the hospitalization to begin HCV management and hospitalization was not mentioned as part of the White House elimination strategy.22
Our report addresses this gap by describing a novel approach of providing HCV care to hospitalized PWID. At our institution, PWID are screened for HCV, but no formal treatment pathway existed. Therefore, we developed a scalable framework to address barriers to HCV treatment during hospitalization, start treatment inpatient when feasible, and utilize telehealth after discharge.
Settings and participants
This was a collaboration between Infectious Diseases (ID) and Addiction Medicine (AM) at University of North Carolina (UNC) Medical Center, an academic quaternary care center serving a large rural and uninsured population.
Program description
The ID and AM consult services see patients with IDU-related infections. Before this project, both services routinely screened PWID for HCV; however, patients were inconsistently referred or connected to care. A quality improvement (QI) team was constituted including an ID fellow and attending, and an AM social worker.
Iterative multidisciplinary discussions identified the following barriers: (1) pre-treatment testing, (2) signatures for manufacturer’s assistance program (MAP) for uninsured patients, and (3) transportation to appointments. Therefore, we created a system to complete necessary testing, vaccinations, and paperwork during hospitalization so patients could be followed by telehealth post-discharge. Hepatology evaluation would be recommended if the patient had decompensated cirrhosis.
Inpatient e-consults for HCV care
We aimed to avoid burdening existing ID consult services by using inpatient e-consults directed to QI team ID providers. Through the e-consult, we reviewed patient labs, imaging, and medications; formally assessed eligibility for the simplified pathway (Supplemental Fig. 1); and recommended indicated testing and vaccinations. The e-consult further served as a vehicle to bill for this service, while typically being completed in under 15 min.
-
2.
Workflow of patient care (Supplemental Fig. 2)
The AM consult team asked patients with detectable HCV RNA if they were interested in treatment and if they were previously treated for HCV. The AM team then recommended an ID e-consult for HCV and obtained any needed signatures. ID QI team members completed the e-consult. Initial outpatient ID telehealth visits were scheduled within 1–2 weeks of discharge. Subsequent appointments were scheduled while on-treatment if needed, then 4 weeks post-treatment completion for counseling and HCV RNA testing, or SVR4. An additional test at or after 12 weeks (SVR ≥ 12) was coordinated if possible, for official test of cure. We initially aimed for SVR4 given the correlation of SVR4 with SVR12 in the clinical trial setting23 and the anticipated difficulties of engaging patients until SVR12. The QI team reviewed active patients monthly in a 30-min meeting.
-
3.
Project evolution and changes over time
During the initial weeks of the project, DAAs were not prescribed until the first outpatient appointment. Many patients made that first appointment but did not ultimately start DAAs. We queried colleagues in other divisions to understand and adapt their approaches to prescribing specialty medications inpatient. As the project progressed, we shifted to prescribing DAAs earlier in the hospitalization by sending DAAs to the outpatient specialty pharmacy, enabling us to address logistical issues (e.g., signature issues on forms) during the admission. DAAs for uninsured patients were pursued through MAPs, a process started for most patients during their inpatient stay. As many hospitalizations exceeded several weeks, DAAs were started inpatient once home medications were received.
Program Evaluation
Our primary outcome was completion of DAA course, given the known efficacy of these medications, or undetectable HCV RNA at or after SVR4 (for those not completing the prescribed treatment course). We assessed retention by creating 3 HCV care cascades: (1) all patients, (2) inpatient DAA initiators, and (3) non-inpatient initiators. Milestones included the following: (1) e-consult performed, (2) linked to care, (3) started treatment, (4) completed treatment, (5) SVR ≥ 4, and (6) SVR ≥ 12. Patients were considered as completing treatment if they met our primary outcome. Inpatient initiators did not require a separate “linked to care” step. Patients not initiating DAAs inpatient were labeled non-inpatient initiators.
Data were kept on a secure server for patient tracking purposes. A shared patient list was also maintained in the electronic medical record to assist in tracking patients. The UNC IRB reviewed this QI project and deemed it not human subject research.
Descriptive results
From August 1, 2021, through August 31, 2022, 28 patients seen by the AM consult service had detectable HCV RNA. All desired treatment. Four additional patients had detectable HCV RNA but were excluded due to leaving the hospital prior to e-consult completion (n = 2) or having decompensated cirrhosis (n = 2).
Of the 28 with completed inpatient e-consults (Table 1), 11 (39%) initiated DAAs inpatient. Overall, patients were young with a median age of 33 years (interquartile range, IQR 30–39), White (23/28, 82%), and uninsured requiring MAP (17/28, 61%). Women comprised 43% (12/28) of the participants but made up 64% (7/11) of inpatient initiators. Most patients were admitted for conditions that typically require long courses of parenteral antibiotics (e.g., endocarditis, osteomyelitis); overall median length of stay was 23 days (IQR 16–43). Patients lived a median distance of 51 miles by car from the ID clinic and as far away as over 150 miles.
Table 1.
Sociodemographic and Clinical Characteristics
| All participants (N = 28) | Inpatient initiators (N = 11) | Non-inpatient initiators (N = 17) | |
|---|---|---|---|
| Median age, years (IQR) | 33 (30, 39) | 33 (29, 36) | 35 (31, 42) |
| Gender, female (%) | 12 (43) | 7 (64) | 5 (29) |
| Race/ethnicity (%) | |||
| Non-Hispanic White | 23 (82) | 9 (82) | 14 (82) |
| Non-Hispanic Black | 4 (14) | 2 (18) | 2 (12) |
| Hispanic | 1 (4) | 0 (0) | 1 (6) |
| Insurance (%) | |||
| Medicaid or managed Medicaid | 9 (32) | 4 (36) | 5 (29) |
| Commercial | 2 (7) | 1 (9) | 1 (6) |
| None (required manufacturer’s assistance) | 17 (61) | 6 (55) | 11 (65) |
| Primary reason for admission (%) | |||
| Endocarditis | 14 (50) | 8 (73) | 6 (35) |
| Osteomyelitis | 6 (22) | 2 (18) | 4 (24) |
| Other infection | 6 (22) | 1 (9) | 5 (29) |
| Other, non-infection | 2 (7) | 0 (0) | 2 (12) |
| Discharge disposition (%) | |||
| Home | 17 (60) | 5 (46) | 12 (70) |
| Other healthcare facility | 3 (11) | 1 (9) | 2 (12) |
| Substance use facility or housing | 3 (11) | 2 (18) | 1 (6) |
| Patient-directed discharge | 5 (18) | 3 (27) | 2 (12) |
| Median length of stay in days (IQR) | 23 (16, 43) | 45 (42, 59) | 17 (8, 24) |
| Median distance from clinic in miles (IQR) | 51 (28, 103) | 53 (33, 81) | 48 (28, 110) |
All patients had uncomplicated HCV infection that met criteria for the AASLD/IDSA simplified treatment pathways (Supplemental Table 1). Most patients (26/28, 93%) were started on MOUD during the hospitalization. Median duration of inpatient DAA treatment was 14 days, though ranged from 2 days to a full 8-week course. Eighty-one percent had their initial outpatient ID appointment by telehealth.
-
2.
Care cascades
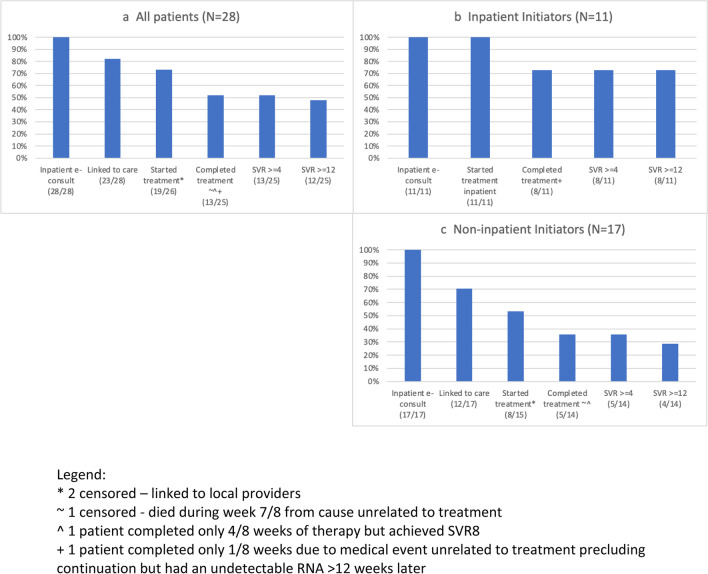
Overall, 82% of patients (23/28) were linked to care, with 73% of patients (19/26) started on DAAs (Fig. 1a). Two patients of the initial 28 were linked to community providers and censored from further analysis. Of those that started DAAs, 68% (13/19) completed treatment and 92% (12/13) of those achieved SVR. Seven patients were lost to follow-up (LTFU) prior to treatment initiation, of whom 5 lacked access to personal cellphones. Of the 4 patients LTFU after starting treatment, 2 did not have cellphones.
Figure 1.
Cascades of HCV care in the overall cohort from 8/2021 through 8/2022, stratified by inpatient initiation of antivirals. Legend: *2 censored — linked to local providers. ~1 censored — died during week 7/8 from cause unrelated to treatment. ^1 patient completed only 4/8 weeks of therapy but achieved SVR8. +1 patient completed only 1/8 weeks due to medical event unrelated to treatment precluding continuation but had an undetectable RNA > 12 weeks later
Of the 11 inpatient initiators (Fig. 1b), 73% (8/11) achieved cure by SVR. The remaining 3 were LTFU and unable to be contacted. Three patients unexpectedly left the hospital but 2 returned for their DAAs.
Among the 17 non-inpatient initiators (Fig. 1c), 71% (12/17) were linked to care with 53% (8/15) starting treatment through our program. Of those that started DAAs, 63% (5/8) completed treatment. SVR ≥ 12 was obtained in 80% (4/5) of patients. One patient was LTFU after starting therapy, and one patient decided to stop treatment after 2 days and did not follow-up.
Four patients were counted as completing treatment in the cascades despite partial treatment, as they had presumed cure with undetectable HCV RNA levels at least 4 weeks after treatment. One patient received one of eight planned weeks of therapy due to a serious medical event unrelated to HCV or HCV treatment but achieved SVR ≥ 12. One patient completed four of eight weeks and had an undetectable HCV RNA eight weeks after stopping therapy, though we were unsuccessful in obtaining subsequent SVR testing. Two patients completed seven of eight weeks of therapy and achieved SVR ≥ 12.
Discussion
Beginning the process of HCV treatment during hospitalization was feasible and effective in curing HCV among inpatients with injection drug-related medical complications. Our model demonstrated that inpatient care coordination and, when possible, inpatient initiation of DAAs, resulted in high rates of treatment initiation and cure. Of 28 patients, nearly 75% started DAAs, and over half completed treatment. Among inpatient initiators, results were more favorable, with greater than 70% finishing treatment or achieving cure.
Although our program included a relatively small number of patients, it successfully leveraged hospitalizations and telehealth to engage patients with numerous barriers to HCV care, including young age, living in rural and underserved areas, and lacking health insurance. Relative to insured patients nationwide where less than 50% initiated DAAs by 1 year,8 our patients achieved exceedingly high rates of DAA initiation (> 70%), completion, and cure.
Our intervention also emphasized engagement in care after discharge. It is well-established that initiating MOUD in the inpatient setting improves adherence and reduces fatal overdose post-discharge.20 Integrating HCV treatment into opioid treatment and harm reduction settings has increased uptake and retention in HCV treatment24, 25 as well initiation of MOUD.26 Our model, which aligns inpatient initiation of MOUD with evaluation for HCV treatment, may improve retention in care for both addiction and ID needs.
Rapid initiation of DAAs in the inpatient setting may help overcome barriers to treatment and cure. The MINMON study demonstrated the effectiveness of providing participants with the full course of DAAs up-front; 95% of participants attained SVR despite minimal on-treatment follow-up.27 In our experience, starting medications inpatient overcame the hurdles of medication deliveries and refills. Lack of cellphone access often delayed or prohibited filling DAAs, which are typically dispensed by mail delivery from specialty pharmacies or MAPs.
Inpatient initiation of DAAs among PWID may also aid in HCV elimination efforts on a population level, now a national priority.17 Modeling studies demonstrate that scaling up HCV treatment among PWID, in addition to harm reduction and opioid treatment services, acts as “treatment-as-prevention” by reducing forward transmission.3 Additionally, the minimum duration of DAAs to cure HCV in young patients with limited liver disease and recent infection is unknown. Our project had several participants that achieved cure despite partial courses of DAAs.
Anticipated prolonged hospitalizations may be an ideal time to start DAAs as there is a period of directly observed therapy prior to discharge during which the magnitude of circulating viremia may be substantially reduced or eradicated altogether. Given the obstacles PWID face in accessing outpatient care, inpatient DAAs could translate into real public health benefits by limiting HCV spread, even if patients do not complete their intended full treatment courses. While concerns of sudden patient-directed discharges may dissuade from starting HCV treatment, we found some patients return to retrieve their DAAs.
Barriers to scaling up hospital-based HCV treatment are structural including DAA cost and unavailability on hospital formularies. A national subscription-based model has been proposed as one approach to enhance DAA access for underserved patient populations.22 Implementing hospital-based screening, treatment initiation, and linkage to follow-up care may be key in reaching vulnerable populations that may not otherwise access outpatient resources. While our model uses physicians to perform e-consults, this could be adapted in other ways, such as utilizing clinical pharmacists. Navigators to link to outpatient care will be key particularly if the full DAA course is not in-hand at time of hospital discharge. To be sustainable and scalable, such infrastructure requires investment from health systems or public health entities.
There are several limitations to this report. First, this was a QI project and not designed to study inpatient versus outpatient DAA initiation. Second, this project took place in an academic center in the Southeast US that serves a population of PWID that is largely rural and uninsured. The model that worked for our institution may not translate to urban settings or less-resourced hospitals. Finally, our processes relied on dedicated providers coordinating care. Without motivated providers and institutional support, this initiative, which takes a novel approach to care delivery, may be unsustainable.
In conclusion, we present a novel and effective model for evaluating and coordinating HCV treatment for patients hospitalized with substance use-related conditions. Our approach was highly successful, particularly when DAAs were started inpatient. This model was successful in engaging a difficult-to-reach patient population with a high prevalence and incidence of HCV infection. This approach may be generalizable to other hospitalized patients with HCV and prolonged admissions.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements:
The authors would like to thank the UNC Institute for Healthcare Quality Improvement, specifically Darren DeWalt, May-Britt Sten, Monecia Thomas, and Joy Martin, for their insight, feedback, and support. They would also like to thank Christopher Hurt for his HCV and e-consult expertise, as well as Greg Rochelle and Keyana Moss with UNC specialty services for their assistance with prior authorizations and MAP.
Funding
AJS received support from NIDA (K23DA049946).
Data Availability
The datasets generated and analyzed during the current study are not publicly available as the data was derived from clinical records as part of a QI program, not explicitly a research study.
Declarations:
Conflict of Interest:
The authors declare that they do not have a conflict of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.2022 Viral Hepatitis National Progress Report | CDC. Published November 7, 2022. Accessed February 19, 2023. https://www.cdc.gov/hepatitis/policy/npr/2022/index.htm
- 2.Recommendations for Testing, Managing, and Treating Hepatitis C | HCV Guidance. Accessed February 19, 2023. https://www.hcvguidelines.org/
- 3.Martin NK, Vickerman P, Grebely J, et al. Hepatitis C virus treatment for prevention among people who inject drugs: Modeling treatment scale-up in the age of direct-acting antivirals. Hepatology. 2013;58(5):1598–1609. doi: 10.1002/hep.26431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falade-Nwulia O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral Direct-Acting Agent Therapy for Hepatitis C Virus Infection: A Systematic Review. Ann Intern Med. 2017;166(9):637–648. doi: 10.7326/M16-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dieterich DT. A Simplified Algorithm for the Management of Hepatitis C Infection. Gastroenterol Hepatol (N Y). 2019;15(5 Suppl 3):1–12. [PMC free article] [PubMed] [Google Scholar]
- 6.US Preventive Services Task Force, Owens DK, Davidson KW, et al. Screening for Hepatitis C Virus Infection in Adolescents and Adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2020;323(10):970. doi:10.1001/jama.2020.1123 [DOI] [PubMed]
- 7.Yehia BR, Schranz AJ, Umscheid CA, Lo Re V. The Treatment Cascade for Chronic Hepatitis C Virus Infection in the United States: A Systematic Review and Meta-Analysis. PLoS One. 2014;9(7):e101554. doi: 10.1371/journal.pone.0101554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson WW. Vital Signs: Hepatitis C Treatment Among Insured Adults — United States, 2019–2020. MMWR Morb Mortal Wkly Rep. 2022;71. 10.15585/mmwr.mm7132e1 [DOI] [PMC free article] [PubMed]
- 9.Carmody MD, Wagner K, Bizstray B, et al. Cascade of Care for Hepatitis C Virus Infection Among Young Adults Who Inject Drugs in a Rural County in New Mexico. Public Health Rep. Published online. 2023;12:333549221143086 . doi: 10.1177/00333549221143086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang X, Parker RL, Vouri SM, et al. Cascade of hepatitis C virus care among patients with substance use disorders. Am J Prev Med. 2021;61(4):576–584. doi: 10.1016/j.amepre.2021.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corcorran MA, Tsui JI, Scott JD, Dombrowski JC, Glick SN. Age and gender-specific hepatitis C continuum of care and predictors of direct acting antiviral treatment among persons who inject drugs in Seattle, Washington. Drug Alcohol Depend. 2021;220:108525. doi: 10.1016/j.drugalcdep.2021.108525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sherbuk JE, Tabackman A, McManus KA, et al. A qualitative study of perceived barriers to hepatitis C care among people who did not attend appointments in the non-urban US South. Harm Reduction Journal. 2020;17(1):64. doi: 10.1186/s12954-020-00409-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grebely J, Dalgard O, Conway B, et al. Sofosbuvir and velpatasvir for hepatitis C virus infection in people with recent injection drug use (SIMPLIFY): an open-label, single-arm, phase 4, multicentre trial. The Lancet Gastroenterology & Hepatology. 2018;3(3):153–161. doi: 10.1016/S2468-1253(17)30404-1. [DOI] [PubMed] [Google Scholar]
- 14.Norton BL, Fleming J, Bachhuber MA, et al. High HCV cure rates for people who use drugs treated with direct acting antiviral therapy at an urban primary care clinic. International Journal of Drug Policy. 2017;47:196–201. doi: 10.1016/j.drugpo.2017.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO-HIV-2016.06-eng.pdf. Accessed July 29, 2023. https://apps.who.int/iris/bitstream/handle/10665/246177/WHO-HIV-2016.06-eng.pdf;jsessionid=60A93ADD1A191FF6A0FA823314D24C43?sequence=1
- 16.Sulkowski M, Cheng WH, Marx S, Sanchez Gonzalez Y, Strezewski J, Reau N. Estimating the Year Each State in the United States Will Achieve the World Health Organization’s Elimination Targets for Hepatitis C. Adv Ther. 2021;38(1):423–440. doi: 10.1007/s12325-020-01535-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.budget_fy2024.pdf. Accessed July 29, 2023. https://www.whitehouse.gov/wp-content/uploads/2023/03/budget_fy2024.pdf
- 18.Capizzi J, Leahy J, Wheelock H, et al. Population-based trends in hospitalizations due to injection drug use-related serious bacterial infections, Oregon, 2008 to 2018. PLOS ONE. 2020;15(11):e0242165. doi: 10.1371/journal.pone.0242165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schranz AJ, Fleischauer A, Chu VH, Wu LT, Rosen DL. Trends in Drug Use-Associated Infective Endocarditis and Heart Valve Surgery, 2007 to 2017: A Study of Statewide Discharge Data. Ann Intern Med. 2019;170(1):31–40. doi: 10.7326/M18-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noam KR, Schmutte TJ, Pirard S, Bourdon C, Langless D, Plant R. Associations Between Inpatient Induction on Medications for Opioid Use Disorder and Postdischarge Medications for Opioid Use Disorder Adherence, Overdose, and Service Use. J Addict Med. Published online October 18, 2022. doi:10.1097/ADM.0000000000001092 [DOI] [PubMed]
- 21.Levander XA, Vega TA, Seaman A, Korthuis PT, Englander H. Utilising an access to care integrated framework to explore the perceptions of hepatitis C treatment of hospital-based interventions among people who use drugs. Int J Drug Policy. 2021;96:103356. doi: 10.1016/j.drugpo.2021.103356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fleurence RL, Collins FS. A National Hepatitis C Elimination Program in the United States: A Historic Opportunity. JAMA. 2023;329(15):1251–1252. doi: 10.1001/jama.2023.3692. [DOI] [PubMed] [Google Scholar]
- 23.Gane E, de Ledinghen V, Dylla DE, et al. Positive predictive value of sustained virologic response 4 weeks posttreatment for achieving sustained virologic response 12 weeks posttreatment in patients receiving glecaprevir/pibrentasvir in Phase 2 and 3 clinical trials. J Viral Hepat. 2021;28(11):1635–1642. doi: 10.1111/jvh.13600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grebely J, Tran L, Degenhardt L, et al. Association between opioid agonist therapy and testing, treatment uptake, and treatment outcomes for hepatitis c infection among people who inject drugs: a systematic review and meta-analysis. Clin Infect Dis. 2021;73(1):e107–e118. doi: 10.1093/cid/ciaa612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenthal ES, Silk R, Mathur P, et al. Concurrent Initiation of Hepatitis C and Opioid Use Disorder Treatment in People Who Inject Drugs. Clin Infect Dis. 2020;71(7):1715–1722. doi: 10.1093/cid/ciaa105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill K, Nussdorf L, Mount JD, et al. Initiation of Low-threshold Buprenorphine in Nontreatment Seeking Patients With Opioid Use Disorder Engaged in Hepatitis C Treatment. J Addict Med. 2022;16(1):10–17. doi: 10.1097/ADM.0000000000000807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Solomon SS, Wagner-Cardoso S, Smeaton L, et al. A minimal monitoring approach for the treatment of hepatitis C virus infection (ACTG A5360 [MINMON]): a phase 4, open-label, single-arm trial. Lancet Gastroenterol Hepatol. 2022;7(4):307–317. doi: 10.1016/S2468-1253(21)00397-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during the current study are not publicly available as the data was derived from clinical records as part of a QI program, not explicitly a research study.