Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a human virus that burst at Wuhan in China and spread quickly over the world, leading to millions of deaths globally. The journey of this deadly virus to different mutant strains is still ongoing. The plethora of drugs and vaccines have been tested to cope up this pandemic. The herbal plants and different spices have received great attention during pandemic, because of their anti-inflammatory, and immunomodulatory properties in treating viruses and their symptoms. Also, it has been shown that nano-formulation of phytochemicals has potential therapeutic effect against COVID-19. Furthermore, the plant derived compound nano-formulation specifically increases its antiviral property by enhancing its bioavailability, solubility, and target-specific delivery system. This review highlights the potentiality of herbal plants and their phytochemical against SARS-CoV-2 utilizing different mechanisms such as blocking the ACE-2 receptors, inhibiting the main proteases, binding spike proteins and reducing the cytokine storms.
Keywords: SARS-CoV-2, Herbal plants, Phytochemicals, Nano formulation
Introduction
In the year 2020, March 11, World Health Organization (WHO) declared the sudden outbreak of COVID-19 as a pandemic disease, because of its rapid transmission worldwide. Almost every country attempted to detect, isolate, trace, and treat the virus in order to stop its spreading. This pandemic has huge impact on world health, social and financial crisis with more than 768 million cases and 6.9 million deaths [1]. The current extraordinary COVID-19 infection proclaimed by WHO has sparked a massive rise in scientific attention around the world [2]. Both animals and human beings are infected by coronavirus, which is generally enclosed within a membrane, contains single strands of ribonucleic acid (RNA) and having big genome size 2632 kb as illustrated in Fig. 1a. On the basis of the genotypic features, virus has been classified in 4 generations: α, β, γ, and δ coronavirus [3]. Corona viruses that can infect humans, belongs mostly to α and β-corona strains [3, 4]. The α-coronaviruses are HCoV-NL63 (Human-CoV-NL63) and HCoV-229E, whereas β coronaviruses are HCoV-OC43 (Human-CoV-OC43), HKU1 (Human CoV), SARS-CoV (Severe Acute Respiratory Syndrome CoV), and MERS-CoV (Middle Eastern Respiratory Syndrome CoV). SARS-CoV in 2002–2003, MERS in 2012, and coronavirus, initially detected in 2019, are three epidemics produced by β CoVs in the last two decades [2]. Since, COVID-19 is a contagious pathogenic virus spread rapidly from one to another individual mostly by suspended air droplets from coughing and sneezing. The symptoms are usually developed in two to fourteen days with an average of 7 days. There are some common symptoms which include high body temperature, dry coughing, sneezing, restlessness and heavy breath [5]. The difficulties may include sore throat, pneumonia, and acute respiratory distress syndrome (ARDS), which is caused by systemic inflammatory reactions (cytokine storm) due to the excessive release of pro-inflammatory cytokines and chemokines by immune effector cells [6].
Biology and Pathogenesis of SARS-CoV-2 into Human
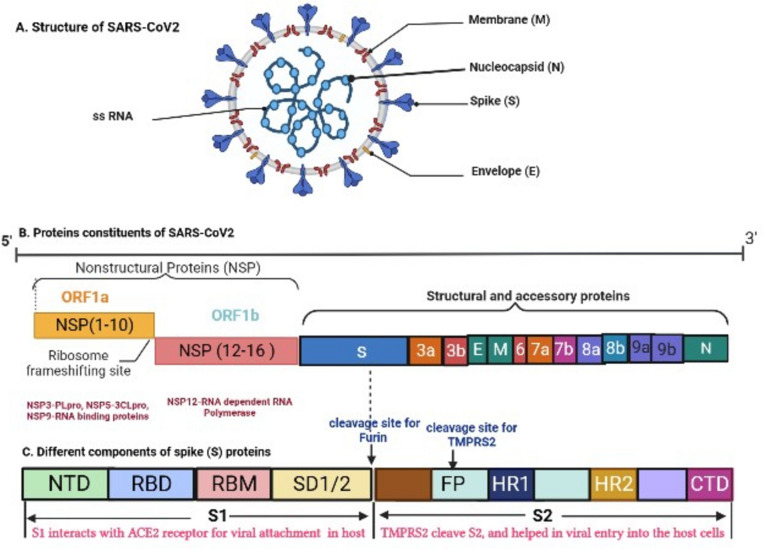
Moreover, this coronavirus has zoonotic (bat) origin, and there is chance of more human CoV outbreaks in the future [7]. DNA recombination has been linked to high-grade glycoproteins that distinguish SARS-CoV (CoVZXC21 or CoVZC45) from other β-CoVs in receptor binding domains (RBD), suggesting it may play a role in interspecies dissemination and infection [8]. This could be due to structural variations in spike (S) proteins. SARS-CoV-2 genome contains 29 proteins [9], which includes structural, non-structural and accessory proteins. The structural component contains four proteins such as spike (S) protein, membrane (M), envelope (E), and nucleocapsid (N) protein, which construct complete virus structure (Fig. 1a) [10]. Whereas, the non-structural component includes 16 proteins and accessory proteins are 9 in number, that also involve in viral replication and protecting it from host immune system (Fig. 1b) [11]. The most crucial protein for virus invading into the host is the S protein, that is present on the virus surface. Spike is a type of class I fusion protein, which has two regions, S1 and S2 with different roles. S1 contain RBD (Fig. 1c). Initially, S1-protein subunit of COVID-19 virus attach to host cell through interacting with ACE2 (angiotensin receptor 2) expressed on alveolar type 2 (AT2) cells in the lung. Moreover, the virus gains entry into host cells through the proteolytic cleavage of the S2 protein by enzymes such as furin, TMPRSS2, lysosomal cysteine proteases, and cathepsins, all of which are found in the host cell [12]. The Envelope (E) proteins of virus are formed in endoplasmic reticulum (ER) and Golgi complex (GC), followed by formation of nucleocapsid (N) utilizing genomic RNA components of the host. Overall, viral particles are synthesized into ER and GC, and the virus particles are secreted through exocytosis process (Fig. 2). Hence, all these steps are very crucial for viral life cycle and could be a potential target to design novel anti-viral against COVID-19 [4, 13].
Fig. 1.
a SARS-CoV-2 structure is composed of structural proteins including envelope protein, membrane protein, spike protein, and nucleocapsid protein. SARS-CoV-2 carries its genetic material in the form of single-stranded RNA (b). SARS-CoV-2 contains a variety of proteins, including structural, non-structural, and accessory proteins. ORF1a and ORF 1b are types of NSPs. Some NSPs such as NSP3, NSP5, NSP9 and NSP12 were known as Papin like proteases (PLpro), 3-chymotrypsin (3-CLpro), RNA binding proteins and RNA dependent RNA polynerases. These NSPs are very important for the replication of SARS-CoV-2. c The different components of spike (S) protein, such as S1 and S2. S1 has receptor binding domains (RBDs), which has specificity to bind ACE2 (Angiotensin Converting Enzyme 2) receptor of host cells. The cleavage of S2 is required for viral entry into the host cells
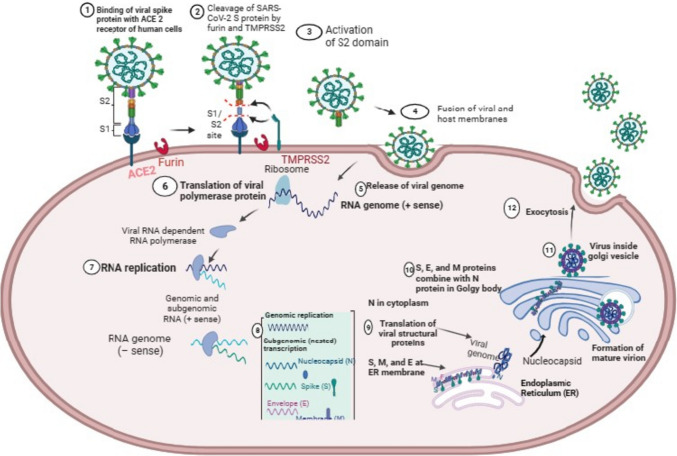
Fig. 2.
The pathogenesis of SARS-CoV-2 in human alveolar host cells. Outside the cells (steps 1 to 4), there are various steps involved in viral attachment and viral entry into the host cells. The replication of SARS-CoV-2 involves different steps, that occurs inside the cell (steps 5 to 12). First, virus is released into the host cell (step 5). The translation of different viral proteins occurs in the cytploasm (step 6), following other steps such as replication of viral RNA (step 8). The assembly of structure proteins such as S, E and M occurs (step 9) in endoplasmic reticullum (ER), wheras the inclusion of nucleopcapsid (N) protein happens in Gogly complex (step 10). The generation of complete SARS-CoV-2 takes place in golgy body (step 11), following exit of virus outside the cells by exocytosis process
Notably, as SARS-CoV-2 transmitted over the world, and the viral genome gained additional mutations with time.. It was studied that during the viral replication in the lung cell, the genome inevitably introduces some errors into their code. Few of the random errors, producing different variants could be either neutral or detrimental to the virus. But, some errors may be beneficial, that may make the virus more contagious and severe [14]. The one that was most noticeable variant till late 2020 was the S protein mutation D614G. This variant was dominant, because of higher infectivity, stability, and transmission compared to the ancestral D614 wild form [15], which resulted to emphasize on configuration of S protein trimer, needed to bind ACE2 receptor [16]. Not unexpectedly, there are numerous SARS-CoV-2 variants found. Some are thought to be particularly important due to their potential for enhanced transmissibility, higher virulence, or decreased vaccination effectiveness [17]. Reports have shown that vaccine-induced immunoglobulins have less neutralization efficiency towards a variety of SARS-CoV-2 variants, particularly the omicron variants, indicating partial immunological escape. Nonetheless, T-cell activity, appear to be sustained across vaccination platforms, regardless of any variants [18]. In March 2023, WHO has recognized Omicron as most concerned variants for human.
Individuals with severe COVID-19 infection have indications of systemic hyper inflammatory response and altered immune reaction. The reports have found the elevated levels of interleukin-2 (IL-2), interleukin-7 (IL-7), interleukin-6 (IL-6), granulocyte–macrophage colony-stimulating factor (GM-CSF), C-X-C Motif Chemokine Ligand 10 (CXCL10), monocyte chemoattractant protein-1 (MCP1), macrophage inflammatory protein-1 α (MIP1α), and tumor necrosis factor α (TNF-α), which are known as cytokine storm in the COVID-19 patients [19]. The cytokines storm has been associated to the severity of the disease and causes ARDS, thrombosis, strokes, myocardial infarction, encephalitis, acute renal injury, and vasculitis [20]. Hence, overcoming cytokine storm could be an effective strategy in the management of COVID-19.
Additionally, It has been shown that the alteration in the level of transcriptional factors p53 and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) have been associated SARS-CoV-2 pathogenesis [21]. Furthermore, interferon-α (IFN-α) plays a dual function in SARS-CoV-2 infection. IFN—α, not only protects human body by eliminating virus-infected cells, but it also involves in promoting COVID-19 infection via upregulating ACE2 expression to easily uptake of SARS-CoV-2. Therefore, the positive and negative competitive action of IFN-α is very crucial in determining the clinical outcome of COVID-19 patients [22]. Along with this, serum level of C-reactive proteins, LDH (lactate dehydrogenase), D-dimers and ferritin have been identified as some important markers for the diagnosis of COVID-19 and ARDS [23]. Moreover, the systemic inflammation causes vasodilation, which allows the infiltration of immune cells such as lymphocytes and monocytes into lung and the heart. Most importantly, granulocyte–macrophage colony-stimulating factor (GM-CSF) secreting T cells have associated with recruiting monocytes, which secrete IL-6, lead to severe lung damage in COVID-19- pathogenesis [19].
COVID-19 Affects Other Body Organs
Commonly, COVID-19 affects the lungs, however, it damages to other organs also in the body, including kidneys, liver, brain, reproductive organs and heart. Primarily, SARS-CoV-2 infects to lungs, because, it has the ACE-2 receptors, through which SARS-CoV-2 spike proteins binds and initiates pathogenesis in the lungs. The basis of infection to other body organs could be virus spill from lungs and may infects to other body organs such as kidney, heart, testis, brains and liver, which expresses ACE-2 receptors. The second possible reason could be cytokine storms, that is developed due to hyper-inflammatory reactions in lung, that affects other body organs [24]. The severe illness in these organs have long term health concerns even after COVID-19. These includes breathing, cardiovascular disease, chronic kidney disease, stroke and Guillain–Barre disorders (causing temporary paralysis) [24]. Some adults and children exposed to COVID-19, have been seen to develop a multisystem inflammatory disorder, resulted into severe disease in particular organs. The plant derived molecules may be beneficial in protecting the various body organs, which has higher risk of damages due to COVID-19.
Phytochemicals Used in Treatment of COVID-19
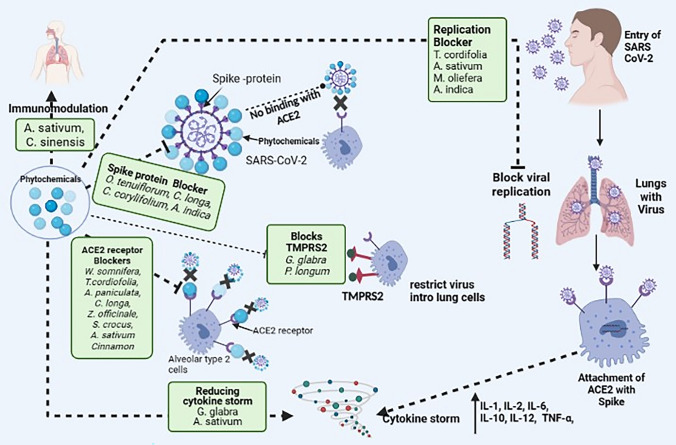
The medicinal plants and their phytoconstituents have been shown as broad-spectrum anti-viral activity for different viral disease. All corona strains, including SARS-CoV-2 and omicron, have shown to cause serious damage to the respiratory system and other body organs [25–27]. Various plants and their metabolites may play an important role in reducing complications in various body organs. Phytochemicals usually target SARS-CoV-2 by various mechanisms such as (a) neutralization of S proteins of virus, (b) targeting ACE2 receptors, furin, TMPRSS2 for stooping viral attachment and entry into the host cell, (c) targeting cathepsin to stop viral replication in host cells, (d) minimization of pro-inflammatory responses and (e) reducing oxidative mediators [28]. The various medicinal plants and their derivatives, which has antiviral activity, have been describe below and picturized in Fig. 3 and Table 1.
Fig. 3.
The different herbal plants and their constituents showing their different mode of action against SARS-CoV-2 via different targets such as ACE-2 receptors, TMPRSS2, S proteins and various cystein proteases invloved in viral replicatoins. The plants and their phytochemicals blocks these targets and inhibits the viral pathogenesis in the human. Some of phytochemicals also reduces the cytokine storm, that is important in management COVID-19
Table 1.
Chemical constituents, parts of plant and uses of herbal plants used in treatment of COVID-19
| Plant (common name) | Plant (botanical name) | Chemical constituents | Parts used | Pharmacological acitivies | Tissue protective activities | Reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Asvagandha |
Withania somnifera (L.) Dunal |
Withaferin, withanone, withanolide | Root, leaf | Anti-covid-19 (suppressing ROS production and TGF-β1 signaling. Inhibit ACE-2 receptor), anti-pyretic, anti-microbial, anti-bacterial, anti-inflammatory (↓ IL-1 β, IL-6, TNFα) | Lung protective, skin protective, neuroprotective | [29] | ||||||||
| Guduchi | Tinospora cordifolia (wild.) Miers | Tinosporin, tinosporide, cordifolide, phenyl prophyl glycoside | Stem, leaf, aerial root | Anti covid-19 (Reduce interaction between ACE-2- RBD complex) Anti-oxidant, immunomodulator, anti-inflammatory (↓ IL-1 β, IL-6, TNFα), anti-rheumatic | Neuroprotective, Lung protective, Immunomodulator | [22] | ||||||||
| Kalamegha | Andrographis paniculata (Burm.f.) | Andrographolide, neoandrographolide, deoxy andrographolide | Whole plant | Anti-viral (supress ACE-2 receptor), anti-pyretic, anti-periodic, immune enhancement | Hepatoprotective, lung protective, immunomodulators | [30] | ||||||||
| Tulasi | Ocimumtenuiflorum L | Bornylacetate, cadinene, eugenol, methyl chavicol, limonene | Leaf, root, seed | Anti-covid19 (potentially inhibit Mpro of SARS-CoV-2) Anti-viral anti-fungal, anti-viral, anti-spasmodic | Cough protective, immunomodulator, lung protective | [29] | ||||||||
| Tvak | Cinnamomum verum Presl | Cinnamaldehyde, eugenol, benzaldehyde, methyl eugenol | Stem bark | Rhinitis, cough, headache, indigestion | Skin protection | [31] | ||||||||
| Haldi | Curcuma longa | curcumin, demethoxycurcumin, and bisdemethoxycurcumin | Root | Anti-covid-19 ( bind with spike proteins and with the ACE-2 receptor), anti-bacterial, anti-viral, anti-pyretic, anti-inflammatory (↓iNOS and cytokine induced neutrophil chemo attractants) | Neuroprotective, skin protective | [32] | ||||||||
| Scurfy pea | cullencorylifolium (l.) medik | corylifol H, epi-bavacoumestan | Flower, seed, leaf | Anti-covid-19(potentially inhibit Mpro of SARS-CoV-2), anti-viral, anti-bacterial, anti-inflammatory (↓ IL-1 β, IL-6, TNFα) | Neuroprotective, Lung protective, Immunomodulator | [33] | ||||||||
| Neem | Azadirachta indica | Azadirachtin, nimbolinin, nimbin, and salannin | Leaves | Anti-inflammatory (↓ IL-1 β, IL-6, TNFα), anti-viral, anti-covid19 (bind with E and M required for assembly of SARS- CoV-2 also inhibit papin like proteases PLpro of SARS-Cov-2) | Neuroprotective, Lung protective, Immunomodulator | [34] | ||||||||
| Meethi | Trigonella foenum graecum linn | Fenugrin B, isoleucine, leucin, graecunins | Seeds | Anti-pyretic, painkiller, anti-inflammation (↓ IL-1 β, IL-6, TNFα) | Hepatoprotective, immunomodulator | [35] | ||||||||
| Adrak | Zingiber officinale roscoe |
Gingerols, shogaols, paradols, 6- gingerol, and 8- gingerol |
Rhizome | Anti-Covid 19 ( inhibit papin like protease (PLpro) and ACE-2 receptor) Respiratory infections, anti-inflammation, expectorant | Cough protective, hepatoprotective, immunomodulator | [36] | ||||||||
| Sarata | Tribulus terrestris | Flavonoids, flavonol, gylcosides steroidal saponins and alkaloids | Whole plant | Anti-covid19 (inhibit papin like protease PLpro), anti-viral, anti-inflammatory (↓iNOS and cytokine induced neutrophil chemo attractants, ↓PGE-2 and reduce the expression of IL-1 β, IL-6, TNFα) | Hepatoprotective, immunomodulator, Neuroprotective, Lung protective | [37] | ||||||||
| Clove | Syzygiumaromaticumlinn | Carvacol, eugenol, thymol,and cinnamaldehyde | Flower and leaves | Immunity enhance, anti-viral, anti-inflammatory (↓ IL-1 β, IL-6, TNFα), throat infection | Cough protective, hepatoprotective, immunomodulator | [38] | ||||||||
| Amla | Emblica officinalis | Ascorbic acid, ellagic acid, chebulinic acid, gallic acid | Fruits | Inhibit replication of virus, immunomodulator, anti-oxidant (reduced glutathione level, increase SOD1 decreased the levels of lipid peroxidation, ROS production) | Neuroprotective, immunomodulator, lung protective | [22] | ||||||||
| Kukum | Mallotusphilippensis | Phenols, diterpenoids, steroid, flavonoids | Fruit and leaves | Anti-covid and anti-viral, anti-inflammatory (↓ IL-1 β, IL-6, TNFα) | Immunomodulator | [22] | ||||||||
| Saffron | Saffron crocus | Carotenoid, crocin, picrocrocin, safranal | Flower | Anti-covid (reduce cytokine cascade and downregulate angiotensin-converting enzyme 2 (ACE2) gene expression), anti-inflammatory, anti-viral | Immunomodulator, neuroprotective, skin protective | [16] | ||||||||
| Rasona | Allium sativum | Allylalcohol, allylthiol, allylpropyldisulphide | Fruit | Anti-covid (supress ACE-2 receptor and main protease PDB6LU7 of SARS-CoV 2 virus and also reduce inflammatory cytokines and adipose tissue derived hormones) | Neuroprotective, immunomodulator, lung protective | [16] | ||||||||
| Black- or chebulic myrobalan | Terminalia chebula | Triterpenes, arjunglucoside, chebuloside | Fruit and flower | Anti-covid (displayed 3CLpro inhibition) | lung protective | [16] | ||||||||
| Long pepper | Piper longum | Alkaloid piperine | Fruit | Anti-covid (inhibit ACE2 and TMPRSS2 receptors) | lung protective | [16] | ||||||||
| Tulip tree | Pulownia tomentosa | flavonoids | bark, fruits, xylem, and leaves | bronchitis, asthmatic attacks, dysentery, anti-covid19 ( inhibit PLpro, 3CLpro) | lung protective, immunomodulator | [39] | ||||||||
| Sage |
Salvia officinalis |
Alkaloids, phenolic compounds, steroids polyacetylen, essential oils |
Fruit, leaves |
Anti-inflammatory (↓ IL-1β, IL-6), Anti-cancer, Anti-diabetic, Anti-microbial, Anti-oxidant |
Neuroprotective, Cardioprotective, Lung protective |
[40] | ||||||||
Cinnamic amides form Tribulus terrestris
Several phytocompounds have been evaluated and studied for its papain-like protease (PLpro) inhibitory activity against SARS-CoV infection. Tribulus terrestris fruits have been known for its utility in preparations of drugs and food supplements. The methanol extract of T. terrestris fruits has demonstrated potent inhibition against papain-like protease (PLpro), an essential proteolytic enzyme in host for protection to pathogenic virus and bacteria. Major bioactive compounds of Tribulus terrestris includes cinnamic amides and ferulic acid, have been reported for inhibition of Papain-like proteinase (PLpro), which is major protein target of COVID-19 and can be used as herbal therapeutics [41]. A study conducted by Song et al., demonstrated that there are six types of cinnamic amides identified in this plant, which has shown inhibitory action against PLpro in dose dependent manner. These includes N-trans-Feruloyloctopamine, N-trans-Coumaroyltyrai-ne, N-trans-Caffeoyltryamine, Terrestrimine, N-trans-Feruloyltryamine, and Terrestriamide [42]. The most potent inhibitory activity was possessed by the Terrestrimine [(E)-N-(1-hydroxy-2-(4 -hydroxyphenyl)-2- oxoethyl)-3-(4-hydroxy-3-methoxypheny) acrylamide]. This enhanced inhibitory action was attributed by the presence of the polar substituents over the methylene groups (C8 and C7) [43].
Tribulusamide D, an ethanolic extract of Tribulus terrestris have been shown to have anti-inflammatory property and inhibited the production of iNOS, PGE2 and reduced the expression of pro-inflammatory cytokines such as IL-6, IL-10 and TNF- α. Hence, this compound could be also used to reduce the cytokine storm during COVID19.
Flavonoids from Cullen corylifolium (L.) Medik
Cullen corylifolium is an erect, annual or short-lived perennial plant growing up to 1.5 m, tall from a taproot. The plant is commonly branched from the base. The seed of this plant has anthelmintic, anti-viral and antibacterial property. Several plants were evaluated for its inhibitory activity over the SARS infection which was demonstrated due to its main chemical constituents namely flavonoids class. This plant possesses majorly six different classes of flavonoids, such as bavachinin, neobavaisoflavone, isobavachalcone, 4′-O-methylbavachalcone, psoralidin and corylifol A which demonstrated inhibitory activity against PLpro in dose dependent manner with IC50 4.2–38.4 μM. Of these, isobavachalcone and psoralidin has shown maximum activity agains PLpro in COVID-19 [44]. In a recent study, it was shown that ethanolic extract from C. corylifolia seeds has good activity against papain-like protease (PLpro), an essential enzyme involved in SARS-CoV replication [45].
Flavonoids from Paulownia tomentosa
Cho et al., evaluated the inhibitory action of the plant Paulownia tomentosa over the SARS-CoV-2 infection [46]. They isolated five novel flavonoids such as tomentin A, tomentin B, tomentin C, tomentin D, and tomentin E, which has shown inhibitory property over the COVID-19 infection. Of these, tomentin E has demonstrated highest inhibition against PLpro with an IC50 of 5.0 ± 0.06uM. The ethanolic extract of the plant depicted inhibition of the SARS-CoV-2 infection in dose dependent manner. Of these Tomentin E showed highest inhibitory action [47]. It was stated that the molecules with the 3, 4-dihydro-2H-pyranmoiety possessed most significant inhibitory action. The antiviral properties of the fruit extract and the isolated compounds from this species have been investigated in vitro against the polyprotein target PLpro, a protein involved in RNA replication [48].
Chalcones from Angelica keiskei (Miq.) Koidz
Angelica Keiskei plant has been demonstrated to have inhibitory property against SARS-CoV-2 infection [49]. The chemical constituents of this plant, which has shown anti-SARS-CoV-2 activity was chalcones. The plant possessed several forms of the alkylated chalcones, of them nine major forms are iso-bavachalcone, 4-hydroxyderricin, xanthoangelol, xanthoangelol F, xanthoangelol D, xanthoangelol E, xanthoangelol B, xanthoangelol G, and xanthokeistal A. All of them have shown anti-viral action against PLpro-SARS-CoV-2 in dose dependent manner with IC50 ranging from 1.2 ± 0.4 to 46.4 ± 7.8 µM. Further investigations over kinetic parameters revealed that the chalcones show non-competitive inhibition, whereas iso-bavachalcone showed mixed type of the inhibition. The studies also revealed that the xanthoangelol E possessed most potent inhibitory effect over 40 times (IC50 = 1.2 ± 0.4 µM) more as compared to other chalcone derivatives and confirmed by the in-silico studies [50].
Tanshinones from Salvia Miltiorrhiza Bunge
It has been reported that plant Salvia Miltiorrhiza demonstrated its inhibitory action against PLpro-SARS-CoV-2 infection [49]. The principal effect of the plant was due to the presence of the chemicals tanshinones and various other phytochemicals also. The n-hexane fraction of this plant extract has revealed seven different constituents, that includes tanshinone IIA, tanshinone IIB, methyl tanshinonate, cryptotanshinone, tanshinone I, dihydrotanshinone I, and rosmariquinone,which has demonstrated inhibitory activities against PLpro [51]. The results also depicted that the cryptotanshinone was the most potent class of chemicals for its inhibitory effect against PLpro with IC50 value of 0.8 ± 0.2 µM. The kinetic parameters confirmed that the rosmariquinone is a mixed type of inhibitor against SARS-CoV infection as compared to other tanshinones, which shows the non-competitive inhibition [52]. Furthermore, the extract of S. miltiorrhiza have been shown to block the interaction of S protein of SARS-CoV-2 with ACE2 receptor on lung cells, and mitigate the inflammatory responses produced due to leukocytes via hindering NFκB signalling [30].
Diaryl heptanoids from Alnus Japonica
The study has shown activity guided fractionation to identify various phytochemicals of the plant Alnus Japonica, which showed therapeutic activities against COVID-19 [42, 53]. Nine phytochemicals of the diaryl heptanooids class, that include platyphyllenone, hirsutenone, platyphyllone, platyphyllonol-5-xylopyranoside, hirsutanonol, oregonin, rubranol, rubranoside B, and rubranoside A were isolated from the ethanolic extract of the herb Alnus Japonica, which has demonstrated inhibitory activity against PLpro. The increased activity of diarylheptanoids against PLpro were shown to be contributed by the presence of α, β-unsaturated carbonyl and catechol groups [42].
Coumarin
Coumarins occur as secondary metabolites in the seeds, roots and leaves of many plant species, notably in high concentration in the tonka bean. Thus, the name comes from a French word, coumarou, for the tonka bean. Research studies suggested that coumarin has role in plant growth regulations, fungistasis, bacteriostasis and, even as waste products [54]. Furthermore, coumarin has been demonstrated to inhibit inflammatory mediators by reducing reactive oxygen species (ROS) and free radical generation [52]. Consequently, coumarins have been suggested as anti-inflammatory properties in the treatment of injury to lungs caused by lipopolysaccharide and other factors [55].
Daphnetin (DAP), a coumarin derivative extracted from Daphne species, is biologically active phytochemical with copious bioactivities including anti-inflammatory, anti-oxidant, neuroprotective, analgesic, anti-pyretic, anti-malarial, anti-bacterial, anti-arthritic, neuroprotective, hepatoprotective, nephroprotective and anti-cancer activities [56]. Further, daphnetin (hydroxycoumarin isolated from Daphne spp.) has shown protective properties against LPS-induced lung damage by reducing the production of IL-6 and TNF cytokines, generated during induction of LPS (100 ng/ml) in lung cancer cell lines [57]. It is well known that, JAK/STATs pathway also regulates generation of pro-inflammatory mediators [58]. Daphnetin has shown the inhibition of LPS-induced cytokine production in mice through downregulating JAK/STAT signalling [57]. Additionally, daphnetin has been also shown to reduced ROS generation [49, 59].
Isofraxidin, a another coumarin, exhibits powerful anti-inflammatory activities, especially in airway inflammation produced by the influenza virus [60]. As a consequence, it reduces the expression of gene cycloxygenase-2 and alleviated lung damage in mice [61]. Additionally, Isofraxidin have been demonstrated to reduce the generation of IL-6 and TNF-α, which help in protecting LPS-induced lung injury. Since, there is huge production of pro-inflamamtory cytokines during COVID-19 pathogenesis [24, 25]. Hence, different types of coumarin may be utilized to overcome the cytokine storm and protecting the lung damages in COVID-19.
Andrographolide from Andrographis paniculata (Burm.f.)
In an emergency condition, the affected COVID-19 person is treated with a combination of hydroxychloroquine and azithromycin [62]. Various available drugs including small compounds Remdesivir has been also tested for the treatment of SARS-CoV-2 virus [62]. Andrographolide has drawn scientific attention due to its remarkable properties of its lipophilicity and higher solubility. It has also shown that andrographolide compound inhibits CYP1A2 but not CYP2C19 or CYP3A4, suggesting, it doesn't affect liver metabolism [42]. Since, andrographolide may liberate phosphate from ATP while simultaneously attaching ADP to the glycoprotein, making it a substrate for p-gp (permeability glycoprotein). The molar refractivity of the chemical (19-norandrographolides 7, 8-dimethoxy-2′-hydroxy-5-O-β-d-glucopyranosyloxyflavone) indicates that it can pass through particular membranes and may remain constant even when there are strong or weak solute–solvent interactions. The results demonstrated both A. paniculata extract and andrographolide inhibited SARS-CoV-2 replication in a dose-dependent manner [62]. Furthermore, in slico study has demonstrated that the several phytoconstituents of A. paniculata has strong binding with different targets of SARS-CoV-2 virus such as spike (S) glycoprotein, 3-chymotrypsin-like protease (3CLpro), PLpro, and RNA-dependent RNA polymerase (RdRp) and ACE2 receptors, which may inhibit pathogenesis of COVID-19 by targeting [63].
Withanolide, Withaferin A, and Withanone from Withania somnifera
There are various phytoconstituents such as Withanolide A and B, Withaferin A, Withanone, and Withanosides available in Withania somnifera (WS), which have been shown for different pharmacological significances [64]. The antimicrobial and anti-viral activity has been assessed for a glycoconstituent of WS, known as Withania somnifera glycoprotein (WSG), which is found in root of WS. It has been shown that WS has the therapeutic potential in the management of COVID-19 via modulating the Th-1/Th-2 immunity. Additionally, WS has been also demonstrated to induce anti-viral immunity by enhancing production of IFN and also reduce the cytokine storm by downregulating the generation of anti-inflammatory cytokines like IL-1, IL-6, and TNF, primary targets relevant to COVID-19 [64]. It has been hypothesized that the major phytoconstituents of WS, such as withanolide-B, withanone, and withaferin-A, will have a lower binding energy than the pharmacological inhibitor, N3. Because of the higher binding that occurs between these phytochemicals and the main protease 3CLpro, there is a possibility that the cleavage of polyproteins to non-structural proteins (NSPs) is reduced, which in turn may inhibit viral replication and transcription [65].
Tulsinol and Dihydrodieuginol from Ocimum sanctum
Ocimum sanctumis is being used in the management of pain, diarrhea, cough and fever, which are the common symptoms of COVID-19. Also, Ocimum sanctum has shown to boosts the immunity of the body and helps to defence the threatening virus and bacteria. Furthermore, Ocimum sanctum extract can be included as a preventive measure against COVID-19 due to its potential to inhibit replication of COVID-19 supported with its immune-modulatory feature and ACE2 blocking properties. Additionally, Ocimum sanctum containing Tulsinol (A, B, C, D, E, F, G) and dihydrodieuginol-B has been demonstrated to inhibit 3CLpro and PLpro [66]. Recently, molecular-docking has shown that phytoconstituents of Ocimum sanctum inhibit the COVID-19 pathogenesis by blocking ACE2 and TMPRSS2 expression in host cells [67].
Phyllaemblicin from Phyllanthus emblica and Constituents of Tinospora cordifolia (Guduchi)
Phyllanthus emblica also has immunomodulatory properties, and may have the potential to bolster health and immunity of the community to fight against SARS-CoV-2 infection [68]. Phyllaemblicin-B and phyllaemblinol from Phyllanthus emblica showed high binding affinity to helicase protein, which is one of the major targets of COVID-19. Phyllaemblicin G7 from Phyllanthus emblica exhibited high binding affinity to the Spike Protein of COVID-19 [69]. The antioxidative and anti-inflammatory properties of Phyllanthus emblica are the key to its therapeutic effect [70].
Tinospora cordifolia is a medicinal plant, that have been known for antioxidant, anti-arthritic, anti-diabetic, anti-inflammatory, anti-malarial, anti-depressant, anti-allergic, and immunomodulatory properties, because of the presence of various types of potential medicinally important phytochemicals [71]. The constituents of T. cordifolia, including berberine (C20H18NO4), choline (C5H14NO), beta-sitosterol (C29H50O), tetrahydropalmatine (C21H25NO), and octacosanol (C28H58O), have been assessed for its anti- SARS-CoV-2 property utilizing an in silico computational approach. Among all tested molecules, molecular docking data revealed that berberine, binds Mpro (main proteases) strongly, which may suppress COVID-19 pathogenesis [72]. Furthermore, It has been demonstrated that the ACE2-RBD complexes were more flexible after addition of phytocompounds, tinocordiside in the mixture [73]. Hence, Tinocordiside of T. cordiofolia may be a therapeutic molecule stopping SARS-CoV-2 entry to lung cells. Additionally, four natural compounds extracted from T. cordifolia such as Berberine, Isocolumbin, Magnoflorine, and Tinocordiside have shown strong binding with surface glycoprotein (6VSB) and the receptor binding domain (6MoJ), which are important molecules in attaching SARS-COV-2 to alveolar cell [74]. Also, these natural phytochemicals have binding affinity for RNA polymerase and proteases, important for replication SARS-CoV-2 in alveolar cells [74]. These data indicate that T. cordifolia and their constituents could be a potential therapeutic option for COVID-19 management.
Glycyrrhetinic acid from Glycyrrhiza glabra (Yashtimadhu)
The natural compound triterpene glycosides (Glycyrrhizin (GL) isolated from the root of Glycyrrhiza glabra has been known for its pharmacological potential including anti-inflammatory, hepatoprotective, anti-carcinogenic and anti-viral capabilities [75]. Glycyrrhetinic acid (GA), an active metabolite of GL has been shown as anti-inflammatory potential through targeting toll like receptor (TLR)-4. Also, GA has demonstrated to block TMPRSS2, which might inhibit the virus uptake. Thus, this data revealed that GL may reduce the severity caused during COVID-19 infection via two mechanisms: (i) by preventing the virus uptake into alveolar cells by blocking TMPRSS2 and, (ii) by reducing the lung inflammation.
Constituents of Allium sativum (Garlic)
Garlic has a well-established historical relevance as a beneficial traditional medicine that was used in the ancient times. There are several biologically active constituents of garlic for e.g., sulfur-containing compounds such as alliin, diallyl-sulfide, diallyl-disulfide, diallyltrisulfide, S-allylcysteine (SAC), and enzymes (alliinase) have been known for its medicinal properties [76]. Recently, the anti-viral property of garlic essential oil has been investigated for its anti-coronavirus activity. The data has shown that the garlic oil constituents such as diallyl-disulfide and diallyl-trisulfide, have the property to suppress ACE2 receptor on alveolar cells. Also it inhibits the main proteases involved viral replication [31]. According to the findings of a study, Allium sativum has the potential to lower levels of inflammatory cytokines and adipose tissue-derived hormones, such as leptin, which have an inflammatory nature. This suggests that Allium sativum has the potential to be used as a preventative measure in the population before individuals are infected with the COVID19 infection [77]. Flavonoids and organosulfurs have been identified as the primary bioactive components responsible for the immunomodulatory effects of the Allium sativum plant. These bioactives have the potential to prevent the COVID-19 outbreak by creating hydrogen bonds with the active sites of serine protease [78]. In addition, a study indicated that alliin, SAC, and other bioactive components of Allium sativum can be utilized as a possible inhibitor candidate for COVID-19 and may be helpful to fight against this pandemic [79].
Constituents of Zingiber officinale (Ginger)
Zingiber officinale (Ginger), has been used as a traditional plant for treating various health conditions, such as nausea, migraine, anticancer and diabetes [80]. Several biologically active constituents of ginger such as Paradols, 5-acetoxy-6-gingerol, gingerdiones, gingerdiols, 6-gingerol, 10-gingerol, 12-gingerol, 6-dehydrogingerols, 6-shogaol, and 3,5-diacetoxy-6-gingerdioal have been known for their pharmacological potential. A recent in silico report demonstrated that rhizome extract of ginger has the strong binding affinity for PLpro, required for replication of SARS-CoV-2 [81]. Additionally, it has been shown that phytoconstituents of Zingiber officinale has significant affinity for S-protein of SARS-CoV-2 and ACE2 on alveolar cells, which might block the entry into host cells. Hence, these phyto-constituents may be used as therapeutics in reducing the viral load in the COVID-patients.
Constituents of Curcuma longa (Turmeric)
In the past, the potential therapeutics of Curcuma longa have mostly been attributed to its main active ingredient, curcumin. Because of its anti-microbial, anti-inflammatory, anti-carcinogenic, and anti-oxidant characteristics, Curcuma longa has a long history of usage as a medicinal herb, particularly for the treatment of pathological disorders [82]. In addition, research conducted in vitro and in vivo shown that curcumin alleviated the symptoms of pneumonia brought by the influenza virus by reducing the severity of lung damage and bringing the levels of inflammatory cytokines produced by macrophages under control [83]. Furthermore, curcumin, a polyphenolic substance, have been demonstrated in the treatment of SARS-CoV-2 infection [84]. In addition, the results of docking study revealed that curcumin has the strongest interaction possible with both the spike protein (− 141.36 kcal/mol) and the ACE2 receptor (− 142.647 kcal/mol) [85]. An investigation revealed that curcumin also displayed substantial binding with the MPro. Based on the results of the molecular docking study, it was found that the binding energy of hydroxychloroquine (− 24.58 kcal/mol) for major protease is higher than that of curcumin (− 20.47 kcal/mol). Curcumin, on the other hand, had a greater binding energy for the S protein receptor binding domain (− 38.84 kcal/mol) compared to hydroxychloroquine (− 35.87 kcal/mol) [86]. According to the findings of the study, curcumin has the potential to be an adjunct medicine that can be used in combination with hydroxychloroquine to impair the structural integrity of the SARS-CoV-2 protein. All of these findings, pointed to utilize the possibility of curcumin as a promising treatment against viral infections such as COVID-19, which causes damage to the lung tissues.
Constituents of Cinnamon (Dalchini)
Cinnamomum verum, Cinnamomum cassia, Cinnamomum burmanii, Cinnamomum zeylanicum, Cinnamomum tamala, Cinnamomum loureirii, and Cinnamomum cordatum are some of the cinnamon species that have been identified as having the potential to be utilized in the food industry as additives. Cinnamaldehyde, trans-cinnamaldehyde, and cinnamic acid are the three chemical constituents of cinnamon that have been shown to have pharmacologically active properties. In the appropriate amounts, these active components provide a number of health benefits, such as antioxidants, anti-microbial agents, anti-inflammatory agents, anti-gastric ulcer agents, and anti-yeast agents, among others [87]. It has shown that the expression of the HSPA5 protein is increased in the majority of viral infections, including SARS-CoV-2, due to ER stress conditions [88]. It is interesting to note that SARS-CoV-2 gets into the host cells via making use of more than one host cell receptor, and that receptors is HSPA5, which is also known as Bip or GRP78 [89]. Under conditions of stress, the HSPA5 protein on the surface of the cell becomes more accessible, hence facilitating the entry of pathogens into the cell. Cinnamon administration has been found to reduce ER stress in a rat obesity mouse model [90], which suggests that it is plausible to suggest that cinnamon administration may also inhibit the translocation of HSPA5 to the cell membrane from cytoplasm and reduce interaction between virus and the host cells, thereby preventing virus entry. Cinnamon was recently shown to have the ability to bind the HSPA5 substrate binding domain (SBD) with a binding energy of -6.25 kcal/mol, and this suggests that it may interfere with the recognition and binding of SARS-CoV-2 [91]. Furthermore, in silico data validation suggested that two compounds, Pavetannin C1 (PAV) and Tenufolin (TEN), among 48 isolates of compounds of cinnamon demonstrate good binding activity with the key proteases and spike proteins of SARS-CoV-2 [92]. Cinnamomum zeylanicum is one of the components of Ayush Kwath that demonstrates anti-inflammatory, anti-oxidant, anti-platelet, and hepatoprotective potential [93]. This potential may be useful in the treatment of COVID19 infection.
Constituents of Moringa oliefera (Drumsticks)
The Moringa oliefera, is a member of family Moringaceae, is widely regarded as one of the healthiest and most nutrient-rich foods due to its abundance of beneficial compounds. It has been used as a traditional herb for hundreds of years, and its pharmacological characteristics may be found in the different distinct parts, including leaf, bark, sap, roots, and flowers [94]. Due to the presence of flavonoids, polyphenols, vitamins, tannins, isothiocyanates, and saponins, M. oliefera has been used as a traditional medicine. Furthermore, in silico data verified that the phytoconstituents of M. oliefera binds strongly with primary protease of SARS-CoV-2 and inhibit its pathogenesis in host cells [95]. Additionally, anthraquinone, a phytochemical in M. oliefera, has been shown as a potential anti-viral agent, which may be used to treat COVID19 [69].
Constituents of Azadirachta indica
Azadirachta indica (neem) is a member of mahogany family also known as muarubaini, and has the ability to heal forty various diseases. In addition to this, it has many health benefits, it has more than 300 phytochemicals including isoprenoids, terpenoids and non-isoprenoids. C-secomeliacins, vilasinins, diterpenoids, and triterpenoids all belong to the class of isoprenoids, whereas coumarin, polyphenolics, sulphur compounds, tannins, aliphatic chemicals, polysaccharides and proteins are all part of the non-isoprenoids group of phytochemicals. Nimbin, is an important triterpenoid in neem, has been shown to fight against various diseases such as fevers, inflammations, infections, allergies, cancer, parasites, and fungi [96]. The neem-derived compounds like nimbolin A, nimocin, and cycloartanols have demonstrated stable and efficient binding with E and M proteins needed for the assembling of SARS-CoV-2 virus. Furthermore, it has been reported that numerous phyto-constituents of neem leaves have the ability to inhibit the Mpro (main proteases) responsible for replication and transcription of SARS-CoV-2 [97]. Additionally, the phytoconstituents of neem has been reported to inhibit papain-like proteases (PLpro) of SARS-CoV-2 [98]. As desacetylgedunin (DCG) of neem has shown the highest binding affinity to PLpro among all tested compounds [98]. In line with this, research has shown that among the many bioactive chemicals found in A. indica, the phytocompounds such as Vepnin, Epiazadiradione, Azadiradione, and Nimbione have the strong ability to inhibit COVID-19 protease inhibitors. Hence, these pieces of evidences indicated that A. indica (neem) may be used as a possible phytotherapy against COVID-19 by binding the proteases which is important for the infectivity and reproduction of SARS-CoV-2 [99].
Constituents of Camellia sinensis (Green tea)
Camellia sinensis, is a member of the Theaceae family, also known as green tea, which is rich of minerals, vitamins, carbs, polyphenols, caffeine, and theanine, make it popular drinks worldwide [100]. Catechins and flavonols are kinds of polyphenols, which are more common than others. Additionally, catechins are found in different forms including epicatechin (EC), epigallocatechin (EGC), gallocatechin (GC), epicatechin gallate (ECG), gallocatechin gallate (GCG), and epigallocatechin gallate (EGCG). It has been reported that green tea is very effective against a various disease including diabetes, obesity, cancer, cardiovascular disease and neurological disorders [100]. Furthermore, L-theanine, a component of green tea has been demonstrated to reduce the organ damage during COVID-19 treatment in dose dependent manner [13]. The molecular docking studies have been shown that different bioactive polyphenolic compounds such as Theaflavin, epigallocatechin 3-gallate, genistein, 1-O-caffeoylquinic acid and ethyl transcaffeate of Camellia sinensis binds to matrix metalloproteinases (MMPs), a main protease against SARS-CoV-2 [101]. Similar reports have indicated that EGCG and theaflavin, especially theaflavin-3, 3'-digallate (TF3), interacts significantly with the receptor binding domain of SARSCoV-2. Interestingly, thearubigins, a component of Camellia sinensis has been reported to have high biding affinity for 3CL pro-proteases and inhibit the SARS-CoV-2 replication [102]. These evidences suggest that the plant green tea may be used as a therapeutic alternative for treating SARS-CoV-2 infection. In addition, Camellia sinensis have been known as an important source of nutritional immunity, which may enhance innate immunity and slow down the spread ofCOVID-19 virus [103].
Herbal Nutraceuticals
The nutraceuticals are called functional food also. It has been utilizing to treat various diseases such as diabetes, atherosclerosis, cardiovascular, cancer and neurological disorders [104]. The nutraceuticals originated from herbs have shown as important in the management of COVID-19. There is various nutraceutical are available, few of them have been discussed here in respect of COVID-19.
Ginseng, Omega-3 Fatty Acid and Quercetin
Ginseng has been shown to enhance the proliferation of B-cells and facilitate the generation of anti- inflammatory cytokines which causes interference against the pathogenies. Additionally, it has been reported to have anti-inflammatory property and inhibit the replication of viruses [29]. However, the immunomodulatory property of ginseng in treating disease has not been explored widely. Though, it was believed that it could be effective in treating respiratory tract infection and reduces the severity of common cold and flu. In COVID-19, there is hyper systemic inflammatory responses, hence due to Ginseng, anti-inflammatory property, it could be utilized to reduce cytokine storm and severity of disease [32].
Omega-3 is a long chain polyunsaturated fatty acid found as an alpha linolenic acid (ALA) in various plants such as walnut, flax, chia, canola, hemp, echium and perilla seed. It was shown as important to maintain the function of various body system such as pulmonary, cardiovascular, endocrine, and immune systems [34]. It affects the immune system via modulating the activity of neutrophils, macrophages, B-cells, T-cells, natural killer cells, and other immune system cells. Additionally, omega-3 fatty acids have been demonstrated to be introduced into entire human body, since it is a part of phospholipid bilayer of the plasma membrane that could lead to reduce production of pro-inflammatory mediators. Moreover, higher doses of omega-3 could be very effective in mediating cytokine storms [35].
Quercetin is a known flavonol, found in dietary supplements from various plants such many fruits, vegetables, leaves, seeds, and grains; capers, red onions, and kale. Additionally, quercetin has been shown to have antioxidant, anti-inflammatory, antiviral and immunoprotective properties in various diseases [36]. Furthermore, quercetin has been reported to have promising therapeutic potential along with vitamin C and bromellin against COVID-19 [38].
The Herbal Plant Constituents Used in Nano-formulation for COVID-19 Treatment
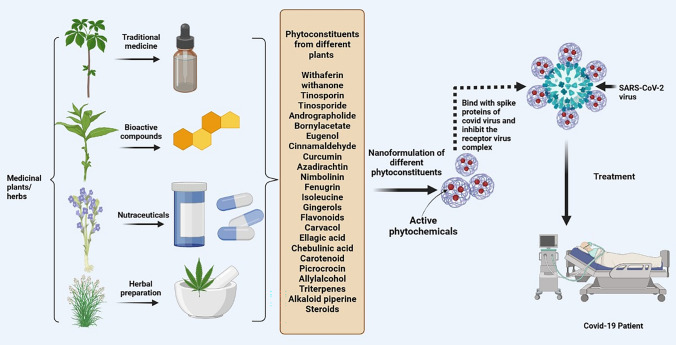
Nanotechnology is the utilization of the nano-size (< 100 nm) particle as a therapeutic agent for the various disease. In the present scenario, there are numerous nano-formulations with potential inhibitory activity over COVID 19 virus. The world has advanced with technology, but viral infections along with their various socioeconomic expressions are continue to exist and contribute to human death. The ability of virus to replicate in the host cell by releasing its own DNA or RNA remains a major roadblock in the development of effective antiviral medicines. The precise type of viral illness is difficult to recognize and diagnose. Because of previous exposure, virus antibodies already existing in the host may become activated, making accidental infections more difficult to detect [40]. The scientific community considered difficulties in prevention, diagnosis, or treatment of COVID-19 virus. Hence, innovative technologies have been developed to address the limitations of present processes. The herbal plants and their constituents used in nano-formulation have been used in the treatment of COVID-19 (Fig. 4).
Fig. 4.
Nanoformulations of various phytochemicals utilized in treatment of COVID-19 patients
Conclusions and Future Direction
The COVID-19 emergence as pandemic, drained the whole world into a devastating scenario. Numerous efforts in the form of the tests, isolations, detections and evaluations started over to cease its impact over the population by the researchers and experts of the field. Since the ancient times, herbal remedies have been the sole weapon against the various pathogens due its low cost, potent, and less toxicity. Several plants were evaluated for their impact over the virus replication cycle and its pathogenesis in the host body. Various studies emphasized the variety of phyto-constituents revealed positive impact on the cessation of SARS-CoV-2. The main phytochemicals categories that showed their inhibitory effect were cinnamic amides, flavonoids, terpenoids, chalcones, tanshinones, diarylheptanoids that were isolated from various plant species and were evaluated with various types of the assay methods. Nano formulations also had a dynamic role in delivering the needful intervention to the population and controlling the situation to a vast extent. Curcumin nano formulations proved to be inhibiting in nature by suppressing the virus binding to its native receptor (ACE2) in the host lung cells.
The review not only appreciates and discloses the herbal remedies, but also gave path for the new ideas over herbal plants for their impact over the virus. The virus keeps on changing its structural features like we are dealing with its new face as delta, and omicron, for which plants with therapeutic and preventive effect need to be considered. Linoleic acid has been shown to bind with S protein, which could be the most remarkable putative drug-lead phytochemicals in viral suppression. The above conclusion indicates that free fatty acids or their derivatives may also play a critical and leading role in the development of COVID-19 infection medications. Some transgenic plant Nicotiana benthamiana has been also investigated for the development of plant-derived corona virus vaccine. These plant based vaccines have been shown to produce less side effects. However, further studies on plant derived potential compounds are required to assess their therapeutic impacts on COVID-19.
Acknowledgements
Sanjay Kumar is highly grateful to Sharda University for proving the resources. Also, biorender software is highly acknowledged for artwork and schemes.
Author Contributions
MS and SHL collected data and prepared original draft of the manuscript. RD, SK, KKC and SK edited and reviewed the manuscript. SK and KKC conceived the idea and supervised the project. All authors have read and agreed to the published version of the manuscript.
Declarations
Conflict of interest
All authors declare no competing interests with the work presented in the manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mansi Singh and Shih-Hsiu Lo have contributed equally and shared first authorship.
Contributor Information
Kundan Kumar Chaubey, Email: kundan2006chaubey@gmail.com.
Sanjay Kumar, Email: drsanjaykumar82@gmail.com.
References
- 1.WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int. Accessed 21 June 2023
- 2.Hafeez A, Ahmad S, Siddqui SA, et al. A review of COVID-19 (Coronavirus Disease-2019) diagnosis, treatments and prevention. Euras J Med Oncol. 2020;4:116–125. doi: 10.14744/ejmo.2020.90853. [DOI] [Google Scholar]
- 3.Yang Y, Peng F, Wang R, et al. The deadly coronaviruses: the 2003 SARS pandemic and the 2020 novel coronavirus epidemic in China. J Autoimmun. 2020;109:102434. doi: 10.1016/j.jaut.2020.102434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thakur V, Bhola S, Thakur P, et al. Waves and variants of SARS-CoV-2: understanding the causes and effect of the COVID-19 catastrophe. Infection. 2022;50:309–325. doi: 10.1007/s15010-021-01734-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fiolet T, Kherabi Y, MacDonald C-J, et al. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review. Clin Microbiol Infect. 2022;28:202–221. doi: 10.1016/j.cmi.2021.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV) Biosci Trends. 2020;14:69–71. doi: 10.5582/bst.2020.01020. [DOI] [PubMed] [Google Scholar]
- 7.Shereen MA, Khan S, Kazmi A, et al. COVID-19 infection: emergence, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sironi M, Hasnain SE, Rosenthal B, et al. SARS-CoV-2 and COVID-19: A genetic, epidemiological, and evolutionary perspective. Infect Genet Evol. 2020;84:104384. doi: 10.1016/j.meegid.2020.104384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sungnak W, Huang N, Bécavin C, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corvillo F, Ceccarini G, Nozal P, et al. Immunological features of patients affected by Barraquer–Simons syndrome. Orphanet J Rare Dis. 2020;15:9. doi: 10.1186/s13023-019-1292-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng X, Hackbart M, Mettelman RC, et al. Coronavirus nonstructural protein 15 mediates evasion of dsRNA sensors and limits apoptosis in macrophages. Proc Natl Acad Sci. 2017;114:E4251–E4260. doi: 10.1073/pnas.1618310114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mani JS, Johnson JB, Steel JC, et al. Natural product-derived phytochemicals as potential agents against coronaviruses: a review. Virus Res. 2020;284:197989. doi: 10.1016/j.virusres.2020.197989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cosar B, Karagulleoglu ZY, Unal S, et al. SARS-CoV-2 mutations and their viral variants. Cytokine Growth Factor Rev. 2022;63:10–22. doi: 10.1016/j.cytogfr.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korber B, Fischer WM, Gnanakaran S, et al. Tracking changes in SARS-CoV-2 Spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;182:812–827.e19. doi: 10.1016/j.cell.2020.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yurkovetskiy L, Wang X, Pascal KE, et al. Structural and functional analysis of the D614G SARS-CoV-2 spike protein variant. Cell. 2020;183:739–751.e8. doi: 10.1016/j.cell.2020.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shahhosseini N, Babuadze GG, Wong G, Kobinger GP. Mutation signatures and in silico docking of novel SARS-CoV-2 variants of concern. Microorganisms. 2021;9:926. doi: 10.3390/microorganisms9050926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLean G, Kamil J, Lee B, et al. The impact of evolving SARS-CoV-2 mutations and variants on COVID-19 vaccines. MBio. 2022;13:e0297921. doi: 10.1128/mbio.02979-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merad M, Blish CA, Sallusto F, Iwasaki A. The immunology and immunopathology of COVID-19. Science. 2022;375:1122–1127. doi: 10.1126/science.abm8108. [DOI] [PubMed] [Google Scholar]
- 20.Gibellini L, De Biasi S, Paolini A, et al. Altered bioenergetics and mitochondrial dysfunction of monocytes in patients with COVID-19 pneumonia. EMBO Mol Med. 2020;12:e13001. doi: 10.15252/emmm.202013001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milani D, Caruso L, Zauli E, et al. p53/NF-kB balance in SARS-CoV-2 infection: from OMICs, genomics and pharmacogenomics insights to tailored therapeutic perspectives (COVIDomics) Front Pharmacol. 2022;13:1. doi: 10.3389/fphar.2022.871583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bastard P, Zhang Q, Zhang S-Y, et al. Type I interferons and SARS-CoV-2: from cells to organisms. Curr Opin Immunol. 2022;74:172–182. doi: 10.1016/j.coi.2022.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang C, Wu Z, Li J-W, et al. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55:105954. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karn V, Ahmed S, Tsai L-W, et al. Extracellular vesicle-based therapy for COVID-19: promises, challenges and future prospects. Biomedicines. 2021;9:1373. doi: 10.3390/biomedicines9101373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagoor Meeran MF, Javed H, Sharma C, et al. Can Echinacea be a potential candidate to target immunity, inflammation, and infection—the trinity of coronavirus disease 2019. Heliyon. 2021;7:e05990. doi: 10.1016/j.heliyon.2021.e05990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jaiswara PK, Shukla SK. Chemotherapy-Mediated Neuronal Aberration. Pharmaceuticals (Basel) 2023;16:1165. doi: 10.3390/ph16081165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shukla SK, Dasgupta A, Mehla K, et al. Silibinin-mediated metabolic reprogramming attenuates pancreatic cancer-induced cachexia and tumor growth. Oncotarget. 2015;6:41146–41161. doi: 10.18632/oncotarget.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fylaktakidou KC, Hadjipavlou-Litina DJ, Litinas KE, Nicolaides DN. Natural and synthetic coumarin derivatives with anti-inflammatory/ antioxidant activities. Curr Pharm Des. 2004;10:3813–3833. doi: 10.2174/1381612043382710. [DOI] [PubMed] [Google Scholar]
- 29.Antonelli M, Donelli D, Firenzuoli F. Ginseng integrative supplementation for seasonal acute upper respiratory infections: a systematic review and meta-analysis. Complement Ther Med. 2020;52:102457. doi: 10.1016/j.ctim.2020.102457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petitjean SJL, Lecocq M, Lelong C, et al. Salvia miltiorrhiza Bunge as a potential natural compound against COVID-19. Cells. 2022;11:1311. doi: 10.3390/cells11081311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thuy BTP, My TTA, Hai NTT, et al. Investigation into SARS-CoV-2 resistance of compounds in garlic essential oil. ACS Omega. 2020;5:8312–8320. doi: 10.1021/acsomega.0c00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silveira D, Prieto-Garcia JM, Boylan F, et al. COVID-19: is there evidence for the use of herbal medicines as adjuvant symptomatic therapy? Front Pharmacol. 2020;11:581840. doi: 10.3389/fphar.2020.581840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lane KE, Wilson M, Hellon TG, Davies IG. Bioavailability and conversion of plant based sources of omega-3 fatty acids - a scoping review to update supplementation options for vegetarians and vegans. Crit Rev Food Sci Nutr. 2022;62:4982–4997. doi: 10.1080/10408398.2021.1880364. [DOI] [PubMed] [Google Scholar]
- 34.Lordan R, Rando HM, COVID-19 Review Consortium, Greene CS (2021) Dietary Supplements and Nutraceuticals under Investigation for COVID-19 Prevention and Treatment. mSystems 6:1. 10.1128/msystems.00122-21 [DOI] [PMC free article] [PubMed]
- 35.Hathaway D, Pandav K, Patel M, et al. Omega 3 fatty acids and COVID-19: a comprehensive review. Infect Chemother. 2020;52:478–495. doi: 10.3947/ic.2020.52.4.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ranucci M, Ballotta A, Di Dedda U, et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost. 2020;18:1747–1751. doi: 10.1111/jth.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aghababaei F, Hadidi M. Recent advances in potential health benefits of quercetin. Pharmaceuticals. 2023;16:1020. doi: 10.3390/ph16071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Önal H, Arslan B, Üçüncü Ergun N, et al. Treatment of COVID-19 patients with quercetin: a prospective, single center, randomized, controlled trial. Turk J Biol. 2021;45:518–529. doi: 10.3906/biy-2104-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song JH, Shim JK, Choi HJ. Quercetin 7-rhamnoside reduces porcine epidemic diarrhea virus replication via independent pathway of viral induced reactive oxygen species. Virol J. 2011;8:460. doi: 10.1186/1743-422X-8-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roh C, Jo SK. Quantitative and sensitive detection of SARS coronavirus nucleocapsid protein using quantum dots-conjugated RNA aptamer on chip. J Chem Technol Biotechnol. 2011;86:1475–1479. doi: 10.1002/jctb.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song YH, Kim DW, Curtis-Long MJ, et al. Papain-Like Protease (PLpro) Inhibitory Effects of Cinnamic Amides from Tribulus terrestris Fruits. Biol Pharm Bull. 2014;37:1021–1028. doi: 10.1248/bpb.b14-00026. [DOI] [PubMed] [Google Scholar]
- 42.Park J-Y, Kim JH, Kim YM, et al. Tanshinones as selective and slow-binding inhibitors for SARS-CoV cysteine proteases. Bioorg Med Chem. 2012;20:5928–5935. doi: 10.1016/j.bmc.2012.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Müller C, Schulte FW, Lange-Grünweller K, et al. Broad-spectrum antiviral activity of the eIF4A inhibitor silvestrol against corona- and picornaviruses. Antiviral Res. 2018;150:123–129. doi: 10.1016/j.antiviral.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Q, Xiang R, Huo S, et al. Molecular mechanism of interaction between SARS-CoV-2 and host cells and interventional therapy. Signal Transduct Target Ther. 2021;6:233. doi: 10.1038/s41392-021-00653-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharifi-Rad J, Kamiloglu S, Yeskaliyeva B, et al. Pharmacological activities of psoralidin: a comprehensive review of the molecular mechanisms of action. Front Pharmacol. 2020;11:1. doi: 10.3389/fphar.2020.571459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cho JK, Curtis-Long MJ, Lee KH, et al. Geranylated flavonoids displaying SARS-CoV papain-like protease inhibition from the fruits of Paulownia tomentosa. Bioorg Med Chem. 2013;21:3051–3057. doi: 10.1016/j.bmc.2013.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ding Y, Wang H, Shen H, et al. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J Pathol. 2003;200:282–289. doi: 10.1002/path.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ebob OT, Babiaka SB, Ntie-Kang F (2021) Natural products as potential lead compounds for drug discovery against SARS-CoV-2. http://www.xml-data.org/YYTRCW/html/2021/6/1639119100996-314880077.htm. Accessed 22 Nov 2022 [DOI] [PMC free article] [PubMed]
- 49.Majnooni MB, Fakhri S, Shokoohinia Y, et al. Isofraxidin: synthesis, biosynthesis, isolation, pharmacokinetic and pharmacological properties. Molecules. 2020;25:2040. doi: 10.3390/molecules25092040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yi L, Li Z, Yuan K, et al. Small molecules blocking the entry of severe acute respiratory syndrome coronavirus into host cells. J Virol. 2004;78:11334–11339. doi: 10.1128/JVI.78.20.11334-11339.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jia Q, Fu J, Liang P, et al. Investigating interactions between chloroquine/hydroxychloroquine and their single enantiomers and angiotensin-converting enzyme 2 by a cell membrane chromatography method. J Sep Sci. 2022;45:456–467. doi: 10.1002/jssc.202100570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benarba B, Pandiella A. Medicinal plants as sources of active molecules against COVID-19. Front Pharmacol. 2020;11:1189. doi: 10.3389/fphar.2020.01189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park J-Y, Yuk HJ, Ryu HW, et al. Evaluation of polyphenols from Broussonetia papyrifera as coronavirus protease inhibitors. J Enzyme Inhib Med Chem. 2017;32:504–515. doi: 10.1080/14756366.2016.1265519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Musa MA, Cooperwood JS, Khan MOF. A review of coumarin derivatives in pharmacotherapy of breast cancer. Curr Med Chem. 2008;15:2664–2679. doi: 10.2174/092986708786242877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seif F, Khoshmirsafa M, Aazami H, et al. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun Signal. 2017;15:23. doi: 10.1186/s12964-017-0177-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Javed M, Saleem A, Xaveria A, Akhtar MF. Daphnetin: a bioactive natural coumarin with diverse therapeutic potentials. Front Pharmacol. 2022;13:1. doi: 10.3389/fphar.2022.993562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu P-J, Li J-R, Zhu Z-G, et al. Praeruptorin D and E attenuate lipopolysaccharide/hydrochloric acid induced acute lung injury in mice. Eur J Pharmacol. 2013;710:39–48. doi: 10.1016/j.ejphar.2013.03.050. [DOI] [PubMed] [Google Scholar]
- 58.Lee H-C, Liu F-C, Tsai C-N, et al. Esculetin ameliorates lipopolysaccharide-induced acute lung injury in mice via modulation of the AKT/ERK/NF-κB and RORγt/IL-17 pathways. Inflammation. 2020;43:962–974. doi: 10.1007/s10753-020-01182-4. [DOI] [PubMed] [Google Scholar]
- 59.Jin L, Ying Z-H, Yu C-H, et al. Isofraxidin ameliorated influenza viral inflammation in rodents via inhibiting platelet aggregation. Int Immunopharmacol. 2020;84:106521. doi: 10.1016/j.intimp.2020.106521. [DOI] [PubMed] [Google Scholar]
- 60.Grant OC, Montgomery D, Ito K, Woods RJ. Analysis of the SARS-CoV-2 spike protein glycan shield reveals implications for immune recognition. Sci Rep. 2020;10:14991. doi: 10.1038/s41598-020-71748-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.D’Aoust M-A, Couture MM-J, Charland N, et al. The production of hemagglutinin-based virus-like particles in plants: a rapid, efficient and safe response to pandemic influenza. Plant Biotechnol J. 2010;8:607–619. doi: 10.1111/j.1467-7652.2009.00496.x. [DOI] [PubMed] [Google Scholar]
- 62.Sa-ngiamsuntorn K, Suksatu A, Pewkliang Y, et al. Anti-SARS-CoV-2 activity of andrographis paniculata extract and its major component andrographolide in human lung epithelial cells and cytotoxicity evaluation in major organ cell representatives. J Nat Prod. 2021;84:1261–1270. doi: 10.1021/acs.jnatprod.0c01324. [DOI] [PubMed] [Google Scholar]
- 63.Intharuksa A, Arunotayanun W, Yooin W, Sirisa-Ard P. A Comprehensive review of Andrographis paniculata (Burm. F.) nees and its constituents as potential lead compounds for COVID-19 drug discovery. Molecules. 2022;27:4479. doi: 10.3390/molecules27144479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sampangi-Ramaiah MH, Vishwakarma R, Shaanker RU. Molecular docking analysis of selected natural products from plants for inhibition of SARS-CoV-2 main protease. Curr Sci. 2020;118:7. doi: 10.18520/cs/v118/i7/1087-1092. [DOI] [Google Scholar]
- 65.Maurya DK, Sharma D. Evaluation of traditional ayurvedic Kadha for prevention and management of the novel Coronavirus (SARS-CoV-2) using in silico approach. J Biomol Struct Dyn. 2022;40(9):3949–3964. doi: 10.1080/07391102.2020.1852119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Varshney KK, Varshney M, Nath B (2020) Molecular modeling of isolated phytochemicals from Ocimum sanctum towards exploring potential inhibitors of SARS coronavirus main protease and papain-like protease to treat COVID-19
- 67.Jindal D, Rani V. In silico studies of phytoconstituents from Piper longum and Ocimum sanctum as ACE2 and TMRSS2 inhibitors: strategies to combat COVID-19. Appl Biochem Biotechnol. 2023;195:2618–2635. doi: 10.1007/s12010-022-03827-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Patwardhan B, Chavan-Gautam P, Gautam M, et al. Ayurveda rasayana in prophylaxis of COVID-19. Curr Sci. 2020;118:3. [Google Scholar]
- 69.Jamal QMS. Antiviral Potential of Plants against COVID-19 during Outbreaks—An Update. Int J Mol Sci. 2022;23:13564. doi: 10.3390/ijms232113564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mondal S, Varma S, Bamola VD, et al. Double-blinded randomized controlled trial for immunomodulatory effects of Tulsi (Ocimum sanctum Linn.) leaf extract on healthy volunteers. J Ethnopharmacol. 2011;136:452–456. doi: 10.1016/j.jep.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 71.Sharma U, Bala M, Kumar N, et al. Immunomodulatory active compounds from Tinospora cordifolia. J Ethnopharmacol. 2012;141:918–926. doi: 10.1016/j.jep.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 72.Chowdhury P. In silico investigation of phytoconstituents from Indian medicinal herb “Tinospora cordifolia (giloy)” against SARS-CoV-2 (COVID-19) by molecular dynamics approach. J Biomol Struct Dyn. 2021;39:6792–6809. doi: 10.1080/07391102.2020.1803968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Balkrishna A, Pokhrel S, Varshney A. Tinocordiside from Tinospora cordifolia (Giloy) may curb SARS-CoV-2 contagion by disrupting the electrostatic interactions between host ACE2 and viral S-protein receptor binding domain. Comb Chem High Throughput Screen. 2021;24:1795–1802. doi: 10.2174/1386207323666201110152615. [DOI] [PubMed] [Google Scholar]
- 74.Sagar V, Kumar AH. Efficacy of natural compounds from Tinospora cordifolia against SARS-CoV-2 protease, surface glycoprotein and RNA polymerase. Biol Eng Med Sci Rep. 2020;6:06–08. doi: 10.5530/bems.6.1.2. [DOI] [Google Scholar]
- 75.Fu X, Wang Z, Li L, et al. Novel chemical ligands to ebola virus and marburg virus nucleoproteins identified by combining affinity mass spectrometry and metabolomics approaches. Sci Rep. 2016;6:29680. doi: 10.1038/srep29680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Padiya R, Banerjee SK. Garlic as an anti-diabetic agent: recent progress and patent reviews. Recent Pat Food Nutr Agric. 2013;5:105–127. doi: 10.2174/18761429113059990002. [DOI] [PubMed] [Google Scholar]
- 77.Donma MM, Donma O. The effects of allium sativum on immunity within the scope of COVID-19 infection. Med Hypotheses. 2020;144:109934. doi: 10.1016/j.mehy.2020.109934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Khubber S, Hashemifesharaki R, Mohammadi M, Gharibzahedi SMT. Garlic (Allium sativum L): a potential unique therapeutic food rich in organosulfur and flavonoid compounds to fight with COVID-19. Nutrit J. 2020;19:124. doi: 10.1186/s12937-020-00643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pandey P, Khan F, Kumar A, et al. Screening of potent inhibitors against 2019 novel coronavirus (Covid-19) from Alliumsativum and Allium cepa: an in silico approach. Biointerface Research in Applied Chemistry. 2021;1:7981–7993. [Google Scholar]
- 80.Ansari M, Porouhan P, Mohammadianpanah M, et al. Efficacy of ginger in control of chemotherapy induced nausea and vomiting in breast cancer patients receiving doxorubicin-based chemotherapy. Asian Pac J Cancer Prev. 2016;17:3877–3880. [PubMed] [Google Scholar]
- 81.Goswami D, Kumar M, Ghosh SK, Das (2020) A natural product compounds in alpinia officinarum and ginger are potent SARS-CoV-2 papain-like protease inhibitors
- 82.Padilla-S L, Rodríguez A, Gonzales MM, et al. Inhibitory effects of curcumin on dengue virus type 2-infected cells in vitro. Arch Virol. 2014;159:573–579. doi: 10.1007/s00705-013-1849-6. [DOI] [PubMed] [Google Scholar]
- 83.Han S, Xu J, Guo X, Huang M. Curcumin ameliorates severe influenza pneumonia via attenuating lung injury and regulating macrophage cytokines production. Clin Exp Pharmacol Physiol. 2018;45:84–93. doi: 10.1111/1440-1681.12848. [DOI] [PubMed] [Google Scholar]
- 84.Zahedipour F, Hosseini SA, Sathyapalan T, et al. Potential effects of curcumin in the treatment of COVID-19 infection. Phytother Res. 2020;34:2911–2920. doi: 10.1002/ptr.6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maurya VK, Kumar S, Prasad AK, et al. Structure-based drug designing for potential antiviral activity of selected natural products from Ayurveda against SARS-CoV-2 spike glycoprotein and its cellular receptor. Virusdisease. 2020;31:179–193. doi: 10.1007/s13337-020-00598-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Khaerunnisa S, Kurniawan H, Awaluddin R, et al (2020) Potential inhibitor of COVID-19 main protease (Mpro) from several medicinal plant compounds by molecular docking study. 10.20944/preprints202003.0226.v1
- 87.Dorri M, Hashemitabar S, Hosseinzadeh H. Cinnamon (Cinnamomum zeylanicum) as an antidote or a protective agent against natural or chemical toxicities: a review. Drug Chem Toxicol. 2018;41:338–351. doi: 10.1080/01480545.2017.1417995. [DOI] [PubMed] [Google Scholar]
- 88.Ibrahim IM, Abdelmalek DH, Elfiky AA. GRP78: a cell’s response to stress. Life Sci. 2019;226:156–163. doi: 10.1016/j.lfs.2019.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ibrahim IM, Abdelmalek DH, Elshahat ME, Elfiky AA. COVID-19 spike-host cell receptor GRP78 binding site prediction. J Infect. 2020;80:554–562. doi: 10.1016/j.jinf.2020.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Neto JGO, Boechat SK, Romão JS, et al. Treatment with cinnamaldehyde reduces the visceral adiposity and regulates lipid metabolism, autophagy and endoplasmic reticulum stress in the liver of a rat model of early obesity. J Nutr Biochem. 2020;77:108321. doi: 10.1016/j.jnutbio.2019.108321. [DOI] [PubMed] [Google Scholar]
- 91.Elfiky AA. Natural products may interfere with SARS-CoV-2 attachment to the host cell. J Biomol Struct Dyn. 2021;39:3194–3203. doi: 10.1080/07391102.2020.1761881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vijayakumar M, Janani B, Kannappan P, et al. In silico identification of potential inhibitors against main protease of SARS-CoV-2 6LU7 from Andrographis panniculata via molecular docking, binding energy calculations and molecular dynamics simulation studies. Saudi J Biol Sci. 2022;29:18–29. doi: 10.1016/j.sjbs.2021.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gautam S, Gautam A, Chhetri S, Bhattarai U. Immunity against COVID-19: Potential role of Ayush Kwath. J Ayurveda Integrat Med. 2022;13:100350. doi: 10.1016/j.jaim.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stohs SJ, Hartman MJ. Review of the Safety and Efficacy of Moringa oleifera. Phytother Res. 2015;29:796–804. doi: 10.1002/ptr.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Umar HI, Josiah SS, Saliu TP, et al. In-silico analysis of the inhibition of the SARS-CoV-2 main protease by some active compounds from selected African plants. J Taibah Univ Med Sci. 2021;16:162–176. doi: 10.1016/j.jtumed.2020.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gupta SC, Prasad S, Tyagi AK, et al. Neem (Azadirachta indica): an indian traditional panacea with modern molecular basis. Phytomedicine. 2017;34:14–20. doi: 10.1016/j.phymed.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 97.Subramanian S (2020) Nearly 20+ compounds in Neem leaves extract exhibit high binding affinity with some of them as high as −14.3 kcal/mol against COVID-19 main protease (Mpro) : a molecular docking study
- 98.Baildya N, Khan AA, Ghosh NN, et al. Screening of potential drug from Azadirachta Indica (Neem) extracts for SARS-CoV-2: an insight from molecular docking and MD-simulation studies. J Mol Struct. 2021;1227:129390. doi: 10.1016/j.molstruc.2020.129390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dwivedi VD, Bharadwaj S, Afroz S, et al. Anti-dengue infectivity evaluation of bioflavonoid from Azadirachta indica by dengue virus serine protease inhibition. J Biomol Struct Dyn. 2021;39:1417–1430. doi: 10.1080/07391102.2020.1734485. [DOI] [PubMed] [Google Scholar]
- 100.Prasanth MI, Sivamaruthi BS, Chaiyasut C, Tencomnao T. A Review of the Role of Green Tea (Camellia sinensis) in Antiphotoaging, Stress Resistance, Neuroprotection, and Autophagy. Nutrients. 2019;11:474. doi: 10.3390/nu11020474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kanbarkar N, Mishra S. Matrix metalloproteinase inhibitors identified from Camellia sinensis for COVID-19 prophylaxis: an in silico approach. Adv Tradit Med. 2021;21:173–188. doi: 10.1007/s13596-020-00508-9. [DOI] [Google Scholar]
- 102.Upadhyay S, Tripathi PK, Singh M, et al. Evaluation of medicinal herbs as a potential therapeutic option against SARS-CoV-2 targeting its main protease. Phytother Res. 2020;34:3411–3419. doi: 10.1002/ptr.6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chowdhury P, Barooah AK. Tea bioactive modulate innate immunity: in perception to COVID-19 pandemic. Front Immunol. 2020;11:1. doi: 10.3389/fimmu.2020.590716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Farzana M, Shahriar S, Jeba FR, et al. Functional food: complementary to fight against COVID-19. Beni Suef Univ J Basic Appl Sci. 2022;11:33. doi: 10.1186/s43088-022-00217-z. [DOI] [PMC free article] [PubMed] [Google Scholar]