Abstract
Health-related quality of life (HRQoL) can be assessed through measures that can be generic or disease specific, encompass several independent scales, or employ holistic assessment (i.e., the derivation of composite scores). HRQoL measures may identify patients with differential risk profiles. However, the usefulness of generic and holistic HRQoL measures in identifying patients at higher risk of death is unclear. The aim of the present study was to undertake a scoping review of generic, holistic assessments of HRQoL as predictors of mortality in general non-patient populations and clinical sub-populations with specified conditions or risk factors in persons 18 years or older. Five databases were searched from 18 June to 29 June 2020 to identify peer-reviewed published articles. The searches were updated in August 2022. Reference lists of included and cited articles were also searched. Of 2552 articles screened, 110 met criteria for inclusion. Over one-third of studies were from North America. Most studies pertained to sub-populations with specified conditions and/or risk factors, almost a quarter for people with cardiovascular diseases. There were no studies pertaining to people with mental health conditions. Nearly three-quarters of the studies used a RAND Corporation QoL instrument, predominantly the SF-36, and nearly a quarter, a utility instrument, predominantly the EQ-5D. HRQoL was associated with mortality in 67 of 72 univariate analyses (92%) and 100 of 109 multivariate analyses (92%). HRQoL was found to be associated with mortality in the general population and clinical sub-populations with physical health conditions. Whether this relationship holds in people with mental health conditions is not known. HRQoL assessment may be useful for screening and/or monitoring purposes to understand how people perceive their health and well-being and as an indicator of mortality risk, encouraging better-quality and timely patient care to support and maximize what may be a patient’s only modifiable outcome.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11606-023-08380-4.
KEYWORDS: health-related quality of life, health states, utility, mortality, predictive model
INTRODUCTION
Quality of life (QoL) as a measure of a patient’s well-being or overall health began to be used for making medical decisions in the 1970s,1 particularly for survival decisions.2 In the 1990s, patients’ own evaluations of their health status came to be considered in relation to the QoL associated with health aspects of the disease and/or its treatment as perceived by the patient.3 The term health-related QoL (HRQoL) was then conceived.3 HRQoL is an important outcome with inherent value when treating patients or improving patient care. Assessing HRQoL can guide decision-making on treatments at population and patient levels,4 and predict the success of treatment.1
A variety of instruments have been developed for assessing HRQoL. Some instruments are generic (for use in populations or sub-populations, irrespective of illness or conditions), while others are disease specific (for use in populations with a specific disease).1 Further, some instruments allow for derivation of a score as a holistic or composite assessment of the individual’s health status, while other instruments employ multiple independent scales that are reported separately. For multi-attribute utility instruments, which provide generic and holistic assessments, a single score or “utility” is obtained through a preference-based assessment of health states determined from responses to multiple items and/or dimensions.
An association between HRQoL with mortality in the general non-patient population has been systematically reviewed.5 However, HRQoL was predominantly assessed as one of multiple possible attributes or dimensions of the individual’s life.5 An association between disease-specific assessments of HRQoL and mortality has been reviewed in patients with heart failure.6,7 The ability of utilities to predict future morbidity and mortality is also being explored. Clarke and colleagues8 reported that index scores derived from the EuroQol Five Dimensions questionnaire (EQ-5D) can be used to independently identify diabetic patients at higher risk of diabetic complications and death, while querying other instruments predictive abilities. Clarke et al. also found cumulative and/or extreme problems were important in identifying high-risk patients, a determination that could only be pursued through a holistic summary measure.
Despite the available evidence, information on the relationship between holistic assessments of health status assessed with generic instruments and mortality in both general non-patient populations and clinical sub-populations has not been reported. Therefore, based on these findings and queries, and previous work on HRQoL in people with psychotic disorders,9,10 a sub-population in which there is significant premature mortality,11 the aim of this scoping review is to map the evidence on generic, holistic assessments of HRQoL as predictors of mortality in general non-patient populations and clinical sub-populations. We also aim to ascertain conditions where this relationship has been examined, instruments and statistical methods employed, findings of those analyses, and in turn identify gaps.
METHODS
The present scoping review follows the methodology of the Joanna Briggs Institute for scoping reviews12,13 and recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for scoping reviews (PRISMA-ScR).14 The protocol of the review was registered with the Open Science Framework (https://osf.io/vdqga) on 18 June 2020.
Data Sources and Searches
Five electronic databases (CINAHL (via EBSCOhost), EMBASE (via Ovid), Science Direct, Web of Science, and PubMed) were searched from 18 to 29 June 2020 and updated in August 2022. Searches were limited to English language with no time restrictions; syntax, filters, and Boolean operators were employed. The search strategy for PubMed is presented in the Supplementary Table 1. Records obtained were exported to EndNote X8 and duplicates deleted. The reference lists of selected articles and literature cited in the “Introduction” section were searched to identify additional articles.
Study Selection
Titles and abstracts of identified studies were screened by two independent reviewers (A.N.F. and A.N.) with full texts of selected articles and then assessed for eligibility. For inclusion, studies needed to be peer-reviewed published articles that considered generic HRQoL scores as predictors of mortality in the general population and/or sub-populations with specified conditions or risk factors, in persons aged 18 years or older. HRQoL scores needed to be assessed with a recognized and complete HRQoL generic instrument, and a holistic score provided. For instruments that assessed mental and physical components separately, both summary scores needed to be provided. Methods for the collection/reporting of mortality needed to be specified. Further, the methodology used for the assessment of predictors had to be detailed and the findings of whether HRQoL scores predicted mortality clearly stated. Studies could be conducted in any contextual setting except prison, and in any geographic location. Study designs included were as follows: case-control; cross-sectional with follow-up; retrospective and prospective cohort; randomized and non-randomized controlled trials; and quasi-experimental studies. Systematic reviews and meta-analysis were excluded.
Data Extraction and Synthesis of Evidence
The data charting form was developed by A.N.F. and A.N.; then, A.N.F. charted the data under the guidance of A.N. During extraction, results were discussed by the two reviewers and the data charting form modified to include other items of relevance to this review. Inconsistencies and disagreements were resolved by consensus; when that was not possible, K.J.C. acted as a third reviewer. Variables identified for extraction a priori were as follows: author(s), year of publication, country of origin, study population, sample size, design, instruments used, statistical method, and key findings (by type of analysis: univariate, multivariate), and during extraction: age, sex, and timeframe (i.e., follow-up). Univariate analyses were included as “proof of concept” of the overall association between HRQoL and mortality. Multivariate analyses were included as evidence of an independent association after adjustment for a range of other factors. Data extracted from each article were grouped with reference to the study population on the basis that health conditions should be given primary consideration when assessing predictors of mortality. In justification, in 2019, nearly half of global deaths (44%) and four-fifths (80%) of the top ten causes of death were due to noncommunicable causes.15 Given the heterogeneity of the results and the objective of this scoping review, a narrative synthesis was then undertaken to synthesize the findings of included studies. Specifically, a descriptive summary of studies is provided to address the review’s aims.12
RESULTS
In June 2020, 2552 records were identified through the database searches; an additional 569 records were identified in August 2022. Of a total of 3121 records, 1404 were duplicates and deleted. Title and abstract of 1717 records were then screened with 190 identified as requiring full-text review. Of these 190 articles, 115 did not meet inclusion criteria, including 54 that used a disease-specific instrument to measure HRQoL and 21 that did not employ holistic assessments; 76 articles were thus eligible for inclusion (Fig. 1). In a final step, 99 references from reference lists of eligible articles and 25 references identified within articles cited in the “Introduction” section of this study were examined, with the full-text obtained and reviewed for 37 and 7 respectively, with 31 and 4 identified as eligible. Thus, 110 articles were included in this review, for which the general characteristics and descriptive summary are presented in Tables 1 and 2, respectively.
Fig. 1.
Prisma diagram of study selection.
Table 1.
General Characteristics of Included Articles (N=110)
| Characteristic | Number | (%) |
|---|---|---|
| Publication year | ||
| ≤2000 | 2 | (1.8%) |
| 2001–2005 | 14 | (12.7%) |
| 2006–2010 | 37 | (33.6%) |
| 2011–2015 | 26 | (23.6%) |
| 2016–2020 | 25 | (22.7%) |
| 2021–2022 | 6 | (5.5%) |
| World region of publication* | ||
| Europe | 42 | (38.2%) |
| North America | 47 | (42.7%) |
| South America | 1 | (0.9%) |
| Western Pacific | 9 | (8.2%) |
| Various countries | 9 | (8.2%) |
| Not available | 2 | (1.8%) |
| Study population | ||
| General population | 7 | (6.4%) |
| Older persons (including veterans) | 16 | (14.5%) |
| Postmenopausal women | 1 | (0.9%) |
| Clinical sub-populations | ||
| Admitted to intensive care/emergency departments | 9 | (8.2%) |
| Cancer-related | 14 | (12.7%) |
| Cardiovascular diseases | 24 | (21.8%) |
| Dementia | 1 | (0.9%) |
| Diabetes | 4 | (3.6%) |
| Human immunodeficiency virus | 1 | (0.9%) |
| Kidney diseases, dialysis, and haemodialysis | 17 | (15.5%) |
| Liver diseases | 2 | (1.8%) |
| Musculoskeletal | 5 | (4.5%) |
| Neurological disorders | 2 | (1.8%) |
| Respiratory diseases | 6 | (5.5%) |
| Systemic lupus erythematosus | 1 | (0.9%) |
| Instrument employed† | ||
| RAND Corporation QoL surveys | 86 | (78.2%) |
| EuroQol five dimensions questionnaire (EQ-5D)‡ | 20 | (18.8%) |
| EQ-5D Visual Analogue Scale | 18 | (16.7%) |
| Health Utilities Index Mark 3 | 2 | (1.8%) |
| Minimum Data Set Health Status Index | 1 | (0.9%) |
| Nottingham Health Profile | 1 | (0.9%) |
| The 15-Dimensional instrument | 1 | (0.9%) |
| Statistical methods (univariate analyses) | ||
| Cox proportional hazards regression | 43 | (39.1%) |
| Logistic regression | 17 | (15.5%) |
| Kaplan–Meier estimator | 9 | (8.2%) |
| Gehan generalized Wilcoxon test | 1 | (0.9%) |
| Linear regression | 1 | (0.9%) |
| Log rank test P value | 1 | (0.9%) |
| Non specified regression | 1 | (0.9%) |
| Not provided | 37 | (33.6%) |
| Statistical methods (multivariate analyses) | ||
| Cox proportional hazards regression | 79 | (71.8%) |
| Logistic regression | 25 | (22.7%) |
| Fine and Gray competing risks regression | 1 | (0.9%) |
| Forecasting models§ | 1 | (0.9%) |
| Logistic regression and Cox proportional hazards | 1 | (0.9%) |
| Non specified regression | 1 | (0.9%) |
| Proportional subdistribution hazards | 1 | (0.9%) |
| Not presented | 1 | (0.9%) |
| Univariate prediction of mortality | ||
| No | 5 | (4.5%) |
| Yes (including those varying by model‖) | 47 | (42.7%) |
| SF physical component score | 19 | (17.3%) |
| SF mental component score | 1 | (0.9%) |
| Not presented | 38 | (34.5%) |
| Multivariate prediction of mortality | ||
| No | 9 | (8.2%) |
| Yes (including those varying by model/instrument) | 71 | (64.5%) |
| SF physical component score | 24 | (21.8%) |
| SF mental component score | 5 | (4.5%) |
| Not presented | 1 | (0.9%) |
*According to the World Health Organization regions
†Sixteen studies used two or more instruments
‡Included predicted EQ-5D
§Deep neural networks model, K nearest neighbor algorithm, support vector machine, naïve Bayes classifier, and Cox regression model
‖Some studies included different models in their analyses with variation in results
Table 2.
Descriptive Summary of Studies Included in the Scoping Review Organized by Year of Publication
| Study characteristics | Prediction of mortality by analysis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| StudyReference year | Location | Sample size, n | Age* | Sex† (%) | Population | Instrument(s) | Timeframe‡ | Univariate/comment | Multivariate/comment | ||
| Deoreo90 1997 | USA | 1000 | 58.2 | 50% | On dialysis | SF-36§ | 2.0 | NA | Y | Only PCS | |
| Rumsfeld et al.79 1999 | USA | 2480 | 63 | 1% | After CABG | SF-36 | 0.6 | Y | Only PCS | Y | Only PCS |
| Curtis et al.65 2002 | USA | 1778 | 64.1 | 24.6% | After CABG | SF-36 | 2.0 | Y | Only PCS | Y | |
| Fan et al.38 2002 | USA | 10 947 | 67.8 | 3% | Veterans on primary care | SF-36 | 2.0 | Y | Y | ||
| Domingo-Salvany et al.118 2002 | Spain | 312 | 65 | 100% | W/ COPD | SF-36 | 4.8 | Y | Only PCS | Y | Only PCS |
| del Aguila et al.114 2003 | USA | 180 | 61.3 | 46.1% | W/ ALS | SF-36 | 4.0 | N | Y | Only PCS | |
| Knight et al.95 2003 | USA | 14 815 | 61 | 47.2% | On haemodial. | SF-36 | 1 & 2 | NA | Y | ||
| Lowrie et al.99 2003 | USA | 13 952 | 59 | 48.6% | On dialysis | SF-36 | 0.6 | NA | Y | ||
| Fan et al.28 2004 | USA | 7702 | 65.4 | 3.4% | Veterans on primary care | SF-36 | 1.0 | Y | Y | ||
| Lopez Revuelta et al.98 2004 | Spain | 318 | 61.9/57.0‖ | 39%/40%‖ | End-stage renal disease | SF-36 | 1 to 3 | Y | Y | Varies by model | |
| Sprenkle et al.121 2004 | USA | 8354 | 65 | 4.4% | W/ asthma or COPD | SF-36 | 1.0 | Y | Y | ||
| DeSalvo et al.26 2005 | USA | 21 762 | 64 | 3.6% | VA patient population | SF-36 | 1.0 | NA | Y | Only PCS | |
| Ho et al.67 2005 | USA | 3160 | 64 |
≥65, 1.8% <65, 1.2% |
After cardiac surgery | SF-36 | 0.5 | NA |
Y N |
Older Young |
|
| Rodriguez-Artalejo et al.45 2005 | Spain | 433 | 77.2 | 56% | Admitted to ED for HF | SF-36 | 0.22¶ | Y | Y | Only PCS | |
| Takaki et al.104 2005 | Japan | 490 | 60.3 | 33.9% | On haemodial. | SF-36 | 3.0 | Y | Y | Only PCS | |
| Singh et al.112 2005 | USA | 34 440 | 64.4 | 4% | W/ arthritis | SF-36 | 1.0 | Y | Y | ||
| Dorr et al.27 2006 | USA | 3042 | 77.9 | 54.9% | Older persons | SF-12 | 2.3 | Y | Y | ||
| Park et al.54 2006 | South Korea | 142 | 62.1 | 50% | W/ terminal Ca | EQ-5D | 18.5 d¶ | Y | Y | ||
| Piotrowicz et al.77 2006 | USA | 1058 | NA | NA | W/ EF ≥30% post-infarction | SF-12 | 1.0 | Y | Y | ||
| Valdés et al.106 2006 | Spain | 199 | 63.5 | 35.2% | On haemodial. | SF-36 | 1.0 | NA | Y | Only MCS | |
| Carusone et al.116 2007 | Canada | 347 | 85.7/84.6# | 69.2% | Respiratory infections | MDS-HSI | 30 d | N | N | ||
| Faller et al.66 2007 | Germany | 231 | 64 | 29.40% | W/ CHF | SF-36 | 2.7 | Y | Y | Varies by model | |
| Fernandez et al.124 2007 | USA | 552 | 36.8 | 89% | W/ SLE | SF-36, SF-6D | 10.0 | Y | SF-6D and SF-36 PCS | N | |
| Grignon et al.52 2007 | USA | 571 | 59.7 | 32.9% | Head/neck Ca | SF-36 | 5.0 | NA | Y | Only PCS | |
| Hofhuis et al.43 2007 | Netherlands | 451 | 71 | 38.8% | ICU admission | SF-36 | 0.5 | NA | Y | ||
| Kaplan et al.22 2007 | Canada | 12 375 | NA | 52% | Canadian people | HUI3 | 9.0 | NA | Y | ||
| Lenzen et al.73 2007 | 31 countries | 3786 | 62.8/69** | 24%/22%** | W/ CAD | EQ-5D, EQ-5D VAS | 1.0 | Y | Y | VAS, EQ-5D NA | |
| Mathews and May123 2007 | USA | 965 | 37 | 12% | HIV-infected adults | EQ-5D VAS | 4.5 | NA | Y | ||
| Tsai et al.33 2007 | Taiwan | 4424 | NA | NA | Older persons | SF-36 | 3.0 | NA | Y | ||
| Esteban119 2008 | Spain | 611 | 67.2 | NA | W/ COPD | SF-36 | 5.0 | NA | N | ||
| Halpin et al.120 2008 | NA | 1834 | 64 | 23% | W/ COPD | SF-36 | 1.0 | Y | Y | ||
| Karvonen-Gutierrez et al.53 2008 | USA | 495 | 58 | 18.4% | Head/neck Ca | SF-36 | 5.1¶ | Y | Only PCS | Y | Only PCS |
| Kroenke et al.18 2008 | USA | 40 337 | NA | 100% | Healthy women | SF-36 | 4.0 | NA | Y | ||
| Steinberg et al.81 2008 | USA | 1016 | 64 | 18% | W/ ventricular arrhythmias | SF-36 | 1.5 ± 1.0 | Y | Only PCS | Y | Only PCS |
| Thombs et al.84 2008 | Canada | 800 | 61.5 | 33.4% | W/ ACS | SF-12 | 1.0 | Y | Only PCS | Y | Varies by model |
| Cella et al.50 2009 | USA | 750 | 62/59†† | 28.5% | Renal cell Ca | EQ-5D VAS | 28 d | NA | Y | ||
| Clarke et al.8 2009 |
Australia, Finland, New Zealand |
7348 | 66.9 | 38% | W/ type 2 diabetes | EQ-5D | 5.0 | NA | Y | ||
| Grande et al.51 2009 | UK | 100 | 71.5/69.2‡‡ | 38.0% | Colorectal or lung Ca | SF-36 | 5.0 | Y | Only MCS | Y | Only MCS |
| Hayashino et al.93 2009 | Japan | 527 | 62.4 | 30.2% | On haemodial. | SF-36 | 1.9¶ | NA | Y | Only PCS | |
| McEwen et al.87 2009 | USA | 7892 | NA | 54.1% | W/ diabetes | EQ-5D | 3.7* | NA | Y | ||
| Sacanella et al.46 2009 | Spain | 230 | 74.5 | 39% | Non-elective ICU admission | EQ-5D VAS | 3.2 | Y | Y | ||
| Zhang et al.86 2009 | USA | 1785 | 53.4 | 41.20% | W/ CAD | SF-36 | 5.0 | Y | Only PCS | Y | Only PCS |
| Ashing-Giwa et al.49 2010 | USA | 353 | 51 | 100% | Cervical Ca survivors | SF-12 | 5.0 | Y | Y | ||
| Issa et al.68 2010 | Netherlands | 503 | 67 | 27% | W/ PAD | EQ-5D | 3.0 | Y | Y | ||
| Kao et al.69 2010 | Canada, USA | 507 | 64.9 | 21.7% | ICD recipients | SF-36 | 1.0 | NA | N | ||
| Kusleikaite et al.96 2010 | Lithuania | 183 | 56.7 | 43.7% | On haemodial. | SF-36 | 6.0 | Y | Y | ||
| Lacson et al.97 2010 | USA | 44 395 | 61.2 | 46.0% | On dialysis | SF-36, SF-12 | 1.0 | Y | Y | ||
| Landman et al.89 2010 | Netherlands | 1353 | 67.8 | 57.6% | W/ type 2 diabetes | SF-36 | 10.0 | NA | Y | ||
| Myint et al.19 2010 | UK | 17 736 | NA | 56.2% | Norfolk residents | SF-6D | 6.5a | NA | Y | ||
| Otero-Rodriguez et al.31 2010 | Spain | 2373 | NA | 57.6% | Older persons | SF-36 | 4 to 6 | Y | Y | Varies by model | |
| Peng et al.101 2010 | Taiwan | 888 | 57.9 | 56.2% | On haemodial. | SF-36 | 7 | Y | Only PCS | Y | Only PCS |
| Tanikella et al.107 2010 | USA | 252 | 54 | 36% | W/ portal hypertension | SF-36 | 422§§ | Y | Only PCS | Y | Only PCS |
| Zuluaga et al.47 2010 | Spain | 416 | 75.3/78.4** | 56% | ED admission | SF-36 | 7.0 | Y | Y | Only MCS | |
| Feroze et al.91 2011 | USA | 705 | 53.5 | 47% | On haemodial. | SF-36 | 6.0 | Y | Y | ||
| Haring et al.16 2011 | Germany | 4259 | 47.0/70.3** | 51% | German citizens | SF-12 | 10.0 | NA | Y | Only PCS | |
| Jerant et al.17 2011 | USA | 22 259 | NA | 53.1% | USA population | SF-6D, EQ-5D, pEQ-5D, EQ-5D VAS | 1.0 | Y | Y | ||
| Muñoz et al.20 2011 | Spain | 3724 | 54.1 | 51.9% | Spanish population | SF-12 | 6.3¶ | Y | Only PCS | Y | Only PCS |
| Pedersen et al.76 2011 | Netherlands | 870 | 62.6 | 27.8% | PCI patients | EQ-5D VAS | 1.0 | Y | Y | ||
| Szekely et al.82 2011 | Austria, Canada, Colombia, France, Germany, Hungary, India, Israel, Italy, Mexico, Poland, Romania, Thailand, Netherlands, UK, USA | 4811 | NA | 20.3% | After CABG | SF-12 | 4.0 | NA | Y | Only MCS | |
| Cavrini et al.25 2012 | Italy | 5256 | 74.5 | 54.6% | Older persons | EQ-5D, EQ-5D VAS | 2.0 | Y | EQ-5D, VAS NA | Y | Both |
| Joyce et al.94 2012 | USA | 439 | NA | 30% | W/ AKI | HUI3 | 1.0 | Y | Y | ||
| Osthus et al.100 2012 | Norway | 252 | 60.2 | 34.1% | On dialysis | SF-36, SF-12 | 4.5 | Y | Both PCS | Y | Both PCS |
| ter Horst et al.83 2012 | Netherlands | 2501 | 65.3 | 21% | Underwent CABG | EQ-5D, EQ-5D VAS | 30 d | N | NA | ||
| Romanus et al.59 2012 | USA | 267 | NA | 44% | Pancreatic Ca | EQ-5D, EQ-5D VAS | 0.2 | Y | Y | ||
| Williams et al.88 2012 | Australia | 9979 | 51 | 55% | W/ diabetes | SF-36 | 7.4 | NA | Y | ||
| Pompili et al.57 2013 | NA | 131 | 68 | 21% | Lung Ca | SF-36 | 3.33¶ | Y | Only PCS | Y | Only PCS |
| Saquib et al.39 2013 | USA | 20 308 | 62.8 | 100% | Postmenopausal women | SF-36 | 3.0 | Y | Y | Only PCS | |
| Naess and Nyland75 2013 | Norway | 188 | 48 | 44% | W/ first-ever cerebral infarction | NHP | 6.0 | Y | Y | ||
| Bukan et al.41 2014 | Denmark | 318 | 68ǁ | 48.4% | ICU admission | SF-36, SF-12 | 90.0 d | Y | Both PCS | Y | Both PCS |
| Chapa et al.64 2014 | Canada, USA | 693 | 72.0/68.5‖‖ | 37.8% | W/ atrial fibrillation | SF-36 | 3.5* | Y | Only Men PCS | Y | Only Men PCS |
| Kikkenborg Berg et al.71 2014 | Denmark | 358 | 65.5 | 65% | ICD patients | EQ-5D VAS | 1.3 | NA | Y | ||
| Ul-Haq et al.21 2014 | Scotland | 5272 | 50 | 54.8% | Scottish population | SF-12 | 8.0 | Y | Y | Varies by model | |
| Wong et al.60 2014 | China | 160 | 62¶ | 45% | Colorectal Ca | SF-6D | 2.0 | Y | Y | ||
| Burns et al.23 2015 | Australia | 14 019 | 73 | 91% | Older persons | SF-36 | 10.0 | Y |
Women both Men MCS |
Y |
Women both Men none |
| Gonzalez-Velez et al.122 2015 | Spain | 412 | 84.7/87.5** | 81.8%/81.9%** | People w/ dementia | EQ-5D | 1.5 | NA |
Y N |
Vary¶¶ | |
| Grincenkov et al.92 2015 | Brazil | 1624 | 58 | 45% | On dialysis | SF-36 | 1.24¶ | Y | Y | ||
| Kielbergerová et al.70 2015 | Czech Republic | 341 | 69 | 41.1% | Post-stroke Patients | SF-36 | 5.0 | Y | Y | ||
| Lizaur-Utrilla et al.113 2015 | Spain | 1529 | 68.2¶ | 76.7% | Consecutive primary TKAs | SF-12 | 10.0 | Y | Y | Only MCS | |
| Pocock et al.42 2015 | Argentina, Belgium, Brazil, Denmark, Finland, France, Germany, Greece, Italy, Luxembourg, Mexico, Norway, Poland, Romania, Slovenia, Spain, Netherlands Turkey, UK, Venezuela | 10 568 | 61.8 | 25.1% | Patients w/ non-fatal ACS | EQ-5D | 1.0 | NA | Y | ||
| Bliemel et al.109 2016 | Germany | 391 | 81 | 72% | W/ hip fracture | EQ-5D | 1.0 | Y | Y | ||
| Hartog et al.29 2016 | Netherlands | 184 | 79.2¶ | 70% | Older persons | RAND-36 | 1.0 | N | N | ||
| Martin-Lesende et al.74 2016 | Spain | 83 | 81.6¶ | 42.2% | W/ HF and/or chronic lung disease | EQ-5D VAS | 5.0 | NA | Y | ||
| Parlevliet et al.44 2016 | Netherlands | 473 | 77.8 | 54.8% | Acutely admitted older patients | EQ-5D | 1.0 | Y | Y | Varies by model | |
| Sexton et al.103 2016 | Ireland | 362 | 63.2 | 59.4% | On haemodial. | EQ-5D VAS | 3.4 | Y | Y | ||
| Perl et al.102 2016 | Australia, Belgium, Canada, France, Germany, Italy, Japan, New Zealand, Spain, Sweden, UK, USA | 13 784 | 62 | 41% | On haemodial. | SF-12 | 0.92¶ | NA | Y | Varies by model | |
| Buecking et al.111 2017 | Germany | 402 | 81 | 73% | W/ hip fracture | EQ-5D | 1.0 | Y | Y | ||
| Cnudde et al.110 2017 | Sweden | 42 862 | 67.7/75.8** | 56.3%/47.0%** | W/ primary hip osteoarthritis | EQ-5D VAS | 5.0 | NA | Y | ||
| Lahoud et al.72 2017 | USA | 7056 | 51.7 | 59.6% | Primary cardiac prevention patients | SF-36 | 8.0 | Y | Only PCS | Y | Only PCS |
| Pinheiro et al.56 2017 | USA | 6290 | NA | 49.8% | W/ lung Ca | SF-36 | 2.0 | NA | Y | ||
| Ramos et al.78 2017 | Portugal | 130 | 69 | 34% | W/ HF | SF-36 | 6.0 | Y | Y | Varies by model | |
| Reyes et al.58 2017 | USA | 3734 | NA | 42% | W/ colorectal Ca | SF-12 | 5.0 | Y | Y | ||
| Stehlik et al.80 2017 | USA | 200 | 63 | 27.0% | W/ HF | EQ-5D VAS | 1.0 | NA | Y | ||
| Bakhru et al.40 2018 | USA | 36 | 64.5 | 47% | ICU admission | SF-36 | 1.0 | NA | N | ||
| Higueras-Fresnillo et al.34 2018 | Spain | 3922 | 71.8 | 56.4% | Older persons | SF-36 | 14.0¶ | NA | Y | Varies by model | |
| Jia et al.30 2018 | USA | 105 473 | 74.6 | 58.0% | Older persons | SF-6D, EQ-5D | 2.0 | NA | Y | ||
| Liira et al.24 2018 | Finland | 3156 | 75 to 85n | 52.4% | Older persons | 15D | 2.0 | Y | Y | Varies by group## | |
| Nater et al.115 2018 | USA | 142 | 59.4 | 42% | Who had spinal decompressive Sx | SF-36, EQ-5D | 1.0 | Y | EQ-5D, SF-36 only PCS | Y | Only SF-36 PCS |
| Pinheiro and Reeve55 2018 | USA | 535 | 75 | 50% | Lung Ca | SF-36 | 2.0 | NA | Y | ||
| Trajceska et al.105 2018 | Republic of Macedonia | 162 | 56.2 | 47% | On haemodial. | SF-36 | 5.0 | Y | Y | ||
| Kikkenborg Berg et al.63 2019 | Denmark | 998 | 63.8 | 20% | W/ ICD | SF-12, EQ-5D VAS | 1.0 | Y | Y | ||
| Rosenberg et al.32 2019 | Canada | 380 | 88.4 | 72% | Older persons | EQ-5D, EQ-5D VAS | 1.5 | Y | Y | Only VAS | |
| van Veen et al.85 2019 | Netherlands | 392 | 58 | 21% | W/ ICD | SF-36 | 7.0 | N | N | ||
| Case et al.117 2020 | USA | 662 | 70¶ | 25.1% | W/ idiopathic pulmonary fibrosis | SF-12, EQ-5D, EQ-5D VAS | 1.0 | Y | All, SF-12 only PCS | N | |
| Kok et al.108 2020 | Canada | 402 | 56.4 | 35.8% | W/ cirrhosis | EQ-5D VAS | 0.5 | Y | Y | ||
| Frendl et al.61 2020 | USA | 2425 | 73 | 0% | W/ prostate Ca | SF-36, VR-12 | 10.0 | NA | N | ||
| Phyo et al.35 2021 | Australia and USA | 19106 | 74 | 56.4% | Older persons | SF-12 | 5.0 | Y | Only PCS | Y | Only PCS*** |
| Phyo et al.36 2021 | Australia and USA | 19106 | 74 | 56.4% | Older persons | SF-12 | 5.0 | Y | Y | ||
| Singh et al.37 2021 | USA | 13900 | 70.1 | 4% | Veterans | SF-12 | 7.0 | NA | Y | Varies by model | |
| Lou et al.62 2022 | Taiwan | 1178 | 52.2 | 100% | W/ breast Ca and breast Ca Sx | SF-36 | 10.0 | Y | Y | ||
| Özyilmaz et al.48 2022 | Turkey | 105 | 58.6 | 48.6% | Consecutive ICU patients | SF-12, EQ-5D | 120 d | NA | Y | ||
ACS acute coronary syndrome, AKI acute kidney injury, ALS amyotrophic lateral sclerosis, Ca cancer, CABG coronary artery bypass graft surgery, CAD coronary artery disease, CHF chronic heart failure, COPD chronic obstructive pulmonary disease, d days, Dx diagnosis, ED emergency department, EF ejection fraction, EQ-5D EuroQol five dimensions questionnaire, haemodial. haemodialysis, HF heart failure, HUI3 Health Utilities Index Mark 3, ICD implantable cardioverter defibrillator, MCS mental component score, MDS-HSI Minimum Data Set Health Status Index, N no, NA not available, NHP Nottingham Health Profile, PAD peripheral artery disease, PCI percutaneous coronary intervention, PCS physical component score, pEQ-5D predicted EQ-5D, RAND-36 36-Item Short Form Health Survey (SF-36), Ref. reference, SF-12 SF 12-Item, SF-6D SF Six-Dimension, SLE systemic lupus erythematosus, sympt. symptoms, Sx surgery, TKA total knee arthroplasty, UK United Kingdom, USA United States of America, VA Veterans Affairs, VAS visual analogue scale, VR-12 Veterans RAND 12 Item Health Survey, W/ with, Y yes
*Mean in years
†Female
‡Follow-up. Time is provided in years unless otherwise specified
§All SF instruments include physical and mental component scores
‖Diabetic/non-diabetic
¶Median
#Hospitalized/not hospitalized
**Alive/deceased
††Treated with sunitinib/treated with interferon alfa
‡‡Colorectal cancer/lung cancer
§§Person-years
‖‖Women/men
¶¶Vary by the presence of depressive symptoms. Score on the Cornell Depression Scale for Dementia <6 or ≥6
##Eight groups were included in this study: home-dwelling cardiovascular patients; former businessmen; home-dwelling people with dementia; spousal caregivers of people with dementia; hospitalized patients with delirium; nursing home residents; older people suffering from loneliness; population sample
***Adjusted by age predicted fatal cardiovascular disease, fatal myocardial infarction, and fatal stroke. Adjusted by age, and other sociodemographic and clinical characteristics, predicted fatal myocardial infarction
Overview of Included Articles
Articles were published between 1997 and 2022, with over one-third (n=42) from the United States of America (USA). Nearly a quarter (n=24) pertained to people with cardiovascular diseases (CVD), followed by kidney disease (n=17), with seven pertaining to the general population. The median number of participants was 879, ranging from 36 to 105,473. Nearly three-quarters of studies (n=81) used at least one of the RAND Corporation QoL surveys: RAND-36/36-Item Short Form Survey (SF)-36, 12-Item SF Survey (SF-12), Veterans RAND 12-Item Health Survey (VR-12), and SF Six-Dimension (SF-6D). Nearly a quarter of studies (n=26) used a utility instrument, predominantly the EQ-5D (n=19); SF-6D (n=5); the Health Utilities Index Mark 3 (HUI3) (n=2); the 15-Dimensional instrument (15D) (n=1); and the Minimum Data Set Health Status Index (MDS-I) (n=1). Two studies used two utility instruments, the EQ-5D and the SF-6D (Table 2). There was variation of timeframes employed for mortality predictions, reported as total, median, and mean follow-up as well as person-years, and ranged from a median follow-up of 18.5 days to a median follow-up of 14 years.
Prediction of Mortality by Instrument
Two-thirds of studies (n=72) undertook univariate analysis and all but one (n=109), multivariate analysis, with all identified instruments employed for each. Cox proportional hazards regression was the most frequently used statistical method for both univariate and multivariate analyses (n=43 and 79 respectively), followed by logistic regression (n=17 and 25 respectively).
HRQoL predicted mortality in all but five univariate analyses, of which two employed utility instruments (EQ-5D and MDS-I). Among studies that used the SF-36 and/or SF-12 and found a univariate association (n=37 and n=15, respectively), over half of each (n=21, n=9, respectively) observed a relationship between both the physical component score (PCS) and mental component score (MCS) and mortality, including one study that used both instruments. Two studies employing the SF-36 found variation in results by sex (Table 2).
In multivariate analyses, most studies (n=100) found HRQoL predicted mortality regardless of the instrument employed. In the nine studies that did not find an association, three employed utility instruments (MHS-I, EQ-5D, and SF-6D). Instruments for which there was always an association included two utility instruments (HUI3, 15D) and the NHP (Table 2). Among studies that used either the SF-36 or the SF-12 and found an association (n=53 and n=19, respectively), over half (n=29 and n=12, respectively) observed a relationship for both the PCS and MCS and mortality, including one study that used both instruments. There were eight studies using the SF-36 that reported variation in their results by sex (n=2); age (older vs younger patients) (n=1); diabetes status (yes/no) (n=1); cause of death (all-cause vs CVD); and model tested (n=1); with inclusion of depressive symptoms (n=2); and with different confounders (n=1). Four studies using the SF-12 reported variation by model tested with inclusion of depressive symptoms; body mass index (BMI); and sociodemographic and clinical characteristics; and by changes in HRQoL over time.
Prediction of Mortality by Population
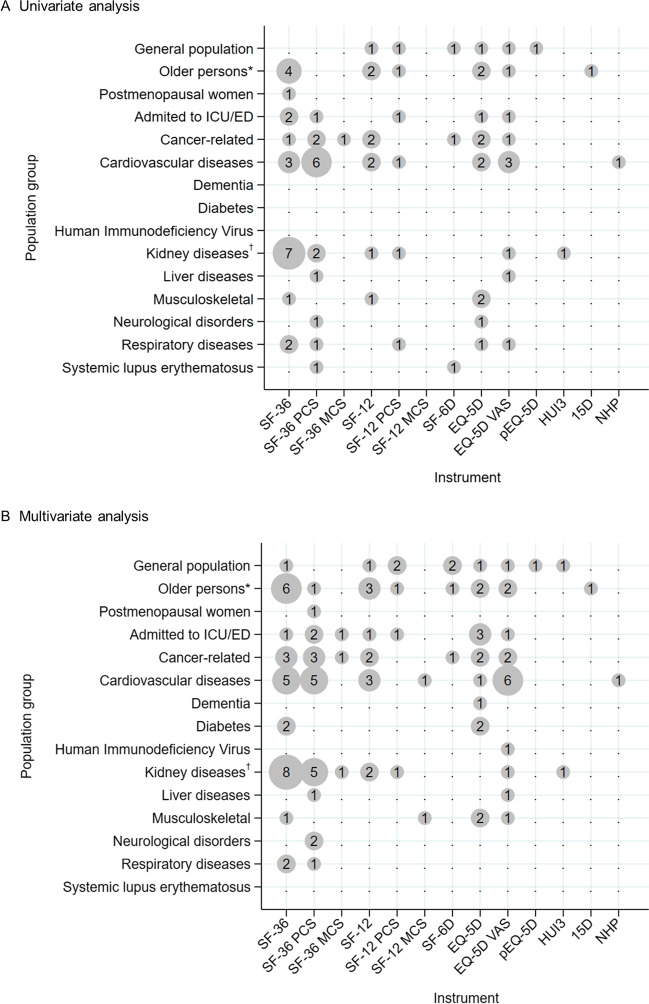
There were seven general population studies16–22 conducted over periods of 1 to 10 years. Three studies provided univariate results, with HRQoL predicting mortality in each17,20,21 (Fig. 2A). The results were consistent in multivariate analyses, although in one study,21 inclusion of BMI led to variation. HRQoL predicted mortality in the four studies that undertook multivariate analysis only (Fig. 2B).
Fig. 2.
Bubble plots of univariate and multivariate predictions of mortality by study population and instrument employed, for identified relationships. Legend: The size of a bubble is proportional to the number of prediction of mortality in the population group and instrument corresponding to the bubble coordinates, reported in the articles included in the review. 15D, 15-Dimensional instrument; ED, emergency department; EQ-5D, EuroQol five dimensions questionnaire; HUI3, Health Utilities Index Mark 3; ICU, intensive care unit; MCS, mental component score; NHP, Nottingham Health Profile; PCS, physical component score; pEQ-5D, predicted EQ-5D; SF-12, Short Form Health Survey 12-Item; SF-36, SF 36-Item; SF-6D, SF Six-Dimension; VAS, visual analogue scale. *Including veterans. †Including dialysis and haemodialysis.
Fifteen studies were conducted in persons 60 years or older,23–37 and another included veterans 50 years or older (average 67.8 years).38 Studies were undertaken over periods of 1 year (total follow-up) to 14 years (median follow-up). Participants included the following: veterans enrolled in general internal medicine clinics; older persons with no specific conditions, with one or more chronic diseases, and admitted to a nursing home; a non-institutionalized population; and people receiving home-based primary care with presence of a frailty syndrome or multiple comorbidities. Of 11 studies that reported univariate analyses,23–25,27–29,31,32,35,36,38 all except one29 found HRQoL predicted mortality. However, in one study,23 results varied by sex (Fig. 2A). Multivariate analyses were undertaken in all studies, with HRQoL predicting mortality in all analyses except for persons admitted to a nursing home,29 as in the univariate analysis (Fig. 2B and Table 2). In five studies, results were found to vary by sex23; model tested31,35; model and cause of death (all-cause vs CVD)34; and eight heterogeneous samples of older persons included.24
A single article reported on postmenopausal women.39 The study was undertaken over a period of 3 years, with HRQoL predicting mortality in both univariate and multivariate analyses (Fig. 2 and Table 2).
Clinical Sub-populations
Nine articles reported on people admitted to intensive care units or emergency departments who were followed up after discharge over periods of 90 days to 7 years.40–48 All except four40,42,43,48 presented univariate analysis results with HRQoL predicting mortality irrespective of instrument employed (Fig. 2A). In multivariate analyses, mortality was predicted by HRQoL in all but one study.40 Further, in a study that assessed HRQoL 3 months after admission,44 mortality was predicted in a model adjusted by age and sex and in the fully adjusted model (including age, sex, and other confounders: delirium, Mini-Mental State Examination score, baseline Katz activity of daily living score, number of geriatric conditions, and Charlson Comorbidity Index score). However, when HRQoL was assessed 12 months after admission, mortality was predicted only in the model adjusted by age and sex (Fig. 2B and Table 2).
Fourteen articles reported on people with cancer49–62 over periods of 18.5 days (median follow-up) to 10 years (total follow-up). Five studies did not provide a univariate analysis.50,52,55,56,61 In all analyses, HRQoL predicted mortality irrespective of instrument employed (Fig. 2A). In multivariate analyses, mortality was predicted by HRQoL in all but one study.61
Twenty-four articles reported on people with a CVD63–86 over periods of 30 days to 8 years. Univariate analysis was undertaken in all but six studies,67,69,71,74,80,82 with HRQoL predicting mortality in all but two.83,85 Two more studies employed two instruments and found associations for each63,73 (Fig. 2A). In another study, results varied by sex, with none of the SF component scores found to predict mortality in women while, in men, the PCS was found to be a significant predictor.64 All but one article83 reported multivariate analyses. With the exception of two studies,69,85 mortality was predicted by HRQoL in all the remaining studies (including two that used two instruments63,73) (Fig. 2B). Three articles reported variation in results with HRQoL no longer predictive of mortality after the inclusion of depressive symptoms.66,78,84 Differences by sex were captured in one study64 and differences by age in another.67
Four articles reported on people with diabetes8,87–89 conducted over periods of 5 to 10 years. No study reported univariate results, with HRQoL predicting mortality in all multivariate analyses (Fig. 2).
Seventeen articles reported on people with a kidney disease involving people on dialysis or haemodialysis90–106 over periods of 6 months to 6 years. Eleven studies found HRQoL predicted mortality based on univariate analysis, including two studies that used two instruments.97,100 The other six studies did not provide univariate results.90,93,95,99,102,106 All 17 studies found HRQoL predicted mortality in multivariate analyses. Variation was found in a study that stratified people with and without diabetes98 and by changes in HRQoL over time102 (Fig. 2 and Table 2).
Only two studies were conducted in people with a liver disease107,108 over periods of 6 months and 3 years, with HRQoL found to predict mortality in univariate and multivariate analyses in both (Fig. 2).
Five studies were conducted in people with a musculoskeletal condition109–113 over periods of 1 to 10 years using different instruments. HRQoL was found to predict mortality through univariate analysis in four studies; the fifth did not provide results.110 In multivariate analyses, HRQoL was found to predict mortality in all studies regardless of instrument (Fig. 2 and Table 2).
Two articles reported on people with a neurological condition114,115 over periods of 1 and 4 years. In Nater et al.,115 the SF-36 PCS and the EQ-5D predicted mortality in univariate analysis, but only the SF-36 PCS in multivariate analysis. In del Aguila and collaborators,114 the SF-36 did not predict mortality in univariate analysis, but the PCS did predict mortality in multivariate analysis.
Six articles reported on people with a respiratory disease116–121 over periods of 30 days to 5 years using a variety of instruments. Four of five studies that provided results of univariate associations found that HRQoL predicted mortality, including a study that employed three instruments117 (Fig. 2A). In multivariate analyses, mortality was predicted by HRQoL in half the studies118,120,121 (Fig. 2B and Table 2).
Three more articles reported on people with dementia,122 adults infected with human immunodeficiency virus (HIV),123 and patients with systemic lupus erythematosus (SLE).124 The first two studies did not provide results from univariate associations,122,123 while the third found an association between HRQoL and mortality (Fig. 2A). Multivariate analyses found that HRQoL only predicted mortality in people with dementia with limited depressive symptoms (<6 on the Cornell Depression Scale for Dementia). HRQoL also predicted mortality in those infected with HIV, but not in people with SLE (Fig. 2 and Table 2).
DISCUSSION
This scoping review is the first to provide an overview of the literature on generic HRQoL scores as predictors of mortality across general populations and clinical sub-populations. Among the 110 studies mapped, nearly a quarter included people with cardiovascular diseases followed by people with kidney disease, dialysis, and haemodialysis. There were no studies investigating diagnosed mental health conditions. Eleven instruments were employed, the SF-36 the most used. Most studies assessed relationships through multivariate analysis using Cox proportional hazards models. For some studies using the SF-36 and SF-12, only the PCS or MCS was found to be associated with mortality in univariate and/or multivariate analyses.
Through this review, a consistent relationship between low HRQoL scores and mortality was observed independent of the generic instrument employed, with consistent findings when multiple instruments were employed.17 The relationship is clearest in the univariate analyses, but also observed in most multivariate analyses. Our finding that lower HRQoL scores are associated with mortality in general non-patient populations is consistent with results from Phyo and collaborators.5 We also found this relationship held in all clinical populations investigated in either univariate or multivariate analysis.
We acknowledge that there is no proposed mechanism through which HRQoL causes death. However, as it is not possible to perfectly measure the degree to which each person’s disease or multiple diseases in combination affect their mortality risk, HRQoL as a holistic assessment of health status appears useful for this purpose. Unadjusted results are of inherent value for standalone screening and monitoring. Additionally, disease-specific HRQoL instruments are not required as predictors of survival125,126 within the populations covered by this review.
There is some evidence of an attenuation of effect between univariate and multivariate analyses, showing the impact of specific health-related factors on the strength of association. For example, in the only two studies including people with CVD that adjusted for depressive symptoms,66,78 neither component score of the SF-36 was significantly associated with survival when depressive symptoms were included in their models. This review also identified the following variables as important to the assessment of an association: sex, cause of death, and the timepoint of assessment (e.g., 3 or 12 months post-admission to intensive care). However, multivariate analysis is not considered as useful for a simple holistic assessment of mortality risk.
Findings also support the importance of choice of timeframe in assessments. For instance, in studies employing short timeframes (up to 30 days), associations between HRQoL and mortality were found for conditions with an anticipated short life expectancy, i.e., renal cancer50 and terminal cancer54 but not otherwise.83,116 In contrast, associations between HRQoL and longer-term mortality were found in study populations with a range of different health conditions,62,65,79,82,117–121 suggesting that generic HRQoL measures can also be indicative of longer-term mortality risk.
Our findings identified that, when the association between mortality and HRQoL was with either the PCS or MCS of an SF survey, the PCS was more frequently associated with mortality. These findings also highlight the likely importance of different factors within holistic assessments across conditions, and the potential benefits of a single overall score as for multi-attribute utility assessments. Extending Clarke and colleagues’ findings on cumulative and/or extreme problems,8 we postulate a detailed understanding of condition-specific cumulative and/or extreme problems associated with mortality (and other events) may provide useful information for screening and/or monitoring within the clinical context.32,102
Clarke and colleagues8 also raised a question about the predictive ability of utility instruments other than the EQ-5D in people with type 2 diabetes; our review suggests this is a moot point as no other utility instruments have been employed in this sub-population. However, relationships between EQ-5D utilities and mortality were identified in all the study populations investigated, with relationships for utilities assessed with other instruments found in five of six study populations investigated.
The results of this study are subject to the review design, the review question, and specific limitations of the included studies. As a scoping review, the quality of studies and appraisal of methodological risk of bias were not undertaken.12 Likewise, due to the exploratory design of this review, the heterogeneity in the instruments used for the assessment of HRQoL, and diversity of study populations assessed, we did not report HRQoL scores. We undertook high-level comparisons only and no summary statistics were assessed. Even so, we have identified a general association between HRQoL scores and mortality, regardless of the instrument or analysis employed, for both the general population and most sub-populations with specified physical health conditions and/or risk factors.
CONCLUSIONS
HRQoL was found to be an indicator of mortality risk in the general population and most clinical sub-populations, independent of the generic instrument employed. However, no studies investigated the relationship between HRQoL and people living with diagnosed mental disorders. This is an important gap, as rates of poor physical health and mortality are much higher in people with mental disorders,127 especially those with severe mental illness,128,129 compared to the general population. In the clinical context, HRQoL assessment may be useful for screening and/or monitoring purposes to understand how people perceive their health and well-being and as an indicator of mortality risk, encouraging better-quality and timely patient care to support and maximize what may be a patient’s only modifiable outcome.
Supplementary Information
(DOCX 21 kb)
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This study was supported by the National Health and Medical Research Council of Australia. Grant number 1102628. A.N. is supported by a Select Foundation Research Fellowship.
Declarations
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fayers PM, Machin D. Quality of life: the assessment, analysis and reporting of patient-reported outcomes. Third ed. Chichester Sussex: Wiley Blackwell; 2015. [Google Scholar]
- 2.Pennacchini M, Bertolaso M, Elvira MM, De Marinis MG. A brief history of the Quality of Life: its use in medicine and in philosophy. Clin Ter. 2011;162(3):e99–e103. [PubMed] [Google Scholar]
- 3.Sosnowski R, Kulpa M, Ziętalewicz U, Wolski JK, Nowakowski R, Bakuła R, Demkow T. Basic issues concerning health-related quality of life. Cent European J Urol. 2017;70(2):206–211. doi: 10.5173/ceju.2017.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Wit M, Hajos T. Health-Related Quality of Life. In: Gellman MD, Turner JR, editors. Encyclopedia of Behavioral Medicine. New York, NY: Springer; 2013. [Google Scholar]
- 5.Phyo AZZ, Freak-Poli R, Craig H, Gasevic D, Stocks NP, Gonzalez-Chica DA, et al. Quality of life and mortality in the general population: a systematic review and meta-analysis. BMC Public Health. 2020;20(1). 10.1186/s12889-020-09639-9 [DOI] [PMC free article] [PubMed]
- 6.Xu J, Sun Y, Gong D, Fan Y. Association between disease-specific health-related quality of life and all-cause mortality in patients with heart failure: a meta-analysis. Curr Probl Cardiol. 2023;48(4):1–18. doi: 10.1016/j.cpcardiol.2023.101592. [DOI] [PubMed] [Google Scholar]
- 7.Mastenbroek MH, Versteeg H, Zijlstra W, Meine M, Spertus JA, Pedersen SS. Disease-specific health status as a predictor of mortality in patients with heart failure: a systematic literature review and meta-analysis of prospective cohort studies. Eur J Heart Fail. 2014;16(4):384–93. doi: 10.1002/ejhf.55. [DOI] [PubMed] [Google Scholar]
- 8.Clarke PM, Hayes AJ, Glasziou PG, Scott R, Shnes J, Keech AC. Using the EQ-5D index score as a predictor of outcomes in patients with type 2 diabetes. Medical care. 2009;47(1):61–8. doi: 10.1097/MLR.0b013e3181844855. [DOI] [PubMed] [Google Scholar]
- 9.Nevarez-Flores AG, Breslin M, Carr VJ, Morgan VA, Waterreus A, Harvey C, et al. Proposing a causal pathway for health-related quality of life in people with psychotic disorders. J. Psychiatr. Res. 2021;138:550–9. doi: 10.1016/j.jpsychires.2021.05.016. [DOI] [PubMed] [Google Scholar]
- 10.Neil AL, Carr VJ, Mackinnon A, Foley DL, Morgan VA. Health-related quality of life in people living with psychotic illness and factors associated with its variation. Value Health. 2018;21(8):1002–9. doi: 10.1016/j.jval.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 11.Hjorthøj C, Stürup AE, McGrath JJ, Nordentoft M. Years of potential life lost and life expectancy in schizophrenia: a systematic review and meta-analysis. The Lancet Psychiatry. 2017;4(4):295–301. doi: 10.1016/S2215-0366(17)30078-0. [DOI] [PubMed] [Google Scholar]
- 12.Peters MDJ, Godfrey C, McInerney P, Munn Z, Tricco AC, Khalil H. Chapter 11: Scoping Reviews (2020 version). In: Aromataris E, Z. M, editors. JBI Manual for Evidence Synthesis: JBI; 2020.
- 13.Peters MDJ, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. JBI Evid Implement. 2015;13(3):141–6. doi: 10.1097/xeb.0000000000000050. [DOI] [PubMed] [Google Scholar]
- 14.Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018;169(7):467–73. doi: 10.7326/m18-0850. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. The top 10 causes of death. WHO, Geneva. 2020. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death. Accessed 21 March 2023.
- 16.Haring R, Feng Y-S, Moock J, Völzke H, Dörr M, Nauck M, et al. Self-perceived quality of life predicts mortality risk better than a multi-biomarker panel, but the combination of both does best. BMC Med Res Methodol. 2011;11(1):103. doi: 10.1186/1471-2288-11-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jerant A, Tancredi DJ, Franks P. Mortality prediction by quality-adjusted life year compatible health measures. Med Care. 2011;49(5):443–50. doi: 10.1097/mlr.0b013e318206c231. [DOI] [PubMed] [Google Scholar]
- 18.Kroenke CH, Kubzansky LD, Adler N, Kawachi I. Prospective change in health-related quality of life and subsequent mortality among middle-aged and older women. Am J Public Health. 2008;98(11):2085–91. doi: 10.2105/ajph.2007.114041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Myint PK, Smith RD, Luben RN, Surtees PG, Wainwright NWJ, Wareham NJ, et al. The Short-Form Six-Dimension utility index predicted mortality in the European Prospective Investigation into Cancer-Norfolk prospective population-based study. J Clin Epidemiol. 2010;63(2):192–8. doi: 10.1016/j.jclinepi.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Muñoz MA, Subirana I, Elosua R, Covas MI, Baena-Diez JM, Ramos R, et al. Utility of a short quality of life questionnaire to predict cardiovascular events. Int J Cardiol. 2011;151(3):392–4. doi: 10.1016/j.ijcard.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Ul-Haq Z, Mackay DF, Pell JP. Association between physical and mental health-related quality of life and adverse outcomes; a retrospective cohort study of 5,272 Scottish adults. BMC Public Health. 2014;14(1):1197. doi: 10.1186/1471-2458-14-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaplan MS, Berthelot J-M, Feeny D, McFarland BH, Khan S, Orpana H. The predictive validity of health-related quality of life measures: mortality in a longitudinal population-based study. Qual Life Res. 2007;16(9):1539–46. doi: 10.1007/s11136-007-9256-7. [DOI] [PubMed] [Google Scholar]
- 23.Burns RA, Butterworth P, Browning C, Byles J, Luszcz M, Mitchell P, et al. Examination of the association between mental health, morbidity, and mortality in late life: findings from longitudinal community surveys. Int Psychogeriatr. 2015;27(5):739–46. doi: 10.1017/s1041610214002051. [DOI] [PubMed] [Google Scholar]
- 24.Liira H, Mavaddat N, Eineluoto M, Kautiainen H, Strandberg T, Suominen M, et al. Health-related quality of life as a predictor of mortality in heterogeneous samples of older adults. Eur Geriatr Med. 2018;9(2):227–34. doi: 10.1007/s41999-018-0029-3. [DOI] [PubMed] [Google Scholar]
- 25.Cavrini G, Broccoli S, Puccini A, Zoli M, Cavrini G, Broccoli S, et al. EQ-5D as a predictor of mortality and hospitalization in elderly people. Qual Life Res. 2012;21(2):269–80. doi: 10.1007/s11136-011-9937-0. [DOI] [PubMed] [Google Scholar]
- 26.DeSalvo KB, Fan VS, McDonell MB, Fihn SD, DeSalvo KB, Fan VS, et al. Predicting mortality and healthcare utilization with a single question. Health Serv Res. 2005;40(4):1234–46. doi: 10.1111/j.1475-6773.2005.00404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dorr DA, Jones SS, Burns L, Donnelly SM, Brunker CP, Wilcox A, et al. Use of health-related, quality-of-life metrics to predict mortality and hospitalizations in community-dwelling seniors. J Am Geriatr Soc. 2006;54(4):667–73. doi: 10.1111/j.1532-5415.2006.00681.x. [DOI] [PubMed] [Google Scholar]
- 28.Fan VS, Au DH, McDonell MB, Fihn SD. Intraindividual change in SF-36 in ambulatory clinic primary care patients predicted mortality and hospitalizations. J Clin Epidemiol. 2004;57(3):277–83. doi: 10.1016/j.jclinepi.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 29.Hartog LC, Landman GWD, Cimzar-Sweelssen M, Knipscheer A, Groenier KH, Kleefstra N, et al. Health-related quality of life, rehabilitation and mortality in a nursing home population. Neth J Med. 2016;74(6):247–56. [PubMed] [Google Scholar]
- 30.Jia HM, Lubetkin EI, DeMichele K, Stark DS, Zack MM, Thompson WW. Comparing the Performance of 2 Health Utility Measures in the Medicare Health Outcome Survey (HOS) Med Decis Mak. 2018;38(8):983–93. doi: 10.1177/0272989x18808494. [DOI] [PubMed] [Google Scholar]
- 31.Otero-Rodriguez A, Leon-Munoz LM, Balboa-Castillo T, Banegas JR, Rodriguez-Artalejo F, Guallar-Castillon P. Change in health-related quality of life as a predictor of mortality in the older adults. Qual Life Res. 2010;19(1):15–23. doi: 10.1007/s11136-009-9561-4. [DOI] [PubMed] [Google Scholar]
- 32.Rosenberg T, Montgomery P, Hay V, Lattimer R. Using frailty and quality of life measures in clinical care of the elderly in Canada to predict death, nursing home transfer and hospitalisation - the frailty and ageing cohort study. BMJ Open. 2019;9(11):10. doi: 10.1136/bmjopen-2019-032712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsai S-Y, Chi L-Y, Lee C-H, Chou P. Health-related quality of life as a predictor of mortality among community-dwelling older persons. Eur J Epidemiol. 2007;22(1):19–26. doi: 10.1007/s10654-006-9092-z. [DOI] [PubMed] [Google Scholar]
- 34.Higueras-Fresnillo S, Cabanas-Sánchez V, García-Esquinas E, Rodríguez-Artalejo F, Martinez-Gomez D. Physical activity attenuates the impact of poor physical, mental, and social health on total and cardiovascular mortality in older adults: a population-based prospective cohort study. Qual Life Res. 2018;27(12):3293–302. doi: 10.1007/s11136-018-1974-5. [DOI] [PubMed] [Google Scholar]
- 35.Phyo AZZ, Ryan J, Gonzalez-Chica DA, Stocks NP, Reid CM, Tonkin AM, et al. Health-related quality of life and incident cardiovascular disease events in community-dwelling older people: A prospective cohort study. Int J Cardiol. 2021;339:170–8. doi: 10.1016/j.ijcard.2021.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phyo AZZ, Ryan J, Gonzalez-Chica DA, Woods RL, Reid CM, Nelson MR, et al. Health-related quality of life and all-cause mortality among older healthy individuals in Australia and the United States: a prospective cohort study. Qual Life Res. 2021;30(4):1037–48. doi: 10.1007/s11136-020-02723-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh JA, Nelson DB, Nichol KL. Recent health-related quality of life, but not change, predicted mortality and healthcare utilization. J Clin Epidemiol. 2021;140:13–21. doi: 10.1016/j.jclinepi.2021.08.023. [DOI] [PubMed] [Google Scholar]
- 38.Fan VS, Au D, Heagerty P, Deyo RA, McDonell MB, Fihn SD. Validation of case-mix measures derived from self-reports of diagnoses and health. J Clin Epidemiol. 2002;55(4):371–80. doi: 10.1016/S0895-4356(01)00493-0. [DOI] [PubMed] [Google Scholar]
- 39.Saquib N, Brunner R, Kubo J, Tindle H, Kroenke C, Desai M, et al. Self-perceived physical health predicts cardiovascular disease incidence and death among postmenopausal women. BMC Public Health. 2013;13:468. doi: 10.1186/1471-2458-13-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bakhru RN, Davidson JF, Bookstaver RE, Kenes MT, Welborn KG, Morris PE, et al. Physical function impairment in survivors of critical illness in an ICU Recovery Clinic. J Crit Care. 2018;45:163–9. doi: 10.1016/j.jcrc.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bukan RI, Møller AM, Henning MAS, Mortensen KB, Klausen TW, Waldau T. Preadmission quality of life can predict mortality in intensive care unit—A prospective cohort study. J Crit Care. 2014;29(6):942–7. doi: 10.1016/j.jcrc.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 42.Pocock S, Bueno H, Licour M, Medina J, Zhang L, Annemans L, et al. Predictors of one-year mortality at hospital discharge after acute coronary syndromes: A new risk score from the EPICOR (longtErm follow uP of antithrombotic management patterns In acute CORonary syndrome patients) study. Eur Heart J Acute Cardiovasc. 2015;4(6):509–17. doi: 10.1177/2048872614554198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hofhuis JGM, Spronk PE, van Stel HF, Schrijvers AJP, Bakker J. Quality of life before intensive care unit admission is a predictor of survival. Crit Care. 2007;11 (no pagination)(R78). 10.1186/cc5970 [DOI] [PMC free article] [PubMed]
- 44.Parlevliet JL, Macneil-Vroomen J, Buurman BM, De Rooij SE, Bosmans JE. Health-related quality of life at admission is associated with postdischarge mortality, functional decline, and institutionalization in acutely hospitalized older medical patients. Eur Heart J Acute Cardiovasc. 2016;64(4):761–8. doi: 10.1111/jgs.14050. [DOI] [PubMed] [Google Scholar]
- 45.Rodriguez-Artalejo F, Guallar-Castillon P, Pascual CR, Otero CM, Montes AO, Garcia AN, et al. Health-related quality of life as a predictor of hospital readmission and death among patients with heart failure. Arch Intern Med. 2005;165(11):1274–9. doi: 10.1001/archinte.165.11.1274. [DOI] [PubMed] [Google Scholar]
- 46.Sacanella E, Perez-Castejon JM, Nicolas J, Masanes F, Navarro M, Castro P, et al. Mortality in healthy elderly patients after ICU admission. Intensive Care Med. 2009;35(3):550–5. doi: 10.1007/s00134-008-1345-8. [DOI] [PubMed] [Google Scholar]
- 47.Zuluaga MC, Guallar-Castillón P, López-García E, Banegas JR, Conde-Herrera M, Olcoz-Chiva M, et al. Generic and disease-specific quality of life as a predictor of long-term mortality in heart failure. Eur J Heart Fail. 2010;12(12):1372–8. doi: 10.1093/eurjhf/hfq163. [DOI] [PubMed] [Google Scholar]
- 48.Özyılmaz E, Kuşçu ÖÖ, Karakoç E, Boz A, Tıraşçı GO, Güzel R, et al. Worse pre-admission quality of life is a strong predictor of mortality in critically ill patients. Turkish J Phys Med Rehabil (2587-1250). 2022;68(1):19-29. 10.5606/tftrd.2022.5287 [DOI] [PMC free article] [PubMed]
- 49.Ashing-Giwa KT, Lim JW, Tang J. Surviving cervical cancer: does health-related quality of life influence survival? Gynecol Oncol. 2010;118(1):35–42. doi: 10.1016/j.ygyno.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 50.Cella D, Cappelleri JC, Bushmakin A, Charbonneau C, Li JZ, Kim ST, et al. Quality of life predicts progression-free survival in patients with metastatic renal cell carcinoma treated with sunitinib versus interferon alfa. J Oncol Pract. 2009;5(2):66–70. doi: 10.1200/jop.0922004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grande GE, Farquhar MC, Barclay SI, Todd CJ. Quality of life measures (EORTC QLQ-C30 and SF-36) as predictors of survival in palliative colorectal and lung cancer patients. Palliat Support Care. 2009;7(3):289–97. doi: 10.1017/s1478951509990216. [DOI] [PubMed] [Google Scholar]
- 52.Grignon LM, Jameson MJ, Karnell LH, Christensen AJ, Funk GF. General health measures and long-term survival in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 2007;133(5):471–6. doi: 10.1001/archotol.133.5.471. [DOI] [PubMed] [Google Scholar]
- 53.Karvonen-Gutierrez CA, Ronis DL, Fowler KE, Terrell JE, Gruber SB, Duffy SA. Quality of life scores predict survival among patients with head and neck cancer. J Clin Oncol. 2008;26(16):2754–60. doi: 10.1200/jco.2007.12.9510. [DOI] [PubMed] [Google Scholar]
- 54.Park SM, Park MH, Won JH, Lee KO, Choe WS, Heo DS, et al. EuroQol and survival prediction in terminal cancer patients: a multicenter prospective study in hospice-palliative care units. Supportive Care in Cancer. 2006;14(4):329–33. doi: 10.1007/s00520-005-0889-1. [DOI] [PubMed] [Google Scholar]
- 55.Pinheiro LC, Reeve BB. Investigating the prognostic ability of health-related quality of life on survival: a prospective cohort study of adults with lung cancer. Supportive care in cancer : official journal of the Multinational Association of Support Care Cancer. 2018;26(11):3925–32. doi: 10.1007/s00520-018-4265-3. [DOI] [PubMed] [Google Scholar]
- 56.Pinheiro LC, Zagar TM, Reeve BB. The prognostic value of pre-diagnosis health-related quality of life on survival: a prospective cohort study of older Americans with lung cancer. Qual Life Res. 2017;26(7):1703–12. doi: 10.1007/s11136-017-1515-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pompili C, Salati M, Refai M, Berardi R, Onofri A, Mazzanti P, et al. Preoperative quality of life predicts survival following pulmonary resection in stage I non-small-cell lung cancer. Eur J Cardiothorac Surg. 2013;43(5):905–10. doi: 10.1093/ejcts/ezs532. [DOI] [PubMed] [Google Scholar]
- 58.Reyes ME, Ye Y, Zhou Y, Liang A, Kopetz S, Rodriquez MA, et al. Predictors of health-related quality of life and association with survival may identify colorectal cancer patients at high risk of poor prognosis. Qual Life Res. 2017;26(2):319–30. doi: 10.1007/s11136-016-1381-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Romanus D, Kindler HL, Archer L, Basch E, Niedzwiecki D, Weeks J, et al. Does health-related quality of life improve for advanced pancreatic cancer patients who respond to gemcitabine? Analysis of a randomized phase III trial of the cancer and leukemia group B (CALGB 80303) J Pain Symptom Manage. 2012;43(2):205–17. doi: 10.1016/j.jpainsymman.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wong CK, Law W-L, Wan Y-F, Poon JT-C, Lam CL-K, Wong CKH. Health-related quality of life and risk of colorectal cancer recurrence and All-cause death among advanced stages of colorectal cancer 1-year after diagnosis. BMC Cancer. 2014;1(1):337. doi: 10.1186/1471-2407-14-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Frendl DM, FitzGerald G, Epstein MM, Allison JJ, Sokoloff MH, Ware JE. Predicting the 10-year risk of death from other causes in men with localized prostate cancer using patient-reported factors: Development of a tool. PLOS ONE. 2020;15(12). 10.1371/journal.pone.0240039 [DOI] [PMC free article] [PubMed]
- 62.Lou SJ, Hou MF, Chang HT, Lee HH, Chiu CC, Yeh SCJ, et al. Breast cancer surgery 10-year survival prediction by machine learning: a large prospective cohort study. Biology (Basel). 2022;11(1). 10.3390/biology11010047 [DOI] [PMC free article] [PubMed]
- 63.Kikkenborg Berg S, Bernholdt Rasmussen T, Elmose Mols E, Brun Thorup C, Borregaard B, Vinggaard Christensen A, et al. Both mental and physical health predicts one year mortality and readmissions in patients with implantable cardioverter defibrillators: findings from the national DenHeart study. Eur J Cardiovasc Nurs. 2019;18(2):96–105. doi: 10.1177/1474515118794598. [DOI] [PubMed] [Google Scholar]
- 64.Chapa DW, Akintade B, Schron E, Friedmann E, Thomas SA. Is health-related quality of life a predictor of hospitalization or mortality among women or men with atrial fibrillation? J Cardiovasc Nurs. 2014;29(6):555–64. doi: 10.1097/jcn.0000000000000095. [DOI] [PubMed] [Google Scholar]
- 65.Curtis LH, Phelps CE, McDermott MP, Rubin HR, Curtis LH, Phelps CE, et al. The value of patient-reported health status in predicting short-term outcomes after coronary artery bypass graft surgery. Med Care. 2002;40(11):1090–100. doi: 10.1097/00005650-200211000-00010. [DOI] [PubMed] [Google Scholar]
- 66.Faller H, Störk S, Schowalter M, Steinbüchel T, Wollner V, Ertl G, et al. Is health-related quality of life an independent predictor of survival in patients with chronic heart failure? J Psychosom Res. 2007;63(5):533–8. doi: 10.1016/j.jpsychores.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 67.Ho PM, Masoudi FA, Peterson PN, Shroyer AL, McCarthy M, Jr, Grover FL, et al. Health-related quality of life predicts mortality in older but not younger patients following cardiac surgery. Am J Geriatr Cardiol. 2005;14(4):176–82. doi: 10.1111/j.1076-7460.2005.04312.x. [DOI] [PubMed] [Google Scholar]
- 68.Issa SM, Hoeks SE, Scholte Op Reimer WJ, Van Gestel YR, Lenzen MJ, Verhagen HJ, et al. Health-related quality of life predicts long-term survival in patients with peripheral artery disease. Vasc Med. 2010;15(3):163–9. doi: 10.1177/1358863x10364208. [DOI] [PubMed] [Google Scholar]
- 69.Kao C-W, Friedmann E, Thomas SA. Quality of life predicts one-year survival in patients with implantable cardioverter defibrillators. Qual Life Res. 2010;19(3):307–15. doi: 10.1007/s11136-010-9596-6. [DOI] [PubMed] [Google Scholar]
- 70.Kielbergerová L, Mayer O, Jr, Vaněk J, Bruthans J, Wohlfahrt P, Cífková R. Quality of life predictors in chronic stable post-stroke patients and prognostic value of SF-36 score as a mortality surrogate. Transl Stroke Res. 2015;6(5):375–83. doi: 10.1007/s12975-015-0418-6. [DOI] [PubMed] [Google Scholar]
- 71.Kikkenborg Berg S, Caspar Thygesen L, Hastrup Svendsen J, Vinggaard Christensen A, Zwisler A-D. Anxiety Predicts Mortality in ICD Patients: Results from the Cross-Sectional National CopenHeartICD Survey with Register Follow-Up. Pacing Clin Electrophysiol. 2014;37(12):1641–50. doi: 10.1111/pace.12490. [DOI] [PubMed] [Google Scholar]
- 72.Lahoud R, Chongthammakun V, Wu YP, Hawwa N, Brennan DM, Cho L. Comparing SF-36 (R) scores versus biomarkers to predict mortality in primary cardiac prevention patients. Eur J Int Med. 2017;46:47–55. doi: 10.1016/j.ejim.2017.05.026. [DOI] [PubMed] [Google Scholar]
- 73.Lenzen MJ, Reimer W, Pedersen SS, Boersma E, Maier W, Widimsky P, et al. The additional value of patient-reported health status in predicting 1-year mortality after invasive coronary procedures: a report from the Euro Heart Survey on Coronary Revascularisation. Heart. 2007;93(3):339–44. doi: 10.1136/hrt.2005.086868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Martin-Lesende I, Recalde E, Viviane-Wunderling P, Pinar T, Borghesi F, Aguirre T, et al. Mortality in a cohort of complex patients with chronic illnesses and multimorbidity: A descriptive longitudinal study. BMC Palliat Care. 2016;15 (1) (no pagination)(42). 10.1186/s12904-016-0111-x [DOI] [PMC free article] [PubMed]
- 75.Naess H, Nyland H. Poor Health-related Quality of Life is Associated with Long-term Mortality in Young Adults with Cerebral Infarction. J Stroke Cerebrovasc Dis. 2013;22(7):E79–E83. doi: 10.1016/j.jstrokecerebrovasdis.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 76.Pedersen SS, Versteeg H, Denollet J, Cheng JM, Serruys PW, van Domburg RT. Patient-rated health status predicts prognosis following percutaneous coronary intervention with drug-eluting stenting. Qual Life Res. 2011;20(4):559–67. doi: 10.1007/s11136-010-9775-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Piotrowicz K, Noyes K, Lyness JM, McNitt S, Andrews ML, Dick A, et al. Physical functioning and mental well-being in association with health outcome in patients enrolled in the Multicenter Automatic Defibrillator Implantation Trial II. Eur Heart J. 2006;28(5):601–7. doi: 10.1093/eurheartj/ehl485. [DOI] [PubMed] [Google Scholar]
- 78.Ramos S, Prata J, Rocha-Goncalves F, Bettencourt P, Coelho R. Quality of Life Predicts Survival and Hospitalisation in a Heart Failure Portuguese Population. Appl Res Qual Life. 2017;12(1):35–48. doi: 10.1007/s11482-016-9449-8. [DOI] [Google Scholar]
- 79.Rumsfeld JS, MaWhinney S, McCarthy M, Jr, Shroyer ALW, VillaNueva CB, O'Brien M, et al. Health-related quality of life as a predictor of mortality following coronary artery bypass graft surgery. JAMA. 1999;281(14):1298–303. doi: 10.1001/jama.281.14.1298. [DOI] [PubMed] [Google Scholar]
- 80.Stehlik J, Estep JD, Selzman CH, Rogers JG, Spertus JA, Shah KB, et al. Patient-Reported Health-Related Quality of Life Is a Predictor of Outcomes in Ambulatory Heart Failure Patients Treated With Left Ventricular Assist Device Compared With Medical Management: Results From the ROADMAP Study (Risk Assessment and Comparative Effectiveness of Left Ventricular Assist Device and Medical Management). Circ Heart Fail. 2017;10(6). 10.1161/circheartfailure.116.003910 [DOI] [PubMed]
- 81.Steinberg JS, Joshi S, Schron EB, Powell J, Hallstrom A, McBurnie M. Psychosocial status predicts mortality in patients with life-threatening ventricular arrhythmias. Heart Rhythm. 2008;5(3):361–5. doi: 10.1016/j.hrthm.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 82.Szekely A, Nussmeier NA, Miao Y, Huang K, Levin J, Feierfeil H, et al. A multinational study of the influence of health-related quality of life on in-hospital outcome after coronary artery bypass graft surgery. Am Heart J. 2011;161(6):1179–85.e2. doi: 10.1016/j.ahj.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 83.ter Horst R, Markou AL, Noyez L. Prognostic value of preoperative quality of life on mortality after isolated elective myocardial revascularization. Interac Cardiovasc Thorac Surg. 2012;15(4):651–4. doi: 10.1093/icvts/ivs184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thombs BD, Ziegelstein RC, Stewart DE, Abbey SE, Parakh K, Grace SL. Physical health status assessed during hospitalization for acute coronary syndrome predicts mortality 12 months later. J Psychosom Res. 2008;65(6):587–93. doi: 10.1016/j.jpsychores.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 85.van Veen B, Andersen CM, Johansen JB, Theuns DAMJ, Pedersen SS. Patient-reported quality of life as a predictor of mortality and ventricular tachyarrhythmia’s during 7 Years’ follow-up in patients with an implantable cardioverter defibrillator (from the MIDAS Study) Am J Cardiol. 2019;123(4):605–10. doi: 10.1016/j.amjcard.2018.11.021. [DOI] [PubMed] [Google Scholar]
- 86.Zhang J-P, Pozuelo L, Brennan DM, Hoar B, Hoogwerf BJ. Association of SF-36 with coronary artery disease risk factors and mortality: a PreCIS study. Prevent Cardiol. 2009:no-no. 10.1111/j.1751-7141.2009.00061.x [DOI] [PubMed]
- 87.McEwen LN, Kim C, Haan MN, Ghosh D, Lantz PM, Thompson TJ, et al. Are health-related quality-of-life and self-rated health associated with mortality? Insights from Translating Research Into Action for Diabetes (TRIAD) Prim Care Diabetes. 2009;3(1):37–42. doi: 10.1016/j.pcd.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Williams ED, Rawal L, Oldenburg BF, Renwick C, Shaw JE, Tapp RJ. Risk of cardiovascular and all-cause mortality: Impact of impaired health-related functioning and diabetes - The Australian Diabetes, Obesity and Lifestyle (AusDiab) study. Diabetes Care. 2012;35(5):1067–73. doi: 10.2337/dc11-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Landman GWD, Van Hateren KJJ, Kleefstra N, Groenier KH, Gans ROB, Bilo HJG. Health-related quality of life and mortality in a general and elderly population of patients with type 2 diabetes (ZODIAC-18) Diabetes Care. 2010;33(11):2378–82. doi: 10.2337/dc10-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Deoreo PB. Hemodialysis patient-assessed functional health status predicts continued survival, hospitalization, and dialysis-attendance compliance. Am J Kidney Dis. 1997;30(2):204–12. doi: 10.1016/s0272-6386(97)90053-6. [DOI] [PubMed] [Google Scholar]
- 91.Feroze U, Noori N, Kovesdy CP, Molnar MZ, Martin DJ, Reina-Patton A, et al. Quality-of-life and mortality in hemodialysis patients: roles of race and nutritional status. Clin J Am Soc Nephrol. 2011;6(5):1100–11. doi: 10.2215/cjn.07690910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Grincenkov FR, Fernandes N, Pereira Bdos S, Bastos K, Lopes AA, Finkelstein FO, et al. Impact of baseline health-related quality of life scores on survival of incident patients on peritoneal dialysis: a cohort study. Nephron. 2015;129(2):97–103. doi: 10.1159/000369139. [DOI] [PubMed] [Google Scholar]
- 93.Hayashino Y, Fukuhara S, Akiba T, Akizawa T, Asano Y, Saito S, et al. Low health-related quality of life is associated with all-cause mortality in patients with diabetes on haemodialysis: the Japan Dialysis Outcomes and Practice Pattern Study. Diabetic Med. 2009;26(9):921–7. doi: 10.1111/j.1464-5491.2009.02800.x. [DOI] [PubMed] [Google Scholar]
- 94.Joyce VR, Smith MW, Johansen KL, Unruh ML, Siroka AM, O'Connor TZ, et al. Health-related quality of life as a predictor of mortality among survivors of AKI. Clin J Am Soc Nephrol. 2012;7(7):1063–70. doi: 10.2215/cjn.00450112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Knight EL, Ofsthun N, Teng M, Lazarus JM, Curhan GC. The association between mental health, physical function, and hemodialysis mortality. Kidney Int. 2003;63(5):1843–51. doi: 10.1046/j.1523-1755.2003.00931.x. [DOI] [PubMed] [Google Scholar]
- 96.Kusleikaite N, Bumblyte IA, Kuzminskis V, Vaiciuniene R. The association between health-related quality of life and mortality among hemodialysis patients. Med Lith. 2010;46(8):531–7. doi: 10.3390/medicina46080076. [DOI] [PubMed] [Google Scholar]
- 97.Lacson E, Xu JL, Lin SF, Dean SG, Lazarus JM, Hakim RM. A Comparison of SF-36 and SF-12 Composite Scores and Subsequent Hospitalization and Mortality Risks in Long-Term Dialysis Patients. Clin J Am Soc Nephrol. 2010;5(2):252–60. doi: 10.2215/cjn.07231009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Katia LR, Garcıa Lopez Fernando J, Fernando d AM, Jordi A, Group obotC Perceived mental health at the start of dialysis as a predictor of morbidity and mortality in patients with end-stage renal disease (CALVIDIA study) Nephrol Dial Transplant. 2004;19(9):2347–53. doi: 10.1093/ndt/gfh392. [DOI] [PubMed] [Google Scholar]
- 99.Lowrie EG, Curtin RB, LePain N, Schatell D, Lowrie EG, Curtin RB, et al. Medical outcomes study short form-36: a consistent and powerful predictor of morbidity and mortality in dialysis patients. Am J Kidney Dis. 2003;41(6):1286–92. doi: 10.1016/S0272-6386(03)00361-5. [DOI] [PubMed] [Google Scholar]
- 100.Osthus TBH, Preljevic VT, Sandvik L, Leivestad T, Nordhus IH, Dammen T, et al. Mortality and health-related quality of life in prevalent dialysis patients: Comparison between 12-items and 36-items short-form health survey. Health Qual Life Outcomes. 2012;10 (no pagination)(46). 10.1186/1477-7525-10-46 [DOI] [PMC free article] [PubMed]
- 101.Peng YS, Chiang CK, Hung KY, Chang CH, Lin CY, Yang CS, et al. Are both psychological and physical dimensions in health-related quality of life associated with mortality in hemodialysis patients: a 7-year Taiwan cohort study. Blood Purif. 2010;30(2):98–105. doi: 10.1159/000319002. [DOI] [PubMed] [Google Scholar]
- 102.Perl J, Karaboyas A, Morgenstern H, Sen A, Rayner HC, Vanholder RC, et al. Association between changes in quality of life and mortality in hemodialysis patients: results from the DOPPS. Nephrol Dial Transplant. 2016:gfw233. 10.1093/ndt/gfw233 [DOI] [PMC free article] [PubMed]
- 103.Sexton DJ, Lowney AC, O'Seaghdha CM, Murphy M, O'Brien T, Casserly LF, et al. Do patient-reported measures of symptoms and health status predict mortality in hemodialysis? An assessment of POS-S Renal and EQ-5D. Hemodial Int. 2016;20(4):618–30. doi: 10.1111/hdi.12415. [DOI] [PubMed] [Google Scholar]
- 104.Takaki J, Nakao M, Yano E. The relationship of quality of life and depression to mortality in hemodialysis patients. Dial Transplant. 2005;34(8):568-+. [Google Scholar]
- 105.Trajceska L, Mladenovska D, Dzekova-Vidimliski P, Sikole A. Quality of life-repeated measurements are needed in dialysis patients. Open Access Maced J Med Sci. 2018;6(8):1410–2. doi: 10.3889/oamjms.2018.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Valdés C, García-Mendoza M, Rebollo P, Ortega T, Ortega F. Mental health at the third month of haemodialysis as a predictor of short-term survival. Nephrol Dial Transplant. 2006;21(11):3223–30. doi: 10.1093/ndt/gfl392. [DOI] [PubMed] [Google Scholar]
- 107.Tanikella R, Kawut SM, Brown RS, Krowka MJ, Reinen J, Dinasarapu CR, et al. Health-related quality of life and survival in liver transplant candidates. Liver Transpl. 2010;16(2):238–45. doi: 10.1002/lt.21984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kok B, Whitlock R, Ferguson T, Kowalczewski J, Tangri N, Tandon P. Health-related quality of life: a rapid predictor of hospitalization in patients with cirrhosis. Am J Gastroenterol. 2020;20. 10.14309/ajg.0000000000000545 [DOI] [PubMed]
- 109.Bliemel C, Sielski R, Doering B, Dodel R, Balzer-Geldsetzer M, Ruchholtz S, et al. Pre-fracture quality of life predicts 1-year survival in elderly patients with hip fracture-development of a new scoring system. Osteoporos Int. 2016;27(6):1979–87. doi: 10.1007/s00198-015-3472-8. [DOI] [PubMed] [Google Scholar]
- 110.Cnudde P, Nemes S, Mohaddes M, Timperley J, Garellick G, Burström K, et al. Is preoperative patient-reported health status associated with mortality after total hip replacement? Int J Environ Res Public Health. 2017;14(8). 10.3390/ijerph14080899 [DOI] [PMC free article] [PubMed]
- 111.Buecking B, Eschbach D, Knobe M, Oberkircher L, Balzer-Geldsetzer M, Dodel R, et al. Predictors of noninstitutionalized survival 1 year after hip fracture. Medicine. 2017;96 (37) (no pagination)(e7820). 10.1097/MD.0000000000007820 [DOI] [PMC free article] [PubMed]
- 112.Singh JA, Nelson DB, Fink HA, Nichol KL. Health-related quality of life predicts future health care utilization and mortality in veterans with self-reported physician-diagnosed arthritis: the veterans arthritis quality of life study. Semin Arthritis Rheum. 2005;34(5):755–65. doi: 10.1016/j.semarthrit.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 113.Lizaur-Utrilla A, Gonzalez-Parreño S, Miralles-Muñoz FA, Lopez-Prats FA. Ten-year mortality risk predictors after primary total knee arthroplasty for osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2015;23(6):1848–55. doi: 10.1007/s00167-015-3502-2. [DOI] [PubMed] [Google Scholar]
- 114.del Aguila MA, Longstreth WT, Jr, McGuire V, Koepsell TD, van Belle G. Prognosis in amyotrophic lateral sclerosis: a population-based study. Neurology. 2003;60(5):813–9. doi: 10.1212/01.wnl.0000049472.47709.3b. [DOI] [PubMed] [Google Scholar]
- 115.Nater A, Tetreault LA, Kopjar B, Arnold PM, Dekutoski MB, Finkelstein JA, et al. Predictive factors of survival in a surgical series of metastatic epidural spinal cord compression and complete external validation of 8 multivariate models of survival in a prospective North American multicenter study. Cancer. 2018;124(17):3536–50. doi: 10.1002/cncr.31585. [DOI] [PubMed] [Google Scholar]
- 116.Carusone SBC, Walter SD, Brazil K, Loeb MB. Pneumonia and lower respiratory infections in nursing home residents: Predictors of hospitalization and mortality. J Am Geriatr Soc. 2007;55(3):414–9. doi: 10.1111/j.1532-5415.2007.01070.x. [DOI] [PubMed] [Google Scholar]
- 117.Case AH, Hellkamp AS, Neely ML, Bender S, Dilling DF, Gulati M, et al. Associations between patient-reported outcomes and death or lung transplant in idiopathic pulmonary fibrosis data from the idiopathic pulmonary fibrosis prospective outcomes registry. Ann Am Thorac Soc. 2020;17(6):699–705. doi: 10.1513/AnnalsATS.201906-437OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Domingo-Salvany A, Lamarca R, Ferrer M, Garcia-Aymerich J, Alonso J, Félez M, et al. Health-related quality of life and mortality in male patients with chronic obstructive pulmonary disease. Am J Resp Crit Care Med. 2002;166(5):680–5. doi: 10.1164/rccm.2112043. [DOI] [PubMed] [Google Scholar]
- 119.Esteban C, Quintana JM, Aburto M, Moraza J, Egurrola M, España PP, et al. Predictors of mortality in patients with stable COPD. J Gen Intern Med. 2008;23(11):1829–34. doi: 10.1007/s11606-008-0783-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Halpin DM, Peterson S, Larsson TP, Calverley PM. Identifying COPD patients at increased risk of mortality: predictive value of clinical study baseline data. Respir Med. 2008;102(11):1615–24. doi: 10.1016/j.rmed.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 121.Sprenkle MD, Niewoehner DE, Nelson DB, Nichol KL. The Veterans Short Form 36 Questionnaire is predictive of mortality and health-care utilization in a population of veterans with a self-reported diagnosis of asthma or COPD. Chest. 2004;126(1):81–9. doi: 10.1378/chest.126.1.81. [DOI] [PubMed] [Google Scholar]
- 122.Gonzalez-Velez AE, Forjaz MJ, Giraldez-Garcia C, Martin-Garcia S, Martinez-Martin P, Quality SRG, L. Quality of life by proxy and mortality in institutionalized older adults with dementia. Geriatr Gerontol Int. 2015;15(1):38–44. doi: 10.1111/ggi.12225. [DOI] [PubMed] [Google Scholar]
- 123.Mathews WC, May S. EuroQol (EQ-5D) measure of quality of life predicts mortality, emergency department utilization, and hospital discharge rates in HIV-infected adults under care. Health Qual Life Outcomes. 2007;5:5-. 10.1186/1477-7525-5-5 [DOI] [PMC free article] [PubMed]
- 124.Fernández M, Alarcón GS, McGwin G, Sanchez ML, Apte M, Vilá LM, et al. Using the Short Form 6D, as an overall measure of health, to predict damage accrual and mortality in patients with systemic lupus erythematosus: XLVII, results from a multiethnic US cohort. Arthritis Rheum. 2007;57(6):986–92. doi: 10.1002/art.22908. [DOI] [PubMed] [Google Scholar]
- 125.Dharma-Wardene M, Au HJ, Hanson J, Dupere D, Hewitt J, Feeny D. Baseline FACT-G score is a predictor of survival for advanced lung cancer. Qual Life Res. 2004;13(7):1209–16. doi: 10.1023/B:QURE.0000037481.36604.eb. [DOI] [PubMed] [Google Scholar]
- 126.Djärv T, Lagergren P. Six-month postoperative quality of life predicts long-term survival after oesophageal cancer surgery. Eur J Cancer. 2011;47(4):530–5. doi: 10.1016/j.ejca.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 127.Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications. JAMA Psychiatry. 2015;72(4):334. doi: 10.1001/jamapsychiatry.2014.2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Morgan VA, Waterreus A, Jablensky A, Mackinnon A, McGrath JJ, Carr V, et al. People living with psychotic illness in 2010: the second Australian national survey of psychosis. The Aust N Z J Psychiatry. 2012;46(8):735–52. doi: 10.1177/0004867412449877. [DOI] [PubMed] [Google Scholar]
- 129.De Hert M, Correll CU, Bobes J, Cetkovich-Bakmas M, Cohen D, Asai I, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52–77. doi: 10.1002/j.2051-5545.2011.tb00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 21 kb)