Abstract
Seven dextran-producing Leuconostoc strains were differentiated by using a modified randomly amplified polymorphic DNA (RAPD) protocol that incorporated specific primers designed from conserved regions of dextransucrase genes. RAPD profiles showed intraspecies differences among the Leuconostoc mesenteroides strains tested. This modified RAPD protocol will aid in the differentiation of polymer-producing leuconostocs, which are currently distinguished by time-consuming analyses of the dextrans they synthesize.
Leuconostocs are heterofermentative lactic acid bacteria that can produce extracellular polymers such as alternan, dextran, and levan from sucrose metabolism (2, 4). Dextrans are high-molecular-weight homopolymers composed of α-d-glucose with predominantly α-(1→6) linkages and have been used in a variety of commercial applications (2). Dextrans are structurally diverse and are characterized according to the percentage, nature, and distribution of their non-α(1→6) linkages (5). The exact molecular structure of a dextran is determined by the specific Leuconostoc strain that synthesizes the polymer (2, 5). Many Leuconostoc strains can produce more than one type of dextran, and the proportions of each polymer synthesized can vary depending on the culture conditions used (5, 19). Since dextran-producing leuconostocs are physiologically similar (4), differentiation of specific strains is often accomplished through unreliable colonial morphological comparisons of the microorganism and structural analyses of the specific polymer produced. Differences in dextran structure are determined by time-consuming and elaborate methods such as gas chromatography-mass spectrometry of methylated dextran derivatives (15), 1H and 13C nuclear magnetic resonance spectrometry (14), or Fourier-transform infrared spectroscopy (13). The development of a convenient method to aid differentiation and identification of dextran-producing Leuconostoc strains would assist both basic and applied research.
Several DNA-based methods for the differentiation of Leuconostoc mesenteroides strains have been developed (6, 17); however, the protocols used either were time-consuming or did not significantly differentiate between strains. Random amplification of polymorphic DNA (RAPD) is a PCR method that incorporates a single arbitrarily designed oligonucleotide primer in the amplification reaction to generate DNA fragment polymorphisms (18, 21). The DNA fragment polymorphisms, or RAPD profiles, have been used to distinguish between closely related bacterial species (1, 11). The RAPD technique is simple and quick, and thus it would serve as a convenient alternative protocol to complement the methods currently used to distinguish dextran-producing Leuconostoc strains. The objective of this study was to determine if dextran-producing Leuconostoc strains could be differentiated by a modified RAPD protocol using primers designed from conserved regions of dextransucrase genes.
All Leuconostoc strains used in this study (NRRL B-512F, B-742, B-1118, B-1142, B-1149, B-1299, and B-1355) were obtained from the Agricultural Research Service culture collection (Peoria, Ill.) and are designated L. mesenteroides except for B-742, which is considered L. citreum (16). These Leuconostoc strains were chosen for this study because they are commonly used in research and for commercial applications (2, 7, 8, 10). Leuconostoc strains were grown in MRS (3) broth containing 2% glucose, and the genomic DNA was isolated according to the method of Pitcher et al. (12). Three oligonucleotide primers were used for RAPD analyses and were designed from conserved sequences of dextransucrase genes from Leuconostoc B-512F and B-1299 (9, 20). Two primers were designed from the N-terminal region of the B-512F dextransucrase gene (9, 20) and were designated 512Fa (5′-GATGCAGTCGACAATGTGGATGCT-3′) and 512Fb (5′-GTCATAAGGATCCTGTGAATGCATA-3′ [9]). Primers 512Fa and 512Fb were originally designed and used by Monchois et al. (9) to clone a dextransucrase gene from L. mesenteroides NRRL B-1299. One primer was designed from the C-terminal region of the B-1299 dextransucrase gene (9) and was designated 1299 [5′-(AGCT)CC(AG)TC(CT)TG(AGCT)CC(AG)AA(AG) TA(AGCT)ACCCA-3′]. (Degenerate positions within primer 1299 are indicated by parentheses.) Primer 1299 was designed degenerate in order to increase the number of DNA fragments detected. The typical 20-μl reaction mixture for RAPD PCR analysis of the Leuconostoc strains contained 1× Pfu buffer (Stratagene, La Jolla, Calif.), 250 μM (each) dATP, dCTP, dGTP, and dTTP (Stratagene), 1 μg of template DNA, 0.25 μg of a single oligonucleotide primer, and 1.5 U of Pfu DNA polymerase (Stratagene). A denaturation step of 95°C for 1 min was performed with the reaction mixture before PCR cycling. The RAPD PCR cycling program consisted of 40 cycles of denaturation at 95°C for 1 min, annealing at 30°C for 1 min, and primer extension at 72°C for 5 min. After the cycling program was finished, the samples were held at 72°C for 10 min to complete the extension of products. PCR amplification was performed in a Progene thermocycler (Techne Incorporated, Princeton, N.J.). The DNA banding patterns were examined by using 1.5% agarose gel electrophoresis, and a DNA ladder (0.25 to 12 kb) was used as size standards (Stratagene).
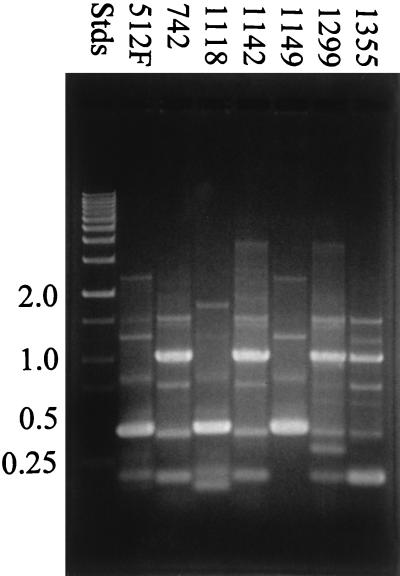
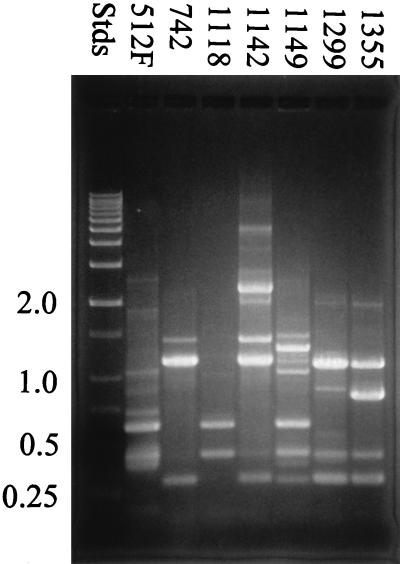
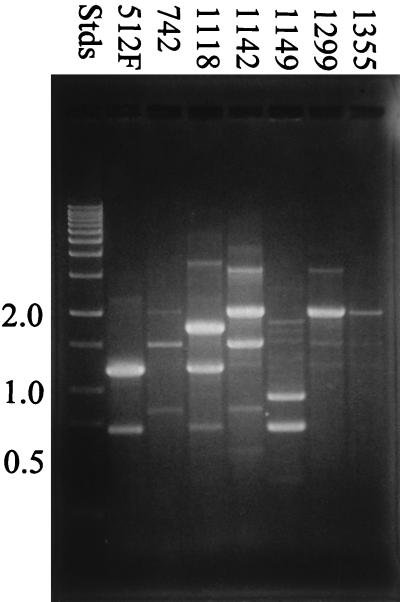
Results from the modified RAPD analysis using each primer are illustrated in Fig. 1 through 3. All seven Leuconostoc strains that were examined by RAPD analysis could be differentiated based on DNA banding patterns when all three oligonucleotide primers were used in separate tests. Many of the strains shared common DNA band sizes (for example, strains B-512F, B-1118, and B-1149 in Fig. 1, strains B-1142 and B-1299 in Fig. 2, and strains B-742 and B-1142 in Fig. 3), which is not unexpected, since all the strains except B-742 are designated L. mesenteroides. All the strains tested showed reproducible RAPD profiles based on DNA band size, number, and intensity after three trials.
FIG. 1.
RAPD analysis of dextran-producing Leuconostoc strains using primer 512Fa. RAPD profiles were examined by using 1.5% agarose gel electrophoresis. Stds, DNA standards.
FIG. 3.
RAPD analysis of dextran-producing Leuconostoc strains using primer 1299. RAPD profiles were examined by using 1.5% agarose gel electrophoresis. Stds, DNA standards.
FIG. 2.
RAPD analysis of dextran-producing Leuconostoc strains using primer 512Fb. RAPD profiles were examined by using 1.5% agarose gel electrophoresis. Stds, DNA standards.
All the dextran-producing Leuconostoc strains tested could be differentiated from each other by RAPD PCR amplification using a combination of primers that were designed from conserved regions of dextransucrase genes. RAPD analysis showed intraspecies differences between the dextran-producing strains examined. Use of all three primers for RAPD analysis would ensure accurate differentiation of dextran-producing Leuconostoc strains when control strains are used. It is not clear why long (24-mer), specific primers produced polymorphic DNA banding patterns, since short, arbitrary primers are usually used in RAPD PCRs. Only two Leuconostoc dextransucrase genes have been cloned (from B-512F [20] and B-1299 [9]), and these displayed 55% amino acid sequence similarity (9). Evidence has indicated that many polymer-producing Leuconostoc strains produce more than one type of dextransucrase (2), which may account for some of the DNA polymorphisms observed during RAPD analysis. L. mesenteroides B-512F, however, has only one type of dextransucrase (2) but still displays DNA polymorphism when the modified RAPD protocol is followed. The presence of multiple dextransucrase genes may not account for all the DNA polymorphism observed when the modified RAPD protocol is used. Although the exact molecular mechanism defining the modified RAPD protocol is unknown, this should not preclude its use as a tool to differentiate polymer-producing Leuconostoc strains.
RAPD analysis was proven to be a simple and reproducible method for differentiation of the dextran-producing Leuconostoc strains compared to other strain characterization methods which involve polymer structural analyses. This study provides a basis for molecular differentiation of dextran-producing Leuconostoc strains that are used in basic and applied research.
Acknowledgments
We thank Richard Greene, Jeffrey Ahlgren, and Timothy Leathers for their contributions.
REFERENCES
- 1.Cocconcelli P S, Porro D, Galandini S, Senini L. Development of RAPD protocol for typing of strains of lactic acid bacteria and enterococci. Lett Appl Microbiol. 1995;21:376–379. doi: 10.1111/j.1472-765x.1995.tb01085.x. [DOI] [PubMed] [Google Scholar]
- 2.Cote G L, Ahlgren J A. Kirk-Othmer encyclopedia of chemical technology. 4th ed. Vol. 16. New York, N.Y: John Wiley & Sons, Inc.; 1995. Microbial polysaccharides; pp. 578–611. [Google Scholar]
- 3.deMan J C, Rogosa M, Sharpe M. A medium for the cultivation of lactobacilli. J Appl Bacteriol. 1960;23:130–135. [Google Scholar]
- 4.Holzapfel W H, Schillinger U. The genus Leuconostoc. In: Balows A, Truper H, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes. 2nd ed. New York, N.Y: Springer-Verlag; 1992. pp. 1500–1535. [Google Scholar]
- 5.Jeanes A, Haynes W C, Wilham C A, Rankin J C, Melvin E H, Austin M J, Cluskey J E, Fisher B E, Tsuchiya H M, Rist C E. Characterization and classification of dextrans from ninety-six strains of bacteria. J Am Chem Soc. 1954;76:5041–5052. [Google Scholar]
- 6.Kelly W J, Asmundson R V, Harrison G L, Huang C M. Differentiation of dextran-producing Leuconostoc strains from fermented rice cake (puto) using pulsed-field gel electrophoresis. Int J Food Microbiol. 1995;26:345–352. doi: 10.1016/0168-1605(94)00146-w. [DOI] [PubMed] [Google Scholar]
- 7.Kim D, Robyt J F. Production and selection of mutants of Leuconostoc mesenteroides constitutive for glucansucrases. Enzyme Microb Technol. 1994;16:659–664. doi: 10.1016/0141-0229(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 8.Martinez-Murcia A J, Collins M D. A phylogenetic analysis of the genus Leuconostoc based on reverse transcriptase sequencing of 16S rRNA. FEMS Microbiol Lett. 1990;70:73–84. doi: 10.1016/0378-1097(90)90106-z. [DOI] [PubMed] [Google Scholar]
- 9.Monchois V, Willemot R-M, Remaud-Simeon M, Croux C, Monsan P. Cloning and sequencing of a gene for a novel dextransucrase from Leuconostoc mesenteroides NRRL-B-1299 synthesizing only α(1→6) and α(1→3) linkages. Gene. 1996;182:23–32. doi: 10.1016/s0378-1119(96)00443-x. [DOI] [PubMed] [Google Scholar]
- 10.Monsan P, Paul F. Enzymatic synthesis of oligosaccharides. FEMS Microbiol Rev. 1995;16:187–192. [Google Scholar]
- 11.Permaul K, Pillay D, Pillay B. Random-amplified polymorphic DNA (RAPD) analysis shows intraspecies differences among Xanthomonas albilineans strains. Lett Appl Microbiol. 1996;23:307–311. doi: 10.1111/j.1472-765x.1996.tb00196.x. [DOI] [PubMed] [Google Scholar]
- 12.Pitcher D G, Saunders N A, Owen R J. Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Lett Appl Microbiol. 1989;8:151–156. [Google Scholar]
- 13.Seymour F R, Julian R L. Fourier-transform, infrared difference spectrometry for structural analysis of dextrans. Carbohydr Res. 1979;74:63–75. [Google Scholar]
- 14.Seymour F R, Knapp R D, Lamberts B L. Structural analysis of soluble d-glucans from strains of Streptococcus mutans by 13C-nuclear magnetic resonance spectrometry. Carbohydr Res. 1980;84:187–195. [Google Scholar]
- 15.Seymour F R, Slodki M E, Plattner R D, Jeanes A. Six unusual dextrans: methylation structural analysis by combined G.L.C.-M.S. of per-o-acetyl-aldononitriles. Carbohydr Res. 1977;53:153–166. [Google Scholar]
- 16.Takahashi M, Okada S, Uchimura T, Kozaki M. Leuconostoc amelibiosum Schillinger, Holzapfel, and Kandler 1989 is a later subjective synonym of Leuconostoc citreum Farrow, Facklam, and Collins 1989. Int J Syst Bacteriol. 1992;42:649–651. [Google Scholar]
- 17.Villani F, Moschetti G, Blaiotta G, Coppola S. Characterization of strains of Leuconostoc mesenteroides by analysis of soluble whole-cell protein pattern, DNA fingerprinting and restriction of ribosomal DNA. J Appl Microbiol. 1997;82:578–588. doi: 10.1111/j.1365-2672.1997.tb03588.x. [DOI] [PubMed] [Google Scholar]
- 18.Welsh J, McClelland M. Fingerprinting genomes using PCR with arbitrary primers. Nucleic Acids Res. 1990;18:7213–7218. doi: 10.1093/nar/18.24.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilham C A, Alexander B H, Jeanes A. Heterogeneity in dextran preparations. Arch Biochem Biophys. 1955;59:61–75. doi: 10.1016/0003-9861(55)90463-x. [DOI] [PubMed] [Google Scholar]
- 20.Wilke-Douglas, M., J. T. Perchorowicz, C. M. Houck, and B. R. Thomas. December 1989. Methods and compositions for altering physical characteristics of fruit and fruit products. Patent WO 89/12386.
- 21.Williams J G K, Kubelik A R, Livak K J, Rafalski J A, Tingey S V. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990;18:6531–6535. doi: 10.1093/nar/18.22.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]