Abstract
The present study investigated the potential ability of Salvia officinalis, one of the oldest medicinal plants, to protect male rats against cadmium reproductive toxicity. Twenty-eight healthy male rats were randomly allocated into four groups (n = 7); control, Salvia-extract treated group, cadmium treated group and a group treated with both Cd and Salvia. Administration of cadmium reduced the relative testis to body weight and significantly affected sperm parameters by decreasing motility, viability, count and increasing morphological aberrations. Comet assay was used to detect DNA fragmentation in sperms of the rats exposed to Cd. Serum levels of testosterone T, follicle stimulating hormone FSH, and luteinizing hormone LH were significantly decreased. The biochemical analysis of testicular tissue showed a significant rise in Malondialdehyde MDA level coupled with a decrease in the activity of antioxidant enzymes (superoxide dismutase SOD, glutathione peroxidase GPx and catalase CAT). The histological examination of testis sections after Cd administration revealed severe degeneration of spermatogenic cells. Seminiferous tubules were filled with homogenous eosinophilic fluid associated with atrophy of other seminiferous tubules. Co-treatment with the Salvia officinalis extract restored the oxidative enzymes activities and decreased the formation of lipid peroxidation byproduct, which in turn ameliorated the effect of Cd on sperm parameters, DNA damage and testis histopathology. Taken together, it can be concluded that the synergistic antioxidant and radical savaging activities of Salvia officinalis prevented the effect of Cd on semen quality, sperm DNA damage, along with the oxidative stress and histological abnormalities in the testis tissues.
Subject terms: Cell biology, Developmental biology, Molecular biology, Zoology
Introduction
Despite revealing many potential root causes for male infertility, the etiology of about 50% of the cases remained obscure1. Exposure to environmental pollutants, including heavy metals, has been linked to male infertility2. Several studies on animal models (specially rodents) and the data collected from human epidemiological studies proved that cadmium (Cd) exposure induces various reproductive health impairments3. It affects male fertility through several routes: by decreasing sperm motility and viability, undermining the endocrine function, impairing spermatogenesis, inducing structural damage of testicular tissue, and sabotage function in Sertoli and Leydig cells4–7.
Some recent studies showed that the bioactive components of Salvia officinalis, one of the oldest used medicinal plants, have potent anti-inflammatory and antioxidant effects8,9 which might result in alleviating the reproductive toxicity of Cd that works mainly through inflammatory and oxidative stress mechanisms4.
Traditional semen analysis is based on sperm concentration, motility, and morphology10,11. Semen examination of poorly fertile males usually reveals sperms with low count, decreased motility, or abnormal morphology12. Strikingly, 15% of males with normal traditional analysis profiles still showed fertility problems. Therefore, to duly diagnose male infertility, it may be necessary to measure other sperm parameters13.
DNA fragmentation is an important factor in the etiology of male infertility. However, its inclusion in routine semen analysis is still under evaluated. DNA fragmentation was shown to be a major indicator of fertility potential, even more than conventional semen parameters13. Comet assay for sperms or testicular cells have been applied in several studies to evaluate effect of reproductive toxins and genotoxins in male human and rats14,15.
Serum levels of FSH, LH and T are used as good indicators of fertility status16,17. Low serum levels of these hormones were previously discovered in male rats exposed to cadmium18.
Histopathological evaluation provides one of the most sensitive methods to identify the probable target cell. Careful examination of the earliest morphological and microscopical changes shed some light on the possible mechanisms of male reproductive system toxicants19,20.
The morphological and molecular damage imposed by Cd in testicular tissue were mainly attributed to the oxidative stress4,21. For assessment of the oxidative stress in testicular tissue, the levels of malondialdehyde (MDA) and the enzyme activities of catalase (CAT), glutathione peroxidase (GPx) and superoxide dismutase (SOD) are analyzed spectrophotometrically22,23. The current study aims at determining the potential ameliorative role of Salvia officinalis against Cd-induced reproductive toxicity in rats.
Materials and methods
Materials
Cadmium chloride (CdCl2) was purchased from Sigma-Aldrich (St. Louis, MO, USA). High quality Salvia officinalis, commonly known as Sage, was purchased from a local shop.
Preparation and identification of Salvia officinalis extract
The bioactive components of the leaves of Salvia officinalis were extracted with methanol at room temperatures using the protocol mentioned by Alkan et al.24. The extract was analysed for phenol content using High Performance Liquid Chromatography (HPLC) (Agilent 1260 infinity, Waldbronn, Germany). All experiments and procedures comply with the IUCN Policy Statement on Research Involving Species at Risk of Extinction and the Convention on the Trade in Endangered Species of Wild Fauna and Flora.
Experimental animals
Twenty-eight adult male Wistar rats of 8 weeks old (weighing 180–200 g) were purchased from the Experimental Animal Research Center, Faculty of Pharmacy, Kafr El sheikh University, Egypt. The animals were maintained in a climate-controlled room under a 12-h alternating light/dark cycle, 20.1 to 21.2 °C temperature and 50 to 55.5% relative humidity. The rats were fed standard chow and water ad libitum. All experiments and procedures were performed in accordance with ARRIVE guidelines and were consented by The Institutional Animal Care and Use Committee (IACUC), Faculty of Science, Cairo University with the approval number (CU/I//F/14/23). All experiments were performed in accordance with relevant guidelines and regulations.
Experimental design
Wistar rats were allotted into four groups, each with seven rats as follows:
Group 1 was injected intraperitoneally (i.p.) with saline (vehicle of CdCl2) and gauged with water (vehicle of Salvia officinalis) to serve as the control group. Group 2 orally received 200 mg/kg of the methanolic extract of Salvia officinalis daily for 8 weeks via an oral tube25. Group 3 received a single dose of 1 mg/kg of CdCl2 dissolved in saline solution through i.p. injection, according to Chen et al.26. Group 4 received a single i.p. dose of Cd as in group 3 simultaneously with the 8 weeks extract treatment as in group 2.
Semen analysis
Sperm motility and count
Epididymal suspension with sperms was poured into counting chamber of the hemocytometer, covered with cover slip, and examined under a phase-contrast microscope with a warming plate at 40× magnification. Sperm motility was calculated according to the procedure described by El-Magd et al.27 and sperm count according to Yokoi et al.28. Approximately, five fields per sample were randomly examined.
Sperm viability and morphology
Sperm viability and abnormalities were examined using eosin-nigrosin (EN) staining (Sigma-Aldrich, Saint Louis, USA). A minimum of 200 spermatozoa were analyzed from each animal under 400× magnification with bright-field microscopy and the proportion of viable sperm cells and morphologically abnormal spermatozoa was recorded according to the criteria described by Okamura et al.29.
Assessment of DNA fragmentation by comet assay (single cell gel electrophoresis, SCGE)
A sperm cell pellet (about 1 g) was homogenized in 1 ml of cold mincing solution then suspended in darkness. The extent of DNA strand breaks in control and treated cells of both cell lines was assessed using the alkaline comet assay, as previously described by Tice et al. (2000)30. Comets were analyzed using Komet 5.0 analysis system developed by Kinetic Imaging, Ltd. (Liverpool, United Kingdom) connected to a charge-coupled device (CCD) camera and 40× objective of fluorescent microscope with excitation filter 420–490 nm (issue 510 nm).
Hormonal assay
Levels of serum hormones were measured using commercial ELISA kits for luteinizing hormone (LH) (Cat. No. BC-1029, BioCheck, Foster city, CA, USA), follicle-stimulating hormone (FSH) (Cat. No. RH-251, DSI, Milan, Italy), and testosterone (Cat. No. CAN-TE- 250, DBC, Ontario, Canada). The procedures were carried out following the manufacturer's protocol and the absorbance was measured at 450 nm.
Estimation of oxidative stress markers
The effect of exposure to cadmium and/or Salvia officinalis on oxidative stress markers in the testicular tissue were measured colorimetrically using commercial kits (Bio-diagnostic, Cairo, Egypt). The procedures were carried out according to the manual provided by the manufacturer to measure the level of malondialdehyde (MDA) (Cat. No. MD 25 29) and the activities of superoxide dismutase (SOD) (Cat. No. SD 25 21), Catalase (CAT) (Cat. No. CA 25 17), and glutathione peroxidase (GPx) (Cat. No. GP 2524).
Histopathological studies
Testis tissue samples from the testis were immediately removed from the euthanized rats and fixed using 10% neutral buffered formalin (Sigma-Aldrich, Saint Louis, USA), sectioned, and stained with Hematoxylin and Eosin (Sigma-Aldrich, Saint Louis, USA) for routine examination31. Histopathological quantitative scoring was assessed according to Venditti et al.32. Testicular tissue was evaluated using the image analysis system Leica QWin DW3000 (LEICA Imaging Systems Ltd., Cambridge, England) for tubular diameter, epithelium thickness, lumen diameter, spermatic cells degeneration, atrophied seminiferous tubules, and seminiferous tubules with oedema. A comprehensive count of 175 seminiferous tubules per group (25/animal) was conducted.
Statistical analysis
Data were analyzed using statistical software package (IBM-SPSS) version 23 software. Kolmogorov–Smirnov test showed that the raw data were normally distributed. One-way ANOVA was applied to study the effect of treatment on the studied parameters. Least significant difference (LSD) test was used to illustrate the statistical differences among the experimental groups. Data is displayed as mean ± standard error of mean (Supplementary table).
Results
Identification of Salvia officinalis methanolic extract using HPLC
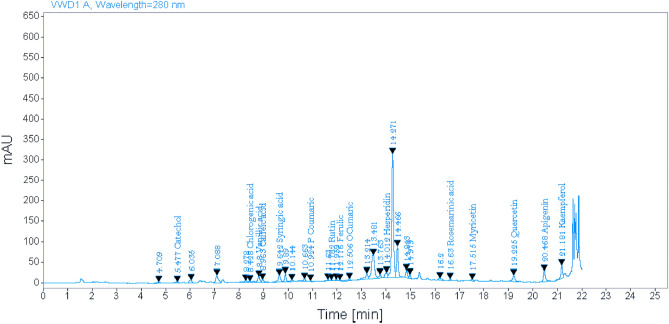
HPLC for the methanolic extract of Salvia officinalis verified the presence of 15 phenolic compounds: catechol, chlorogenic acid, vanillic acid, caffeic acid, syringic acid, p-coumaric acid, rutin, ferulic, o-coumaric acid, hesperidin, rosemarinic acid, myricetin, quercetin, apigenin, and kaempferol (Fig. 1).
Figure 1.
HPLC analysis for methanolic extract of Salvia officinalis.
Relative testis to body weight (g/g)
The examined rats of all the four groups (control group, Salvia officinalis treated group, Cd treated group, Cd + Salvia treated group) did not show any obvious sign of toxicity or abnormal behavior. However, rats of group 3 and 4 recorded a slightly less body weight gain and a notable decrease in testis weight. As shown in Table. 1, the relative testis weight in Cd group (0.005 ± 0.0005) was significantly decreased (P ≤ 0.05) compared to the control group (0.015 ± 0.001). Rats treated with Salvia extract showed no significant change in relative testis weight (0.017 ± 0.001) compared to the control group. The co-administration of Salvia and Cd showed a significant testis weight ratio decrease (0.010 ± 0.001) compared to the control group, and a significant increase when compared to the average weight recorded in Cd group.
Table 1.
Average testis to body weight for all rats in the four studied groups.
| Group | Mean of relative testis/body weight (g/g) |
|---|---|
| Control (G1) | 0.015 ± 0.001 |
| Salvia officinalis extract treated group (G2) | 0.017 ± 0.001 |
| P1 = 0.18 | |
| Cadmium treated group (G3) | 0.005 ± 0.0005* |
| P1 = 0.00 | |
| Cadmium and extract treated group (G4) | 0.010 ± 0.001*# |
| P1 = 0.02, P2 = 0.01 |
*Significant difference (P1), as compared to the control group.
#Significant difference (P2), as compared to the Cd-treated group.
Semen analysis
Sperm motility (%)
The percentage of sperm motility in the control group was 91.25 ± 3.27. In the rats treated with Salvia extract, the sperm motility was 90.42 ± 3.54 showing no significant change compared to the control group. In the group treated with cadmium, the recorded sperm motility (62.31 ± 2.26) was significantly decreased (P ≤ 0.05) compared to the control one. The co-administration of Salvia with Cd showed a significant decrease in sperm motility (76.27 ± 3.12) compared to the control group, and a significant increase when compared to the percentage of sperm motility recorded in Cd group (Table 2).
Table 2.
Effect of cadmium or/and Salvia officinalis on sperm parameters in the semen collected from all rats in the four studied groups.
| Group | Mean of percentage of sperm motility (%) | Mean of sperm count (106/ml) | Mean of percentage viability of sperm (%) | Mean of sperm abnormalities (%) |
|---|---|---|---|---|
| Control (untreated) (G1) | 91.25 ± 3.27 | 74.48 ± 5.02 | 93.37 ± 3.1 | 8.66 ± 0.52 |
| Salvia officinalis extract treated group (G2) | 90.42 ± 3.54 | 72.56 ± 4.11 | 92.42 ± 3.54 | 8.15 ± 0.46 |
| P1 = 0.86 | P1 = 0.73 | P1 = 0.83 | P1 = 0.68 | |
| Cadmium treated group (G3) | 62.31 ± 2.26* | 36.51 ± 2.32* | 62.31 ± 2.26* | 20.28 ± 1.40* |
| P1 = 0.00 | P1 = 0.00 | P1 = 0.00 | P1 = 0.00 | |
| Cadmium and extract treated group (G4) | 76.27 ± 3.12*# | 51.6 ± 3.05*# | 76.27 ± 3.12*# | 14.36 ± 0.72*# |
| P1 = 0.01, P2 = 0.01 | P1 = 0.00, P2 = 0.02 | P1 = 0.00, P2 = 0.01 | P1 = 0.00, P2 = 0.00 |
*Significant difference (P1), as compared to the control group.
#Significant difference (P2), as compared to the Cd-treated group.
Sperm count (106/ml)
The mean value of sperm count in semen samples of rats in the control group was 74.48 ± 5.02. In the group treated with Salvia extract, the mean value of sperm count was 72.56 ± 4.11 showing no significant change compared to the control group. In the group treated with cadmium, the recorded sperm count mean (36.51 ± 2.32) was significantly decreased (P ≤ 0.05) compared to the control group. Despite showing a significant decrease in sperm count (51.6 ± 3.05) in the fourth group (treated with Cd and Salvia) compared to the control group, the value was significantly higher than the mean value of sperm count recorded in Cd treated group (Table 2).
Sperm viability (%)
The percentage of sperm viability in the control group was 93.37 ± 3.1. In the group treated with Salvia extract, the viability percentage was 92.42 ± 3.54 showing no significant change compared to the control group. In the group treated with cadmium, the recorded viability percentage (62.31 ± 2.26) was significantly decreased (P ≤ 0.05) compared to the control group. The co-administration of Salvia with Cd showed a significant decrease in percentage of viability (76.27 ± 3.12) compared to the control group, and a significant increase when compared to the sperm viability recorded in Cd exposed group (Table 2).
Sperm abnormalities (%)
The recorded percentage of sperm abnormalities in control group was 8.66 ± 0.52. In the group treated with Salvia extract, the percentage of sperm abnormalities was 8.15 ± 0.46 showing no significant change compared to the control group. In the group treated with cadmium, the recorded percentage of sperm abnormalities (20.28 ± 1.4) was significantly increased (P ≤ 0.05) compared to the control group. In Salvia + Cd group showed a significant decrease in percentage of sperm abnormalities (14.36 ± 0.72) compared to Cd group. However, it was still significantly higher than the percentage of sperm abnormalities recorded in the control group (Table 2).
Assessment of DNA fragmentation in sperms by comet assay (Single cell gel electrophoresis (SCGE)
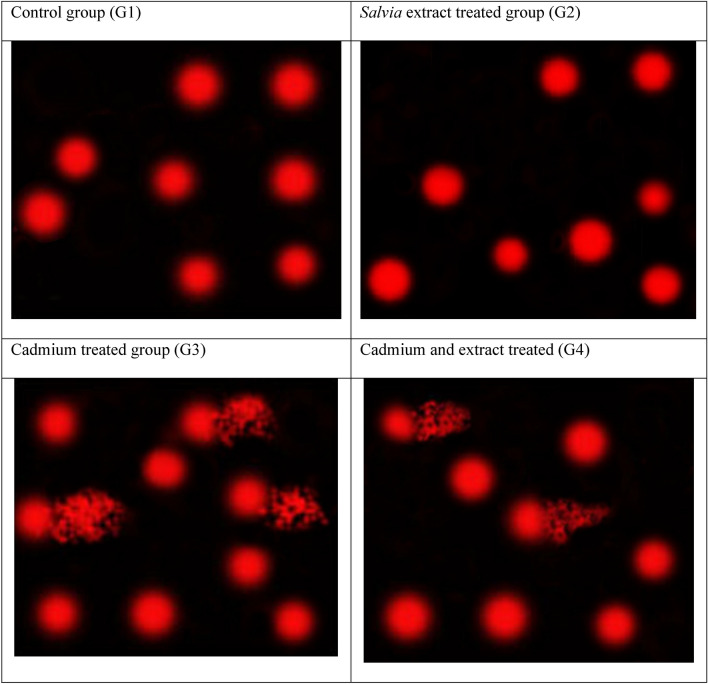
In Salvia extract treated group, the average tail length was 2.11 ± 0.10 showing no significant change compared to the control group (1.62 ± 0.12). In the group treated with cadmium, the recorded average tail length (8.35 ± 0.31) was significantly increased (P ≤ 0.05) compared to the control group. The co-administration of Salvia + Cd showed a significant increase in tail length (7.03 ± 0.23) when compared to the control group, and a significant decrease when compared to Cd group (Table 3, Fig. 2).
Table 3.
Comet assay parameters obtained by image analysis for sperms collected from all rats in the four groups.
| Group | Tailed % | Untailed % | Tails length µm | Tail DNA% | Tail moment (arbitrary units) |
|---|---|---|---|---|---|
| Control (untreated) (G1) | 2 | 98 | 1.62 ± 0.12 | 1.64 ± 0.05 | 2.66 ± 0.03 |
| Salvia officinalis extract treated group (G2) | 3 | 97 | 2.11 ± 0.10 | 1.99 ± 0.11 | 4.20 ± 0.12 |
| P1 = 0.13 | P1 = 0.14 | P1 = 0.46 | |||
| Cadmium treated group (G3) | 25 | 75 | 8.35 ± 0.31* | 6.93 ± 0.22* | 57.86 ± 2.23* |
| P1 = 0.00 | P1 = 0.00 | P1 = 0.00 | |||
| Cadmium and extract treated group (G4) | 18 | 82 | 7.03 ± 0.23*# | 5.28 ± 0.16*# | 37.12 ± 1.67*# |
| P1 = 0.00, P2 = 0.00 | P1 = 0.00, P2 = 0.00 | P1 = 0.00, P2 = 0.00 |
*Significant difference (P1), as compared to the control group.
#Significant difference (P2), as compared to the Cd-treated group.
Figure 2.
Photomicrographs showing the extent of DNA damage in sperms of rats in all groups as detected by comet assay. G1: Control, G2: Salvia extract treated group, G3: Cadmium treated group, G4: Cadmium and extract treated group.
Serum hormonal levels
The average serum concentrations of FSH, LH and testosterone in the control group (G1) were 6.21 ± 0.31 U/ml, 33.48 ± 0.56 U/ml, and 7.52 ± 0.59 ng/ml respectively. The administration of Salvia extract alone did not significantly affect the serum levels of any of the hormones recording 6.83 ± 0.27 U/ml for FSH, 32.17 ± 0.58 U/ml for LH, and 6.93 ± 0.64 ng/ml for T. The cadmium administration resulted in a significant decrease in the serum concentration levels of all of the three hormones compared to the control group recording 3.36 ± 0.16 U/ml for FSH, 24.33 ± 0.36 U/ml for LH, and 1.45 ± 0.08 ng/ml for T. Meanwhile, in Salvia + Cd group, despite recording serum level concentrations significantly higher than those recorded for Cd group (5.02 ± 0.19 U/ml, 28.42 ± 0.31 U/ml, and 4.04 ± 0.24 ng/ml) respectively, the values were still significantly less than those recorded in the control group (Table 4).
Table 4.
Levels of follicle stimulating hormone (FSH), Luteinizing hormone (LH) and testosterone (T) in serum samples collected from all rats in the four studied groups.
| Group | Means of serum level of FSH (U/ml) | Means of serum level of LH (U/ml) | Means of serum level of T (ng/ml) |
|---|---|---|---|
| Control (G1) | 6.21 ± 0.31 | 33.48 ± 0.56 | 7.52 ± 0.59 |
| Salvia officinalis extract treated group (G2) | 6.83 ± 0.27 | 32.17 ± 0.58 | 6.93 ± 0.64 |
| P1 = 0.11 | P1 = 0.08 | P1 = 0.38 | |
| Cadmium treated group (G3) | 3.36 ± 0.16* | 24.33 ± 0.36* | 1.45 ± 0.08* |
| P1 = 0.00 | P1 = 0.00 | P1 = 0.00 | |
| Cadmium and extract treated group (G4) | 5.02 ± 0.19*# | 28.42 ± 0.31*# | 4.04 ± 0.24*# |
| P1 = 0.01, P2 = 0.00 | P1 = 0.00, P2 = 0.00 | P1 = 0.00, P2 = 0.00 |
*Significant difference (P1), as compared to the control group.
#Significant difference (P2), as compared to the Cd-treated group.
Detection of oxidative stress markers
The average level of MDA in the testicular tissue of control group rats was 15.11 ± 0.74 nmol/g tissue. While in the group treated with Salvia extract, the average level of MDA was 14.02 ± 0.77 nmol/g tissue showing no significant change compared to the control group. In the group treated with cadmium, the recorded average MDA level (28.48 ± 1.25 nmol/g tissue) was significantly increased (P ≤ 0.05) compared to the control group. The co-administration of Salvia showed a significant increase in MDA level (19.35 ± 0.92 nmol/g tissue) compared to the control group, and a significant decrease when compared to the average MDA-level recorded in Cd group (Table 5).
Table 5.
Malondialdehyde (MDA) levels in testes of rats in the four studied groups (nmol/g tissue).
| Group | Means of testicular levels of MDA (nmol/g) |
|---|---|
| Control (G1) | 15.11 ± 0.74 |
| Salvia officinalis extract treated group (G2) | 14.02 ± 0.77 |
| P1 = 0.44 | |
| Cadmium treated group (G3) | 28.48 ± 1.25* |
| P1 = 0.00 | |
| Cadmium and extract treated group (G4) | 19.35 ± 0.92*# |
| P1 = 0.01, P2 = 0.00 |
*Significant difference (P1), as compared to the control group.
#Significant difference (P2), as compared to the Cd-treated group.
The average testicular levels of superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx) in the control group rats were (1117.26 ± 74.5, 251.6 ± 74.5 and 12.41 ± 0.72 U/g tissue) respectively. In the group exposed to Salvia extract alone, the testicular levels of the enzymes were not significantly affected recording 1206.93 ± 71.34 U/g for SOD, 278.75 ± 15.36 U/g for CAT, and 13 ± 0.75 U/g for GPx. The testis tissue of the cadmium group showed a significant decrease in the activities of the three enzymes compared to the control group recording 648.6 ± 40.37 U/g for SOD, 128.17 ± 6.42 U/g for CAT, and 7.14 ± 0.37 U/g for GPx. The co-administration of Salvia + Cd despite recording testicular activities significantly less than the control group (906.34 ± 52.65, 182.54 ± 8.7, and 10.25 ± 0.42 U/g tissue) respectively, they were significantly higher than the Cd group testicular activities (Table 6).
Table 6.
The mean value for testicular activity of superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx) in the four studied groups (U/g tissue).
| Group | SOD (U/g tissue) | CAT (U/g tissue) | GPx (U/g tissue) |
|---|---|---|---|
| Control (G1) | 1117.26 ± 74.5 | 251.6 ± 13.49 | 12.41 ± 0.72 |
| Salvia officinalis extract treated group (G2) | 1206.93 ± 71.34 | 278.75 ± 15.36 | 13.00 ± 0.75 |
| P1 = 0.33 | P1 = 0.14 | P1 = 0.50 | |
| Cadmium treated group (G3) | 648.6 ± 40.37* | 128.17 ± 6.42* | 7.14 ± 0.37* |
| P1 = 0.00 | P1 = 0.00 | P1 = 0.00 | |
| Cadmium and extract treated group (G4) | 906.34 ± 52.65*# | 182.54 ± 8.70*# | 10.25 ± 0.42*# |
| P1 = 0.04, P2 = 0.02 | P1 = 0.00, P2 = 0.01 | P1 = 0.03, P2 = 0.01 |
*Significant difference (P1), as compared to the control group.
#Significant difference (P2), as compared to the Cd-treated group.
Histopathology of testis tissue
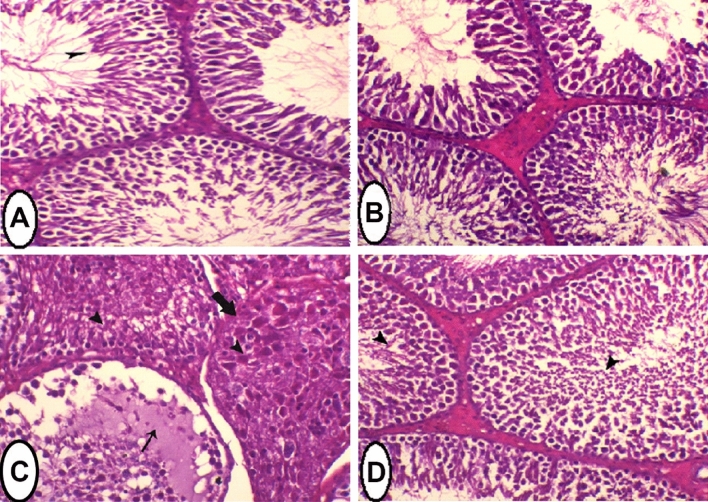
In the control group, the testis sections showed normal appearance of seminiferous tubules, spermatogenic cells with active spermatogenesis and normal interstitial cells. Sections taken from rats treated with Salvia extract showed an increase in the number of seminiferous tubules within the normal limits. In Cd treated group, the testis sections showed severe degeneration of spermatogenic cells. Seminiferous tubules were filled with homogenous eosinophilic fluid associated with complete atrophy of some of the seminiferous tubules. These histopathological changes were reduced after co-administration of Salvia, and the testes tissue showed improvements in spermatogenesis accompanied with seminiferous tubules regeneration with only mild spermatid layer degeneration (Fig. 3). The histological examination was validated by the recording of six morphometric parameters (Table 7). The tubular diameter, epithelial thickness, lumen diameter, numbers of degenerated spermatogenic cells, atrophied seminiferous tubules, and seminiferous tubules with eosinophilic fluid in the control group (G1) were 278.49 ± 0.72, 187.41 ± 2.65, 91.08 ± 2.74, 5.43 ± 0.48, 00.00 ± 0.00, and 00.00 ± 0.00, respectively. The administration of Salvia extract alone (G2) did not significantly affect these parameters recording 274.02 ± 1.14 for tubular diameter, 180.67 ± 3.16 for epithelial thickness, 93.35 ± 3.77 for lumen diameter, 8.86 ± 0.63 for the number of degenerated spermatogenic cells, and 00.00 ± 0.00 for the numbers of atrophied seminiferous tubules and seminiferous tubules with eosinophilic fluid. In Cd treated group, a significant decrease in the tubular diameter (221.99 ± 9.87) and epithelial thickness (98.24 ± 2.06) was recorded along with a non-significant increase in lumen diameter (123.76 ± 10.89). Moreover, the numbers of degenerated spermatogenic cells (109.29 ± 3.62), atrophied seminiferous tubules (4.71 ± 0.57), and seminiferous tubules with eosinophilic fluid (3.29 ± 0.36) were significantly elevated compared to the control group. On the other hand, the Cd + Salvia treated group demonstrated a significant increase in tubular diameter and epithelial thickness (244.67 ± 3.52 and 146.23 ± 5.62, respectively) compared to Cd group but still significantly lower than control group. Despite the significant reduction in the lumen diameter (98.44 ± 7.64), numbers of degenerated spermatogenic cells (32.29 ± 3.15), atrophied seminiferous tubules (1.29 ± 0.18), and seminiferous tubules with eosinophilic fluid (1.14 ± 0.26) compared to cadmium group, these values were still significantly higher than those recorded in control group.
Figure 3.
Sections in testis of rats of all the four groups stained with H & E. X = 400. (A) Testis of control (normal) rat showing normal seminiferous tubules with active spermatogenesis, arrowhead indicates free sperm. (B) Testis of rat treated with Salvia extract showing several seminiferous tubules within the normal limits. (C) Testis of rat treated with cadmium showing severe degeneration of spermatogenic cells (arrowhead). Seminiferous tubules were filled with homogenous eosinophilic fluid (thin arrow) associated with atrophy of other seminiferous tubules (thick arrow). (D) Testis of cadmium-intoxicated rat treated with Salvia extract showing improvement in spermatogenesis accompanied with seminiferous tubules regeneration with only mild spermatid layer degeneration (arrowheads).
Table 7.
Testicular morphometric parameters from all rats in the four studied groups.
| Group | Tubular diameter (µm) | Epithelium thickness (µm) | Lumen diameter (µm) | Number of degenerated spermatogenic cells | Number of atrophied seminiferous tubules | Number of seminiferous tubules with eosinophilic fluid |
|---|---|---|---|---|---|---|
| Control (untreated) (G1) | 278.49 ± 0.72 | 187.41 ± 2.65 | 91.08 ± 2.74 | 5.43 ± 0.48 | 00.00 ± 0.00 | 00.00 ± 0.00 |
| Salvia officinalis extract treated group (G2) | 274.02 ± 1.14 | 180.67 ± 3.16 | 93.35 ± 3.77 | 8.86 ± 0.63 | 00.00 ± 0.00 | 00.00 ± 0.00 |
| P1 = 0.93 | P1 = 0.57 | P1 = 0.99 | P1 = 0.75 | P1 = 1.00 | P1 = 1.00 | |
| Cadmium treated group (G3) | 221.99 ± 9.87* | 98.24 ± 2.06* | 123.76 ± 10.89* | 109.29 ± 3.62* | 4.71 ± 0.57* | 3.29 ± 0.36* |
| P1 = 0.00 | P1 = 0.00 | P1 = 0.002 | P1 = 0.00 | P1 = 0.00 | P1 = 0.00 | |
| Cadmium and extract treated group (G4) | 244.67 ± 3.52*# | 146.23 ± 5.62*# | 98.44 ± 7.64# | 32.29 ± 3.15*# | 1.29 ± 0.18*# | 1.14 ± 0.26*# |
| P1 = 0.001, P2 = 0.03 | P1 = 0.00, P2 = 0.00 | P1 = 0.8, P2 = 0.08 | P1 = 0.00, P2 = 0.00 | P1 = 0.03, P2 = 0.00 | P1 = 0.007, P2 = 0.00 |
*Significant difference (P1), as compared to the control group.
#Significant difference (P2), as compared to the Cd-treated group.
Discussion
In the current study, administration of Cd reduced relative testis to body weight in the treated male rats. The decreased testis weight might be attributed to the necrotic and degenerative cadmium-induced changes7. Similar results were found by Blanco et al.20, when they found a significant decrease in testis weight of mice after cadmium exposure. We also found that the co-administration of Salvia officinalis ameliorated the effect of Cd, and the testis weight was significantly increased. Similar results were obtained by Chouabia et al.33 against cypermethrin- induced reprotoxicity in male Wistar rats. This weight gain might be due to the regulation of signal transduction pathways of cell growth and proliferation by the phenolic compounds of Salvia officinalis34.
We found that the administration of Cd resulted in a significant decrease in sperm count, motility, viability and altered sperm morphology. These results are consistent with the fact that heavy metals cause degenerative changes in the seminiferous tubules with loss of spermatogenesis35,36. Several previous studies have reported decrease in sperm count, motility and viability of mice, rats, and humans as a result of exposure to cadmium37–40. However, Salvia officinalis extract significantly improved sperm parameters when administrated with Cd. In line with our findings, Al-chalabi et al.41 discovered that Salvia officinalis causes a significant elevation in sperm activity along with a significant reduction in sperm mortality and abnormalities in diabetic male albino rats. This increase might be attributed to ability of Salvia officinalis extract to stimulate the growth of testes and enhance the proliferation, maturation, and differentiation of spermatozoa due to the presence of saponin and alkaloids42,43.
In the current study, exposure to cadmium caused significant DNA damage as detected by the single-cell gel electrophoresis assay. In a previous study, Comet assay in epididymal sperms of adult male SD rats demonstrated a significant difference in the lengths of the head and comet in all the three Cd treated groups, indicating damage in the heritable DNA44. In our study we observed that administration of Salvia officinalis with Cd protects the sperm cells from DNA damage. The protective effect of Salvia officinalis is consistent with our previous findings of the ability of Salvia officinalis to increase the levels of oxidative stress enzymes and reduce the level of MDA. Our results are in accordance with the findings of Aherne et al.45, who described the DNA-protective activity of Salvia officinalis against H2O2-induced DNA damage in Caco-2 cells. Kozics et al.46 showed that Salvia officinalis protects against oxidative stress and DNA damage through the elevation of glutathione peroxidase activity.
Our study revealed that cadmium administration resulted in a significant decrease in the circulating levels of follicle stimulating hormone (FSH), luteinizing hormone (LH) and testosterone (T). Similar results were found in previous work as they reported that cadmium significantly reduced the levels of LH, FSH and T in blood of rats47,48. They explained their finding that cadmium is an endocrine disruptor that interferes with the synthesis and/or regulation of the circulating levels of several hormones, including T, FSH, and LH4,48. The co-administration of Salvia officinalis with Cd increased the circulating levels of LH, FSH and testosterone. In consistency with our results, previous studies found that the level of T, LH and FSH were significantly increased in the serum of albino rats after treatment with Salvia officinalis41,49.
Upon administration of Cd, we recorded a significant increase in MDA level and a significant decrease in activities of antioxidant enzymes (SOD, GPx and CAT) which might be due to the inactivation of cellular antioxidants by the lipid peroxides and reactive oxygen species (ROS) produced by Cd intoxication39,50. It was also reported that Cd might disrupt the expression of these enzymes during the transcriptional stage51. Same results were recorded in the liver of female albino rats after Cd administration52. We also found that co-administration of Salvia officinalis with Cd led to a decrease in MDA level and increased the activities of SOD, GPx and CAT. These results agree with previous studies where they concluded that flavonoids and vanillin components of Salvia officinalis mediated the lipid peroxidation reduction because of their antioxidant properties and their ability of removing free radicals and chelating divalent cations53–55.
Upon examining the testis’s tissues, we found that administration of cadmium caused several histopathologic changes. The examination revealed severe degeneration of spermatogenic cells. Seminiferous tubules were filled with homogenous eosinophilic fluid associated with atrophy of some seminiferous tubules. Similar findings were described in previous studies where they found that Cd treatment caused degenerative changes, severe necrosis of seminiferous tubules and absence of spermatogenic cells39,56. Various studies have reported that testicular tissue is more vulnerable to Cd toxicity than any other organ57. The described histopathological damage might be attributed to the generation of ROS and oxidative stress induced by cadmium58 which in turn damages DNA and causes lipid and protein peroxidation59. When we co-administrated Salvia officinalis with Cd, the tissue examination showed a normal overall structure of the testes, regeneration of seminiferous tubules, and improvement in spermatogenesis compared to Cd group. Studies of Chouabia et al.33 and Al-Syaad60 showed similar results, as they found improvement in testicular histopathology of rats exposed to cypermethrin or doxorubicin after administration of Salvia officinalis. Bioactive constituents in Salvia officinalis extract have antioxidant and radical scavenging activities, which account for the plant's protective effect52.
Conclusion
This study concludes that cadmium exposure negatively affects the integrity and quality of the sperm and the levels of reproductive hormones as well as testicular Oxidative stress markers and histology. Salvia officinalis opposes the toxic effects of cadmium exposure by significant improvement of spermatogenesis, oxidative stress, DNA fragmentation, and testicular histopathology which contributes to increasing the reproductive activity.
Supplementary Information
Author contributions
W.T.B.: The corresponding author. He made substantial contributions to the conception, design of the work, and the acquisition, analysis, and interpretation of data. He also revised the manuscript. A.M. I.: Pivotal role in design of the work, data analysis and interpretation. She also revised the work. O.A. A.: substantial contributions in acquisition, analysis, and interpretation of data. A.R. O.: Pivotal role in design of the work, data acquisition, analysis and interpretation. She also revised the work.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Data availability
Data supporting this study are included within the article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-45193-1.
References
- 1.Gao Y, Mruk DD, Cheng CY. Sertoli cells are the target of environmental toxicants in the testis—A mechanistic and therapeutic insight. Expert Opin. Ther. Targets. 2015;19(8):1073–1090. doi: 10.1517/F14728222.2015.1039513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wirth JJ, Mijal RS. Adverse effects of low level heavy metal exposure on male reproductive function. Syst. Biol. Reprod. Med. 2010;56(2):147–167. doi: 10.3109/19396360903582216. [DOI] [PubMed] [Google Scholar]
- 3.Mehrdad RR, Mehravar RR, Sohrab K, Ali-akbar M. Cadmium toxicity and treatment: An update. Caspian J. Intern. Med. 2017;8(3):135–145. doi: 10.22088/cjim.8.3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Angelis C, Galdiero M, Pivonello C, Salzano C, Gianfrilli D, Piscitelli P, Lenzi A, Colao A, Pivonello R. The environment and male reproduction: The effect of cadmium exposure on reproductive function and its implication in fertility. Reprod. Toxicol. 2017;73:105–127. doi: 10.1016/j.reprotox.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 5.Pavlova, E. & Atanassova, N. Impact of cadmium on male fertility. Acta Morphol. Anthropol. 25(1–2), 108–116 (2018). http://www.iempam.bas.bg/journals/acta/arhiv/acta25a/108-116.pdf.
- 6.Genchi, G., Sinicropi, M.S., Lauria, G., Carocci, A. & Catalano, A. The effects of cadmium toxicity. Int. J. Environ. Res. Public Health17(11), 3782. (2020). https://pubmed.ncbi.nlm.nih.gov/32466586. [DOI] [PMC free article] [PubMed]
- 7.Zhu Q, Li X, Ge RS. Toxicological effects of cadmium on mammalian testis. Front. Genet. 2020;11:527. doi: 10.3389/fgene.2020.00527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghorbani A, Esmaeilizadeh M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 2017;7(4):433–440. doi: 10.1016/j.jtcme.2016.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma Y, Fagan J, Schaefer J. Ethnobotany, phytochemistry, cultivation and medicinal properties of garden sage (Salvia officinalis L.) J. Pharmacogn. Phytochem. 2019;8(3):3139–3148. [Google Scholar]
- 10.Hall, J.E. Guyton and Hall Textbook of Medical Physiology e-Book. (Elsevier, 2010). https://edisciplinas.usp.br/pluginfile.php/4914524/mod_resource/content/1/Guyton-and-Hall-Textbook-of-Medical-Physiology-12th-Ed.pdf.
- 11.Ghosal, S., Chakraborty, I. & Pradhan, N.K. Jussiaea repens (L) induced morphological alterations in epididymal spermatozoa of rat. Int. J. Pharm. Sci. Rev. Res. 22(2), 288–295 (2013). https://globalresearchonline.net/journalcontents/v22-2/52.pdf.
- 12.Kumar N, Singh AK. Trends of male factor infertility, an important cause of infertility: A review of literature. J. Hum. Reprod. Sci. 2015;8(4):191–196. doi: 10.4103/0974-1208.170370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright C, Milne S, Leeson H. Sperm DNA damage caused by oxidative stress: modifiable clinical, lifestyle and nutritional factors in male infertility. Reprod. Biomed. Online. 2014;28(6):684–703. doi: 10.1016/j.rbmo.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Codrington AM, Hales BF, Robaire B. Spermiogenic germ cell phase-specific DNA damage following cyclophosphamide exposure. J. Androl. 2004;25(3):354–362. doi: 10.1002/j.1939-4640.2004.tb02800.x. [DOI] [PubMed] [Google Scholar]
- 15.Baumgartner A, Cemeli E, Anderson D. The comet assay in male reproductive toxicology. Cell Biol. Toxicol. 2007;25(1):81–98. doi: 10.1007/s10565-007-9041-y. [DOI] [PubMed] [Google Scholar]
- 16.Oduwole OO, Peltoketo H, Huhtaniemi IT. Role of follicle-stimulating hormone in spermatogenesis. Front. Endocrinol. (Lausanne) 2018;9:763. doi: 10.3389/fendo.2018.00763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simoni M, Huhtaniemi I, Santi D, Casarini L. Editorial: Follicle stimulating hormone: Fertility and beyond. Front. Endocrinol. (Lausanne) 2019;10:610. doi: 10.3389/fendo.2019.00610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lafuente A, González-Carracedo A, Romero A, Cano P, Esquifino AI. Cadmium exposure differentially modifies the circadian patterns of norepinephrine at the median eminence and plasma LH, FSH and testosterone levels. Toxicol. Lett. 2004;146(2):175–182. doi: 10.1016/j.toxlet.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Mangelsdorf I, Buschmann J, Orthen B. Some aspects relating to the evaluation of the effects of chemicals on male fertility. Regul. Toxicol. Pharmacol. 2003;37(3):356–369. doi: 10.1016/s0273-2300(03)00026-6. [DOI] [PubMed] [Google Scholar]
- 20.Blanco A, Moyano R, Vivo J, Flores-Acuna R, Molina A, Blanco C, Aguera E, Monterde JG. Quantitative changes in the testicular structure in mice exposed to low doses of cadmium. Environ. Toxicol. Pharmacol. 2007;23(1):96–101. doi: 10.1016/j.etap.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Ali W, Ma Y, Zhu J, Zou H, Liu Z. Mechanisms of cadmium-induced testicular injury: A risk to male fertility. Cells. 2022;11(22):3601. doi: 10.3390/cells11223601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mouro VGS, Martins ALP, Silva J, Menezes TP, Gomes MLM, Oliveira JA, Melo FCSA, Matta SLP. Subacute testicular toxicity to cadmium exposure intraperitoneally and orally. Oxid. Med. Cell Longev. 2019 doi: 10.1155/2019/3429635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi X, Fu L. Piceatannol inhibits oxidative stress through modification of Nrf2-signaling pathway in testes and attenuates spermatogenesis and steroidogenesis in rats exposed to cadmium during adulthood. Drug Des. Dev. Ther. 2019;13:2811–2824. doi: 10.2147/dddt.s198444.PMID:31496657;PMCID:PMC6698161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alkan ÜF, Gürsel EF, Ateş A, Özyürek M, Güçlü K, Altun M. Protective effects of Salvia officinalis extract against cyclophosphamide-induced genotoxicity and oxidative stress in rats. Turk. J. Vet. Anim. Sci. 2012;36(6):646–654. doi: 10.3906/vet-1105-36. [DOI] [Google Scholar]
- 25.Jedidi S, Selmi H, Aloui F, Rtibi K, Sammari H, Abbes C, Sebai H. Antioxidant properties, phytoactive compounds and potential protective action of Salvia officinalis flowers against combined gastro-intestinal ulcer and diarrhea experimentally induced in rat. Dose Response. 2022 doi: 10.1177/15593258221102313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen, L., Zhou, J., Gao, W. & Jiang, Y.Z. Action of NO and TNF-alpha release of rats with cadmium loading in malfunction of multiple system organ. Sheng Li Xue Bao [Acta Physiol. Sin.]. 55(5), 535–540 (2003). https://pubmed.ncbi.nlm.nih.gov/14566400/. [PubMed]
- 27.El-Magd MA, Kahilo KA, Nasr NE, Kamal T, Shukry M, Saleh AA. A potential mechanism associated with lead-induced testicular toxicity in rats. Andrologia. 2016;49(9):e12750. doi: 10.1111/and.12750. [DOI] [PubMed] [Google Scholar]
- 28.Yokoi K, Uthus EO, Nielsen FH. Nickel deficiency diminishes sperm quantity and movement in rats. Biol. Trace Elem. Res. 2003;93(1–3):141–154. doi: 10.1385/bter:93:1-3:141. [DOI] [PubMed] [Google Scholar]
- 29.Okamura A, Kamijima M, Shibata E, Ohtani K, Takagi K, Ueyama J, Nakajima T. A comprehensive evaluation of the testicular toxicity of dichlorvos in Wistar rats. Toxicology. 2005;213(1–2):129–137. doi: 10.1016/j.tox.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 30.Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, Miyamae Y, Rojas E, Ryu JC, Sasaki YF. Single cell gel/comet assay: Guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. 2000;35(3):206–221. doi: 10.1002/(sici)1098-2280(2000)35:3<206::aid-em8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 31.Bancroft, J.D., Layton, C. & Suvarna, S.K. Bancroft’s Theory and Practice of Histological Techniques. 173–186 (Churchill Livingstone Elsevier, 2013). 10.1016/b978-0-7020-4226-3.00010-x.
- 32.Venditti M, Ben Rhouma M, Romano MZ, Messaoudi I, Reiter RJ, Minucci S. Altered expression of DAAM1 and PREP induced by cadmium toxicity is counteracted by melatonin in the rat testis. Genes. 2021;12(7):1016. doi: 10.3390/genes12071016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chouabia A, Djemli S, Abdennour C, Mallem L, Kahalerras L, Arkoub FZ, Bouabdallah N, Tahraoui A. Protective effect of Salvia officinalis against cypermethrininduced reprotoxicity in male Wistar rats. Pharmacogn. J. 2021;13(6):1413–1421. doi: 10.5530/pj.2021.13.179. [DOI] [Google Scholar]
- 34.Aron PM, Kennedy JA. Flavan-3-ols: Nature, occurrence and biological activity. Mol. Nutr. Food Res. 2008;52(1):79–104. doi: 10.1002/mnfr.200700137. [DOI] [PubMed] [Google Scholar]
- 35.Martin LJ, Chen H, Liao X, Allayee H, Shih DM, Lee GS, Hovland DN, Robbins WA, Carnes K, Hess RA, Lusis AJ, Collins MD. FK506, a calcineurin inhibitor, prevents cadmium-induced testicular toxicity in mice. Toxicol. Sci. 2007;100(2):474–485. doi: 10.1093/toxsci/kfm229. [DOI] [PubMed] [Google Scholar]
- 36.De Souza PF, Diamante MA, Dolder H. Testis response to low doses of cadmium in Wistar rats. Int. J. Exp. Pathol. 2010;91(2):125–131. doi: 10.1111/j.1365-2613.2009.00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ige S, Olaleye S, Akhigbe R, Akanbi T, Oyekunle O, Udoh UA. Testicular toxicity and sperm quality following cadmium exposure in rats: Ameliorative potentials of Allium cepa. J. Hum. Reprod. Sci. 2012;5(1):37–42. doi: 10.4103/0974-1208.97798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ban, T.S. Effects of cadmium on sperm parameters, histological and hormonal changes in testes of mature rats. Iraqi J. Embr. Infertil. Res. 3(6), 38–43 (2013). https://www.iasj.net/iasj/article/131889.
- 39.Babaknejad N, Bahrami S, Moshtaghie AA, Nayeri H, Rajabi P, Iranpour FG. Cadmium testicular toxicity in male Wistar rats: Protective roles of zinc and magnesium. Biol. Trace Elem. Res. 2017;185(1):106–115. doi: 10.1007/s12011-017-1218-5. [DOI] [PubMed] [Google Scholar]
- 40.Krzastek, S.C., Farhi, J., Gray, M. & Smith, R.P. Impact of environmental toxin exposure on male fertility potential. Transl. Androl. Urol.9(6), 2797–2813 (2020). 10.21037/tau-20-685. [DOI] [PMC free article] [PubMed]
- 41.Al-Chalabi, M., Shukri, M., Raña, I.M. & Khalid, L. Effect of Salvia officinalis L. (Sage) aqueous extract on liver and testicular function of diabetic albino male rats. J. Babylon Uni Pure Appl. Sci. 24(2), 390–399 (2016). https://www.iasj.net/iasj/download/4ee91c5f245ed365.
- 42.Homady M, Hussain HH, Tarawneh KA, Shakhanbeh JM, Al-Raheil IA, Brain PF. Effects of oral application of some medicinal plants extract used in Jordan on social aggression as well as testicular and preputial gland structures in male mice. Pak. J. Bio Sci. 2000;3(3):398–415. doi: 10.3923/pjbs.2000.398.402. [DOI] [Google Scholar]
- 43.Lima CF, Femandes-Ferreira M, Pereira-Wilson C. Drinking of Salvia officinalis tea increases CCl4 iduced hepatotoxicity in mice. Food Chem. Toxicol. 2007;45(3):456–464. doi: 10.1016/j.fct.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 44.Iqbal T, Cao M, Zhao Z, Zhao Y, Chen L, Chen T, Li C, Zhou X. Damage to the testicular structure of rats by acute oral exposure of cadmium. Int. J. Environ. Res. Public Health. 2021;18(11):6038. doi: 10.3390/ijerph18116038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aherne SA, Kerry JP, O’Brien NM. Effects of plant extracts on antioxidant status and oxidant-induced stress in Caco-2 cells. Br. J. Nutr. 2007;97(2):321–328. doi: 10.1017/s0007114507250469. [DOI] [PubMed] [Google Scholar]
- 46.Kozics K, Klusova V, Rančíková A, Mučaji P, Slameňová D, Hunáková L, Barbara K, Horváthová E. Effects of Salvia officinalis and Thymus vulgaris on oxidant-induced DNA damage and antioxidant status in HepG2 cells. Food Chem. 2013;141(3):2198–2206. doi: 10.1016/j.foodchem.2013.04.089. [DOI] [PubMed] [Google Scholar]
- 47.Al-Azemi M, Omu FE, Kehinde EO, Anim JT, Oriowo MA, Omu AE. Lithium protects against toxic effects of cadmium in the rat testes. J. Assist. Reprod. Genet. 2010;27(8):469–476. doi: 10.1007/s10815-010-9426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ahmed MM, El-Shazly SA, Alkafafy ME, Mohamed AA, Mousa AA. Protective potential of royal jelly against cadmium-induced infertility in male rats. Andrologia. 2018;50(5):e12996. doi: 10.1111/and.12996. [DOI] [PubMed] [Google Scholar]
- 49.Alshubaily, F.A. & Jambi, E.J. The possible protective effect of Sage (Salvia officinalis L.) water extract against testes and heart tissue damages of hypercholesterolemic rats. Int. J. Pharm. Phyto Res. 8(1), 62–68 (2018). https://eijppr.com/5tQy7ZS.
- 50.Andjelkovic M, Buha DA, Antonijevic E, Antonijevic B, Stanic M, Kotur-Stevuljevic J, Spasojevic-Kalimanovska V, Jovanovic M, Boricic N, Wallace D, Bulat Z. Toxic effect of acute cadmium and lead exposure in rat blood, liver, and kidney. Int. J. Environ. Res. Public Health. 2019;16(2):274. doi: 10.3390/ijerph16020274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kumar A, Nikhat JS, Alrashood ST, Khan HA, Dubey A, Sharma B. Protective effect of eugenol on hepatic inflammation and oxidative stress induced by cadmium in male rats. Biomed. Pharmacother. 2021;139:111588. doi: 10.1016/j.biopha.2021.111588. [DOI] [PubMed] [Google Scholar]
- 52.Rashwan HM, Mohammed HE, El-Nekeety AA, Hamza ZK, Abdel-Aziem SH, Hassan NS, Abdel-Wahhab MA. Bioactive phytochemicals from Salvia officinalis attenuate cadmium-induced oxidative damage and genotoxicity in rats. Environ. Sci. Pollut. Res. 2021;28(48):68498–68512. doi: 10.1007/s11356-021-15407-y. [DOI] [PubMed] [Google Scholar]
- 53.Zhao D, Sun J, Sun B, Zhao M, Zheng F, Huang M, Li H. Intracellular antioxidant effect of vanillin, 4- methylguaiacol and 4-ethylguaiacol: Three components in Chinese Baijiu. RSC Adv. 2017;7(73):46395–46405. doi: 10.1039/C7RA09302K. [DOI] [Google Scholar]
- 54.Sun J, Wang H, Liu B, Shi W, Shi J, Zhang Z, Xing J. Rutin attenuates H2O2-induced oxidation damage and apoptosis in Leydig cells by activating PI3K/Akt signal pathways. Biomed. Pharmacother. 2017;88:500–506. doi: 10.1016/j.biopha.2017.01.066. [DOI] [PubMed] [Google Scholar]
- 55.Brindisi M, Bouzidi C, Frattaruolo L, Loizzo MR, Cappello MS, Dugay A, Deguin B, Lauria G, Cappello AR, Tundis R. New insights into the antioxidant and anti-inflammatory effects of italian Salvia officinalis leaf and flower extracts in lipopolysaccharide and tumor-mediated inflammation models. Antioxidants. 2021;10(2):311. doi: 10.3390/antiox10020311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.El-Refaiy AI, Eissa FI. Histopathology and cytotoxicity as biomarkers in treated rats with cadmium and some therapeutic agents. Saudi J. Biol. Sci. 2013;20(3):265–280. doi: 10.1016/j.sjbs.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Siu ER, Mruk DD, Porto CS, Yan CC. Cadmium-induced testicular injury. Toxicol. Appl. Pharmacol. 2009;238(3):240–249. doi: 10.1016/j.taap.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu H, Li K, Liang J, Zhang J, Wu Q. Changes in the levels of DNA methylation in testis and liver of SD rats neonatally exposed to 5-aza-2'-deoxycytidine and cadmium. J. Appl. Toxicol. 2011;31(5):484–495. doi: 10.1002/jat.1673. [DOI] [PubMed] [Google Scholar]
- 59.Horri E, Moghadam AE, Amiri FT, Ebrahimzadeh MA. Protective effect of Feijoa sellowianan fruit on testicular toxicity-induced by cadmium chloride. Andrologia. 2020;53(e13926):1–9. doi: 10.1111/and.13926. [DOI] [PubMed] [Google Scholar]
- 60.Al-Syaad K. Effects of aqueous extract of Salvia officinalis on some parameters of sperms and histopathological changes in testes of rats treated with Doxorubicin. Egypt. Acad. J. Biol. Sci. Zool. 2014;6(1):27–40. doi: 10.21608/eajbsz.2014.13491. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting this study are included within the article.