Summary
Background
Immune checkpoint inhibitors (ICI) plus platinum-based chemotherapy has been recognized as a standard first-line therapy in non-small cell lung cancer (NSCLC); however, no prospective clinical trials of docetaxel (DTX) plus ramucirumab (RAM) following first-line ICI plus platinum-based chemotherapy has been reported.
Methods
In this multicentre, open-label, single-arm, phase 2 trial, we enrolled patients with NSCLC from eight centres in Japan. Patients with metastatic NSCLC with disease progression after platinum-based chemotherapy plus ICI were eligible for the study. Patients were intravenously treated with 60 mg/m2 of DTX and 10 mg/kg of RAM on day 1 with a strong recommendation of pegfilgrastim administration on day 2 every 3 weeks. The primary end point was objective response rate (ORR) in efficacy analysis population. Safety was assessed in all patients treated at least one dose. The ORR of the null and alternative hypotheses were 10% and 30%, with α error of 0.1 and β error of 0.1. This trial is registered with the Japan Registry for Clinical Trials, jCRTs041190077.
Findings
Between 16 January, 2020, and 24 August, 2021, 33 patients (median age 66 [range 42–79] years) were enrolled. Thirteen patients (41%) had Eastern Cooperative Oncology Group performance status of 1. Twenty-five patients (78%) had an interval of <60 days after the last administration of ICI. In the efficacy analysis population (n = 32), the primary endpoint was met as 11 patients achieved partial response (PR), with ORR of 34.4% (80% CI, 23.1–47.2). Grade ≥3 anaemia and febrile neutropenia were observed in 2 (6%) and 3 (9%) patients, respectively. No treatment-related deaths and no new safety signals were observed.
Interpretation
DTX plus RAM demonstrated encouraging antitumor activity with a manageable safety profile in patients who have progressed on front-line ICIs plus platinum-based chemotherapy. The results of this trial can be a helpful reference in conducting further phase III trials of new second-line treatment options.
Funding
Eli Lilly Japan K.K.
Keywords: Non-small cell lung cancer, Docetaxel, Ramucirumab, Second-line treatments, First-line immune-therapy
Research in context.
Evidence before this study
Although docetaxel plus ramucirumab demonstrated a survival benefit over docetaxel after platinum-based chemotherapy for non-small cell lung cancer (NSCLC), immune checkpoint inhibitors (ICI) combined with platinum-based chemotherapy has become one of the front-line standards of care. The clinical benefit of docetaxel plus ramucirumab is possibly modulated by the residual effect of prior ICIs. We searched PubMed for reports published in English using the terms (non small cell lung cancer) and (ramucirumab) and (docetaxel) and (PD-1 or PD-L1 or CTLA4), excluding review articles, published from database inception up to May 4, 2023. The search yielded eight articles. Of these, one article was not related to docetaxel and ramucirumab (articles focusing on nivolumab), and one article was a clinical study design paper of docetaxel plus ramucirumab with pembrolizumab treatment. The other six articles were observational studies on docetaxel plus ramucirumab following ICI administration. However, no prospective clinical trial investigating the efficacy and safety of docetaxel plus ramucirumab following ICI plus platinum-based chemotherapy, could be found.
Added value of this study
To the best of our knowledge, this study is the first phase II clinical trial that reports the efficacy and safety of docetaxel plus ramucirumab following front-line ICI plus platinum-based chemotherapy. This study showed promising efficacy and manageable safety of docetaxel plus ramucirumab.
Implications of all the available evidence
Docetaxel plus ramucirumab is still the effective second-line standard of care regimen in the era of ICI front-line use. There are several on-going phase III trials of second-line new strategies such as anti-angiogenesis therapy plus ICI and antibody-drug conjugates such as datopotamab deruxtecan and telisotuzumab vedotin for NSCLC patients treated with prior ICIs and platinum-based chemotherapy in combination or sequentially, and in these trials the control arm is docetaxel monotherapy. Considering these circumstances, the order of precedence regarding second-line regimen including docetaxel plus ramucirumab should be discussed in the era of ICI front-line use.
Introduction
Lung cancer is the primary cause of cancer-related deaths worldwide. Non-small cell lung cancer (NSCLC) accounts for approximately 80% of all lung cancer cases. In clinical development for advanced NSCLC, immune checkpoint inhibitors (ICI) have led to remarkable survival improvements. In addition to the evidence of the efficacy of first-line pembrolizumab or atezolizumab for Programmed death ligand-1 (PD-L1) high expression NSCLC,1,2 several phase III studies have shown the survival superiority of platinum-based chemotherapy combined with ICI to platinum-based chemotherapy alone.3, 4, 5, 6, 7, 8 Based on the evidence from multiple phase III trials, ICI (Programmed death receptor-1 (PD-1)/PD-L1 inhibitors with or without cytotoxic T-lymphocyte associated antigen 4 (CTLA-4) inhibitors) plus platinum-based chemotherapy for advanced NSCLC are established as the first-line standard treatment regardless of the tumour PD-L1 expression status. Approximately 10% of patients treated with ICI plus platinum-based chemotherapy achieved progression-free survival (PFS) over 5 years; however, over 80% of patients experience disease progression, which required subsequent second-line chemotherapy.9,10
Docetaxel with or without ramucirumab is the standard of care in the second-line setting for advanced NSCLC. In the REVEL study, a phase III study comparing docetaxel plus ramucirumab versus docetaxel in patients with stage IV NSCLC who had disease progression during or after prior first-line platinum-based chemotherapy, docetaxel plus ramucirumab significantly prolonged overall survival (OS) compared to docetaxel alone.11 However, the REVEL study had been conducted before the regulatory approval of first-line ICI; thus, the efficacy and safety of docetaxel plus ramucirumab following ICI plus platinum-based chemotherapy have not been adequately studied previously, and we need to consider the residual effect of ICIs. The immune tumour microenvironment (TME) modified by first-line ICI treatment possibly exerts long lasting effect that can impact clinical response to second-line therapies. Indeed, the residual efficacy of nivolumab is observed beyond 20 weeks following the last dose of nivolumab.12 In addition, ramucirumab, which binds with high affinity to the endothelial growth factor receptor-2 (VEGFR-2), blocks not only tumour-associated angiogenesis but also reverts immunosuppressive signals within the TME.
The safety of docetaxel plus ramucirumab after ICI is an issue that needs further investigation. In addition to the reported late-onset immune-related adverse events (AEs) caused by PD-1 inhibitors after the cessation of PD-1 inhibitors,13 several studies have revealed that prior ICI administration affected the possible increased risk of subsequent treatment-related AEs especially in driver-gene targeted tyrosine kinase inhibitors. The incidence of osimertinib-induced pneumonitis and liver toxicity caused by sotorasib were possibly increased immediately after prior PD-1 blockade therapy.14,15 Although case reports of pneumonitis caused by docetaxel plus ramucirumab after ICIs administration are reported,16 no clinical trials have focused on the safety of docetaxel plus ramucirumab after ICI plus platinum-based chemotherapy.
Under these circumstances of treatment strategy in advanced NSCLC, here, we firstly report the activity and safety of docetaxel plus ramucirumab in patients who showed disease progression on or after first-line ICI plus platinum-based chemotherapy.
Methods
Study design and participants
We conducted a multicentre, open-label, single-arm, phase 2 trial evaluating the efficacy and safety of docetaxel plus ramucirumab following ICI plus platinum-based chemotherapy. Key eligibility criteria were as follows: (1) NSCLC confirmed by histology or cytology, (2) stage III/IV of cancer or recurrence, (3) confirmed progression after first-line treatment with concurrent platinum-based chemotherapy plus ICIs, (4) age ≥20 years, (5) Eastern Cooperative Oncology Group performance status 0 or 1, (6) at least one measurable lesion as defined by the Response Evaluation Criteria in Solid Tumour (RECIST) Guidelines version 1.1. In patients with driver gene alteration, prior molecular-targeted therapy was allowed before starting ICI plus platinum-based chemotherapy. Further details of the inclusion and exclusion criteria are shown in the clinical protocol (Supplementary Information).
This study was conducted in accordance with the Declaration of Helsinki, and the study protocol was approved by Nagoya University Central Review Board (Clinical trial information: jCRTs041190077). All participants provided written informed consent.
Procedures
Patients received docetaxel 60 mg/m2 and ramucirumab 10 mg/kg on day 1 intravenously every 21-day cycle. In the REVEL trial, the mid-trial protocol amendment was performed to reduce the dose of docetaxel for East Asia patients from 75 mg/m2 to 60 mg/m2 because of the higher rate of febrile neutropenia and neutropenia in East Asia patients compared to non-East Asia patients.11 Based on these results of the REVEL trial, the JVCG trial showed the efficacy and safety of 60 mg/m2 of docetaxel with ramucirumab in Japanese patients,17 and the approved docetaxel dose for advanced NSCLC is 60 mg/m2 in Japan. Under these evidences, we set the docetaxel dose as 60 mg/m2. Subcutaneous injection of pegfilgrastim 3.6 mg was strongly recommended on day 2 of each cycle (allowance of pegfilgrastim was set as 24–72 h at the end of docetaxel plus ramucirumab therapy). Study treatment was discontinued in case of confirmed disease progression, unacceptable toxicity, or patient's refusal to continuing study treatment. Radiological assessment of the tumour was planned to every 6 weeks, and the objective tumour response was evaluated in accordance with RECIST version 1.1. Adverse event (AE)s were stratified using CTCAE criteria version 4.0.
Outcomes
The primary endpoint of this study was the objective response rate (ORR; the proportion of patients with a complete response (CR) or partial response (PR) to the treatment). Confirmation of PR and CR (duration not less than 4 weeks) was required. A central review for radiological evaluation was performed to determine the overall best tumour response in each patient. The criteria for stable disease (SD) determination required at least one radiological evaluation after the start of treatment at a minimum interval of 6 weeks. The secondary endpoints were progression-free survival (PFS; the time from registration until disease progression or death), OS (time from registration until death from any cause), and safety.
The study was conducted based on the concept of a possible positive effect of prior ICI administration on the clinical outcomes of docetaxel plus ramucirumab; thus, subgroup analyses of ORR, PFS, OS and DOR were preplanned to perform according to tumour response of previous ICI plus platinum-based chemotherapy (responders; patients with CR/PR vs. non-responders; patients with SD/PD).
Statistical analysis
The target sample size was determined based on the ORR estimation using an exact binomial distribution. The ORRs of the null and alternative hypotheses were set as 10% and 30%, respectively, with a one-sided α error of 0.1 and β error of 0.1, and the required number of patients was calculated to be 29. The target number of patients was set at 32 accounting for ineligible cases. In an exploratory phase II study setting, one-sided alphas are generally set between 0.05 and 0.1, thus one-sided alpha level of 0.1 in our study is considered to be acceptable. For instance, the same one-sided alpha level of 0.1 was adopted in the Lung-MAP S1800A study18 and a phase II trial of pembrolizumab for patients with melanoma or non-small cell lung cancer.19 The decision of one-sided alpha level in this phase II study was made according to the consultation with the biostatistician of our study (Y.K. Ph.D., Department of Advanced Medicine, Nagoya University Hospital, Nagoya, Japan). If the lower limit of the 80% CI of the response rate exceeded the threshold value of 10%, it was considered an effective treatment.
PFS and OS were calculated by the Kaplan–Meier method, and Greenwood's formula was used for interval estimation. The analysis of the disease control rate (DCR) and duration of response (DOR) was planned in advance. All statistical analyses including subset analysis were performed based on the pre-specified statistical analysis plan by Y.K (statistical manager of this study). Stata version 17.0 (StataCorp, TX) was used for the analyses.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. MM had access to dataset and had final responsibility for the decision to submit for publication.
Results
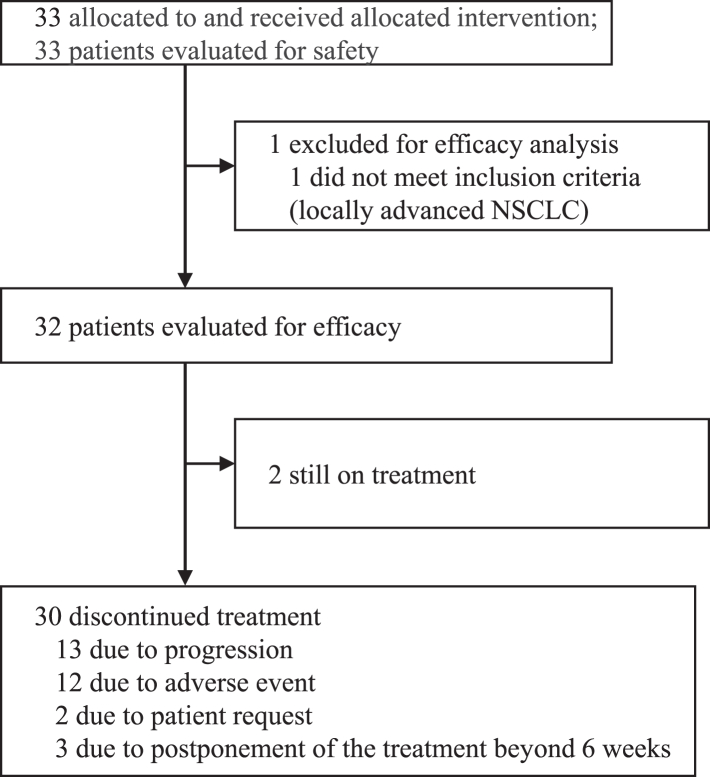
Patient flow is shown in Fig. 1. A total of 33 patients were enrolled from eight institutions between 16 January 2020 (the first patient was enrolled) and 24 August 2021 (the last patient was enrolled). All enrolled patients started the study protocol treatment with docetaxel and ramucirumab. One patient was found to have stage III NSCLC and received concurrent chemo radiation therapy followed by PD-L1 inhibitor as prior first-line treatment. The patient was excluded from the efficacy analysis, and the remaining 32 patients constituted the efficacy analysis population. The safety analysis included all 33 patients. At the date of data cutoff (25 May 2022), two patients were still on the study treatment without progression, and 30 patients discontinued the study protocol treatment because of disease progression (n = 13), AEs (n = 12), patient request (n = 2) and postponement of the treatment beyond 6 weeks (n = 3). The AEs prompting discontinuation of study protocol treatment was as follows; fluid retention/oedema in 4 patients, drug-induced pneumonitis in 3 patients, bleeding events (gastrointestinal bleeding:1, bronchopulmonary hemorrhage:1) in 2 patients, and prolonged thrombocytopenia, increased creatinine, delirium/cognitive impairment in one patient each.
Fig. 1.
Trial consort diagram. NSCLC; non-small cell lung cancer.
Baseline characteristics of the 32 patients of the efficacy analysis population are shown in Table 1. Median age was 66 years (range, 42–79), 13 (41%) had Eastern Cooperative Oncology Group performance status of 1, and 28 (88%) had non-squamous NSCLC. Seven patients had epidermal growth factor receptor (EGFR) activating mutation, and one patient had anaplastic lymphoma kinase (ALK) fusion. Eight patients (25%) were previously treated with bevacizumab and 12 (37%) with taxane agents (paclitaxel or nab-paclitaxel). The median time to discontinuation of the previous therapy was 5.0 months (95% CI: 4.2–7.6).
Table 1.
Patient characteristics.
| Number = 32 | % | |
|---|---|---|
| Age, median (range) | 66 (42–79) | |
| <65 years | 15 | 47 |
| 65–75 | 11 | 34 |
| ≥75 years | 6 | 19 |
| Sex | ||
| Male | 25 | 78 |
| Female | 7 | 22 |
| ECOG performance status | ||
| 0 | 19 | 59 |
| 1 | 13 | 41 |
| Smoking history, Brinkman indexa, median (range) | 880 (360–3000) | |
| Ever | 24 | 75 |
| Never | 8 | 25 |
| Histological subtype | ||
| Non-squamous | 28 | 88 |
| Squamous | 4 | 12 |
| EGFR mutation status | ||
| Wild type | 24 | 75 |
| Mutant | 7 | 22 |
| Unknown | 1 | 3 |
| Best response to platinum-based plus ICI combination therapy | ||
| CR/PR | 15 | 47 |
| SD/PD | 17 | 53 |
| Time to discontinuation of previous therapyb, Median months (95% CI) | 5.0 (4.2–7.6) | |
| Previous taxane | ||
| Yes | 12 | 37 |
| No | 20 | 63 |
| Previous bevacizumab treatment | ||
| Yes | 8 | 25 |
| No | 24 | 75 |
| Period since last ICI | ||
| <60 days | 25 | 78 |
| ≥60 days | 7 | 22 |
| Previous radiation therapy | ||
| Yes | 5 | 16 |
| No | 27 | 84 |
ECOG; Eastern Cooperative Oncology Group, EGFR; epidermal growth factor receptor, ICI: immune-checkpoint inhibitor, CR; complete response, PR; partial response, SD; stable disease, PD; progressive disease, CI; confidence interval.
Brinkman Index was defined as the number of cigarettes smoked per day multiplied by the number of years of smoking.
Time to discontinuation was defined as the time from the start of the treatment until the last dose or death.
At the date of data cutoff, 11 of 32 patients had achieved partial response (PR) though no patient achieved complete response (CR); the ORR was 34.4% (80% CI: 23.1–47.2, 95% CI: 18.6–53.2). The lower limit of 80% confidence interval exceeded the predefined threshold (10%), resulting in the primary endpoint met (Table 2). Further, 15 patients achieved SD, and the disease control rate was 81.3% (95% CI 63.6–92.8). In this study, four patients met the exclusion criteria for safety (the last dose of any anticancer agent should be at least 28 days before registration). Therefore, excluding these four patients, further analysis of ORR was performed, and the ORR (n = 28) was 32.1% (80% CI 20.4–45.9).
Table 2.
Overall response.
| Best response | N = 32 | % |
|---|---|---|
| CR | 0 | 0 |
| PR | 11 | 34.4 |
| SD | 15 | 46.9 |
| PD | 6 | 18.8 |
| NE | 0 | 0 |
| ORR | 34.4% (80% CI 23.1–47.2, 95% CI 18.6–53.2) | |
| DCR | 81.3% (95% CI 63.6–92.8) | |
CR; complete response, PR; partial response, SD; stable disease, PD; progressive disease, NE; not examined, ORR; objective response rate, CI; confidence interval, DCR; disease control rate.
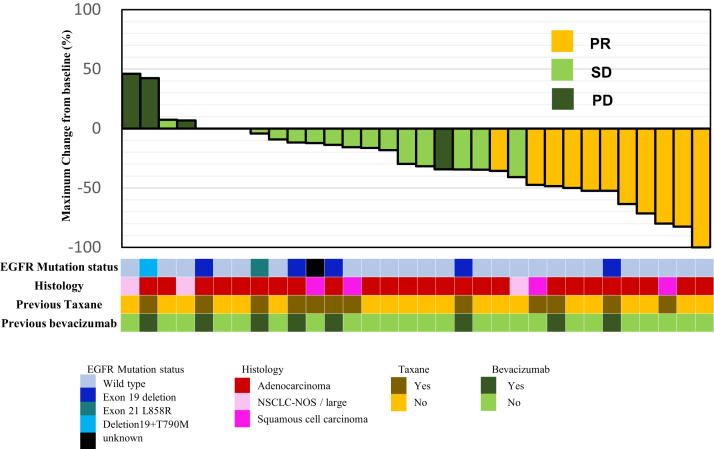
The maximum size changes in the target lesion from baseline according to RECIST version 1.1 are shown in Fig. 2. Of 32 patients, 25 (78%) showed tumour shrinkage. Tumour shrinkage by docetaxel plus ramucirumab was observed regardless of the presence or absence of EGFR activating mutation and the histological subtype (non-squamous cell carcinoma or squamous cell carcinoma).
Fig. 2.
Maximum size change in the target lesion from baseline (n = 32). The presence and type of EGFR mutations, histology, and whether the use of taxane and bevacizumab in the previous therapy are shown in the bottom row. PR; partial response, SD; stable disease, PD; progressive disease, EGFR; epidermal growth factor receptor, NSCLC-NOS; non-small cell lung cancer, not-otherwise specified.
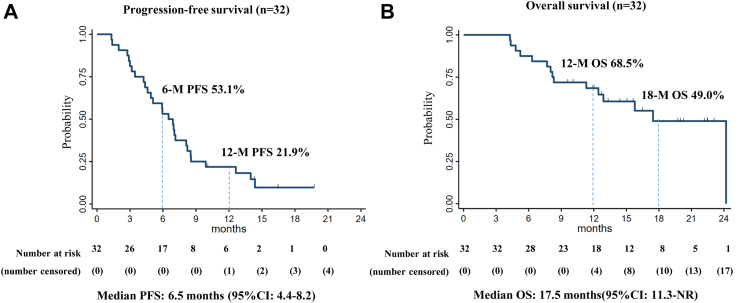
The Kaplan–Meier analyses of the PFS and OS are shown in Fig. 3. The median PFS was 6.5 months (95% CI: 4.4–8.2), the 6-month PFS rate was 53.1%, and the 12-month PFS rate was 21.9%, respectively (Fig. 3A). The median OS was 17.5 months (95% CI 11.3–not reached [NR]), and the 18-month OS rate was 49.0% (Fig. 3B), respectively. Among 11 patients who achieved PR, the median DOR was 5.1 months (95% CI: 2.9–11.2 months), and the 6-month DOR rate was 50.0% (Appendix p3).
Fig. 3.
Kaplan–Meier analysis of progression-free survival and overall survival. Kaplan–Meier curves of (A) progression-free survival and (B) overall survival. The 95% confidential intervals of the medians are described in brackets. PFS; progression-free survival, OS; overall survival, CI; confidence interval, NR; not reached.
For treatment effects according to tumour response of previous treatment as a pre-specified subgroup analysis, the ORRs were 40.0% (95% CI: 16.3–67.7) and 29.4% in (95% CI: 10.3–56.0) responders and non-responders, respectively (Appendix p4). The median PFS of responders was 6.9 months (95% CI: 3.0–12.6) and that of non-responders was 6.0 months (95% CI: 4.2–8.5), and their 12-month PFS rates were 33.3% and 11.8%, respectively (Appendix p5). The median OS of responders and non-responders and the median DOR of those were shown in Appendix p5 and Appendix p6, respectively.
Safety was evaluated for all 33 enrolled patients. Overall, treatment-emergent AEs of any grade were reported in 33 (100%), and 19 (58%) patients developed grade 3 or 4 AE. Grade 4 AEs were all neutropenia (n = 4 [12%]), and the other AEs were grade 3 or lower. The AE profile is shown in Table 3. The most common non-haematological AEs were malaise (n = 21 [64%]), anorexia (n = 21 [64%]), and alopecia (n = 19 [58%]). The rate of grade 3 febrile neutropenia was 9%. Any bleeding events were detected in 11 (33%) patients, and grade 3 in 2 (6%) patients. Pneumonitis was observed in 4 (12%) patients, with grade 3 in 3 (9%).
Table 3.
Adverse events.
| Adverse events, n (%) | Any grade | Grade 1-2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Any | 33 (100) | 19 (58)a | ||
| Malaise | 21 (64) | 21 (64) | ||
| Anorexia | 21 (64) | 16 (49) | 5 (15) | |
| Alopecia | 19 (58) | 19 (58) | ||
| Edema limbs | 16 (48) | 14 (42) | 2 (6) | |
| Stomatitis | 13 (39) | 12 (36) | 1 (3) | |
| Constipation | 11 (33) | 11 (33) | ||
| Aspartate aminotransferase increased | 11 (33) | 11 (33) | ||
| Weight loss | 10 (30) | 10 (30) | ||
| Alanine aminotransferase increased | 8 (24) | 7 (21) | 1 (3) | |
| Peripheral sensory/motor neuropathy | 8 (24) | 8 (24) | ||
| Fever | 6 (18) | 6 (18) | ||
| Weight gain | 6 (18) | 5 (15) | 1 (3) | |
| Diarrhea | 5 (15) | 5 (15) | ||
| Vomiting | 5 (15) | 5 (15) | ||
| Creatinine increased | 5 (15) | 5 (15) | ||
| Pleural effusion | 5 (15) | 5 (15) | ||
| Hypoalbuminemia | 4 (12) | 4 (12) | ||
| Hyponatremia | 4 (12) | 1 (3) | 3 (9) | |
| Dysgeusia | 4 (12) | 4 (12) | ||
| Pneumonitis | 4 (12) | 1 (3) | 3 (9) | |
| Rash maculo-papular | 4 (12) | 4 (12) | ||
| Thrombocytopenia | 22 (67) | 21 (64) | 1 (3) | |
| Anemia | 18 (55) | 16 (48) | 2 (6) | |
| Neutropenia | 13 (39) | 5 (15) | 4 (12) | 4 (12) |
| White cell count decreased | 9 (27) | 8 (24) | 1 (3) | |
| Febrile neutropenia | 3 (9) | 3 (9) | ||
| Bleeding or hemorrhage | 11 (33) | 9 (27) | 2 (6) | |
| Oral hemorrhage | 1 (3) | 1 (3) | ||
| Epistaxis | 7 (21) | 7 (21) | ||
| Pulmonary hemorrhage events | 2 (6) | 1 (3) | 1 (3) | |
| Gastrointestinal hemorrhage | 1 (3) | 1 (3) | ||
| Proteinuria | 8 (24) | 7 (21) | 1 (3) | |
| Hypertension | 13 (39) | 11 (33) | 2 (6) | |
| Arterial/Venous thromboembolic event | 0 (0) | 0 (0) | 0 (0) |
AE; adverse event.
19 (58%) patients developed grade 3 or 4 AE. No grade 5 AEs was observed.
Nineteen serious AEs were reported; however, no treatment-related deaths (grade 5 AEs) occurred (Appendix p1). Clinical baseline characteristics of patients who developed grade 3 pneumonitis are shown in Appendix p 2. All three patients were male with smoking history. One patient simultaneously developed grade 3 dyspnoea and grade 2 hypoxia related with oedema and fluid retention (Appendix p1, patient No. 11). All serious AEs were improved by receiving appropriate supportive treatment.
Discussion
The SCORPION study demonstrated an encouraging antitumor activity of docetaxel plus ramucirumab with a manageable safety profile in patients who have failed front-line ICI plus platinum-based chemotherapy. The study met its primary endpoint with 34.4% of ORR and 6.5 months of median PFS. To the best of our knowledge, this study is the first phase II trial which reports the efficacy and safety of docetaxel plus ramucirumab following front-line ICI plus platinum-based chemotherapy. A previous retrospective study of docetaxel plus ramucirumab following front-line ICI administration showed 32.5% of ORR.20 Our study would further enhance the evidence of second-line docetaxel plus ramucirumab in the front-line ICI paradigm.
In the REVEL study, the prior therapy was platinum-based chemotherapy without ICIs (the ORR: 23% and median PFS: 4.5 months).11 The ORR and median PFS of this study appear to be better than those of docetaxel plus ramucirumab in the REVEL study. However, it is difficult to compare findings of the current small phase II single-arm study to large scale phase III REVEL study, especially given differences in geography and molecular genotypes, and further investigations with larger sample size are warranted. Although it was an exploratory analysis with small sample size, responder to prior ICI plus platinum-based chemotherapy showed ORR with 40% with 33.3% of 12 months PFS rate. Recent review of anti-angiogenic strategy for NSCLC following first-line immunotherapy described ICI combination with anti-angiogenic agent as one of the promising second-line strategy following ICI plus platinum-based chemotherapy.21 A phase III trial of lenvatinib plus pembrolizumab (NCT03976375) following prior ICIs plus platinum-based chemotherapy in combination or sequentially is ongoing. Further, in the interim analysis of VARGADO, nintedanib (a multi-kinase inhibitor targeting VEGF receptors, fibroblast growth factor receptors, and platelet-derived growth factor receptors α) plus docetaxel demonstrated encouraging activity with 37.5% of ORR.22 These treatment strategies focusing on the positive effect of VEGF inhibition after first-line ICI would be similar to our study concept. Further, several phase III studies of antibody–drug conjugates such as datopotamab deruxtecan (NCT04656652) and telisotuzumab vedotin (NCT04928846) are also under investigation. All these studies enrol patients with NSCLC prior ICIs plus platinum-based chemotherapy in combination or sequentially, and their control arm is docetaxel monotherapy. If these strategies are approved for the second-line standard of care in the future, our study can be a reference data of docetaxel plus ramucirumab in the front-line ICI era.
In this study, over 75% of patients started docetaxel plus ramucirumab <60 days after the last administration of ICIs; however, the safety profile was considered manageable. The incidence of pneumonitis related to docetaxel plus ramucirumab in this study should be discussed (any grade: 4 patients (12%), grade 3: 3 patients (9%)). The incidence of any grade and grade ≥3 pneumonitis was 10.5% and 2.3% in the JVCG study.17 In real-world data of docetaxel plus ramucirumab after platinum-based chemotherapy with ICI, the incidence of any grade and grade ≥3 pneumonitis was 10.9% and 4.9%, respectively.23 At this point, we could not conclude whether prior ICI administration increase the incidence of grade ≥3 pneumonitis related to docetaxel plus ramucirumab or not, but we should carefully monitor the development of pneumonitis. At the timing of planning this study, there was no prospective safety data of docetaxel with ICI administration, however, well tolerability of the combination of pembrolizumab plus docetaxel is now reported as a result of the PROLUNG study.24 Collectively, docetaxel and ramucirumab immediately after ICI administration is considered to be well tolerated. Our study protocol strongly recommended the use of primary prophylactic pegfilgrastim treatment, and the frequency of grade ≥3 neutropenia/febrile neutropenia (24%/9%) appears to be reduced compared with that following docetaxel plus ramucirumab arm in REVEL11 and JVCG.17
This study has several limitations. First, this is a single-arm phase II trial of docetaxel plus ramucirumab; thus, we could not determine the actual super-added effect of ramucirumab to docetaxel monotherapy under the effect of prior ICIs. Second, patients treated with PD-1/PD-L1 inhibitors and CTLA-4 inhibitors in combination with platinum-based chemotherapy were eligible; however, the prior regimen choice had been PD-1/PD-L1 inhibitors plus platinum-based chemotherapy for all patients. Therefore, it is unclear if this favourable activity can be adapted for patients treated with PD-1/PD-L1 inhibitors and CTLA-4 inhibitors in combination with platinum-based chemotherapy. Third, the study included eight EGFR or ALK positive patients who progressed on ICI plus platinum-based chemotherapy. Because the incidence of EGFR activating mutation is higher in East Asian population compared to that of Caucasian population, the efficacy results of our study should be interpreted with careful caution.
In conclusion, docetaxel plus ramucirumab demonstrated encouraging antitumor activity with a manageable safety profile in patients who have failed with front-line ICIs plus platinum-based chemotherapy. The results of this phase II trial can be a helpful reference in conducting further phase III trials of new second-line treatment options of the front-line ICI paradigm.
Contributors
Conceptualisation: RM and MM, data curation: RM, MM and YK (These three authors accessed and verified the underlying data.), formal analysis: YK, funding acquisition: MM, investigation: all authors, methodology: RM and MM, project administration: RM and MM, resources: all authors, software: YK, supervision: MM and MI, writing-original draft: RM and MM, and writing-review & editing: all authors.
Data sharing statement
All data could be requested from corresponding author. Qualified researchers should submit a proposal to the corresponding author outlining the reasons for requiring the data. Use of data must also comply with the requirements of our institutes. A signed data access agreement with the sponsor is required before accessing shared data. Patient level data will not be made available.
Declaration of interests
RM received personal fees from Boehringer Ingelheim, AstraZeneca, Pfizer, Eli Lilly, Chugai Pharmaceutical, Merk Sharp & Dohme (MSD), Ono Pharmaceutical, and Taiho Pharmaceutical. MM received grants from Boehringer Ingelheim and Eli Lilly; personal fees from Eli Lilly, Chugai, Astra Zeneca, Ono, Pfizer, and MSD; non-financial support from F. Hoffmann-La Roche; others from Chugai, Astra Zeneca, Pfizer, Merk Serono, Kissei, Taiho, and Novartis. KI received personal fees from Boehringer Ingelheim, AstraZeneca, Pfizer, Eli Lilly, Chugai, MSD, Ono, Taiho, Takeda, and Daiichi Sankyo. OH received grants from AstraZeneca, Novartis, MSD, Daiichi Sankyo, Bayer Yakuhin, AbbVie, Eli Lilly, Janssen, and Ono; personal fees from AstraZeneca, Chugai, Takeda, MSD, Merck, Daiichi Sankyo, Eli Lilly, AbbVie, Boehringer Ingelheim, Nippon Kayaku, and Taiho. KT received personal fees from AstraZeneca, Boehringer Ingelheim, Chugai, Eli Lilly, GlaxoSmithKline (GSK), MSD, Nippon Kayaku, Novartis, Pfizer, Taiho, and Takeda. YG received personal fees from Taiho, Boehringer Ingelheim, Chugai, MSD, GSK, Pfizer, Takeda, Nippon Kayaku, Novartis, Bristol-Meyers Squibb, Kyowa Hakko Bio, and Eli Lilly. HI received personal fees from Eli Lilly. TY received personal fees from Ono, Chugai, Eli Lilly, Taiho, AstraZeneca, MSD, and Bristol-Meyers Squibb. YZ received grants from AstraZeneca, MSD, Daiichi-Sankyo, Merck and Amgen; personal fees from AstraZeneca, Eli Lilly, Chugai, Ono, Bristol-Meyers Squibb, Takeda, Boehringer-Ingelheim, Taiho, MSD, Novartis, Pfizer, Nihon Kayaku, Kyowa Kirin, and Amgen. MO received grants from AbbVie, Chugai, GSK, MSD, Parexel, Sanofi, AstraZeneca, Fujifilm Toyama Chemical, Janssen, Ono and Pfizer; personal fees from AMCO, Canon Medical Systems, Fujifilm Toyama Chemical, Merit Medical Japan, Olympus, AstraZeneca, Chugai, Kaneka Medix, Novartis, and Sanofi. MI received personal fees from AstraZeneca, GlaxoSmithKline, Pfizer, Shionogi, Sanofi, Insmed, Asahi Kasei Pharma, Kyorin pharmaceutical, Chugai pharmaceutical, Boehringer Ingelheim, Eli Lilly, Daiichi Sankyo, MSD, Abbott, Taiho pharmaceutical, Amgen, and Novartis.
Acknowledgements
This study was investigator initiated trial financially supported by Eli Lilly Japan K.K. This study received the study management support by the Department of Advanced Medicine, Nagoya University Hospital as an academic research organization and EPS Corporation, Tokyo. We thank Megumi Tanabe, clinical research coordinator of Nagoya University, all participants, their families.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102303.
Appendix A. Supplementary data
References
- 1.Reck M., Rodriguez-Abreu D., Robinson A.G., et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 2.Herbst R.S., Giaccone G., de Marinis F., et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N Engl J Med. 2020;383(14):1328–1339. doi: 10.1056/NEJMoa1917346. [DOI] [PubMed] [Google Scholar]
- 3.Socinski M.A., Jotte R.M., Cappuzzo F., et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 4.Gandhi L., Rodriguez-Abreu D., Gadgeel S., et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 5.Paz-Ares L., Luft A., Vicente D., et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379(21):2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 6.West H., McCleod M., Hussein M., et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(7):924–937. doi: 10.1016/S1470-2045(19)30167-6. [DOI] [PubMed] [Google Scholar]
- 7.Nishio M., Barlesi F., West H., et al. Atezolizumab plus chemotherapy for first-line treatment of nonsquamous NSCLC: results from the randomized phase 3 IMpower132 trial. J Thorac Oncol. 2021;16(4):653–664. doi: 10.1016/j.jtho.2020.11.025. [DOI] [PubMed] [Google Scholar]
- 8.Paz-Ares L., Ciuleanu T.E., Cobo M., et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(2):198–211. doi: 10.1016/S1470-2045(20)30641-0. [DOI] [PubMed] [Google Scholar]
- 9.Garassino M.C., Gadgeel S., Speranza G., et al. Pembrolizumab plus pemetrexed and platinum in nonsquamous non-small-cell lung cancer: 5-year outcomes from the phase 3 KEYNOTE-189 study. J Clin Oncol. 2023;41(11):1992–1998. doi: 10.1200/JCO.22.01989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Novello S., Kowalski D.M., Luft A., et al. Pembrolizumab plus chemotherapy in squamous non-small-cell lung cancer: 5-year update of the phase III KEYNOTE-407 study. J Clin Oncol. 2023;41(11):1999–2006. doi: 10.1200/JCO.22.01990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garon E.B., Ciuleanu T.E., Arrieta O., et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384(9944):665–673. doi: 10.1016/S0140-6736(14)60845-X. [DOI] [PubMed] [Google Scholar]
- 12.Osa A., Uenami T., Koyama S., et al. Clinical implications of monitoring nivolumab immunokinetics in non-small cell lung cancer patients. JCI Insight. 2018;3(19) doi: 10.1172/jci.insight.59125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimura H., Sone T., Araya T., et al. Late-onset programmed cell death protein-1 inhibitor-induced pneumonitis after cessation of nivolumab or pembrolizumab in patients with advanced non-small cell lung cancer: a case series. Transl Lung Cancer Res. 2021;10(3):1576–1581. doi: 10.21037/tlcr-20-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gemma A., Kusumoto M., Sakai F., et al. Real-world evaluation of factors for interstitial lung disease incidence and radiologic characteristics in patients with EGFR T790M-positive NSCLC treated with osimertinib in Japan. J Thorac Oncol. 2020;15(12):1893–1906. doi: 10.1016/j.jtho.2020.08.025. [DOI] [PubMed] [Google Scholar]
- 15.Chour A., Denis J., Lafitte C., et al. Sotorasib-induced liver and non-liver toxicity associated with sequential sotorasib following anti-PD(L)1 in KRAS(G12C) mutant lung cancer. Ann Oncol. 2022;33:S49–S50. [Google Scholar]
- 16.Okeya K., Kawagishi Y., Yamoto M., Shimizu M., Imizuda T., Tsuji H. Interstitial lung disease induced by docetaxel and ramucirumab chemotherapy after nivolumab treatment. Respirol Case Rep. 2020;8(5) doi: 10.1002/rcr2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoh K., Hosomi Y., Kasahara K., et al. A randomized, double-blind, phase II study of ramucirumab plus docetaxel vs placebo plus docetaxel in Japanese patients with stage IV non-small cell lung cancer after disease progression on platinum-based therapy. Lung Cancer. 2016;99:186–193. doi: 10.1016/j.lungcan.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 18.Reckamp K.L., Redman M.W., Dragnev K.H., et al. Phase II randomized study of ramucirumab and pembrolizumab versus standard of care in advanced non–small-cell lung cancer previously treated with immunotherapy—lung-MAP S1800A. J Clin Oncol. 2022;40(21):2295–2307. doi: 10.1200/JCO.22.00912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldberg S.B., Gettinger S.N., Mahajan A., et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2016;17(7):976–983. doi: 10.1016/S1470-2045(16)30053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brueckl W.M., Reck M., Rittmeyer A., et al. Efficacy of docetaxel plus ramucirumab as palliative second-line therapy following first-line chemotherapy plus immune-checkpoint-inhibitor combination treatment in patients with non-small cell lung cancer (NSCLC) UICC stage IV. Transl Lung Cancer Res. 2021;10(7):3093–3105. doi: 10.21037/tlcr-21-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reck M., Popat S., Grohe C., et al. Anti-angiogenic agents for NSCLC following first-line immunotherapy: Rationale, recent updates, and future perspectives. Lung Cancer. 2023;179 doi: 10.1016/j.lungcan.2023.03.009. [DOI] [PubMed] [Google Scholar]
- 22.Grohe C., Wehler T., Dechow T., et al. Nintedanib plus docetaxel after progression on first-line immunochemotherapy in patients with lung adenocarcinoma: Cohort C of the non-interventional study, VARGADO. Transl Lung Cancer Res. 2022;11(10):2010–2021. doi: 10.21037/tlcr-21-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamura A., Yamaguchi O., Mori K., et al. Multicentre real-world data of ramucirumab plus docetaxel after combined platinum-based chemotherapy with programmed death-1 blockade in advanced non-small cell lung cancer: NEJ051 (REACTIVE study) Eur J Cancer. 2023;184:62–72. doi: 10.1016/j.ejca.2023.01.025. [DOI] [PubMed] [Google Scholar]
- 24.Arrieta O., Barron F., Ramirez-Tirado L.A., et al. Efficacy and safety of pembrolizumab plus docetaxel vs docetaxel alone in patients with previously treated advanced non-small cell lung cancer: the PROLUNG phase 2 randomized clinical trial. JAMA Oncol. 2020;6(6):856–864. doi: 10.1001/jamaoncol.2020.0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.