Abstract
In the present study, the new data of the infinite dilution activity coefficient for 32 different solutes in {1-ethyl-1-methylpyrrolidinium bromide +1,5-pentanediol}, [[EMPYR] Br + 1,5-PDO] DES, were measured using the gas liquid chromatography (GLC) method with pre-saturation of the helium gas. The list of selected solutes included alkanes, alkenes, alkynes, cycloalkanes, cycloalkenes, aromatics, ketones, alcohols, and water. Because the solvents were volatile at the temperatures used for measurements, pre-saturation was deemed necessary. The measurements were taken at temperatures T = (313.15–343.15) K and atmospheric pressure. Values of partial molar properties, i.e., enthalpy, entropy, and Gibbs free energy, were computed at a reference temperature of Tref = 333.15 K. Moreover, the values of capacity and selectivity relating to [[EMPYR] Br + 1,5-PDO] DES for different sets of binary systems that are normally problematic in the separation through solvent extraction or distillation were also computed. These include cyclohexane/benzene; acetone/methanol; and hexane/benzene. The obtained data in the present work was then compared to the literature data, at similar temperatures. Thus, the thermodynamical data is important for pre-selecting solvents for industrial purposes.
Keywords: Ionic liquids; Deep eutectic solvents; 1,5-Pentanediol; Infinite dilution activity coefficient; Excess thermodynamic properties
1. Introduction
The United Nations Commission on Environment and Development presented rules and guidelines to meet the needs of the present generation without compromising the ability of future generations to meet their needs, “sustainable development” [1,2]. The sustainable development goals demand that all developments be environmentally acceptable, socially equitable, and economically viable [2,3]. Thus, chemistry, as a central science, covering chemical engineering, environmental science, agricultural science, forensic science, atmospheric science, solid-state physics, and biochemistry, to name a few, is key to this requirement. Furthermore, this brings forward the benefits of modern technology, which include the principles of green chemistry as well as green engineering [[4], [5], [6]]. To meet up with green chemistry and engineering requirements, green solvents were presented as future alternative over traditional solvents, such as N-methyl-2-pyrrolidone, N-formaylmorpholine, acetone, glycerol, etc., in the chemical industry for various purposes [[7], [8], [9], [10]]. This initiative was due to discovery of many drawbacks related to traditional solvents, which include non-recyclability, low thermal stability, low production yield, flammability, high purchasing costs, high energy, high volatility, and toxicity, in addition to many other environmental impact issues [11,12]. Green solvents are designed and developed based on green chemistry selection principles and guidelines to help mitigate the large number of drawbacks posed by industrial solvents. Several studies have reported on green chemistry selection guidelines, including [[11], [12], [13]]. Once more, the design and development of ionic liquids (ILs) and, lately, deep eutectic solvents (DESs) are based on green chemistry selection criteria.
Ionic liquids (ILs) have been known since 1914. They are a class of salts with low melting points that can be achieved through the high bulkiness and asymmetry of the ions, thus avoiding the molecule packing from promoting crystallization [[14], [15], [16], [17]]. Typically, ILs are made of large-size of nitrogen-containing cations such as ammonium, pyrrolidinium, pyridinium, imidazolium, piperidinium, or a phosphorus-containing cation such as phosphonium, mixed with an anion of weak coordination properties, e.g., organic anions and halogens [18,19]. The above list of cations allow for prominent characteristics of these solvents, including in-flammability, low volatility, low thermal stability, low vapour pressure, and some have low toxicity and biodegradability, as well as many environmentally friendly benefits [[20], [21], [22]]. Nonetheless, lately scientific researchers have reported few limitations linked to ILs. These include high viscosity, toxicity, low-biodegradability, and high-cost synthesis [18,20,[23], [24], [25]]. Thus, this led to the proposition of deep eutectic solvents as a better alternative to both ILs and traditional solvents.
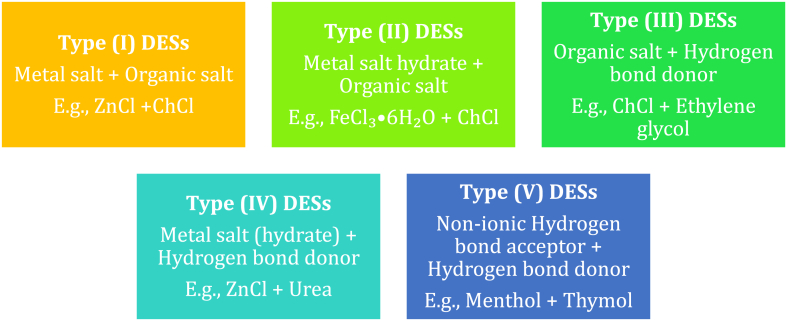
Abbott and co-authors [26] invented deep eutectic solvents as an upgrade and/or a possible replacement to both ionic liquids (ILs) and traditional solvents. Deep eutectic solvents are a new-fangled class of solvent mixtures combining the characteristics of both ILs and traditional solvents, which include low volatility, non-toxicity, and biodegradability, to name a few [[27], [28], [29], [30]]. Deep eutectic solvents are classified into five groups or types, as displayed in Fig. 1. On the list of DESs, type III has drawn immense attention from scientific research due to the fact that type III is formed through hydrogen bonds, where the charge delocalization takes place through hydrogen bonds amongst the hydrogen bond donor (HBD) and the halide, resulting in a decrease in the freezing point of the mixture [31]. These solvents are made by pairing a complexing agent, generally a hydrogen bond donor (HBD), e.g., glycerol, ethylene glycol, urea, etc., with a quaternary ammonium salt (QAS), e.g., ammonium IL, etc., to attain ambient temperatures [[32], [33], [34], [35], [36], [37]]. For these solvents to be adopted for various industrial purposes, the knowledge on their properties is required. As a result, the search aims to add data on the properties of these solvents. Moreover, thermodynamic properties as imperative tools for screening for suitable solvents in chemical industry are presented in this work.
Fig. 1.
Classification of deep eutectic solvents.
The experimental data on thermodynamic properties, particularly infinite dilution activity coefficients (IDACs) of organic solutes in deep eutectic solvents, is inadequate. Few studies have stated on excess thermodynamic data covering deep eutectic solvents [28,38,39]. Thus, this work presents the measurements of infinite dilution activity coefficients for 32 different organic solutes, including alkanes, alkenes, alkynes, cycloalkanes, cycloalkenes, heterocyclics, alcohols, ketones, and water in {1-ethyl-1-methypyrrolidinium bromide +1,5-pentanediol} DES at a molar ratio of 1:2. As mentioned, thermodynamic properties are imperative for the preselection of extracting solvents for various industrial purposes. The present study adopted the method from the previous work [28,29,39].
Moreover, the research of Li [39], the activity coefficients at infinite dilution of solutes in [choline chloride + 1,5-pentanediol] stationary phase using gas liquid chromatography (GLC), encouraged the use of 1,5-pentanediol (1,5-PDO) as a hydrogen bond donor (HBD) for this work.
2. Experimental materials and methods
2.1. Materials
Table 1 displays all materials used in the experiments. The organic solutes were utilized exactly as supplied by the vendors because gas liquid chromatography (GLC) eliminates any unwanted components from the column.
Table 1.
List of chemical reagents, suppliers, and purity.
| Chemical reagents | Suppliers | CAS No | Purity | Method Analysis |
|---|---|---|---|---|
| 1-pentyne | Alfa Aesar | 627-19-0 | ≥0.99 | GC |
| 1-hexyne | Alfa Aesar | 693-02-7 | ≥0.98 | GC |
| 1-heptyne | Alfa Aesar | 628-71-7 | ≥0.99 | GC |
| 1-pentene | Merck | 109-67-1 | ≥0.99 | GC |
| 1-hexene | Merck | 592-41-7 | ≥0.99 | GC |
| 1-nonane | Sigma Aldrich | 124-11-8 | ≥0.99 | GC |
| 1-decene | Sigma Aldrich | 872-05-9 | ≥0.99 | GC |
| 2,2-Dimethylbutane | Sigma Aldrich | 75-83-2 | ≥0.99 | GC |
| Pentane | Fluka | 109-66-0 | ≥0.98 | GC |
| Hexane | Sigma Aldrich | 110-54-3 | ≥0.99 | GC |
| Heptane | Sigma Aldrich | 142-82-5 | ≥0.99 | GC |
| Octane | Sigma Aldrich | 111-65-9 | ≥0.99 | GC |
| n-nonane | Sigma Aldrich | 111-84-2 | ≥0.99 | GC |
| n-decane | Sigma Aldrich | 124-18-5 | ≥0.99 | GC |
| Cyclopentane | Sigma Aldrich | 287-92-3 | ≥0.98 | GC |
| Cyclohexane | Sigma Aldrich | 110-82-7 | ≥0.995 | GC |
| Methylcyclohexane | Alfa Aesar | 108-87-2 | ≥0.99 | GC |
| Cyclooctane | Merck | 292-64-8 | ≥0.99 | GC |
| Cyclopentene | Sigma Aldrich | 142-29-0 | ≥0.96 | GC |
| Cyclohexene | Sigma Aldrich | 110-83-8 | ≥0.99 | GC |
| o-xylene | Fluka | 95-47-6 | ≥0.99 | GC |
| m-xylene | Fluka | 108-88-3 | ≥0.99 | GC |
| p-xylene | Fluka | 106-42-3 | ≥0.99 | GC |
| Benzene | Alfa Aesar | 71-43-2 | ≥0.99 | GC |
| Toluene | Sigma Aldrich | 108-88-3 | ≥0.995 | GC |
| Ethylbenzene | Fluka | 100-41-4 | ≥0.99 | GC |
| Methanol | Sigma Aldrich | 67-56-1 | ≥0.999 | HPLC |
| Ethanol | Sigma Aldrich | 64-17-5 | ≥0.998 | GC |
| 2-propanol | Sigma Aldrich | 71-23-8 | ≥0.99 | GC |
| Butan-1-ol | Saarchem | 71-36-3 | ≥0.99 | GC |
| Acetone | Sigma Aldrich | 67-64-1 | ≥0.998 | HPLC |
| 2-butanone | Allied Signal | 78-93-3 | ≥0.999 | GC |
| Water | Sigma Aldrich | 7732-18-5 | ≥0.999 | HPLC |
| 1,5-pentanediol | Merck | ≥0.99 | GC | |
| 1-ethyl-1-methypyrrolidinium bromide | Sigma Aldrich | 69227-51-6 | ≥0.99 | HPLC |
| Helium | Afrox SA | – | ≥0.999 | GC |
2.2. Deep eutectic solvent preparation
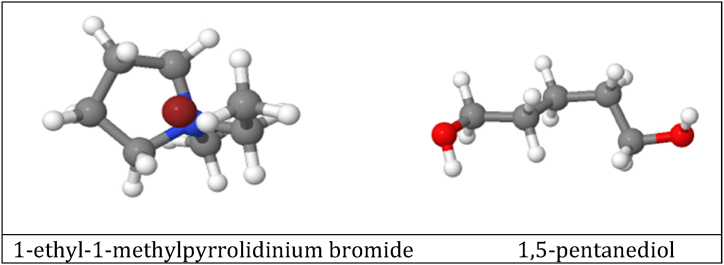
The {[EMPYR]Br + 1,5-PDO} DES was prepared as a separating agent based on previous research [17,28,36,39]. Fig. 2 shows the structures of the components used during the preparation of the investigated deep eutectic solvent. A 1:2 mol fraction of 1-ethyl-1-methylpyrrolidinium bromide and 1,5-pentanediol were mixed at room temperature to produce {[EMPYR]Br + 1,5-PDO} DES. The two components were carefully weighed using an analytical balance, and they were thoroughly blended into a round-bottom flask. A clear, homogeneous DES was produced after 4 h of vigorous mixing at 363.15 K. After that, any volatile species in the produced DES were removed using a rotary evaporator. The DES was kept in an airtight chamber to keep moisture out. The moisture content of the prepared DES was determined to be less than 350 x 10−4 in mass fraction, by the Karl Fischer Auto Titrator. Lastly, the physical properties, density, and velocity of [[EMPYR] Br + 1,5-PDO] DES at different temperatures were compared between this work and the literature, to confirm the nature of the produced DES.
Fig. 2.
Components used to synthesise the deep eutectic solvent.
2.3. Preparing a column
The method used to prepare the columns was discovered in the literature by Refs. [29,40]. Based on this method, the chromosorb was spread out and blended with deep eutectic solvent using dichloromethane prior to packing. Dichloromethane was evaporated using a rotating evaporator. For packaging, a 1 m long stainless-steel column with a 4 mm internal diameter was used. The columns were cleaned with hot soapy water, then rinsed with distilled water, and finally dried with acetone.
3. Infinite dilution activity coefficient data collection
A gas chromatography equipped with a thermal conductivity detector was utilized to measure retention data for all 32 selected solutes in {[EMPYR] Br + 1,5-PDO} DES. The flow rate of the carrier gas, helium, was measured using a soapy bubble flow meter. Using an analytical balance, the masses of components, i.e., [EMPYR] Br, [1,5-PDO], and chromosorb (solid support material) were weighed with an accuracy of 0.0001 g. As in our earlier work, the two columns were prepared with the DES mass loadings of 28.9 and 32.4 (both by mass %), and this was sufficient to prevent any undesired solute adsorption on the columns [29,40,41]. Furthermore, the columns were conditioned by passing helium gas for 6 h at a flow rate of 2.0 cm3 s−1 and at 360 K. A digital barometer with a 0.1 hPa inaccuracy was used to measure the air pressure. The pressure drop was calculated with a 0.1 hPa inaccuracy using a pressure transducer in gas liquid chromatography. Sample solute injections (0.2–0.3) μL at each analyzed temperature were performed twice for each sample to ensure reproducibility of the experimental data. The approximate overall error in infinite dilution activity coefficient was within 5 % for all the investigated solutes, except for alkynes (gave 8 %), when considering potential uncertainties in measuring retention time, column packing, and vapour pressure.
4. Theoretical sources
Infinite dilution activity coefficient data can be used to determine the pre-selection of extracting solvents for certain separation applications. This also reveals the types of interactions that exist between the solutes under investigation as well as the deep eutectic solvent used under investigation. The detailed equations for determining infinite dilution activity coefficients were previously published by various studies [28,[41], [42], [43], [44]].
5. Discussion of the results
5.1. Measured physical properties
The density and sound velocity of the manmade deep eutectic solvent were measured simultaneously at T = (298.15–313.15) K at atmospheric pressure using the density and sound velocity meter (Anton Paar DSA 5000 M). Physical quantities that were measured are listed in Table 2. As shown in Table 2, the obtained results were comparable with the previously investigated deep eutectic solvents [43,45], this includes deep eutectic solvent prepared with a similar hydrogen bond acceptor (organic salt) [45]. Additionally, this confirmed the nature and cleared the way for further investigation into the deep eutectic solvent's infinite dilution activity coefficients.
Table 2.
Physical properties, density () and velocity () of [[EMPYR] Br + 1,5-PDO] DES at different temperatures compared between this work and the literature.
| Authors | Temperature (T/K) | Density (/g. cm−3) | Speed of sound (/m. s−1) |
|---|---|---|---|
| [[EMPYR] Br + 1,5-PDO] (This work) | 293.15 | 1.1547 | 1815.7 |
| 298.15 | 1.1502 | 1803.3 | |
| 303.15 | 1.1476 | 1791.7 | |
| 308.15 | 1.1434 | 1779.4 | |
| 313.15 | 1.1407 | 1769.2 | |
| [[EMPYR] Br + 1,6-HDO] [1] | 293.15 | 1.2543 | 1915.5 |
| 298.15 | 1.2504 | 1903.4 | |
| 303.15 | 1.2473 | 1891.8 | |
| 308.15 | 1.2432 | 1879.3 | |
| 313.15 | 1.2404 | 1869.1 | |
| [[BMIM] Cl + Gly] [2] | 293.15 | 1.1709 | 1851.3 |
| 298.15 | 1.1678 | 1838.8 | |
| 303.15 | 1.1647 | 1826.5 | |
| 308.15 | 1.1616 | 1814.4 | |
| 313.15 | 1.1585 | 1802.4 |
Standard uncertainties were u () = 0.005 g cm−3; u () = 0.5 m. s−1; u (T) = 0.01K
5.2. Measured infinite dilution activity coefficients (IDACs)
The experimental IDAC data for 33 various solutes in a stationary phase of {1-ethyl-1-methylpyrrolidinium bromide + 1,5-pentanediol}, {[EMPYR] Br + 1,5-PDO} deep eutectic solvent as an extracting solvent were determined from retention time data that was measured in a gas liquid chromatography (GLC). The parameters such as temperature and pressure were monitored at fixed range, T = (313.15–343.15) K and 101.31 kPa, respectively.
The results are presented in Table 3, Table 4 as well as displayed in supporting information, S. Figs. 1–8. In this investigation, the uncertainties and the reproducibility of the calculated results and variables were considered. The experimentally obtained infinite dilution activity coefficient's error margin was calculated to be 5 % for all investigated solutes and 8 % for alkynes using an examination of error propagation. For all studied functional groups, the IDAC values were directly related to the experimental temperature as well as the solutes' alkyl chains. This is a pragmatic effect, as per numerous studies [28,41,46,47]. The infinite dilution activity coefficients of the nonpolar solutes are higher than those of the polar solutes at the same temperature. N-decane has the highest infinite dilution activity coefficient of the 33 organic solutes examined, whereas methanol and water have the lowest. For the alkanes, as the length of the carbon chain increases, the infinite dilution activity coefficients increase. For cycloalkanes and aromatics, the infinite dilution activity coefficients increase as the substituted alkane chain lengthens. For example, the infinite dilution activity coefficient of cyclohexane is lower than that of methylcyclohexane, and the infinite dilution activity coefficient of toluene is lower than that of ethylbenzene. This is because the –CH2- will make the solutes more lipophilic and less resistant to the solvent's pulling power [31,39,48]. The infinite dilution activity coefficients of alkanes are higher than the obtained infinite dilution activity coefficients for alkenes, cycloalkanes, and aromatics. The obtained order is as follows: alkanes > alkenes > cycloalkanes > aromatics. For example, at T = 313.15 K, hexane gave = 2.91, 1-hexene gave = 2.29, cyclohexane gave = 2.55, and benzene gave = 1.19. Branched chains, cyclization, and double bonds all increase the polarity of organic solutes with the same amount of carbon atoms while decreasing the infinite dilution activity coefficient. This is due to the fact that adding cyclization, or a double-stranded structure will make the solutes' charge distribution asymmetrical and increase their polarity, which will increase the interaction between the solute molecule and the DES component [49]. The infinite dilution activity coefficients of 2,2-methylbutane are slightly lower than those of hexane. For example, at T = 323.15 K, 2,2-dimethylbutane gave = 2.92 and hexane gave = 2.99. This is because the interaction between 2,2-methylbutane and the DES is increased by the introduction of branched structures [49,50]. In addition, because of the six delocalized electronic structures of aromatics, which cause them to interact strongly with solvent molecules, the infinite dilution activity coefficients of aromatics are lower than those of alkanes [46,47]. O-xylene has the lowest value for xylene. This occurs because of the component molecules' centers of gravity deviating due to the difference between the generated dipole moment and the nearby methyl groups on the o-xylene molecule.
Table 3.
Average infinite dilution activity coefficient values for different organic solutes in [1-ethyl-1-methylpyrrolidinium bromide + 1,5-pentanediol] deep eutectic solvent at different temperatures and atmospheric pressures.
| Average infinite dilution activity coefficient values at T (K) |
||||
|---|---|---|---|---|
| Organic solutes | T = 313.15 K | T = 323.15 K | T = 333.15 K | T = 343.15 K |
| 1-pentyne | 1.70 | 1.78 | 1.84 | 1.89 |
| 1-hexyne | 2.06 | 2.12 | 2.17 | 2.20 |
| 1-heptyne | 2.60 | 2.66 | 2.67 | 2.71 |
| 1-pentane | 2.05 | 2.18 | 2.31 | 2.44 |
| 1-hexene | 2.29 | 2.42 | 2.56 | 2.69 |
| 1-nonene | 2.53 | 2.65 | 2.76 | 2.83 |
| 1-decane | 3.12 | 3.38 | 3.48 | 3.60 |
| 2,2-Dimethylbutane | 2.90 | 2.92 | 2.94 | 2.97 |
| Pentane | 2.81 | 2.86 | 2.89 | 2.94 |
| Hexane | 2.91 | 2.99 | 3.09 | 3.18 |
| Heptane | 2.99 | 3.18 | 3.33 | 3.51 |
| Octane | 3.24 | 3.50 | 3.72 | 3.92 |
| n-nonane | 3.74 | 3.99 | 4.29 | 4.52 |
| n-decane | 3.95 | 4.27 | 4.61 | 4.91 |
| Cyclopentane | 2.50 | 2.55 | 2.61 | 2.64 |
| Cyclohexane | 2.55 | 2.60 | 2.64 | 2.69 |
| Methylcyclohexane | 2.66 | 2.72 | 2.78 | 2.84 |
| Cyclooctane | 2.61 | 2.66 | 2.72 | 2.77 |
| Cyclopentene | 1.45 | 1.51 | 1.59 | 1.66 |
| Cyclohexene | 1.63 | 1.67 | 1.70 | 1.74 |
| o-xylene | 1.35 | 1.48 | 1.60 | 1.73 |
| m-xylene | 1.45 | 1.60 | 1.71 | 1.82 |
| p-xylene | 1.54 | 1.64 | 1.74 | 1.85 |
| Benzene | 1.19 | 1.30 | 1.40 | 1.50 |
| Toluene | 1.21 | 1.31 | 1.42 | 1.54 |
| Ethylbenzene | 1.39 | 1.53 | 1.65 | 1.79 |
| Methanol | 0.17 | 0.25 | 0.34 | 0.46 |
| Ethanol | 0.22 | 0.30 | 0.41 | 0.54 |
| 2-propanol | 0.32 | 0.39 | 0.47 | 0.63 |
| Butan-1-ol | 0.40 | 0.48 | 0.58 | 0.70 |
| Acetone | 1.31 | 1.42 | 1.51 | 1.58 |
| 2-butanone | 1.33 | 1.45 | 1.54 | 1.63 |
| Water | 0.17 | 0.25 | 0.34 | 0.45 |
The investigated solutes standards uncertainties (u) are = 5 %, (u) T = 0.01 K, and (u) p = 1 kPa, except for alkynes which gave (u) are = 8 %, (u) T = 0.01 K, and (u) p = 1 kPa.
Table 4.
Correlation constants a, b, R2, as well as , at T = 298.15 K for [[EMPYR] Br + 1,5-PDO] DES.
| Solutes | a | b | R2 | |
|---|---|---|---|---|
| 1-pentyne | 1.74 | −378.2 | 0.995 | 1.61 |
| 1-hexyne | 1.49 | −237.9 | 0.994 | 2.01 |
| 1-heptyne | 1.43 | −146.6 | 0.997 | 2.38 |
| 1-pentene | 2.69 | −615.8 | 1.000 | 1.92 |
| 1-hexene | 2.63 | −563.9 | 1.000 | 2.16 |
| 1-nonane | 2.22 | −402.7 | 0.999 | 2.39 |
| 1-decene | 2.32 | −357.1 | 0.998 | 2.97 |
| 2,2-dimethylbutane | 1.27 | −85.20 | 0.994 | 2.87 |
| Pentane | 1.50 | −144.7 | 0.957 | 2.77 |
| Hexane | 2.18 | −349.9 | 0.999 | 2.80 |
| Heptane | 2.88 | −556.7 | 0.999 | 2.82 |
| Octane | 3.28 | −657.1 | 0.999 | 2.98 |
| n-nonane | 3.53 | −693.7 | 0.997 | 3.51 |
| n-decane | 3.88 | −783.7 | 1.000 | 3.66 |
| Cyclopentane | 1.55 | −197.7 | 1.000 | 2.44 |
| Cyclohexane | 1.51 | −178.4 | 0.995 | 2.49 |
| Methylcyclohexane | 1.65 | −216.0 | 1.000 | 2.60 |
| Cyclooctane | 1.71 | −230.1 | 1.000 | 2.55 |
| Cyclopentene | 1.83 | −455.0 | 1.000 | 1.40 |
| Cyclohexene | 1.26 | −241.0 | 0.998 | 1.58 |
| o-xylene | 3.09 | −872.3 | 1.000 | 1.23 |
| m-xylene | 2.62 | −695.5 | 0.999 | 1.31 |
| p-xylene | 2.55 | −665.3 | 0.999 | 1.45 |
| Benzene | 2.84 | −833.2 | 0.999 | 1.09 |
| Toluene | 2.95 | −864.8 | 1.000 | 1.12 |
| Ethylbenzene | 3.19 | −893.3 | 1.000 | 1.25 |
| Methanol | 9.28 | −3449 | 1.000 | 0.10 |
| Ethanol | 8.73 | −3206 | 1.000 | 0.13 |
| 2-propanol | 6.44 | −2372 | 0.999 | 0.23 |
| Butan-1-ol | 5.63 | −2053 | 0.999 | 0.31 |
| Acetone | 2.54 | −748.5 | 0.999 | 1.22 |
| 2-butanone | 2.59 | −721.6 | 0.994 | 1.25 |
| Water | 9.23 | −3436 | 0.999 | 0.09 |
For ketone solutes, such as acetone and 2-butanone, the infinite dilution activity coefficients are lower than those of alkanes, alkenes, alkynes, cycloalkanes, cycloalkenes, and aromatics, signifying that the solutes interact closely with the DES components. This is due to the presence of O atoms in these organic solutes, which have significant interactions with the DES components. For alcohols and water, their infinite dilution activity coefficients in the investigated DES are minimal because of the fact that the lone pair electron of the oxygen atom in the hydroxyl group can interact with components in the DES and that acidic protons have a strong binding capacity with halides, making them more soluble in the solvent [[51], [52], [53], [54]]. Like the law of alkanes, the intermolecular force of alcohols will increase as the carbon chain length increases, and the force between alcohols and DESs will decrease as a result. For this reason, straight-chain alcohols' infinite dilution activity coefficients similarly increase as carbon chain lengthens [[55], [56], [57]].
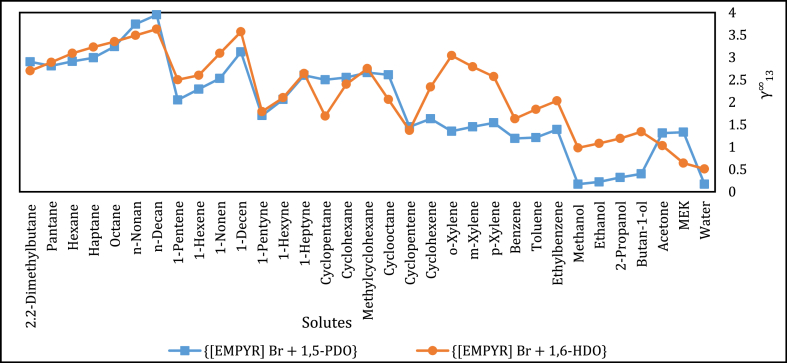
Moreover, the IDAC literature values for the solutes in {[EMPYR]Br + 1,5-PDO} at similar temperatures to those at which measurements were conducted in this study were compared to the DESs with related components, i.e., {[EMPYR]Br + 1,6-PDO} [45] and {[ChCl] + 1,5-PDO} [51], as well as the recent studied ionic liquid, [C7MIm] [Cl] [58]. The data comparison of few selected solutes is presented in Table 5 as well as plotted and displayed in Fig. 3. For {[EMPYR]Br + 1,6-HDO}, which contains similar HBA to that of the investigated DES, the values are highly comparable, while for {[ChCl] + 1,5-PDO} which contains similar HBD, the values are much differing, and this is a similar case to the ionic liquid. Consequently, the investigated DES revealed promising data as compared to the literature of comparison.
Table 5.
Comparison of infinite dilution activity coefficient values for this work, {[EMPYR]Br + 1,5-PDO}, and the studies from the literature, including {[EMPYR]Br + 1,6-PDO}, {[ChCl] + 1,5-PDO]}, and [C7MIm] [Cl] at T = 313.15 K
| Infinite dilution activity coefficients () |
||||
|---|---|---|---|---|
| Solutes | {[EMPYR]Br + 1,5-PDO} (This work) | {[EMPYR]Br + 1,6-PDO} [1] | {[ChCl] + 1,5-PDO]} [3] |
[C7MIm] [Cl] [4] |
| Hexane | 2.91 | 3.09 | 251 | 65.1 |
| Heptane | 2.88 | 3.23 | 312 | 70.3 |
| 1-hexene | 2.29 | 2.60 | 108 | – |
| Cyclohexane | 2.55 | 2.40 | 209 | 34.3 |
| Ethylbenzene | 1.39 | 2.03 | 90.3 | 6.96 |
| Ethanol | 0.22 | 1.08 | 2.13 | 0.24 |
| Acetone | 1.31 | 1.03 | 10.2 | 3.19 |
| Water | 0.17 | 0.51 | – | – |
Fig. 3.
Comparison of at T = 313.15 K for investigated solutes in DESs: {[EMPYR]Br + 1,6-HDO} and {[EMPYR]Br + 1,5-PDO}.
In addition, the energy of solute dissociation and the energy-breaking interaction between the solute and DES components cause the infinite dilution activity coefficients of organic solutes to be very temperature-dependent and fluctuate with temperature. The method outlined below demonstrates how thermodynamic laws, particularly those that depend on temperature, can be simplified into enthalpy and entropy components.
5.3. Partial molar excess properties
The partial molar excess properties, viz., enthalpy, entropy, and Gibbs free energy for 32 different organic solutes, were calculated from the infinite dilution activity coefficient values at T = 313.15 K to further discusses the kinds of intermolecular interactions occurring between the selected organic solutes and DES components, i.e., [[EMPYR] Br and [1,5-PDO].
Equation (1) below was used for computation of excess Gibbs free energy values.
| (1) |
Equation (1) can be rearranged to obtain equation (2).
| (2) |
While equation (3) was used for fittings of IDAC values to obtain the constants.
| (3) |
where a and b are correlation coefficients and can subsequently be used to obtain both partial molar enthalpy and partial molar entropy.
As presented in Table 6, the obtained partial molar enthalpy values are negative for all the selected organic solutes. For example, at T = 313.15 K, pentane ( −1.621 kJ mol−1), cyclopentane ( −1.645 kJ mol−1), toluene ( −7.165 kJ mol−1), o-xylene ( −7.551 kJ mol−1), ethanol ( −26.62 kJ mol−1), acetone ( −5.569 kJ mol−1), and water ( −28.66 kJ mol−1). This informs about stronger interactions between the DES components (i.e., [[EMPYR] Br and [1,5-PDO]) and the selected organic solutes [28,46,59]. In addition, these values are related to the properties of ideal solution, and they result from the differences of interactions between like and unlike molecules [41,47].
Table 6.
Partial molar properties, enthalpy (), Gibbs free energy (), and entropy () for the various organic solutes in [[EMPYR] Br + 1,5-DPO] at the reference temperature, .
| Organic solutes | (kJ. mol−1) | (kJ. mol−1) | (kJ. mol−1) |
|---|---|---|---|
| 1-pentyne | −4.23 | 5.81 | −10.0 |
| 1-hexyne | −3.03 | 6.17 | −9.20 |
| 1-heptyne | −2.47 | 5.91 | −8.38 |
| 1-pentene | −5.56 | 2.83 | −8.39 |
| 1-hexene | −4.68 | 3.73 | −8.41 |
| 1-nonene | −3.59 | 5.04 | −8.63 |
| 1-decene | −4.23 | 5.82 | −10.1 |
| 2,2-dimethylbutane | −0.72 | 8.26 | −8.98 |
| Pentane | −1.62 | 6.95 | −8.57 |
| Hexane | −2.90 | 5.70 | −8.60 |
| Heptane | −4.69 | 4.03 | −8.71 |
| Octane | −5.64 | 3.44 | −9.08 |
| n-nonane | −5.67 | 3.30 | −8.97 |
| n-decane | −6.48 | 2.32 | −8.80 |
| Cyclopentane | −1.65 | 6.45 | −8.10 |
| Cyclohexane | −1.60 | 6.64 | −8.23 |
| Methylcyclohexane | −1.91 | 6.75 | −8.66 |
| Cyclooctane | −1.68 | 6.76 | −8.44 |
| Cyclopentene | −3.99 | 10.2 | −14.2 |
| Cyclohexene | −2.31 | 8.05 | −10.4 |
| o-xylene | −7.55 | −2.66 | −4.89 |
| m-xylene | −5.76 | −0.71 | −5.06 |
| p-xylene | −5.55 | −0.47 | −5.08 |
| Benzene | −6.93 | −1.93 | −5.01 |
| Toluene | −7.17 | −2.14 | −5.03 |
| Ethylbenzene | −7.47 | −2.33 | −5.14 |
| Methanol | −28.8 | −35.1 | 6.35 |
| Ethanol | −26.6 | −31.5 | 4.90 |
| 2-Propanol | −19.7 | −23.4 | 3.69 |
| Butan-1-ol | −19.2 | −24.1 | 3.23 |
| Acetone | −5.57 | −0.97 | −4.60 |
| 2-butanone | −6.03 | −2.61 | −3.43 |
| Water | −28.7 | −24.9 | −3.78 |
Standard uncertainties are u (T) = 0.01 K and (p) = 1 kPa.
The obtained partial molar Gibbs free energy values are positive for all the selected alkanes, alkenes, and alkynes as well as their corresponding cyclic compounds, whereas they are negative for all selected aromatics as well as for all the selected polar compounds, including water. These values are related to the properties of ideal solution, and they result from the differences of interactions between like and unlike molecules [[60], [61], [62]]. Moreover, the negative partial molar Gibbs free energy values for alcohols, ketones, and water suggest the formation of intermolecular hydrogen bonds and dipole forces, respectively, with the investigated DES [28,51,63].
On the other hand, the partial molar entropy values are all negative except for the alcohols; thus, this indicates arrangements of solute molecules in the cavities of [[EMPYR] Br + 1,5-PDO] DES. Furthermore, the obtained values are related to the properties of ideal solution, and they result from the differences of interactions and entropies between like and unlike molecules [29,63].
The data revealed that high temperatures are favourable for separation of some selected compounds, especially the polar compounds with investigated DES. An endotherm was revealed for most selected solutes. Lastly, DES data is crucial as these solvents promise desirable characteristics for industrial purposes, particularly for separation duties.
5.4. Selectivity and capacity
The extraction performance of [[EMPYR] Br + 1,5-PDO] DES is evaluated through extracting parameters, i.e., selectivity and capacity at infinite dilution. The separation problems are cyclohexane/benzene, acetone/methanol, and hexane/benzene all at T = 333.5 K. Moreover, the separation problems data is then compared with the literature to determine the ability of the investigated DES (Table 7). Lastly, the selectivity and capacity values are imperative for giving a clear view regarding specific separation problems. In addition, these parameters can be computed through equations (4), (5) below.
| (4) |
| (5) |
Thus, and denote selectivity and capacity at infinite dilution, respectively, while and are IDAC values for solutes, i and j, respectively.
Table 7.
Comparison of capacity and Selectivity values at infinite dilution for separation problems, hexane/toluene, ethanol/cyclohexane, and cyclohexane/hexane in [[EMPYR] Br + 1,5-PDO] DES as separating solvent at T = 333.15 K
| Extracting solvents |
Selectivity () |
Capacity () |
|||||
|---|---|---|---|---|---|---|---|
| Deep Eutectic Solvents | Hexane/toluene | Ethanol/cyclohexane | Cyclohexane/hexane | Toluene | Cyclohexane | Hexane | Reference |
| [[EMPYR] Br + 1,5-PDO] | 2.17 | 0.16 | 0.85 | 0.70 | 0.38 | 0.32 | This work |
| [[EMPYR] Br + Gly] | 1.70 | 0.03 | 0.82 | 0.02 | 0.01 | 0.01 | [5] |
| [[TMAM] Cl + EG] | 43.4 | 0.01 | 0.18 | 0.03 | 0.01 | 0.00 | [6] |
| [[BMIM] Cl + Gly] | 2.80 | 0.01 | 1.00 | 0.02 | 0.01 | 0.01 | [2] |
| Ionic Liquids | |||||||
| 6.96 | 0.09 | 0.28 | 0.47 | 0.24 | 0.07 | [7] | |
| 7.31 | 0.31 | 0.63 | 0.99 | 0.22 | 0.14 | [8] | |
| 13.1 | 0.09 | 0.53 | 0.49 | 0.07 | 0.04 | [9] | |
| Traditional solvents | |||||||
| Diethylene glycol | 8.53 | 0.03 | 0.45 | 0.09 | 0.02 | 0.01 | [10] |
| 2-pyrrolidone | 8.10 | 0.07 | 0.59 | 0.24 | 0.07 | 0.03 | [11] |
| Sulfolane | 10.7 | 0.15 | 0.45 | 0.30 | 0.06 | 0.03 | [12] |
As known, the number of equilibrium steps for a specific separation problem can be determined through the selectivity values, whereas the volume of separation solvent can be determined through capacity values [41,64,65]. In the present study, relatively high selectivity value of 2.17 and low-capacity value of 0.70, were obtained for the separation problem hexane/toluene. These values are comparable to the literature. This includes all the studies, the DESs, ILs, and traditional solvents for this separation problem apart from [66], which gave a capacity value of 0.99.
While the separation problem of ethanol/cyclohexane gave relatively low values for both selectivity and capacity parameters, as well as for all the literature studies. Overall, the ethanol/cyclohexane obtained data is as follows: The selectivity values for the DESs [[EMPYR] Br + 1,5-PDO] (this work); [[EMPYR] Br + Gly] [29]; [[TMAM] Cl + EG] [42]; and [[BMIM] Cl + Gly] [43] are 0.16, 0.03, 0.01, and 0.01, respectively, while the capacity values are 0.38, 0.01, 0.01, and 0.01. The ionic liquids { [67];
[66]; and [68]} selectivity values are 0.09, 0.38, and 0.09, respectively, while the capacity values are 0.24, 0.22, and 0.07, respectively. The traditional solvents' {DEG [69]; 2-PYR [70]; and sulfolane [71]} selectivity values are 0.02, 0.07, and 0.06, respectively. This is a suggestion that the investigated DES with a molar ratio of 1:2 won't be an appropriate or suitable solvent for this separation problem.
In the meantime, the separation problem, cyclohexane/hexane gave slightly lower selectivity values, i.e., 0.85, 0.82, and 1.00, for the DESs, including [[EMPYR] Br + 1,5-PDO], [[EMPYR] Br + Gly], and [[TMIM] Cl + Gly], respectively, apart from [[TMAM] Cl + EG], which gave a selectivity value of 0.18. Better than both ionic liquids and traditional solvents which obtained relatively low selectivity values for this separation problem. In addition, the present study, and the literature studies, they all gave relatively low-capacity values for this separation problem.
As mentioned, selectivity and capacity parameters are imperative as they determine the efficiency of separation and solutes distribution between the phases.
6. Conclusion
The thermodynamic functions, including infinite dilution activity coefficients () of 32 different organic solutes and water, were investigated in [[EMPYR] Br + 1,5-PDO] DES as an extracting solvent. This was done at a molar ratio of 1:2 for hydrogen bond accepter (1-ethyl-1-methylpyrrolidinium bromide) and hydrogen bond donor (1,5-pentanediol), respectively. The experiments were conducted at a temperature sequence of T = 313.15–343.15 K. The infinite dilution activity coefficients were determined from the retentions data obtained using the gas liquid chromatography. Before the experiments, the physical properties, i.e., density, viscosity, and sound velocity values were measured to obtain new data of [[EMPYR] Br + 1.5-PDO] DES. These were measured using the Anton Paar DSA 5000 M, the results were comparable to the literature. Partial molar properties, i.e., enthalpy (), entropy (), and Gibbs free energy (), were calculated from the experimental infinite dilution activity coefficients at T = 313.13 K to further detail possible intermolecular interactions between the selected organic solutes as well as [[EMPYR] Br + 1,5-PDO] component molecules. Moreover, selectivity () and capacity () values were also calculated and compared with the literature for separation problems, hexane/toluene, ethanol/cyclohexane, and hexane/cyclohexane.
The infinite dilution activity coefficients and partial molar properties revealed solvent-solute characteristics with the selected solutes, especially the nonpolar compounds, i.e., alkanes, alkenes, aromatics, cycloalkanes, and cycloalkenes. Nevertheless, the obtained selectivity and capacity values for the studied separation problems revealed satisfactory values for some problems (e.g., hexane/toluene) and unsatisfactory values (e.g., ethanol/cyclohexane). Hence, it is known that the picking of a DES for the separation process can be impacted by numerous factors. These include the nature and ratio of each selected component as well as temperature, which could also impact production yield.
In addition, deep eutectic solvents are expected to compete with and outclass both ionic liquids as well as traditional solvents, so more experimental data is required for inclusive comparison.
CRediT authorship contribution statement
Lindokuhle Manyoni: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. Gan Redhi: Funding acquisition, Project administration, Resources, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e21516.
Contributor Information
Lindokuhle Manyoni, Email: mlindoh94@gmail.com.
Gan Redhi, Email: redhigg@mweb.co.za.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Olabi A., Obaideen K., Elsaid K., Wilberforce T., Sayed E.T., Maghrabie H.M., Abdelkareem M.A. Assessment of the pre-combustion carbon capture contribution into sustainable development goals SDGs using novel indicators. Renew. Sustain. Energy Rev. 2022;153 [Google Scholar]
- 2.Carlsen L., Bruggemann R. The 17 United Nations' sustainable development goals: a status by 2020. Int. J. Sustain. Dev. World Ecol. 2022;29(3):219–229. [Google Scholar]
- 3.Wang X., Khurshid A., Qayyum S., Calin A.C. The role of green innovations, environmental policies and carbon taxes in achieving the sustainable development goals of carbon neutrality. Environ. Sci. Pollut. Control Ser. 2022;29(6):8393–8407. doi: 10.1007/s11356-021-16208-z. [DOI] [PubMed] [Google Scholar]
- 4.Bryan M.C., Dalton C., Díaz-Rodríguez A., Doerfler J., Engl O.D., Ferguson P., Molina A.G., Han Z.S., Hosford J., Howell G.P. Green chemistry articles of interest to the pharmaceutical industry. Org. Process Res. Dev. 2022;26(2):251–262. [Google Scholar]
- 5.Armenta S., Esteve-Turrillas F.A., Garrigues S., de la Guardia M. Alternative green solvents in sample preparation. Green Analytical Chemistry. 2022;1 [Google Scholar]
- 6.Sajid M., Płotka-Wasylka J. Green analytical chemistry metrics: a review. Talanta. 2022;238 doi: 10.1016/j.talanta.2021.123046. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X.f., Zhang Q., Xue H., Zhang J., Wang X. A green and highly efficient method of extracting polyphenols from Lilium davidii var. unicolor Salisb using deep eutectic solvents. Chem. Eng. Commun. 2022;209(2):271–280. [Google Scholar]
- 8.Abdussalam-Mohammed W., Ali A., Errayes A. Green chemistry: principles, applications, and disadvantages. Chem. Methodol. 2020;4:408–423. [Google Scholar]
- 9.Sheldon R.A. The greening of solvents: towards sustainable organic synthesis. Curr. Opin. Green Sustainable Chem. 2018;18:13–19. [Google Scholar]
- 10.Singh S., Bahadur I., Naidoo P., Redhi G., Ramjugernath D. Application of 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl) imide ionic liquid for the different types of separations problem: activity coefficients at infinite dilution measurements using gas-liquid chromatography technique. J. Mol. Liq. 2016;220:33–40. [Google Scholar]
- 11.Curzons A., Constable D., Cunningham V. Solvent selection guide: a guide to the integration of environmental, health and safety criteria into the selection of solvents. Clean Prod. Process. 1999;1(2):82–90. [Google Scholar]
- 12.Prat D., Wells A., Hayler J., Sneddon H., McElroy C.R., Abou-Shehada S., Dunn P.J. CHEM21 selection guide of classical-and less classical-solvents. Green Chem. 2016;18(1):288–296. [Google Scholar]
- 13.Prat D., Hayler J., Wells A. A survey of solvent selection guides. Green Chem. 2014;16(10):4546–4551. [Google Scholar]
- 14.Chen Y., Mu T. Revisiting greenness of ionic liquids and deep eutectic solvents. Green Chemical Engineering. 2021;2(2):174–186. [Google Scholar]
- 15.Paiva A., Craveiro R., Aroso I., Martins M., Reis R.L., Duarte A.R.C. Natural deep eutectic solvents–solvents for the 21st century. ACS Sustain. Chem. Eng. 2014;2(5):1063–1071. [Google Scholar]
- 16.Piper S.L., Kar M., MacFarlane D.R., Matuszek K., Pringle J.M. Ionic liquids for renewable thermal energy storage–a perspective. Green Chem. 2022;24(1):102–117. [Google Scholar]
- 17.Chandran K., Kait C.F., Wilfred C.D., Zaid H.F.M. A review on deep eutectic solvents: physiochemical properties and its application as an absorbent for sulfur dioxide. J. Mol. Liq. 2021;338 [Google Scholar]
- 18.de Jesus S.S., Maciel Filho R. Are ionic liquids eco-friendly? Renew. Sustain. Energy Rev. 2022;157 [Google Scholar]
- 19.Kaur G., Kumar H., Singla M. Diverse applications of ionic liquids: a comprehensive review. J. Mol. Liq. 2022 [Google Scholar]
- 20.Marchel M., Cieśliński H., Boczkaj G. Deep eutectic solvents microbial toxicity: current state of art and critical evaluation of testing methods. J. Hazard Mater. 2022;425 doi: 10.1016/j.jhazmat.2021.127963. [DOI] [PubMed] [Google Scholar]
- 21.Pei Y., Zhang Y., Ma J., Fan M., Zhang S., Wang J. Ionic liquids for advanced materials. Materials Today Nano. 2022;17 [Google Scholar]
- 22.Kaur G., Kumar H., Singla M. Diverse applications of ionic liquids: a comprehensive review. J. Mol. Liq. 2022;351 [Google Scholar]
- 23.Vanda H., Dai Y., Wilson E.G., Verpoorte R., Choi Y.H. Green solvents from ionic liquids and deep eutectic solvents to natural deep eutectic solvents. Compt. Rendus Chem. 2018;21(6):628–638. [Google Scholar]
- 24.Jenkin G.R., Al-Bassam A.Z., Harris R.C., Abbott A.P., Smith D.J., Holwell D.A., Chapman R.J., Stanley C.J. The application of deep eutectic solvent ionic liquids for environmentally-friendly dissolution and recovery of precious metals. Miner. Eng. 2016;87:18–24. [Google Scholar]
- 25.Chabib C.M., Ali J.K., Abi Jaoude M., Alhseinat E., Adeyemi I.A., Al Nashef I.M. Application of deep eutectic solvents in water treatment processes: a review. J. Water Proc. Eng. 2022;47 [Google Scholar]
- 26.Abbott A.P., Capper G., Davies D.L., Rasheed R.K., Tambyrajah V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003;(1):70–71. doi: 10.1039/b210714g. [DOI] [PubMed] [Google Scholar]
- 27.Andruch V., Makoś-Chełstowska P., Płotka-Wasylka J. Remarks on use of the term “deep eutectic solvent” in analytical chemistry. Microchem. J. 2022;179 [Google Scholar]
- 28.Manyoni L., Redhi G.G. Measurements of infinite dilution activity coefficient for aromatic and aliphatic hydrocarbons in Deep Eutectic Solvent, 1-ethyl-1-methylpyrrolidinium bromide+ ethylene glycol at different temperatures and a stated molar ratio. Chemical Thermodynamics and Thermal Analysis. 2022;7 [Google Scholar]
- 29.Manyoni L., Kabane B., Redhi G.G. Deep eutectic solvent as a possible entrainer for industrial separation problems: pre-screening tool for solvent selection. Fluid Phase Equil. 2022;553 [Google Scholar]
- 30.Shaibuna M., Theresa L.V., Sreekumar K. Neoteric deep eutectic solvents: history, recent developments, and catalytic applications. Soft Matter. 2022;18(14):2695–2721. doi: 10.1039/d1sm01797g. [DOI] [PubMed] [Google Scholar]
- 31.Zhekenov T., Toksanbayev N., Kazakbayeva Z., Shah D., Mjalli F.S. Formation of type III Deep Eutectic Solvents and effect of water on their intermolecular interactions. Fluid Phase Equil. 2017;441:43–48. [Google Scholar]
- 32.Smith E.L., Abbott A.P., Ryder K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014;114(21):11060–11082. doi: 10.1021/cr300162p. [DOI] [PubMed] [Google Scholar]
- 33.El Achkar T., Greige-Gerges H., Fourmentin S. Basics and properties of deep eutectic solvents: a review. Environ. Chem. Lett. 2021;19(4):3397–3408. [Google Scholar]
- 34.Tome L.I., Baiao V., da Silva W., Brett C.M. Deep eutectic solvents for the production and application of new materials. Appl. Mater. Today. 2018;10:30–50. [Google Scholar]
- 35.Taghizadeh M., Taghizadeh A., Vatanpour V., Ganjali M.R., Saeb M.R. Deep eutectic solvents in membrane science and technology: fundamental, preparation, application, and future perspective. Separ. Purif. Technol. 2021;258 [Google Scholar]
- 36.Li X., Row K.H. Development of deep eutectic solvents applied in extraction and separation. J. Separ. Sci. 2016;39(18):3505–3520. doi: 10.1002/jssc.201600633. [DOI] [PubMed] [Google Scholar]
- 37.Li X., Choi J., Ahn W.-S., Row K.H. Preparation and application of porous materials based on deep eutectic solvents. Crit. Rev. Anal. Chem. 2018;48(1):73–85. doi: 10.1080/10408347.2017.1383881. [DOI] [PubMed] [Google Scholar]
- 38.Wang C., Wang Q., Yalikun N., Fu J., Wang B. Infinite dilution activity coefficients and thermodynamic properties of various organic solutes in a choline chloride + oxalic acid deep eutectic solvent. J. Chem. Eng. Data. 2022;67(5):1070–1077. [Google Scholar]
- 39.Li Y., Wang W., Wang Q., Yalikun N., Tang J. Thermodynamic parameters and infinite dilution activity coefficients for organic solutes in deep eutectic solvent: choline Chloride + 1,5-Pentanediol. J. Chem. Therm. 2022;170 [Google Scholar]
- 40.Domanska U., Marciniak A. Measurements of activity coefficients at infinite dilution of aromatic and aliphatic hydrocarbons, alcohols, and water in the new ionic liquid [EMIM][SCN] using GLC. J. Chem. Therm. 2008;40(5):860–866. [Google Scholar]
- 41.Kabane B., Redhi G.G. Application of trihexyltetradecylphosphonium dicyanamide ionic liquid for various types of separations problems: activity coefficients at infinite dilution measurements utilizing GLC method. Fluid Phase Equil. 2019;493:181–187. [Google Scholar]
- 42.Nkosi N., Tumba K., Ramsuroopa S. Measurements of activity coefficient at infinite dilution for organic solutes in tetramethylammonium chloride + ethylene glycol deep eutectic solvent using gas-liquid chromatography. Fluid Phase Equil. 2018;462:31–37. [Google Scholar]
- 43.Kabane B., Redhi G.G. Thermodynamic properties and activity coefficients at infinite dilution for different solutes in deep eutectic solvent: 1-butyl-3-methylimidazolium chloride + glycerol. J. Mol. Liq. 2020;311 [Google Scholar]
- 44.Manyoni L., Kabane B., Redhi G.G. Excess thermodynamic functions of phosphonium-based deep eutectic solvent for various organic solutes at different temperatures. J. Taiwan Inst. Chem. Eng. 2022;138 [Google Scholar]
- 45.Manyoni L., Redhi G. 1.6-Hexanediol based deep eutectic solvent and their excess data at infinite dilution. Chemical Thermodynamics and Thermal Analysis. 2022;8 [Google Scholar]
- 46.Arumugam V., Kabane B., Moodley K.G., Gao Y., Redhi G.G. Activity coefficients at infinite dilution of organic solutes, using novel N-(2′, 3′-epoxypropyl)-N-methyl-2-oxopyrrolidinium chloride ionic liquid by GLC. Fluid Phase Equil. 2020;505 [Google Scholar]
- 47.Kabane B., Chokkareddy R., Redhi G.G. Separation of (water/butan-1-ol) binary systems based on activity coefficients at infinite dilution with phosphonium ionic liquid. J. Chem. Therm. 2019;137:7–12. [Google Scholar]
- 48.Verevkin S.P., Sazonova A.Y., Frolkva A.K., Zaitsau D.H., Prikhodko I.V., Held C. Separation performance of BioRenewable deep eutectic solvents. Ind. Eng. Chem. Res. 2015;54(13):3498–3504. [Google Scholar]
- 49.Buchowski H., Ksiazczak A., Pietrzyk S. Solvent activity along a saturation line and solubility of hydrogen-bonding solids. J. Phys. Chem. 1980;84(9):975–979. [Google Scholar]
- 50.Liu Y., Li M., Sahoo S., Ma X. Solubility determination and thermodynamic analysis of organic zinc supported by β-diimine ligands in pure solvents. J. Mol. Liq. 2022;348 [Google Scholar]
- 51.Li Y., Wang W., Wang Q., Yalikun N., Tang J. Thermodynamic parameters and infinite dilution activity coefficients for organic solutes in deep eutectic solvent: choline Chloride+ 1, 5-Pentanediol. J. Chem. Therm. 2022;170 [Google Scholar]
- 52.Li J., Wang Q., Tian L., Li Z., Li Y., Hu Y., Wang B. Application potential of N-hexylpyridinium bromide for separation azeotrope: thermodynamic properties measurements. Fluid Phase Equil. 2022;557 [Google Scholar]
- 53.Qin S., Jiang S., Li J., Balaprakash P., Van Lehn R., Zavala V. Capturing molecular interactions in graph neural networks: a case study in multi-component phase equilibrium. 2022;2:138–151. [Google Scholar]
- 54.Cotroneo-Figueroa V.P., Gajardo-Parra N.F., López-Porfiri P., Leiva Á., Gonzalez-Miquel M., Garrido J.M., Canales R.I. Hydrogen bond donor and alcohol chain length effect on the physicochemical properties of choline chloride based deep eutectic solvents mixed with alcohols. J. Mol. Liq. 2022;345 [Google Scholar]
- 55.Zheng C., Shen Z., Zhou J., Pei Y., Yang B. Influence of the anions on the interaction energy between water and ionic liquids. Chem. Eng. Technol. 2022;45(2):266–274. [Google Scholar]
- 56.Li Q., Yan H., Lin S., Han Y., Han M., Fan W. Liquid-liquid phase equilibrium and interaction exploration for separation 2-Methoxy-phenol and water with different solvents. J. Mol. Liq. 2022 [Google Scholar]
- 57.Assis G.P., Derenzo S., Bernardo A. Solid-liquid equilibrium of nicotinamide in water-ethanol and water-propylene glycol mixtures. J. Mol. Liq. 2022;345 [Google Scholar]
- 58.Chen D.-W., Zhang C., Zhang Z.-Y., Peng X.-M., Ren R.-Z., Ge M.-L. Measurements and correlation of activity coefficients at infinite dilution for organic solutes in 1-heptyl-3-methylimidazolium chloride. Fluid Phase Equil. 2023;567 [Google Scholar]
- 59.Mbatha B.P., Ngema P.T., Nkosi N., Ramsuroop S. Infinite dilution activity coefficient measurements for 1-Methyl-4-(1-methylethenyl)-cyclohexene as a green solvent for separation. J. Chem. Eng. Data. 2022;67(4):966–974. [Google Scholar]
- 60.Kaneko K., Kitawaki K., Mori T., Yoshimura Y., Shimizu A. Molar and partial molar thermodynamic functions of [BMIm] BF4/water mixture (Gibbs free energy, entropy and enthalpy) Phys. Chem. Liq. 2022;60(4):582–597. [Google Scholar]
- 61.Tosti S., Marrelli L. Classical thermodynamic analysis of deuterium-based fusion reactions. Hydro. 2022;3(1):53–61. [Google Scholar]
- 62.Sultana S., Islam K., Hasan M.A., Khan H.J., Khan M.A.R., Deb A., Al Raihan M., Rahman M.W. Adsorption of crystal violet dye by coconut husk powder: isotherm, kinetics and thermodynamics perspectives. Environ. Nanotechnol. Monit. Manag. 2022;17 [Google Scholar]
- 63.Mgxadeni N., Mmelesi O., Kabane B., Bahadur I. Influence of hydrogen bond donor on zinc chloride in separation of binary mixtures: activity coefficients at infinite dilution. J. Mol. Liq. 2022;351 [Google Scholar]
- 64.Domańska U., Zawadzki M., Królikowska M., Tshibangu M.M., Ramjugernath D. Measurements of activity coefficients at infinite dilution of organic compounds and water in isoquinolinium-based ionic liquid [C8iQuin][NTf2] using GLC. J. Chem. Therm. 2011;43(3):499–504. [Google Scholar]
- 65.Domańska U., Lukoshko E.V. Measurements of activity coefficients at infinite dilution for organic solutes and water in the ionic liquid 1-butyl-1-methylpyrrolidinium tricyanomethanide. J. Chem. Therm. 2013;66:144–150. [Google Scholar]
- 66.Acree W.E., Jr., Baker G.A., Revelli A.-L., Moise J.-C., Mutelet F. Activity coefficients at infinite dilution for organic compounds dissolved in 1-alkyl-1-methylpyrrolidinium bis (trifluoromethylsulfonyl) imide ionic liquids having six-, eight-, and ten-carbon alkyl chains. J. Chem. Eng. Data. 2012;57(12):3510–3518. [Google Scholar]
- 67.Kabane B., Arumugam V., Chokkareddy R., Redhi G.G. Assessment of pyrrolidinium-based ionic liquid for the separation of binary mixtures based on activity coefficients at infinite dilution. J. Chem. Eng. Data. 2019;64(12):5105–5112. [Google Scholar]
- 68.Nebig S., Liebert V., Gmehling J. Measurement and prediction of activity coefficients at infinite dilution, vapor–liquid equilibria (VLE) and excess enthalpies (HE) of binary systems with 1,1-dialkyl-pyrrolidinium bis(trifluoromethylsulfonyl)imide using mod. UNIFAC (Dortmund) Fluid Phase Equil. 2009;277:61–67. [Google Scholar]
- 69.Williams-Wynn M., Letcher T.M., Naidoo P., Remjugernath D. Activity coefficients at infinite dilution of organic solutes in diethyleneglycol and triethylene glycol from gas–liquid chromatography. J. Chem. Therm. 2013;65:120–130. [Google Scholar]
- 70.Gruber D., Topphoff M., Gmehling J. Measurement of activity coefficients at infinite dilution using gas-liquid chromatography. 9. Results for various solutes with the stationary phases 2-pyrrolidone and N-methylformamide. J. Chem. Eng. Data. 1998;43(6):935–940. [Google Scholar]
- 71.Mollmann C., Gmehling J. Measurement of activity coefficients at infinite dilution using gas-liquid chromatography. 5. Results for N-methylacetamide, N,N-dimethylacetamide, N,N-dibutylformamide, and sulfolane as stationary phases. J. Chem. Eng. Data. 1997;42(1):35–40. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.