Summary
Background & Aims:
Non-alcoholic fatty liver disease (NAFLD) can develop in individuals who are not overweight. Whether lean persons with NAFLD have lower mortality and lower incidence of cirrhosis, cardiovascular diseases (CVD), diabetes mellitus (DM) and cancer than overweight/obese persons with NAFLD remains in-conclusive. We compared mortality and incidence of cirrhosis, CVD, DM and cancer between lean versus non-lean persons with NAFLD.
Methods:
This is a retrospective study of adults with NAFLD in a single centre from 2012 to 2021. Primary outcomes were mortality and new diagnosis of cirrhosis, CVD, DM and cancer. Outcomes were modelled using competing risk analysis and Cox proportional hazards regression analysis.
Results:
A total of 18,594 and 13,420 patients were identified for cross-sectional and longitudinal analysis respectively: approximately 11% lean, 25% overweight, 28% class 1 obesity and 35% class 2–3 obesity. The median age was 51.0 years, 54.6% were women. The median follow-up was 49.3 months. Lean patients had lower prevalence of metabolic diseases at baseline and lower incidence of cirrhosis and DM than non-lean patients and no difference in CVD, any cancer or obesity-related cancer during follow-up. However, lean patients had significantly higher mortality with incidence per 1000 person-years of 16.67, 10.11, 7.37 and 8.99, respectively, in lean, overweight, obesity class 1 and obesity class 2–3 groups respectively.
Conclusions:
Lean patients with NAFLD had higher mortality despite lower incidence of cirrhosis and DM, and similar incidence of CVD and cancer and merit similar if not more attention as non-lean patients with NAFLD.
1 |. INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is the leading cause of chronic liver disease, affecting 25% of the population worldwide, and its clinical burden is expected to rise.1 NAFLD is strongly associated with obesity and its comorbidities but can also develop in individuals who are not overweight (‘lean NAFLD’).2 The prevalence of NAFLD among lean individuals in the general population is 10%–20% with most studies reported from Asian countries, and the highest prevalence reported in Mexico (37%).3–6
NAFLD is associated with a wide spectrum of extrahepatic diseases, such as cardiovascular diseases (CVD), metabolic diseases (diabetes mellitus [DM], hypertension, dyslipidaemia), chronic kidney diseases (CKD) or cancers.7,8 Several cross-sectional studies have evaluated prevalence of hepatic and extra-hepatic diseases among lean versus obese individuals with NAFLD. A cross-sectional analysis of the TARGET-NASH study cohort with 3386 NAFLD patients in the United States found a lower prevalence of cirrhosis, DM and CVD in lean individuals compared to overweight/obese individuals.2 Another study from Austria with 4091 NAFLD participants had similar findings with lower prevalence of metabolic diseases (DM, metabolic syndrome, hypertension, dyslipidaemia) and lower Framingham risk score for CVD but no significant difference in prevalence of coronary artery disease in lean patients compared with overweight/ obese patients.9 By contrast, a recent Korean study with 4786 NAFLD patients found higher atherosclerotic CVD scores among lean persons.10 However, cross-sectional studies can only show an association but not a cause–effect relationship.
Data on the impact of baseline body mass index (BMI) on the incidence of outcomes such as cirrhosis, CVD, cancers and mortality among patients with NAFLD are limited. One longitudinal study on the natural history of NAFLD in lean subjects included 1090 U.S. participants with biopsy-proven NAFLD followed for a mean of 133 ± 81.3 months demonstrated an increased overall mortality in lean patients compared to non-lean patients despite lower prevalence of metabolic diseases and advanced fibrosis.11 A retrospective cohort study from the Olmsted county database with 4834 NAFLD patients with the median follow-up of 6.4 years showed similar findings with higher overall mortality in the lean persons and no significant differences in risk of cirrhosis, hepatic decompensation, CVD events or malignancy between lean and obese persons.12 Finally, a recent multi-centre study from Western countries with 1339 participants with biopsy-proven NAFLD with the median follow-up of 94 months, demonstrated a lower prevalence of non-alcoholic steatohepatitis (NASH), advanced fibrosis and DM among lean versus obese individuals but no significant differences in overall mortality or incidence of liver-related events between lean versus non-lean participants.5 Notably, the criteria for inclusion and the definition of outcomes in these published studies were not uniform. Given these inconsistencies, we conducted a comprehensive evaluation of multiple outcomes in a large cohort of patients with NAFLD with the aim to compare the mortality and incidence of cirrhosis, CVD, DM and cancers between lean versus overweight and obese individuals with NAFLD.
2 |. METHODS
2.1 |. Study population
This is a retrospective cohort study of adult patients diagnosed with NAFLD at the University of Michigan Health System between 1 January 2012 and 1 December 2021. The study was approved by the institutional review board of the University of Michigan.
Inclusion criteria were the presence of NAFLD defined as hepatic steatosis without an alternative aetiology (Table S1). We identified patients with hepatic steatosis on imaging or biopsy based on a validated natural language processing algorithm previously described,13 or vibration-controlled transient elastography (VCTE) showing controlled attenuation parameter (CAP) >250 db/m.14 In brief, the algorithm screened imaging or biopsy reports for the terms ‘fatty’ or ‘steato’ appearing in the same sentence as ‘liver’ or ‘hepat’, then excluded reports with terms of negation (‘no hepatic steatosis’) or evaluation (‘rule out hepatic steatosis’). This algorithm had been previously shown to have a positive predictive value (PPV) >95%.13 Over 95% of our cohort had an initial diagnosis of NAFLD at our centre based on imaging, 3.4% based on transient elastography and 1.6% on liver biopsy.
The study has two components: a cross-sectional analysis to compare prevalent/baseline clinical characteristics and comorbidities of lean versus non-lean patients with NAFLD, and a longitudinal analysis to determine mortality and incidence of clinical outcomes of lean versus non-lean patients. The index date was defined as the date when a patient was first diagnosed with NAFLD at our centre. Follow-up time was defined as the interval from the index date to a clinical event of interest (for non-mortality outcomes), the date of the last clinical encounter, death or study end date (1 December 2021), whichever was earliest. For both cross-sectional and longitudinal analyses, we excluded participants with (i) age <18 or >80 years (n = 1627); (ii) other causes of liver disease (n = 4577); (iii) significant alcohol consumption defined as >7 drinks/week in women and >14 drinks/week in men (n = 690)15; (iv) BMI <18.5 kg/m2 (n = 199); (v) cancer diagnosis other than non-melanoma skin cancer up to 1 year after the index date (n = 8535) since low BMI in these patients may be due to the cancer; (vi) previous history of bariatric surgery (n = 550); (vii) unknown race/ethnicity data, or missing important laboratory data such as aspartate aminotransferase (AST), alanine aminotransferase (ALT) or platelet (n = 17,087) within 12 months of the index date or (viii) baseline cirrhosis with portal hypertension, ascites, oesophageal varices or hepatic encephalopathy since ascites may affect BMI (n = 1247). For the longitudinal analysis, we further excluded those with a follow-up time of less than 365 days. (Figure 1).
FIGURE 1.
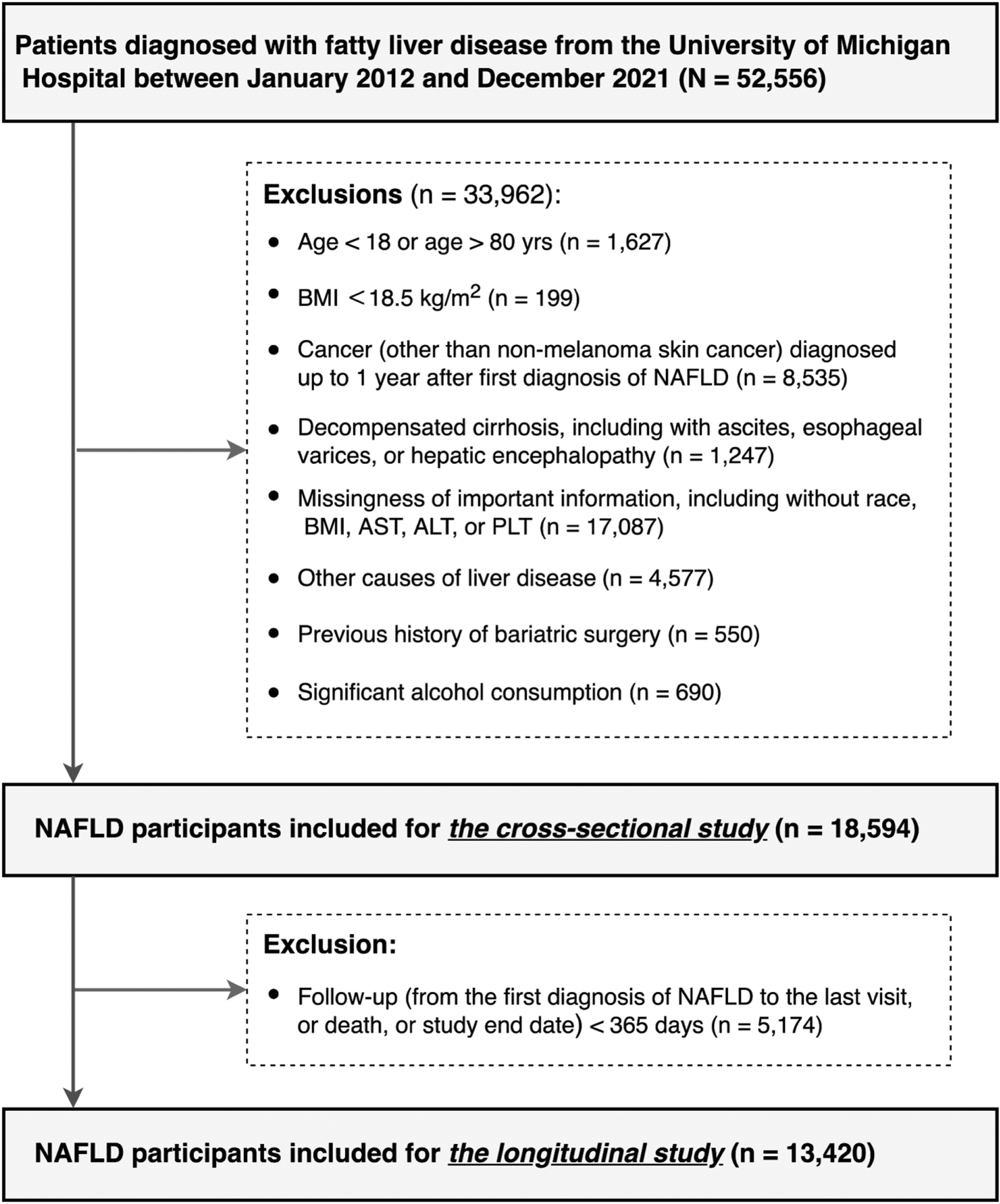
Study flow chart.
2.2 |. Study outcomes and variable definitions
For the cross-sectional analysis, the primary outcomes were the prevalence of cirrhosis, CVD, metabolic diseases and chronic kidney disease (CKD) stage 3–5. Specifically, CVD comprised four types of diseases: coronary artery disease (CAD), cerebrovascular accident (CVA), peripheral arterial disease (PAD) and congestive heart failure (CHF). Metabolic diseases included DM, dyslipidaemia and hypertension. The prevalence of diseases of interest was defined as any diagnosis before or up to 90 days after the index date.
For the longitudinal analysis, the primary outcomes were overall mortality and incidence of CVD, cirrhosis, liver-related events (LREs) (ascites, variceal bleeding, hepatic encephalopathy or HCC), DM, obesity-related cancer and any cancer. We excluded patients with baseline cirrhosis from LREs incidence analysis because the interest of our study was identifying progression from non-cirrhotic NAFLD to LREs, rather than from compensated cirrhosis to decompensation. Obesity-related cancer included malignant neoplasms of gastrointestinal tract and liver, breast, thyroid gland, uterus, ovary, kidney, as well as multiple myeloma.16 We evaluated the association between Fibrosis-4 (FIB-4) as non-invasive fibrosis score and the incidence of liver cirrhosis and LREs among NAFLD patients stratified by BMI category.17 Mortality was identified based on linkage to the Michigan Department of Vital and Health Records database; all deaths occurring in the state of Michigan are required to be reported to the Department. The cause of death was mainly based on the International Classification of Diseases (ICD-10-CM) code provided. Causes of death were then grouped into organ/system-level cause. We manually reviewed the medical records of 20 patients who died at our hospital and found that the ICD-10 code assigned by the Michigan Death Index corresponded with the primary or a contributing cause of death in 19 (95%) cases. Incident disease was defined as any new diagnosis after 365 days from the index date (to avoid misclassification of patients with prevalent disease as having incident disease) among patients with no prevalent disease.
The majority of outcomes and comorbidities were diagnosed based on ICD codes16 (Table S1). For cirrhosis, we demonstrated that the ICD codes used had a positive predictive value (PPV) of 86%.18 We additionally assessed the sensitivity of ICD codes for cirrhosis and found that 46% of 582 NAFLD patients with evidence of cirrhosis on imaging had at least one ICD code for cirrhosis within 6 months of the imaging with no meaningful differences in sensitivity (41%–51%) or specificity (≥98%) across BMI categories. Previous studies have validated the accuracy of diagnosis of CVD based on ICD codes with PPV greater than 90%19–21 and large epidemiological studies found PPV of 80% for the diagnosis of all malignant tumours by ICD coding.22–25 Chronic kidney disease (CKD) stage 3–5 was based on an estimated glomerular filtration rate of less than 60 mL/min/1.73m2.26
2.3 |. Body mass index
The NAFLD cohort was stratified into four subgroups based on average BMI values within a 6-month period around the index date. The World Health Organization recommendations for Asian and non-Asian BMI cut-offs27,28 were applied to categorise patients into lean, overweight, class 1 obesity and class 2–3 obesity.
Given that weight and therefore BMI category may change during follow-up, we conducted a sensitivity analysis in which only patients whose weight category did not change during follow-up were included.
2.4 |. Genotyping and genetic analysis
A subset of our cohort was enrolled in the Michigan Genomics Initiative, a prospective cohort of Michigan Medicine patients undergoing genotyping for research purposes.29 Subjects were genotyped using an Illumina HumanCoreExome v.12.1 array. Among these patients, we compared the distribution of PNPLA3-rs738409 genotype (defined as 0, 1, or 2 copies of the risk allele PNPLA3-rs738409-G). The chi-square test was used to compare the genotype distribution among NAFLD patients based on BMI category.
2.5 |. Statistical analyses
Baseline characteristics were compared between groups by the Chi-square test for categorical variables and the Kruskal–Wallis test for continuous variables. For descriptive statistics, the baseline characteristics were reported by number and percentages for categorical variables and median and interquartile range (IQR) for continuous variables. We used multivariable logistic regression analyses to determine the independent associations between the prevalence of diseases and NAFLD after adjusting for confounders including age, sex, race, smoking status, alcohol consumption, type 2 DM, hypertension, dyslipidaemia and use of aspirin or statin.
The cumulative incidence of death by BMI category was calculated using Kaplan–Meier estimates. For evaluation of incident events, we required >1 year of follow-up. Time at risk was defined as the time from the index date to the date of recorded death or last visit. The log-rank test was applied to compare differences in the cumulative incidence of death between groups. For the outcome of mortality, we evaluated the effect of the BMI category using a Cox proportional hazards model. For all other outcomes (CVD, cirrhosis, etc.), we evaluated the cumulative incidence and the effects of the BMI category using a Fine-Grey competing risk model, with death without the outcome of interest as a competing event. For both model types, we adjusted for the same covariates as in the prevalent disease analyses. We performed additional analysis to assess the association between PNPLA3-rs738409 genotype and risk of mortality and incidences of diseases among NAFLD patients stratified by BMI category adjusted for the same covariates with genetic principal components to account for race and ethnicity. We also performed sensitivity analysis among NAFLD patients with no change in BMI category throughout follow-up and separate analysis for smokers and non-smokers. For cause of death analysis, F-test was used to compare the differences between the lean and non-lean groups.
All analyses were performed in RStudio with R version 4.1.2. A two-tailed p ≤ 0.05 was considered statistically significant.
3 |. RESULTS
3.1 |. Cross-sectional analysis
A total of 18,594 patients with NAFLD were included in the cross-sectional analysis: 2137 lean, 4692 overweight, 5234 with class 1 obesity and 6531 with class 2–3 obesity. As shown in Table 1, lean individuals were more often female, more likely to have active tobacco use and prior CVA, and had a lower prevalence of metabolic abnormalities including DM, hypertension, dyslipidaemia, CKD stage 3–5 and CAD than non-lean individuals. The lean individuals also had lower levels of liver enzymes, total cholesterol, low-density lipoprotein and triglycerides and higher level of high-density lipoprotein compared with non-lean individuals (p < 0.05 for all). There was no significant difference in race distribution between lean and overweight/obese groups, though our cohort was predominantly Caucasian.
TABLE 1.
Baseline characteristics, laboratory values and prevalence of diseases among 18,594 patients with NAFLDa.
| Non-lean (n = 16,457) | |||||
|---|---|---|---|---|---|
| Characteristics | Lean (n = 2137) | Overweight (n = 4692) | Obesity class 1 (n = 5234) | Obesity class 2–3 (n = 6531) | p value (lean vs. non-lean) |
| Age, years | 51.0 (36, 63) | 52.5 (41, 63) | 52.0 (40, 61) | 48.0 (37, 58) | 0.70 |
| Sex (male, %) | 881 (41.2%) | 2574 (54.9%) | 2761 (52.8%) | 2551 (39.1%) | <0.0001 |
| Race, % | 0.89 | ||||
| Asian | 106 (5.0%) | 375 (8.0%) | 270 (5.2%) | 161 (2.5%) | |
| African American | 178 (8.3%) | 323 (6.9%) | 410 (7.8%) | 664 (10.2%) | |
| Caucasian | 1724 (80.7%) | 3661 (78.0%) | 4227 (80.8%) | 5305 (81.2%) | |
| Other/Unknown | 129 (6.0%) | 333 (7.1%) | 327 (6.3%) | 401 (6.1%) | |
| Smoking, % | <0.0001 | ||||
| Never | 1475 (69.0%) | 3283 (70.0%) | 3580 (68.4%) | 4508 (69.0%) | |
| Former | 376 (17.6%) | 1016 (21.7%) | 1181 (22.6%) | 1420 (21.7%) | |
| Current | 286 (13.4%) | 393 (8.4%) | 473 (9.0%) | 603 (9.2%) | |
| ALT (U/L) | 27 (18, 49) | 36 (24, 59) | 39 (26, 62) | 38 (25, 61) | <0.0001 |
| AST (U/L) | 28 (22, 44) | 30 (23, 42) | 31 (24, 43) | 30 (23, 44) | <0.0001 |
| Platelet (K/μL) | 239 (192, 291) | 236 (195, 283) | 239 (197, 286) & | 251 (206, 299) | 0.01 |
| HbA1c (%) | 5.8 (5.4, 6.7) | 5.8 (5.5, 6.5) | 5.9 (5.5, 6.7) | 6.1 (5.6, 7.2) | <0.0001 |
| Total cholesterol (mg/dL) | 173 (143, 210) | 184 (155, 213) | 180 (150, 212) | 176 (148, 206) | 0.003 |
| HDL (mg/dL) | 51 (38, 66) | 45 (38, 55) | 43 (36, 52) | 42 (36, 50) | <0.0001 |
| LDL (mg/dL) | 91 (66, 116) | 101 (76, 125) | 99 (75, 123) | 95 (72, 119) | <0.0001 |
| Triglyceride (mg/dL) | 125 (88, 189) | 153 (107, 226) | 163 (115, 240) | 164 (119, 240) | <0.0001 |
| Cirrhosis | 27 (1.3%) | 61 (1.3%) | 77 (1.5%) | 165 (2.5%) | 0.07 |
| Cardiovascular diseases | |||||
| Coronary artery disease | 206 (9.6%) | 562 (12.0%) | 607 (11.6%) | 660 (10.1%) | 0.04 |
| Congestive heart failure | 73 (3.4%) | 158 (3.4%) | 195 (3.7%) | 268 (4.1%) | 0.45 |
| Cerebrovascular accident | 135 (6.3%) | 243 (5.2%) | 266 (5.1%) | 279 (4.3%) | 0.003 |
| Peripheral arterial disease | 124 (5.8%) | 249 (5.3%) | 283 (5.4%) | 267 (4.1%) | 0.07 |
| Any cardiovascular disease | 370 (17.3%) | 855 (18.2%) | 965 (18.4%) | 1099 (16.8%) | 0.65 |
| Metabolic diseases | |||||
| Diabetes mellitus | 288 (13.5%) | 828 (17.7%) | 1138 (21.7%) | 1972 (30.2%) | <0.0001 |
| Hypertension | 608 (28.5%) | 1689 (36.0%) | 2187 (41.8%) | 3042 (46.6%) | <0.0001 |
| Dyslipidaemia | 473 (22.1%) | 1626 (34.7%) | 1928 (36.8%) | 2195 (33.6%) | <0.0001 |
| Any metabolic disease | 845 (39.5%) | 2467 (52.6%) | 2995 (57.2%) | 3933 (60.2%) | <0.0001 |
| CKD (stage 3–5) | 261 (12.3%) | 754 (16.3%) | 867 (16.7%) | 953 (14.7%) | <0.0001 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CKD, chronic kidney disease; HbA1c, haemoglobin A1c; HDL, high-density lipoprotein; IQR, interquartile range; LDL, low-density lipoprotein.
Data are expressed as median (interquartile range) for continuous variables or number (percentage) for categorical variables.
Multivariate logistic regression analysis demonstrated that the overweight group had a significantly lower prevalence of CVA and PAD with adjusted odds ratio (OR) and 95% confidence interval (CI) of 0.68 (95% CI 0.54–0.86, p = 0.001) and 0.73 (95% CI 0.58–0.93, p = 0.01), respectively, and the obese groups (class 1 and class 2–3) had a significantly lower prevalence of CAD, CVA, PAD and any CVD (all p values < 0.001), compared to the lean group (Table 2). The prevalence of cirrhosis and CKD stage 3–5 was significantly higher in the obesity class 2–3 group compared with the lean group, with an adjusted OR 1.66 (95% CI 1.11–2.58, p = 0.019) and 1.22 (95% CI 1.03–1.44, p = 0.02) respectively (Table 2).
TABLE 2.
Logistic regression analysis of factors associated with the prevalence of cardiovascular disease, cirrhosis and chronic kidney disease.
| Any cardiovascular disease | Coronary artery disease | Peripheral artery disease | Cerebrovascular accident | Congestive heart failure | Cirrhosis | Chronic kidney disease stage ≥3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMI category | OR (95% CI)a | p value | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value |
| Lean | Referent | Referent | Referent | Referent | Referent | Referent | Referent | |||||||
| Overweight | 0.88 (0.75–1.02) | 0.08 | 0.91 (0.75–1.12) | 0.38 | 0.73 (0.58–0.93) | 0.01 | 0.68 (0.54–0.86) | 0.001 | 0.76 (0.56–1.02) | 0.07 | 0.96 (0.61–1.55) | 0.87 | 1.09 (0.92–1.28) | 0.34 |
| Obesity class 1 | 0.75 (0.65–0.88) | <0.001 | 0.77 (0.63–0.94) | 0.009 | 0.70 (0.56–0.89) | 0.003 | 0.61 (0.48–0.77) | <0.001 | 0.76 (0.57–1.02) | 0.06 | 1.04 (0.67–1.66) | 0.86 | 1.13 (0.96–1.34) | 0.14 |
| Obesity class 2–3 | 0.77 (0.66–0.89) | <0.001 | 0.72 (0.59–0.87) | <0.001 | 0.58 (0.46–0.74) | <0.001 | 0.51 (0.40–0.64) | <0.001 | 0.88 (0.66–1.17) | 0.36 | 1.66 (1.11–2.58) | 0.02 | 1.22 (1.03–1.44) | 0.02 |
Abbreviations: CI, confidence interval; OR, odds ratio.
OR was adjusted for age, sex, race, smoking status, type 2 diabetes, alcohol consumption, hypertension, dyslipidaemia and use of aspirin and statin.
3.2 |. Subgroup analysis of CVD prevalence among smokers and non-smokers
Since lean persons with NAFLD were more likely to be current smokers than non-lean persons with NAFLD (p < 0.0001), we performed a subgroup analysis to compare the prevalence of CVD across BMI categories separately for never smokers (n = 12,846), former smokers (n = 3993) and current smokers (n = 1755) (Table S2). Among never smokers, multivariate logistic regression analysis demonstrated that the odds of CVD prevalence were significantly lower in non-lean than lean persons, with adjusted OR of 0.82 (95% CI 0.68–0.99, p = 0.04) for overweight individuals, 0.72 (95% CI 0.60–0.87, p < 0.001) for obesity class 1 and 0.78 (95% CI 0.65–0.94, p = 0.007) for obesity class 2–3 versus lean persons. Among former smokers and current smokers, there were no significant differences in CVD prevalence between lean and non-lean individuals (all p values > 0.05). Overall, the higher prevalence of CVD among lean subjects with NAFLD compared to non-lean subjects was mainly attributed to never smokers.
3.3 |. Longitudinal analysis
A total of 13,420 patients with NAFLD were included in the longitudinal analysis: 1454 lean, 3373 overweight, 3830 class 1 obesity and 4763 class 2–3 obesity. The median (IQR) follow-up time was 49.3 (29.2–79.8) months and a total follow-up of 64,149 person-years. Roughly half (54.6%) of the patients were female, 80.7% were Caucasian, 8.6% African American, 4.9% Asian and 5.8% other/ mixed races.
3.4 |. Risk of overall mortality and causes of death
During follow-up, a total of 616 patients died, including 111 lean, 162 overweight, 136 obesity class 1 and 207 obesity class 2–3 individuals, with incidence per 1000 person-years of 16.67, 10.11, 7.37 and 8.99 respectively (p < 0.0001). The cumulative incidence of mortality is shown in Figure 2A. Compared to lean individuals, overweight (adjusted HR 0.54, 95% CI 0.42–0.69, p < 0.001), obesity class 1 (adjusted HR 0.40, 95% CI 0.31–0.52, p < 0.001) and obesity class 2–3 (adjusted HR 0.52, 95% CI 0.41–0.66, p < 0.001) individuals had significantly lower mortality after adjusting for confounders (Table 3). After adjusting for Charlson Comorbidity Index, overweight (adjusted HR 0.44, 95% CI 0.27–0.71, p < 0.001), obesity class 1 (adjusted HR 0.34, 95% CI 0.21–0.56, p < 0.001) and obesity class 2–3 (adjusted HR 0.30, 95% CI 0.19–0.49, p < 0.001) individuals still had significantly lower mortality. The causes of death were mainly CVD (190, 30.8%), neoplasms (103, 16.7%) and respiratory diseases (75, 12.2%) with no statistically significant differences in causes of death between lean and non-lean groups (Table 4). CVD-related deaths were reported in 26.1%, 28.4%, 33.1% and 33.8% of all deaths in lean, overweight, obesity class 1 and obesity class 2–3 groups respectively. Compared with the non-lean group, the lean group tended to die more often from respiratory (15.3% vs. 11.5%), metabolic (8.1% vs. 5.3%), liver (7.2% vs. 5.0%), mental-neurological (8.1% vs. 6.1%) and infectious (5.4% vs. 4.2%) diseases, though the differences were not statistically significant. None of the lean persons with NAFLD died from DM-related causes in this cohort.
FIGURE 2.
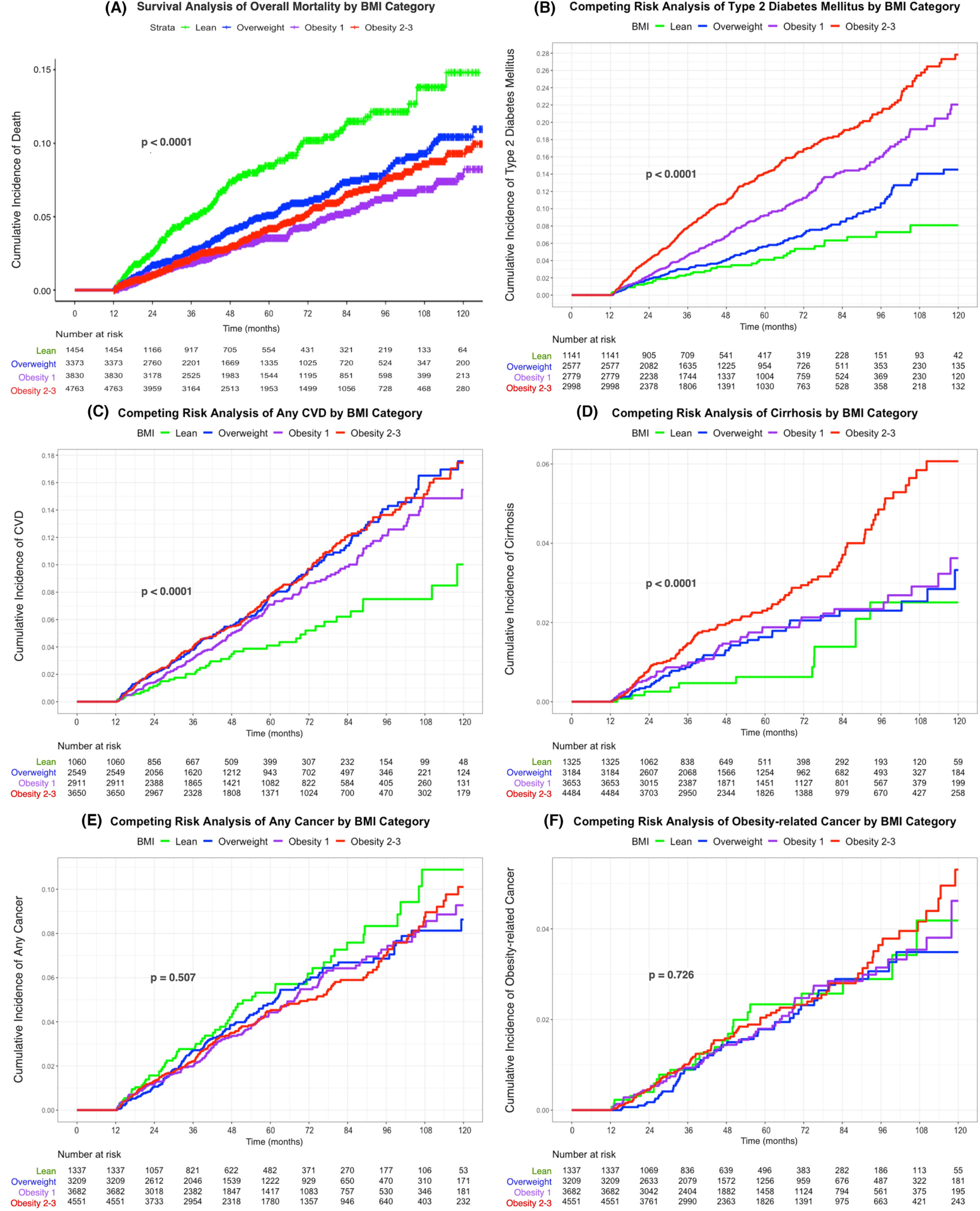
Cumulative incidence of death and diseases among NAFLD by BMI category. (A) Cumulative incidence of death by BMI category. (B) Cumulative incidence of type 2 diabetes mellitus by BMI category. (C) Cumulative incidence of any cardiovascular disease by BMI category. (D) Cumulative incidence of cirrhosis by BMI category. (E) Cumulative incidence of any cancer by BMI category. (F) Cumulative incidence of obesity-related cancer by BMI category. *(B–F) Used competing risk analysis.
TABLE 3.
Competing risk analysis for death and incidence of diseases by BMI categorya.
| Weight category | Deathb | Any cardiovascular diseases | Cirrhosis | Liver-related events | Type 2 diabetes Mellitusc | Obesity-related cancer | Any cancer | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| Lean | Referent | Referent | Referent | Referent | Referent | Referent | Referent | |||||||
| Overweight | 0.54 (0.42–0.69) | <0.001 | 1.40 (1.00–1.95) | 0.051 | 1.53 (0.81–2.88) | 0.19 | 1.08 (0.42–2.78) | 0.88 | 1.22 (0.87–1.71) | 0.25 | 0.87 (0.53–1.42) | 0.58 | 0.76 (0.56–1.02) | 0.07 |
| Obesity class 1 | 0.40 (0.31–0.52) | <0.001 | 1.08 (0.77–1.50) | 0.66 | 1.50 (0.80–2.81) | 0.20 | 0.77 (0.30–2.01) | 0.60 | 1.78 (1.29–2.45) | <0.001 | 0.97 (0.60–1.56) | 0.90 | 0.75 (0.56–1.00) | 0.049 |
| Obesity class 2–3 | 0.52 (0.41–0.66) | <0.001 | 1.27 (0.91–1.76) | 0.15 | 2.19 (1.20–4.01) | 0.011 | 1.15 (0.48–2.76) | 0.76 | 2.85 (2.08–3.91) | <0.001 | 1.02 (0.64–1.62) | 0.93 | 0.78 (0.59–1.04) | 0.09 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
HR was adjusted for age, sex, race, smoking status, alcohol consumption, type 2 diabetes mellitus, hypertension, dyslipidaemia and use of aspirin and statin.
Cox proportional hazards model was performed for death analysis.
For type 2 diabetes mellitus, HR was adjusted with the same confoundersa excluding type 2 diabetes mellitus.
TABLE 4.
Causes of death of all deceased patients in each BMI group.
| Causes of death | Total (n = 616) | Lean (n = 111) | Non-lean (n = 505) | p value (lean vs. non-lean) | ||
|---|---|---|---|---|---|---|
| Overweight (n = 162) | Obesity 1 (n = 136) | Obesity 2–3 (n = 207) | ||||
| Overall death | 616/13,420 (4.6%) | 111/1454 (7.6%) | 162/3373 (4.8%) | 136/3830 (3.6%) | 207/4763 (4.3%) | <0.0001 |
| Cardiovascular diseases | 190 (30.8%) | 29 (26.1%) | 46 (28.4%) | 45 (33.1%) | 70 (33.8%) | 0.24 |
| Neoplasms | 103 (16.7%) | 14 (12.6%) | 31 (19.1%) | 21 (15.4%) | 37 (17.9%) | 0.20 |
| Digestive and liver cancers | 33 (5.4%) | 3 (2.7%) | 7 (4.3%) | 12 (8.8%) | 11 (5.3%) | 0.17 |
| Other neoplasmsa | 70 (11.3%) | 11 (9.9%) | 24 (14.8%) | 9 (6.6%) | 26 (12.6%) | 0.59 |
| Respiratory diseases | 75 (12.2%) | 17 (15.3%) | 15 (9.3%) | 18 (13.2%) | 25 (12.1%) | 0.26 |
| Digestive diseases | 48 (7.8%) | 11 (9.9%) | 13 (8.0%) | 8 (5.9%) | 16 (7.7%) | 0.36 |
| Liver diseases | 33 (5.4%) | 8 (7.2%) | 8 (4.9%) | 6 (4.4%) | 11 (5.3%) | 0.34 |
| Digestive diseases other than liver diseases | 15 (2.4%) | 3 (2.7%) | 5 (3.1%) | 2 (1.5%) | 5 (2.4%) | 0.84 |
| External causeb | 45 (7.3%) | 6 (5.4%) | 19 (11.7%) | 12 (8.8%) | 8 (3.9%) | 0.40 |
| Mental-neurological diseases | 40 (6.5%) | 9 (8.1%) | 13 (8.0%) | 8 (5.9%) | 10 (4.8%) | 0.45 |
| Metabolic diseasesc | 36 (5.8%) | 9 (8.1%) | 6 (3.7%) | 6 (4.4%) | 15 (7.3%) | 0.26 |
| Diabetes mellitus | 7 (1.1%) | 0 (0.0%) | 2 (1.2%) | 1 (0.7%) | 4 (1.9%) | 0.22 |
| Infectious diseases | 27 (4.4%) | 6 (5.4%) | 8 (4.9%) | 3 (2.2%) | 10 (4.8%) | 0.56 |
| Genitourinary diseases | 25 (4.1%) | 1 (0.9%) | 4 (2.5%) | 9 (6.62%) | 11 (5.3%) | 0.06 |
| Musculoskeletal and connective tissue diseases | 9 (1.5%) | 3 (2.7%) | 2 (1.2%) | 3 (2.2%) | 1 (1.5%) | 0.23 |
| Otherd | 18 (2.9%) | 6 (5.4%) | 5 (3.1%) | 3 (2.2%) | 4 (1.9%) | 0.09 |
Note: The cause of death was categorised based on the ICD-10-CM codes.
Other neoplasms refer to neoplasms that are other than digestive cancers, mainly including (1) C15-C26 Malignant neoplasms of digestive organs; (2) C30-C39 Malignant neoplasms of respiratory and intrathoracic organs; (3) C50-C50 Malignant neoplasms of breast; (4) C69-C72 Malignant neoplasms of eye, brain and other parts of central nervous system; (5) C81-C96 Malignant neoplasms of lymphoid, haematopoietic and related tissue; (6) D10-D36 Benign neoplasms, except benign neuroendocrine tumours and (7) D37-D48 Neoplasms of uncertain behaviour, polycythaemia vera and myelodysplastic syndromes.
External cause comprises (1) S00-T88 Injury, poisoning and certain other consequences of external causes and (2) V00-Y99 External causes of morbidity.
Metabolic diseases refer to E00-E89 Endocrine, nutritional and metabolic diseases.
Other includes (1) D50-D89 Diseases of the blood and blood-forming organs and certain disorders involving the immune mechanism; (2) Q00-Q99 Congenital malformations, deformations and chromosomal abnormalities and (3) R263 unknown.
We also performed subgroup analysis to compare mortality between non-obese (lean and overweight) versus obese patients with NAFLD. Compared to non-obese patients, obese individuals had significantly lower mortality after adjusting for confounders (adjusted HR 0.71, 95% CI 0.60–0.83, p < 0.001) (Figure S1).
3.5 |. Risk of type 2 DM
There were 846 incident cases of type 2 DM, of which 45 were in lean, 254 in overweight, 393 in obesity class 1 and 154 in obesity class 2–3 individuals, with the incidence per 1000 person-years of 8.62, 12.50, 18.89 and 27.05 respectively (p < 0.0001). The cumulative incidence of type 2 DM among the four groups is displayed in Figure 2B. The risks of incident DM among patients in obesity class 1 and obesity class 2–3 were significantly higher than the lean patients, with adjusted HR 1.78 (95% CI 1.29–2.45, p < 0.001) and 2.85 (95% CI 2.08–3.91, p < 0.001) (Table 3).
3.6 |. Risk of CVD
There were 719 events of any CVD during the study, with 43 in lean, 195 in overweight, 286 in obesity class 1 and 195 in obesity class 2–3 patients. The incidence per 1000 person-years of CVD in each weight category was 8.67, 15.95, 13.85 and 16.22 respectively (p < 0.001). The cumulative incidence of any CVD is displayed in Figure 2C. In the competing risk analysis, compared to lean individuals with NAFLD, there was a marginally significant increased risk of any CVD in overweight (adjusted HR 1.40, 95% CI 1.00–1.95, p = 0.051) but not in obese individuals (Table 3). We stratified by smoking status and found no difference in CVD incidence in lean versus non-lean persons among never smokers, ever smokers or current smokers (Table S3).
3.7 |. Risk of cirrhosis and LREs
Of the 12,646 NAFLD individuals with no cirrhosis at study entry, 254 developed cirrhosis during follow-up, with the incidence per 1000 person-years of 1.97, 3.35, 3.62 and 5.81 in lean, overweight, obesity class 1 and obesity class 2–3 groups respectively (p < 0.0001). The cumulative incidence of cirrhosis based on the competing risk analysis is displayed in Figure 2D. Compared with lean individuals, obesity class 2–3 individuals were more likely to develop cirrhosis with adjusted HR 2.19 (95% CI 1.20–4.01, p = 0.01) (Table 3). In FIB-4 category 1.3–2.67, the incidence of cirrhosis was significantly higher among patients with obesity class 2–3 compared to lean patients only as well as to non-obese (lean + overweight) patients (Table S4). Patients with obesity class 2–3 also had a higher incidence of cirrhosis compared with overweight patients in those with FIB-4 > 2.67. There was no significant difference in LREs between lean and non-lean persons with NAFLD (Table 3) regardless of FIB-4 category (Table S4).
3.8 |. Risk of any cancer and obesity-related cancer
For any cancer, there were 556 total events with incidence per 1000 person-years of 9.10, 10.82, 9.05, 8.93 and 8.80 in lean, overweight, obesity class 1 and obesity class 2–3 groups respectively (p = 0.51). The cumulative incidence of any cancer is shown in Figure 2E and the competing risk analysis of any cancer displayed in Table 3 shows no significant difference across the groups.
There were 243 obesity-related cancers, with 24 in lean, 71 in overweight, 94 in obesity class 1 and 54 in obesity class 2–3 groups, and incidence per 1000 person-years of 3.94, 3.54, 4.01 and 4.29 events respectively (p = 0.73). The cumulative incidence of obesity-related cancers among the four groups is illustrated in Figure 2F. There was no statistically significant difference in risk of obesity-related cancers among overweight (adjusted HR 0.87, p = 0.58), obesity class 1 (adjusted HR 0.97, p = 0.90) and obesity class 2–3 (adjusted HR 1.02, p = 0.93) individuals compared to lean individuals (Table 3).
3.9 |. Sensitivity analysis among patients with no change in BMI category during the follow-up period
We conducted a sensitivity analysis of subjects whose BMI category did not change throughout the follow-up period of this study. A total of 3773 (28.1%) patients were included (370 lean, 680 overweight, 538 obesity class 1 and 2185 obesity class 2–3). The cumulative incidence of any CVD, CAD, type 2 DM, obesity-related cancer and all cancer using the competing risk model are shown in Figure S2. Among the patients who stayed in the same BMI category, cumulative incidences of any CVD (p = 0.03), cirrhosis (p < 0.001) and type 2 DM (p < 0.001) were significantly different among different BMI categories with the persistently lean group having lower incidences of these outcomes. However, the mortality in the persistently lean group remained significantly higher than in the non-lean groups (Table S5).
3.10 |. Sensitivity analysis among smokers versus non-smokers during the follow-up period
A total of 13,420 patients were included—9113 never smokers, 1275 current smokers and 3032 former smokers. Among never and former smokers, the lean subgroups had a significantly higher cumulative incidence of death compared with the non-lean subgroups (both p < 0.05), and a trend towards a higher cumulative incidence of death in lean versus non-lean subgroups among current smokers (p = 0.057) (Figure S3).
3.11 |. Sensitivity analysis among patients with available PNPLA3-rs738409 genotype
A total of 3039 patients with NAFLD: 259 lean, 652 overweight and 2128 obese, followed for a median (IQR) of 54.7 (28.0–86.6) months and a total follow-up of 15,311 person-years were studied. 84.1% were Caucasian, 6.4% African American and 3.3% Asian. PNPLA3 genotype was CC, CG and GG in 52.7%, 38.1% and 9.2% of patients overall; 43.2%, 38.0% and 8.7% of obese patients and 51.4%, 38.3% and 10.3% of lean/overweight patients (p > 0.05). NAFLD patients with PNPLA3 GG, but not those with CG genotype, had a higher incidence of cirrhosis with adjusted HR 2.23 (95% CI 1.25–4.00, p-value = 0.007) compared to patients with CC genotype (Table S6). This was true in both obese and non-obese patients. PNPLA3 GG was significantly associated with LREs in the overall cohort with adjusted HR 1.87 (95%CI 1.04–3.34, p-value = 0.04), and in obese patients with adjusted HR 6.26 (95%CI 1.81–21.65, p-value = 0.004) but not in the non-obese (lean/overweight) patients, compared to patients with CC genotype. PNPLA3 genetic variants were not associated with all-cause mortality or incidence of CVD, DM or cancer (Table S6).
4 |. DISCUSSION
In this study of more than 10,000 patients with NAFLD including nearly 1500 lean persons followed for up to 10 years, we found that lean persons with NAFLD had a lower incidence of cirrhosis and DM and a similar incidence of CVD than overweight/obese persons with NAFLD. Despite similar or lower incidence of cirrhosis, DM and CVD and a lower prevalence of metabolic diseases at initial diagnosis, lean persons with NAFLD had significantly higher overall mortality. The finding of a significantly higher mortality among lean persons with NAFLD compared to overweight/obese persons with NAFLD remained true in a sensitivity analysis of the subgroup who stayed in the same weight category throughout the duration of follow-up and among the subgroups of smokers and non-smokers. This finding is relevant to a large proportion of patients with NAFLD, as 27% patients with NAFLD are lean and this proportion is even higher in Asian (27%–36%) than Western populations (10%–23%).30
We found that lean individuals in our cohort were more likely to be smokers than overweight/obese individuals. However, the prevalence of CVD in particular, PAD and CVA, and to a lesser extent CAD were significantly higher in lean individuals, even after adjusting for smoking status. The explanation for the higher prevalence of CVD among lean individuals with NAFLD is not clear. One study found that lean Caucasians with NAFLD showed distinct metabolomics profiles compared to overweight/obese individuals with NAFLD.31 Another hypothesis that has been proposed is that lean persons with NAFLD are more prone to have sarcopenia and expansion of visceral adipose tissue may play a role not only in the pathogenesis of NAFLD but also atherosclerosis and CVD.32,33
In our longitudinal analysis, we found a lower incidence of cirrhosis and DM among lean individuals with NAFLD compared to overweight/obese individuals with NAFLD with no significant differences in incident CVD, all cancers or obesity-related cancers. A prospective cohort study from United States with 394 NAFLD patients (diagnosed with ultrasound or histology) with a median follow-up of 5.7 years also found no significant difference in incidence of CVD between obese versus non-obese patients with NAFLD.34 In contrast, a recent study from the Olmsted county database and a multicentre-study from four countries (Italy, United Kingdom, Spain and Australia) showed no significant differences in the incidence of both liver and non-liver events: cirrhosis, liver-related events/decompensation, DM and CVD between lean and obese subjects with NAFLD.5,12 Non-invasive fibrosis scores such as FIB-4 have been proposed to risk stratify patients with NAFLD for liver-related outcomes.35 We found that patients with obesity class 2–3 had significantly higher incident cirrhosis than lean and non-obese (lean + overweight) patients among those with intermediate FIB-4 score 1.3–2.67. In addition, among patients with FIB-4 score >2.67, obesity class 2–3 subjects had a significantly higher risk of cirrhosis than overweight persons indicating an urgency in weight control among patients with intermediate or high FIB-4 scores.
An intriguing finding from this study is a significantly higher mortality among lean individuals with NAFLD compared with non-lean individuals, though we did not find significant differences in the distribution of causes of death between the two groups. We also found a significantly higher mortality among non-obese (lean and overweight) individuals compared with obese individuals. A nationwide study of 646 patients with biopsy-confirmed NAFLD in Sweden followed for a mean of 20 years demonstrated that patients with normal BMI had significantly higher liver-related death than overweight and obese patients (8.1% vs. 3.0% and 8.1% vs. 4.3%, respectively, p-value < 0.001), and a higher incidence of severe liver disease defined as a diagnosis of cirrhosis, decompensated liver disease, hepatocellular carcinoma or liver failure, compared with overweight patients (15.5% vs. 9.3%, p-value < 0.001).36 A population-based study from Olmsted County with 4834 NAFLD individuals identified by ICD-code with/without imaging diagnosis also demonstrated a higher all-cause mortality in lean individuals compared with obese individuals with HR 1.96 (95% 1.52–2.51) and no significant difference in distribution of deaths.12 A multicentre study from Europe and Australia of 1339 patients biopsy-confirmed NAFLD found no significant difference in overall mortality between lean versus non-lean patients but obese patients had significantly higher overall mortality compared to non-obese patients.5 A longitudinal U.S. population-based study using National Health and Nutrition Examination Survey with 4711 persons identified as having NAFLD based on US fatty liver index (3183 obese, 1299 overweight and 229 lean) ≥30, found that lean individuals had significantly higher 15-year cumulative all cause-mortality (76.3% vs. 27.2%, p-value < 0.001) and CVD-related mortality (16.9% vs. 5.6%, p < 0.05) than obese individuals.37 The discrepancies in mortality among these studies may be due to differences in definition of NAFLD, that is, liver histology5,36 versus USFLI37 versus ICD codes,12 prevalence of metabolic and CV diseases and severity of liver disease at baseline and average duration of follow-up (5.7–20 years). A recently published systematic review and meta-analysis including 10 cohort studies with 109,151 NAFLD patients found no difference in overall mortality between lean patients and non-lean patients (relative risk 1.09, 95% CI 0.66–1.90), though there was marked heterogeneity (I2 = 97%).38 The difference in the mortality finding between our study and the meta-analysis is likely due to differences in patient population, race (our study included mainly Caucasians while 3 studies39–41 in the meta-analysis were conducted in Asia and likely included exclusively Asian), age of the lean patients (median 51 years in our study vs. 41–48 years), sex (58.8% female in the lean group vs. 24.6%–45.2%), definition of lean (race-specific BMI cut-off for lean in our study vs. a mix of race-specific and standard BMI cut-off) and methods of NAFLD diagnosis (predominantly imaging in our study vs. a mix of predominantly biopsy, imaging or transient elastography). Of note, the meta-analysis found significantly increased liver-related mortality in lean patients38 and we found a trend towards higher liver-related mortality in lean patients.
Mechanisms underlying potentially increased mortality in lean persons with NAFLD remain unclear. We speculate that sarcopenia which has been demonstrated to be more common in lean persons with NAFLD and an inactive lifestyle may partially explain the higher mortality in this group.33,42–44 Our results support the recent clinical practice update from the American Gastroenterology Association stating that lean persons with NAFLD should be evaluated and treated for modifiable CVD risk factors including DM, hypertension and dyslipidaemia similar to overweight and obese persons with NAFLD.45
The PNPLA3 -rs738409-G genetic variant is associated with hepatic steatosis, cirrhosis and HCC.46 However, the data on the prevalence of the PNPLA3 risk variant in obese versus non-obese persons with NAFLD are limited and it is not clear if the association between PNPLA3 genotype and liver-related outcomes is true in both obese and non-obese persons and if PNPLA3 genotype is also associated with non-liver outcomes. Similar to a previous study, we found no difference in PNPLA3-rs738409 genotype distribution between lean and overweight/obese subjects with NAFLD.5 However, the majority of NAFLD population in our study and all NAFLD patients in the study cited above5 were Caucasian in whom the prevalence of PNPLA3 GG genotype is lower, whether there is a difference in PNPLA3 genotype distribution among lean versus overweight/obese Asian and Hispanic patients with NAFLD remain to be determined.46,47 Our study showed that PNPLA3 GG genotype was associated with a higher incidence of liver cirrhosis, but not overall mortality or non-liver outcomes, in both obese and non-obese individuals. In addition, we found that PNPLA3 GG genotype was associated with a higher incidence of LREs in obese patients with NAFLD; the lack of association in non-obese patients may be related to the small number of events in lean NAFLD patients. A recent study from Sweden with 20 years of follow-up also found that PNPLA3 GG genotype was associated with an increased risk of cirrhosis, but not mortality, compared with CC genotype among 546 imaging/biopsy-proven NAFLD patients (data for obese vs. non-obese patients was not provided).48 Our study found that increasing BMI amplified the risk of PNPLA3 GG genotype (vs. CC genotype), with the odds of cirrhosis of 3.2 among non-obese subjects, and 6.3 in obese NAFLD patients compared to lean patients, similar to findings from a study in Denmark.49 Our data suggest that future NAFLD treatment interventions should stratify for PNPLA3 genotype and therapies targeting PNPLA3 variant-induced pathophysiology are needed particularly among obese patients who are at higher baseline risk.
This study has several strengths. First, we included a large number of patients with NAFLD, nearly 20,000 patients in the cross-sectional analysis and more than 13,000 in the longitudinal analysis. We applied minimal exclusion criteria to capture a largely unselected cohort with hepatic steatosis seen in our health system for a variety of reasons and did not limit the cohort to patients seen in specialist clinics or were selected for liver biopsy. Second, the diagnosis of NAFLD used a validated natural language processing algorithm that relied primarily on imaging, rather than ICD codes or histology to minimise disease-spectrum bias. Third, access to medical history, laboratory, imaging and histological data from medical records provided more in-depth clinical information that is not available in large insurance claims databases or national epidemiological databases. These detailed data also allowed us to adjust the prevalence and incidence of outcomes for multiple confounding factors including host factors (age, sex and race), behavioural risk factors (smoking and alcohol consumption), genetic risk factors and metabolic risk factors (DM, hypertension and dyslipidaemia). Fourth, PNPLA3 genotype data were available in a large number (3039) of patients allowing us to examine the effect of the GG variant on outcomes in obese versus non-obese patients.
The current study also has limitations. First, this is a single tertiary referral centre study in the United States with predominantly Caucasians and the data may not be generalised to other NAFLD populations. Of note, multiple epidemiological studies50,51 demonstrated that obese adults had worse survival than those with normal BMI. The finding of higher mortality in lean patients with NAFLD in our study should be cautiously interpreted because it is a single-centre study with potential for referral bias. Second, diagnosis of metabolic and cardiovascular diseases and causes of death were mainly based on ICD codes which may not be accurate though this approach is widely used in large cohort studies and population studies. Although we found 95% accuracy of causes of death, only 20 medical records were manually reviewed and the possibility of misclassification or misdiagnosis based on ICD codes cannot be excluded. We acknowledge that onset or severity of disease cannot be gleaned from ICD codes and that even though we adjusted for multiple major confounders that may affect the outcomes of interest, unmeasured confounders such as diet, body composition and physical activity were not available in this retrospective study. Third, most of our patients had an initial diagnosis of NAFLD based on imaging suggesting that the diagnosis of NAFLD was incidental and their outcomes may be different from those who present with liver disease. Fourth, it is possible that some lean patients with NAFLD may have been previously overweight or obese but lost weight due to illnesses which led to worse outcomes (reverse causation) though we excluded patients with comorbidities that might cause significant weight changes such as baseline cancer or prior bariatric surgery. In addition, we performed sensitivity analyses for mortality adjusted for Charlson Comorbidity Index and still found significantly higher mortality in the lean group.
In conclusion, this study found that lean individuals with NAFLD in the United States had significantly higher mortality and higher prevalence of CVD and a trend towards a higher incidence of cancers compared with overweight or obese individuals with NAFLD despite a lower incidence of DM and cirrhosis. These findings emphasise that lean individuals with NAFLD merit the same attention in clinical practice as their overweight/obese counterparts. Further studies are warranted to understand the pathogenic mechanisms of NAFLD in lean persons (such as genetic variants, distinct metabolomic profiles, body fat distribution and sarcopenia) and the cause of increased mortality in these persons to design specific interventions to reverse the course and improve clinical outcomes.
Supplementary Material
FUNDING INFORMATION
Karn Wijarnpreecha was a recipient of the American Association for the Study of Liver Diseases (AASLD) Advanced/Transplant Hepatology Fellowship Award. Vincent L Chen was supported in part by an AASLD Clinical, Translational and Outcomes Research Award and an NIDDK K08 DK132312.
Footnotes
CONFLICT OF INTEREST STATEMENT
Vincent Chen receives research grant funding from KOWA and Astra Zeneca. Anna Lok receives research grant funding from KOWA, Astra Zeneca and TARGET, and serves on Data and Safety Monitoring Board for Novo Nordisk.
SUPPORTING INFORMATION
Additional supporting information will be found online in the Supporting Information section.
REFERENCES
- 1.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. 10.1002/hep.28431 [DOI] [PubMed] [Google Scholar]
- 2.Albhaisi S, Chowdhury A, Sanyal AJ. Non-alcoholic fatty liver disease in lean individuals. JHEP Rep. 2019;1(4):329–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ye Q, Zou B, Yeo YH, Li J, Huang DQ, Wu Y, et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5(8):739–52. [DOI] [PubMed] [Google Scholar]
- 4.Fracanzani AL, Petta S, Lombardi R, Pisano G, Russello M, Consonni D, et al. Liver and cardiovascular damage in patients with lean non-alcoholic fatty liver disease, and association with visceral obesity. Clin Gastroenterol Hepatol. 2017;15(10):1604–1611.e1. [DOI] [PubMed] [Google Scholar]
- 5.Younes R, Govaere O, Petta S, Miele L, Tiniakos D, Burt A, et al. Caucasian lean subjects with non-alcoholic fatty liver disease share long-term prognosis of non-lean: time for reappraisal of BMI-driven approach? Gut. 2022;71(2):382–90. [DOI] [PubMed] [Google Scholar]
- 6.Weinberg EM, Trinh HN, Firpi RJ, Bhamidimarri KR, Klein S, Durlam J, et al. Lean Americans with nonalcoholic fatty liver disease have lower rates of cirrhosis and comorbid diseases. Clin Gastroenterol Hepatol. 2021;19(5):996–1008.e6. [DOI] [PubMed] [Google Scholar]
- 7.Li AA, Ahmed A, Kim D. Extrahepatic manifestations of nonalcoholic fatty liver disease. Gut Liver. 2020;14(2):168–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams LA, Anstee QM, Tilg H, Targher G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. 2017;66(6):1138–53. [DOI] [PubMed] [Google Scholar]
- 9.Semmler G, Wernly S, Bachmayer S, Wernly B, Schwenoha L, Huber-Schönauer U, et al. Nonalcoholic fatty liver disease in lean subjects: associations with metabolic dysregulation and cardiovascular risk-a single-center cross-sectional study. Clin Transl Gastroenterol. 2021;12(4):e00326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim Y, Han E, Lee JS, Lee HW, Kim BK, Kim MK, et al. Cardiovascular risk is elevated in lean subjects with nonalcoholic fatty liver disease. Gut Liver. 2022;16(2):290–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cruz ACD, Bugianesi E, George J, Day CP, Liaquat H, Charatcharoenwitthaya P, et al. 379 characteristics and long-term prognosis of lean patients with nonalcoholic fatty liver disease. Gastroenterology. 2014;146(5):S-909–S, 909. [Google Scholar]
- 12.Ahmed OT, Gidener T, Mara KC, Larson JJ, Therneau TM, Allen AM. Natural history of nonalcoholic fatty liver disease with Normal body mass index: a population-based study. Clin Gastroenterol Hepatol. 2022;20(6):1374–1381.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen VL, Du X, Chen Y, Kuppa A, Handelman SK, Vohnoutka RB, et al. Genome-wide association study of serum liver enzymes implicates diverse metabolic and liver pathology. Nat Commun. 2021;12(1):816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu C, Caussy C, Imajo K, Chen J, Singh S, Kaulback K, et al. Magnetic resonance vs transient elastography analysis of patients with nonalcoholic fatty liver disease: a systematic review and pooled analysis of individual participants. Clin Gastroenterol Hepatol. 2019;17(4):630–637.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hagström H, Adams LA, Allen AM, Byrne CD, Chang Y, Grønbæk H, et al. Administrative coding in electronic health care record-based research of NAFLD: an expert panel consensus statement. Hepatology. 2021;74(1):474–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–38. [DOI] [PubMed] [Google Scholar]
- 17.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43(6):1317–25. [DOI] [PubMed] [Google Scholar]
- 18.Burkholder DA, Moran IJ, DiBattista JV, Lok AS, Parikh ND, Chen VL. Accuracy of international classification of diseases-10 codes for cirrhosis and portal hypertensive complications. Dig Dis Sci. 2022;67(8):3623–31. [DOI] [PubMed] [Google Scholar]
- 19.Cozzolino F, Montedori A, Abraha I, Eusebi P, Grisci C, Heymann AJ, et al. A diagnostic accuracy study validating cardiovascular ICD-9-CM codes in healthcare administrative databases. The Umbria Data-Value Project. PLoS One. 2019;14(7):e0218919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Floyd JS, Blondon M, Moore KP, Boyko EJ, Smith NL. Validation of methods for assessing cardiovascular disease using electronic health data in a cohort of Veterans with diabetes. Pharmacoepidemiol Drug Saf. 2016;25(4):467–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ando T, Ooba N, Mochizuki M, Koide D, Kimura K, Lee SL, et al. Positive predictive value of ICD-10 codes for acute myocardial infarction in Japan: a validation study at a single center. BMC Health Serv Res. 2018;18(1):895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Webber BJ, Rogers AE, Pathak SR, Robbins AS. Positive predictive value of an algorithm used for cancer surveillance in the U.S. Armed Forces. MSMR. 2019;26(12):18–22. [PubMed] [Google Scholar]
- 23.Cozzolino F, Bidoli E, Abraha I, Fusco M, Giovannini G, Casucci P, et al. Accuracy of colorectal cancer ICD-9-CM codes in Italian administrative healthcare databases: a cross-sectional diagnostic study. BMJ Open. 2018;8(7):e020630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hwang YJ, Kim N, Yun CY, Yoon H, Shin CM, Park YS, et al. Validation of administrative big database for colorectal cancer searched by international classification of disease 10th codes in Korean: a retrospective big-cohort study. J Cancer Prev. 2018;23(4):183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abraha I, Serraino D, Montedori A, Fusco M, Giovannini G, Casucci P, et al. Sensitivity and specificity of breast cancer ICD-9-CM codes in three Italian administrative healthcare databases: a diagnostic accuracy study. BMJ Open. 2018;8(7):e020627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, et al. New creatinine-and cystatin C-based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 2000;894:1–253, i-xii. [PubMed] [Google Scholar]
- 28.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–63. [DOI] [PubMed] [Google Scholar]
- 29.Dey R, Schmidt EM, Abecasis GR, Lee S. A fast and accurate algorithm to test for binary phenotypes and its application to PheWAS. Am J Hum Genet. 2017;101(1):37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang A, Ng CH, Phang PH, Chan KE, Chin YH, Fu CE, et al. Comparative burden of metabolic dysfunction in lean NAFLD vs. non-lean NAFLD—a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2022. 10.1016/j.cgh.2022.06.029 [DOI] [PubMed] [Google Scholar]
- 31.Feldman A, Eder SK, Felder TK, Kedenko L, Paulweber B, Stadlmayr A, et al. Clinical and metabolic characterization of lean Caucasian subjects with non-alcoholic fatty liver. Am J Gastroenterol. 2017;112(1):102–10. [DOI] [PubMed] [Google Scholar]
- 32.Kang MK, Park JG. Low skeletal muscle mass is a risk factor for sub-clinical atherosclerosis in patients with nonalcoholic fatty liver disease. Diagnostics (Basel). 2021;11(5):854. 10.3390/diagnostics11050854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuchay MS, Martínez-Montoro JI, Choudhary NS, Fernández-García JC, Ramos-Molina B. Non-alcoholic fatty liver disease in lean and non-obese individuals: current and future challenges. Biomedicine. 2021;9(10):1346. 10.3390/biomedicines9101346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arvind A, Henson JB, Osganian SA, Nath C, Steinhagen LM, Memel ZN, et al. Risk of cardiovascular disease in individuals with nonobese nonalcoholic fatty liver disease. Hepatol Commun. 2022;6(2):309–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanwal F, Shubrook JH, Adams LA, Pfotenhauer K, Wai-Sun Wong V, Wright E, et al. Clinical care pathway for the risk stratification and management of patients with nonalcoholic fatty liver disease. Gastroenterology. 2021;161(5):1657–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hagström H, Nasr P, Ekstedt M, Hammar U, Stål P, Hultcrantz R, et al. Risk for development of severe liver disease in lean patients with nonalcoholic fatty liver disease: a long-term follow-up study. Hepatol Commun. 2018;2(1):48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zou B, Yeo YH, Nguyen VH, Cheung R, Ingelsson E, Nguyen MH. Prevalence, characteristics and mortality outcomes of obese, non-obese and lean NAFLD in the United States, 1999–2016. J Intern Med. 2020;288(1):139–51. [DOI] [PubMed] [Google Scholar]
- 38.Ha J, Yim SY, Karagozian R. Mortality and liver-related events in lean versus non-lean nonalcoholic fatty liver disease: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2022;26. 10.1016/j.cgh.2022.11.019 [DOI] [PubMed] [Google Scholar]
- 39.Chang Y, Cho YK, Cho J, Jung HS, Yun KE, Ahn J, et al. Alcoholic and nonalcoholic fatty liver disease and liver-related mortality: a cohort study. Am J Gastroenterol. 2019;114(4):620–9. [DOI] [PubMed] [Google Scholar]
- 40.Hirose S, Matsumoto K, Tatemichi M, Tsuruya K, Anzai K, Arase Y, et al. Nineteen-year prognosis in Japanese patients with biopsy-proven nonalcoholic fatty liver disease: lean versus overweight patients. PLoS One. 2020;15(11):e0241770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leung JCF, Loong TCW, Wei JL, Wong GLH, Chan AWH, Choi PCL, et al. Histological severity and clinical outcomes of non-alcoholic fatty liver disease in nonobese patients. Hepatology. 2017;65(1):54–64. [DOI] [PubMed] [Google Scholar]
- 42.Jang DK, Lee JS, Lee JK, Kim YH. Independent association of physical activity with nonalcoholic fatty liver disease and alanine aminotransferase levels. J Clin Med Res. 2019;8(7):1013. 10.3390/jcm8071013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Golabi P, Gerber L, Paik JM, Deshpande R, de Avila L, Younossi ZM. Contribution of sarcopenia and physical inactivity to mortality in people with non-alcoholic fatty liver disease. JHEP Rep. 2020;2(6):100171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moon JH, Koo BK, Kim W. Non-alcoholic fatty liver disease and sarcopenia additively increase mortality: a Korean nationwide survey. J Cachexia Sarcopenia Muscle. 2021;12(4):964–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Long MT, Noureddin M, Lim JK. AGA clinical practice update: diagnosis and management of nonalcoholic fatty liver disease in lean individuals: expert review. Gastroenterology. 2022;163:764–774. e1. 10.1053/j.gastro.2022.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dong XC. PNPLA3-a potential therapeutic target for personalized treatment of chronic liver disease. Front Med (Lausanne). 2019;6:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Y, Xing C, Cohen JC, Hobbs HH. Genetic variant in PNPLA3 is associated with nonalcoholic fatty liver disease in China. Hepatology. 2012;55(1):327–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holmer M, Ekstedt M, Nasr P, Zenlander R, Wester A, Tavaglione F, et al. Effect of common genetic variants on the risk of cirrhosis in non-alcoholic fatty liver disease during 20 years of follow-up. Liver Int. 2022;42(12):2769–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stender S, Kozlitina J, Nordestgaard BG, Tybjærg-Hansen A, Hobbs HH, Cohen JC. Adiposity amplifies the genetic risk of fatty liver disease conferred by multiple loci. Nat Genet. 2017;49(6):842–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Masters RK, Reither EN, Powers DA, Yang YC, Burger AE, Link BG. The impact of obesity on US mortality levels: the importance of age and cohort factors in population estimates. Am J Public Health. 2013;103(10):1895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Byers T Body weight and mortality. N Engl J Med. 1995;333(11): 723–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


