Abstract
Thiamine, also known as vitamin B1, is an essential nutrient that plays a crucial role in energy metabolism and overall health. It is a water-soluble vitamin that plays an important role in the conversion of carbohydrates into energy in the body. Thiamine is essential for the proper functioning of the nervous system, heart and muscles. Thiamine deficiency is a life-threatening disease that leads to various disorders and lesions in the nerves and brain, at least in vertebrates. Several thiamine precursors with higher bioavailability have been developed to compensate for thiamine deficiency, including benfotiamine. Benfotiamine is more bioavailable and has higher tissue penetration than thiamine. Studies have shown its antioxidant and anti-inflammatory potential in activated immune and glial cells. It also improves complications observed in type 2 diabetes and has beneficial effects in mouse models of neurodegenerative disease. Benfotiamine represents an off-the-shelf agent used to support nerve health, promote healthy aging and support glucose metabolism. Accordingly, the present review aimed to provide an overview of the neuroprotective effects of thiamine/benfotiamine in the context of inflammation and oxidative stress.
Keywords: Thiamine deficiency, Benfotiamine, Neurological disease
1. Introduction
Thiamine (vitamin B1) is important for the proper functioning of the nervous system. It is the precursor of thiamine diphosphate (ThDP), an important coenzyme for transketolase, pyruvate dehydrogenase, and 2-oxoglutarate dehydrogenase [1]. Thus, it is essential for brain energy metabolism. Thiamine deficiency (TD) can cause severe damage to the nervous system, including disturbed neurotransmitter synthesis, nucleic acid synthesis, and synthesis of steroids and fatty acids [2]. The disorders caused by TD are quite complex in diagnosis and can take numerous forms. TD provoked by malnutrition often leads to syndromes such as beriberi; alcohol-related thiamine deficiency leads to acute neurological condition (Wernicke's encephalopathy) with a possibly fatal outcome, and chronic neurological condition (Korsakoff's syndrome), an impairment memory disorder. In the absence of irreparable brain damage, TD responds to thiamine treatment [3]. However, intestinal intake of thiamine, which requires specific transporters, is rate-limiting. Benfotiamine (S-benzoylthiamine-O-monophosphate) is a synthetic S-acyl derivative of thiamine (vitamin B1) characterized by higher bioavailability. Although often referred to as a lipid-soluble analog of thiamine, benfotiamine is virtually insoluble in hydrophobic and organic solvents [4]. When administered orally, benfotiamine is dephosphorylated by alkaline phosphatases in the intestine to S-benzoylthiamine, which is lipophilic and can cross cell membranes. S-Benzoylthiamine diffuses through the intestinal epithelium into the bloodstream, where it is converted to free thiamine by erythrocytes [5]. Because thiamine absorption is limited by thiamine transporters, oral administration of benfotiamine results in higher plasma concentrations of thiamine than oral administration of equal concentrations of thiamine itself [6,7]. For this reason, benfotiamine is frequently used in conditions characterized by thiamine deficiency (TD) [5,[8], [9], [10]]. However, numerous studies also show beneficial effects of benfotiamine in various in vitro cell dysfuction model not caused by TD [[11], [12], [13], [14], [15], [16], [17], [18], [19]]. Benfotiamine has been shown to be protective in animal models of neurodegeneration [[20], [21], [22], [23]]. In addition, benfotiamine has been shown to be safe and potentially effective in improving cognitive decline in patients with mild Alzheimer's disease, although these studies need to be verified in larger numbers of patients [24,25].
The benefits of taking thiamine and benfotiamine are well known. However, because of the additional properties of benfotiamine, it may be even more helpful for certain health needs. Benfotiamine is a promising therapeutic agent for improving cognitive function and protecting against inflammation and oxidative stress-induced cell death in the CNS. Therefore, the aim of this review is to provide a comprehensive overview of the various beneficial therapeutic effects of thiamine and benfotiamine demonstrated in cellular, animal, and human studies, with a focus on benfotiamine. We note that benfotiamine deserves further research because of its ability to downregulate pathology in inflammatory and neuroinflammatory conditions. To summarize the existing data on this topic, PubMed, Google, and Scopus were used for the literature search.
2. Methods
We conducted a systematic review of manuscripts (original papers and reviews) by searching the Medline database on PubMed and Google (Google Scholar) and Scopus search engines from inception to December 2022. The selection criteria were applied independently by two authors. We used as search terms (thiamine and/or benfotiamine; thiamine deficiency) OR (thiamine/benfotiamine, inflammation) AND (thiamine/benfotiamine, nervous system) AND (thiamine/benfotiamine, oxidative stress).
2.1. Thiamine (vitamin B1, aneurin)
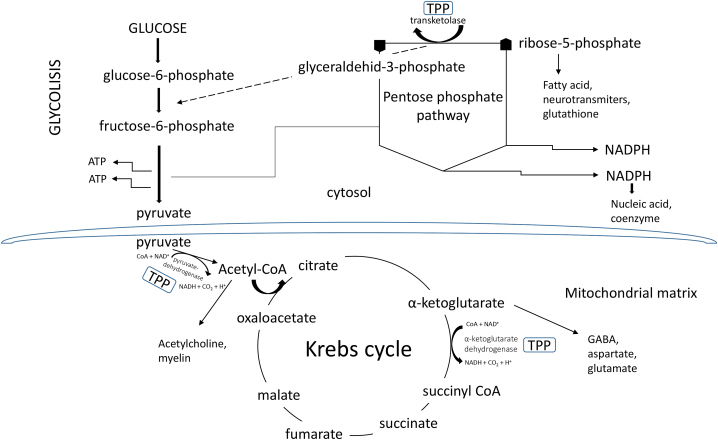
Thiamine is a water-soluble essential micronutrient that cannot be synthesized endogenously, so dietary intake is the only source of this vitamin. Foods rich in thiamine include meats such as pork and beef, fish, whole grains, wheat germ, eggs, legumes, and nuts [26]. In contrast, certain foods such as coffee, tea, raw fish and shellfish contain thiaminases - enzymes that destroy thiamine. Unlike humans, microbes such as Salmonella enterica and Escherichia coli are capable of synthesizing thiamine de novo [27]. Because thiamine is a hydrophilic vitamin, it cannot pass freely across cell membranes and relies on thiamine transporters to enter the cell. Thiamine is taken up in the small intestine by the THTR1 and THTR2 transporters, which are encoded by the SLC19A2 and SLC19A3 genes and belong to the solute carrier (SLC) family [28]. Unlike thiamine, lipophilic analogs diffuse freely in the intestine and across cell membranes, bypassing rate-limiting transporters and producing higher concentrations of thiamine. They are rapidly converted to thiamine in the cell by enzymatic or nonenzymatic processes [4]. Thiamine exists in free form and in its phosphorylated derivatives thiamine monophosphate (ThMP), thiamine diphosphate/thiamine pyrophosphate (ThDP, TPP), thiamine triphosphate (ThTP), and adenosine thiamine triphosphate (AThTP) [29]. Thiamine (Fig. 1) in phosphate-free form has no immediate biological significance. However, the active form of thiamine is ThDP, which acts as a coenzyme for three important enzymes - transketolase (TKT), a key enzyme of the pentose phosphate pathway important for nucleic acid and lipid biosynthesis, pyruvate dehydrogenase complex (PDHC), an enzyme complex that converts pyruvate to acetyl-CoA and links glycolysis to the citric acid cycle, and α-ketoglutarate dehydrogenase complex (OGDHC), a complex involved in the TCA cycle [30]. Considering that these enzymes are crucial for glucose oxidation, thiamine plays an essential role in energy metabolism (Fig. 2), and its deficiency has several negative effects on the whole organism, but especially on organ systems that are major energy consumers, such as the nervous system and the heart [31]. ThTP plays a critical role in several other biological processes, such as gene expression (it regulates the activity of enzymes that modify chromatin), redox reactions, immune system activation, signaling, and maintenance processes [32]. Importantly, thiamine is involved in the production of neurotransmitters and is therefore critical for healthy brain function. It plays a key role in cognitive function, memory, and mood [33]. Thiamine also plays an unknown role in the transmission of nerve impulses and in the maintenance of the myelin sheath [34].
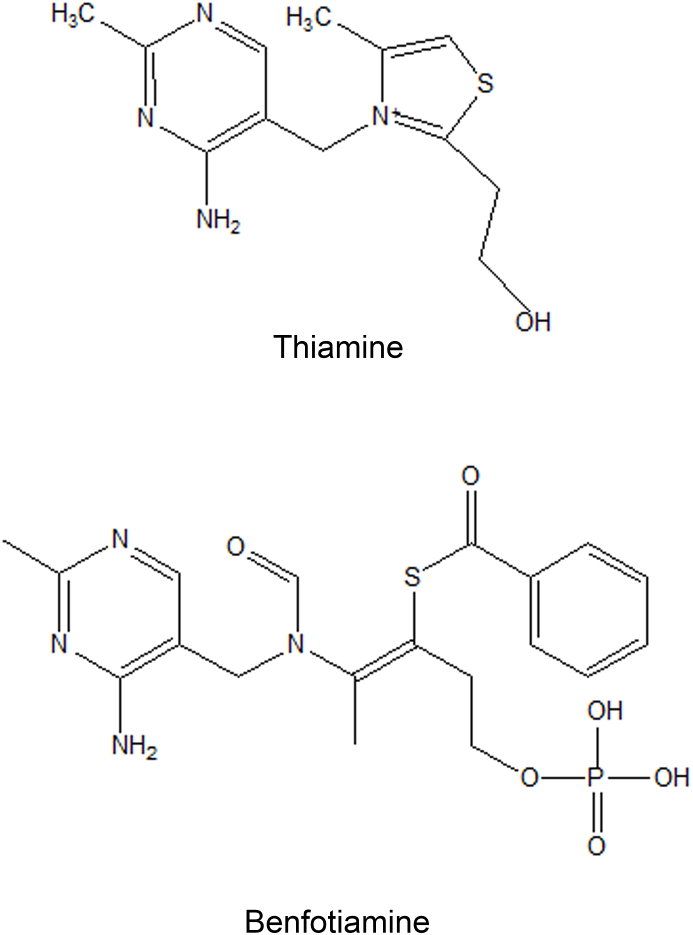
Fig. 1.
Chemical structure of thiamine and benfotiamine.
Fig. 2.
The key carbohydrate metabolic pathways involving thiamine phosphate derivate-thiamine pyrophosphate (TPP)-dependent enzymes. TPP is an essential cofactor for steps regulating glycolysis, the pentose phosphate cycle, and the Krebs cycle.
2.2. Thiamine in inflammatory conditions
Thiamine has been found to modulate the immune response by reducing inflammation and oxidative stress, two processes that can damage immune cells and contribute to the development of various diseases. Thiamine is an important cofactor in the immune system and has multiple functions in regulating and controlling immune cell function of various immune cells, including T cells, B cells, and natural killer (NK) cells [35]. In a study on a rat model of rheumatoid arthritis, thiamine was found to reduce joint inflammation and hyperalgesia, along with reduction in production of the proinflammatory cytokines TNF-α and IL -1β [36]. In addition, thiamine has been shown to have antioxidant effects and is able to protect immune cells from oxidative damage. It increases the production of glutathione, an important antioxidant. In a study in a rat model of acute ethanol exposure, thiamine was found to increase glutathione levels and reduce liver inflammation [37]. In a study in a mouse model of sepsis, thiamine was found to reduce the production of pro-inflammatory cytokines and improve survival [38].
2.3. Thiamine deficiency
Thiamine deficiency (TD) can lead to a number of health problems, including beriberi, Wernicke-Korsakoff syndrome, and other neurological disorders. Therefore, it is important to ensure adequate thiamine intake through a balanced diet or supplements, if necessary. Thiamine deficiency impairs brain activity, which is highly dependent on oxidative glucose metabolism [39]. As a result, free radical production is increased, leading to oxidative stress.
In humans and animals, thiamine deficiency has been shown to decrease myelin fiber density and consequently lead to myelin sheath damage with selective neuronal death and glutamate-related excitotoxicity [[40], [41], [42]]. Indeed, thiamine deficiency leads to damage of thalamic regions with marked lesions in the cortex, cerebellum, and hippocampus [43,44], resulting in cognitive and motor impairments [[45], [46], [47]]. In addition, thiamine is known to be involved in the uptake of serotonin and GABA, which, when deficient, leads to cellular abnormalities that affect cerebellar, hypothalamic, and hippocampal activity. Also, thiamine deficiency affects the peripheral nervous system.
In general, TD in the brain leads to oxidative stress, lactic acidosis, neuroinflammation, and excitotoxicity as a result of a decrease in the activity of ThDP-dependent enzymes, as well as other thiamine-dependent processes [48]. The early signs of TD are nonspecific and are easily overlooked or misdiagnosed-lack of appetite, nausea, fatigue, memory problems, sleep disturbances, abdominal pain, weakness, and anorexia. If left untreated, they can lead to diseases such as beriberi and Wernicke-Korsakoff syndrome. Historically, beriberi is the term for nutritional thiamine deficiency and is divided into wet and dry beriberi based on the amount of fluid that accumulates in the body as a result of heart and kidney damage [49]. Thus, wet beriberi is characterized by heart failure and edema, whereas dry beriberi is characterized by polyneuropathy, decreased reflexes, and muscle weakness that can lead to paralysis of limbs [48,49]. Wernicke-Korsakoff syndrome develops as a result of TD and is most commonly associated with alcoholism. The first stage of the disease is Wernicke's encephalopathy with symptoms including general confusion, ophthalmoplegia, and ataxia. If untreated, this acute condition can progress to Korsakoff syndrome, in which severe lesions occur in the thalamus and mammillary bodies, leading to memory loss, learning deficits, and personality changes [48]. Patients respond well to thiamine if it is administered early in the disease, otherwise irreversible lesions form and the damage becomes permanent. In addition to inadequate intake and alcoholism, TD can be caused by a variety of factors, including increased need or excretion, a diet rich in simple carbohydrates, consumption of foods or drugs containing antithiamine components, or genetic mutations in transporters/enzymes involved in thiamine uptake or metabolism [26]. Thiamine requirements also vary between different populations. For example, several studies have shown low thiamine status in the elderly [[50], [51], [52]], diabetics [53], people with HIV/AIDS [54,55], cancer patients [56], and patients with depression [47]. In addition, pregnant and lactating women require higher thiamine intakes than the recommended daily allowance [49], so subclinical thiamine deficiency is likely more common than previously thought, even in countries where malnutrition is not a widespread problem [48]. In addition, TD is difficult to diagnose because there are no cheap and reliable laboratory tests to detect the deficiency-serum thiamine levels are not adequate to determine the body's supply of thiamine; instead, transketolase activity in erythrocytes or the concentration of thiamine diphosphate ester in erythrocytes are used [32].
Although thiamine has been used to treat TD disorders due to its low bioavailability, several lipophilic thiamine derivatives have been developed.
2.4. Lipophilic thiamine derivatives
The first lipophilic thiamine derivative was found in garlic and was named allithiamine for the Allium genus to which garlic belongs [57]. Afterwards, several lipophilic analogs were synthesized, including fursultiamine, sulbutiamine and benfotiamine. This work was mostly done in Japan in the 1950s and 1960s, because beriberi was a widespread health problem in Japan [58]. Other than raising thiamine levels, some of these compounds have shown other beneficial health effects. For example, sulbutiamine was shown to have antiasthenic properties, although more studies need to be conducted to prove its efficiency [58]. Structurally these compounds differ between each other - allithiamine, fursultiamine and sulbutiamine are disulfides, while benfotiamine is a thioester. Out of these compounds, benfotiamine is best known and most widely used and studied for its anti-inflammatory, antioxidative and other beneficial effects. It has been investigated as a treatment for diabetic complications, neurodegenerative diseases and inflammatory complications. An overview of these effects is given in Table 1.
Table 1.
An overview of the effects of benfotiamine in the treatment of diabetic complications, neurodegenerative diseases and inflammatory complications.
| Disease | Proposed mechanisms of action | Clinical trials | In vivo models | In vitro models |
|---|---|---|---|---|
| Diabetes | Inhibition of hexosamine pathway, AGE formation, DAG –PKC pathway (Hammes et al., 2003) Inhibition of NF-κB (Hammes et al., 2003) |
Randomized, double-blind, placebo controlled study in progress, to evaluate benfotiamine effects on diabetic sensorimotor polyneuropathy (Bonhof et al., 2022) Enhanced thiamine levels in a small randomized, double-blind, placebo-controlled trial, but without the effect on urinary albumin excretion and tubular damage marker kidney injury molecule-1 (Alkhalaf et al., 2010) No changes in plasma or urinary AGEs, plasma markers of inflammation and endothelial dysfunction after 12 weeks treatment in a randomized, placebo-controlled study (Alkhalaf et al., 2012) No effect on peripheral nerve function or soluble markers of inflammation in patients with type I diabetes in a small, randomized, placebo-controlled study (Fraser et al., 2012) |
Inhibition of three important biochemical pathways (hexosamine pathway, AGE formation, DAG –PKC pathway) implicated in pathogenesis of diabetes and inhibition of NF-κB in retinas of streptozotocin induced diabetic rats (Hammes et al., 2003) Decrease in inflammatory and neuropathic pain in a rat model of diabetes (Sanchez-Ramirez et al., 2006) Reduction in cerebral oxidative stress in diabetic mice (Wu and Ren, 2006) Increase in motor nerve conduction velocity, decrease in AGEs, inhibition of glycoxidation products in diabetic rats (Stracke et al., 2001) |
Inhibition of three important biochemical pathways (hexosamine pathway, AGE formation, DAG –PKC pathway) implicated in pathogenesis of diabetes and inhibition of NF-κB in aortic endothelial cells (Hammes et al., 2003) |
| Alzheimerˈs disease | Decreased GSK-3 activity (Pan et al., 2010; Moraes et al., 2020) Suppression of ERK1/2 activity (Moraes et al., 2020) Antioxidative and anti-inflammatory activity, reduced mitochondrial dysfunction, activation of Nrf2 (Tapias et al., 2018) |
Improved cognitive ability in small, uncontrolled clinical trial performed on 5 patients (Pan et al., 2016) Improved cognitive ability in small, double-blind, placebo controlled study (Gibson et al., 2020) |
Improved cognitive function in APP/presenilin 1 transgenic mice Reduced amyloid plaque numbers and tau phosphorylation levels (Pan et al., 2010) Increased lifespan, improved behavioral deficits, decreased neurofibrillary tangles, prevented motor neuron death (Tapias et al., 2018) |
Decreased GSK-3 activity Suppression of β-amyloid production in HEK cells (Sun et al., 2012) |
| Alcoholism | Increased thiamine levels Anti-oxidative effects |
Improved alcoholic polyneuropathy in a small, randomized, placebo-controlled study (Woelk et al., 1998) Reduced alcohol consumption in women in a small, double-blind, randomized, placebo-controlled study (Manzardo et al., 2013) |
Reduced hepatic alcohol concentrations and oxidative stress markers after ethanol gavage in rats (Portari et al., 2016) |
2.5. Benfotiamine metabolism and mechanism of action
Benfotiamine (Fig. 1) shows better bioavailability and absorption than thiamine, and results in at least five times higher plasma concentrations than an equivalent dose of thiamine [59]. Most human studies have shown that benfotiamine is safe and well tolerated, even in higher concentrations [24,25,[60], [61], [62]]. A study examining the pharmacokinetics of benfotiamine treatment in Alzheimer's patients showed that maximum thiamine concentrations in the blood were reached 1–2h after a single dose of benfotiamine, while ThMP and ThDP peaked at later time points, between 3,5 and 8h for ThMP and 8–24h for ThDP. Also, thiamine and ThDP were moderately accumulated after repeated treatments of benfotiamine [60].
Following oral administration, benfotiamine is dephosphorylated to S-benzoylthiamine by ecto-alkaline phosphatases in the small intestine. S-benzoylthiamine is lipophilic and passes easily through intestinal and endothelial cells and enters the bloodstream where erythrocytes convert it to free thiamine [5]. Part of S-benzoylthiamine is hydrolyzed in the liver to thiamine and benzoic acid by thioesterases [4]. Because dephosphorylation is necessary in order to obtain the lipophilic S-benzoylthiamine, oral route of administration is deemed to be the most effective [31]. Volvert et al. showed that in mice, oral treatment of benfotiamine resulted in maximum thiamine concentrations in the liver after 1 h, while maximum concentrations in the blood are reached after 2 h, which led to the conclusion that S-benzoylthiamine from the blood is taken up by the liver and converted to thiamine [4]. Higher concentrations of thiamine monophosphate (ThMP) and ThDP were also found in the blood and liver after benfotiamine ingestion [4]. This study did not find elevated thiamine levels in the brain neither after acute nor chronic (14 days) treatment with benfotiamine in mice. However, several other studies found higher thiamine levels in the brain after oral benfotiamine treatment in mice [20,21] and rats [22,63]. Specifically, after longer benfotiamine treatment (10 days) Pan et al. found higher levels of thiamine in the brain, but no difference in ThMP or ThDP concentrations. Moraes et al. had a model of chronic benfotiamine treatment (30 days) after which they found higher ThDP concentrations in the hippocampus and entorhinal cortex [22]. Also, many studies have found neuroprotective effects of benfotiamine, without measuring benfotiamine metabolites in the brain tissue. The situation is further complicated because analytical methods that are used register only the metabolites with an intact thiazolium ring [64]. Thus, to what extent thiamine and other benfotiamine metabolites enter the brain is still inconclusive, however its neuroprotective effects have been corroborated by a number of studies.
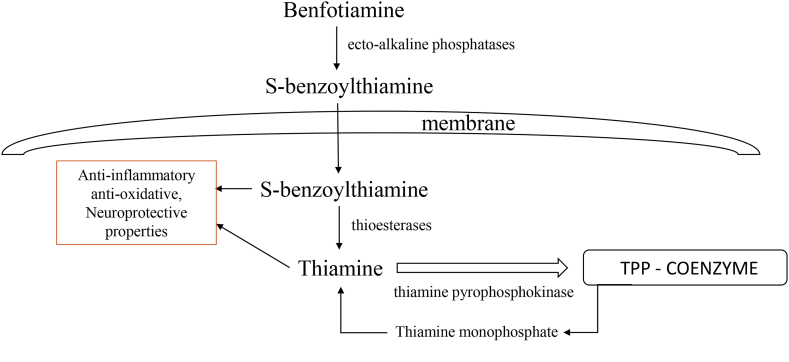
Study by Sambon et al. done on neuroblastoma cells reported that benfotiamine does not pass through the cell membrane in a considerable amount, which is in line with previous research and the fact that benfotiamine has a hydrophilic phosphate group. The authors propose that benfotiamine is dephosphorylated to S-benzoylthiamine by phosphatases present on the cell membrane or in the culture medium. S-benzoylthiamine then enters the cell and is converted to thiamine by thioesterases. A portion of thiamine is further phosphorylated by thiamine pyrophosphokinase to ThDP (Fig. 3) [64]. The authors of this study propose that protective and antioxidative effects of benfotiamine are mediated by thiamine or its metabolites, because benfotiamine, sulbutiamine and higher concentrations of thiamine all protected the cells from oxidative stress. However, when investigating the effect of benfotiamine and thiamine on Nrf2/ARE pathway, master regulator of cellular antioxidative response, Tapias et al. found that benfotiamine and its metabolites activate this pathway [21]. However, this is not true for thiamine, suggesting that antioxidative effects of benfotiamine are not exerted through thiamine, or that potentially there is another mechanism involved.
Fig. 3.
The metabolic pathways of benfotiamine. Benfotiamine is dephosphorylated to S-benzoylthiamine by phosphatases present on the cell membrane. S-benzoylthiamine then enters the cell and is converted to thiamine by thioesterases. A portion of thiamine is further phosphorylated by thiamine pyrophosphokinase to TPP that serves as coenzyme in glycolysis, Krebs cycle and pentose phosphate cycle. Thiamine and benfotiamine metabolites show anti-inflammatory, antioxidative and neuroprotective effects.
2.6. Neuroprotective effects of benfotiamine
Neuroprotective effects of benfotiamine were first observed when it was used in the treatment of neuropathies associated with diabetes [[65], [66], [67]]. Patients that received benfotiamine had a significant improvement in the neuropathy score when compared to placebo [67]. Furthermore, benfotiamine was shown to be effective in treating patients with alcoholic polyneuropathy [68]. This was further investigated in 2013, when Manzardo et al. conducted a double-blind, randomized placebo-controlled clinical trial investigating benfotiamine efficacy in treating alcohol dependence. Participants received 600 mg of benfotiamine or placebo, once a day for 24 weeks. Even though alcohol consumption decreased for both groups, the reduction was significantly greater in benfotiamine treated women, leading the authors to conclude that benfotiamine could be examined as an effective adjuvant therapy in treating alcoholism [62]. However, no follow-up clinical trial was conducted afterwards.
Several neurodegenerative diseases have been associated with diminished activity of thiamine-dependent enzymes, including Alzheimerˈs disease (AD), Parkinsonˈs disease, Huntington's disease, Wernicke–Korsakoff syndrome, and others [69]. In AD patients, the reductions in thiamine-dependent processes have been correlated with clinical symptoms of dementia [70]. However, treatment with thiamine hydrochloride did not lead to improvement in symptoms of AD [71]. The first study that linked benfotiamine to treatment of AD was published in 2010 [20]. The authors showed that chronic (8 week) treatment of benfotiamine improved spatial memory, and reduced amyloid plaque and phosphorylated tau levels in amyloid precursor protein (APP)/presenilin-1 transgenic mice. These effects were specifically attributed to benfotiamine, considering that neither fursultiamine nor high doses of thiamine produced a similar result. The authors found that benfotiamine enhanced phosphorylation of glycogen synthase kinase (GSK), which leads to the suppression of its activity, and they proposed that this is the mechanism through which benfotiamine exerts its protective effects in the AD model, seeing that GSK has been involved in tau phosphorylation [72] and the production and accumulation of β-amyloid in APP overexpressing mice [20]. Subsequently, other studies also found that benfotiamine inhibits GSK [22,73,74]. This mechanism was further examined in in vitro model, where it was shown that benfotiamine suppresses β-amyloid production in HEK cells, while also enhancing the ratio of phosphorylated GSK/GSK, thus inhibiting its activity [73]. In streptozotocin-induced model of AD benfotiamine reduced cognitive deficit, following the increase in ThDP concentrations in hippocampus and entorhinal cortex, brain regions important for memory. These effects were contributed to increase in energy metabolism, insulin signaling pathway and suppression of GSK3α/β and ERK 1/2 activity. Additionally, benfotiamine increased the expression of glutamate subunit 2B (GluN2B) of NMDA-type receptors in the hippocampus and entorhinal cortex, important for long-term potentiation and memory formation [22]. In a transgenic mouse model of tauopathy, benfotiamine also showed neuroprotective effects, increasing lifespan, improving behavioral deficits, decreasing generation of neurofibrillary tangles and preventing death of motor neurons. Furthermore, benfotiamine decreased inflammation, oxidative stress and mitochondrial dysfunction. When investigating the underlying mechanism, the authors did not find changes in GSK phosphorylation, but showed that benfotiamine exerts its effects by activating Nrf2, master regulator of oxidative stress response, to translocate to the nucleus and activate transcription of antioxidative genes [21]. Besides the effect on cognitive function, benfotiamine suppressed changes in hypothalamic insulin signaling in a rat model of AD, which resulted in improvement of the metabolic profile [23].
These promising results in animal studies led to clinical trials of benfotiamine treatment to investigate whether it could be beneficial for patients with AD. In 2016, Pan et al. conducted a small, uncontrolled clinical study on five patients with mild to moderate AD. They found that daily supplementation with 300 mg of benfotiamine during 18 months had improved the cognitive abilities of patients, independently of brain amyloid accumulation [24]. In 2020, Gibson et al. performed double-blind early phase II randomized placebo controlled clinical trial to assess whether benfotiamine is safe, efficacious and feasible for patients with mild cognitive impairment or mild dementia. Patients were treated with 300 mg of benfotiamine or placebo, twice a day for 12 months. Benfotiamine treatment was safe and effective in raising thiamine concentrations in the periphery. Importantly, benfotiamine was effective in suppressing cognitive decline, as measured by Alzheimerˈs disease assessment scale and clinical dementia rating. It also reduced increases in advanced glycation end products [25]. These encouraging results warrant a larger clinical trial to confirm these findings.
Other than treatment for AD, benfotiamine has been shown to be effective in reducing stress induced behavioral and molecular pathologies in animal models [[74], [75], [76]]. Specifically, Markova et al. showed that benfotiamine improved cognition in a mouse model of predator stress and forced swimming and these changes were connected to its effect on GSK-3β activity [74]. Another study showed that benfotiamine prevents stress-impaired adult hippocampal neurogenesis, as well as stress-induced bodyweight loss, anxiety-like behavior and oxidative stress in mice [75]. Additionally, it was shown that benfotiamine prevented ultrasound-induced aggression in a mouse model, while also ameliorating changes in glutamate subunit A1 and A2 and decreasing oxidative stress [76]. Results of these animal studies suggest that benfotiamine could be explored as a neuroprotective agent in various neurological and psychiatric diseases.
2.7. Benfotiamine in the treatment of diabetic complications
Diabetes may be regarded as a thiamine deficient state, specifically because of the important role of ThDP as a cofactor for crucial enzymes in intracellular glucose metabolism that is amplified in this disease [77]. Lower blood thiamine levels, along with increased renal clearance of thiamine was reported in patients with type 1 and type 2 diabetes [78,79]. Because thiamine is water-soluble and cannot be stored in the body, increasing the daily amount of thiamine intake could be beneficial for diabetic patients and enhance their glucose metabolism. In a randomized, double-blind, placebo-controlled pilot clinical trial thiamine was given orally to 24 patients with type 2 diabetes mellitus for 1 month and was found to decrease fasting blood glucose levels and leptin concentrations [80]. Another small clinical trial was done on patients with gestational diabetes where it was shown that thiamine supplementation for 6 weeks decreased levels of certain markers of inflammation and oxidative stress, such as serum C-reactive protein, gene expression of TNF-α and plasma MDA levels [81]. However, these studies were not followed by large-scale clinical trials which could unambiguously estimate whether thiamine should be given as a treatment for diabetic patients.
As a lipid soluble analogue of thiamine, benfotiamine has been extensively studied in the context of diabetic complications. In 2003, Hammes et al. found that benfotiamine inhibits three major pathways implicated in hyperglycemic vascular damage - the hexosamine pathway, the advanced glycation end product (AGE) formation pathway and the diacylglycerol (DAG)–protein kinase C (PKC) pathway. Benfotiamine managed this by activating transketolase which converts glyceraldehyde-3-phosphate (GA3P) and fructose-6-phosphate (F6P) into pentose-5-phosphates and other sugars, thus decreasing the levels of GA3P and F6P which activate the three detrimental pathways in hyperglycemia. Benfotiamine also decreased activity of NF-κB and prevented experimental diabetic retinopathy [8]. Further, positive effects of benfotiamine treatment have been shown in several animal models of diabetes. Sanchez-Ramirez et al. found that benfotiamine decreases inflammatory and neuropathic pain in a rat model of diabetes [82]. Another study showed that benfotiamine reduces cerebral oxidative stress associated with diabetes in mice. Interestingly, this effect was not in connection with AGE pathway, considering AGE levels remained the same after benfotiamine treatment [9]. Benfotiamine also improved motor nerve conduction velocity, decreased formation of neural imidazole-type AGEs and prevented the formation of glycoxidation products in diabetic rats [83].
Several smaller size clinical trials with benfotiamine treatment in diabetic patients have been performed and reported inconclusive results. In a double blind, placebo-controlled, phase III study, benfotiamine was effective in lowering the neuropathy symptom score, even though total symptom score was not changed after treatment. The improvement was more pronounced in the group that received higher dose of benfotiamine – 600 mg [65]. However, two studies performed in 2012 found no improvement after shorter (12 weeks) and longer (24 months) benfotiamine treatment. Specifically, benfotiamine did not reduce plasma or urinary AGEs or plasma markers of inflammation and endothelial dysfunction after treatment for 12 weeks [84]. It was also shown that 24 month long treatment did not result in changes in peripheral nerve function or biomarkers of inflammation [85]. Recently, a randomized double-blind, placebo-controlled clinical trial has been approved in order to assess the efficacy of benfotiamine on morphometric, neurophysiological and clinical measures in patients with type 2 diabetes, in the period of 12 months [61]. Thus, investigations of benfotiamine for diabetic complications are still under way.
2.8. Benfotiamine and neuroinflammation
Thiamine deficiency is characterized by neuroinflammation, showing a link between thiamine levels and inflammatory conditions in the CNS. Activation of microglia and increased production of pro-inflammatory cytokines have been found in various brain regions [30]. Not suprisingly, anti-inflammatory actions of benfotiamine have been shown in diverse in vivo and in vitro model systems. Experiments in cell culture have been helpful in elucidating possible mechanisms of benfotiamine effects and anti-inflammatory effects have been shown in cultures of macrophages [14,15], microglia [18], dendritic cells [86]. In macrophages benfotiamine reduced the expression of proinflammatory cytokines and chemokines, induced with lipopolysacharide (LPS) treatment. Inflammation markers - inducible nitric oxide synthase and cyclooxygenase 2, as well as their products NO and PGE2 were also reduced under benfotiamine treatment [14]. These effects were contributed to benfotiamine's action on transcription factors NF-κB and Egr-1, arachidonic acid pathway, protein kinase C and members of MAPK signaling cascade – p38, ERK1/2, SAPK/JNK [14,15].
In addition, our group found that benfotiamine mitigated morphological alterations of BV-2 cells, induced by LPS stimulation. Microglia are extremely mobile cells that quickly change their shape in order to exert their functions, and rearrangement of the cytoskeletal proteins, specifically actin microfilaments, is crucial in this process [87]. We found that activation of microglial cells with LPS led to the expansion of the cell body, reorganization of the actin cytoskeleton and formation of the membrane bound stress fibers, while benfotiamine reduced these changes, returned the cell body size to the level of control cells and promoted LPS-induced actin stress fibers to relocate throughout the cell [18]. We found similar effect of benfotiamine on morphological changes in dendritic cells which also undergo enlargement of the cell body and reorganization of the actin cytoskeleton after LPS stimulation, however benfotiamine reversed these changes, returning them to control levels [86]. Muller-Krebs et al., also found that benfotiamine restores reorganization of actin cytoskeleton of podocytes that were exposed to high glucose concentrations [88]. Cytoskeleton reorganization in activation of microglia has been attributed to activation of MAPK signaling cascade, specifically ERK1/2, p38 and JNKs were found to be partly responsible for morphological activation of microglia [89]. JNKs catalyze phoshporylation of proteins in the cytoskeleton [89], and induce actin expression by actvating c-Jun [90]. Indeed, in our study on microglial cells we found that benfotiamine reduces activation of ERK1/2 and JNK, that being the probable route by which benfotiamine exerts its effects on actin cytoskeleton reorganization.
In both cell culture systems that we studied, microglial and dendritic cells, morphological alterations were connected with proinflammatory signaling [18,86]. In dendritic cells, reorganzation of the cytoskeleton is important for expression of MHC class II and CD86 molecules, both necessary for antigen presentation [91]. Furthermore, a decrease in morphological alterations in dendritic cells that were treated with benfotiamine was paralleled with a decrease in expression of MHC II and CD86, leading to a conclusion that benfotiamine could reduce antigen presentation in dendritic cells [86]. In microglial cells benfotiamine reduced the formation of membrane rufflings that are crucial for chemotaxis and activation of microglial cells [18,92]. These changes in morphology towards a more quiescent phenotype were accompanied by a reduction in production of proinflammatory cytokines and mediators. Benfotiamine reduced production of NO, by inhibiting LPS-induced expression of inducible nitric oxide synthase (iNOS). Benfotiamine also inhibited gene and protein expression of cyclooxygenase 2 (COX-2) that was induced by LPS stimulation. Furthermore, benfotiamine suppressed production of master proinflammatory cytokines TNF-α and IL-6, while enhancing the production of anti-inflammatory cytokine IL-10. Analysis of signaling pathways that underlie microglial activation and production of proinflammatory mediators showed that benfotiamine suppressed activation of ERK1/2 and JNK members of MAPK signaling pathway, as well as protein kinase B/Akt. Using specific inhibitors for ERK1/2, JNK and Akt pathways showed that JNK1/2 and Akt signaling was responsible for benfotiamine mediated suppression of NO, while ERK1/2, JNK and Akt signaling was at the bottom of production of proinflammatory cytokines. Importantly, benfotiamine decreased activation of NF-κB transcription factor, a master regulator of inflammatory actions in microglia [18,93]. Similarly, in dendritic cells benfotiamine suppressed the release of proinflammatory cytokines TNF-α and IL-1β and prevented translocation of NF-κB to the nucleus [86].
Benfotiamine has also shown strong anti-inflammatory actions in various animal models. It has been shown that it is beneficial in treating inflammatory and neuropathic pain in rats [82]. Furthermore, in a rat model of myocardial infarction, benfotiamine reduced expression of inflammatory markers such as protein kinase C, metalloproteinase-9 and NF-κB, together with markers of oxidative stress and apoptosis [94]. Yadav et al. have shown that benfotiamine reduces ocular inflammation in endotoxin-induced uveitis in rats. In particular, benfotiamine reduced the number of infiltrating leukocytes, levels of proinflammatory cytokines and chemokines and protein concentrations in aqueous humor of rats with uveitis. In ciliary body and retinal wall, levels of proinflammatory enzymes iNOS and COX-2 and activation of PKC and NF-κB were also reduced in groups that underwent benfotiamine treatment [13]. Benfotiamine has also proven to be neuroprotective and beneficial in suppresing inflammation in mouse model of tauopathy [21]. Specifically, it reduced the expression of iNOS, COX-2, TNF-α, IL-1β and NF-κB p65 in transgenic mice that overexpress tau gene that has P301S mutation that leads to frontotemporal dementia in men. Additionally, benfotiamine mitigated oxidative stress and mitochondrial dysfunction and activated Nrf2/ARE pathway in this model, showing its potential to be used in treatment for diseases with tau pathology [21].
2.9. Benfotiamine in oxidative stress pathology
When thiamine is deficient in the body, other than an increase in inflammatory processes, there is also an increase in production of reactive oxygen species (ROS) and oxidative stress [30]. A wide range of markers, such as heme oxygenase (HO-1), superoxide dismutase (SOD), intercellular adhesion molecule 1 (ICAM-1), endothelial nitric oxide synthase (eNOS), inducible nitric oxide synthase (iNOS), ferritin and malondialdehyde were found to be increased in TD [26,69]. TD is also accompanied by increased apoptosis [30], lipid peroxydation and changes in the levels of antioxidant enzymes [26]. In addition, thiamine has been shown to reverse oxidative stress pathology in conditions that are not caused by TD [69]. For example, thiamine suppressed lipid peroxidation and enhanced glutathione reductase activity in experimental cardiac hypertrophy in rats [95]. Recently, Ma and colleagues showed that thiamine suppresses oxidative stress induced by high concentrate diet in goats [96]. Sambon and colleagues showed that thiamine, benfotiamine and sulbutiamine protected neuroblastoma cell line against paraquat and β-amyloid induced oxidative stress [64]. Their results demonstrate that antioxidative properties of these compounds are all related to thiamine (or its metabolite) and are not a result of direct ROS scavenging activity, but instead of an antioxidative signaling mechanism that could possibly be involved. A number of other studies done both in vivo and in vitro have also shown antioxidative properties of benfotiamine.
Our group has demonstrated that benfotiamine suppresses oxidative stress and upregulates antioxidative system in activated BV-2 microglial cells [19]. LPS activated microglial cells had upregulated levels of oxidative stress markers, such as NO, superoxide anion and malondialdehyde (MDA), while also upregulating antioxidative defense system – enzymes involved in antioxidative defense and glutathione, non-enzymatic antioxidant. Benfotiamine decreased production of NO, superoxide anion and MDA, and increased levels and activities of superoxide dismutase, catalase and glutathione. Thus, benfotiamine was shown to be effective in suppressing both inflammation and oxidative stress, two hallmarks of chronically activated microglia [19]. Similarly, benfotiamine suppressed ROS levels in LPS treated macrophages [14], as well as levels of protein-HNE adducts and lipid hydroperoxides [15]. Antioxidative effects of benfotiamine were also shown in three different kidney cell lines [12].
In addition, benfotiamine has shown antioxidative properties in various in vivo experiments. In streptozotocin – induced diabetes mellitus, benfotiamine alleviated oxidative stress in the brain and heart, without affecting AGE levels [9,97]. Benfotiamineˈs antioxidative effects were also shown in models of vascular endothelial dysfunction where it suppressed aortic superoxide anion generation and serum thiobarbituric acid reactive substances, formed as a byproduct of lipid peroxidation [11,16]. Chronic dietary treatment with benfotiamine was neuroprotective in transgenic mice with tauopathy, with benfotiamine suppressing oxidative damage [21]. Furthermore, the authors of this study proposed that benfotiamine and its metabolites are exerting antioxidative effects through stimulation of the Nuclear factor erythroid 2-related factor 2 (Nrf2)/antioxidant response element (ARE) pathway. Nrf2 is a transcription factor that regulates the expression of multiple antioxidative enzymes that are key in elimination of oxidative stress. When activated, Nrf2 dissociates from Kelch-like ECH-associated protein (Keap1) and translocates to the nucleus where it binds the ARE promoter and transcription of many antioxidative genes ensues [98]. In transgenic tauopathy model benfotiamine and its metabolites stimulated the expression of Nrf2/ARE dependent genes in the mouse brain of transgenic and wild type animals, but not Nrf2-deficient fibroblasts. Additionally, this study showed that benfotiamine and its metabolites directly affect Nrf2 stability by interacting with Keap1 and thus releasing Nrf2 to translocate to the nucleus and activate the transcription of antioxidative genes [21]. Another mechanism that could be at play here is the previously described effect of benfotiamine and thiamine on the activity of GSK-3β [20,22,73,74]. Even though Tapias et al. did not find changes in expression of phospho- GSK-3β, benfotiamine has been shown to enhance phosphorylation of GSK-3β both in vitro [73] and in vivo [20,22]. GSK-3β phosporylates Nrf2 which leads to its ubiquitination and subsequent degradation, thus when GSK-3β is inactived Nrf2 is upregulated and leads to higher expression of antioxidative enzymes [99]. Taken together, results of these studies indicate that there are multiple pathways through which benfotiamine could exert its antioxidative action.
3. Conclusions
Overall, while benfotiamine is converted to thiamine in the body, it has a different structure than thiamine and can have different effects on the body. Benfotiamine has demonstrated promising therapeutic potential in various disorders, including those related to high blood sugar and oxidative stress.
One of the key differences between thiamine and benfotiamine is their ability to prevent or reverse complications associated with high blood sugar levels. Benfotiamine has been shown to be more effective than thiamine in preventing diabetic complications, such as neuropathy and nephropathy. This is due, in part, to benfotiamine's ability to increase intracellular levels of thiamine pyrophosphate, a coenzyme involved in glucose metabolism.
Another difference between thiamine and benfotiamine is their ability to protect against oxidative stress-induced damage. Benfotiamine has been shown to be more effective than thiamine in reducing oxidative stress and preventing cell damage in various experimental models.
In conclusion, benfotiamine has shown beneficial effects in treatment of various disorders, most notably thiamine deficiency, diabetes, alcoholism and neurodegenerative diseases including Alzheimerˈs disease. These effects have been investigated in a plethora of in vitro and in vivo models. Importantly, results of clinical trials investigating benfotiamine in Alzheimerˈs disease, diabetes and alcoholism show promising results, but warrant further studies with larger number of patients.
Overall, benfotiamine is a promising therapeutic agent for improving neuronal function and protecting against inflammation and oxidative stress-induced cell death in the CNS. However, further research is needed to fully understand the mechanisms underlying these effects and to determine the optimal dosage and treatment regimen for various neurological disorders.
Funding statement
This study is supported by Ministry of Science, Technological Development and Innovations of the Republic of Serbia, Project No. 451-03-47/2023-01/200007.
Data availability
No data was used for the research described in the article.
CRediT authorship contribution statement
Iva Bozic: Conceptualization, Writing – original draft. Irena Lavrnja: Supervision, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Iva Bozic, Email: ivabo1@yahoo.com.
Irena Lavrnja, Email: irenam@ibiss.bg.ac.rs.
References
- 1.Bettendorff L. The chemistry, biochemistry and metabolism of thiamin (vitamin B1) B vitamins and folate: chemistry, analysis, function and effects. R Soc Chem. 2012:71–92. doi: 10.1039/9781849734714-00071. [DOI] [Google Scholar]
- 2.Chandrakumar A., Bhardwaj A., t Jong G.W. Review of thiamine deficiency disorders: Wernicke encephalopathy and Korsakoff psychosis. J. Basic Clin. Physiol. Pharmacol. 2018;30(2):153–162. doi: 10.1515/jbcpp-2018-0075. [DOI] [PubMed] [Google Scholar]
- 3.Sriram K., Manzanares W., Joseph K. Thiamine in nutrition therapy. Nutr. Clin. Pract. 2012;27(1):41–50. doi: 10.1177/0884533611426149. [DOI] [PubMed] [Google Scholar]
- 4.Volvert M.L., Seyen S., Piette M., Evrard B., Gangolf M., Plumier J.C., et al. Benfotiamine, a synthetic S-acyl thiamine derivative, has different mechanisms of action and a different pharmacological profile than lipid-soluble thiamine disulfide derivatives. BMC Pharmacol. 2008;8:10. doi: 10.1186/1471-2210-8-10. Pmc2435522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balakumar P., Rohilla A., Krishan P., Solairaj P., Thangathirupathi A. The multifaceted therapeutic potential of benfotiamine. Pharmacol. Res. 2010;61(6):482–488. doi: 10.1016/j.phrs.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Bitsch R., Wolf M., Möller J., Heuzeroth L., Grüneklee D. Bioavailability assessment of the lipophilic benfotiamine as compared to a water-soluble thiamin derivative. Ann. Nutr. Metab. 1991;35(5):292–296. doi: 10.1159/000177659. [DOI] [PubMed] [Google Scholar]
- 7.Xie F., Cheng Z., Li S., Liu X., Guo X., Yu P., et al. Pharmacokinetic study of benfotiamine and the bioavailability assessment compared to thiamine hydrochloride. J. Clin. Pharmacol. 2014;54(6):688–695. doi: 10.1002/jcph.261. [DOI] [PubMed] [Google Scholar]
- 8.Hammes H.P., Du X., Edelstein D., Taguchi T., Matsumura T., Ju Q., et al. Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat Med. 2003;9(3):294–299. doi: 10.1038/nm834. [DOI] [PubMed] [Google Scholar]
- 9.Wu S., Ren J. Benfotiamine alleviates diabetes-induced cerebral oxidative damage independent of advanced glycation end-product, tissue factor and TNF-alpha. Neurosci. Lett. 2006;394(2):158–162. doi: 10.1016/j.neulet.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 10.Raj V., Ojha S., Howarth F.C., Belur P.D., Subramanya S.B. Therapeutic potential of benfotiamine and its molecular targets. Eur. Rev. Med. Pharmacol. Sci. 2018;22(10):3261–3273. doi: 10.26355/eurrev_201805_15089. [DOI] [PubMed] [Google Scholar]
- 11.Balakumar P., Sharma R., Singh M. Benfotiamine attenuates nicotine and uric acid-induced vascular endothelial dysfunction in the rat. Pharmacol. Res. 2008;58(5–6):356–363. doi: 10.1016/j.phrs.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 12.Schmid U., Stopper H., Heidland A., Schupp N. Benfotiamine exhibits direct antioxidative capacity and prevents induction of DNA damage in vitro. Diabetes Metab Res Rev. 2008;24(5):371–377. doi: 10.1002/dmrr.860. [DOI] [PubMed] [Google Scholar]
- 13.Yadav U.C., Subramanyam S., Ramana K.V. Prevention of endotoxin-induced uveitis in rats by benfotiamine, a lipophilic analogue of vitamin B1. Invest. Ophthalmol. Vis. Sci. 2009;50(5):2276–2282. doi: 10.1167/iovs.08-2816. Pmc2685466Nihms115303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yadav U.C., Kalariya N.M., Srivastava S.K., Ramana K.V. Protective role of benfotiamine, a fat-soluble vitamin B1 analogue, in lipopolysaccharide-induced cytotoxic signals in murine macrophages. Free Radic. Biol. Med. 2010;48(10):1423–1434. doi: 10.1016/j.freeradbiomed.2010.02.031. Pmc2856750Nihms182894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shoeb M., Ramana K.V. Anti-inflammatory effects of benfotiamine are mediated through the regulation of the arachidonic acid pathway in macrophages. Free Radic. Biol. Med. 2012;52(1):182–190. doi: 10.1016/j.freeradbiomed.2011.10.444. Pmc3249497Nihms334342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verma S., Reddy K., Balakumar P. The defensive effect of benfotiamine in sodium arsenite-induced experimental vascular endothelial dysfunction. Biol. Trace Elem. Res. 2010;137(1):96–109. doi: 10.1007/s12011-009-8567-7. [DOI] [PubMed] [Google Scholar]
- 17.Harisa G.I. Benfotiamine enhances antioxidant defenses and protects against cisplatin-induced DNA damage in nephrotoxic rats. J. Biochem. Mol. Toxicol. 2013;27(8):398–405. doi: 10.1002/jbt.21501. [DOI] [PubMed] [Google Scholar]
- 18.Bozic I., Savic D., Laketa D., Bjelobaba I., Milenkovic I., Pekovic S., et al. Benfotiamine attenuates inflammatory response in LPS stimulated BV-2 microglia. PLoS One. 2015;10(2) doi: 10.1371/journal.pone.0118372. Pmc4335016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bozic I., Savic D., Stevanovic I., Pekovic S., Nedeljkovic N., Lavrnja I. Benfotiamine upregulates antioxidative system in activated BV-2 microglia cells. Front. Cell. Neurosci. 2015;9:351. doi: 10.3389/fncel.2015.00351. Pmc4559599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan X., Gong N., Zhao J., Yu Z., Gu F., Chen J., et al. Powerful beneficial effects of benfotiamine on cognitive impairment and beta-amyloid deposition in amyloid precursor protein/presenilin-1 transgenic mice. Brain. 2010;133(Pt 5):1342–1351. doi: 10.1093/brain/awq069. [DOI] [PubMed] [Google Scholar]
- 21.Tapias V., Jainuddin S., Ahuja M., Stack C., Elipenahli C., Vignisse J., et al. Benfotiamine treatment activates the Nrf2/ARE pathway and is neuroprotective in a transgenic mouse model of tauopathy. Hum. Mol. Genet. 2018;27(16):2874–2892. doi: 10.1093/hmg/ddy201. Pmc6077804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moraes R.C.M., Singulani M.P., Gonçalves A.C., Portari G.V., Torrão A.D.S. Oral benfotiamine reverts cognitive deficit and increase thiamine diphosphate levels in the brain of a rat model of neurodegeneration. Exp. Gerontol. 2020;141 doi: 10.1016/j.exger.2020.111097. [DOI] [PubMed] [Google Scholar]
- 23.Moraes R.C.M., Lima G.C.A., Cardinali C., Gonçalves A.C., Portari G.V., Guerra-Shinohara E.M., et al. Benfotiamine protects against hypothalamic dysfunction in a STZ-induced model of neurodegeneration in rats. Life Sci. 2022;306 doi: 10.1016/j.lfs.2022.120841. [DOI] [PubMed] [Google Scholar]
- 24.Pan X., Chen Z., Fei G., Pan S., Bao W., Ren S., et al. Long-term cognitive improvement after benfotiamine administration in patients with Alzheimer's disease. Neurosci. Bull. 2016;32(6):591–596. doi: 10.1007/s12264-016-0067-0. Pmc5567484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibson G.E., Luchsinger J.A., Cirio R., Chen H., Franchino-Elder J., Hirsch J.A., et al. Benfotiamine and cognitive decline in Alzheimer's disease: results of a randomized placebo-controlled phase IIa clinical trial. J Alzheimers Dis. 2020;78(3):989–1010. doi: 10.3233/jad-200896. Pmc7880246Nihms1666597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu D., Ke Z., Luo J. Thiamine deficiency and neurodegeneration: the interplay among oxidative stress, endoplasmic reticulum stress, and autophagy. Mol. Neurobiol. 2017;54(7):5440–5448. doi: 10.1007/s12035-016-0079-9. Pmc5337452Nihms814798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bazurto J.V., Farley K.R., Downs D.M. An unexpected route to an essential cofactor: Escherichia coli relies on threonine for thiamine biosynthesis. mBio. 2016;7(1) doi: 10.1128/mBio.01840-15. Pmc4725005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao R., Goldman I.D. Folate and thiamine transporters mediated by facilitative carriers (SLC19A1-3 and SLC46A1) and folate receptors. Mol Aspects Med. 2013;34(2–3):373–385. doi: 10.1016/j.mam.2012.07.006. Pmc3831518Nihms399003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tylicki A., Łotowski Z., Siemieniuk M., Ratkiewicz A. Thiamine and selected thiamine antivitamins - biological activity and methods of synthesis. Biosci. Rep. 2018;38(1) doi: 10.1042/bsr20171148. Pmc6435462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hazell A.S., Faim S., Wertheimer G., Silva V.R., Marques C.S. The impact of oxidative stress in thiamine deficiency: a multifactorial targeting issue. Neurochem. Int. 2013;62(5):796–802. doi: 10.1016/j.neuint.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 31.Sambon M., Wins P., Bettendorff L. Neuroprotective effects of thiamine and precursors with higher bioavailability: focus on benfotiamine and dibenzoylthiamine. Int. J. Mol. Sci. 2021;22(11) doi: 10.3390/ijms22115418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manzetti S., Zhang J., van der Spoel D. Thiamin function, metabolism, uptake, and transport. Biochemistry. 2014;53(5):821–835. doi: 10.1021/bi401618y. [DOI] [PubMed] [Google Scholar]
- 33.Kennedy D.O. B vitamins and the brain: mechanisms, dose and efficacy--A review. Nutrients. 2016;8(2):68. doi: 10.3390/nu8020068. Pmc4772032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wiley K.D., Gupta M. StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022. Vitamin B1 Thiamine Deficiency. StatPearls. Treasure Island (FL) [Google Scholar]
- 35.Spinas E., Saggini A., Kritas S.K., Cerulli G., Caraffa A., Antinolfi P., et al. Crosstalk between vitamin B and immunity. J. Biol. Regul. Homeost. Agents. 2015;29(2):283–288. [PubMed] [Google Scholar]
- 36.Zaringhalam J., Akbari A., Zali A., Manaheji H., Nazemian V., Shadnoush M., et al. Long-term treatment by vitamin B(1) and reduction of serum proinflammatory cytokines, hyperalgesia, and paw edema in adjuvant-induced arthritis. Basic Clin. Neurosci. 2016;7(4):331–340. doi: 10.15412/j.bcn.03070406. Pmc5102562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Portari G.V., Ovidio P.P., Deminice R., Jordão A.A., Jr. Protective effect of treatment with thiamine or benfotiamine on liver oxidative damage in rat model of acute ethanol intoxication. Life Sci. 2016;162:21–24. doi: 10.1016/j.lfs.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 38.Costa N.A., Pereira A.G., Sugizaki C.S.A., Vieira N.M., Garcia L.R., de Paiva S.A.R., et al. Insights into thiamine supplementation in patients with septic shock. Front. Med. 2021;8 doi: 10.3389/fmed.2021.805199. Pmc8832096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gibson G.E., Blass J.P. Thiamine-dependent processes and treatment strategies in neurodegeneration. Antioxid Redox Signal. 2007;9(10):1605–1619. doi: 10.1089/ars.2007.1766. [DOI] [PubMed] [Google Scholar]
- 40.Ke Z.J., Gibson G.E. Selective response of various brain cell types during neurodegeneration induced by mild impairment of oxidative metabolism. Neurochem. Int. 2004;45(2–3):361–369. doi: 10.1016/j.neuint.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 41.Hazell A.S., Butterworth R.F. Update of cell damage mechanisms in thiamine deficiency: focus on oxidative stress, excitotoxicity and inflammation. Alcohol Alcohol. 2009;44(2):141–147. doi: 10.1093/alcalc/agn120. [DOI] [PubMed] [Google Scholar]
- 42.Polegato B.F., Pereira A.G., Azevedo P.S., Costa N.A., Zornoff L.A.M., Paiva S.A.R., et al. Role of thiamin in health and disease. Nutr. Clin. Pract. 2019;34(4):558–564. doi: 10.1002/ncp.10234. [DOI] [PubMed] [Google Scholar]
- 43.Bâ A. Metabolic and structural role of thiamine in nervous tissues. Cell. Mol. Neurobiol. 2008;28(7):923–931. doi: 10.1007/s10571-008-9297-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chatterton B.J., Nunes P.T., Savage L.M. The effect of chronic ethanol exposure and thiamine deficiency on myelin-related genes in the cortex and the cerebellum. Alcohol Clin. Exp. Res. 2020;44(12):2481–2493. doi: 10.1111/acer.14484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu Q., Liu H., Sang S. Thiamine deficiency contributes to synapse and neural circuit defects. 2018;51(1):35. doi: 10.1186/s40659-018-0184-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bâ A., N'Douba V., D'Almeida M.A., Seri B.V. Effects of maternal thiamine deficiencies on the pyramidal and granule cells of the hippocampus of rat pups. Acta Neurobiol. Exp. 2005;65(4):387–398. doi: 10.55782/ane-2005-1567. [DOI] [PubMed] [Google Scholar]
- 47.Mikkelsen K., Stojanovska L., Prakash M., Apostolopoulos V. The effects of vitamin B on the immune/cytokine network and their involvement in depression. Maturitas. 2017;96:58–71. doi: 10.1016/j.maturitas.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 48.Bettendorff L. Elsevier; 2020. Chapter 10: Thiamine. Present Knowledge in Nutrition. [Google Scholar]
- 49.Dhir S., Tarasenko M., Napoli E., Giulivi C. Neurological, psychiatric, and biochemical aspects of thiamine deficiency in children and adults. Front Psychiatry. 2019;10:207. doi: 10.3389/fpsyt.2019.00207. Pmc6459027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bettendorff L., Mastrogiacomo F., Kish S.J., Grisar T. Thiamine, thiamine phosphates, and their metabolizing enzymes in human brain. J. Neurochem. 1996;66(1):250–258. doi: 10.1046/j.1471-4159.1996.66010250.x. [DOI] [PubMed] [Google Scholar]
- 51.O'Keeffe S.T., Tormey W.P., Glasgow R., Lavan J.N. Thiamine deficiency in hospitalized elderly patients. Gerontology. 1994;40(1):18–24. doi: 10.1159/000213570. [DOI] [PubMed] [Google Scholar]
- 52.Nichols H.K., Basu T.K. Thiamin status of the elderly: dietary intake and thiamin pyrophosphate response. J. Am. Coll. Nutr. 1994;13(1):57–61. doi: 10.1080/07315724.1994.10718372. [DOI] [PubMed] [Google Scholar]
- 53.Page G.L., Laight D., Cummings M.H. Thiamine deficiency in diabetes mellitus and the impact of thiamine replacement on glucose metabolism and vascular disease. Int. J. Clin. Pract. 2011;65(6):684–690. doi: 10.1111/j.1742-1241.2011.02680.x. [DOI] [PubMed] [Google Scholar]
- 54.Butterworth R.F., Gaudreau C., Vincelette J., Bourgault A.M., Lamothe F., Nutini A.M. Thiamine deficiency and Wernicke's encephalopathy in AIDS. Metab. Brain Dis. 1991;6(4):207–212. doi: 10.1007/bf00996920. [DOI] [PubMed] [Google Scholar]
- 55.Müri R.M., Von Overbeck J., Furrer J., Ballmer P.E. Thiamin deficiency in HIV-positive patients: evaluation by erythrocyte transketolase activity and thiamin pyrophosphate effect. Clin Nutr. 1999;18(6):375–378. doi: 10.1016/s0261-5614(99)80019-3. [DOI] [PubMed] [Google Scholar]
- 56.Zastre J.A., Sweet R.L., Hanberry B.S., Ye S. Linking vitamin B1 with cancer cell metabolism. Cancer Metab. 2013;1(1):16. doi: 10.1186/2049-3002-1-16. Pmc4178204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fujiwara M., Watanabe H., Matsui K. “ALLITHIAMINE” a newly found derivative of vitamin B1: I. Discovery of allithiamine. The Journal of Biochemistry. 1954;41(1):29–39. doi: 10.1093/oxfordjournals.jbchem.a126421. [DOI] [Google Scholar]
- 58.Starling-Soares B., Carrera-Bastos P., Bettendorff L. Role of the synthetic B1 vitamin sulbutiamine on health. J Nutr Metab. 2020;2020 doi: 10.1155/2020/9349063. PMC7210561 Scientifique-FNRS, Belgium. The authors declare that they have no conflicts of interest. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Loew D. Pharmacokinetics of thiamine derivatives especially of benfotiamine. Int J Clin Pharmacol Ther. 1996;34(2):47–50. [PubMed] [Google Scholar]
- 60.Sheng L., Cao W., Lin P., Chen W., Xu H., Zhong C., et al. vol. 15. 2021. pp. 1101–1110. (Safety, Tolerability and Pharmacokinetics of Single and Multiple Ascending Doses of Benfotiamine in Healthy Subjects). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bönhof G.J., Sipola G., Strom A., Herder C., Strassburger K., Knebel B., et al. BOND study: a randomised double-blind, placebo-controlled trial over 12 months to assess the effects of benfotiamine on morphometric, neurophysiological and clinical measures in patients with type 2 diabetes with symptomatic polyneuropathy. BMJ Open. 2022;12(2) doi: 10.1136/bmjopen-2021-057142. Pmc8814806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Manzardo A.M., He J., Poje A., Penick E.C., Campbell J., Butler M.G. Double-blind, randomized placebo-controlled clinical trial of benfotiamine for severe alcohol dependence. Drug Alcohol Depend. 2013;133(2):562–570. doi: 10.1016/j.drugalcdep.2013.07.035. Pmc3818307Nihms514760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Netzel M., Ziems M., Jung K.H., Noll E., Borsch C., Bitsch I. Effect of high-dosed thiamine hydrochloride and S-benzoyl-thiamine-O-monophosphate on thiamine-status after chronic ethanol administration. Biofactors. 2000;11(1–2):111–113. doi: 10.1002/biof.5520110133. [DOI] [PubMed] [Google Scholar]
- 64.Sambon M., Napp A., Demelenne A., Vignisse J., Wins P., Fillet M., et al. Thiamine and benfotiamine protect neuroblastoma cells against paraquat and β-amyloid toxicity by a coenzyme-independent mechanism. Heliyon. 2019;5(5) doi: 10.1016/j.heliyon.2019.e01710. Pmc6520661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stracke H., Gaus W., Achenbach U., Federlin K., Bretzel R.G. Benfotiamine in diabetic polyneuropathy (BENDIP): results of a randomised, double blind, placebo-controlled clinical study. Exp. Clin. Endocrinol. Diabetes. 2008;116(10):600–605. doi: 10.1055/s-2008-1065351. [DOI] [PubMed] [Google Scholar]
- 66.Winkler G., Pál B., Nagybéganyi E., Ory I., Porochnavec M., Kempler P. Effectiveness of different benfotiamine dosage regimens in the treatment of painful diabetic neuropathy. Arzneimittelforschung. 1999;49(3):220–224. doi: 10.1055/s-0031-1300405. [DOI] [PubMed] [Google Scholar]
- 67.Haupt E., Ledermann H., Köpcke W. Benfotiamine in the treatment of diabetic polyneuropathy--a three-week randomized, controlled pilot study (BEDIP study) Int J Clin Pharmacol Ther. 2005;43(2):71–77. doi: 10.5414/cpp43071. [DOI] [PubMed] [Google Scholar]
- 68.Woelk H., Lehrl S., Bitsch R., Köpcke W. Benfotiamine in treatment of alcoholic polyneuropathy: an 8-week randomized controlled study (BAP I Study) Alcohol Alcohol. 1998;33(6):631–638. doi: 10.1093/alcalc/33.6.631. [DOI] [PubMed] [Google Scholar]
- 69.Gibson G.E., Zhang H. Interactions of oxidative stress with thiamine homeostasis promote neurodegeneration. Neurochem. Int. 2002;40(6):493–504. doi: 10.1016/s0197-0186(01)00120-6. [DOI] [PubMed] [Google Scholar]
- 70.Gibson G.E., Hirsch J.A., Cirio R.T., Jordan B.D., Fonzetti P., Elder J. Abnormal thiamine-dependent processes in Alzheimer's Disease. Lessons from diabetes. Mol. Cell. Neurosci. 2013;55:17–25. doi: 10.1016/j.mcn.2012.09.001. Pmc3609887Nihms431588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nolan K.A., Black R.S., Sheu K.F., Langberg J., Blass J.P. A trial of thiamine in Alzheimer's disease. Arch. Neurol. 1991;48(1):81–83. doi: 10.1001/archneur.1991.00530130093025. [DOI] [PubMed] [Google Scholar]
- 72.Takashima A. GSK-3 is essential in the pathogenesis of Alzheimer's disease. J Alzheimers Dis. 2006;9(3 Suppl):309–317. doi: 10.3233/jad-2006-9s335. [DOI] [PubMed] [Google Scholar]
- 73.Sun X.J., Zhao L., Zhao N., Pan X.L., Fei G.Q., Jin L.R., et al. Benfotiamine prevents increased β-amyloid production in HEK cells induced by high glucose. Neurosci. Bull. 2012;28(5):561–566. doi: 10.1007/s12264-012-1264-0. Pmc5561916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Markova N., Bazhenova N., Anthony D.C., Vignisse J., Svistunov A., Lesch K.P., et al. Thiamine and benfotiamine improve cognition and ameliorate GSK-3β-associated stress-induced behaviours in mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2017;75:148–156. doi: 10.1016/j.pnpbp.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 75.Vignisse J., Sambon M., Gorlova A., Pavlov D., Caron N., Malgrange B., et al. Thiamine and benfotiamine prevent stress-induced suppression of hippocampal neurogenesis in mice exposed to predation without affecting brain thiamine diphosphate levels. Mol. Cell. Neurosci. 2017;82:126–136. doi: 10.1016/j.mcn.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 76.Gorlova A., Pavlov D., Anthony D.C., Ponomarev E.D., Sambon M., Proshin A., et al. Thiamine and benfotiamine counteract ultrasound-induced aggression, normalize AMPA receptor expression and plasticity markers, and reduce oxidative stress in mice. Neuropharmacology. 2019;156 doi: 10.1016/j.neuropharm.2019.02.025. [DOI] [PubMed] [Google Scholar]
- 77.Beltramo E., Berrone E., Tarallo S., Porta M. Effects of thiamine and benfotiamine on intracellular glucose metabolism and relevance in the prevention of diabetic complications. Acta Diabetol. 2008;45(3):131–141. doi: 10.1007/s00592-008-0042-y. [DOI] [PubMed] [Google Scholar]
- 78.Thornalley P.J., Babaei-Jadidi R., Al Ali H., Rabbani N., Antonysunil A., Larkin J., et al. High prevalence of low plasma thiamine concentration in diabetes linked to a marker of vascular disease. Diabetologia. 2007;50(10):2164–2170. doi: 10.1007/s00125-007-0771-4. Pmc1998885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Al-Daghri N.M., Alharbi M., Wani K., Abd-Alrahman S.H., Sheshah E., Alokail M.S. Biochemical changes correlated with blood thiamine and its phosphate esters levels in patients with diabetes type 1 (DMT1) Int. J. Clin. Exp. Pathol. 2015;8(10):13483–13488. Pmc4680506. [PMC free article] [PubMed] [Google Scholar]
- 80.González-Ortiz M., Martínez-Abundis E., Robles-Cervantes J.A., Ramírez-Ramírez V., Ramos-Zavala M.G. Effect of thiamine administration on metabolic profile, cytokines and inflammatory markers in drug-naïve patients with type 2 diabetes. Eur. J. Nutr. 2011;50(2):145–149. doi: 10.1007/s00394-010-0123-x. [DOI] [PubMed] [Google Scholar]
- 81.Amirani E., Aghadavod E., Shafabakhsh R., Asemi Z., Tabassi Z., Panahandeh I., et al. Anti-inflammatory and antioxidative effects of thiamin supplements in patients with gestational diabetes mellitus. J. Matern. Fetal Neonatal Med. 2022;35(11):2085–2090. doi: 10.1080/14767058.2020.1779212. [DOI] [PubMed] [Google Scholar]
- 82.Sánchez-Ramírez G.M., Caram-Salas N.L., Rocha-González H.I., Vidal-Cantú G.C., Medina-Santillán R., Reyes-García G., et al. Benfotiamine relieves inflammatory and neuropathic pain in rats. Eur. J. Pharmacol. 2006;530(1–2):48–53. doi: 10.1016/j.ejphar.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 83.Stracke H., Hammes H.P., Werkmann D., Mavrakis K., Bitsch I., Netzel M., et al. Efficacy of benfotiamine versus thiamine on function and glycation products of peripheral nerves in diabetic rats. Exp. Clin. Endocrinol. Diabetes. 2001;109(6):330–336. doi: 10.1055/s-2001-17399. [DOI] [PubMed] [Google Scholar]
- 84.Alkhalaf A., Kleefstra N., Groenier K.H., Bilo H.J., Gans R.O., Heeringa P., et al. Effect of benfotiamine on advanced glycation endproducts and markers of endothelial dysfunction and inflammation in diabetic nephropathy. PLoS One. 2012;7(7) doi: 10.1371/journal.pone.0040427. Pmc3391239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fraser D.A., Diep L.M., Hovden I.A., Nilsen K.B., Sveen K.A., Seljeflot I., et al. The effects of long-term oral benfotiamine supplementation on peripheral nerve function and inflammatory markers in patients with type 1 diabetes: a 24-month, double-blind, randomized, placebo-controlled trial. Diabetes Care. 2012;35(5):1095–1097. doi: 10.2337/dc11-1895. Pmc3329837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Djedovic N., Božić I., Đ Miljković, Lavrnja I. Benfotiamine reduces dendritic cell inflammatory potency. Endocr., Metab. Immune Disord.: Drug Targets. 2021;21(7):1344–1351. doi: 10.2174/1871530320999200905114135. [DOI] [PubMed] [Google Scholar]
- 87.Franco-Bocanegra D.K., McAuley C., Nicoll J.A.R., Boche D. Molecular mechanisms of microglial motility: changes in ageing and Alzheimer's disease. Cells. 2019;8(6) doi: 10.3390/cells8060639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Müller-Krebs S., Nissle K., Tsobaneli J., Zeier M., Kihm L.P., Kender Z., et al. Effect of benfotiamine in podocyte damage induced by peritoneal dialysis fluid. Front. Med. 2015;2:10. doi: 10.3389/fmed.2015.00010. Pmc4354337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Waetzig V., Czeloth K., Hidding U., Mielke K., Kanzow M., Brecht S., et al. c-Jun N-terminal kinases (JNKs) mediate pro-inflammatory actions of microglia. Glia. 2005;50(3):235–246. doi: 10.1002/glia.20173. [DOI] [PubMed] [Google Scholar]
- 90.Bishopric N.H., Jayasena V., Webster K.A. Positive regulation of the skeletal alpha-actin gene by Fos and Jun in cardiac myocytes. J. Biol. Chem. 1992;267(35):25535–25540. [PubMed] [Google Scholar]
- 91.Jaksits S., Bauer W., Kriehuber E., Zeyda M., Stulnig T.M., Stingl G., et al. Lipid raft-associated GTPase signaling controls morphology and CD8+ T cell stimulatory capacity of human dendritic cells. J. Immunol. 2004;173(3):1628–1639. doi: 10.4049/jimmunol.173.3.1628. [DOI] [PubMed] [Google Scholar]
- 92.Ohsawa K., Imai Y., Kanazawa H., Sasaki Y., Kohsaka S. Involvement of Iba1 in membrane ruffling and phagocytosis of macrophages/microglia. J. Cell Sci. 2000;113(Pt 17):3073–3084. doi: 10.1242/jcs.113.17.3073. [DOI] [PubMed] [Google Scholar]
- 93.Milosevic K., Stevanovic I., Bozic I.D., Milosevic A., Janjic M.M., Laketa D., et al. Agmatine mitigates inflammation-related oxidative stress in BV-2 cells by inducing a pre-adaptive response. Int. J. Mol. Sci. 2022;23(7) doi: 10.3390/ijms23073561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ahmed L.A., Hassan O.F., Galal O., Mansour D.F., El-Khatib A. Beneficial effects of benfotiamine, a NADPH oxidase inhibitor, in isoproterenol-induced myocardial infarction in rats. PLoS One. 2020;15(5) doi: 10.1371/journal.pone.0232413. Pmc7209119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tolstykh O.I., Khmelevskiĭ Iu V. [The role of alpha-tocopherol and thiamine in the correction of lipid peroxidation in compensatory myocardial hypertrophy] Vopr. Pitan. 1991;(3):38–42. [PubMed] [Google Scholar]
- 96.Ma Y.E.M., Wang C., Li Z., Zhang H., He B., Zhao X., Zhang Z., Wang H. Thiamine supplementation alleviates lipopolysaccharide-triggered adaptive inflammatory response and modulates energy state via suppression of NFκB/p38 MAPK/AMPK signaling in rumen epithelial cells of goats. Antioxidants. 2022;11(10) doi: 10.3390/antiox11102048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ceylan-Isik A.F., Wu S., Li Q., Li S.Y., Ren J. High-dose benfotiamine rescues cardiomyocyte contractile dysfunction in streptozotocin-induced diabetes mellitus. J. Appl. Physiol. 2006;100(1):150–156. doi: 10.1152/japplphysiol.00988.2005. 1985. [DOI] [PubMed] [Google Scholar]
- 98.Ngo V., Duennwald M.L. Nrf2 and oxidative stress: a general overview of mechanisms and implications in human disease. Antioxidants. 2022;11(12) doi: 10.3390/antiox11122345. Pmc9774434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cuadrado A., Kügler S., Lastres-Becker I. Pharmacological targeting of GSK-3 and NRF2 provides neuroprotection in a preclinical model of tauopathy. Redox Biol. 2018;14:522–534. doi: 10.1016/j.redox.2017.10.010. Pmc5681345. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.