ABSTRACT
B-lymphocytes recognize antigen via B-cell antigen receptors (BCRs). This binding induces signaling, leading to B-cell activation, proliferation and differentiation. Early events of BCR signaling include reorganization of actin and membrane spreading, which facilitates increased antigen gathering. We have previously shown that the gap junction protein connexin43 (Cx43; also known as GJA1) is phosphorylated upon BCR signaling, and its carboxyl tail (CT) is important for BCR-mediated spreading. Here, specific serine residues in the Cx43 CT that are phosphorylated following BCR stimulation were identified. A chimeric protein containing the extracellular and transmembrane domains of CD8 fused to the Cx43 CT was sufficient to support cell spreading. Cx43 CT truncations showed that the region between amino acids 246-307 is necessary for B-cell spreading. Site-specific serine-to-alanine mutations (S255A, S262A, S279A and S282A) resulted in differential effects on both BCR signaling and BCR-mediated spreading. These serine residues can serve as potential binding sites for actin remodeling mediators and/or BCR signaling effectors; therefore, our results may reflect unique roles for each of these serines in terms of linking the Cx43 CT to actin remodeling.
Keywords: B-lymphocyte, B-cells, Connexin43, Cx43, Cytoskeletal-dependent processes, B-cell spreading
Summary: Mutation of specific serine residues of connexin43 affects B-cell spreading, suggesting that this protein can be considered as a regulator of B-cell responses.
INTRODUCTION
B-cells play a major role in the adaptive immune system by producing antibodies in response to infection. Signaling induced cytoskeletal-dependent processes are required for both B-cell development and the immune response. Processes such as B-cell antigen receptor (BCR) mobility and membrane spreading that are essential steps for immune synapse formation, an early event leading to B-cell activation, are examples of processes that depend on cytoskeletal rearrangements (Batista et al., 2001; Fleire et al., 2006; Treanor et al., 2010; Freeman et al., 2015; Bolger-Munro et al., 2019 preprint). Cross-linking of the BCR results in activation of multiple signaling pathways, including those involving tyrosine kinases and serine kinases (Campbell and Sefton, 1990; Gold et al., 1990; Kurosaki et al., 1994; DeFranco, 1997; Kurosaki, 1997; Reth and Wienands, 1997; Tamir and Cambier, 1998), which lead to responses such as cytoskeleton remodeling. Cytoskeleton-dependent processes and their importance for B-cell activation have been reviewed over the years (Penninger and Crabtree, 1999; Harwood and Batista, 2011; Song et al., 2014; Tolar, 2017; Li et al., 2019a). Previous work has shown that the gap junction protein connexin43 (Cx43; also known as GJA1), influences cytoskeletal-dependent processes in B-cells (Machtaler et al., 2011, 2014; Falk et al., 2014) and in other cell types (Bates et al., 2007; Behrens et al., 2010; Cina et al., 2009; Crespin et al., 2010; Dbouk et al., 2009; Kameritsch et al., 2015; Matsuuchi and Naus, 2013; Moorby, 2000; Xu, 2006). The Cx43 carboxyl tail (CT) is important for inhibiting fibroblast mobility (Moorby, 2000), reducing glioma invasion (Bates et al., 2007; Crespin et al., 2010), regulating neural migration during brain development (Cina et al., 2009) and filopodia formation (Kameritsch et al., 2015). We have previously shown that Cx43 is phosphorylated after BCR crosslinking, and that the Cx43 CT (amino acids 246-382) is important for B-cell adhesion and spreading (Machtaler et al., 2011). Furthermore, mutagenesis studies indicated two potential phosphorylation sites, Y247 and Y265, that are important for BCR-mediated cell spreading (Falk et al., 2014). However, these previous studies did not examine many additional residues within the Cx43 CT that could also be important.
Signal transduction downstream of the BCR is influenced by multiple adaptor proteins in terms of the signaling precision, and in positive and negative feedback effects (Kelly and Chan, 2000; Kurosaki, 2002; Leo and Schraven, 2001). Adaptor proteins, including B-cell linker protein (BLNK), B-cell adaptor for phosphoinositide 3-kinase (BCAP) and GRB2-associated binding protein (GAB) contain protein–protein interaction domains, including but not limited to SRC homology 2 (SH2)-binding (phosphorylated tyrosine) and SH3-binding (proline-rich) domains, which are important for regulation of BCR signaling (Kurosaki, 2002). The CT of Cx43 also contains SH2- and SH3-binding domains, which could function similarly to an adaptor protein, influencing BCR signaling and supporting spreading by recruiting protein interactors via these domains.
In this report, we expand on our previous studies, which focused on the tyrosine residues in the Cx43 CT (Falk et al., 2014). We investigated the serine residues as other potentially important sites. Using a mutational approach, individual or multiple serines of the Cx43 CT were changed to alanine and expressed in B-cell lines. The effects of these mutations on BCR signaling and BCR-induced membrane spreading were assessed using a total phospho-tyrosine profile and cell spreading assay, respectively. These studies have helped identify key regions of the Cx43 CT that act in a channel-independent manner to influence cytoskeletal rearrangements in B-cells.
RESULTS
Identification of Cx43 CT amino acids phosphorylated after BCR stimulation
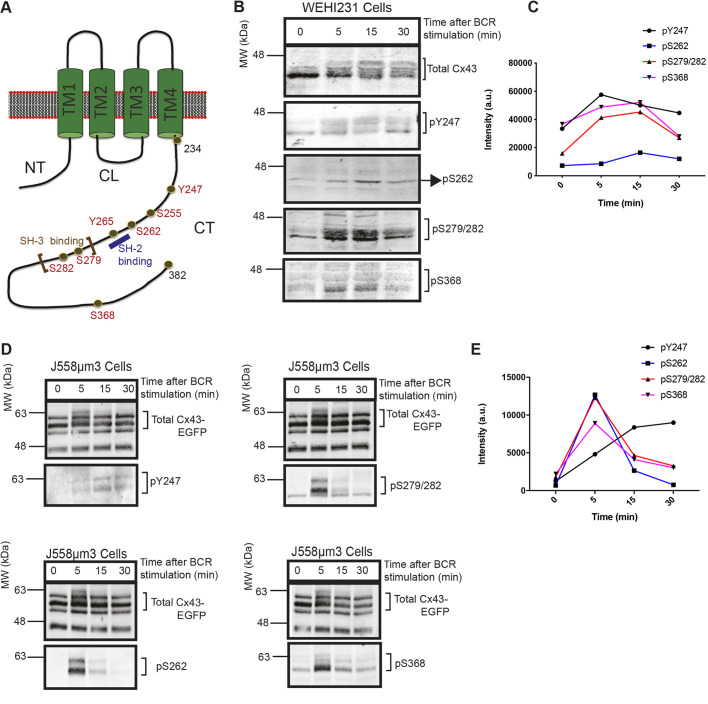
The CT of the rodent Cx43 contains ∼150 amino acids (aa). Within the CT, there are sites of post-translational modification, such as phosphorylation sites, as well as protein–protein interaction sites (Chen et al., 2013; Kanemitsu et al., 1997; Lampe et al., 2000; Lau et al., 1996; Solan and Lampe, 2008; Swenson et al., 1990; Warn-Cramer et al., 1996) (reviewed by Giepmans, 2004; Solan and Lampe, 2005, 2009) (Fig. 1A). We previously showed that Rattus norvegicus Cx43 (hereafter referred as rat Cx43) exhibited a phosphatase-sensitive shift in molecular weight (MW) upon BCR and chemokine (CXCL12) receptor signaling in both WEHI231 (endogenous Cx43 expression) and J558μm3 (transduced Cx43 expression) B-cell lines (Machtaler et al., 2011, 2014) (Fig. S1A,B). Using phospho-specific antibodies developed by the Lampe lab (Solan and Lampe, 2008), phosphorylation of Cx43 upon BCR stimulation was found, similar to the modifications found in other cell types (Lampe and Lau, 2004; Solan and Lampe, 2009). In response to BCR signaling, the serine residues S262, S279, S282 and S368, and the tyrosine at position Y247 of the Cx43 CT were phosphorylated in WEHI231 (Fig. 1B,C) as well as in J558μm3 cells that were transduced and expressed WTCx43-EGFP (Fig. 1D,E). These modifications of the Cx43 CT in B-lymphocytes are consistent with well-characterized modifications in other cell types (Solan and Lampe, 2005, 2009).
Fig. 1.
Serine (S) and tyrosine (Y) residues of the carboxyl tail of Cx43 are phosphorylated following BCR stimulation. (A) Schematic of a Cx43 monomer at the plasma membrane showing the amino terminus (NT), the four transmembrane domains (TM1-4), the cytoplasmic loop (CL) and the carboxyl tail (CT). The residues depicted in red are some of the potential phosphorylation sites. An SH-2 binding domain is shown in blue; Y265 and SH3-binding domain (proline-rich region) is indicated with brown brackets from Proline (P) 274 to P284. (B) Western blots showing the phosphorylation of CT residues of endogenous Cx43, Y247, S262, S278, S282 and S368, in response to BCR stimulation by anti-IgM crosslinking for the times indicated, in the immature mouse B lymphoma cell line WEHI231. Top panel shows total Cx43 and lower panels are individual blots detecting pY247, pS262, pS279/282 and pS368. (C) Sum of band densities presented in B were quantified using ImageJ and plotted using GraphPad Prism7. (D) Western blots showing the phosphorylation of CT residues of transduced Cx43-EGFP, Y247, S262, S278, S282 and S368 in response to BCR stimulation by anti-IgM crosslinking for the times indicated, in the BCR-positive mouse B plasmacytoma cell line J558μm3. (E) Sum of band densities presented in D were quantified using ImageJ and plotted using GraphPad Prisim7. All data shown are representative of two independent experiments.
The CT of Cx43 supports BCR-mediated cell spreading independent of the rest of the protein
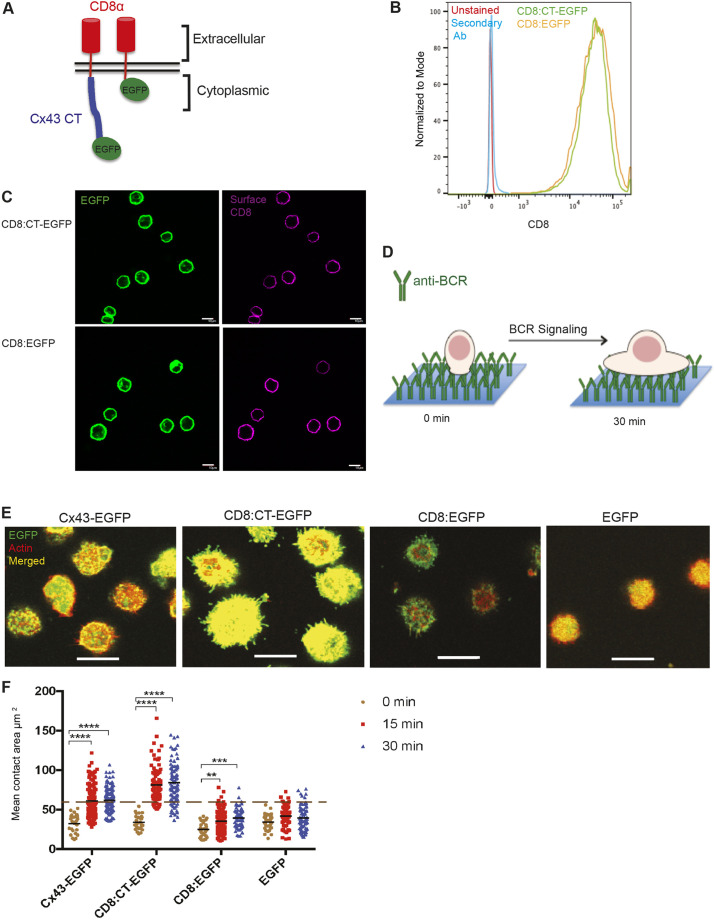
Chimeric proteins have been used successfully before to study the importance of certain domains (Hanissian and Geha, 1997; Jabara et al., 1998; Morio et al., 1999; Sutherland et al., 1999). In order to determine the importance of Cx43 CT in the absence of other domains of the protein, a DNA construct encoding a chimeric protein was made, comprising human CD8α extracellular and transmembrane domains fused in-frame to the CT of rat Cx43, and expressed in B-cell lines (Fig. 2A). First, using the WT Cx43-expressing WEHI231 B lymphoma cells, the chimeric protein CD8α:CT-EGFP was characterized in terms of phosphorylation in response to BCR signaling (Fig. S2A,B). Then, the proteins were expressed in the Cx43-negative B-cell line J558µm3 and characterized, followed by testing for their ability to spread in response to BCR signaling. For characterization, the cell surface localization of the chimeric CD8α:CT-EGFP and the control CD8α:EGFP protein was examined using flow cytometry. Cells were visualized with the OKT8 antibody, which recognizes the extracellular domain of CD8α and a fluorophore-conjugated secondary antibody (Fig. 2B). Cellular localization of the chimeric protein was also assessed by immunofluorescence (IF) using confocal microscopy. The chimeric protein showed a similar localization pattern to that of WT Cx43, as a fluorescent rim at the surface of non-permeabilized cells (Fig. 2C). These findings suggest that the chimeric protein was successfully expressed and trafficked to the cell surface similar to the WT Cx43 protein. Continuing with the characterization of the chimeric protein, we tested if the CD8α:Cx43 CT-EGFP is modified in the same way as WT Cx43. We have previously shown that there is a shift in MW of Cx43 upon BCR stimulation, as a result of phosphorylation. CD8α:Cx43 CT-EGFP-expressing WEHI231 cells also showed a shift in MW, as well as site-specific phosphorylation after BCR stimulation (Fig. S2A,B). This occurred only when cells were stimulated with anti-BCR (anti-IgM) and not with anti-CD8α and anti-IgG (Fig. S2A). Additionally, the CT domain in the chimeric protein showed phosphorylation following BCR stimulation (Fig. S2C) on the same residue as the WT Cx43 (Fig. 1B,D). The chimeric protein containing Cx43 CT and the control protein was successfully expressed in J558µm3 and showed similar localization and modification to the WT protein. Next, the ability of the chimeric protein in supporting B-cell spreading was tested.
Fig. 2.
Expression, characterization and effects of CD8α:Cx43 CT-EGFP in J558µm3 and WEHI231 cells. (A) Schematic of the chimeric proteins at the plasma membrane. Human CD8α (containing the extracellular, transmembrane with the first 4 aa of the cytoplasmic domain) fused to the CT of rat Cx43 (aa 242-382) and to an EGFP tag is illustrated on the left. The control protein composed of human CD8α, as explained above, fused to EGFP is shown on the right. (B) Cell surface localization of CD8α:Cx43 CT-EGFP (labeled CD8:CT-EGFP) and CD8α:EGFP (CD8:EGFP) in J558μm3 cells by flow cytometry. Cells expressing the two proteins were stained with OKT8 mouse antibody (anti-human CD8α) followed by an anti-mouse Alexa Fluor 647 secondary antibody. Unstained and secondary antibody alone cells were used as controls. (C) Cellular localization of CD8:Cx43 CT-EGFP and CD8:EGFP in transduced J558μm3 cells by IF to detect the EGFP and surface CD8 (staining without permeabilization) using confocal microscopy. Optical sections from the middle of the cells were obtained using a laser-scanning confocal microscope. (D) Schematic of the spreading assay. Glass coverslips were coated with anti-BCR antibody overnight. Cells were incubated on the coverslips for different times then fixed and stained for IF. BCR signaling results in B-cell spreading. (E) Representative images of the spread area of J558μm3 cells expressing CD8:Cx43 CT-EGFP and controls WTCx43-EGFP, CD8:EGFP and EGFP at 15 min time point. Number of cells was >100 for each time point and the majority of the cells had a similar phenotype to those shown. Cells were stained for actin using Rhodamine-Phalloidin. Cx43=green; CD8 when indicated=green; actin=red; merged=yellow. Images were obtained at the interface of the coverslips and the cells (contact site=spreading area) using a laser-scanning confocal microscope. The green color for cells expressing EGFP was enhanced slightly because of dimmer levels of EGFP alone on AP2 vector compared with NAP2 vector (also applies for same conditions in other figures). (F) Quantification of the spreading area at time points after BCR stimulation using Image Pro Plus 6.2 analysis software (Media Cybernetics, Rockville, MD, USA). The dashed line indicates average spreading area for cells expressing WT Cx43 at 15 min, when maximum spreading area is usually reached under these conditions. The significance of the data was identified based on two-way ANOVA and a Tukey's test: **P≤0.01; ***P≤0.001; ****P<0.0001. The data shown are representative of more than three independent experiments. Scale bars: 10 μm.
BCR signaling initiated by antigen encounter induces cytoskeletal remodeling that results in membrane spreading across antigen-presenting cells. B-cell spreading is considered an important early step in activation since it allows for enhanced antigen gathering and amplified BCR signaling to overcome the threshold for B-cell activation (Fleire et al., 2006; Harwood and Batista, 2011). We assessed the effects of the CD8α:Cx43 CT-EGFP chimera in supporting BCR-mediated cell spreading using a well-established cell-spreading assay (Fig. 2D), and the spreading area was imaged using a laser-scanning confocal microscope (Santos-Argumedo et al., 1997; Lin et al., 2008; Machtaler et al., 2011, 2014; Falk et al., 2014). J558μm3 B-cells expressing CD8α:Cx43 CT-EGFP spread in response to BCR signaling to a similar extent as cells expressing WT Cx43, whereas cells expressing CD8α:EGFP or EGFP alone did not spread so far (Fig. 2E,F). Using flow cytometry, the sizes of the cells were compared to ensure that differences in the cell spreading area were not influenced by different cell sizes (Fig. S2E). Moreover, surface BCR and EGFP levels of the cells were checked using flow cytometry (Fig. S2F). The chimeric protein allowed us to show that the CT of Cx43 supports B-cell spreading independent of the other domains of the Cx43 protein.
The region of the CT of Cx43 from amino acids 246 to 307 is important for supporting BCR-mediated cell spreading
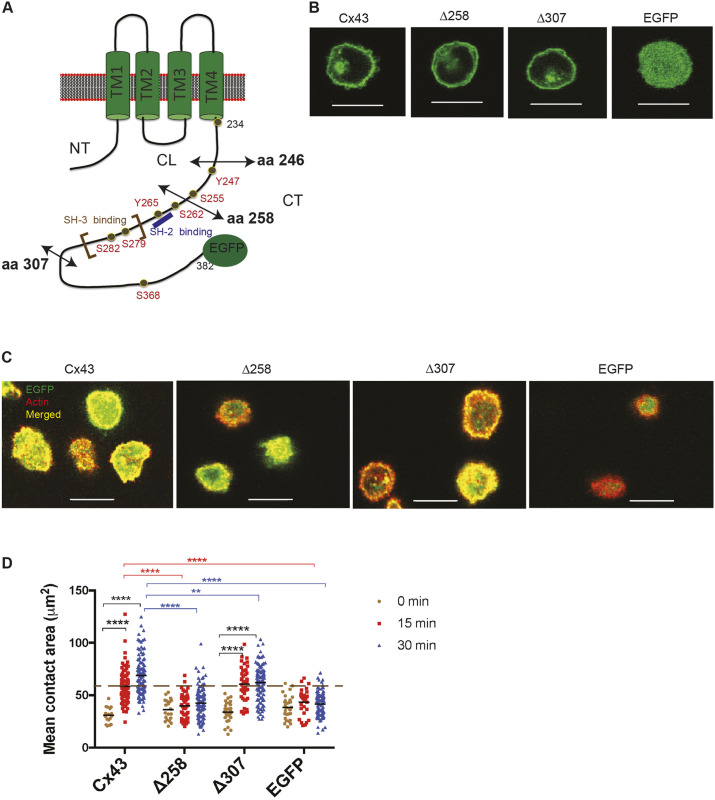
As we showed in Fig. 2, Cx43 CT can support BCR-mediated cell spreading independent of the other domains of the Cx43 protein. We also showed previously that the region of the Cx43 CT that includes aa 246-382 is necessary for spreading (Machtaler et al., 2011). It is important to better define the specific region within the Cx43 CT that is critical for this effect; therefore, we generated additional deletions in the CT (Fig. 3A) to identify more precisely the region important for cell spreading. Expression vectors containing Cx43 cDNA encoding deletions at aa 258 (Δ258 Cx43-EGFP) and aa 307 (Δ307 Cx43-EGFP) were expressed in J558μm3 cells and protein expression was determined by monitoring the EGFP using fluorescence microscopy (Fig. 3B). Localization of the truncated Cx43 proteins was similar to that of the WT Cx43-EGFP. We asked how the Cx43 CT deletions would affect BCR-mediated spreading of J558μm3 cells. As controls, the size of the transduced cells was compared using flow cytometry to ensure that any difference in cell spreading was not influenced by initial differences in cell size (Fig. S3A). Similarly, cell surface levels of the BCR and EGFP levels were compared using flow cytometry to ensure similar protein amounts in cells expressing different ΔCT Cx43-EGFP constructs (Fig. S3B). The cell spreading assay was performed as described (Fig. 2F). Cells expressing Δ258 Cx43-EGFP did not increase in spreading area on anti-BCR-coated coverslips. However, cells expressing Δ307 Cx43-EGFP showed a significant increase in the spreading area over the same time course, with spreading areas similar to the cells expressing WT Cx43-EGFP (Fig. 3C,D). Since we previously showed the importance of Y247 for B-cell spreading (Falk et al., 2014), we suggest that the region of the CT between aa 246-307 is important for B-cell spreading. However, the residues before aa 258 cannot support B-cell spreading in the absence of the rest of the CT. Crosslinking of BCR results in activation of multiple tyrosine kinases (PTK), which are essential for regulation of BCR signaling effectors. Evidence also suggests that adaptor proteins are involved in fine-tuning the activity of PTKs (Brdicka et al., 2000; Takeuchi et al., 2000; Yamadori et al., 1999; reviewed by Kurosaki, 2002). Since we hypothesize that Cx43 CT functions as an adaptor protein downstream of BCR signaling, we studied how different Cx43 CT truncations affect the phospho-tyrosine (P-Tyr) profile of the cells. Results showed that total P-Tyr level was less when Δ246, Δ258 and Δ307 CT Cx43-EGFP were expressed; but was similar to the WT protein by 15 min with Δ307 CT Cx43-EGFP (Fig. S3C-F). This shows the importance of Cx43 CT for proper BCR signal transduction. Next, we focused on some of the key residues of the CT contained in the region between aa 246 and aa 307.
Fig. 3.
The carboxyl tail of Cx43 between aa 246 and aa 307 is important for BCR-mediated cell spreading. (A) Schematic showing the location of different deletions within the CT of Cx43 (double-headed arrows). Each of these deletions removes potential modification sites and protein–protein interaction sites. (B) Localization of Δ258 Cx43-EGFP and Δ307 Cx43-EGFP expressed in J558μm3. Cells were plated on anti-BCR-coated stimulatory coverslips for the cell spreading assay. At the zero time point, optical sections were obtained from the middle of the cells. In B and C, all the proteins are EGFP tagged. (C) Representative images of membrane spreading of J558μm3 cells expressing Δ258 Cx43-EGFP and Δ307 Cx43-EGFP at 15 min. Images were obtained using a laser scanning confocal microscope at the contact site of the cells and stimulatory (anti-BCR coated) coverslips at 0, 15 and 30 min time points. Number of cells was >100 for each time point. Cells were stained for actin using Rhodamine-Phalloidin. Cx43=green, actin=red, merged=yellow. (D) Quantification of the spreading area at different time points as described for Fig. 2. Dashed line indicates average spreading area for cells expressing WT Cx43 at 15 min for comparison. The significance of the data was identified based on two-way ANOVA and a Tukey's test: **P≤0.01; ****P<0.0001. Data shown are representative of three independent experiments. Scale bars: 10 μm.
Serines 279 and 282 of the Cx43 CT are important for BCR-mediated cell spreading
Expression vectors containing Cx43 cDNA encoding individual serine (S) to alanine (A) point mutations (S255A, S262A, S279A and S282A) with EGFP fused in-frame to the Cx43 CT were expressed in J558μm3 cells and protein expression was checked by monitoring EGFP using fluorescence microscopy (Fig. 4A). As controls, the size of the transduced cells and cell surface levels of the BCR and Cx43-EGFP were compared using flow cytometry (Fig. S4A,B). Cells expressing the S279A or S282A Cx43-EGFP mutants showed a reduced spreading response and did not show a significant increase in cell area when plated on anti-BCR-coated coverslips. However, cells expressing the S255A or S262A Cx43-EGFP mutants showed a significant increase in the spreading area in response to BCR signaling, similar to the cells expressing WT Cx43-EGFP (Fig. 4B,C). The 3D reconstruction of the optical slices showed an inclusive localization of Cx43-EGFP and the mutants throughout the cells. However, a small aggregate of the WT Cx43 and the mutants that did not affect cell spreading (S255A and S262A) was noted at the contact site of cells with anti-BCR-coated coverslips (Fig. S4C). The localization of Cx43 and mutants was studied at the 15 min time point, where usually the maximum spreading area is reached. The WT Cx43 and mutants colocalize with the actin at the cell-stimulatory coverslip contact site (Fig. 4B and Fig. S4D). In Fig. 4B, this is shown by the yellow color at the contact site emerging from Cx43 (green) and actin (red). Additionally, the colocalization of Cx43 and the mutants with actin is visualized in Fig. S4D. Detecting a difference in colocalization of WT Cx43 versus different mutants with actin requires more advanced techniques such as super-resolution microscopy which is beyond the scope of this paper. We looked at how the P-Tyr profile of the cells changed in the presence of these mutations. The results showed that cells expressing S255A or S262A had similar P-Tyr levels to WT Cx43 whereas S279A- and S282A-expressing cells had lower P-Tyr levels (Fig. S4E-I). These results suggest that S279 and S282 of the Cx43 CT are important for BCR-mediated cell spreading, while S255 and S262 had no effect. The next step was to see how a combination of these mutations would affect B-cell spreading.
Fig. 4.
Single serine mutations of the Cx43 carboxyl tail have different effects on BCR-mediated cell spreading. (A) Localization of WT Cx43, the S255A, S262A, S279A, S282 Cx43-EGFP mutants, and EGFP expressed in J558μm3 cells. Optical sections obtained from the middle of the cells showing EGFP at the cell periphery and intracellular staining that we have previously shown was the ER. (B) Membrane spreading of J558μm3 cells expressing WT, each one of the S255A, S262A, S279A, S282A Cx43-EGFP mutants, and EGFP. Images were obtained using a laser scanning confocal microscope at the contact site of the cells and stimulatory coverslips at 0 and 15 min time points. Number of cells was >100 for each time point. Cells were stained for actin using Rhodamine-Phalloidin. Cx43-EGFP=green, actin=red, merged=yellow. (C) Quantification of the spreading area at the different time points as described for Fig. 2. Dashed line represents average spreading area for cells expressing WT Cx43 at 15 min. The significance of the data was identified based on two-way ANOVA and a Tukey's test: **P≤0.01; ****P<0.0001. Data shown are representative of three independent experiments. Scale bars: 10 μm.
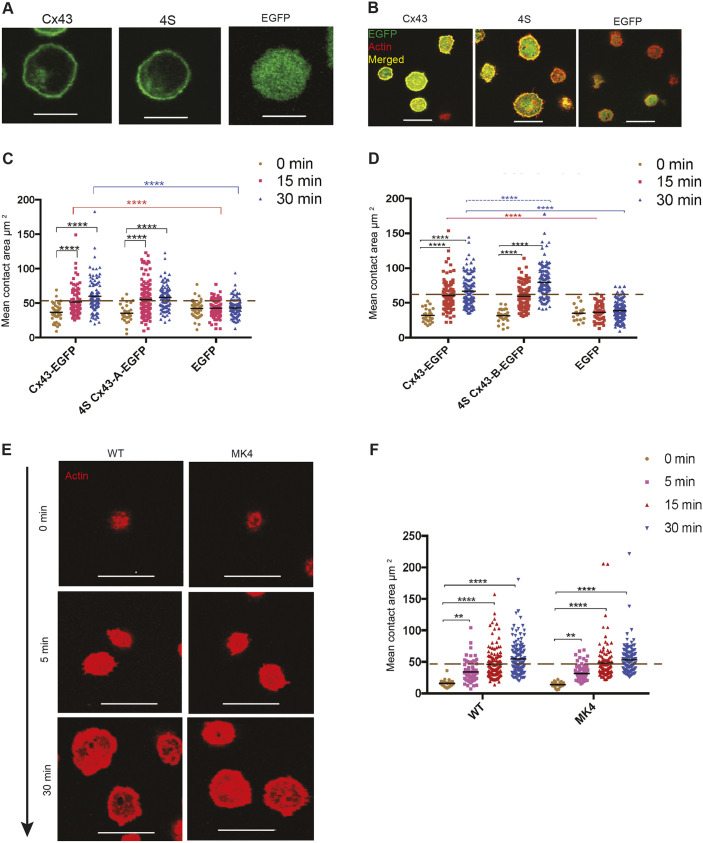
Quadruple serine-to-alanine mutations at amino acids 255, 262, 279 and 282 of Cx43 CT did not interfere with BCR-mediated cell spreading
After investigating the effect of the individual serine mutants, we examined the effect of mutating all four serine residues to alanine (4S), which has been shown to affect gap junction function (Johnstone et al., 2012). An expression vector containing Cx43 cDNA encoding S255/262/279/282A Cx43-EGFP (4S) was transduced in two different cell populations of J558μm3 cells (population A and B) and expression was monitored by assessing EGFP using fluorescence microscopy (Fig. 5A). Two populations were made to confirm the results. Using flow cytometry, the size of the transduced cells and the surface levels of BCR and Cx43 were compared (Fig. S5A,B). Both cell populations expressing 4S Cx43-EGFP (populations A and B) responded with a significant increase in spreading area over the standard time course, similar to WT Cx43-EGFP (Fig. 5B-D). A 3D reconstruction of the optical slices showed an inclusive localization of Cx43-EGFP and the 4S mutant throughout the cells, with a small aggregate of the WT and 4S Cx43 at the contact site (Fig. S5C) similar to that observed for S255A and S262A in Fig. S4C. This localization was studied at the 15 min time point. The WT Cx43 and 4S mutant colocalize with the actin at contact site (Fig. 5B and Fig. S5D). In Fig. 5B, the colocalization is shown by the yellow color emerging from Cx43 (green) and actin (red). Furthermore, the colocalization of Cx43 and the 4S mutant with actin by means of showing individual channels is visualized in Fig. S5D. The P-Tyr profile for cells expressing the 4S Cx43 was also similar to the WT protein (Fig. S5C,D). Primary splenic B-cells were obtained from MK4 mice (Johnstone et al., 2012; a generous gift from the lab of Christian Naus, UBC), which have the 4S serine-to-alanine mutation in Cx43 expressed in all cell types. These splenic B-cells were tested for spreading in response to BCR signaling as described and showed a significant increase in the spreading area similar to splenic B-cells from mice expressing WT Cx43 (Fig. 5E,F). To better understand how Cx43 mutants affect actin dynamics underlying the spreading response, colocalization of Cx43 with HS1, an actin regulatory protein, was assessed. HS1 is leukocyte-specific homolog of cortactin, which interacts with the Arp2/3 complex (Hao et al., 2005), an actin nucleator complex organizing actin filaments into branch network. HS1 is recruited and is important for F-actin organization at the immune synapse of T Lymphocytes (Gomez et al., 2006). HS1 was localized at the cell–coverslip contact site and an enhanced localization around the cell periphery was noted (Fig. S5G). Colocalization of Cx43 and HS1 at the contact site and around the cell periphery was observed, which was more enhanced for cells expressing WT, S255A, S262A and 4S Cx43-EGFP compared with S279A and S282A Cx43-EGFP expressing cells (Fig. S5G). Detection of any detailed difference in colocalization of WT Cx43 versus different mutants with HS1 requires more advanced techniques such as super-resolution microscopy, which is beyond the scope of this paper. Although individual serine-to-alanine mutations had different effects on B-cell spreading, the combination of these four mutations did not affect B-cell spreading. Colocalization of Cx43 with actin regulatory proteins such as HS1 can provide an insight into how Cx43 affects the actin dynamics during the spreading response.
Fig. 5.
Quadruple serine mutation of Cx43 carboxyl tail does not affect BCR-mediated cell spreading. (A) Localization of WT, 4S Cx43-EGFP population A and EGFP alone expressed in J558μm3 cells. Optical sections were obtained from the middle of the cells, showing the green EGFP at the cell periphery in WT- and 4S Cx43-expressing cells, and intracellularly when EGFP is expressed alone. (B) Membrane spreading of J558μm3 cells expressing 4S Cx43-EGFP, population A and controls. Images were obtained using a laser-scanning confocal microscope at the contact site of the cells and stimulatory coverslips at 15 min time points. Cells were stained for actin using Rhodamine-Phalloidin. Cx43-EGFP=green, actin=red, merged=yellow. (C,D) Quantification of BCR-mediated cell spreading of J558μm3 cells expressing WT, 4S Cx43-EGFP populations A and B (respectively), and EGFP for 0, 15 and 30 min time points after plating. Dashed line indicates average spreading area for cells expressing WT Cx43-EGFP at 15 min. (E) BCR-mediated cell spreading of primary splenic B-cells from the MK4 and WT mice. Actin=red. (F) Quantification of the spreading area at the different time points as described. The significance of the data was identified based on two-way ANOVA and a Tukey's test: **P≤0.01; ****P<0.0001. Data shown are representative of two or more independent experiments. Number of cells was >100 for each time point. Scale bars: 10 μm.
Triple mutants of the Cx43 CT containing S255/262A with S279A or S282A mutations had mixed effects on BCR-mediated spreading within B-cell populations
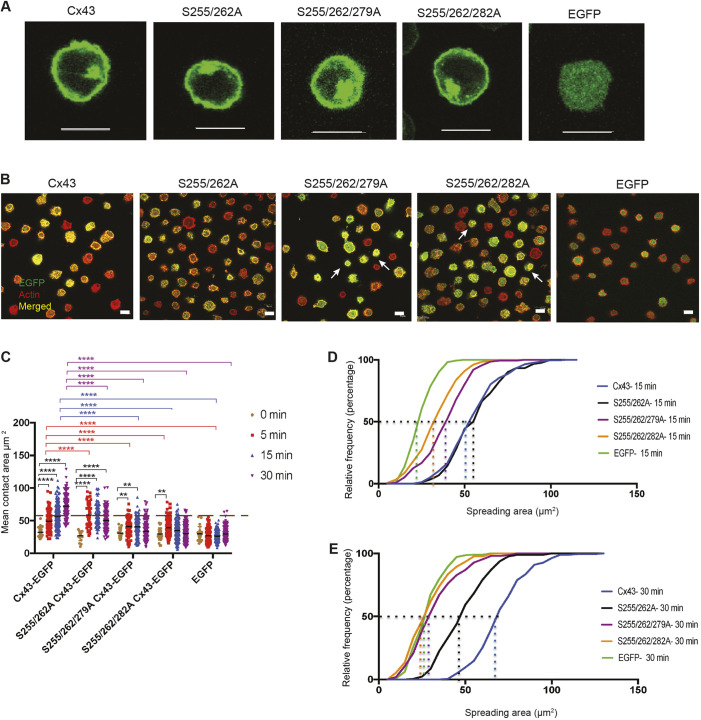
Since cells expressing 4S Cx43 were able to spread in response to BCR signaling despite the presence of S279A and S282A, which impeded cell spreading, we next studied the effect of double and triple serine mutations on cell spreading. These experiments were done to gain further insight into the importance of the serine residues of Cx43 CT for BCR-mediated cell spreading. The serine mutations that did not affect cell spreading (S255A and S262A) were studied together or with an added serine mutation that impaired cell spreading (S279A or S282A). These mutants are henceforward referred to as Group 1 mutants. Protein expression of the mutated proteins was monitored by checking for EGFP using IF, and cellular localization of the mutated proteins was similar to the WT protein (Fig. 6A). The size of the transduced cells and cell surface levels of the BCR and of the Cx43-EGFP were compared (Fig. S6A,B) using flow cytometry. Cells expressing the double mutant showed a significant increase in the spreading area over time; however, the area was smaller when compared with the cells expressing the WT protein (Fig. 6B,C). Cells expressing the triple mutants showed a mixed response to BCR signaling in terms of spreading: some cells had a large spreading area resembling that of WT Cx43 cells and other cells had a small spread, resembling EGFP-, S279A- or S282A-expressing cells (Fig. 6B,C). The relative frequency of the cells with respect to the spreading area for each cell type was calculated. The results showed that a larger percentage of cells expressing WT Cx43 had a larger spreading area (at both 15 and 30 min after BCR stimulation) than the cells expressing mutated Cx43. The double and triple mutants showed a smaller percentage of the cells with large spreading area when compared with the WT Cx43-expressing cells. The P-Tyr profile of the cells expressing S255/262A Cx43-EGFP and the Group 1 triple mutants were similar to WT Cx43-EGFP (Fig. S6C-F). These results showed that Group 1 triple serine mutations of the Cx43 CT had a different effect compared with the individual serine mutations on BCR-mediated cell spreading. However, there was no detectable change in total P-Tyr profile. We next studied the effect of the other set of double and triple mutations.
Fig. 6.
Double and triple mutations of Cx43 carboxyl tail have mixed effects on BCR-mediated spreading within B-cell populations. (A) Localization of Group 1 double and triple mutants S255/262A, S255/262/279A and S255/262/282A along with WT Cx43-EGFP and EGFP expressed in J558μm3 cells. (B) Membrane spreading of J558μm3 cells expressing WT Cx43, Group 1 triple mutants and cells expressing EGFP. Images were obtained at the contact site of the cells and stimulatory coverslips at 15 min time points using a laser-scanning confocal microscope as described. Arrows indicate non-spreading cells in triple-mutant-expressing cells. Cells were stained for actin using Rhodamine-Phalloidin. Number of cells >100 for each time point. Cx43-EGFP=green, actin=red, merged=yellow. (C) Quantification of the spreading area at the different time points as described for Fig. 2. The significance of the data was identified based on two-way ANOVA and a Tukey's test: **P≤0.01; ****P<0.0001. Data shown are representative of three independent experiments. (D,E) Relative frequency (percentages) of cells expressing Group 1 mutants with respect to the spreading area at the 15 min (D) and 30 min (E) time points. Data shown are representative of three independent experiments. Scale bars: 10 μm.
Triple mutants of the Cx43 CT containing S279/282A with S255A or S262A mutations had mixed effects on BCR-mediated spreading within B-cell populations
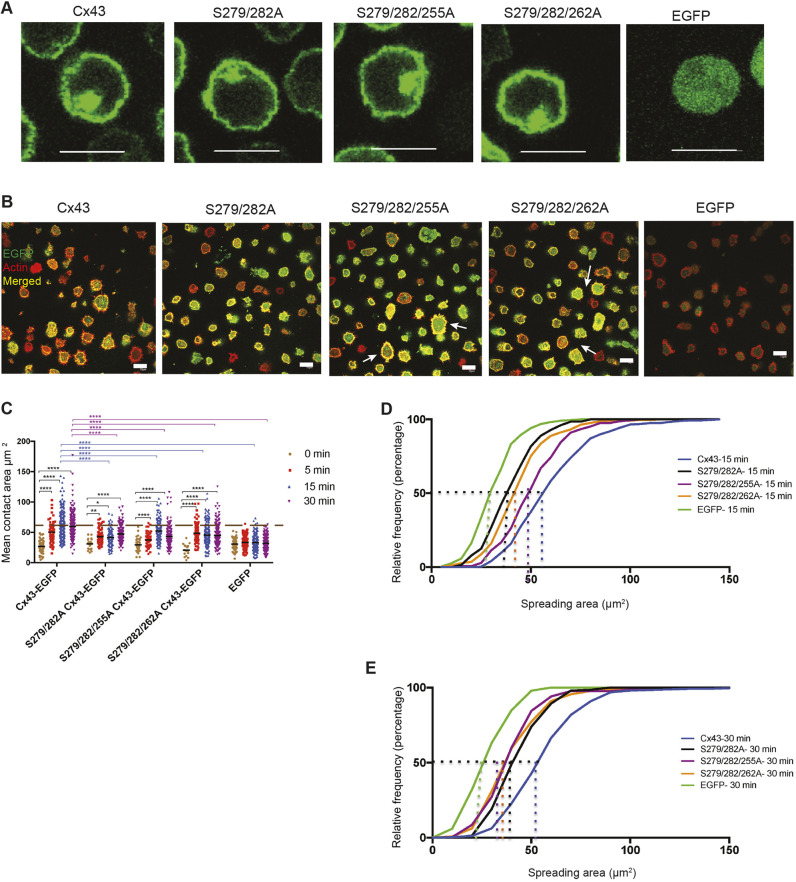
The serine mutations that affected cell spreading (S279A and S282A) were studied together with an added serine mutation that did not affect BCR-mediated cell spreading when expressed alone (S255A or S262A). These mutants are referred to as Group 2 mutants. Protein expression levels of the mutated Cx43-EGFPs were checked as described earlier and the cellular localization of the mutated proteins was shown to be similar to the WT protein (Fig. 7A). The size of the transduced cells and cell surface levels of the BCR and of the Cx43-EGFP were compared (Fig. S7A,B) using flow cytometry. Cells expressing the double mutant (S279/282A Cx43-EGFP) did not show a significant increase in the spreading area compared with cells expressing the WT protein (Fig. 7B,C). Cells expressing the Group 2 triple mutants, showed a mixed response to BCR signaling over the time course, similar to Group 1 triple mutants. These cells showed different responses, including cells with very small spreading area similar to S279A-, S282A- or EGFP-expressing cells, and cells with large spreading area similar to the WT Cx43- and S255A- or S262A-expressing cells (Fig. 7B,C). The relative frequency of the cells with respect to the spreading area for each cell type was calculated. The results showed that Group 2 triple mutants had a large percentage of cells with larger spreading area (at both 15 and 30 min after BCR stimulation) very similar to cells expressing WT Cx43. The majority of the cells expressing the double and triple mutants showed a very small spreading area when compared with the WT Cx43-expressing cells (Fig. 6E and Fig. 7E). The P-Tyr level for this group was slightly higher compared with WT Cx43-expressing cells (Fig. S7C-F). These results showed that Group 2 triple serine mutations of the Cx43 CT had a different effect compared with the individual serine mutations in the BCR-mediated cell-spreading assay while their total P-Tyr was somewhat higher.
Fig. 7.
Double and triple mutations of Cx43 carboxyl tail have mixed effects on BCR-mediated spreading in transduced B-cell populations. (A) Localization of Group 2 double and triple mutants S279/282A, S279/282/255A and S279/282/262A along with WT Cx43-EGFP and EGFP expressed in J558μm3 cells. (B) Membrane spreading of J558μm3 cells expressing WT Cx43, Group 2 triple mutants and cells expressing EGFP. Images were obtained at the contact site of the cells and stimulatory coverslips at 15 min time points using a laser-scanning confocal microscope as described. Number of cells was >100 for each time point. Cells were stained for actin using Rhodamine-Phalloidin. Arrows indicate spreading cells in triple-mutant-expressing cells. Cx43-EGFP=green, actin=red, merged=yellow. (C) Quantification of the spreading area at the different time points as described for Fig. 2. The significance of the data was identified based on two-way ANOVA and a Tukey's test. Asterisks (*) denote significant differences determined by P-values (P: 0.01 to 0.05): **P≤0.01; ****P<0.0001. Data shown are representative of three independent experiments. (D,E) Relative frequency (percentages) of cells expressing Group 2 mutants with respect to the spreading area at the 15 min (D) and 30 min (E) time points. Data shown are representative of three independent experiments. Scale bars: 10 μm.
DISCUSSION
In this study, we show that the CT of Cx43 is phosphorylated on specific residues following BCR stimulation and the CT is sufficient to support BCR-mediated membrane spreading independent of the rest of Cx43 protein. We also identified a key region and a number of residues of the CT that are important for BCR-mediated membrane spreading. We showed site-specific phosphorylation of the Cx43 CT on S262, S279, S282, S368 and Y247 in B-cell lines after BCR signaling using phospho-specific antibodies (Solan and Lampe, 2008). These sites are well-characterized modifications of the Cx43 CT in various cell types (Márquez-Rosado et al., 2012; Solan and Lampe, 2005, 2009). Using a chimeric protein that consists of the extracellular and transmembrane domains of the -cell protein, CD8, fused to the Cx43 CT, we studied the effects of the CT as part of a chimeric protein that is located at the plasma membrane. The chimeric protein was localized at the plasma membrane, phosphorylated similar to WT Cx43, and was able to support BCR-mediated cell spreading. Studying a series of Cx43 CT truncations showed that the region between aa 246-307 is necessary for B-cell spreading. The examination of additional point mutations of the Cx43 CT revealed that S279 and S282 were important for BCR-mediated spreading, while S255 and S262 were not. The serine mutations were studied by grouping them in different combinations, and both showed mixed effects in terms of supporting spreading among the populations of B-cells. Lastly, mutation of all four serine residues to alanine did not affect B-cell spreading. The total P-Tyr profile after BCR stimulation was lower in cells expressing the Cx43 CT mutations that did not support B-cell spreading compared with the WT protein. These results show the importance of the CT of Cx43 for B-cell spreading and define certain regions and residues that are critical for these responses.
Signaling through the BCR is essential for B-cell development, survival and immune responses. In our experiments B-cell stimulation by clustering the BCR represents antigen-induced signaling, which results in survival or activation of B-cells depending on their developmental stage (Lebien and Tedder, 2008; Pieper et al., 2013; Yam-Puc et al., 2018 preprint). BCR-induced signaling starts by phosphorylation of the signaling subunit of the BCR (Igα/β), which in turn creates additional binding motifs for recruitment of signaling molecules, including protein kinases such as Src family kinases, to the receptor (Gold et al., 1990; Kurosaki et al., 1994; Rolli et al., 2002). This leads to the activation of other signaling pathways, including the mitogen-activated protein kinase (MAPK) pathway (Cambier et al., 1994; Defranco et al., 1995; Craxton et al., 1999; Gold et al., 2000; Gold, 2002; Yokozeki et al., 2003). Both Src kinases and MAPKs have been shown to phosphorylate the CT of Cx43 in different cell types (Márquez-Rosado et al., 2012; Solan and Lampe, 2005, 2009, 2016). Stimulation of a membrane receptor resulting in phosphorylation of Cx43 was previously reported with respect to epidermal growth factor receptor (EGFR) and vascular endothelial growth factor receptor (VEGFR) in different cell types (Kanemitsu and Lau, 1993; Abdelmohsen et al., 2003; Fong et al., 2014; Nimlamool et al., 2015). Here, we showed phosphorylation of the Cx43 CT on S262, S279, S282, S368 and Y247 residues upon BCR-induced signaling. We speculate that the kinases activated and recruited to the plasma membrane upon BCR stimulation, phosphorylate the CT of Cx43 on those residues.
BCR signaling initiated by antigen encounter induces cytoskeletal rearrangements that allow for membrane spreading across the antigen-presenting cells. The BCR-mediated cell spreading is an important early step towards B-cell activation since the process enhances antigen gathering and signal amplification (Fleire et al., 2006; Harwood and Batista, 2011; Li et al., 2019; Weber et al., 2008). We previously showed that the CT of Cx43 (aa 246-382) and also the Y247 and Y265 residues are important for B-cell spreading (Falk et al., 2014; Machtaler et al., 2014). However, there are additional domains in the CT that could influence B-cell spreading. By expressing different Cx43 CT truncations or mutations in B-cell lines and testing for the ability of the cells to spread upon BCR signaling, we showed that the domain between aa 246-307 of the CT, as well as S279 and S282 are important for B-cell spreading. While mutations at S255 and S262 of the Cx43 CT did not affect B-cell spreading, in the presence of quadruple mutations containing these two and mutations at S279, S282, which impeded B-cell spreading when expressed individually, B-cell spreading response was comparable to that in WT Cx43-expressing cells. This might suggest that S255A and S262A mutations counteract the effect of S279A and S282A. Mutations at S255 and S262 might influence conformational changes and/or availability of the interaction sites caused by S279 S282A mutations. Using the chimeric protein, it was shown that the CT of Cx43 could support B-cell spreading in the absence of the other domains of the protein, such as the channel, the cytoplasmic loop and the amino terminus. Our findings are consistent with previous reports on the non-channel functions of Cx43 with respect to cytoskeleton-dependent cellular processes in different cell types (Kameritsch et al., 2012; Matsuuchi and Naus, 2013; Zhou and Jiang, 2014) indicating that the CT of Cx43 is the important domain for migration (Bates et al., 2007; Behrens et al., 2010; Cina et al., 2009; Kameritsch et al., 2015). Thus, together, all these results suggest that the CT of Cx43 is the domain that is important for cytoskeletal-dependent cellular processes.
The CT of Cx43 can function as an adaptor protein by providing binding sites for actin regulating proteins close to the plasma membrane. The region of Cx43 CT between aa 246-307 contains multiple modification and interaction sites (Giepmans, 2004; Laird, 2006; Hervé et al., 2007; Olk et al., 2009; Solan and Lampe, 2009; Palatinus et al., 2012; Rhett and Gourdie, 2012; Ambrosi et al., 2016; Sorgen et al., 2018). Protein interactions at these sites with cytoskeleton linker and adaptor proteins are possible and can lead to cytoskeleton remodeling. Some of the interesting domains located within this region are a SH2-binding domain (Y265) and a SH3-binding domain (proline-rich region P274-P284) and a PY motif (xPPxY) (aa 282-286). The serine residues 279 and 282 (MAPK phosphorylation sites) are located within the proline-rich region, while S282 is also part of the PY motif (Solan and Lampe, 2009). Previous reports suggest that phosphorylation of these residues may affect protein interactions with Cx43 (Leykauf et al., 2006). Cortactin, an actin binding protein known to play an important role in adhesion, spreading, migration and endocytosis in different cell types (Bryce et al., 2005; Ammer and Weed, 2008; Lai et al., 2009; He et al., 2015; Schnoor et al., 2018), is identified to associate with Cx43 (Squecco et al., 2006; Vitale et al., 2009). This interaction was found to be dependent on activation of p38 MAPK, suggesting that phosphorylation of Cx43 is important (Squecco et al., 2006). Since cortactin contains an SH3 domain (Schnoor et al., 2018; Weed and Parsons, 2001), it is possible that this key interaction happens at the region we found to be important for B-cell spreading. B-lymphocytes do not express cortactin; however, they do express a hematopoietic homolog of the protein, HS1, which is important for immune synapse formation and function (Gomez et al., 2006). We detected colocalization of Cx43 and HS1 at the cell–coverslip contact site. However, teasing apart the difference of colocalization in presence of various Cx43 mutants requires more advanced techniques such as super-resolution microscopy. Another actin binding protein, Drebrin, is also shown to interact with the CT of Cx43 in the region that overlaps with aa 246-307. Additionally, tyrosine phosphorylation of Cx43 is also shown to affect these interactions (Ambrosi et al., 2016; Butkevich et al., 2004; Zhou and Jiang, 2014). Drebrin and its hematopoietic homolog, HIP55, are important for immune synapse formation and function in T cells (Larbolette et al., 1999; Le Bras et al., 2004; Rocha-perugini et al., 2017). Multiple reports have shown indirect association of Cx43 with F-actin and it was suggested that the association is affected by phosphorylation of Cx43; in other words, removing or preventing phosphorylation reduced F-actin and Cx43 association (Li et al., 2005; Squecco et al., 2006; Elias et al., 2007). Therefore, we suggest that the interactions of Cx43 with residues within aa 246-307 of the CT with cytoskeleton linker and adaptor proteins could lead to cytoskeleton remodeling. Our findings suggest that phosphorylation of CT might be important for the interactions.
The CT of Cx43 can function as an adaptor protein by providing binding sites for kinases and phosphatases close to the plasma membrane; hence, affecting the fine tuning of BCR signaling and downstream effects. Adaptor proteins containing SH2- and SH3-binding domains, have been shown to affect function of kinases and phosphatases downstream of BCR signaling (Kurosaki, 2002). Here, we showed that the total P-Tyr profile of the B-cells is different in the presence of different Cx43 CT truncations and mutations. This could suggest the involvement of Cx43 in regulation of kinases and phosphatases downstream of BCR signaling. This function can serve in terms of recruitment of molecules and their stability at the plasma membrane as well as formation protein complexes. Fine tuning and spatio-temporal precision of BCR signal transduction is important in terms of regulation of BCR signaling during B-cell development and responses. The Cx43 CT can potentially function as an adaptor protein within the BCR signal transduction in terms of regulating kinases and phosphatases.
The CT of Cx43 is widely accepted as a platform that Cx43 binding protein can interact with to promote signaling (Laird, 2006; Olk et al., 2009; Palatinus et al., 2012; Solan and Lampe, 2009; Sorgen et al., 2018). Therefore, we propose that the protein interactions at the CT, which might be mediated by phosphorylation of Cx43, could influence BCR signaling, cytoskeleton remodeling and membrane dynamics, which are all important for BCR-mediated cell spreading. The CT could function as a scaffold close to the plasma membrane that provides an anchoring site for the machinery required for cytoskeleton remodeling and membrane expansion.
MATERIALS AND METHODS
Antibodies and fluorescent reagents
Polyclonal goat anti-mouse IgM (μ chain specific, #115-005-020) used for cell stimulation (20 µg ml−1), and for coating glass coverslips (2.5 µg cm−2) for the cell spreading assay was from Jackson ImmunoResearch (JIR) Laboratories (West Grove, PA, USA). Monoclonal phycoerythin (PE)-conjugated rat anti-mouse IgM (12-5790-81) from eBiosciences (San Diego, CA, USA) was used for flow cytometry analysis (2 μg ml−1). Rabbit anti-mouse Cx43 CT (amino acids 363-382) (C6219) was from Sigma-Aldrich (St Louis, MO, USA) (1:10,000 dilution for western blot). Phospho-specific antibodies against different serine residues were custom prepared by the Lampe Lab, Fred Hutchinson Cancer Centre, Seattle, WA, USA (Solan and Lampe, 2008) (1:1000 dilution for western blot). The mouse anti-mouse-p-Tyr (4G10) was from the 4G10 hybridoma (clone 4G10) (Gold et al., 1990) prepared in the Matsuuchi lab. Mouse anti-mouse actin (691001) (1:3000 dilution for western blot) was from Fisher Scientific (Waltham, MA, USA). Goat anti-rabbit IgG (H+L) HRP conjugated and goat anti-mouse IgG (H+L) HRP conjugated (170-6515 and 170-6516, respectively) were from Bio-Rad (Hercules, CA, USA). Both these antibodies were used as secondary Ab at 1:3000 dilution. The OKT8 Ab used for flow cytometry (30 μg ml−1) and IF (1:100 dilution) to detect CD8 was prepared from hybridomas from the American Type Culture collection (ATCC) (CRL-8014) (Manassas, VA, USA). The secondary Ab used for flow cytometry (5 μg ml−1) and IF (1:100 dilution) microscopy was donkey anti-mouse IgG (H+L) Alexa Fluor 647 conjugated (A31571) from Invitrogen (Carlsbad, CA, USA). Rabbit Anti-HS1 (rodent specific) Ab (4557S) form Cell Signaling (Danvers, MA) was used as primary Ab for IF (1:200 dilution). The secondary Ab used for IF (1:400 dilution) was goat anti-rabbit IgG (H+L) Alexa Fluor 647 conjugated (A21244) from Invitrogen (Carlsbad, CA, USA). Monoclonal phycoerythrin (PE)-conjugated rat anti-mouse IgM (12-5790-81) from eBiosciences was used for flow cytometry analysis and fluorescence-activated cell sorting (FACS) (2 μg ml−1). Rhodamine-Phalloidin (415) from Life Technologies (Carlsbad, CA, USA) was used to stain F-actin for IF imaging (1:200 and 1:400 dilution, respectively).
Cell lines and primary cells
The J558μm3 mouse plasmacytoma B-cell line expressing a transfected, 4-chain BCR at the cell surface [membrane IgM (µ, λ), Ig-α and Ig-β] was from Dr Louis Justement (University of Alabama, Birmingham, AL, USA) (Reth et al., 1987; Hombach et al., 1988; Justement et al., 1990; Machtaler et al., 2011; Falk et al., 2014). The WEHI231, mouse B-cell lymphoma (immature), cell line was obtained from ATCC (CRL-1702). The BOSC23 retroviral packaging cell line was a gift from Dr Warren S. Pear (Massachusetts Institute of Technology, Cambridge, MA, USA) (Pear et al., 1993). Primary murine B-cells were obtained from the spleen of 8- to 10-week-old MK4 mice (Johnstone et al., 2012) (received from John Bechberger and Dr Christian Naus, UBC, Vancouver, BC, Canada). Isolation of primary B-lymphocytes was done using the EasySep® mouse B-cell enrichment kit (Stem Cell Technologies, Vancouver, BC, Canada). All the cell lines were routinely checked for contamination.
Mice
C57/Bl6 (8-10 weeks, male) and MK4 (knock in S255A, S262A, S279A and S282) (8-10 weeks, male) (Johnstone et al., 2012), received from John Bechberger and Dr Christian Naus (UBC, Vancouver, BC, Canada). Animals were treated and used according to the University of British Columbia Animal Care and Use Committee guidelines.
Plasmids, primers and construction of Cx43 mutants
The NAP2 retroviral expression vector encoding cDNA for WT rat Cx43 with EGFP fused in-frame to the Cx43 CT, the Cx43 CT truncated after codon corresponding to amino acid 246 (Δ246 Cx43-EGFP) and the AP2 retroviral expression vector encoding EGFP were from Dr Christian Naus and have been described previously (Galipeau et al., 1999; Mao et al., 2000; Bates et al., 2007; Machtaler et al., 2011; Falk et al., 2014). Additional NAP2 plasmids with Cx43 cDNAs that contained deletions (Δ258 and Δ307) or point mutations at different serine residues were created using site-directed mutagenesis and custom designed primers as described previously (Farnaz Pournia, MSc thesis, UBC, 2015) (Pournia, 2015). To make the Δ258- and Δ307 Cx43-EGFP-encoding plasmids, first a BglII restriction enzyme (RE) cut site was inserted right before the EGFP start codon using custom primer pairs (Fr: 5′-CTGGATCCACCGGTCAGATCTATGGTGAGCAAGGGC-3′). The only existing BglII site in the NAP2 expression vector was previously changed to an XbaI site using custom primer pairs; therefore, BglII digestion only occurred at the desired locations. The insertion was confirmed by sequencing and the new construct was used as a template for insertion of a BglII site after codon 258 (258-BglII/BglII-EGFP) and in a separate reaction after codon 307 (307-BglII/BglII-EGFP). This was done as described above using the following custom primer pairs (Fr 258-BglII: 5′-CCACTGAGCCCATCAAAAAGATCTGGATCTCCAAAATACGCC-3′ and Fr 307-BglII; 5′-AACAAGCAAGCTAGCGAGAGATCTTGGGCGAACTACAGCGCA-3′). The newly constructed 258-BglII/BglII-EGFP and 307-BglII/BglII-EGFP (confirmed by sequencing) were digested by BglII RE (R0144S, New England BioLabs, lpswich, MA, USA) and re-ligated using T4 DNA Ligase (15224041, Invitrogen). Thus, Δ258 Cx43-EGFP and Δ307 Cx43-EGFP encoding plasmids were made and confirmed by sequencing.
Different serine residues were replaced with alanine using site-directed mutagenesis and the following custom primer pairs (Fr S255A: 5′-CCACTGGCCCACTGGCTCCATCAAAAGACTGC-3′; Fr S262A: 5′-CCCATCAAAAGACTGCGGAGCTCCAAAATACGCCTACTTC-3′; Fr S279A: 5′-CACTCGCGCCTATGTCTCCTCCTGGGTACAAGC-3′; Fr S282A: 5′-CTCCACTCTCGCCTATGGCTCCTCCTGGGTACAAGCTG-3′). The mutations were confirmed by sequencing, then, the new constructs and the same primers were used to make double and multiple mutations. For S279/282A, the following primer was used (Fr: 5′-CACTCGCGCCTATGGCTCCTCCTGGGTACAAGC-3′).
Retroviral transduction and enrichment of cells
The J558μm3 or WEHI231 cells were transduced with retrovirus containing WT Cx43, various mutated Cx43-EGFPs, CD8: Cx43CT-EGFP, CD8: EGFP cDNA, or the AP2 plasmid, using the retroviral packing cell line BOSC23 using a standard protocol (Falk et al., 2014; Krebs et al., 1999; Machtaler et al., 2011). Stably transduced cells were sorted 1 week after transduction using FACS at the UBC FACS Facility, selecting for EGFP- and IgM-positive cells. These sorted stable cell lines were frozen, and samples were thawed and cultured 1 week prior to experiments.
Cell culture and B-cell isolation
All B-cell lines were cultured in supplemented RPMI-160 medium (Gibco by Thermo Fisher Scientific, Waltham, MA, USA) and adherent BOSC23 cells in supplemented DMEM (Gibco) as described (Machtaler et al., 2011; Falk et al., 2014). Cells were maintained in a direct heat incubator at 37°C and 5% CO2 atmosphere. Primary B-lymphocytes were isolated from the spleen of mice using the EasySep® mouse B-cell Enrichment kit (Stem Cell Technologies, Vancouver, BC, Canada). All the cell lines were routinely checked for contamination.
Cell stimulation
Stimulation of B-cells by BCR cross-linking using 20 µg ml−1 of goat anti-mouse IgM (μ chain specific) Ab per time point was done as described previously (Machtaler et al., 2011).
Western blot quantification
SDS Polyacrylamide gels were used to separate cellular proteins and then the contents of the gels were transferred for western blotting using standard procedures (Machtaler et al., 2011; Falk et al., 2014). Protein bands were scanned (for data presented in Figs S3-S7) using a C-digit blot scanner (Li-cor, Lincoln, NE, USA) and the quantification was done using Image Studio Lite (Li-cor). First, the band intensity of each lane was normalized to their respective loading control. One sample was chosen as a reference and all normalized intensities were further normalized to the chosen reference so that all bands are relative to this one with a value of 1. The adjusted relative densities from three experiments were averaged, analyzed and plotted with Prism 7 software (GraphPad, La Jolla, CA, USA). Blots presented in Fig. 1 were developed and then scanned using an EPSON Perfection V37 scanner. Quantification was done using ImageJ software (NIH, Bethesda, MD, USA) and plotted with Prism 7.
Surface BCR and chimeric protein expression
One million cells per sample were suspended in 2% FACS buffer (PBS+2% FBS) and stained with PE-conjugated anti-IgM (2 μg ml−1) for surface BCR detection using standard procedures (Falk et al., 2014). Cells were kept on ice and stained with OKT8 (30 μg ml−1) and anti-mouse Ig Alexa-647 (5 μg ml−1) primary and secondary antibodies, respectively, for detection of surface expression of the chimeric proteins. Data were collected using an LSR II Flow Cytometer (Becton Dickinson, Franklin Lakes, NJ, USA) at the UBC Flow Cytometry Facility.
Surface expression of the chimeric protein by immunofluorescence microscopy
Two hundred thousand cells per cell type were added to anti-IgM-coated coverslips and incubated at 37°C for 5 min. Cells were fixed with 4% paraformaldehyde and stained with OKT8 (1:100 dilution) and anti-mouse Ig Alexa-647 secondary antibodies (1:100 dilution) without permeabilization. Images were obtained using Olympus (Tokyo, Japan) Fluoview1000 confocal microscope (100× objective) (Machtaler et al., 2014).
Localization of truncated and mutated Cx43-EGFP
Cells were plated on anti-BCR-coated stimulatory coverslips for the cell spreading assay. Images were obtained at the zero time point. Optical sections were obtained from the middle of the cells using an Olympus Fluoview1000 confocal microscope with the 100× objective lens. All imaging for this study was performed at UBC Life Sciences Institute Imaging Core facility (LSI IMAGING). The green color for cells expressing EGFP had to be enhanced slightly because of the dimmer expression of EGFP alone on AP2 compared with NAP2 vector.
Cell spreading and statistical analysis
The assay was performed as described (Santos-Argumedo et al., 1997; Lin et al., 2008; Machtaler et al., 2011; Falk et al., 2014). Modifications include coating the glass coverslips with 2.5 μg cm−2 of the stimulating Ab overnight at 4°C. For each time point, 25-35×104 J558μm3 cells or 105 WEHI231 cells were resuspended in 100 μl RPMI medium and added to the coverslips. At the end of the time point, an equal volume of 4% paraformaldehyde was directly added to the coverslips to fix the cells. The spreading area at the cell–coverslip contact site was imaged using an Olympus Fluoview1000 confocal microscope (100× objective). Again, we needed to enhance green color slightly to visualize cells expressing EGFP because of the dimmer expression of EGFP alone on AP2 compared with NAP2 vector, as mentioned above. This enhancement did not interfere with quantification of the spreading area, which was done based on actin (red). The areas were quantified using Image Pro Plus 6.2 analysis software (Media Cybernetics, Rockville, MD, USA) as described (Machtaler et al., 2011; Falk et al., 2014). To analyze significance, two-way ANOVA and a Tukey's test were used to compare the means to either a positive or negative control using GraphPad Prism 6 software. Significance was based on 95% confidence interval: *P=0.01 to 0.05; **P=0.001 to 0.01); ***P= 0.0001-0.001; ****P<0.0001.
3D localization of Cx43-EGFP using the spreading assay
Two hundred thousand cells per cell type were plated on anti-IgM-coated coverslips and incubated at 37°C for 15 min. Cells were fixed with 4% paraformaldehyde. Optical slices were collected from the cell–coverslip contact site up to the top of the cells using a Zeiss (Jena, Germany) Spinning Disk confocal microscope (100× objective lens). The 3D reconstruction was done using SlideBook 6 3i software (Denver, CO, USA).
Colocalization of Cx43 with actin or HS1 at cell–coverslip contact site
Cells were plated and fixed as described above and stained with Rhodamine-Phalloidin for actin visualization. For HS1 colocalization, fixed cells were stained with anti-HS1 (1:200 dilution) anti-rabbit Ig Alexa-647 secondary antibodies (1:400 dilution). In order to better visualize the actin structures at the contact site, a lower concentration of Rhodamine-Phalloidin was used (1:400 dilution compared with 1:200 dilution used for original spreading assays). The spreading area at the cell–coverslip contact site was imaged using a Zeiss spinning disk confocal microscope (100× objective).
Supplementary Material
Acknowledgements
We thank Dr Michael R. Gold for constant advice and input on project plan and experiments set up and performance. We also thank Dr Christian C. Naus and John Bechberger for providing the MK4 mice and input and advice. We acknowledge the support of UBC Life Sciences Institute Imaging Core facility (LSI IMAGING) and UBC FLOW Cytometry Facility.
Footnotes
Author contributions
Conceptualization: L.M., F.P.; Methodology: L.M., F.P., M.D., K.C., V.M., P.D.L.; Validation: L.M., F.P., M.D., K.C., V.M., P.D.L.; Formal analysis: L.M., F.P., M.D., K.C., V.M., P.D.L.; Investigation: L.M., F.P., M.D., K.C., V.M., P.D.L.; Resources: L.M., P.D.L.; Data curation: L.M., F.P.; Writing - original draft: L.M., F.P.; Writing - review & editing: L.M., F.P., K.C., V.M., P.D.L.; Visualization: L.M., F.P.; Supervision: L.M.; Project administration: L.M., M.D.; Funding acquisition: L.M., P.D.L.
Funding
The authors would like to acknowledge financial support from the Canadian Institutes of Health Research (CIHR) [MOP-86486 and MOP-111079] and Natural Sciences and Engineering Research Council of Canada (NSERC) [RGPIN-2019-04911] to L.M. and the National Institutes of Health [GM55632] to P.D.L. Trainee awards from the Cell and Developmental Biology Graduate Program, University of British Columbia to F.P. and V.M. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://jcs.biologists.org/lookup/doi/10.1242/jcs.237925.supplemental
Peer review history
The peer review history is available online at https://jcs.biologists.org/lookup/doi/10.1242/jcs.237925.reviewer-comments.pdf
References
- Abdelmohsen, K., Gerber, P. A., von Montfort, C., Sies, H. and Klotz, L.-O. (2003). Epidermal growth factor receptor is a common mediator of quinone-induced signaling leading to phosphorylation of connexin-43. Role of glutathione and tyrosine phosphatases. J. Biol. Chem. 278, 38360-38367. 10.1074/jbc.M306785200 [DOI] [PubMed] [Google Scholar]
- Ambrosi, C., Ren, C., Spagnol, G., Cavin, G., Cone, A., Grintsevich, E. E., Sosinsky, G. E. and Sorgen, P. L. (2016). Connexin43 forms supramolecular complexes through non-overlapping binding sites for drebrin, tubulin, and ZO-1. PLoS ONE 11, 1-23. 10.1371/journal.pone.0157073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammer, A. G. and Weed, S. A. (2008). Cortactin branches out: Roles in regulating protrusive actin dynamics.
- Bates, D. C., Sin, W. C., Aftab, Q. and Naus, C. C. (2007). Connexin43 enhances glioma invasion by a mechanism involving the carboxy terminus. Glia 55, 1554-1564. 10.1002/glia.20569 [DOI] [PubMed] [Google Scholar]
- Batista, F. D., Iber, D. and Neuberger, M. S. (2001). B cells acquire antigen from target cells after synapse formation. Nature 411, 489-494. 10.1038/35078099 [DOI] [PubMed] [Google Scholar]
- Behrens, J., Kameritsch, P., Wallner, S., Pohl, U. and Pogoda, K. (2010). The carboxyl tail of Cx43 augments p38 mediated cell migration in a gap junction-independent manner. Eur. J. Cell Biol. 89, 828-838. 10.1016/j.ejcb.2010.06.003 [DOI] [PubMed] [Google Scholar]
- Bolger-Munro, M., Choi, K., Scurll, J., Abraham, L., Chappell, R., Sheen, D., Dang-Lawson, M., Wu, X., Priatel, J. J., Coombs, D., et al. (2019). Arp2/3 complex-driven spatial patterning of the B cell receptor (BCR) enhances immune synapse formation, BCR signaling, and cell activation. bioRxiv, 490698. 10.7554/elife.44574.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brdicka, T., Pavlistová, D., Leo, A., Bruyns, E., Korínek, V., Angelisová, P., Scherer, J., Shevchenko, A., Hilgert, I., Cerný, J., et al. (2000). Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adaptor protein, binds the protein tyrosine kinase csk and is involved in regulation of T cell activation. J. Exp. Med. 191, 1591-1604. 10.1084/jem.191.9.1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryce, N. S., Clark, E. S., Leysath, J. M. L., Currie, J. D., Webb, D. J. and Weaver, A. M. (2005). Cortactin promotes cell motility by enhancing lamellipodial persistence. Curr. Biol. 15, 1276-1285. 10.1016/j.cub.2005.06.043 [DOI] [PubMed] [Google Scholar]
- Butkevich, E., Hülsmann, S., Wenzel, D., Shirao, T., Duden, R. and Majoul, I. (2004). Drebrin is a novel connexin-43 binding partner that links gap junctions to the submembrane cytoskeleton. Curr. Biol. 14, 650-658. 10.1016/j.cub.2004.03.063 [DOI] [PubMed] [Google Scholar]
- Cambier, J. C., Pleiman, C. M. and Clark, M. R. (1994). Signal transduction by the B cell antigen receptor and its coreceptors. Annu. Rev. Immunol. 12, 457-486. 10.1146/annurev.iy.12.040194.002325 [DOI] [PubMed] [Google Scholar]
- Campbell, M. A. and Sefton, B. M. (1990). Protein tyrosine phosphorylation is induced in murine B lymphocytes in response to stimulation with anti-immunoglobulin. EMBO J. 9, 2125-2131. 10.1002/j.1460-2075.1990.tb07381.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, V. C., Gouw, J. W., Naus, C. C. and Foster, L. J. (2013). Connexin multi-site phosphorylation: Mass spectrometry-based proteomics fills the gap. Biochim. Biophys. Acta Biomembr. 1828, 23-34. 10.1016/j.bbamem.2012.02.028 [DOI] [PubMed] [Google Scholar]
- Cina, C., Maass, K., Theis, M., Willecke, K., Bechberger, J. F. and Naus, C. C. (2009). Involvement of the cytoplasmic C-terminal domain of Connexin43 in neuronal migration. J. Neurosci. 29, 2009-2021. 10.1523/JNEUROSCI.5025-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craxton, A., Jiang, A., Kurosaki, T. and Clark, E. A. (1999). Syk and Bruton's tyrosine kinase are required for B cell antigen receptor-mediated activation of the kinase Akt. J. Biol. Chem. 274, 30644-30650. 10.1074/jbc.274.43.30644 [DOI] [PubMed] [Google Scholar]
- Crespin, S., Bechberger, J., Mesnil, M., Naus, C. C. and Sin, W.-C. (2010). The carboxy-terminal tail of connexin43 gap junction protein is sufficient to mediate cytoskeleton changes in human glioma cells. J. Cell. Biochem. 110, 589-597. 10.1002/jcb.22554 [DOI] [PubMed] [Google Scholar]
- Dbouk, H. A., Mroue, R. M., El-Sabban, M. E. and Talhouk, R. S. (2009). Connexins: A myriad of functions extending beyond assembly of gap junction channels. Cell Commun. Signal. 7, 4. 10.1186/1478-811X-7-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFranco, A. L. (1997). The complexity of signaling pathways activated by the BCR. Curr. Opin. Immunol. 9, 296-308. 10.1016/S0952-7915(97)80074-X [DOI] [PubMed] [Google Scholar]
- DeFranco, A. L., Richards, J. D., Blum, J. H., Stevens, T. L., Law, D. A., Chan, V. W.-F., Datta, S. K., Foy, S. P., Hourihane, S. L., Gold, M. R., et al. (1995). Signal transduction by the B-cell antigen receptor. Ann. N. Y. Acad. Sci. 766, 195-201. 10.1111/j.1749-6632.1995.tb26662.x [DOI] [PubMed] [Google Scholar]
- Elias, L. A. B., Wang, D. D. and Kriegstein, A. R. (2007). Gap junction adhesion is necessary for radial migration in the neocortex. Nature 448, 901-907. 10.1038/nature06063 [DOI] [PubMed] [Google Scholar]
- Falk, L., Dang-Lawson, M., Vega, J. L., Pournia, F., Choi, K., Jang, C., Naus, C. C. and Matsuuchi, L. (2014). Mutations of Cx43 that affect B cell spreading in response to BCR signaling. Biol. Open 3, 185-194. 10.1242/bio.20147328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleire, S. J., Goldman, J. P., Carrasco, Y. R., Weber, M., Bray, D. and Batista, F. D. (2006). B cell ligand discrimination through a spreading and contraction response. Science 312, 738-741. 10.1126/science.1123940 [DOI] [PubMed] [Google Scholar]
- Fong, J. T., Nimlamool, W. and Falk, M. M. (2014). EGF induces efficient Cx43 gap junction endocytosis in mouse embryonic stem cell colonies via phosphorylation of Ser262, Ser279/282, and Ser368. FEBS Lett. 588, 836-844. 10.1016/j.febslet.2014.01.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman, S. A., Jaumouillé, V., Choi, K., Hsu, B. E., Wong, H. S., Abraham, L., Graves, M. L., Coombs, D., Roskelley, C. D., Das, R., et al. (2015). Toll-like receptor ligands sensitize B-cell receptor signalling by reducing actin-dependent spatial confinement of the receptor. Nat. Commun. 6, 7015. 10.1038/ncomms8015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galipeau, J., Li, H., Paquin, A., Sicilia, F., Karpati, G. and Nalbantoglu, J. (1999). Vesicular stomatitis virus G pseudotyped retrovector mediates effective in vivo suicide gene delivery in experimental brain cancer. Cancer Res. 59, 2384-2394. [PubMed] [Google Scholar]
- Giepmans, B. N. G. (2004). Gap junctions and connexin-interacting proteins. Cardiovasc. Res. 62, 233-245. 10.1016/j.cardiores.2003.12.009 [DOI] [PubMed] [Google Scholar]
- Gold, M. R. (2002). To make antibodies or not: signaling by the B-cell antigen receptor. Trends Pharmacol. Sci. 23, 316-324. 10.1016/S0165-6147(02)02045-X [DOI] [PubMed] [Google Scholar]
- Gold, M. R., Law, D. A. and DeFranco, A. L. (1990). Stimulation of protein tyrosine phosphorylation by the B-lymphocyte antigen receptor. Nature 345, 810-813. 10.1038/345810a0 [DOI] [PubMed] [Google Scholar]
- Gold, M. R., Ingham, R. J., McLeod, S. J., Christian, S. L., Scheid, M. P., Duronio, V., Santos, L. and Matsuuchi, L. (2000). Targets of B-cell antigen receptor signaling: the phosphatidylinositol 3-kinase/Akt/glycogen synthase kinase-3 signaling pathway and the Rap1 GTPase. Immunol. Rev. 176, 47-68. 10.1034/j.1600-065X.2000.00601.x [DOI] [PubMed] [Google Scholar]
- Gomez, T. S., McCarney, S. D., Carrizosa, E., Labno, C. M., Comiskey, E. O., Nolz, J. C., Zhu, P., Freedman, B. D., Clark, M. R., Rawlings, D. J., et al. (2006). HS1 functions as an essential actin-regulatory adaptor protein at the immune synapse. Immunity 24, 741-752. 10.1016/j.immuni.2006.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanissian, S. H. and Geha, R. S. (1997). Jak3 is associated with CD40 and is critical for CD40 induction of gene expression in B cells. Immunity 6, 379-387. 10.1016/S1074-7613(00)80281-2 [DOI] [PubMed] [Google Scholar]
- Hao, J.-J., Zhu, J., Zhou, K., Smith, N. and Zhan, X. (2005). The coiled-coil domain is required for HS1 to bind to F-actin and activate Arp2/3 complex. J. Biol. Chem. 280, 37988-37994. 10.1074/jbc.M504552200 [DOI] [PubMed] [Google Scholar]
- Harwood, N. E. and Batista, F. D. (2011). The cytoskeleton coordinates the early events of B-cell activation. Cold Spring Harb. Perspect. Biol. 3, a002360. 10.1101/cshperspect.a002360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, Y., Ren, Y., Wu, B., Decourt, B., Lee, A. C., Taylor, A. and Suter, D. M. (2015). Src and cortactin promote lamellipodia protrusion and filopodia formation and stability in growth cones. Mol. Biol. Cell 26, 3229-3244. 10.1091/mbc.e15-03-0142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervé, J. C., Bourmeyster, N., Sarrouilhe, D. and Duffy, H. S. (2007). Gap junctional complexes: From partners to functions. Prog. Biophys. Mol. Biol. 94, 29-65. 10.1016/j.pbiomolbio.2007.03.010 [DOI] [PubMed] [Google Scholar]
- Hombach, J., Leclercq, L., Radbruch, A., Rajewsky, K. and Reth, M. (1988). A novel 34-kd protein co-isolated with the IgM molecule in surface IgM-expressing cells. EMBO J. 7, 3451-3456. 10.1002/j.1460-2075.1988.tb03219.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabara, H. H., Buckley, R. H., Roberts, J. L., Lefranc, G., Loiselet, J., Khalil, G. and Geha, R. S. (1998). Role of JAK3 in CD40-mediated signaling. Blood 92, 2435-2440. 10.1182/blood.V92.7.2435 [DOI] [PubMed] [Google Scholar]
- Johnstone, S. R., Kroncke, B. M., Straub, A. C., Best, A. K., Dunn, C. A., Mitchell, L. A., Peskova, Y., Nakamoto, R. K., Koval, M., Lo, C. W., et al. (2012). MAPK phosphorylation of connexin 43 promotes binding of cyclin e and smooth muscle cell proliferation. Circ. Res. 111, 201-211. 10.1161/CIRCRESAHA.112.272302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justement, L. B., Wienands, J., Hombach, J., Reth, M. and Cambier, J. C. (1990). Membrane IgM and IgD molecules fail to transduce Ca2+ mobilizing signals when expressed on differentiated B lineage cells. J. Immunol. 144, 3272-3280. [PubMed] [Google Scholar]
- Kameritsch, P., Pogoda, K. and Pohl, U. (2012). Channel-independent influence of connexin 43 on cell migration. Biochim. Biophys. Acta Biomembr. 1818, 1993-2001. 10.1016/j.bbamem.2011.11.016 [DOI] [PubMed] [Google Scholar]
- Kameritsch, P., Kiemer, F., Beck, H., Pohl, U. and Pogoda, K. (2015). Cx43 increases serum induced filopodia formation via activation of p21-activated protein kinase 1. Biochim. Biophys. Acta Mol. Cell Res. 1853, 2907-2917. 10.1016/j.bbamcr.2015.08.004 [DOI] [PubMed] [Google Scholar]
- Kanemitsu, M. Y. and Lau, A. F. (1993). Epidermal growth factor stimulates the disruption of gap junctional communication and connexin43 phosphorylation independent of 12-0-tetradecanoylphorbol 13-acetate-sensitive protein kinase C: the possible involvement of mitogen-activated protein kinase. Mol. Biol. Cell 4, 837-848. 10.1091/mbc.4.8.837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemitsu, M. Y., Loo, L. W. M., Simon, S., Lau, A. F. and Eckhart, W. (1997). Tyrosine phosphorylation of Connexin 43 by v-Src is mediated by SH2 and SH3 domain interactions. J. Biol. Chem. 272, 22824-22831. 10.1074/jbc.272.36.22824 [DOI] [PubMed] [Google Scholar]
- Kelly, M. E. and Chan, A. C. (2000). Regulation of B cell function by linker proteins. Curr. Opin. Immunol. 12, 267-275. 10.1016/S0952-7915(00)00086-8 [DOI] [PubMed] [Google Scholar]
- Krebs, D. L., Yang, Y., Dang, M., Haussmann, J. and Gold, M. R. (1999). Rapid and efficient retrovirus-mediated gene transfer into B cell lines. Methods Cell Sci. 21, 57-68. 10.1023/A:1009843325770 [DOI] [PubMed] [Google Scholar]
- Kurosaki, T. (1997). Molecular mechanisms in B cell antigen receptor signaling. Curr. Opin. Immunol. 9, 309-318. 10.1016/S0952-7915(97)80075-1 [DOI] [PubMed] [Google Scholar]
- Kurosaki, T. (2002). Regulation of B-cell signal transduction by adaptor proteins. Nat. Rev. Immunol. 2, 354-363. 10.1038/nri801 [DOI] [PubMed] [Google Scholar]
- Kurosaki, T., Takata, M., Yamanashi, Y., Inazu, T., Taniguchi, T., Yamamoto, T. and Yamamura, H. (1994). Syk activation by the Src-family tyrosine kinase in the B cell receptor signaling. J. Exp. Med. 179, 1725-1729. 10.1084/jem.179.5.1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, F. P. L., Szczodrak, M., Oelkers, J. M., Ladwein, M., Acconcia, F., Benesch, S., Auinger, S., Faix, J., Small, J. V., Polo, S., et al. (2009). Cortactin promotes migration and platelet-derived growth factor-induced actin reorganization by signaling to Rho-GTPases. Mol. Biol. Cell 20, 3209-3223. 10.1091/mbc.e08-12-1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird, D. W. (2006). Life cycle of connexins in health and disease. Biochem. J. 394, 527-543. 10.1042/BJ20051922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe, P. D. and Lau, A. F. (2004). The effects of connexin phosphorylation on gap junctional communication. Int. J. Biochem. Cell Biol. 36, 1171-1186. 10.1016/S1357-2725(03)00264-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe, P. D., TenBroek, E. M., Burt, J. M., Kurata, W. E., Johnson, R. G. and Lau, A. F. (2000). Phosphorylation of connexin43 on serine368 by protein kinase C regulates gap junctional communication. J. Cell Biol. 149, 1503-1512. 10.1083/jcb.149.7.1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larbolette, O., Wollscheid, B., Schweikert, J., Nielsen, P. J. and Wienands, J. (1999). SH3P7 is a cytoskeleton adapter protein and is coupled to signal transduction from lymphocyte antigen receptors. Mol. Cell. Biol. 19, 1539-1546. 10.1128/MCB.19.2.1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau, A. F., Kurata, W. E., Kanemitsu, M. Y., Loo, L. W. M., Warn-Cramer, B. J., Eckhart, W. and Lampe, P. D. (1996). Regulation of connexin43 function by activated tyrosine protein kinases. J. Bioenerg. Biomembr. 28, 359-368. 10.1007/BF02110112 [DOI] [PubMed] [Google Scholar]
- Le Bras, S., Foucault, I., Foussat, A., Brignone, C., Acuto, O. and Deckert, M. (2004). Recruitment of the actin-binding protein HIP-55 to the immunological synapse regulates T cell receptor signaling and endocytosis. J. Biol. Chem. 279, 15550-15560. 10.1074/jbc.M312659200 [DOI] [PubMed] [Google Scholar]
- Lebien, T. W. and Tedder, T. F. (2008). B lymphocytes: how they develop and function. Blood 112, 1570-1580. 10.1182/blood-2008-02-078071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo, A. and Schraven, B. (2001). Adapters in lymphocyte signalling. Curr. Opin. Immunol. 13, 307-316. 10.1016/S0952-7915(00)00220-X [DOI] [PubMed] [Google Scholar]
- Leykauf, K., Salek, M., Bomke, J., Frech, M., Lehmann, W.-D., Dürst, M. and Alonso, A. (2006). Ubiquitin protein ligase Nedd4 binds to connexin43 by a phosphorylation-modulated process. J. Cell Sci. 119, 3634-3642. 10.1242/jcs.03149 [DOI] [PubMed] [Google Scholar]
- Li, W., Hertzberg, E. L. and Spray, D. C. (2005). Regulation of connexin43-protein binding in astrocytes in response to chemical ischemia/hypoxia. J. Biol. Chem. 280, 7941-7948. 10.1074/jbc.M410548200 [DOI] [PubMed] [Google Scholar]
- Li, J., Yin, W., Jing, Y., Kang, D., Yang, L., Cheng, J., Yu, Z., Peng, Z., Li, X., Wen, Y., et al. (2019). The coordination between B cell receptor signaling and the actin cytoskeleton during B cell activation. Front. Immunol. 10, 1-13. 10.3389/fimmu.2018.03096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, K. B. L., Freeman, S. A., Zabetian, S., Brugger, H., Weber, M., Lei, V., Dang-Lawson, M., Tse, K. W. K., Santamaria, R., Batista, F. D., et al. (2008). The Rap GTPases regulate B cell morphology, immune-synapse formation, and signaling by particulate B cell receptor ligands. Immunity 28, 75-87. 10.1016/j.immuni.2007.11.019 [DOI] [PubMed] [Google Scholar]
- Machtaler, S., Dang-Lawson, M., Choi, K., Jang, C., Naus, C. C. and Matsuuchi, L. (2011). The gap junction protein Cx43 regulates B-lymphocyte spreading and adhesion. J. Cell Sci. 124, 2611-2621. 10.1242/jcs.089532 [DOI] [PubMed] [Google Scholar]
- Machtaler, S., Choi, K., Dang-Lawson, M., Falk, L., Pournia, F., Naus, C. C. and Matsuuchi, L. (2014). The role of the gap junction protein connexin43 in B lymphocyte motility and migration. FEBS Lett. 588, 1249-1258. 10.1016/j.febslet.2014.01.027 [DOI] [PubMed] [Google Scholar]
- Mao, A. J., Bechberger, J., Lidington, D., Galipeau, J., Laird, D. W. and Naus, C. C. G. (2000). Neuronal differentiation and growth control of neuro-2a cells after retroviral gene delivery of connexin43. J. Biol. Chem. 275, 34407-34414. 10.1074/jbc.M003917200 [DOI] [PubMed] [Google Scholar]
- Márquez-Rosado, L., Solan, J. L., Dunn, C. A., Norris, R. P. and Lampe, P. D. (2012). Connexin43 phosphorylation in brain, cardiac, endothelial and epithelial tissues. Biochim. Biophys. Acta Biomembr. 1818, 1985-1992. 10.1016/j.bbamem.2011.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuuchi, L. and Naus, C. C. (2013). Gap junction proteins on the move: Connexins, the cytoskeleton and migration. Biochim. Biophys. Acta Biomembr. 1828, 94-108. 10.1016/j.bbamem.2012.05.014 [DOI] [PubMed] [Google Scholar]
- Moorby, C. D. (2000). A connexin 43 mutant lacking the carboxyl cytoplasmic domain inhibits both growth and motility of mouse 3T3 fibroblasts. Mol. Carcinog. 28, 23-30. [DOI] [PubMed] [Google Scholar]
- Morio, T., Hanissian, S. H., Bacharier, L. B., Teraoka, H., Nonoyama, S., Seki, M., Kondo, J., Nakano, H., Lee, S.-K., Geha, R. S., et al. (1999). Ku in the cytoplasm associates with CD40 in human B cells and translocates into the nucleus following incubation with IL-4 and anti-CD40 mAb. Immunity 11, 339-348. 10.1016/S1074-7613(00)80109-0 [DOI] [PubMed] [Google Scholar]
- Nimlamool, W., Andrews, R. M. K. and Falk, M. M. (2015). Connexin43 phosphorylation by PKC and MAPK signals VEGF-mediated gap junction internalization. Mol. Biol. Cell 26, 2755-2768. 10.1091/mbc.E14-06-1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olk, S., Zoidl, G. and Dermietzel, R. (2009). Connexins, cell motility, and the cytoskeleton. Cell Motil. Cytoskelet. 66, 1000-1016. 10.1002/cm.20404 [DOI] [PubMed] [Google Scholar]
- Palatinus, J. A., Rhett, J. M. and Gourdie, R. G. (2012). The connexin43 carboxyl terminus and cardiac gap junction organization. Biochim. Biophys. Acta Biomembr. 1818, 1831-1843. 10.1016/j.bbamem.2011.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pear, W. S., Nolan, G. P., Scott, M. L. and Baltimore, D. (1993). Production of high-titer helper-free retroviruses by transient transfection. Proc. Natl. Acad. Sci. USA 90, 8392-8396. 10.1073/pnas.90.18.8392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninger, J. M. and Crabtree, G. R. (1999). The actin cytoskeleton and lymphocyte activation. Cell 96, 9-12. 10.1016/S0092-8674(00)80954-X [DOI] [PubMed] [Google Scholar]
- Pieper, K., Grimbacher, B. and Eibel, H. (2013). B-cell biology and development. J. Allergy Clin. Immunol. 131, 959-971. 10.1016/j.jaci.2013.01.046 [DOI] [PubMed] [Google Scholar]
- Pournia, F. (2015). The role of the tyrosines in the carboxyl tail of connexin43 in regulating cytoskeletal rearrangements in B-Lymphocytes. MSc thesis. University of British Columbia. 10.14288/1.0167176 [DOI]
- Reth, M. and Wienands, J. (1997). Initiation and processing of signals from the B cell antigen receptor. Annu. Rev. Immunol. 15, 453-479. 10.1146/annurev.immunol.15.1.453 [DOI] [PubMed] [Google Scholar]
- Reth, M., Petrac, E., Wiese, P., Lobel, L. and Alt, F. W. (1987). Activation of V kappa gene rearrangement in pre-B cells follows the expression of membrane-bound immunoglobulin heavy chains. EMBO J. 6, 3299-3305. 10.1002/j.1460-2075.1987.tb02649.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhett, J. M. and Gourdie, R. G. (2012). The perinexus: a new feature of Cx43 gap junction organization. Hear. Rhythm 9, 619-623. 10.1016/j.hrthm.2011.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha-Perugini, V., Gordon-Alonso, M. and Sánchez-madrid, F. (2017). Role of Drebrin at the immunological synapse. Drebrin 1006, 271-280. 10.1007/978-4-431-56550-5_15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolli, V., Gallwitz, M., Wossning, T., Flemming, A., Schamel, W. W. A., Zürn, C. and Reth, M. (2002). Amplification of B cell antigen receptor signaling by a Syk/ITAM positive feedback loop. Mol. Cell 10, 1057-1069. 10.1016/S1097-2765(02)00739-6 [DOI] [PubMed] [Google Scholar]
- Santos-Argumedo, L., Kincade, P. W., Partida-Sánchez, S. and Parkhouse, R. M. (1997). CD44-stimulated dendrite formation (‘spreading’) in activated B cells. Immunology 90, 147-153. 10.1046/j.1365-2567.1997.00126.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoor, M., Stradal, T. E. and Rottner, K. (2018). Cortactin: cell functions of a multifaceted actin-binding protein. Trends Cell Biol. 28, 79-98. 10.1016/j.tcb.2017.10.009 [DOI] [PubMed] [Google Scholar]
- Solan, J. L. and Lampe, P. D. (2005). Connexin phosphorylation as a regulatory event linked to gap junction channel assembly. Biochim. Biophys. Acta Biomembr. 1711, 154-163. 10.1016/j.bbamem.2004.09.013 [DOI] [PubMed] [Google Scholar]
- Solan, J. L. and Lampe, P. D. (2008). Connexin43 in LA-25 cells with active v-src is phosphorylated on Y247, Y265, S262, S279/282, and S368 via multiple signaling pathways. Cell Commun. Adhes. 15, 75-84. 10.1080/15419060802014016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solan, J. L. and Lampe, P. D. (2009). Connexin43 phosphorylation: structural changes and biological effects. Biochem. J. 419, 261-272. 10.1042/BJ20082319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solan, J. L. and Lampe, P. D. (2016). Kinase programs spatiotemporally regulate gap junction assembly and disassembly: effects on wound repair. Semin. Cell Dev. Biol. 50, 40-48. 10.1016/j.semcdb.2015.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, W., Liu, C. and Upadhyaya, A. (2014). The pivotal position of the actin cytoskeleton in the initiation and regulation of B cell receptor activation. Biochim. Biophys. Acta Biomembr. 1838, 569-578. 10.1016/j.bbamem.2013.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorgen, P. L., Trease, A. J., Spagnol, G., Delmar, M. and Nielsen, M. S. (2018). Protein–protein interactions with connexin 43: regulation and function. Int. J. Mol. Sci. 19, E1428. 10.3390/ijms19051428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squecco, R., Sassoli, C., Nuti, F., Martinesi, M., Chellini, F., Nosi, D., Zecchi-Orlandini, S., Francini, F., Formigli, L. and Meacci, E. (2006). Sphingosine 1-phosphate induces myoblast differentiation through Cx43 protein expression: a role for a gap junction-dependent and -independent function. Mol. Biol. Cell 17, 4896-4910. 10.1091/mbc.e06-03-0243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland, C. L., Krebs, D. L. and Gold, M. R. (1999). An 11-amino acid sequence in the cytoplasmic domain of CD40 is sufficient for activation of c-Jun N-terminal kinase, activation of MAPKAP kinase-2, phosphorylation of I kappa B alpha, and protection of WEHI-231 cells from anti-IgM-induced growth arrest. J. Immunol. 162, 4720-4730. [PubMed] [Google Scholar]
- Swenson, K. I., Piwnica-Worms, H., McNamee, H. and Paul, D. L. (1990). Tyrosine phosphorylation of the gap junction protein connexin43 is required for the pp60v-src-induced inhibition of communication. Mol. Biol. Cell 1, 975-1067. 10.1091/mbc.1.13.989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi, S., Takayama, Y., Ogawa, A., Tamura, K. and Okada, M. (2000). Transmembrane phosphoprotein Cbp positively regulates the activity of the carboxyl-terminal Src kinase, Csk. J. Biol. Chem. 275, 29183-29186. 10.1074/jbc.C000326200 [DOI] [PubMed] [Google Scholar]
- Tamir, I. and Cambier, J. C. (1998). Antigen receptor signaling: integration of protein tyrosine kinase functions. Oncogene 17, 1353-1364. 10.1038/sj.onc.1202187 [DOI] [PubMed] [Google Scholar]
- Tolar, P. (2017). Cytoskeletal control of B cell responses to antigens. Nat. Rev. Immunol. 17, 621-634. 10.1038/nri.2017.67 [DOI] [PubMed] [Google Scholar]
- Treanor, B., Depoil, D., Gonzalez-Granja, A., Barral, P., Weber, M., Dushek, O., Bruckbauer, A. and Batista, F. D. (2010). The membrane skeleton controls diffusion dynamics and signaling through the B cell receptor. Immunity 32, 187-199. 10.1016/j.immuni.2009.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale, M. L., Akpovi, C. D. and Pelletier, R.-M. (2009). Cortactin/tyrosine-phosphorylated cortactin interaction with connexin 43 in mouse seminiferous tubules. Microsc. Res. Tech. 72, 856-867. 10.1002/jemt.20771 [DOI] [PubMed] [Google Scholar]
- Warn-Cramer, B. J., Lampe, P. D., Kurata, W. E., Kanemitsu, M. Y., Loo, L. W. M., Eckhart, W. and Lau, A. F. (1996). Characterization of the mitogen-activated protein kinase phosphorylation sites on the connexin-43 gap junction protein. J. Biol. Chem. 271, 3779-3786. 10.1074/jbc.271.7.3779 [DOI] [PubMed] [Google Scholar]
- Weber, M., Treanor, B., Depoil, D., Shinohara, H., Harwood, N. E., Hikida, M., Kurosaki, T. and Batista, F. D. (2008). Phospholipase C-γ2 and Vav cooperate within signaling microclusters to propagate B cell spreading in response to membrane-bound antigen. J. Exp. Med. 205, 1243-1243. 10.1084/jem.20072619040408c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed, S. A. and Parsons, J. T. (2001). Cortactin: coupling membrane dynamics to cortical actin assembly. Oncogene 20, 6418-6434. 10.1038/sj.onc.1204783 [DOI] [PubMed] [Google Scholar]
- Xu, X. (2006). Connexin 43-mediated modulation of polarized cell movement and the directional migration of cardiac neural crest cells. Development 133, 3629-3639. 10.1242/dev.02543 [DOI] [PubMed] [Google Scholar]
- Yam-Puc, J. C., Zhang, L., Zhang, Y. and Toellner, K.-M. (2018). Role of B-cell receptors for B-cell development and antigen-induced differentiation. F1000Research 7, 429. 10.12688/f1000research.13567.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamadori, T., Baba, Y., Matsushita, M., Hashimoto, S., Kurosaki, M., Kurosaki, T., Kishimoto, T. and Tsukada, S. (1999). Bruton's tyrosine kinase activity is negatively regulated by Sab, the Btk-SH3 domain-binding protein. Proc. Natl. Acad. Sci. USA 96, 6341-6346. 10.1073/pnas.96.11.6341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokozeki, T., Adler, K., Lankar, D. and Bonnerot, C. (2003). B cell receptor-mediated Syk-independent activation of phosphatidylinositol 3-kinase, Ras, and mitogen-activated protein kinase pathways. J. Immunol. 171, 1328-1335. 10.4049/jimmunol.171.3.1328 [DOI] [PubMed] [Google Scholar]
- Zhou, J. Z. and Jiang, J. X. (2014). Gap junction and hemichannel-independent actions of connexins on cell and tissue functions - an update. FEBS Lett. 588, 1186-1192. 10.1016/j.febslet.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.