Abstract
Introduction
Although catheter ablation (CA) of tachycardia-bradycardia syndrome (TBS) in patients with atrial fibrillation (AF) is considered to be an effective treatment strategy, pacemaker implantations (PMIs) are often required even after a successful CA. This study aimed to elucidate the clinical predictors of a PMI after CA.
Methods
From 2011 to 2020, 103 consecutive patients diagnosed with TBS were retrospectively enrolled in the study. Among the 103 patients, 54 underwent a PMI and 49 CA of AF. During 47.4 ± 35.4 months after 1.4 ± 0.6 CA sessions, 37 (75.5%) of 49 patients were free from atrial arrhythmia recurrences. PMIs were performed in 11 patients (PMI group) and the remaining 38 did not receive a PMI (non-PMI group).
Results
When comparing the PMI and non-PMI groups, there were no differences in the basic mean heart rate (P = 0.36), maximum pauses detected by 24-hour Holter-monitoring (P = 0.61), and other clinical parameters between the two groups while the right atrial area index was larger (42.1 ± 24.0 vs. 21.8 ± 8.4 cm2/m2 P = 0.002) in the PMI group than non-PMI group. The ROC curve analysis showed that the optimal cutoff point of the ratio of the right atrial area index to the left atrial area index for predicting a PMI following CA was 0.812 (Sensitivity 72.7%, specificity 71.1%, positive predictive value 42.1%, negative predictive value 90.0%, diagnostic accuracy 71.4%, AUC = 0.81).
Conclusion
Right atrial enlargement prior to CA was considered to be one of the risk factors for a PMI after CA of AF.
Keywords: Tachycardia bradycardia syndrome, Catheter ablation, Pacemaker implantation, Right atrium volume index
1. Introduction
Tachycardia-bradycardia syndrome (TBS) refers to prolonged sinus pauses after the termination of supraventricular tachycardia, mostly paroxysmal atrial fibrillation (AF) [1]. TBS is often associated with syncope or light headless resulting in trauma such as a head injury or pacemaker implantation (PMI), which has been the conventional treatment for TBS [2]. Although PMIs are considered as a first-line therapy for TBS, device-related problems become issues. As the number of device implantations is increasing due to the aging population, the risk of device-related complications including infections, lead failures, and perioperative risks during PMIs is rising.
Recently, catheter ablation (CA) has been shown to be a curative therapy for atrial tachyarrhythmias (ATs) and has emerged as an alternative treatment strategy for TBS [3], [4]. The CA may eliminate the triggers of tachycardia, mostly ectopic beats from the pulmonary veins, and eventually prevent bradycardia. The guidelines have determined that CA could be an alternative treatment for patients with TBS in order to avoid a PMI [5], [6], and previous studies have shown that greater than 85% of the patients may avoid a PMI when CA of AF is successfully performed [7], [8]. Successful CA results in “no tachyarrhythmias lead to no sinus pauses”. However, the long-term outcomes of CA are unclear and some of the patients eventually require a PMI even after a successful CA, and whether CA should be considered as the first-line therapy for TBS in AF patients remains controversial. The purpose of this study was to elucidate the clinical predictors of a PMI after CA for TBS.
2. Methods
2.1. Study population
From March 2011 to December 2020, 103 consecutive patients diagnosed with TBS due to AF were retrospectively enrolled in the study. TBS was defined as symptomatic (presyncope or syncope) prolonged sinus pauses of at least three seconds on termination of AF. The study protocol conformed to the Declaration of Helsinki and the present study was conducted with the prior approval of the Ethics Committee of Nippon Medical School Hospital (Approved No. B-2022-569). All the patients gave written informed consent for the PMI and CA. Patients who had received CA or cardiac surgery in the past were excluded from the study.
2.2. Catheter ablation and pacemaker implantation
All antiarrhythmic drugs were discontinued for at least five half-lives before the ablation. The electrophysiological study and CA were performed in a fasting state under conscious sedation after an appropriate dose of anticoagulation for at least one month. All patients underwent transesophageal echocardiography or multidetector computed tomography with contrast medium within one month prior to the CA to exclude left atrial thrombi.
The intracardiac electrograms and surface electrocardiograms were continuously monitored and recorded using the EP Workmate (St. Jude Medical, Minneapolis, Minnesota, USA). A 20-polar catheter with 2–2–2 mm interelectrode spacing (BeeAT, Japan Lifeline Co., Ltd, Tokyo, Japan) was introduced from the right internal jugular vein and advanced into the coronary sinus. A transseptal puncture was performed using an RF needle (Baylis Medical, Montreal, Quebec, Canada) inserted through a long sheath (SL 0 and/or SL 8.5, St. Jude Medical). Heparin was initiated before the transseptal puncture and an activated clotting time of 300–350 s was maintained with a continuous administration of heparin during catheter manipulation and the ablation procedure in the left atrium (LA). After transseptal access, one or two 7-Fr duo-decapolar circular mapping catheters (Lasso, Biosense Webster, Diamond Bar, CA, USA, or Optima, Abbott, Abbot Park, IL, USA) and an irrigated ablation catheter (SmartTouch, Biosense Webster Inc. or FlexAbility, Abbott) were inserted into the LA. A steerable sheath (Agilis, St. Jude Medical, Minneapolis, Minnesota, USA) was used for the ablation catheter in all procedures. The procedures were guided with an electroanatomical mapping system (CARTO system [Biosense Webster Inc.] or Ensite Velocity system [Velocity, Abbott]) in which the 3-dimensional reconstructed computerized tomography images of the LA and pulmonary veins (PV) were merged with real-time anatomical maps. The radiofrequency power was delivered at 30–40 W to the LA and reduced to 20 W inside the coronary sinus. In patients who underwent cryoballoon ablation, the 28 mm cryoballoon catheter (Arctic Front Advance, Medtronic) was utilized through the steerable sheath into the LA with a 20-mm small-diameter circular mapping catheter inserted in the central lumen of the cryoballoon and used as a guidewire. Cryoablation with a minimum ablation duration of 3 min was utilized. Lesions that failed to isolate the vein within 60 s or achieve a temperature colder than −40 ℃ after 60 s of ablation were considered ineffectual and terminated. Thereafter the balloon and/or guidewire was re-positioned, and a new lesion delivered. The procedural endpoint was bidirectional conduction block of all PVs after a 10-minute observation period. If a reconnection of a PV was observed, a repeat ablation was performed until the block was achieved.
The PMIs were performed using a standard technique under local anesthesia with sedative drugs. All the patients who underwent a PMI received a dual-chamber PM. The right atrial bipolar and right ventricular bipolar screw-in leads were inserted via the left or right subclavian vein and placed in the right ventricular appendage or right ventricular apex. The lower rate limit was programmed at 60–70 beats per minute (bpm), and the maximal tracking rate was programmed to 130 bpm.
2.3. Follow-up
After discharge from the hospital, the patients were seen in the outpatient clinic every month for the first three months with a 12-lead electrocardiogram and every two or three months thereafter with 24-hour Holter monitoring. The patient data such as bradyarrhythmia-related symptoms, recurrence of AF, repeated CA, usage of medications, and the incidence of hospitalizations were collected during the follow-up. In the case of an AF recurrence, which was defined as any AT of at least 30 s occurring after the three-month blanking period, the patients were offered to choose a permanent pacemaker implantation or repeat ablation. The repeat CA consisted of a re-isolation of reconnected PVs and adding linear lesions.
2.4. Statistical analysis
The continuous variables are presented as the mean ± standard deviation and compared using an independent sample t-test, and the categorical variables are presented as the count and percentage and analyzed using the chi-square test. All tests were two-sided and a P value of <0.05 was considered statistically significant. A Kaplan-Meier analysis and log-rank test were performed to analyze the probability of the freedom from an arrhythmia recurrence after CA. Receiver operator characteristic curves were generated to determine the sensitivity, specificity, and positive and negative predictive values of the top performing features. The statistical analyses were conducted using SPSS 28.0 software (IBM Inc., Armonk, NY, USA).
3. Results
3.1. Clinical outcomes of AF ablation
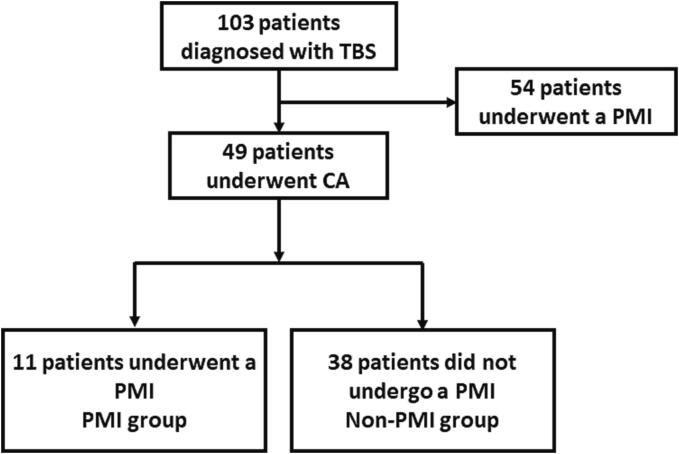
Among the 103 patients diagnosed with TBS, 54 underwent a PMI and the remaining 49 underwent CA of AF (Fig. 1). The patient characteristics are shown in Table 1. The average age was significantly younger in the CA group than PMI group (P < 0.001). Among the 49 patients, the ablation was performed by radiofrequency CA in 42 patients and cryoablation in 7. All patients underwent a successful circumferential PV isolation. Additional linear ablation was performed in 35 of 49 (71.4%) patients that included a cavotricuspid isthmus line (N = 27), roof line (N = 28), inferior line (N = 28), mitral isthmus line (N = 6) and superior vena cava isolation (N = 2). Procedure-related complications occurred in two patients; one patient had a stroke and another had pericarditis with a small pericardial effusion, which was successfully managed without pericardiocentesis.
Fig. 1.
The patient flow chart of the study. CA = catheter ablation, PMI = pacemaker implantation, TBS = tachycardia-bradycardia syndrome.
Table 1.
Clinical characteristics of the study subjects.
| Underwent CA N = 49 |
Underwent a PMI N = 54 |
P value | |
|---|---|---|---|
| Age (years) | 74.2 ± 8.3 | 82.1 ± 6.3 | <0.001 |
| Female (n, %) | 25 (51.0%) | 35 (64.8%) | 0.16 |
| BMI (kg/m2) | 23.5 ± 3.9 | 21.5 ± 3.1 | 0.002 |
| Basic heart rate (beats/min) | 58.9 ± 7.0 | 59.1 ± 6.8 | 0.84 |
| Diabetes (n, %) | 10 (20.4%) | 11 (20.4%) | 0.99 |
| Hypertension (n, %) | 37 (75.5%) | 42 (77.8%) | 0.79 |
| Coronary heart disease (n, %) | 0 (0%) | 7 (13.0%) | 0.009 |
| Stroke (n, %) | 6 (12.2%) | 13 (24.1%) | 0.12 |
| AF duration (years) | 3.6 ± 6.0 | 2.9 ± 4.7 | 0.20 |
| Longest pause (second) | 4.8 ± 2.1 | 6.8 ± 2.5 | <0.001 |
| Syncope (n, %) | 20 (40.8%) | 30 (55.6%) | 0.14 |
| LAD (mm) | 36.8 ± 6.3 | 39.3 ± 6.6 | 0.05 |
| LVEDD (mm) | 44.0 ± 5.4 | 43.8 ± 5.6 | 0.57 |
| LVEF (%) | 70.0 ± 7.7 | 70.5 ± 9.9 | 0.31 |
| CHA2DS2-VASc score | 3.2 ± 1.6 | 4.4 ± 1.7 | <0.001 |
| Antiarrhythmic medication (n, %) | 21 (42.9%) | 16 (29.6%) | 0.16 |
| Pilsicainide (n, %) | 11 (22.4%) | 7 (13.0%) | 0.21 |
| Flecainide (n, %) | 3 (6.1%) | 0 (0%) | 0.07 |
| Amiodarone (n, %) | 1 (2.0%) | 8 (14.8%) | 0.022 |
| d-Sotalol (n, %) | 2 (4.1%) | 0 (0%) | 0.13 |
| Bepridil (n, %) | 1 (2.0%) | 0 (0%) | 0.29 |
| β-blockers (n, %) | 20 (40.8%) | 23 (42.6%) | 0.86 |
| Calcium channel blocker (n, %) | 21 (42.9%) | 23 (42.6%) | 0.98 |
AF atrial fibrillation, BMI body mass index, CA catheter ablation, CHA2DS2-VASc congestive heart failure, hypertension, age, diabetes mellitus, stroke, vascular disease, age, sex category, LAD left atrial diameter, LVEDD left ventricular end diastolic diameter, LVEF left ventricular ejection fraction, PMI pacemaker implantation.
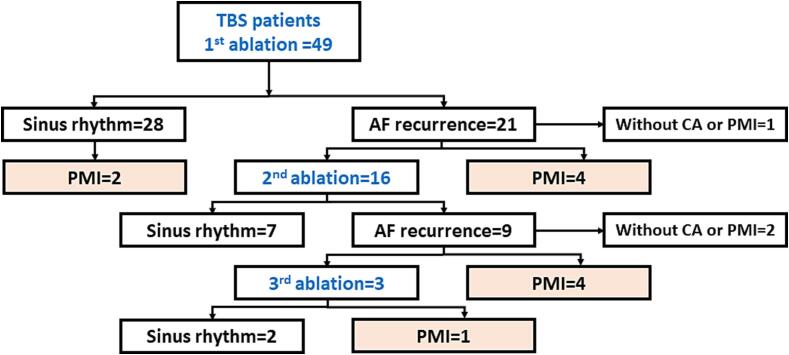
During a mean follow-up of 51.2 ± 36.4 months after the initial CA, 28 (57.1%) patients remained free from AT recurrences. Average heart rate was significantly increased after 1 month from CA as compared with baseline (58.4 ± 7.4 bpm vs. 64.6 ± 8.4 bpm p = 0.002). The number of patients who underwent a repeat procedure is shown in Fig. 2. Eventually, during a mean follow-up period of 47.4 ± 35.4 months after 1.4 ± 0.6 CA sessions, sinus rhythm was maintained in 37 (75.5%) patients.
Fig. 2.
The flowchart of the ablation outcomes after each procedure. AF = atrial fibrillation, CA = catheter ablation, PMI = pacemaker implantation, TBS = tachycardia-bradycardia syndrome.
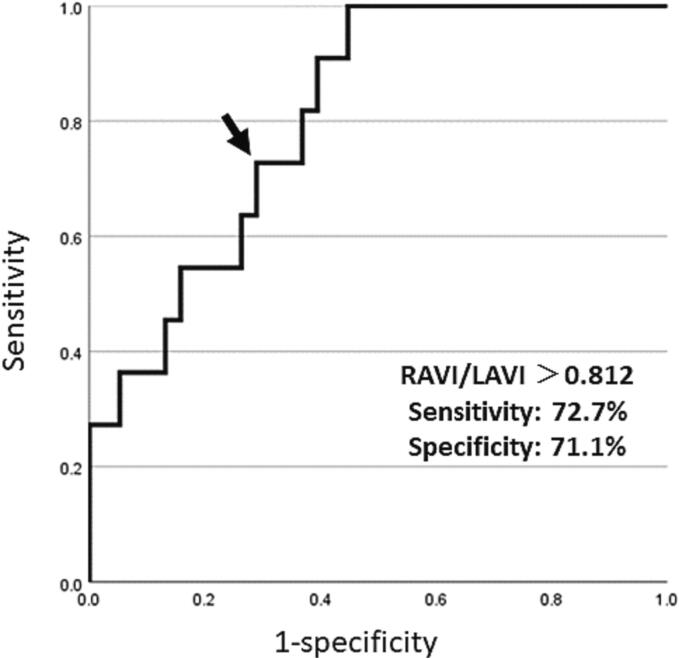
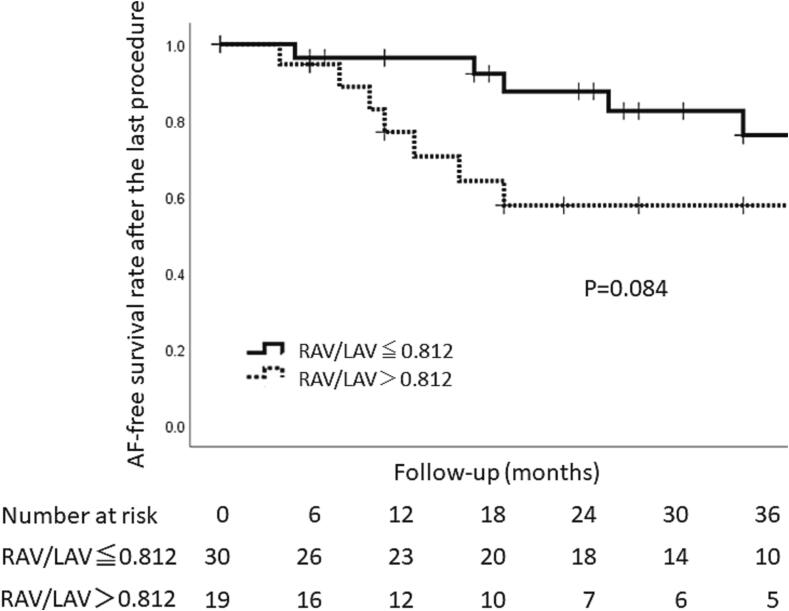
After the CA, a PMI was performed in 2 patients without arrhythmia recurrences and 9 with AT recurrences (PMI group), however, the remaining 38 patients did not receive a PM (non-PMI group). All the subjects in the PMI group were implanted with a dual-chamber pacemaker without preprocedural complications 15.2 ± 11.7 months after the initial CA. A comparison of the clinical characteristics between the two groups is shown in Table 2. The gender, age, baseline heart rate, and usage of β blockers and antiarrhythmic drugs (AADs) were similar between the two groups. The mean AF duration (2.6 ± 2.9 years vs. 3.9 ± 6.8 years P = 0.81) and CHA2DS2-VASc score (3.6 ± 1.6 vs. 3.1 ± 1.5P = 0.27) did not significantly differ between the two groups. Regarding the echo parameters, the right atrial volume index (RAVI) (42.1 ± 24.0 vs. 21.8 ± 8.4 cm3/m2 P = 0.002) and RAVI/LAVI (1.06 ± 0.11 vs. 0.67 ± 0.05 cm3/m2 P = 0.002) were significantly larger in the PMI group than non-PMI group while the LA volume index (LAVI) (39.1 ± 13.1 vs. 35.1 ± 14.1 cm3/m2 P = 0.24) was similar between the two groups. A receiver operating characteristic curve analysis of the RAVI/LAVI for predicting a PMI following CA exhibited an area under curve of 0.81 (CI 95% [0.68–0.94]) and identified an RAVI/LAVI of more than 0.812 to have a 72.7% sensitivity, 71.1% specificity, 42.1% positive predictive value, 90.0% negative predictive value, and 71.4% diagnostic accuracy (Fig. 3). The arrhythmia free rate after CA had a tendency to be lower in patients with an RAV/LAV > 0.812 cm3/m2 as compared to those with an RAV/LAVI ≤ 0.182 cm3/m2 (63.2% vs. 83.3% Log-rank P = 0.084) (Fig. 4).
Table 2.
Comparison of the clinical characteristics between the PMI group and non-PMI group.
| Variables | PMI group N = 11 | Non-PMI group N = 38 |
P value |
|---|---|---|---|
| Age (years) | 77.5 ± 5.3 | 73.3 ± 8.9 | 0.08 |
| Female (n, %) | 7 (63.6%) | 18 (47.4%) | 0.34 |
| BMI (kg/m2) | 23.5 ± 3.6 | 23.5 ± 4.1 | 0.98 |
| Basic mean heart rate (beats/min) | 60.6 ± 5.4 | 58.4 ± 7.4 | 0.36 |
| Diabetes (n, %) | 2 (18.2%) | 8 (21.1%) | 0.84 |
| Hypertension (n, %) | 9 (81.8%) | 28 (73.7%) | 0.58 |
| Coronary heart disease (n, %) | 0 (0%) | 0 (0%) | 1.00 |
| Stroke (n, %) | 1 (9.1%) | 5 (13.2%) | 0.72 |
| AF duration (years) | 2.6 ± 2.9 | 3.9 ± 6.8 | 0.81 |
| Longest pause (second) | 4.8 ± 1.5 | 4.7 ± 2.1 | 0.61 |
| Syncope (n, %) | 5 (45.5%) | 15 (39.5%) | 0.72 |
| LAD (mm) | 36.5 ± 5.8 | 36.9 ± 6.6 | 0.86 |
| LVEDD (mm) | 43.1 ± 6.7 | 44.2 ± 5.2 | 0.58 |
| LVEF (%) | 69.2 ± 10.7 | 69.8 ± 6.8 | 0.81 |
| LA volume index | 39.1 ± 13.1 | 35.1 ± 14.1 | 0.24 |
| RA volume index | 42.1 ± 24.0 | 21.8 ± 8.4 | 0.002 |
| RAVI/LAVI | 1.06 ± 0.11 | 0.67 ± 0.05 | 0.002 |
| TRPG (mmHg) | 23.3 ± 4.1 | 23.9 ± 8.6 | 0.74 |
| CHA2DS2-VASc score | 3.6 ± 1.6 | 3.1 ± 1.5 | 0.27 |
| Antiarrhythmic medication (n, %) | 3 (27.3%) | 18 (47.4%) | 0.24 |
| Pilsicainide (n, %) | 0 (0%) | 11 (28.9%) | 0.04 |
| Flecainide (n, %) | 2 (18.2%) | 1 (2.6%) | 0.06 |
| Amiodarone (n, %) | 1 (9.1%) | 0 (0%) | 0.06 |
| d-Sotalol (n, %) | 0 (0%) | 2 (5.3%) | 0.44 |
| Bepridil (n, %) | 0 (0%) | 1 (2.6%) | 0.59 |
| β-blockers (n, %) | 4 (36.4%) | 16 (42.1%) | 0.73 |
| Calcium channel blocker (n, %) | 6 (54.5%) | 15 (39.5%) | 0.37 |
| Number of CA | 1.5 ± 0.7 | 1.3 ± 0.6 | 0.31 |
AF atrial fibrillation, BMI body mass index, CA catheter ablation, CHA2DS2-VASc congestive heart failure, hypertension, age, diabetes mellitus, stroke, vascular disease, age, sex category, LAD left atrial diameter, LAVI left atrial volume index, LVEDD left ventricular end diastolic diameter, LVEF left ventricular ejection fraction, PMI pacemaker implantation, RAVI right atrial volume index, TRPG tricuspid regurgitant pressure gradient.
Fig. 3.
The ROC curve of the RAVI/LAVI was more than 1.23 for predicting a pacemaker implantation following catheter ablation of atrial fibrillation.
Fig. 4.
Kaplan-Meier analysis of the freedom from recurrent AF after the last catheter ablation procedure. Events were not counted during the first 3 month blanking period. The solid line and dotted line indicate the arrhythma free rate in patients whose ratio of the right atrial area index to left atrial area index was 0.812 or more and in those whose index was less than 0.812, respectively. RAVI = right atrium volume index, LAVI = left atrium volume index.
The three patients with recurrence of AF did not receive a pacemaker implantation as their normal daily activity was not affected by symptoms related to AF. The patient characteristics in 11 patients in the PMI group are shown in Table 3. The reasons for the pacemaker indications were sinus bradycardia (N = 4), recurrence of TBS due to post ablation AT (N = 6), and development of complete atrioventricular block (N = 1). All the subjects had arrhythmia related symptoms including syncope (N = 7) and dizziness (N = 4). The 9 patients with recurrence included AF recurrences (N = 4) and AT recurrences (N = 5). The pacemaker implantation was performed due to atrial tachyarrhythmia recurrence and bradycardia suggested symptoms (dizziness or syncope) without ECG recording after CA in patient No. 6 and 9 (Table 3).
Table 3.
Patients characteristics in the PMI group.
| Age (year) | Sex | RAVI/LAVI | LA size (mm) | Longest pause (seconds) | CHA2DS2-VASc score | AAD after ablation | No. of CA procedure | AF or AT recurrence after CA | Symptom after CA | Sinus arrest/sinus brady/AVB | Pause after CA (seconds) | Time interval from CA to PMI (days) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 85 | M | 1.45 | 40 | 5.0 | 5 | None | 1 | AT recurrence | Syncope | sinus brady | 4.7 | 9 |
| 2 | 81 | M | 1.29 | 30 | 3.0 | 2 | Bepridil | 2 | AF recurrence | Syncope | sinus arrest | 12.0 | 436 |
| 3 | 75 | F | 1.60 | 41 | 4.0 | 4 | None | 1 | AF recurrence | Syncope | sinus arrest | 8.0 | 463 |
| 4 | 78 | F | 0.74 | 38 | 4.9 | 3 | None | 1 | SR | Dizziness | sinus arrest | 5.8 | 151 |
| 5 | 79 | F | 0.82 | 43 | 6.0 | 4 | None | 3 | AT recurrence | Dizziness | sinus arrest | 7.6 | 971 |
| 6 | 78 | F | 0.66 | 43 | 7.0 | 7 | None | 1 | AF recurrence | Dizziness | None | 1.6 | 203 |
| 7 | 72 | F | 0.84 | 27 | 5.0 | 3 | None | 2 | AT recurrence | Syncope | sinus arrest | 12.1 | 348 |
| 8 | 65 | M | 0.61 | 31 | 3.0 | 1 | None | 2 | AF recurrence | Dizziness | sinus arrest | 8.8 | 1024 |
| 9 | 79 | M | 1.13 | 42 | 3.9 | 3 | None | 1 | AT recurrence | Syncope | None | 2.3 | 59 |
| 10 | 81 | F | 1.49 | 31 | 7.5 | 4 | None | 2 | AT recurrence | Syncope | sinus arrest | 7.6 | 968 |
| 11 | 80 | F | 1.04 | 36 | 3.5 | 4 | Bisoprolol | 1 | SR | Syncope | AVB | 2.2 | 669 |
AAD: antiarrhythmic drug, AF: atrial fibrillation, AT: atrial tachycardia, AVB: atrioventricular block, CA: catheter ablation, LA: left atrium, LAVI: left atrium volume index, PMI: pacemaker implantation, SR: sinus rhythm, RAVI: right atrium volume index,
3.2. Clinical outcomes between PMI group and non-PMI group
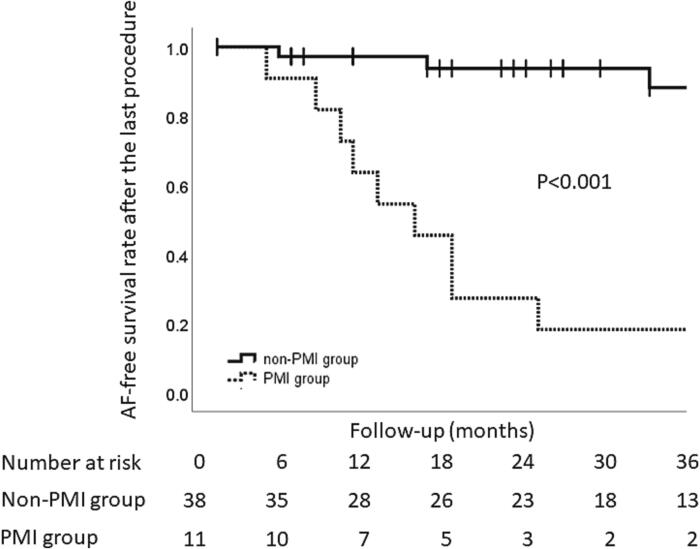
During a mean follow-up of 51.2 ± 36.4 months, 1 patient in the non-PMI group underwent coronary artery revascularization. Among the PMI group, there were heart failure hospitalizations (N = 2), ischemic strokes (N = 2), and death (N = 1) (Table 4). At the end of the follow-up, 7 patients (18.4 %) in the non-PMI group and 7 (63.6 %) in the PMI group were on AADs (P = 0.003). Although the usage of AADs was higher in the PMI group, the arrhythmia free rate was significantly lower in the PMI group than non-PMI group (18.2% vs. 92.1% Log-rank P<0.001) (Fig. 5). In the non-PM group, one patient progressed to persistent AT.
Table 4.
Comparison of the clinical outcomes between the PMI group and non-PMI group.
| Variables | PMI group N = 11 | Non-PMI group N = 38 | P value |
|---|---|---|---|
| Follow-up (months) | 66.3 ± 28.5 | 46.8 ± 37.2 | 0.07 |
| Syncope (n, %) | 1 (9.1%) | 0 (0%) | 0.06 |
| Freedom from AF (n, %) | 2 (18.2%) | 35 (92.1%) | <0.001 |
| AF progression (n, %) | 1 (9.1%) | 0 (0%) | 0.06 |
| AADs use (n, %) | 7 (63.6%) | 7 (18.4%) | 0.003 |
| Flecainide (n, %) | 2 (18.2%) | 0 (0%) | 0.007 |
| Amiodarone (n, %) | 1 (9.1%) | 1 (2.6%) | 0.34 |
| d-Sotalol (n, %) | 0 (0%) | 4 (10.5%) | 0.26 |
| Bepridil (n, %) | 4 (36.4%) | 2 (5.3%) | 0.006 |
| β-blockers (n, %) | 4 (36.4%) | 5 (13.2%) | 0.08 |
| Cardiovascular related hospitalization (n, %) | 10 (90.9%) | 14 (36.8%) | 0.002 |
| Repeat ablation (n, %) | 6 (54.5%) | 13 (34.2%) | 0.22 |
| Coronary artery disease (n, %) | 0 (0%) | 1 (2.6%) | 0.59 |
| Heart failure (n, %) | 2 (18.2%) | 0 (0%) | 0.007 |
| Stroke (n, %) | 2 (18.2%) | 0 (0%) | 0.007 |
| Death (n, %) | 1 (9.1%) | 0 (0%) | 0.06 |
AAD antiarrhythmic drug, AF atrial fibrillation, PMI pacemaker implantation.
Fig. 5.
Kaplan-Meier analysis of the freedom from recurrent AF after the last catheter ablation procedure. Events were not counted during the first 3 month blanking period. The solid line and dotted line indicate the arrhythma free rate in the non-PMI group and PMI group, respectively. PMI = pacemaker implantatiuon.
4. Discussion
The main findings of the study can be summarized as follows: (1) CA of AF in patients with TBS who did not receive a pacemaker included 77.6% of the subjects, (2) patients who underwent a PMI after AF ablation had a significantly larger right atrium, and (3) the AF free survival rate was significantly higher in the patient who did not receive a PMI after the AF ablation than in those who received a PMI among the patients with TBS.
4.1. Catheter ablation for TBS
Several previous studies examined the effectiveness of CA of AF in patients with TBS. The sinus rhythm maintenance rate ranged from 70.9% to 86.5%, and the patients who require a PMI after CA are reported to be 2.3% to 12.6% [7], [8], [9], [10]. The reasons for the PMIs after CA were a progression of the sinus bradycardia and/or a recurrence of TBS. In the present study, although sinus rhythm was maintained in 37 (75.5%) patients, which was similar to the previous studies, PMIs were required in 11 (22.4%) of the subjects, which was higher as compared to the previous findings [7], [8], [9], [10]. There are several reasons for that. First, not all of the patients underwent a repeat ablation after the recurrence of AF. The arrhythmia free rate after a single CA of PAF from the recent studies is reported to be 59% to 64% [11], [12], therefore the number of CA sessions in the present study might not have been enough to achieve suppression of the TBS triggering AF. As the decision to perform a repeat ablation depended on the clinical symptoms of the subjects, some patients had chosen a PMI mostly due to the concern of the risk of syncope. Second, the age of the subjects in the present study was 74.2 ± 8.3 years, which was older than in the previous studies [7], [8], [9], [10]. As aging is one of the major factors of sinus node dysfunction, the different patient background might have affected the sinus node function, which eventually lead to a higher requirement of a PMI. Third, the follow up interval of the present study was longer than that of the previous studies. Even after the successful CA of AF, a longer follow up might result in sinus pauses or AF recurrences requiring a PMI especially in the aging population. Fourth, 65 out of 103 patients had implanted pacemaker in the total enrolled patients. The patient was enrolled from 2011 to 2022 in the present study. Therefore, the first half of the study period, the effectiveness of catheter ablation therapy for TBS was less established and majority of the patients chose PMI. Moreover, successful ablation rate was 57.1% by first CA session which was relatively lower success rate. Recent advancement of the technology associated with CA in terms of efficacy and safety procedure might increase the successful ratio of CA.
4.2. The predictors of PMI after AF ablation
In the present study, 11 out of 49 patients were implanted with a pacemaker 482 ± 376 days after the CA. All of them experienced syncope or dizziness after the CA. Notably, 4 out of 11 patients had sinus node dysfunction that was associated with bradycardia-related symptoms. As AF and sinus node dysfunction are strongly related [13], the progression of sinus node dysfunction could occur in patients with TBS even if there is no recurrence of AF, and a pacemaker implantation is eventually required. Previous studies reported that long sinus pauses upon termination of AF, an anterior line ablation, and the E/e’ are independent predictors for a PMI after AF ablation in patients with AF and sinus node dysfunction [10], [14]. In the present study, the RA area index was larger in the patients in the PMI group than non-PMI group. Morton et al. [15] reported that chronic RA stretching causes electrical remodeling with modest increases in the RA effective refractory period, conduction delays at the crista terminalis, and sinus node dysfunction. Cho Et al. [16] reported that in patients who underwent mitral valve surgery and concomitant maze procedures, post-operative sinus node dysfunction requiring a PMI was more commonly observed in patients with moderate to severe tricuspid regurgitation than in those without. Those studies suggest that RA stretching might have contributed to the sinus node remodeling, and a dilated RA may represent an impaired intrinsic sinus function. In the present study, patients who underwent cardiac surgery were excluded and the number of patients with chronic obstructive pulmonary disease was similar between the two groups, which indicated the above clinical background did not affect the difference in the RA size. The other mechanism of the dilated RA that would be a predictor of a PMI may be the higher recurrence rate after AF ablation in patients with an enlarged RA. In the present study, the arrhythmia free rate was higher in the patients with a dilated RA. Previous studies showed that the RA diameter can affect early recurrence of AF after ablation [17] and RA anatomical remodeling predicts recurrence of AF after ablation irrespective of the AF classification [18]. Patients with an enlarged RA might have had advanced structural remodeling of the RA and subsequently contained more AF initiating extra PV triggers and substrates, which eventually would have led to a higher recurrence rate. The effect of CA with the current strategy including a pulmonary vein isolation was limited in terms of preventing AF recurrence in the patients presenting with an enlarged RA.
In the present study, more than half of the patients with AT recurrences had post-ablation AT. Post-ablation AT is often observed from less than 5 % to 40 % of the subjects after AF ablation [19]. The previous studies reported the risk factors of post-ablation AT which included the linear ablation and the complex fractionated atrial electrograms ablation [20], [21]. An AT has a fixed arrhythmia circuit and tachycardia cycle length which causes sinus pause after termination of tachyarrhythmia more frequently than AF. In the present study, five (55.5%) patients had a recurrence of AF as a post-ablation AT, which was higher than that in the previous findings. The higher prevalence may have been due to the advanced structural remodeling expressed as RA dilation and the additional linear lesions. Aldaas et al. [22] reported that an anterior line is a predictor of a PMI after AF ablation that may have been caused by a higher AT recurrence. As additional linear lesions cause post-ablation AT, creating a pulmonary vein isolation alone may be a better ablation strategy in patients with TBS. In the present study, additional linear ablation beyond the pulmonary vein isolation was performed in 71.4% of the patients. Aggressive CA might increase iatrogenic ATs requiring additional ablation procedures.
We found that the RAV/LAV ratio predicted a future PMI with a sensitivity and specificity of 72.7% and 71.1%, respectively. This parameter might be useful for a precise decision making to choose the therapeutic options for patients with TBS. The AF-free survival rate was significantly lower in the PMI group than non-PMI group. The progressive nature of atrial remodeling as an arrhythmogenic substrate rather than the PVs might be associated with recurrence of AF after CA. Therefore, both the progression of sinus node dysfunction and the development of an arrhythmogenic substrate of the atrium are considered to be the main cause of PMIs after CA in patients with TBS. Careful follow-up will be needed in patients with a larger RA volume even after a successful CA of AF.
5. Limitations
There are several limitations to the present study. First, this was a retrospective single-center study with a small study population. There may have been a selection bias during the selection of the treatment strategies. Choice of the treatment for TBS was not randomized and was mostly by shared decision making with patient’s preference. Due to the retrospective design of the present study, a further prospective study will be needed to validate the RAVI/LAVI ratio for predicting a PMI. Second, the freedom from AF more than 3 months after the ablation might possibly have been overestimated, as asymptomatic episodes of recurrent AF could not have been evaluated by regular twelve-lead electrocardiograms and 24-hour Holter monitoring.
6. Conclusions
Right atrial enlargement is considered to be one of the risk factors for a PMI after CA of AF. The patients with TBS undergoing CA of AF should be carefully followed up when there is an enlarged RA.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank John Martin for his linguistic assistance.
Footnotes
This manuscript has not been published or presented elsewhere in part or in entirety and is not under consideration by another journal. All authors have read and agree with the final version of the manuscript. The study design was approved by the appropriate ethics review board. We have read and understood your journal’s policies, and we believe that neither the manuscript nor the study violates any of these. There are no conflicts of interest to declare.
References
- 1.Kaplan B.M., Langendorf R., Lev M., Pick A. Tachycardia-bradycardia syndrome (so-called “sick sinus syndrome”). Pathology, mechanisms and treatment. Am. J. Cardiol. 1973;31(4):497–508. doi: 10.1016/0002-9149(73)90302-0. [DOI] [PubMed] [Google Scholar]
- 2.Nogami A., Kurita T., Abe H., Ando K., Ishikawa T., Imai K., Usui A., Okishige K., Kusano K., Kumagai K., Goya M., Kobayashi Y., Shimizu A., Shimizu W., Shoda M., Sumitomo N., Seo Y., Takahashi A., Tada H., Naito S., Nakazato Y., Nishimura T., Nitta T., Niwano S., Hagiwara N., Murakawa Y., Yamane T., Aiba T., Inoue K., Iwasaki Y., Inden Y., Uno K., Ogano M., Kimura M., Sakamoto S.I., Sasaki S., Satomi K., Shiga T., Suzuki T., Sekiguchi Y., Soejima K., Takagi M., Chinushi M., Nishi N., Noda T., Hachiya H., Mitsuno M., Mitsuhashi T., Miyauchi Y., Miyazaki A., Morimoto T., Yamasaki H., Aizawa Y., Ohe T., Kimura T., Tanemoto K., Tsutsui H., Mitamura H. JCS/JHRS Joint Working Group, CORRIGENDUM: JCS/JHRS 2019 guideline on non-pharmacotherapy of cardiac arrhythmias. Circ. J. 2021;85(9):1692–1700. doi: 10.1253/circj.CJ-66-0196. [DOI] [PubMed] [Google Scholar]
- 3.Khaykin Y., Marrouche N.F., Martin D.O., Saliba W., Schweikert R., Wexman M., Strunk B., Beheiry S., Saad E., Bhargava M., Burkhardt J.D., Joseph G., Tchou P., Natale A. Pulmonary vein isolation for atrial fibrillation in patients with symptomatic sinus bradycardia or pauses. J. Cardiovasc. Electrophysiol. 2004;15(7):784–789. doi: 10.1046/j.1540-8167.2004.03279.x. [DOI] [PubMed] [Google Scholar]
- 4.Hocini M., Sanders P., Deisenhofer I., Jais P., Hsu L.F., Scavee C., Weerasoriya R., Raybaud F., Macle L., Shah D.C., Garrigue S., Le Metayer P., Clementy J., Haissaguerre M. Reverse remodeling of sinus node function after catheter ablation of atrial fibrillation in patients with prolonged sinus pauses. Circulation. 2003;108(10):1172–1175. doi: 10.1161/01.CIR.0000090685.13169.07. [DOI] [PubMed] [Google Scholar]
- 5.Nogami A., Kurita T., Kusano K., Goya M., Shoda M., Tada H., Naito S., Yamane T., Kimura M., Shiga T., Soejima K., Noda T., Yamasaki H., Aizawa Y., Ohe T., Kimura T., Kohsaka S., Mitamura H. Japanese Circulation Society / the Japanese Heart Rhythm Society Joint Working Group, JCS/JHRS 2021 guideline focused update on non-pharmacotherapy of cardiac arrhythmias. Circ. J. 2022;86(2):337–363. doi: 10.1253/circj.CJ-21-0162. [DOI] [PubMed] [Google Scholar]
- 6.Calkins H., Hindricks G., Cappato R., Kim Y.H., Saad E.B., Aguinaga L., Akar J.G., Badhwar V., Brugada J., Camm J., Chen P.S., Chen S.A., Chung M.K., Nielsen J.C., Curtis A.B., Davies D.W., Day J.D., d'Avila A., de Groot N., Di Biase L., Duytschaever M., Edgerton J.R., Ellenbogen K.A., Ellinor P.T., Ernst S., Fenelon G., Gerstenfeld E.P., Haines D.E., Haissaguerre M., Helm R.H., Hylek E., Jackman W.M., Jalife J., Kalman J.M., Kautzner J., Kottkamp H., Kuck K.H., Kumagai K., Lee R., Lewalter T., Lindsay B.D., Macle L., Mansour M., Marchlinski F.E., Michaud G.F., Nakagawa H., Natale A., Nattel S., Okumura K., Packer D., Pokushalov E., Reynolds M.R., Sanders P., Scanavacca M., Schilling R., Tondo C., Tsao H.M., Verma A., Wilber D.J., Yamane T. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. Heart Rhythm. 2017;14(10):e445–e494. doi: 10.1016/j.hrthm.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 7.Inada K., Yamane T., Tokutake K., Yokoyama K., Mishima T., Hioki M., Narui R., Ito K., Tanigawa S., Yamashita S., Tokuda M., Matsuo S., Shibayama K., Miyanaga S., Date T., Sugimoto K., Yoshimura M. The role of successful catheter ablation in patients with paroxysmal atrial fibrillation and prolonged sinus pauses: outcome during a 5-year follow-up. Europace. 2014;16(2):208–213. doi: 10.1093/europace/eut159. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y.W., Bai R., Lin T., Salim M., Sang C.H., Long D.Y., Yu R.H., Tang R.B., Guo X.Y., Yan X.L., Nie J.G., Du X., Dong J.Z., Ma C.S. Pacing or ablation: which is better for paroxysmal atrial fibrillation-related tachycardia-bradycardia syndrome? Pacing Clin. Electrophysiol. 2014;37(4):403–411. doi: 10.1111/pace.12340. [DOI] [PubMed] [Google Scholar]
- 9.Zhang S., Yang Y., Xia Y., Gao L., Zhang X., Tse G., Yin X., Dai S., Chang D. Long-term effect of catheter ablation on tachycardia-bradycardia syndrome: evidenced by 10 years follow up. Acta Cardiol. 2020;75(6):537–543. doi: 10.1080/00015385.2019.1630055. [DOI] [PubMed] [Google Scholar]
- 10.Kim D.H., Choi J.I., Lee K.N., Ahn J., Roh S.Y., Lee D.I., Shim J., Kim J.S., Lim H.E., Park S.W., Kim Y.H. Long-term clinical outcomes of catheter ablation in patients with atrial fibrillation predisposing to tachycardia-bradycardia syndrome: a long pause predicts implantation of a permanent pacemaker. BMC Cardiovasc. Disord. 2018;18(1):106. doi: 10.1186/s12872-018-0834-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuck K.H., Brugada J., Furnkranz A., Metzner A., Ouyang F., Chun K.R., Elvan A., Arentz T., Bestehorn K., Pocock S.J., Albenque J.P., Tondo C. FIRE AND ICE Investigators, Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N. Engl. J. Med. 2016;374(23):2235–2245. doi: 10.1056/NEJMoa1602014. [DOI] [PubMed] [Google Scholar]
- 12.Natale A., Reddy V.Y., Monir G., Wilber D.J., Lindsay B.D., McElderry H.T., Kantipudi C., Mansour M.C., Melby D.P., Packer D.L., Nakagawa H., Zhang B., Stagg R.B., Boo L.M., Marchlinski F.E. Paroxysmal AF catheter ablation with a contact force sensing catheter: results of the prospective, multicenter SMART-AF trial. J. Am. Coll Cardiol. 2014;64(7):647–656. doi: 10.1016/j.jacc.2014.04.072. [DOI] [PubMed] [Google Scholar]
- 13.Jackson L.R., 2nd, Rathakrishnan B., Campbell K., Thomas K.L., Piccini J.P., Bahnson T., Stiber J.A., Daubert J.P. Sinus node dysfunction and atrial fibrillation: a reversible phenomenon? Paci. Clin. Electrophysiol. 2017;40(4):442–450. doi: 10.1111/pace.13030. [DOI] [PubMed] [Google Scholar]
- 14.Hwang T.H., Yu H.T., Kim T.H., Uhm J.S., Kim J.Y., Joung B., Lee M.H., Pak H.N. Permanent pacemaker implantations after catheter ablation in patients with atrial fibrillation associated with underlying sinus node dysfunction. Korean Circ. J. 2020;50(4):346–357. doi: 10.4070/kcj.2019.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morton J.B., Sanders P., Vohra J.K., Sparks P.B., Morgan J.G., Spence S.J., Grigg L.E., Kalman J.M. Effect of chronic right atrial stretch on atrial electrical remodeling in patients with an atrial septal defect. Circulation. 2003;107(13):1775–1782. doi: 10.1161/01.CIR.0000058164.68127.F2. [DOI] [PubMed] [Google Scholar]
- 16.Cho M.S., Heo R., Jin X., Lee J.B., Lee S., Kim D.H., Kim J.B., Kim J., Jung S.H., Choo S.J., Song J.M., Nam G.B., Choi K.J., Kang D.H., Chung C.H., Lee J.W., Kim Y.H., Song J.K. Sick sinus syndrome after the maze procedure performed concomitantly with mitral valve surgery. J. Am. Heart Assoc. 2018;7(19):e009629. doi: 10.1161/JAHA.118.009629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moon J., Hong Y.J., Shim J., Hwang H.J., Kim J.Y., Pak H.N., Lee M.H., Joung B. Right atrial anatomical remodeling affects early outcomes of nonvalvular atrial fibrillation after radiofrequency ablation. Circ. J. 2012;76(4):860–867. doi: 10.1253/circj.cj-11-1232. [DOI] [PubMed] [Google Scholar]
- 18.Takagi T., Nakamura K., Asami M., Toyoda Y., Enomoto Y., Moroi M., Noro M., Sugi K., Nakamura M. Impact of right atrial structural remodeling on recurrence after ablation for atrial fibrillation. J. Arrhythm. 2021;37(3):597–606. doi: 10.1002/joa3.12541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veenhuyzen G.D., Knecht S., O'Neill M.D., Phil D., Wright M., Nault I., Weerasooriya R., Miyazaki S., Sacher F., Hocini M., Jais P., Haissaguerre M. Atrial tachycardias encountered during and after catheter ablation for atrial fibrillation: part I: classification, incidence, management. Pacing Clin. Electrophysiol. 2009;32(3):393–398. doi: 10.1111/j.1540-8159.2008.02250.x. [DOI] [PubMed] [Google Scholar]
- 20.Estner H.L., Hessling G., Biegler R., Schreieck J., Fichtner S., Wu J., Jilek C., Zrenner B., Ndrepepa G., Schmitt C., Deisenhofer I. Complex fractionated atrial electrogram or linear ablation in patients with persistent atrial fibrillation–a prospective randomized study. Pacing Clin. Electrophysiol. 2011;34(8):939–948. doi: 10.1111/j.1540-8159.2011.03100.x. [DOI] [PubMed] [Google Scholar]
- 21.Chugh A., Oral H., Lemola K., Hall B., Cheung P., Good E., Tamirisa K., Han J., Bogun F., Pelosi F., Jr., Morady F. Prevalence, mechanisms, and clinical significance of macroreentrant atrial tachycardia during and following left atrial ablation for atrial fibrillation. Heart Rhythm. 2005;2(5):464–471. doi: 10.1016/j.hrthm.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 22.Aldaas O.M., Lupercio F., Lin A.Y., Han F.T., Hoffmayer K.S., Raissi F., Ho G., Krummen D., Feld G.K., Hsu J.C. Ablation of mitral annular flutter ablation utilizing a left atrial anterior line versus a lateral mitral isthmus line: a systematic review and meta-analysis. J. Interv. Card. Electrophysiol. 2022;63(1):87–95. doi: 10.1007/s10840-021-00943-x. [DOI] [PMC free article] [PubMed] [Google Scholar]