Abstract
Background:
The aims of this study were to evaluate the protective effects of agmatine against cisplatin-induced cellular apoptosis in an auditory cell line and to prove the protective mechanism of agmatine.
Methods:
The House Ear Institute-Organ of Corti 1 cells were co-treated with agmatine at different concentrations and 15 µM of cisplatin for 48 hours. Cell viability and proliferation were measured. Annexin V-fluorescein isothiocyanate /propidium iodide staining was performed to analyze apoptosis. The levels of intracellular reactive oxygen species were measured using flow cytometry. The expression of BCL2-associated X protein and the enzymatic activity of caspase-3 was measured to examine the pathway of apoptosis induction.
Results:
In normal conditions, the maximal protective effect occurred with 10 mM of agmatine. However, in the presence of cisplatin, the maximal protective effect was observed from 8 mM of agmatine. Thus, 8 mM was chosen as the ideal agmatine concentration for the analysis of protective effects against cisplatin-induced cytotoxicity. Agmatine exerted a significant protective effect against 15 µM of cisplatin when applied for 48 hours and reduced the proportion of necrotic and late apoptotic cells. Agmatine did not significantly reduce the cisplatin-induced increase in reactive oxygen species but decreased the expression of BCL2-associated X protein and the activity of caspase-3.
Conclusion:
Agmatine protected against cisplatin-induced cellular apoptosis in an auditory cell line. These effects were mediated by the protection of mitochondrial function and inhibition of apoptosis.
Keywords: Agmatine, cisplatin, ototoxicity, mitochondria, apoptosis
Main Points
Agmatine (8 mM) exerted significant protective effects against cisplatin-induced cellular apoptosis in House Ear Institute-Organ of Corti 1 auditory cells.
Agmatine (8 mM) significantly reduced the proportion of cells in necrosis and late apoptosis induced by cisplatin.
Agmatine (8 mM) did not show a significant reduction of reactive oxygen species in cisplatin-induced cytotoxicity.
Agmatine (8 mM) significantly reduced apoptosis via the inhibition of BCL2-associated X protein related to cisplatin-induced apoptosis.
Agmatine (8 mM) inhibited the expression of caspase 3 activity.
Introduction
Ototoxicity refers to damage to the inner ear, which is caused by various therapeutic agents. Aminoglycoside, loop diuretics, and platinum-based chemotherapeutic agents are known to cause ototoxicity. In addition, the clinical use of these agents can cause sensorineural hearing loss, tinnitus, and dizziness.1 Therefore, several studies focused on the pharmacologic prevention of ototoxicity have been conducted. However, to date, no agents have been used clinically to cure or prevent ototoxicity.2
The House Ear Institute-Organ of Corti 1 (HEI-OC1) cells are the widely used auditory cell line in the study of the ototoxicity.3 The House Ear Institute-Organ of Corti 1 cells express several molecular markers that are characteristic of the organ of Corti sensory cells and are extremely sensitive to ototoxic drugs. It is well known that cisplatin causes potentially irreversible damage to auditory hair cells; clinically, this may lead to sensorineural hearing loss, tinnitus, and dizziness.4 The sensorineural hearing loss appears to be dose-related, cumulative, bilateral, and usually permanent; initially, it occurs only in the higher frequencies.5,6 In a recent systematic review study, in addition to the cumulative dose, age, genetics, and use of other ototoxic drugs were revealed as risk factors for sensorineural hearing loss.7 One known mechanism of cisplatin ototoxicity is the generation of excessive reactive oxygen species (ROS). Reactive oxygen species can deplete antioxidant enzymes in the cochlear tissue and can increase calcium influx and apoptosis in the hair cells of the cochlea.5 Reactive oxygen species activates BCL2-associated X protein (BAX) in the cytosol, translocating it to the mitochondria and allowing cytochrome c release into the cytosol; it can also activate caspase-3 and -9, which induce cellular apoptosis.8,9
Agmatine (decarboxylated arginine) is an endogenous polyamine synthesized via decarboxylation of l-arginine by arginine decarboxylase.10,11 As shown in a previous study, agmatine has oxygen radical-scavenging effects (antioxidant activity), protects mitochondrial function, and confers resistance to cellular apoptosis.12 The agmatine-induced protection of mitochondria is presumed to be a key mechanism of action, and agmatine effectively inhibits ROS-induced activation of BAX. Therefore, numerous studies have been conducted in the field of neuroscience to examine its effect as a neuromodulator for protecting the central nervous system (CNS) in several models of cellular damage.10-14 Clinically, there is a growing interest in the role of agmatine in epilepsy, Parkinson’s disease, Alzheimer’s disease, depression, spinal cord injury, and neuropathic diseases.15,16 However, there has been no investigation in the use of agmatine to prevent ototoxicity.
Thus, this study aimed to evaluate the protective effects of agmatine against cisplatin-induced cellular apoptosis in the HEI-OC1 auditory cell line. We hypothesized that agmatine may reduce several molecular markers that are released by cisplatin-induced cellular apoptosis in the HEI-OC1 auditory cell line. Therefore, the primary endpoint of the present study was to investigate the exact dose range for the protective effects of agmatine and to confirm the potential of agmatine to scavenge ROS. The second endpoint was to investigate the protective mechanism in the cellular metabolism by using BAX, a representative apoptosis regulator.
Materials and Methods
Chemicals
Agmatine (CAS no. 2482-0-0) and cisplatin (CAS no. 15663-27-1) were obtained from Sigma Aldrich Corporation (St Louis, Mo, USA). Agmatine and cisplatin were prepared as 5 mM stock solutions in cell culture medium and diluted to the appropriate concentrations.
Auditory Cell Culture
The HEI-OC1 cell line was used as the auditory cell line in this study. The HEI-OC1 cell line is a transgenic immortomouse auditory cell line and was provided by F. Kalinec (House Ear Institute, Los Angeles, Calif, USA). The cells were cultured in high-glucose Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco BRL, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS; JRH Bioscience, Lexena, KS, USA) and 50 U/mL interferon-γ without antibiotics at 33.8ºC and in 10% CO2 in the air. Cell culture practices used a previously reported method.17
Cell Viability Assay
To examine the effect of agmatine on HEI-OC1 cells, we used the water soluble tetrazolium salt (WST)-based cell viability/cytotoxicity assay kit (EZ-CYTOX, DOGEN, Korea) to measure cell viability in the cell proliferation and cytotoxicity assays. The House Ear Institute-Organ of Corti 1 cells (2 × 104 cells/well in 48-well microplates) were incubated with 2-20 mM agmatine for 48 hours. After incubation, the culture medium was removed and replaced with a fresh culture medium; subsequently, 10 μL of assay solution was added to each well of the microplate, and the microplates were incubated for 2 hours at 33.8ºC and in 10% CO2 in the air. The optical density of the contents of each well was measured at 450 nm by using a microplate reader (Spectra Max, Molecular Devices, Sunnyvale, Calif, USA).
To examine the protective effect of agmatine against cisplatin-induced cellular apoptosis, we co-treated the cells with 2-20 mM agmatine and 15 µM cisplatin (a concentration that was shown to result in a 50% decrease in cell viability in our previous studies) for 48 hours. After 48 hours of incubation, cell viability was measured by using EZ-CYTOX.
Cell Proliferation Assay
To examine the cell proliferation effect of agmatine on HEI-OC1 cells, we used the 5-bromo-2-deoxyuridine (BrdU) cell proliferation assay kit (Biovision, Milpitas, Calif, USA). For assay, HEI-OC1 cells (2 × 104 cells/well in 48-well microplates) were incubated with 8 mM agmatine and 15 µM cisplatin for 48 hours. 5-bromo-2-deoxyuridine was added to cell at 10 µL/mL in culture media. After cell incubation for 1 hour, it was fixing and denaturing. Incubation was done with the BrdU detection antibody for 1 hour and reacted with the anti-mouse horseradish peroxidase (HRP)-linked antibody. After washing, 3,3’,5,5’-tetramethylbenzidine (TMB) substrate was added to each well for color development. To stop the color development, a stop solution was added to each well. The plates were measured for absorbance at 450 nm using a microplate reader (SpectraMax, Molecular Devices, Sunnyvale, Calif, USA).
Flow Cytometric Assay of Apoptosis
Annexin V-fluorescein isothiocyanate (FITC) (Ezway Annexin V-FITC Apoptosis Detection Kit, Komabiotech, Seoul, Korea) was used to determine the patterns of cellular apoptosis. The specific binding of annexin V-FITC occurred during incubation of the cells for 15 minutes at room temperature in binding buffer containing annexin V-FITC and propidium iodide (PI) at saturating concentrations. A minimum of 10 000 cells was analyzed by FACScan flow cytometry (BD Biosciences, Heidelberg, Germany).
Intracellular ROS Measurement
The fluorescent dye, 2',7'-dichlorofluorescein diacetate (DCFH-DA, Calbiochem, San Diego, Calif, USA), was used to measure the intracellular ROS level. In the presence of oxidizing agents, DCFH was converted to the highly fluorescent 2',7'-dichlorofluorescein (DCF). For the analysis, HEI-OC1 cells were incubated in the dark with 50 µm DCFH-DA for 30 minutes at 37ºC. A FACScan flow cytometer was used to analyze the fluorescence at an excitation wavelength of 495 nm and an emission wavelength of 530 nm, with gating at 10 000 cells/sample.
Western Blotting Analysis of BAX (Bcl-2-associated X Protein)
The primary antibodies (BAX (cat no. 2772), beta-actin (cat no. 4967)) were obtained from Cell Signaling Technology (Danvers, Mass, USA). Cell lysates were resuspended in radioimmune precipitation assay buffer, and equal amounts of protein (20 µg/sample) were immediately heated at 100ºC for 5 minutes and resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The isolated proteins were transferred to nitrocellulose membranes, and Western blotting was done using gel-loading and protein transfer system kits (Bio-Rad, Hercules, Calif, USA). Non-specific binding to the membrane was subsequently blocked by incubation with 5% skim milk in Tris buffer solution containing 0.1% Tween (TBST). The membrane was incubated in solutions of primary polyclonal antibodies prepared at a final dilution of 1:1000. After 3 washes in TBST, the membranes were incubated with peroxidase-conjugated secondary antibodies in blocking buffer (final dilution, 1:2000) for 1 hour and then washed again. A chemiluminescent solution was applied to the membranes (ECL solution; Gendepot, Barker Tex, USA), and bound antibodies were detected using the ChemiDOC touch imaging system (Bio-Rad, Hercules, Calif, USA). The relative band densities were computed using ImageJ software (Imagej.nih.gov/ij/index.html).
Measurement of Caspase-3 Activity
Caspase-3 activity was analyzed by using a caspase 3/CPP32 fluorometric assay kit (Biovision, Milpitas, Calif, USA). The House Ear Institute-Organ of Corti 1cell lysates were obtained by using a lysis buffer on ice within 10 minutes and were centrifuged at 14 000 rpm for 5 minutes. The protein concentration of each lysate was measured. The catalytic activity of caspase-3 in the cell lysates was determined by measuring the proteolytic cleavage of 50 mM DEVD-AFC and fluorometric substrates at 37ºC for 2 hours. Cell lysate mixture incubated without DEVD-AFC was used as the negative control. The plates were analyzed using a microplate reader with a 400 nm excitation filter and a 505 nm emission filter (SpectraMax, Molecular Devices, Sunnyvale, Calif, USA). Caspase-3 activity was measured using a previously reported method.3
Statistical Analysis
All values are presented as the mean ± standard deviation using the Statistical Program for the Social Sciences 22.0 statistical program (IBM SPSS Corp.; Armonk, NY, USA). The paired t-test was used to analyze data pairs, and P values < .05 indicated statistical significance.
Results
Agmatine Significantly Inhibited Cisplatin-Induced Cytotoxicity on HEI-OC1 Cells Without Cell Proliferation
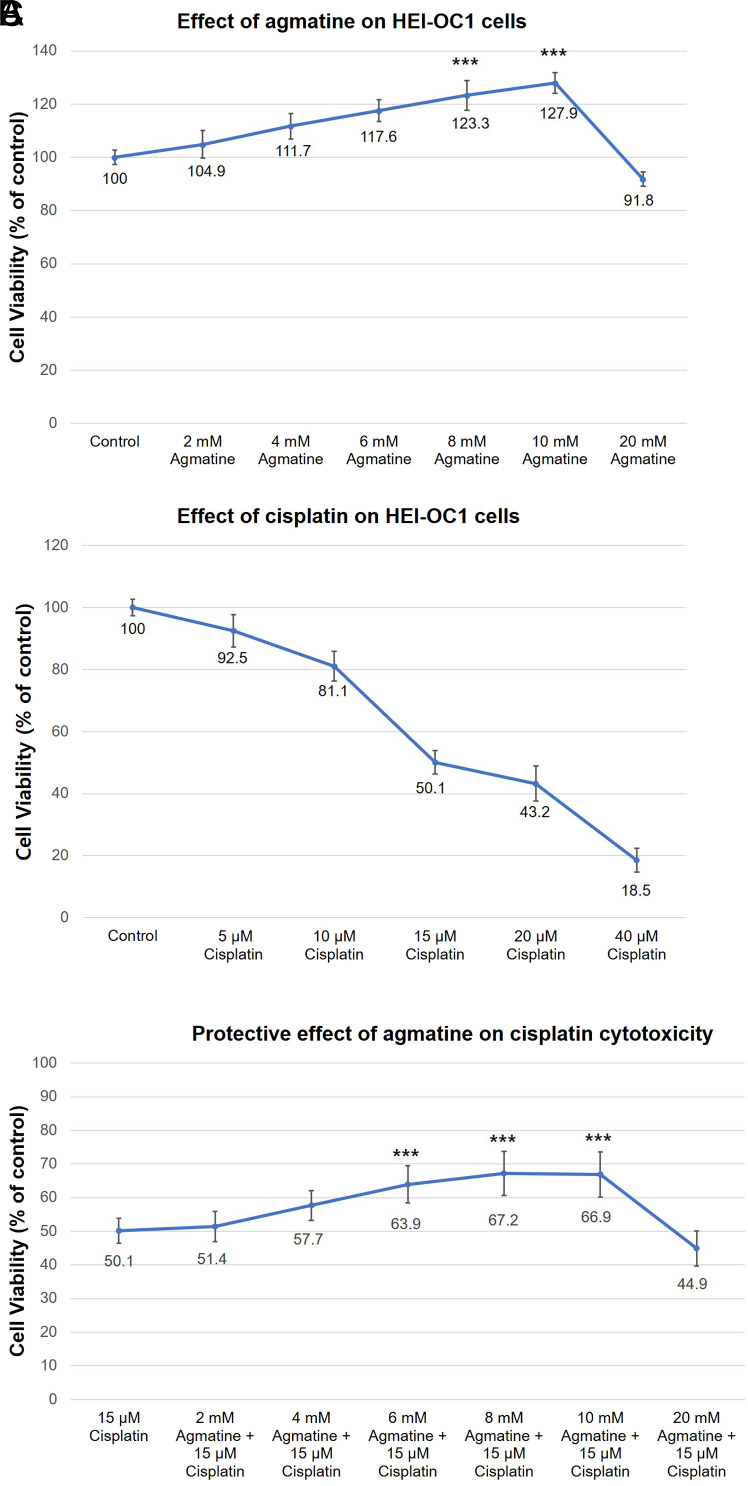
The House Ear Institute-Organ of Corti 1 cells were co-treated with 2-20 mM agmatine for 48 hours and cell viability was measured by the EZ-CYTOX assay. The maximal protective effect was observed for 10 mM agmatine, with significant protective effects relative to the control were found from 8 and 10 mM agmatine (*P < .05; **P < .01; ***P < .001, respectively). Agmatine concentrations above 20 mM resulted in lower cell viability than the control. The results were obtained from 5 separate experiments, each performed in triplicate (Figure 1A).
Figure 1. a-c.
Effect of agmatine and cisplatin on HEI-OC1 cells. HEI-OC1, House Ear Institute-Organ of Corti 1.
The protective effect of agmatine on cisplatin-induced cytotoxicity in HEI-OC1 cells was measured. The House Ear Institute-Organ of Corti 1 cells were co-treated with variable concentrations of agmatine (2-10 mM) with 15 µM cisplatin for 48 hours. Agmatine (6-10 mM) provided significant protection against the cytotoxic effects of 15 µM cisplatin (vs. 50.1% ± 3.7% viability in the cisplatin group, ***P < .001, respectively) (Figure 1B). In normal conditions, the maximal protective effect occurred with 10 mM agmatine. However, in the presence of cisplatin, the maximal protective effect was observed from 8 mM agmatine. Thus, 8 mM was chosen as the ideal agmatine concentration for the analysis of the protective effects against cisplatin-induced cytotoxicity. The results were obtained from 5 separate experiments, each performed in triplicate (Figure 1C).
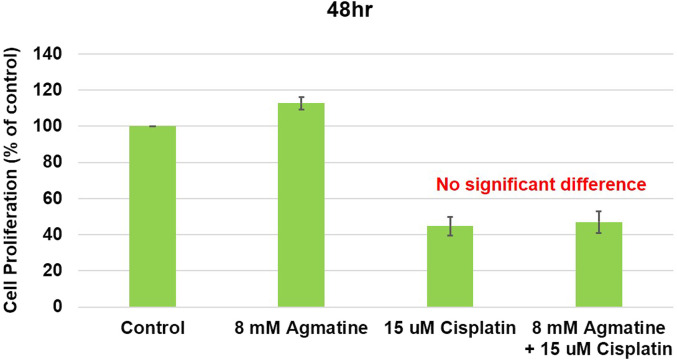
The cell proliferation effect of agmatine on HEI-OC1 cells was measured. In the cisplatin group, cell proliferation was decreased by 44.7% ± 5.1% compared to that in the control group. Agmatine co-treatment (8 mM) resulted in a reduction in cell proliferation, and the difference was not statistically significant. (47.0% ± 6.0% vs. 44.7% ± 5.1% decreases, respectively, compared with the control group; P = .7 for the agmatine vs. cisplatin comparison) (Figure 2). It was estimated that agmatine inhibits cisplatin-induced cytotoxicity without cell proliferation. The results were obtained from 5 separate experiments.
Figure 2.
Cell proliferation study of agmatine on HEI-OC1 cells. HEI-OC1, House Ear Institute-Organ of Corti 1.
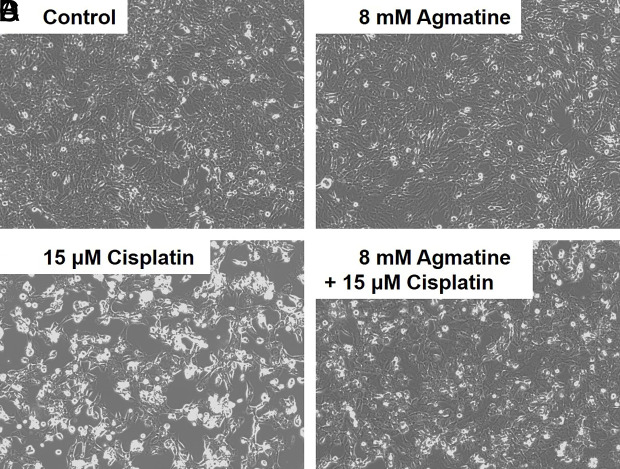
Representative microscopy images showed the protective effect of agmatine against cisplatin-induced cytotoxicity in HEI-OC1 cells. In the 8 mM agmatine group, a small level of cellular growth was observed, similar to that in the control group (Figure 2A and 2B). In the 15 µM cisplatin group, significant necrotic debris and a decreased cell population were observed on the surface of the cell culture (Figure 2C). Co-treatment with 8 mM agmatine resulted in cell sizes within the normal range and a decrease in necrotic debris compared to those in the cisplatin group (Figure 2D).
Agmatine Significantly Reduced the Proportion of Cells in Necrosis and Late Apoptosis Induced by Cisplatin
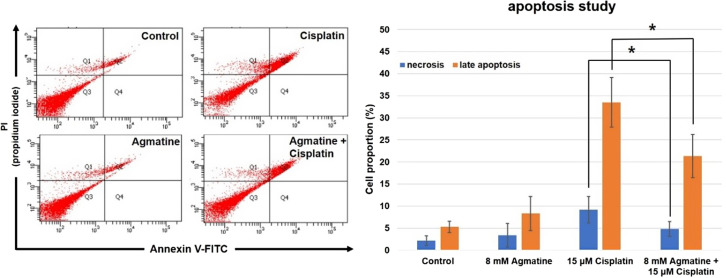
Flow cytometric analysis was used to identify necrotic or late apoptotic cells (Figure 3A). In both the control and 8 mM agmatine groups, the percentages of necrotic and late apoptotic cells were similar (2.2% ± 1.1% and 5.3% ± 1.3% in the control group; 3.4% ± 2.7% and 8.3% ± 3.9% in the agmatine group). In the 15 µm cisplatin group, significantly higher densities were observed for both necrosis and late apoptosis areas (9.2% ± 3.0% and 33.5 ± 5.6%, respectively) than in the control and agmatine groups (*P < .05). Agmatine treatment significantly reduced both necrosis and late apoptosis (Figure 3B): necrosis was reduced from 9.2% to 4.8% ± 1.7% (*P < .05) and late apoptosis was reduced from 33.5% to 21.3% ± 4.9% (**P < .05). The results were obtained from 5 separate experiments.
Figure 3. a-d.
Representative microscopy images showing the protective effects of agmatine against cisplatin-induced cytotoxicity in cultured auditory cells.
Agmatine Did Not Show Significant Reduction of ROS in Cisplatin-Induced Cytotoxicity
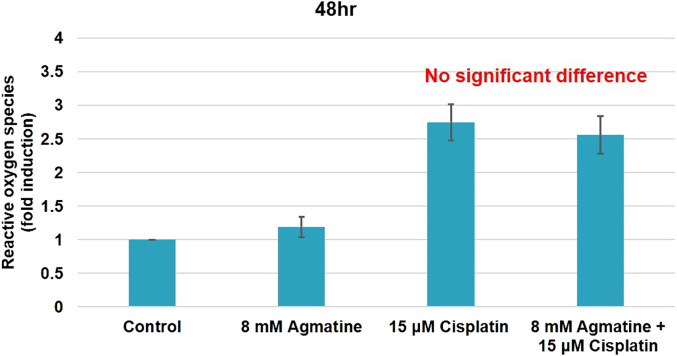
To investigate the effects of agmatine on the intracellular ROS generation induced by cisplatin, HEI-OC1 cells were treated with 15 µM cisplatin in the presence or absence of 8 mM agmatine for 48 hours. Unlike the control group, the cisplatin group showed a significant increase in ROS generation (2.75 ± 0.27-fold, ***P < .001). Co-treatment with 8 mM agmatine showed reduction in ROS, but it did not show statistical significance as expected (2.56 ± 0.28-fold vs. 2.75 ± 0.27-fold increases, respectively, compared with the control group; P = .3 for the agmatine vs. cisplatin comparison) (Figure 4). The results were obtained from 5 separate experiments.
Figure 4.
Study of effect of agmatine on apoptosis.
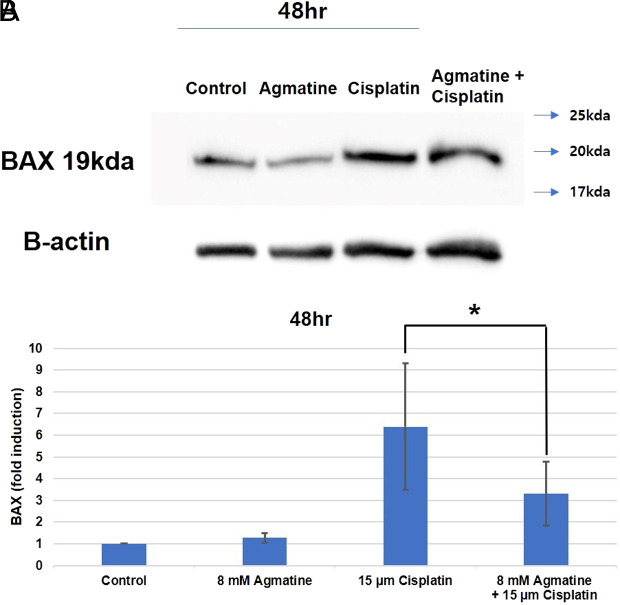
Agmatine Significantly Reduced Apoptosis Via the Inhibition of BAX Related to Cisplatin-induced Apoptosis
BAX is a known pro-apoptotic protein in the mitochondrial apoptosis pathway. In the cisplatin group, the activity of BAX increased compared to that in the control group (***P < .001). Co-treatment of HEI-OC1 cells with 8 mM agmatine and cisplatin significantly decreased the activity of BAX unlike cisplatin treatment alone (*P < .05) (Figure 5). The results were obtained from 5 separate experiments.
Figure 5.
Production of intracellular reactive oxygen species (ROS).
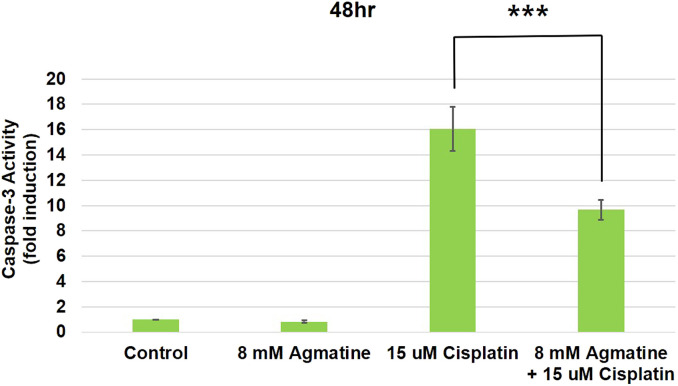
Agmatine Inhibited the Expression of Caspase-3 Activity
Caspase-3 activity is involved in cisplatin-induced toxicity and is related to the apoptotic changes that occur in cisplatin ototoxicity. The administration of 15 µM cisplatin increased the activity of caspase-3 (16.04 ± 1.74-fold compared with control cells). Co-treatment of HEI-OC1 cells with 8 mM agmatine and cisplatin significantly reduced caspase-3 activity (9.68 ± 0.78-fold compared with the normal control) compared to that in cells treated with cisplatin alone (***P < .001) (Figure 6). The results were obtained from 5 separate experiments.
Figure 6.
Western blotting for BCL2-associated X protein (BAX).
Discussion
To the best of our knowledge, this is the first study to investigate the protective effects of agmatine on auditory cells. Our results showed that agmatine exhibited protective effects at 8 and 10 mM. In addition, our results showed that 8 mM agmatine may confer effective protection against the cellular apoptosis induced by cisplatin. Although 8 mM agmatine did not significantly reduce ROS, it was found to inhibit cellular apoptosis via protective effects on the mitochondrial function of HEI-OC1 cells, as shown by the changes in BAX expression and caspase-3 activity.
The various beneficial effects of agmatine on human health are documented.18 Agmatine is a natural product that was discovered more than 100 years ago; however, research into this compound is continuing. The known clinical effects include neuroprotection, neuropathic pain reduction, nerve regeneration, and antidepressant and antioxidant effects.18 Therefore, although agmatine is currently a compound of interest in the field of neuroscience, its potential activities related to otology have not yet been explored. In previous studies, agmatine was shown to exert protective effects against cell damage and cellular apoptosis in human neuroblastoma cells, hippocampal neuronal cells, and retinal neuronal cells.10-14,19,20 Although this study investigated the protective effects of agmatine on auditory cells, previous studies have mainly shown its protective effects on neuronal cells. Thus, the authors expect that the protective effect of agmatine on spiral ganglion neuronal cell will be determined in future studies.
According to previous studies on the protective effect of agmatine in other cell lines, a concentration of 250 nM-100 µM is required to exert a protective effect.10,11,14,19,20 The agmatine concentrations used in this study (8 mM) were relatively higher than those used in previous studies; however, this is presumed to be due to the different toxic agents and cell lines used. The authors will perform an animal study to investigate the optimal dose required for protective effects in vivo. As mentioned above, agmatine did not reduce the excess ROS induced by cisplatin in this study but was considered to protect auditory cells from apoptosis through a reduction in the expression of BAX. Song et al21 reported that diphenyleneiodonium reduced cellular apoptosis in kidney proximal tubular epithelial cells without a reduction in ROS owing to the upregulation of Bcl2. Cellular apoptosis was reduced by maintaining mitochondrial membrane permeabilization through Bcl2 family regulation; the results of our present study are considered to be similar to those of that study.
A limitation of this study was that the experiments were performed on an auditory cell line and were not in vivo experiments. Our results confirmed that agmatine protected auditory cells against cisplatin-induced cytotoxicity; however, this may not indicate definitively that agmatine can protect against ototoxicity in humans. In the future, we intend to investigate whether agmatine protects auditory cells against cisplatin-induced cytotoxicity in vivo study.
Conclusion
Agmatine exerted significant protective effects against cisplatin-induced cellular apoptosis in HEI-OC1 auditory cells. These effects were mediated by the protection of mitochondrial function and the inhibition of apoptosis via the regulation of BAX.
Figure 7.
Measurement of caspase-3 activity.
Footnotes
Ethics Committee Approval: N/A.
Informed Consent: N/A.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – E.P.; Design – E.P., S.H.L., G.J.I.; Supervision – H.H.J., G.J.I.; Funding – E.P., H.H.J., G.J.I.; Materials – E.P., S.H.L., G.J.I.; Data Collection and/or Processing – E.P., S.H.L.; Analysis and/or Interpretation – E.P., S.H.L., G.J.I.; Literature Review – E.P., S.H.L., G.J.I.; Writing – E.P., S.H.L., G.J.I.; Critical Review – E.P., S.H.L., H.H.J., G.J.I.
Declaration of Interests: The authors have no conflict of interest to declare.
Funding: This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2021R1I1A1A01052753), the National Research Grant funded by a Korea Medical Device Development Fund grant, which was funded by the Korea government (Ministry of Science and ICT, Ministry of Trade, Industry and Energy, Ministry of Health & Welfare, Ministry of Food and Drug Safety) (Project Numbers: KMDF_PR_20200901_0183-2021-02, 1711138387), and Korea University Research Fund (K2100821, K2125741). These funding sources provided only financial support and played no specific scientific role in this study.
The content of this study is published in the preprint. We will inform you of the URL as below.
References
- 1. Rybak LP, Ramkumar V. Ototoxicity. Kidney Int. 2007;72(8):931 935. 10.1038/sj.ki.5002434) [DOI] [PubMed] [Google Scholar]
- 2. Im GJ, Chang J, Lee S.et al. Protective role of edaravone against cisplatin-induced ototoxicity in an auditory cell line. Hear Res. 2015;330(A):113 118. 10.1016/j.heares.2015.08.004) [DOI] [PubMed] [Google Scholar]
- 3. Kim SK, Im GJ, An YS, Lee SH, Jung HH, Park SY. The effects of the antioxidant α-tocopherol succinate on cisplatin-induced ototoxicity in HEI-OC1 auditory cells. Int J Pediatr Otorhinolaryngol. 2016;86:9 14. 10.1016/j.ijporl.2016.04.008) [DOI] [PubMed] [Google Scholar]
- 4. Alam SA, Ikeda K, Oshima T.et al. Cisplatin-induced apoptotic cell death in Mongolian gerbil cochlea. Hear Res. 2000;141(1-2):28 38. 10.1016/s0378-5955(99)00211-7) [DOI] [PubMed] [Google Scholar]
- 5. Rybak LP. Mechanisms of cisplatin ototoxicity and progress in otoprotection. Curr Opin Otolaryngol Head Neck Surg. 2007;15(5):364 369. 10.1097/MOO.0b013e3282eee452) [DOI] [PubMed] [Google Scholar]
- 6. Bokemeyer C, Berger CC, Hartmann JT.et al. Analysis of risk factors for cisplatin-induced ototoxicity in patients with testicular cancer. Br J Cancer. 1998;77(8):1355 1362. 10.1038/bjc.1998.226) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gon çalves LF, Paiva KM, Haas P. Ototoxic effects of antineoplastic drugs: a systematic review. Braz J Otorhinolaryngol. 2022;88(1):130 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Watanabe K, Inai S, Jinnouchi K, Baba S, Yagi T. Expression of caspase-activated deoxyribonuclease (CAD) and caspase 3 (CPP32) in the cochlea of cisplatin (CDDP)-treated guinea pigs. Auris Nasus Larynx. 2003;30(3):219 225. 10.1016/s0385-8146(03)00049-x) [DOI] [PubMed] [Google Scholar]
- 9. Wang J, Ladrech S, Pujol R, Brabet P, Van De Water TR, Puel JL. Caspase inhibitors, but not c-Jun NH2-terminal kinase inhibitor treatment, prevent cisplatin-induced hearing loss. Cancer Res. 2004;64(24):9217 9224. 10.1158/0008-5472.CAN-04-1581) [DOI] [PubMed] [Google Scholar]
- 10. Song J, Lee B, Kang S.et al. Agmatine ameliorates high glucose-induced neuronal cell senescence by regulating the p21 and p53 signaling. Exp Neurobiol. 2016;25(1):24 32. 10.5607/en.2016.25.1.24) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Han N, Yu L, Song Z, Luo L, Wu Y. Agmatine protects Muller cells from high-concentration glucose-induced cell damage via N-methyl-D-aspartic acid receptor inhibition. Mol Med Rep. 2015;12(1):1098 1106. 10.3892/mmr.2015.3540) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Condello S, Currò M, Ferlazzo N, Caccamo D, Satriano J, Ientile R. Agmatine effects on mitochondrial membrane potential and NF-kappaB activation protect against rotenone-induced cell damage in human neuronal-like SH-SY5Y cells. J Neurochem. 2011;116(1):67 75. 10.1111/j.1471-4159.2010.07085.x) [DOI] [PubMed] [Google Scholar]
- 13. Amiri E, Ghasemi R, Moosavi M. Agmatine protects Against 6-OHDA-induced apoptosis, and ERK and Akt/GSK disruption in SH-SY5Y cells. Cell Mol Neurobiol. 2016;36(6):829 838. 10.1007/s10571-015-0266-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Condello S, Calabrò E, Caccamo D.et al. Protective effects of agmatine in rotenone-induced damage of human SH-SY5Y neuroblastoma cells: fourier transform infrared spectroscopy analysis in a model of Parkinson's disease. Amino Acids. 2012;42(2-3):775 781. 10.1007/s00726-011-0994-z) [DOI] [PubMed] [Google Scholar]
- 15. Zomkowski AD, Hammes L, Lin J, Calixto JB, Santos AR, Rodrigues AL. Agmatine produces antidepressant-like effects in two models of depression in mice. NeuroReport. 2002;13(4):387 391. 10.1097/00001756-200203250-00005) [DOI] [PubMed] [Google Scholar]
- 16. Moretti M, Matheus FC, de Oliveira PA.et al. Role of agmatine in neurodegenerative diseases and epilepsy. Front Biosci (Elite Ed). 2014;6(2):341 359. 10.2741/E710) [DOI] [PubMed] [Google Scholar]
- 17. Lee SH, Kim HS, An YS, Chang J, Choi J, Im GJ. Protective effect of resveratrol against cisplatin-induced ototoxicity in HEI-OC1 auditory cells. Int J Pediatr Otorhinolaryngol. 2015;79(1):58 62. 10.1016/j.ijporl.2014.11.008) [DOI] [PubMed] [Google Scholar]
- 18. Piletz JE, Aricioglu F, Cheng JT.et al. Agmatine: clinical applications after 100 years in translation. Drug Discov Today. 2013;18(17-18):880 893. 10.1016/j.drudis.2013.05.017) [DOI] [PubMed] [Google Scholar]
- 19. Freitas AE, Egea J, Buendía I.et al. Agmatine induces Nrf2 and protects against corticosterone effects in hippocampal neuronal cell line. Mol Neurobiol. 2015;51(3):1504 1519. 10.1007/s12035-014-8827-1) [DOI] [PubMed] [Google Scholar]
- 20. Iizuka Y, Hong S, Kim CY, Yang WI, Lee JE, Seong GJ. Protective mechanism of agmatine pretreatment on RGC-5 cells injured by oxidative stress. Braz J Med Biol Res. 2010;43(4):356 358. 10.1590/S0100-879X2010007500018) [DOI] [PubMed] [Google Scholar]
- 21. Song H, Han IY, Kim Y.et al. The NADPH oxidase inhibitor DPI can abolish hypoxia-induced apoptosis of human kidney proximal tubular epithelial cells through Bcl2 up-regulation via ERK activation without ROS reduction. Life Sci. 2015;126:69 75. 10.1016/j.lfs.2015.02.004) [DOI] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a