Abstract
Background
The optimal timing of birth for women with an otherwise uncomplicated twin pregnancy at term is uncertain, with clinical support for both elective delivery at 37 weeks, as well as expectant management (awaiting the spontaneous onset of labour).
Objectives
To assess a policy of elective delivery from 37 weeks' gestation compared with an expectant approach for women with an otherwise uncomplicated twin pregnancy.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (12 December 2013).
Selection criteria
Randomised controlled trials with reported data that compared outcomes in mothers and babies who underwent elective delivery from 37 weeks' gestation in a twin pregnancy with outcomes in controls who were managed expectantly.
Data collection and analysis
At least two review authors independently assessed trial eligibility, trial quality and extracted data from the included trials.
Main results
Two randomised controlled trials comparing elective birth at 37 weeks for women with an uncomplicated twin pregnancy, with expectant management were included, involving 271 women and 542 infants. One trial was at an overall low risk of bias, and one trial was at unclear risk of selection bias, performance bias and detection bias.
There were no statistically significant differences identified between a policy of elective birth at 37 weeks' gestation and expectant management with regards to birth by caesarean section (two studies; 271 participants; risk ratio (RR) 1.05; 95% confidence interval (CI) 0.83 to 1.32); perinatal death or serious perinatal morbidity (two studies; 542 infants; RR 0.34; 95% CI 0.01 to 8.35); or maternal death or serious maternal morbidity (one study; 235 women; RR 0.29; 95% CI 0.06 to 1.38).
There were no statistically significant differences identified for the pre‐specified secondary maternal and infant review outcomes reported by these two trials between the two treatment policies (including for: haemorrhage requiring blood transfusion; instrumental vaginal birth; meconium‐stained liquor; Apgar score less than seven at five minutes; admission to neonatal intensive care; birthweight less than 2500 g; neonatal encephalopathy; and respiratory distress syndrome). While not a pre‐specified review outcome, elective birth at 37 weeks, compared with expectant management, was shown to significantly reduce the risk of infants being born with a birthweight less than the third centile (one study; 470 infants; RR 0.30; 95% CI 0.13 to 0.68).
Authors' conclusions
Early birth at 37 weeks' gestation compared with ongoing expectant management for women with an uncomplicated twin pregnancy does not appear to be associated with an increased risk of harms, findings which are consistent with the United Kingdom's National Institute for Health and Care Excellence (NICE) recommendations which advocate birth for women with a dichorionic twin pregnancy at 37 + 0 weeks' gestation. It is unlikely that sufficient clinical equipoise exists to allow for the randomisation of women to a later gestational age at birth.
Plain language summary
Elective birth of women with an uncomplicated twin pregnancy from 37 weeks' gestation
The optimal timing of birth for women with a twin pregnancy is uncertain, with clinical support for both elective delivery at 37 weeks' gestation (either by induction of labour or caesarean birth), and for waiting for labour to start spontaneously (expectant management).
Two randomised controlled trials were included in this review involving a total of 271 women with twin pregnancies at 37 weeks' gestation. One of the two trials (involving 235 women) was of high quality, and the quality of the second trial (involving 36 women) was unclear. There were no differences shown between the group of women who had an elective birth at 37 weeks' gestation and the group of women who waited for labour to start spontaneously for the outcomes: birth by caesarean section, perinatal (fetal or neonatal) death or serious perinatal morbidity, or maternal death or serious maternal morbidity. No other differences between the two groups of women were shown for other pregnancy and birth complications or for complications for the infant.
Elective birth at 37 weeks' gestation compared with ongoing expectant management for women with uncomplicated twin pregnancies does not appear be associated with an increased risk of harms.
Background
There are a number of clinical situations where elective induction of labour has been advocated with the aim of reducing adverse outcomes for both mother and baby. These situations have included induction to reduce the risks associated with the development of macrosomia (large‐for‐gestational‐age infant) in women requiring insulin therapy for diabetes (Boulvain 2001); induction of labour where a clinical suspicion of macrosomia exists (Irion 1998); and induction of labour in women with an otherwise low‐risk singleton pregnancy after 41 weeks (Gülmezoglu 2012). On the basis of these systematic reviews, induction of labour after 41 weeks is the only intervention associated with a reduction in perinatal mortality.
Description of the condition
Although women with a twin pregnancy are more likely to give birth preterm, just under half will give birth after 37 weeks' gestation (Law 2009). The optimal timing of birth for women with an otherwise uncomplicated twin pregnancy at term is uncertain, with clinical support for both elective delivery at 37 weeks, as well as expectant management (awaiting the spontaneous onset of labour).
Luke and colleagues (Luke 1993) retrospectively reviewed 163 women with a twin pregnancy, and developed several models of the 'ideal twin pregnancy'. Using multivariate logistic regression, the best model of intrauterine growth and lowest perinatal morbidity was at an earlier gestation for twins than for singletons. Using length of stay and growth restriction criteria, 70% of women with an 'ideal' twin pregnancy gave birth between 35 and 38 weeks' gestation. Cincotta and colleagues retrospectively reviewed data from Queensland (Australia), over a 10‐year period in 6328 women with a twin pregnancy, to establish the gestational age‐specific stillbirth risk for both twins and singleton gestations (Cincotta 2001). On the basis of this information, the authors concluded that the gestation‐specific rise in stillbirth rate seen in singletons at 40 weeks and beyond occurs in twins from 36 weeks' gestation and onwards. Minakami and Sato have suggested that the estimated date of confinement in women with a multiple pregnancy is between 37 and 38 weeks' gestation (Minakami 1996). This is based on retrospective information obtained from almost 89,000 infants born to women with a multiple pregnancy in Japan between 1989 and 1993. This study found a mean gestation at birth for twins of 37 weeks, with the risk of stillbirth and early neonatal death increasing after 38 weeks' gestation. The lowest risk of perinatal death in multiple pregnancies at 38 weeks' gestation corresponded to that observed in singleton pregnancies at 43 weeks' gestation. Cheung and colleagues obtained similar data from the Swedish Medical Birth Registry, for women with a twin pregnancy giving birth between 1982 and 1995 (Cheung 2000). The models used identified a higher mortality rate among twins born after 37 weeks when compared with singleton infants at a similar gestational age. Hartley and colleagues retrospectively analysed the birth and death certificates, and hospital discharge data for 8150 twin pairs born in Washington State between 1987 and 1997 (Hartley 2001). The lowest perinatal mortality rate for twin gestations was found with birth at 37 weeks' gestation.
In retrospective data from South Australia obtained between 1991 and 2000, the stillbirth rate for women with a twin pregnancy was found to be higher than for singletons at each week of gestational age (Dodd 2003). An increase in stillbirth rate was seen with singleton pregnancies rising from 40 weeks' to 42 weeks' gestation. A similar trend was noted with twin pregnancies, but was seen at an earlier gestational age, rising from 36 to 38 weeks' gestation. The contribution of unexplained stillbirths was greater for singletons than for twins, but the proportion was greater in twin pregnancies after 32 weeks' gestation when the effects of preterm labour, fetal abnormalities, infection and pathology specific to twin pregnancies (e.g., twin‐twin transfusion syndrome) were less.
The risk of stillbirth and early neonatal death in twin gestations have been shown to correlate with that seen beyond 42 weeks in singleton gestations (Bakr 2006; Cheung 2000; Dodd 2003; Hartley 2001; Kato 2006; Luke 1993; Minakami 1996). Induction of labour in women with singleton pregnancies beyond 41 weeks has been shown to reduce perinatal mortality (Gülmezoglu 2012). The questions to then be considered relate to defining the 'post‐dates' twin pregnancy, and assessing the role of elective delivery in reducing perinatal mortality.
Description of the intervention
Elective birth at 37 weeks' gestation for women with a twin pregnancy where there is no contraindication to continuing the pregnancy with appropriate surveillance of fetal well being, compared with ongoing expectant management with a plan for birth at a later time.
How the intervention might work
The potential advantages of elective delivery in women with a twin pregnancy from 37 weeks' gestation include a reduction in perinatal mortality and morbidity. This has to be balanced against any associated increase in the risk of caesarean section, as well as the potential risks for the infants associated with early birth, including respiratory distress syndrome, and need for admission to the neonatal unit. Women's views on elective delivery versus continued antenatal surveillance should be considered.
Why it is important to do this review
Just under 50% of women with a twin pregnancy will reach 37 weeks' gestation and beyond and the risk of perinatal mortality (stillbirth and neonatal death) and neonatal morbidity associated with twin pregnancies increases with advancing gestational age (Bakr 2006; Cheung 2000; Dodd 2003; Hartley 2001; Kato 2006; Luke 1993; Minakami 1996). Elective birth at 37 weeks' gestation may be a safe and effective way to reduce the perinatal mortality and morbidity in twins. Women and their caregivers need unbiased information best provided by meta‐analysis of high‐quality randomised controlled trials about the risks and benefits of elective timing of birth to enable informed healthcare choices to be made.
Objectives
To assess a policy of elective delivery from 37 weeks' gestation compared with an expectant approach for women with an otherwise uncomplicated twin pregnancy. The primary outcomes relate to caesarean section, maternal and neonatal morbidity, and maternal and perinatal mortality.
Methods
Criteria for considering studies for this review
Types of studies
All published, unpublished, and ongoing randomised controlled trials and quasi‐randomised controlled trials with reported data that compared outcomes in mothers and babies who underwent elective delivery from 37 weeks' gestation in a twin pregnancy with outcomes in controls who were managed expectantly. We planned to include cluster‐randomised trials and exclude cross‐over trials.
Types of participants
Women with an otherwise uncomplicated twin pregnancy who reach 37 weeks' gestation.
Types of interventions
Elective birth by induction of labour or caesarean section at 37 weeks' gestation compared with expectant management.
Types of outcome measures
Primary outcomes
Caesarean section (all cases and for fetal distress)
Perinatal death or serious perinatal morbidity (e.g., growth restriction; seizures; birth asphyxia defined by trialists; neonatal encephalopathy; disability in childhood)
Maternal death or serious maternal morbidity (e.g., uterine rupture; admission to intensive care unit; septicaemia; postpartum haemorrhage and need for blood transfusion)
Uterine rupture included all clinically significant ruptures of unscarred or scarred uteri. Trivial scar dehiscence noted incidentally at the time of surgery was excluded.
Perinatal and maternal morbidity are composite outcomes. This is not an ideal solution because some components are clearly less severe than others. It is possible for one intervention to cause more deaths but fewer babies with severe morbidity. However, in the context of elective delivery at term this is unlikely. All these events will be rare, and a modest change in their incidence will be easier to detect if composite outcomes are presented. In assessment of a composite outcome, each mother and baby will be considered only once even though there may be more than one adverse outcome. The incidence of individual outcomes was explored as secondary outcomes.
Secondary outcomes
Secondary outcomes related to pregnancy outcomes, complications, satisfaction and costs.
Pregnancy and birth outcomes
Admission to intensive care unit
Infection requiring intravenous antibiotics
Haemorrhage requiring blood transfusion
Uterine rupture
Instrumental vaginal birth
Meconium‐stained liquor
Randomisation to delivery interval
Complications for infant and child (one or both)
Apgar score less than seven at five minutes
Need for neonatal intensive care unit admission
Birthweight less than 2500 g
Infant weight less than the third centile (small‐for‐gestational age (SGA))*
Neonatal encephalopathy
Respiratory distress syndrome
Parameters of birth asphyxia (neonatal irritability, neonatal seizures, neonatal hypotonia, abnormal level of consciousness, neonatal apnoea, tube feeding greater than 48 hours)
Neonatal jaundice requiring phototherapy
Disability at childhood follow‐up
Measures of satisfaction included
Woman not satisfied
Caregiver not satisfied
Woman and caregiver preferences for care
Costs included
Costs associated with expectant management versus elective delivery
Length of maternal hospitalisation
Length of neonatal hospitalisation
Outcomes were included in the analysis if data were available according to original allocation and reasonable measures were taken to minimise observer bias. Only outcomes with available data appear in the analysis tables. In order to minimise the risk of bias, the conclusions are based solely on the pre‐specified review outcomes.
*This outcome was not pre‐specified in the original protocol for the review.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (12 December 2013).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of Embase;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
We did not apply any language restrictions.
Data collection and analysis
For the methods used when assessing the trials identified in the previous version of this review, seeDodd 2003a.
For this update we used the following methods when assessing the reports identified by the updated search.
Selection of studies
At least two of the four review authors (JM Dodd, AR Deussen, RM Grivell and CA Crowther) independently assessed for inclusion all the potential studies we identified as a result of the search strategy. Disagreement was resolved through discussion.
We created a Study flow diagram to map out the number of records identified, included and excluded (Figure 1).
1.
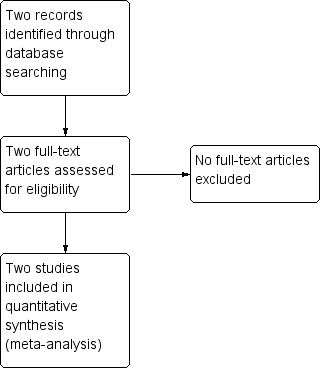
Study flow diagram.
Data extraction and management
We designed a form to extract data. For eligible studies, at least two review authors extracted data using the agreed form. Data from the study by Dodd et al (Dodd 2012a) were extracted by two review authors (AR Deussen and RM Grivell) who were not authors on this trial. We resolved discrepancies through discussion, or if required, we consulted a third assessor. We entered data into Review Manager software (RevMan 2012) and checked for accuracy.
Assessment of risk of bias in included studies
Two of the four review authors (JM Dodd, AR Deussen, RM Grivell and CA Crowther) independently assessed risk of bias for each study using criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreement by discussion or by involving the third review author. The study by Dodd et al (Dodd 2012a) was assessed by two review authors (AR Deussen and RM Grivell) who were not authors on this trial.
Two of the four review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We will assess the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or supplied by the trial authors, we will re‐include missing data in the analyses which we undertake.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we consider it was likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we have presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we planned to present the mean difference if outcomes were measured in the same way between trials, and the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
We have included individually‐randomised trials and plan to include cluster‐randomised controlled trials if subsequently identified in future updates of this review. We will adjust their sample sizes using the methods described in the Handbook using an estimate of the intra cluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
Cross‐over trials will not be included in this review.
Other unit of analysis issues
Dealing with missing data
For included studies, we noted levels of attrition. We planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either the Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
In future updates of this review if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2012). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average of the range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials.
Where we have used random‐effects analyses, the results are presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
The denominator for maternal outcomes was the number of women randomised. The denominator for neonatal outcomes was the number of babies.
Clustering for twins was adjusted for in the included studies.
Subgroup analysis and investigation of heterogeneity
If we had identified substantial heterogeneity, we planned to investigate it using subgroup analyses and sensitivity analyses. We planned to consider whether an overall summary was meaningful, and if it was, used random‐effects analysis to produce it.
We planned to carry out the following subgroup analyses.
Birth by elective caesarean section versus elective induction of labour
Chorionicity of the twin pregnancy (monochorionic versus dichorionic)
Parity (primiparous women versus multiparous women)
We planned to use only primary outcomes in subgroup analyses.
We planned to assess subgroup differences by interaction tests available within RevMan (RevMan 2012). We planned to report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Due to the paucity of data, however, we were unable to perform subgroup analyses in this update of the review.
Sensitivity analysis
We planned to carry out sensitivity analyses to explore the effects of trial quality assessed by allocation concealment and random sequence generation (considering selection bias), by omitting studies rated as 'high risk of bias' or 'unclear risk of bias' for these components. We planned to restrict this to the primary outcomes. However due to the paucity of data, we were unable to conduct sensitivity analyses in this review update.
Results
Description of studies
Results of the search
For this review update the search identified one newly completed study (Dodd 2012a), which was classified as 'ongoing' in the previous version of this review.
Included studies
We have included two randomised controlled trials comparing elective birth at 37 weeks for women with an uncomplicated twin pregnancy, with expectant management (Dodd 2012a; Suzuki 2000) in the review. A total of 271 women were recruited and randomised, with 133 women allocated to elective birth at 37 weeks' gestation, and 138 women allocated to ongoing expectant management. The trial conducted by Suzuki and colleagues (Suzuki 2000) involved 36 women, and compared elective induction of labour at 37 weeks with ongoing expectant management. The trial conducted by Dodd and colleagues (Dodd 2012a) recruited 235 women, and compared elective birth at 37 weeks' gestation, either by induction of labour or planned caesarean birth, with ongoing expectant management.
Excluded studies
We have not excluded any studies from this review.
Risk of bias in included studies
Details of risk of bias for the included studies are presented in the Characteristics of included studies, see Figure 2 and Figure 3. Overall, the Dodd 2012a trial was judged to be at a low risk of bias, and the Suzuki 2000 trial was judged to be at an unclear risk of bias.
2.
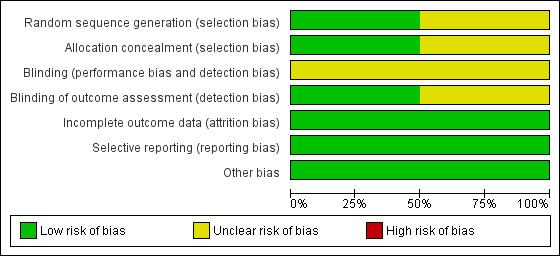
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
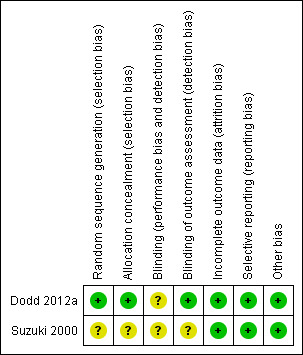
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The risk of selection bias was judged as unclear in the Suzuki 2000 trial, with no details provided on the methods used for sequence generation and allocation concealment. The Dodd 2012a trial, however was judged to be at a low risk of selection bias, using a computer‐generated randomisation sequence, and a central telephone randomisation service.
Blinding
The risks of performance and detection bias, due to lack of blinding of participants, caregivers and outcome assessors were judged to be unclear in Suzuki 2000, with no information provided regarding blinding. In Dodd 2012a, while it was not possible to blind women and caregivers due to the nature of the intervention, outcome assessors were blind to group allocation, and thus the trial was judged at a low risk of detection bias.
Incomplete outcome data
The two trials were judged to be at a low risk of attrition bias, with outcome data provided for all randomised participants (Dodd 2012a; Suzuki 2000).
Selective reporting
The two trials were judged to be at a low risk of reporting bias, with each trial reporting on all pre‐specified outcomes, according to the manuscript methods (Suzuki 2000), or published trial protocol (Dodd 2012a).
Other potential sources of bias
No other obvious risk of bias was identified in either of the included trials (Dodd 2012a; Suzuki 2000).
Effects of interventions
Elective birth at 37 weeks' gestation versus expectant management
Two randomised trials comparing elective birth at 37 weeks for women with an uncomplicated twin pregnancy were identified. A total of 271 women were recruited to the two studies with 133 women randomised to elective birth at 37 weeks and 138 women to the ongoing expectant management (Dodd 2012a; Suzuki 2000).
Primary outcomes
There were no statistically significant differences identified between the two management groups with regards to birth by caesarean section (two studies; 271 participants; risk ratio (RR) 1.05; 95% confidence interval (CI) 0.83 to 1.32) (Analysis 1.1), or caesarean performed for non‐reassuring fetal heart rate tracing (two studies; 271 participants; RR 0.79; 95% CI 0.29 to 2.15) (Analysis 1.2). There were no statistically significant differences identified between birth at 37 weeks and ongoing expectant management for the outcomes perinatal death or serious perinatal morbidity (two studies; 542 infants; RR 0.34; 95% CI 0.01 to 8.35) (Analysis 1.3), or maternal death or serious maternal morbidity (one study; 235 women; RR 0.29; 95% CI 0.06 to 1.38) (Analysis 1.4). There were no maternal deaths.
1.1. Analysis.
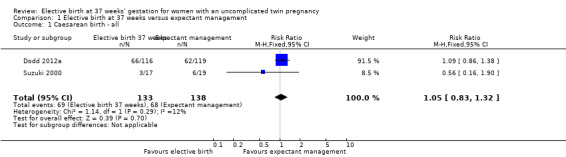
Comparison 1 Elective birth at 37 weeks versus expectant management, Outcome 1 Caesarean birth ‐ all.
1.2. Analysis.
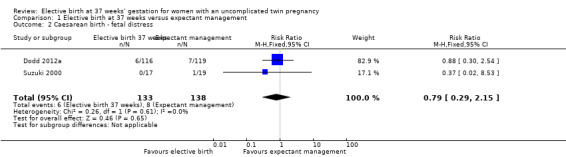
Comparison 1 Elective birth at 37 weeks versus expectant management, Outcome 2 Caesarean birth ‐ fetal distress.
1.3. Analysis.
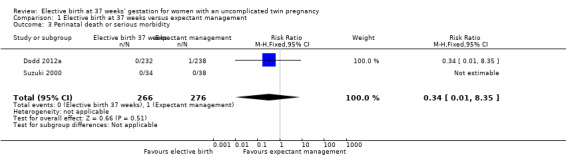
Comparison 1 Elective birth at 37 weeks versus expectant management, Outcome 3 Perinatal death or serious morbidity.
1.4. Analysis.
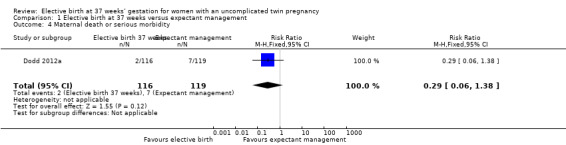
Comparison 1 Elective birth at 37 weeks versus expectant management, Outcome 4 Maternal death or serious morbidity.
Secondary outcomes
Pregnancy and birth complications
There were no statistically significant differences identified in risk of haemorrhage requiring blood transfusion (two studies; 271 women; RR 0.48; 95% CI 0.11 to 2.08) (Analysis 1.5), instrumental birth (one study; 470 women; RR 0.63; 95% CI 0.35 to 1.15) (Analysis 1.6), or meconium‐stained liquor (two studies; 542 women; average RR 0.52; 95% CI 0.04 to 7.35) (Analysis 1.7). For the outcome meconium‐stained liquor, substantial statistical heterogeneity was observed (T² = 2.64; I² = 68%), and thus a random‐effects meta‐analysis was used.
1.5. Analysis.
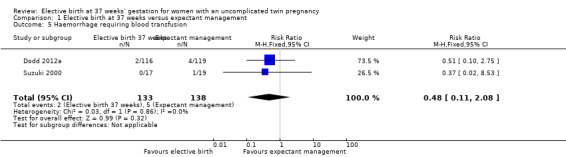
Comparison 1 Elective birth at 37 weeks versus expectant management, Outcome 5 Haemorrhage requiring blood transfusion.
1.6. Analysis.
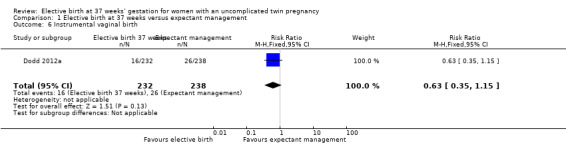
Comparison 1 Elective birth at 37 weeks versus expectant management, Outcome 6 Instrumental vaginal birth.
1.7. Analysis.
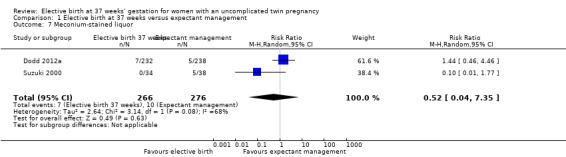
Comparison 1 Elective birth at 37 weeks versus expectant management, Outcome 7 Meconium‐stained liquor.
Neither of the two included trials reported on the secondary review outcomes: admission to the intensive care unit, infection requiring intravenous antibiotics, uterine rupture, or randomisation to delivery interval.
Complications for the infants
There were no statistically significant differences identified for the secondary infant outcomes, Apgar score of less than seven at five minutes (two studies; 542 infants; RR 0.15; 95% CI 0.01 to 2.82) (Analysis 1.8), admission to the neonatal intensive care unit (one study; 470 infants; RR 1.03; 95% CI 0.37 to 2.88) (Analysis 1.9), birthweight less than 2500 g (two studies; 542 infants; RR 1.28; 95% CI 0.93 to 1.75) (Analysis 1.10), neonatal encephalopathy (one study; 470 infants; RR 3.08; 95% CI 0.13 to 75.16) (Analysis 1.11) and respiratory distress syndrome (one study; 470 infants; RR 2.05; 95% CI 0.19 to 22.47) (Analysis 1.12). Neither of the two included trials reported on parameters of birth asphyxia, neonatal jaundice requiring phototherapy, or disability at childhood follow‐up.
1.8. Analysis.
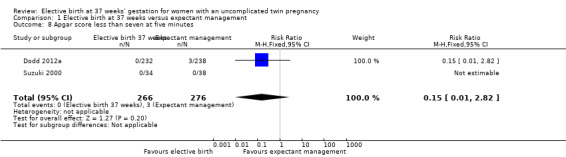
Comparison 1 Elective birth at 37 weeks versus expectant management, Outcome 8 Apgar score less than seven at five minutes.
1.9. Analysis.
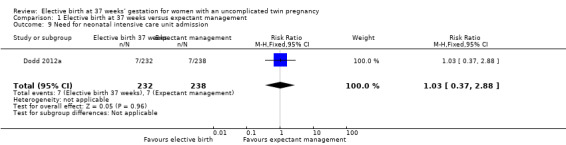
Comparison 1 Elective birth at 37 weeks versus expectant management, Outcome 9 Need for neonatal intensive care unit admission.
1.10. Analysis.
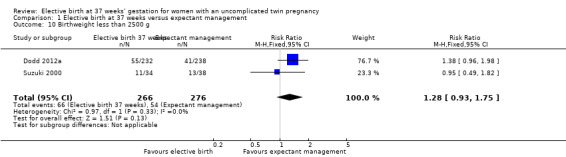
Comparison 1 Elective birth at 37 weeks versus expectant management, Outcome 10 Birthweight less than 2500 g.
1.11. Analysis.
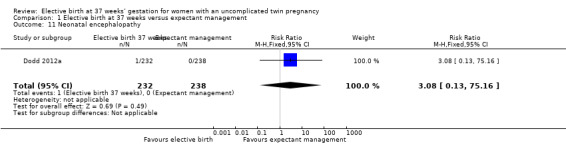
Comparison 1 Elective birth at 37 weeks versus expectant management, Outcome 11 Neonatal encephalopathy.
1.12. Analysis.
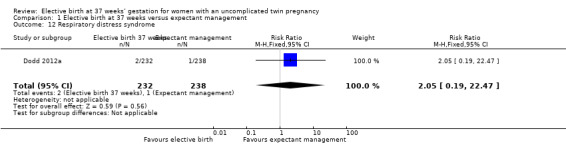
Comparison 1 Elective birth at 37 weeks versus expectant management, Outcome 12 Respiratory distress syndrome.
Infants born at 37 weeks' gestation were significantly less likely to have a birthweight less than the third centile for gestational age and infant sex (one study; 470 infants; RR 0.30; 95% CI 0.13 to 0.68) (Analysis 1.13). While this was reported in a single study (Dodd 2012a), it was not a pre‐specified outcome for this review.
1.13. Analysis.
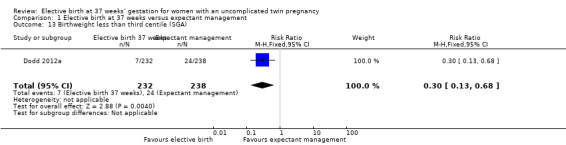
Comparison 1 Elective birth at 37 weeks versus expectant management, Outcome 13 Birthweight less than third centile (SGA).
Other secondary outcomes
The included studies did not report outcomes for women's satisfaction with care, caregiver satisfaction, timing of birth preferences for women and caregivers and costs associated with elective birth from 37 weeks' gestation compared with expectant management.
Discussion
This systematic review identified and included two randomised trials addressing the question of optimal timing of birth for women with an otherwise uncomplicated twin pregnancy at term, involving 271 women and 542 infants. For the primary outcomes of the review, there were no statistically significant differences identified in risk of caesarean birth, perinatal death or serious infant morbidity, or maternal death or serious maternal morbidity. There were no statistically significant differences identified for the pre‐specified secondary maternal and infant outcomes. Neither trial reported on maternal quality of life, the economic implications of elective timing of birth, or on longer‐term childhood outcomes. However, there appear to be no short‐term harms associated with earlier birth.
It was not possible to conduct subgroup analyses based on chorionicity of the pregnancy. Since the inception and reporting of both trials, there has been increasing clinical recognition of the need to identify early in pregnancy whether a twin pregnancy is monochorionic (the twins sharing a placental circulation) or dichorionic (the twins have separate placental circulations). The risk of both mortality and morbidity for monochorionic diamniotic twin infants is greater than for dichorionic twins, at all gestational ages (Hack 2008; Smith 2010).
The data available from the two included randomised trials are relatively underpowered to detect differences in significant infant morbidity. Furthermore, evaluation of differences in perinatal mortality would require a trial with a sample size of over 20,000 women with an uncomplicated twin pregnancy at term (Dodd 2012a; Dodd 2012b). However, given the current United Kingdom's National Institute for Health and Care Excellence (NICE) recommendations, which advocate birth for women with a monochorionic twin pregnancy at 36 + 0 weeks’ gestation, and for women with a dichorionic twin pregnancy at 37 + 0 weeks’ gestation (NICE 2011), it is unlikely that sufficient equipoise exists in clinical practice to allow randomisation of women to a later gestational age at birth.
Authors' conclusions
Implications for practice.
Early birth at 37 weeks' gestation compared with ongoing expectant management for women with an uncomplicated twin pregnancy does not appear to be associated with an increased risk of harms. Furthermore, there may be possible benefits in earlier birth (including a reduction in infants being born small‐for‐gestational age; although this outcome was not pre‐specified in this review). The findings of no increased risk of harms with birth at 37 weeks' gestation is consistent with the NICE recommendations advocating birth for women with a dichorionic twin pregnancy at 37 + 0 weeks' gestation.
Implications for research.
The available randomised data are relatively underpowered to address differences in either serious markers of perinatal morbidity, or perinatal death. However, in view of the current NICE recommendations, it is unlikely that sufficient clinical equipoise exists to allow randomisation of women to a later gestational age at birth.
What's new
| Date | Event | Description |
|---|---|---|
| 12 December 2013 | New citation required but conclusions have not changed | Search updated. One new trial that was previously classified as 'ongoing' has now been included in the review (Dodd 2012a). Methods updated. |
| 12 December 2013 | New search has been performed | Review updated. |
History
Protocol first published: Issue 2, 2002 Review first published: Issue 1, 2003
| Date | Event | Description |
|---|---|---|
| 2 September 2008 | Amended | Converted to new review format. |
| 22 October 2004 | New search has been performed | Search updated. No new trials identified. |
| 30 December 2003 | New search has been performed | Search rerun but no new trials identified. The 'Potential conflict of interest' section has been updated. |
Acknowledgements
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team) and the Group's Statistical Adviser.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pregnancy and Childbirth Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Data and analyses
Comparison 1. Elective birth at 37 weeks versus expectant management.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean birth ‐ all | 2 | 271 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.83, 1.32] |
| 2 Caesarean birth ‐ fetal distress | 2 | 271 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.29, 2.15] |
| 3 Perinatal death or serious morbidity | 2 | 542 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.35] |
| 4 Maternal death or serious morbidity | 1 | 235 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.06, 1.38] |
| 5 Haemorrhage requiring blood transfusion | 2 | 271 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.11, 2.08] |
| 6 Instrumental vaginal birth | 1 | 470 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.35, 1.15] |
| 7 Meconium‐stained liquor | 2 | 542 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.04, 7.35] |
| 8 Apgar score less than seven at five minutes | 2 | 542 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.82] |
| 9 Need for neonatal intensive care unit admission | 1 | 470 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.37, 2.88] |
| 10 Birthweight less than 2500 g | 2 | 542 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.93, 1.75] |
| 11 Neonatal encephalopathy | 1 | 470 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.08 [0.13, 75.16] |
| 12 Respiratory distress syndrome | 1 | 470 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.05 [0.19, 22.47] |
| 13 Birthweight less than third centile (SGA) | 1 | 470 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.13, 0.68] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Dodd 2012a.
| Methods | Randomised controlled trial. | |
| Participants | 235 women were randomised. Inclusion criteria: women with a twin pregnancy at gestational age 36 + 6 weeks or more, with no contraindication to continuation of their pregnancy who present to participating centres were eligible for participation. Exclusion criteria: women with fetal death of 1 or both fetuses at the time of trial entry, in active labour, with a non‐reassuring fetal heart rate tracing, or with maternal or fetal compromise precluding continued antenatal surveillance were excluded from participation. |
|
| Interventions | "Elective delivery group" (n = 116) Women randomised to the "elective delivery group" underwent elective birth at 37 weeks' gestation (either by induction of labour or caesarean section as assessed and determined by the woman and her caregiver). "Standard care group" (n = 119) Women randomised to the "standard care group" had their care according to local hospital guidelines. Where there was a plan for vaginal birth this involved induction of labour after 38 weeks' gestation, or awaiting the spontaneous onset of labour. Where caesarean section was the preferred mode of birth, this was booked after 38 weeks and as close to 39 weeks' gestation as was possible. |
|
| Outcomes | The primary outcome was a composite of perinatal mortality and morbidity, including perinatal death after trial entry, and serious neonatal morbidity (including birth trauma, Apgar score < 4 at 5 minutes, neonatal encephalopathy, ventilation requirement greater than 24 hours). Secondary outcomes included antenatal medical and obstetric complications, labour and birth complications, other adverse outcomes for the infant, and serious adverse outcomes for the woman. |
|
| Notes | Nil. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation schedule was computer‐generated by a researcher not involved in the study, using balanced variable blocks, and stratification for collaborating centre and planned mode of birth (planned caesarean birth or planned vaginal birth). |
| Allocation concealment (selection bias) | Low risk | Central telephone randomisation service. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not possible to blind this clinical intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes available for all participants. |
| Selective reporting (reporting bias) | Low risk | Outcomes specified in published protocol have been reported. |
| Other bias | Low risk | Recruitment ceased before sample size reached. |
Suzuki 2000.
| Methods | Randomised controlled trial. | |
| Participants | 36 women were randomised. Inclusion criteria: women with a twin pregnancy at 37 weeks' gestation, with the first twin in a cephalic presentation. |
|
| Interventions | Induction group (n = 17) Induction of labour at 37 weeks' gestation with vaginal prostaglandin E2 gel followed by ARM and oxytocin infusion as required. Expectant management group (n = 19) Women continued pregnancy surveillance until spontaneous onset of labour, provided no antenatal complications developed and fetal well being confirmed (involved daily CTG and twice‐weekly ultrasound examination). |
|
| Outcomes | Gestational age at birth; incidence of PROM; uterine hyperactivity or meconium‐stained amniotic fluid; caesarean delivery rates; indication for caesarean section; total blood loss; need for maternal blood transfusion; infant birthweight; infant Apgar scores. | |
| Notes | No reported sample size calculation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "eligible patients were then randomised to one of two management groups". |
| Allocation concealment (selection bias) | Unclear risk | No information was given on allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information given on who remained blinded to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data available on all randomised participants. No post‐randomisation exclusions. No losses to follow‐up reported. |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in methods are reported. There is no published protocol. |
| Other bias | Low risk | None identified. |
ARM: artificial rupture of membranes CTG: cardiotocography PROM: premature rupture of membrane
Differences between protocol and review
The title has been changed from 'Elective delivery of women with a twin pregnancy from 37 weeks' gestation' to 'Elective birth at 37 weeks’ gestation for women with an uncomplicated twin pregnancy'. We have added infant birthweight less than the third centile (small‐for‐gestational age) as a secondary infant outcome.
Contributions of authors
Jodie Dodd wrote the initial version and subsequent drafts of the protocol and review. Caroline Crowther contributed to the drafting of the protocol, review and its subsequent revisions. This update was written and reviewed by Jodie Dodd, Andrea Deussen, Rosalie Grivell and Caroline Crowther. All authors were involved in assessment of studies, data extraction and entry. Assessment of, data extraction and risk of bias assessment for the Dodd 2012a trial was completed by Andrea Deussen and Rosalie Grivell.
Sources of support
Internal sources
The University of Adelaide, Discipline of Obstetrics and Gynaecology, Australia.
External sources
No sources of support supplied
Declarations of interest
Two of the review authors (Jodie Dodd and Caroline Crowther) were chief investigators of a randomised trial assessing the optimal timing of birth for women with a twin pregnancy at term, that has been included in this review (Dodd 2012a).
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Dodd 2012a {published data only}
- Dodd J. ACTOTTAB Twins: Timing of birth at term. Perinatal Trials Report (http://www.ctc.usyd.edu.au/6registry/PTO426.htm) (accessed 7 April 2004).
- Dodd J, Crowther C, Haslam R, Robinson J, for the Twins Timing of Birth Trial Group. Elective birth at 37 weeks of gestation versus standard care for women with an uncomplicated twin pregnancy at term: the Twins Timing of Birth Randomised Trial. British Journal of Obstetrics and Gynaecology 2012;119(8):964‐74. [DOI: 10.1111/j.1471-0528.2012.03356.x; PUBMED: 22691051] [DOI] [PubMed] [Google Scholar]
- Dodd J, Crowther C, Robinson J, Haslam R. Twins: timing of birth at term ‐ a randomised controlled trial. Perinatal Society of Australia and New Zealand (www.psanz.org.au) (accessed 4 October 2006).
- Dodd JM, Crowther CA, Haslam RR, Robinson JS. Timing of birth for women with a twin pregnancy at term: a randomised controlled trial. BMC Pregnancy and Childbirth 2010;10:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd JM, Crowther CA, Haslam RR, Robinson JS. What is the optimal time of birth for women with an uncomplicated twin pregnancy at term? The twins timing of birth randomised trial. Journal of Paediatrics and Child Health 2011;47(Suppl 1):51. [Google Scholar]
Suzuki 2000 {published data only}
- Suzuki S, Otsubo Y, Sawa R, Yoneyama Y. Clinical trial of induction of labor versus expectant management in twin pregnancy. Gynecologic and Obstetric Investigation 2000;49:24‐7. [DOI] [PubMed] [Google Scholar]
Additional references
Bakr 2006
- Bakr AF, Karkour T. What is the optimal gestational age for twin delivery. BMC Pregnancy Childbirth 2006;6:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boulvain 2001
- Boulvain M, Stan CM, Irion O. Elective delivery in diabetic pregnant women. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD001997] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheung 2000
- Cheung YB, Yip P, Karlberg J. Mortality of twins and singletons by gestational age: a varying‐coefficient approach. American Journal of Epidemiology 2000;152(12):1117‐9. [DOI] [PubMed] [Google Scholar]
Cincotta 2001
- Cincotta RB, Flenady V, Hockey R, King J. When should twins be delivered? ‐ gestational age‐specific stillbirth risk of twins vs singletons. Perinatal Society of Australia and New Zealand 5th Annual Congress; 2001 March 13‐16; Canberra, Australia, 2001. Canberra, 2001:22.
Dodd 2003
- Dodd JM, Robinson JS, Crowther CA, Wilkinson C, Chan A, Keane R. Stillbirth and neonatal outcomes in twin and singleton pregnancies in South Australia, 1991‐2000. Perinatal Society of Australia and New Zealand 6th Annual Congress; 2002 March 9‐13; Australia, 2002. Australia, 2002.
Dodd 2012b
- Dodd J, Crowther C, Haslam R, Robinson J. Authors' response to: Elective birth at 37 weeks of gestation versus standard care for women with an uncomplicated twin pregnancy at term: the Twins Timing of Birth Randomised Trial. British Journal of Obstetrics and Gynaecology 2012;119(13):1676‐7. [DOI] [PubMed] [Google Scholar]
Gülmezoglu 2012
- Gülmezoglu AM, Crowther CA, Middleton P, Heatley E. Induction of labour for improving birth outcomes for women at or beyond term. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD004945.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hack 2008
- Hack KE, Derks JB, Elias SG, Franx A, Roos EJ, Voerman SK. Increased perinatal mortality and morbidity in monochorionic versus dichorionic twin pregnancies: clinical implications of a large Dutch cohort study. British Journal of Obstetrics and Gynaecology 2008;115(1):58‐67. [DOI] [PubMed] [Google Scholar]
Hartley 2001
- Hartley RS, Emanuel I, Hitti J. Perinatal mortality and neonatal morbidity rates among twin pairs at different gestational ages: optimal delivery timing at 37 to 38 weeks gestation. American Journal of Obstetrics and Gynecology 2001;184(3):451‐8. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Irion 1998
- Irion O, Boulvain M. Induction of labour for suspected fetal macrosomia. Cochrane Database of Systematic Reviews 1998, Issue 2. [DOI: 10.1002/14651858.CD000938] [DOI] [PubMed] [Google Scholar]
Kato 2006
- Kato N, Matsuda T. Estimation of optimal birth weights and gestational ages for twin births in Japan. BMC Public Health 2006;6:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Law 2009
- Law P, Sullivan EA. Australia's Mothers and Babies 2007. Perinatal Statistics Series No 23. Cat No. PER 48. AIHW National Perinatal Statistics Unit, 2009. [Google Scholar]
Luke 1993
- Luke B, Minogue J, Witter F, Keith L, Johnson T. The ideal twin pregnancy: patterns of weight gain, discordancy, and length of gestation. American Journal of Obstetrics and Gynecology 1993;169:588‐97. [DOI] [PubMed] [Google Scholar]
Minakami 1996
- Minakami H, Sato I. Re‐estimating the date of delivery in multifetal pregnancies. JAMA 1996;275:1432‐4. [PubMed] [Google Scholar]
NICE 2011
- National Institute for Health and Clinical Excellence (NICE). Multiple Pregnancy: the Management of Twin and Triplet Pregnancies in the Antenatal Period. London: RCOG Press, 2011. [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Smith 2010
- Smith NA, Wilkins‐Haug L, Santolaya‐Forgas J, Acker D, Economy KE, Benson CB. Contemporary management of monochorionic diamniotic twins: outcomes and delivery recommendations revisited. American Journal of Obstetrics and Gynecology 2010;203(133):e1‐e6. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Dodd 2002
- Dodd JM, Crowther CA. Elective delivery of women with a twin pregnancy from 37 weeks' gestation. Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI: 10.1002/14651858.CD003582] [DOI] [PubMed] [Google Scholar]
Dodd 2003a
- Dodd JM, Crowther CA. Elective Delivery of women with a twin pregnancy from 37 weeks' gestation. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD003582] [DOI] [PubMed] [Google Scholar]


