Abstract
Aims
The guideline‐directed medical therapy (GDMT) has been recommended for heart failure (HF) with reduced ejection fraction (HFrEF) based on the accumulating clinical evidence. However, it is difficult to implement all the trial‐proven medications for every patient in the real world.
Methods and results
A simple GDMT score was created, according to the combination of GDMT drugs (renin–angiotensin system inhibitors, beta‐blockers, mineralocorticoid receptor antagonists, and sodium–glucose transporter 2 inhibitors) administration and their dosage (0–9 points). Its impact on the prognosis of HF patients was investigated. Admitted HF patients [HFrEF and HF with mildly reduced ejection fraction (HFmrEF), n = 1054] were retrospectively analysed (excluding those with in‐hospital death and dialysis). A simple GDMT score ≥5, but not the number of medications, was significantly associated with a reduction of all‐cause death, HF readmission, and composite outcome (HF readmission and all‐cause death) (P < 0.001). Subgroup analysis showed that almost all groups with a simple GDMT score of 5 or higher had a better prognosis.
Conclusions
The developed simple GDMT score was associated with prognosis in HFrEF and HFmrEF patients. Even if all four drugs cannot be introduced for some reason, a regimen with a simple GDMT score ≥5 may lead to a prognosis in HF patients.
Keywords: Heart failure, Simple GDMT score, Prognosis
Introduction
Guideline‐directed medical therapy (GDMT) for heart failure (HF), particularly HF with reduced ejection fraction (HFrEF), has progressed dramatically in recent years. Indeed, angiotensin receptor neprilysin inhibitor (ARNI) showed a significant benefit in the PARADIGM trial. 1 The benefit of sodium–glucose transporter 2 (SGLT2) inhibitors is also established in EMPEROR‐Reduced 2 and Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA‐HF) 3 trials. The combination of ARNI, SGLT2 inhibitors, together with the beta‐blocker (BB) and mineralocorticoid receptor antagonist (MRA) that have been shown to be useful, is known as the ‘fantastic 4’. 4 , 5 Each of these drugs is classified as Class I recommendation in HFrEF in national guidelines, 6 , 7 , 8 and several analyses have shown the efficacy of the combination of these four drugs. 9 , 10 The global recommendation is to aim for a four‐drug regimen in HFrEF patients whenever possible. In practice, however, the implementation of GDMTs is still inadequate, as shown by the registry studies (CHAMP‐HF 11 and EVOLUTION‐HF 12 ). The GUIDE‐IT trial also reported that despite a protocol‐driven approach, optimal GDMT could not be achieved. 13 In actual clinical practice, we often face with the difficulty of fully implementing GDMT because of the individual problems in each patient, such as hypotension, bradycardia, renal function, and electrolyte abnormalities, which are the reasons why GDMT is not fully implemented. In recent years, scoring methods have been proposed for the implementation of these GDMTs to adjust patient background and thus clarify the significance of newly added drugs in large clinical trials. The use of the optimal medical therapy (OMT) score developed by the Heart Failure Collaboratory and the Academic Research Consortium 14 , 15 , 16 and, more recently, the new GDMT score including SGLT2 inhibitors, vericiguat, and ivabradine has been recommended. 17 However, there are few reports on how such scoring systems themselves relate to the prognosis of HF patients. In this study, we developed a simple GDMT score, which is a modification of the previously proposed GDMT score, 17 and investigated whether this score is related to the prognosis of HF patients. The rationale for proposing a simple GDMT score in the present study was to provide a practical and easily applicable scoring system that could be readily implemented in clinical practice. Because the GDMT score previously proposed by the Heart Failure Collaboratory and the Academic Research Consortium is valuable and based on rigorous analysis, the aim of the present study was to create a simpler scoring system that could effectively guide clinicians in the initiation of GDMTs.
Materials and methods
Simple guideline‐directed medical therapy score
We created a simple GDMT score based on previously proposed GDMT scores. 17 First, vericiguat and ivabradine were excluded from this study because sample size was very small (only a few cases), and hydralazine/nitrates were excluded because they cannot be prescribed in Japan. Renin–angiotensin system (RAS) inhibitors were scored 0 if not initiated, 1 if <50% of target dose, and 2 if 50–100% of target dose; ARNIs were scored 3 regardless of dose; BBs were scored 0 if not initiated, 1 if <50% of target dose, and 2 if 50–100% of target dose; and MRAs and SGLT2 inhibitors were scored 0 if not initiated and 2 if initiated regardless of dose. MRAs and SGLT2 inhibitors were scored 0 if not initiated and 2 if initiated regardless of dose, resulting in a total score of 0–9 (Figure 1 A ).
Figure 1.
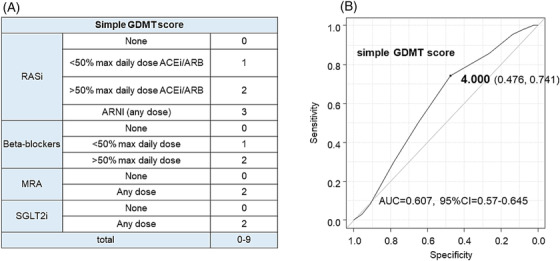
(A) Design of simple guideline‐directed medical therapy (GDMT) score. (B) Receiver operating characteristic curve of association between simple GDMT score and the composite outcome. ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; AUC, area under curve; CI, confidence interval; MRA, mineralocorticoid receptor antagonist; RASi, renin–angiotensin system inhibitor; SGLT2i, sodium–glucose transporter 2 inhibitor.
Study design
This is a single‐centre, retrospective cohort study to determine whether the GDMT scoring system is useful in predicting prognosis in acute decompensated HF (ADHF) patients. We retrospectively analysed 1782 consecutive patients with ADHF at our hospital from April 2015 to March 2022. ADHF was diagnosed according to Framingham's HF criteria. 18 One thousand fifty‐four patients with HFrEF and HF with mildly reduced ejection fraction (HFmrEF) were selected, excluding 61 patients who died in hospital, 53 patients on dialysis, and 614 patients with HF with preserved ejection fraction (HFpEF) (Table 1 ).
Table 1.
Characteristics of total patients
| Total (n = 1054) | |
|---|---|
| Backgrounds | |
| Age (years) | 75.2 ± 14.0 |
| Male (%) | 688 (65.3) |
| BNP (pg/mL) | 1094.1 ± 1101.4 |
| NYHA class | 3.0 ± 0.7 |
| NYHA 1–2 (%) | 203 (19.3) |
| NYHA 3 (%) | 569 (54.0) |
| NYHA 4 (%) | 282 (26.8) |
| EF (%) | 30.9 ± 10.3 |
| HFrEF (%) | 784 (74.6) |
| HFmrEF (%) | 270 (25.4) |
| Hx of HF (%) | 319 (30.3) |
| ICM (%) | 418 (39.7) |
| sBP (mmHg) | 139.4 ± 34.5 |
| dBP (mmHg) | 84.1 ± 24.2 |
| HR (b.p.m.) | 94.8 ± 26.5 |
| BMI (kg/m2) | 23.3 ± 13.8 |
| Comorbidity | |
| AF (%) | 433 (41.1) |
| DM (%) | 373 (35.4) |
| COPD (%) | 71 (6.7) |
| Pneumonia (%) | 134 (12.7) |
| Laboratory data | |
| BUN (mg/dL) | 29.3 ± 17.5 |
| Cr (mg/dL) | 1.55 ± 1.23 |
| eGFR (mL/min/1.73 m2) | 44.9 ± 22.2 |
| UA (mg/dL) | 6.9 ± 2.2 |
| Na (mmol/L) | 139.0 ± 4.4 |
| K (mmol/L) | 4.37 ± 0.73 |
| Hb (g/dL) | 12.3 ± 2.5 |
| Alb (g/dL) | 3.5 ± 0.5 |
| In‐hospital use | |
| Vasodilator (%) | 244 (23.2) |
| Carperitide | 155 (14.7) |
| Loop diuretics (%) | 772 (73.2) |
| TLV (%) | 303 (28.7) |
| Catecholamine (%) | 196 (18.6) |
| NPPV (%) | 168 (15.9) |
| At discharge | ||
|---|---|---|
| Hospital stay (days) | 21.7 ± 16.4 | |
| No drugs | 2.4 ± 1.0 | |
| GDMT score (pts) | 4.1 ± 2.0 | |
| RASi (%) | 869 (82.3) | |
| ACEi/ARB (%) | 808 (76.6) | ACEi = 63.2%, ARB = 36.8% |
| ARNI (%) | 61 (5.7) | |
| ARNI dose (mg) | 165.5 ± 99.3 | |
| BB (%) | 868 (82.3) | Carvedilol = 49.3%, bisoprolol = 50.7% |
| BB dose (mg) | 8.6 ± 6.9 | |
| MRA (%) | 613 (58.1) | Spironolactone = 89.3%, eplerenone = 10.7% |
| MRA dose (mg) | 25.2 ± 9.6 | |
| SGLT2i (%) | 188 (17.8) | Empagliflozin = 47.8%, dapagliflozin = 52.2% |
| Loop (%) | 681 (64.6) | |
| Loop dose (mg) | 14.6 ± 14.4 | |
| TLV (%) | 248 (23.5) | |
| TLV dose (mg) | 7.3 ± 4.1 | |
| PDEIIIi (%) | 103 (9.7) | |
| CRT/ICD (%) | 59 (5.6) | |
| Clinical outcomes | ||
|---|---|---|
| Composite outcome (%) | 243 (23.0) | |
| HF readmission (%) | 197 (18.6) | |
| All‐cause death (%) | 70 (6.6) | CV death = 44.2% |
ACEi, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; Alb, albumin; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; BB, beta‐blocker; BMI, body mass index; BNP, brain natriuretic peptide; BUN, blood urea nitrogen; COPD, chronic obstructive pulmonary disease; Cr, creatinine; CRT, cardiac resynchronization therapy; CV, cardiovascular; dBP, diastolic blood pressure; DM, diabetes mellitus; EF, ejection fraction; eGFR, estimated glomerular filtration rate; GDMT, guideline‐directed medical therapy; Hb, haemoglobin; HFmrEF, heart failure with mildly reduced ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, heart rate; Hx of HF, history of heart failure; ICD, implantable cardiac defibrillator; ICM, ischaemic cardiomyopathy; K, potassium; MRA, mineralocorticoid receptor antagonist; Na, sodium; NPPV, non‐invasive positive pressure ventilation; NYHA, New York Heart Association; PDEIIIi, phosphodiesterase III inhibitor; RASi, renin–angiotensin system inhibitor; sBP, systolic blood pressure; SGLT2i, sodium–glucose transporter 2 inhibitor; TLV, tolvaptan; UA, uric acid.
First, the simple GDMT score was calculated in these patients. Then, the association with clinical outcomes (composite outcome: HF readmission or all‐cause death, HF readmission, and all‐cause death) was examined. Based on the cut‐off value of the relationship between the composite outcome and the simple GDMT score, patients were divided into two groups: those with high score (≥5 points) and those with low score (≦4 points). Clinical outcomes were compared between the two groups. The study design is shown in Figure 2 .
Figure 2.
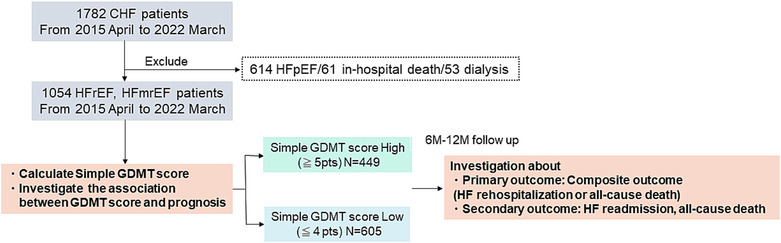
Illustration of the study protocol. Retrospective analysis of the association with the prognosis of heart failure (HF) with reduced ejection fraction (HFrEF) and HF with mildly reduced ejection fraction (HFmrEF) patients and simple guideline‐directed medical therapy (GDMT) score. CHF, congestive HF; HFpEF, HF with preserved ejection fraction.
Statistical analyses
All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). More specifically, it is a modified version of the R Commander designed to add statistical functions commonly used in biostatistics. 19
Continuous variables in the high/low GDMT score groups were compared using the unpaired t‐test or Mann–Whitney U test, as appropriate. Categorical variables in the high/low groups were compared using the χ 2 test or Fisher's exact test, as appropriate. Freedom from composite outcomes, HF readmission, and all‐cause death was analysed using the Kaplan–Meier curve with the log‐rank test and Cox proportional hazards regression analysis.
Variables with P < 0.05 in the univariate analysis and those associated with clinical outcomes were used in the Cox proportional hazards regression analysis for each as shown in Table 2 and Supporting Information, Tables S2A and S2B . Univariate and multivariate analyses were performed using logistic regression analysis for association with low GDMT scores. Variables with P < 0.1 in the univariate analysis were used in the Cox proportional hazards regression analysis for each as shown in Table 4 .
Table 2.
Association with the composite outcome
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P value | Hazard ratio | 95% CI | P value | |
| Backgrounds | ||||||
| Age (years) | 1.024 | 1.014–1.035 | <0.001 | 1.014 | 1.001–1.027 | 0.031 |
| Male (%) | 0.988 | 0.759–1.286 | 0.928 | 1.162 | 0.997–1.557 | 0.314 |
| BNP | 1.000 | 0.999–1.000 | 0.471 | |||
| NYHA class | 1.309 | 1.088–1.574 | 0.004 | 1.210 | 0.989–1.480 | 0.063 |
| EF (%) | 1.008 | 0.996–1.020 | 0.189 | 0.996 | 0.981–1.011 | 0.615 |
| Hx of HF (%) | 1.948 | 1.511–2.511 | <0.001 | 1.307 | 0.977–1.748 | 0.071 |
| ICM (%) | 1.584 | 1.232–2.037 | <0.001 | 1.248 | 0.947–1.644 | 0.115 |
| sBP (mmHg) | 0.997 | 0.993–1.001 | 0.127 | |||
| dBP (mmHg) | 0.990 | 0.985–0.996 | 0.001 | 0.999 | 0.992–1.006 | 0.778 |
| HR (b.p.m.) | 0.992 | 0.987–0.997 | 0.004 | 0.998 | 0.992–1.004 | 0.557 |
| BMI (kg/m2) | 0.970 | 0.944–0.996 | 0.026 | 0.998 | 0.980–1.015 | 0.805 |
| Comorbidity | ||||||
| AF (%) | 1.110 | 0.861–1.431 | 0.419 | |||
| DM (%) | 1.175 | 0.907–1.521 | 0.221 | 1.120 | 0.844–1.486 | 0.432 |
| COPD (%) | 1.285 | 0.813–2.030 | 0.282 | 1.301 | 0.802–2.109 | 0.286 |
| Pneumonia (%) | 0.875 | 0.586–1.306 | 0.514 | |||
| Laboratory data | ||||||
| BUN (mg/dL) | 1.018 | 1.012–1.024 | <0.001 | 0.998 | 0.988–1.009 | 0.801 |
| eGFR (mL/min/1.73 m2) | 0.979 | 0.973–0.985 | <0.001 | 0.990 | 0.981–1.000 | 0.054 |
| UA (mg/dL) | 1.029 | 0.973–1.088 | 0.308 | |||
| Na (mmol/L) | 0.972 | 0.947–0.998 | 0.035 | 0.994 | 0.965–1.023 | 0.718 |
| K (mmol/L) | 1.218 | 1.052–1.412 | 0.008 | 1.034 | 0.862–1.239 | 0.718 |
| Hb (g/dL) | 0.887 | 0.843–0.933 | <0.001 | 0.994 | 0.929–1.065 | 0.879 |
| Alb (g/dL) | 0.670 | 0.539–0.833 | <0.001 | 0.853 | 0.663–1.098 | 0.218 |
| In‐hospital use | ||||||
| Vasodilator (%) | 0.949 | 0.701–1.285 | 0.737 | |||
| Carperitide | 0.872 | 0.601–1.266 | 0.473 | |||
| In‐hospital loop diuretics (%) | 1.040 | 0.780–1.385 | 0.790 | |||
| In‐hospital TLV (%) | 1.829 | 1.416–2.364 | <0.001 | 1.118 | 0.743–1.682 | 0.591 |
| Catecholamine (%) | 1.426 | 1.060–1.918 | 0.019 | 1.041 | 0.706–1.534 | 0.838 |
| NPPV (%) | 1.198 | 0.861–1.665 | 0.283 | |||
| At discharge | ||||||
| Hospital stay (days) | 1.009 | 1.003–1.015 | 0.002 | 1.003 | 0.995–1.010 | 0.507 |
| No drugs | 0.735 | 0.653–0.827 | <0.001 | 0.929 | 0.403–1.121 | 0.445 |
| GDMT score ≧5 | 0.432 | 0.324–0.576 | <0.001 | 0.606 | 0.403–0.912 | 0.016 |
| ACEi/ARB (%) | 0.817 | 0.612–1.091 | 0.170 | |||
| ARNI (%) | 0.137 | 0.034–0.551 | 0.005 | |||
| BB (%) | 0.692 | 0.513–0.935 | 0.016 | |||
| MRA (%) | 0.561 | 0.436–0.722 | <0.001 | |||
| SGLT2i (%) | 0.707 | 0.485–1.032 | 0.072 | |||
| Loop (%) | 1.240 | 0.944–1.627 | 0.121 | |||
| Loop dose (mg) | 1.013 | 1.006–1.021 | <0.001 | 1.005 | 0.997–1.012 | 0.190 |
| TLV (%) | 2.193 | 1.690–2.846 | <0.001 | 1.386 | 0.887–2.164 | 0.151 |
| PDEIIIi (%) | 1.406 | 0.959–2.060 | 0.080 | 0.891 | 0.566–1.402 | 0.618 |
| CRT/ICD (%) | 1.664 | 1.053 | 0.029 | 1.263 | 0.738–2.161 | 0.393 |
ACEi, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; Alb, albumin; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; BB, beta‐blocker; BMI, body mass index; BNP, brain natriuretic peptide; BUN, blood urea nitrogen; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; dBP, diastolic blood pressure; DM, diabetes mellitus; EF, ejection fraction; eGFR, estimated glomerular filtration rate; GDMT, guideline‐directed medical therapy; Hb, haemoglobin; HR, heart rate; Hx of HF, history of heart failure; ICD, implantable cardiac defibrillator; ICM, ischaemic cardiomyopathy; K, potassium; MRA, mineralocorticoid receptor antagonist; Na, sodium; NPPV, non‐invasive positive pressure ventilation; NYHA, New York Heart Association; PDEIIIi, phosphodiesterase III inhibitor; sBP, systolic blood pressure; SGLT2i, sodium–glucose transporter 2 inhibitor; TLV, tolvaptan; UA, uric acid.
Table 4.
Association with low simple GDMT score
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P value | Odds ratio | 95% CI | P value | |
| Backgrounds | ||||||
| Age (years) | 1.050 | 1.040–1.060 | <0.001 | 1.030 | 1.020–1.040 | <0.001 |
| Male (%) | 0.604 | 0.465–0.785 | <0.001 | 0.991 | 0.720–1.370 | 0.957 |
| BNP | 1.000 | 1.000–1.000 | 0.057 | 1.000 | 1.000–1.000 | 0.603 |
| NYHA class | 1.200 | 1.000–1.420 | 0.045 | 1.310 | 1.050–1.630 | 0.014 |
| EF (%) | 1.040 | 1.030–1.060 | <0.001 | 1.030 | 1.010–1.050 | <0.001 |
| Hx of HF (%) | 1.270 | 0.971–1.660 | 0.080 | 0.830 | 0.594–1.160 | 0.272 |
| ICM (%) | 1.180 | 0.917–1.510 | 0.200 | |||
| sBP (mmHg) | 0.996 | 0.992–1.000 | 0.026 | 0.998 | 0.991–1.010 | 0.595 |
| dBP (mmHg) | 0.988 | 0.983–0.993 | <0.001 | 0.999 | 0.989–1.010 | 0.924 |
| HR (b.p.m.) | 0.993 | 0.988–0.998 | 0.002 | 0.997 | 0.991–1.000 | 0.418 |
| BMI (kg/m2) | 0.929 | 0.905–0.953 | <0.001 | 0.992 | 0.973–1.010 | 0.424 |
| Comorbidity | ||||||
| AF (%) | 1.180 | 0.922–1.520 | 0.186 | |||
| DM (%) | 0.712 | 0.552–0.918 | 0.008 | 0.682 | 0.503–0.924 | 0.013 |
| COPD (%) | 1.150 | 0.703–1.880 | 0.577 | |||
| Pneumonia (%) | 1.330 | 0.917–1.940 | 0.132 | |||
| Laboratory data | ||||||
| BUN (mg/dL) | 1.040 | 1.030–1.040 | <0.001 | 1.010 | 0.999–1.030 | 0.071 |
| eGFR (mL/min/1.73 m2) | 0.977 | 0.971–0.982 | <0.001 | 0.992 | 0.983–1.000 | 0.092 |
| UA (mg/dL) | 1.010 | 0.950–1.060 | 0.863 | |||
| Na (mmol/L) | 0.974 | 0.947–1.000 | 0.060 | 1.000 | 0.969–1.040 | 0.900 |
| K (mmol/L) | 1.530 | 1.270–1.850 | <0.001 | 1.480 | 1.170–1.870 | 0.001 |
| Hb (g/dL) | 0.795 | 0.753–0.839 | <0.001 | 0.927 | 0.860–0.999 | 0.047 |
| Alb (g/dL) | 0.664 | 0.529–0.835 | <0.001 | 0.841 | 0.628–1.130 | 0.245 |
| In‐hospital use | ||||||
| Vasodilator (%) | 0.693 | 0.520–0.924 | 0.012 | 0.702 | 0.483–1.020 | 0.063 |
| Carperitide | 0.941 | 0.667–1.330 | 0.729 | |||
| In‐hospital loop diuretics (%) | 0.978 | 0.742–1.290 | 0.874 | |||
| In‐hospital TLV (%) | 1.080 | 0.824–1.420 | 0.575 | |||
| Catecholamine (%) | 1.070 | 0.778–1.460 | 0.690 | |||
| NPPV (%) | 0.932 | 0.669–1.300 | 0.679 | |||
| At discharge | ||||||
| Hospital stay (days) | 1.000 | 0.993–1.010 | 0.835 | |||
| Loop (%) | 1.110 | 0.859–1.430 | 0.427 | |||
| Loop dose (mg) | 1.000 | 0.995–1.010 | 0.365 | |||
| TLV (%) | 1.320 | 0.984–1.770 | 0.063 | 0.983 | 0.682–1.420 | 0.927 |
| PDEIIIi (%) | 0.835 | 0.556–1.260 | 0.388 | |||
| CRT/ICD (%) | 0.812 | 0.480–1.370 | 0.438 | |||
AF, atrial fibrillation; Alb, albumin; BMI, body mass index; BNP, brain natriuretic peptide; BUN, blood urea nitrogen; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; dBP, diastolic blood pressure; DM, diabetes mellitus; EF, ejection fraction; eGFR, estimated glomerular filtration rate; GDMT, guideline‐directed medical therapy; Hb, haemoglobin; HR, heart rate; Hx of HF, history of heart failure; ICD, implantable cardiac defibrillator; ICM, ischaemic cardiomyopathy; K, potassium; Na, sodium; NPPV, non‐invasive positive pressure ventilation; NYHA, New York Heart Association; PDEIIIi, phosphodiesterase III inhibitor; sBP, systolic blood pressure; TLV, tolvaptan; UA, uric acid.
Unless otherwise specified, all data are expressed as the mean ± standard deviation or median [95% confidence interval (CI)]. The probability was two‐tailed, with P values of <0.05 considered as statistically significant.
Ethical standards
This study was approved by the Ethics Committee of Japanese Red Cross Fukuoka Hospital (Approval No. 404) and was conducted in accordance with the ethical standards of the 1964 Declaration of Helsinki and its subsequent amendments. Informed consent for data handling was obtained at admission, and informed consent for the study was obtained opt‐out.
Results
Characteristics of study patients
The analysis included 1054 patients with HFrEF and HFmrEF among 1782 HF patients admitted between April 2015 and March 2022. The mean age was 75.2 ± 14.0 years, 65.3% were male, 80.8% were in New York Heart Association (NYHA) Classes 3–4, mean ejection fraction (EF) was 30.9 ± 10.3%, 74.6% had HFrEF, and 30.3% had a history of HF. RAS inhibitors were prescribed at discharge in 82.3% of patients, ARNIs in 5.7% of patients, BBs in 82.3% of patients, MRAs in 58.1% of patients, and SGLT2 inhibitors in 17.8% of patients (Table 1 ).
Relationship between clinical outcomes and guideline‐directed medical therapy score
One‐year events included composite outcome in 243 patients (23.0%), HF readmission in 197 patients (18.6%), and all‐cause death in 70 patients (6.6%). First, a simple GDMT score was calculated for each of these patients. The content of the prescription for each score is shown in Supporting Information, Table S1 . The cut‐off value for the association between the composite outcome and the simple GDMT score was 4 points [area under curve (AUC) = 0.607, 95% CI = 0.57–0.64] using the receiver operating characteristic (ROC) curve (Figure 2 B ).
Univariate and multivariate analyses were performed for each clinical parameter. In a multivariate analysis, older age [cut‐off: 80 years, hazard ratio (HR) = 1.039, 95% CI = 1.010–1.067, P = 0.001], chronic obstructive pulmonary disease (COPD) (HR = 2.205, 95% CI = 1.010–3.810, P = 0.047), potassium (K) values (cut‐off: 4.87, HR = 1.535, 95% CI = 1.127–2.091, P = 0.006), albumin (Alb) value (cut‐off: 3.4 g/dL, HR = 0.474, 95% CI = 0.300–0.748, P = 0.001), hospital stay (cut‐off: 20 days, HR = 1.012, 95% CI = 1.002–1.022, P = 0.015), and GDMT score ≥5 (HR = 0.250, 95% CI = 0.093–0.669, P = 0.005) were strongly associated with all‐cause mortality (Supporting Information, Table S2A ). Older age (cut‐off: 64 years, HR = 1.036, 95% CI = 1.009–1.064, P = 0.009), higher heart rate (cut‐off: 83 b.p.m., HR = 1.011, 95% CI = 1.001–1.021, P = 0.038), higher blood urea nitrogen (BUN) (cut‐off: 28.5 mg/dL, HR = 1.016, 95% CI = 1.000–1.032, P = 0.047), and GDMT score ≥5 (HR = 0.207, 95% CI = 0.077–0.558, P = 0.001) were strongly associated with HF readmission (Supporting Information, Table S2B ). Older age (cut‐off: 64 years, HR = 1.014, 95% CI = 1.001–1.027, P = 0.031) and GDMT score ≥5 (HR = 0.606, 95% CI = 0.403–0.912, P = 0.016) were strongly associated with the composite outcome.
Characteristics between groups with high and low guideline‐directed medical therapy scores
The low‐score group was older, more female, and had higher BNP, NYHA class, and EF than the high‐score group. Blood pressure, heart rate, and body mass index (BMI) were lower in the low‐score group. In terms of comorbidities, diabetes mellitus (DM) was less common and laboratory data showed higher BUN, lower estimated glomerular filtration rate (eGFR), higher K, lower haemoglobin (Hb), and lower Alb in the low‐score group. In addition, a lower percentage of vasodilators were used in the inpatient setting, but otherwise there were no differences (Table 3 ).
Table 3.
Characteristics between low and high simple GDMT scores
| Low GDMT score | High GDMT score | P value | |
|---|---|---|---|
| n | 605 | 449 | |
| Backgrounds | |||
| Age (years) | 78.8 ± 12.2 | 70.3 ± 14.8 | <0.001 |
| Male (%) | 366 (60.5) | 322 (71.7) | <0.001 |
| BNP | 1152.2 ± 1045.9 | 1016.9 ± 1167.9 | <0.001 |
| NYHA class | 3.1 ± 0.7 | 3.0 ± 0.7 | 0.045 |
| NYHA 1–2 (%) | 105 (17.4) | 98 (21.8) | 0.070 |
| NYHA 3 (%) | 326 (53.9) | 243 (54.1) | 0.950 |
| NYHA 4 (%) | 174 (28.8) | 108 (24.1) | 0.092 |
| EF (%) | 32.8 ± 10.0 | 28.4 ± 10.0 | <0.001 |
| HFrEF (%) | 417 (68.9) | 367 (81.7) | <0.001 |
| HFmrEF (%) | 185 (30.6) | 83 (18.5) | <0.001 |
| Hx of HF (%) | 196 (32.4) | 123 (27.4) | 0.090 |
| ICM (%) | 250 (41.3) | 168 (37.4) | 0.204 |
| sBP (mmHg) | 137.4 ± 33.6 | 142.2 ± 35.4 | 0.026 |
| dBP (mmHg) | 81.2 ± 22.1 | 88.1 ± 26.2 | <0.001 |
| HR (b.p.m.) | 92.6 ± 26.6 | 97.6 ± 26.1 | 0.002 |
| BMI (kg/m2) | 22.1 ± 4.0 | 24.9 ± 20.5 | <0.001 |
| Comorbidity | |||
| AF (%) | 259 (42.8) | 174 (38.8) | 0.205 |
| DM (%) | 194 (32.1) | 179 (39.9) | 0.009 |
| COPD (%) | 43 (7.1) | 28 (6.2) | 0.620 |
| Pneumonia (%) | 85 (14.0) | 49 (10.9) | 0.136 |
| Laboratory data | |||
| BUN (mg/dL) | 33.0 ± 18.8 | 24.3 ± 14.0 | <0.001 |
| Cr (mg/dL) | 1.74 ± 1.41 | 1.29 ± 0.89 | <0.001 |
| eGFR (mL/min/1.73 m2) | 40.2 ± 21.6 | 51.4 ± 21.5 | <0.001 |
| UA (mg/dL) | 6.9 ± 2.1 | 6.9 ± 2.3 | 0.863 |
| Na (mmol/L) | 138.8 ± 4.7 | 139.3 ± 4.0 | 0.049 |
| K (mmol/L) | 4.46 ± 0.72 | 4.25 ± 0.73 | <0.001 |
| Hb (g/dL) | 11.7 ± 2.4 | 13.1 ± 2.4 | <0.001 |
| Alb (g/dL) | 3.4 ± 0.5 | 3.5 ± 0.5 | <0.001 |
| In‐hospital use | |||
| Vasodilator (%) | 123 (20.4) | 121 (26.9) | 0.015 |
| Carperitide | 87 (14.4) | 68 (15.1) | 0.726 |
| In‐hospital loop diuretics (%) | 442 (73.1) | 330 (73.5) | 0.888 |
| In‐hospital TLV (%) | 178 (29.4) | 125 (27.8) | 0.583 |
| Catecholamine (%) | 115 (19.0) | 81 (18.0) | 0.749 |
| NPPV (%) | 94 (15.5) | 74 (16.5) | 0.734 |
| At discharge | |||
| Hospital stay (days) | 21.8 ± 15.7 | 21.6 ± 17.2 | 0.835 |
| No drugs | 1.75 ± 0.77 | 3.28 ± 0.46 | <0.001 |
| Loop (%) | 397 (65.6) | 284 (63.3) | 0.435 |
| Loop dose (mg) | 25.7 ± 16.4 | 24.5 ± 17.2 | 0.364 |
| TLV (%) | 155 (25.6) | 93 (20.7) | 0.067 |
| PDEIIIi (%) | 55 (9.1) | 48 (10.7) | 0.403 |
| ICD/CRT (%) | 31 (5.1) | 28 (6.2) | 0.498 |
AF, atrial fibrillation; Alb, albumin; BMI, body mass index; BNP, brain natriuretic peptide; BUN, blood urea nitrogen; COPD, chronic obstructive pulmonary disease; Cr, creatinine; dBP, diastolic blood pressure; DM, diabetes mellitus; EF, ejection fraction; eGFR, estimated glomerular filtration rate; GDMT, guideline‐directed medical therapy; Hb, haemoglobin; HFmrEF, heart failure with mildly reduced ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, heart rate; Hx of HF, history of heart failure; ICD, implantable cardiac defibrillator; ICM, ischaemic cardiomyopathy; K, potassium; Na, sodium; NPPV, non‐invasive positive pressure ventilation; NYHA, New York Heart Association; PDEIIIi, phosphodiesterase III inhibitor; sBP, systolic blood pressure; TLV, tolvaptan; UA, uric acid.
Incidence of all‐cause death, HF readmission, and composite outcome between groups with high and low guideline‐directed medical therapy scores
The group with higher GDMT score had fewer events for all‐cause death (HR = 0.241, 95% CI = 0.102–0.568, P = 0.001), HF readmission (HR = 0.476, 95% CI = 0.324–0.701, P < 0.001), and composite outcome (HR = 0.431, 95% CI = 0.295–0.630, P < 0.001) (Figure 3 ). Stratification analysis of low and high GDMT scores into 0–2, 3–4, 5–6, and 7–9 points showed no significant differences in the occurrence of each event within the low‐ and high‐score groups (Figure 4 ).
Figure 3.
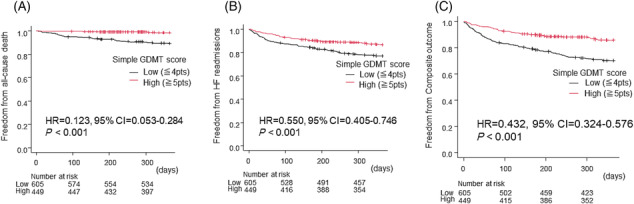
Survival curves of freedom from (A) 1 year all‐cause death, (B) 1 year heart failure (HF) readmissions, and (C) 1 year composite outcome between the groups with low and high simple guideline‐directed medical therapy (GDMT) scores by a Kaplan–Meier analysis. Hazard ratio (HR) and 95% confidence interval (CI) were analysed by Cox proportional hazards regression analysis.
Figure 4.
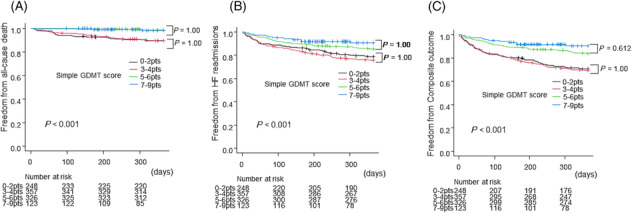
Survival curves of freedom from (A) 1 year all‐cause death, (B) 1 year heart failure (HF) readmissions, and (C) 1 year composite outcome between the groups with simple guideline‐directed medical therapy (GDMT) scores of 0–2, 3–4, 5–6, and 7–9 points by a Kaplan–Meier analysis. Hazard ratio (HR) and 95% confidence interval (CI) were analysed by Cox proportional hazards regression analysis.
Subgroup analysis
Subgroup analyses were performed for the composite outcome by age, gender, NYHA class, systolic blood pressure (sBP), EF, underlying disease, history of HF, presence of DM, eGFR, K level, Hb level, and ARNI period (defined based on when ARNI became available in Japan; pre‐ARNI: from April 2015 to August 2020, post‐ARNI: from November 2020 to March 2022). In these subgroups, a simple GDMT score of 5 or higher was consistently associated with a good prognosis (Figure 5 ).
Figure 5.
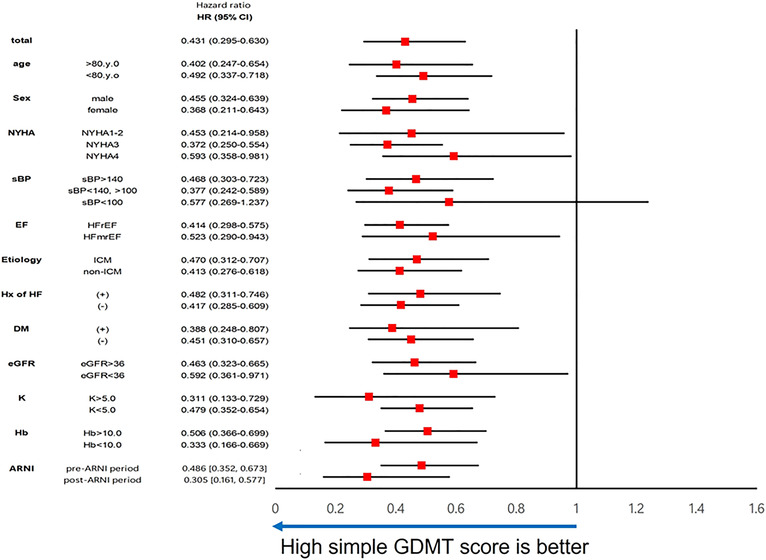
Subgroup analysis between patients with the high simple guideline‐directed medical therapy (GDMT) score and low score by Cox proportional hazards model presented by a forest plot. ARNI, angiotensin receptor neprilysin inhibitor; CI, confidence interval; DM, diabetes mellitus; EF, ejection fraction; eGFR, estimated glomerular filtration rate; Hb, haemoglobin; HFmrEF, heart failure with mildly reduced ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, hazard ratio; Hx of HF, history of heart failure; ICM, ischaemic cardiomyopathy; K, potassium; NYHA, New York Heart Association class; sBP, systolic blood pressure.
Association with low guideline‐directed medical therapy score
We also examined the association with low GDMT score of 4 or less.
A multivariate analysis showed that older age, higher NYHA class, higher EF, no DM, hyperkalaemia, and anaemia were associated with the GDMT score of 4 or less (Table 4 ).
Discussion
The present study is the first to report that a simple GDMT score calculated on the basis of RAS inhibitors, BBs, MRAs, and SGLT2 inhibitors is associated with the prognosis of HF patients. A sub‐analysis of DAPA‐HF using a similar scoring has recently been reported regarding on the use of SGLT2 inhibitors. 20 This study separated backgrounds according to their scores and reported that the effect of dapagliflozin was constant regardless of the score. A study of Danish nationwide registries reported that higher GDMT scores were associated with improved mortality in patients with HF, similar to the results of the present study. 21 The four drugs used for scoring in this study are RAS inhibitors, BB, MRA, and ivabradine, and did not include SGLT2 inhibitors, which is the current standard therapy for HFrEF. However, this study did not examine HF rehospitalization, another important clinical outcome. Therefore, the present study is the first to examine the prognosis of patients with acute HF regarding the medicated status of the current standard therapy using a scoring system. Subgroup analyses showed that 5 or more scoring points were associated with better prognosis in various settings of the patient subgroups.
As the use of vericiguat and ivabradine was extremely low in this study, a score based on the four standard drugs was developed. The prognostic value of combination therapy, including the fantastic 4, has been reported in many studies, and there is no doubt of its benefit, with Class I guidelines recommending the use of four drugs whenever possible. Therefore, it seems highly appropriate to create a simple score based on the four standard drugs in the present study. Ivabradine and vericiguat have also been shown to improve prognosis in patients with HFrEF. 22 , 23 Further analysis using a new scoring system with the addition of these two drugs will be necessary in the future.
HF readmission and mortality rates in representative registries of acute HF patients have been reported as follows: ESC‐HF‐LT (2011–2015) 24 —25.9% HF readmission rate and 14.3% mortality rate, JROADHF (2013) 25 —29.4% HF readmission rate and 14.2% mortality rate, and REALITY‐AHF (2014–2015) 26 —22.9% HF readmission rate and 14.8% mortality rate. Compared with these results, the HF readmission and mortality rates in our study were lower. However, it is important to consider that our study included a period when ARNIs and SGLT2 inhibitors were available for use and excluded patients with HFpEF and dialysis patients. These factors may have contributed to the observed differences. The present study analysed data from 2015 to 2022, and when subgroup analyses were performed for the pre‐ and post‐ARNI periods, the benefit of a simple GDMT score of 5 or higher remained the same in both periods. Acute HF patients are at high risk of readmission and mortality, and repeated readmissions are associated with a worse prognosis. 27 Preventing the HF readmissions is therefore an important challenge. In our study, the HF readmission rate and mortality rate in the group with a score of 4 or less were similar to previous reports, at around 22.8% and 10.6%, respectively. However, in the group with a score of 5 or above, the HF readmission rate decreased to about 13.1% and the mortality rate decreased to 1.3%. This highlights the importance of implementing GDMT during hospitalization.
It has been reported that GDMTs have not yet been fully implemented in practice, and we often find ourselves unable to implement them for a variety of reasons. Therefore, we undertook this study to confirm the usefulness of the proposed GDMT score by adapting it to actual clinical practice and to help improve the uptake of GDMT in the future. It was shown that if a simple GDMT score of 5 or more could be achieved in this study, the prognosis could be improved. Therefore, even in patients who cannot be fully introduced to all four drugs, aiming for a score of 5 or higher by designing combinations may lead to an improved prognosis for HF patients. For example, in the case of a patient with low blood pressure, it appears that a combination of SGLT2 inbibitors+MRA+low dose BB is beneficial for achieving an improvement of 5 points or more, while the presence of bradycardia, a combination of ARNI+SGLT2 inhibitors+MRA seems to be effective.
In addition to hypotension and bradycardia as described above, other possible barriers to GDMT induction include older age, chronic kidney disease (CKD), and hyperkalaemia. In fact, older age, higher NYHA class, higher EF, absence of DM, hyperkalaemia, and anaemia were strongly associated with lower GDMT scores in this study. Further subgroup analysis showed a consistent prognostic benefit of a GDMT score of 5 or higher, regardless of renal function, K level, or presence of anaemia.
Of these factors that interfere with GDMT induction, hyperkalaemia and anaemia are factors that can be corrected by our intervention. For example, hyperkalaemia is a known factor that prevents the introduction of renin–angiotensin–aldosterone system (RAAS) inhibitors as much as possible. However, the American Heart Association (AHA) guidelines recommend that RAAS inhibitors should be continued for as long as possible with pottasium‐lowering agents and so forth, rather than discontinued in HF patients with hyperkalaemia. 6 , 7 Therefore, in the future, it may be necessary to aim for at least 5 points on the GDMT score whenever possible, with vigorous intervention on those factors that can be corrected.
The major limitation of the present study is its retrospective, observational nature. The second limitation is the low use of ARNIs and SGLT2 inhibitors (5.7% and 17.8%, respectively) and the inclusion of a time bias, as these drugs were not available at the time of the study. The third limitation is that the decision to prescribe depends on the judgement of the attending physician, and selection bias cannot be excluded. Prospective studies using such GDMT scores should be conducted. The fourth limitation is that HFpEF and dialysis patients were excluded in the present study. It should also be noted that our simple GDMT score, which has a smaller number of components compared with components proposed by the Heart Failure Collaboratory and the Academic Research Consortium, may have a different impact for each component. Consequently, a 1‐point increase in our simple GDMT score may not directly correspond to the same change as outlined in the original. We acknowledge this limitation and emphasize that the original score was developed based on cut‐off values calculated using ROC curves to compare prognosis between the two groups. This discrepancy in the number of components warrants caution in interpreting the magnitude of change represented by a 1‐point increase in our simple GDMT score.
Conclusions
A high simple GDMT score was associated with better prognosis in HFrEF and HFmrEF patients. In future GDMT introduction strategies for patients with HFrEF and HFmrEF, if, for some reason, all four drugs cannot be introduced, targeting for a combination with a simple GDMT score of 5 or higher may help improve patient prognosis.
Conflict of interest
R.M. received lecture fees from Otsuka Pharmaceutical, AstraZeneca, Novartis Japan, Ono Pharmaceutical, and Boehringer Ingelheim. The remaining authors have nothing to disclose.
Funding
This research received no grant from any funding agency in the public, commercial, or not‐for‐profit sectors.
Supporting information
Table S1. The GDMT prescription details for each simple GDMT score. ACEi/ARB; angiotensin converting enzyme inhibitor/angiotensin receptor blocker, ARNI; angiotensin receptor neprilysin inhibitor, MRA; mineralocorticoid receptor antagonist, SGLT2i; sodium‐glucose transporter 2 inhibitor.
Table S2A. Association with all‐cause death.
Table S2B. Association with HF readmissions.
Matsukawa, R. , Okahara, A. , Tokutome, M. , Itonaga, J. , Koga, E. , Hara, A. , Kisanuki, H. , Sada, M. , Okabe, K. , Kawai, S. , Ogawa, K. , Matsuura, H. , and Mukai, Y. (2023) A scoring evaluation for the practical introduction of guideline‐directed medical therapy in heart failure patients. ESC Heart Failure, 10: 3352–3363. 10.1002/ehf2.14524.
References
- 1. McMurray JJV, Packer M, Desai AS, Gong MPH, Lefkowitz MP, Rizkala AR, et al. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993‐1004. doi: 10.1056/NEJMoa1409077 [DOI] [PubMed] [Google Scholar]
- 2. Packer M, Anker SD, Anker SD, Butler J, Filippatos G, Pocock SJ, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413‐1424. doi: 10.1056/NEJMoa2022190 [DOI] [PubMed] [Google Scholar]
- 3. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995‐2008. doi: 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 4. Johann B. Heart failure drug treatment: The fantastic four. Eur Heart J 2021;42:681‐683. doi: 10.1093/eurheartj/ehaa1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bhatt AS, Abraham AS, Lindenfeld J, Bristow M, Carson PE, Felker GM, et al. Treatment of HF in an era of multiple therapies: Statement from the HF Collaboratory. JACC Heart Fail 2021;9:1‐12. doi: 10.1016/j.jchf.2020.10.014 [DOI] [PubMed] [Google Scholar]
- 6. Authors/Task Force Members , McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2022;24:4‐131. doi: 10.1002/ejhf.2333 [DOI] [PubMed] [Google Scholar]
- 7. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: Executive summary: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022;145:e876‐e894. doi: 10.1161/CIR.0000000000001062 [DOI] [PubMed] [Google Scholar]
- 8. Tsutsui H, Ide T, Ito H, Kihara Y, Kinugawa K, Kinugawa S, et al. JCS/JHFS 2021 guideline focused update on diagnosis and treatment of acute and chronic heart failure. J Card Fail 2021;27:1404‐1444. doi: 10.1016/j.cardfail.2021.04.023 [DOI] [PubMed] [Google Scholar]
- 9. Vaduganathan M, Claggett BL, Jhund PS, Cunningham JW, Ferreira JP, Zannad F, et al. Estimating lifetime benefits of comprehensive disease‐modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: A comparative analysis of three randomised controlled trials. Lancet 2020;396:121‐128. doi: 10.1016/S0140-6736(20)30748-0 [DOI] [PubMed] [Google Scholar]
- 10. Tromp J, Ouwerkerk W, van Veldhuisen DJ, Hillege HL, Richards AM, van der Meer P, et al. A systematic review and network meta‐analysis of pharmacological treatment of heart failure with reduced ejection fraction. JACC Heart Fail 2022;10:73‐84. doi: 10.1016/j.jchf.2021.09.004 [DOI] [PubMed] [Google Scholar]
- 11. Greene SJ, Butler J, Albert NM, DeVore AD, Sharma PP, Duffy CI, et al. Medical therapy for heart failure with reduced ejection fraction: The CHAMP‐HF registry. J Am Coll Cardiol 2018;72:351‐366. doi: 10.1016/j.jacc.2018.04.070 [DOI] [PubMed] [Google Scholar]
- 12. Savarese G, Kish T, Vardeny O, Eryd SA, Bodegård J, Lund LH, et al. Heart failure drug treatment—Inertia, titration, and discontinuation: A multinational observational study (EVOLUTION HF). JACC Heart Fail 2023;11:1‐14. doi: 10.1016/j.jchf.2022.08.009 [DOI] [PubMed] [Google Scholar]
- 13. Fiuzat M, Ezekowitz J, Alemayehu W, Westerhout CM, Sbolli M, Cani D, et al. Assessment of limitations to optimization of guideline‐directed medical therapy in heart failure from the GUIDE‐IT trial: A secondary analysis of a randomized clinical trial. JAMA Cardiol 2020;5:757‐764. doi: 10.1001/jamacardio.2020.0640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abraham WT, Psotka MA, Fiuzat M, Filippatos G, Lindenfeld J, Mehran R, et al. Standardized definitions for evaluation of heart failure therapies: Scientific expert panel from the Heart Failure Collaboratory and Academic Research Consortium. JACC Heart Fail 2020;8:961‐972. doi: 10.1016/j.jchf.2020.10.002 [DOI] [PubMed] [Google Scholar]
- 15. DeFilippis EM, Fiuzat M. Putting the “optimal” in optimal medical therapy. JACC Heart Fail 2021;9:39‐41. doi: 10.1016/j.jchf.2020.08.016 [DOI] [PubMed] [Google Scholar]
- 16. Hong KN. The Heart Failure Collaboratory medical therapy score: Quantifying the quality of goal‐directed medical therapy. JACC Heart Fail 2022;10:556‐558. doi: 10.1016/j.jchf.2022.05.001 [DOI] [PubMed] [Google Scholar]
- 17. Fiuzat M, Hamo CE, Butler J, Abraham WT, DeFilippis EM, Fonarow GC, et al. Optimal background pharmacological therapy for heart failure patients in clinical trials: JACC review topic of the week. J Am Coll Cardiol 2022;79:504‐510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: The Framingham study. N Engl J Med 1971;285:1441‐1446. doi: 10.1056/NEJM197112232852601 [DOI] [PubMed] [Google Scholar]
- 19. Kanda Y. Investigation of the freely available easy‐to‐use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013;48:452‐458. doi: 10.1038/bmt.2012.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Butt JH, Dewan P, DeFilippis EM, Biering‐Sørensen T, Docherty KF, Jhund PS, et al. Effects of dapagliflozin according to the Heart Failure Collaboratory medical therapy score: Insights from DAPA‐HF. JACC Heart Fail 2022;10:543‐555. doi: 10.1016/j.jchf.2022.03.009 [DOI] [PubMed] [Google Scholar]
- 21. Johansen ND, Vaduganathan M, Zahir D, Fiuzat M, DeFilippis EM, Januzzi JL, et al. A composite score summarizing use and dosing of evidence‐based medical therapies in heart failure: A nationwide cohort study. Circ: Heart Failure 2023;0:e009729. doi: 10.1161/CIRCHEARTFAILURE.122.009729 [DOI] [PubMed] [Google Scholar]
- 22. Swedberg K, Komajda M, Böhm M, Borer JS, Ford I, Dubost‐Brama A, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): A randomised placebo‐controlled study. Lancet 2010;376:875‐885. doi: 10.1016/S0140-6736(10)61198-1 [DOI] [PubMed] [Google Scholar]
- 23. Armstrong PW, Pieske B, Anstrom KJ, Ezekowitz J, Hernandez AF, Butler J, et al. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med 2020;382:1883‐1893. doi: 10.1056/NEJMoa1915928 [DOI] [PubMed] [Google Scholar]
- 24. Crespo‐Leiro MG, Anker SD, Maggioni AP, Coats AJ, Filippatos G, Ruschitzka F, et al. European Society of Cardiology Heart Failure Long‐Term Registry (ESC‐HF‐LT): 1‐year follow‐up outcomes and differences across regions. Eur J Heart Fail 2016;18:613‐625. doi: 10.1002/ejhf.566 [DOI] [PubMed] [Google Scholar]
- 25. Ide T, Kaku H, Matsushima S, Tohyama T, Enzan N, Funakoshi K, et al. Clinical characteristics and outcomes of hospitalized patients with heart failure from the large‐scale Japanese Registry Of Acute Decompensated Heart Failure (JROADHF). Circ J 2021;85:1438‐1450. doi: 10.1253/circj.CJ-20-0947 [DOI] [PubMed] [Google Scholar]
- 26. Kagiyama N, Kitai T, Hayashida A, Yamaguchi T, Okumura T, Kida K, et al. Prognostic value of BNP reduction during hospitalization in patients with acute heart failure. J Card Fail 2019;25:712‐721. doi: 10.1016/j.cardfail.2019.04.004 [DOI] [PubMed] [Google Scholar]
- 27. Setoguchi S, Stevenson LW, Schneeweiss S, Setoguchi S, Stevenson LW, Schneeweiss S. Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J 2007;154:260‐266. doi: 10.1016/j.ahj.2007.01.041 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The GDMT prescription details for each simple GDMT score. ACEi/ARB; angiotensin converting enzyme inhibitor/angiotensin receptor blocker, ARNI; angiotensin receptor neprilysin inhibitor, MRA; mineralocorticoid receptor antagonist, SGLT2i; sodium‐glucose transporter 2 inhibitor.
Table S2A. Association with all‐cause death.
Table S2B. Association with HF readmissions.


